Integration of Transcriptomics and Proteomics Analysis Reveals the Molecular Mechanism of Eriocheir sinensis Gills Exposed to Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Acute Heat Stress Treatment
2.2. Transcriptome Sequencing and Analysis
2.3. Proteome Sequencing and Analysis
2.4. Screening of Genes and Proteins Related to Antioxidation
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
3. Results
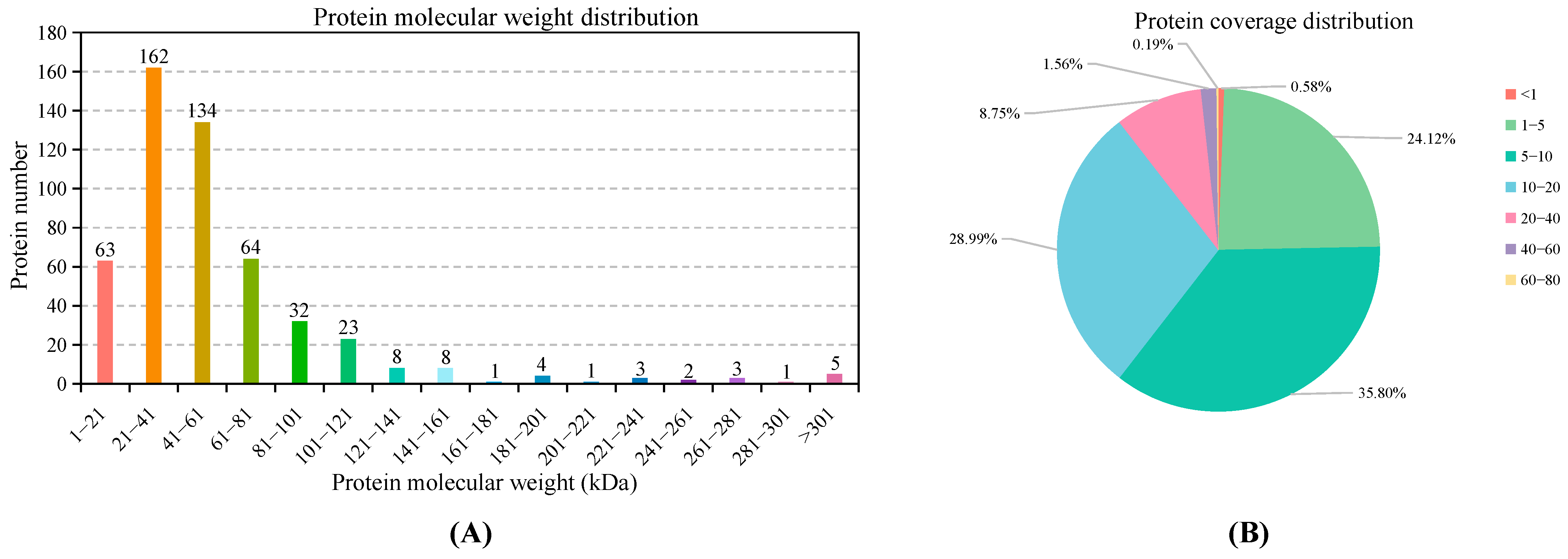
3.1. Overall Statistics for Transcriptomic and Proteomic Sequencing
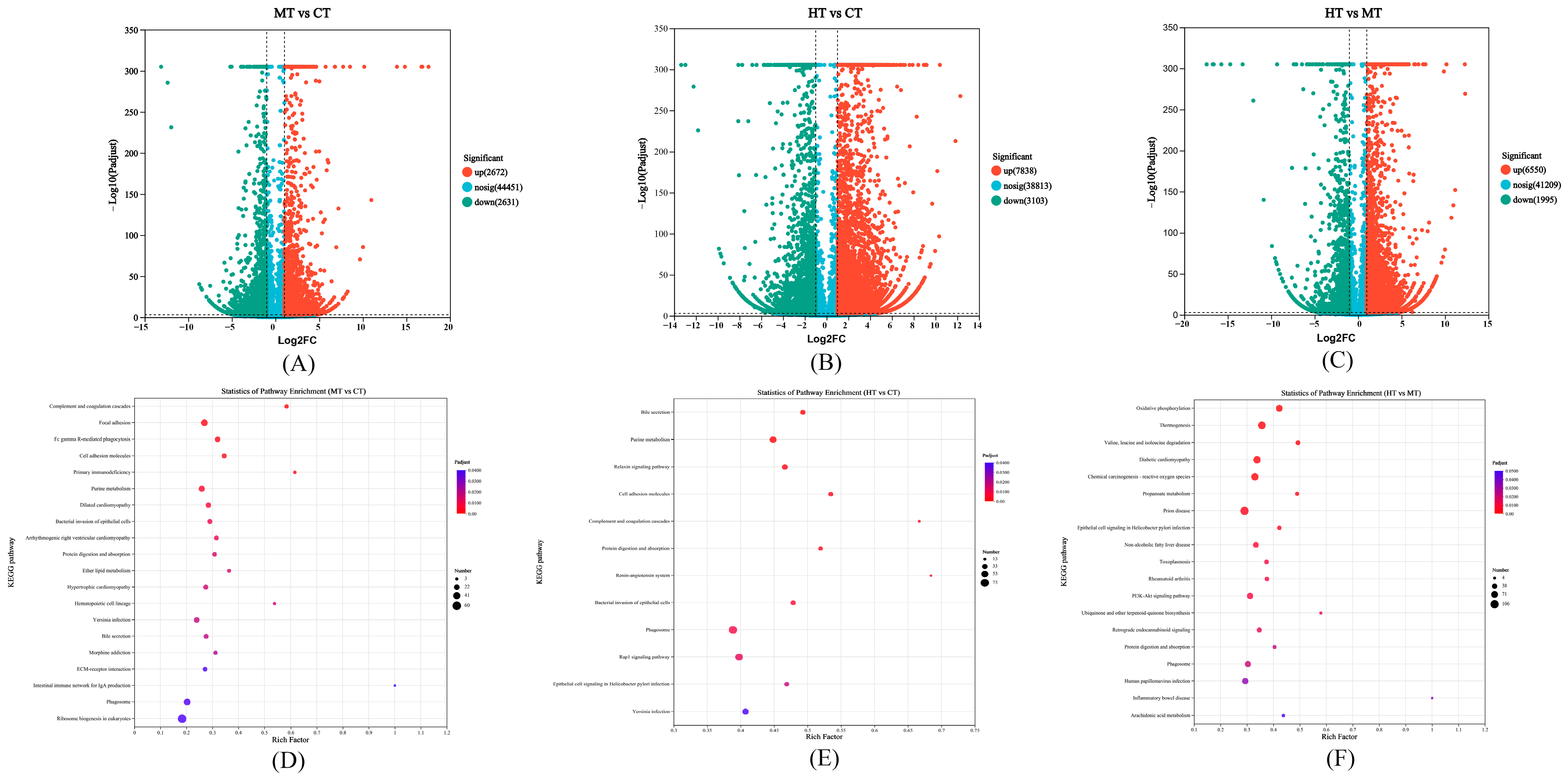
3.2. Comparison and Enrichment Analysis of DEGs
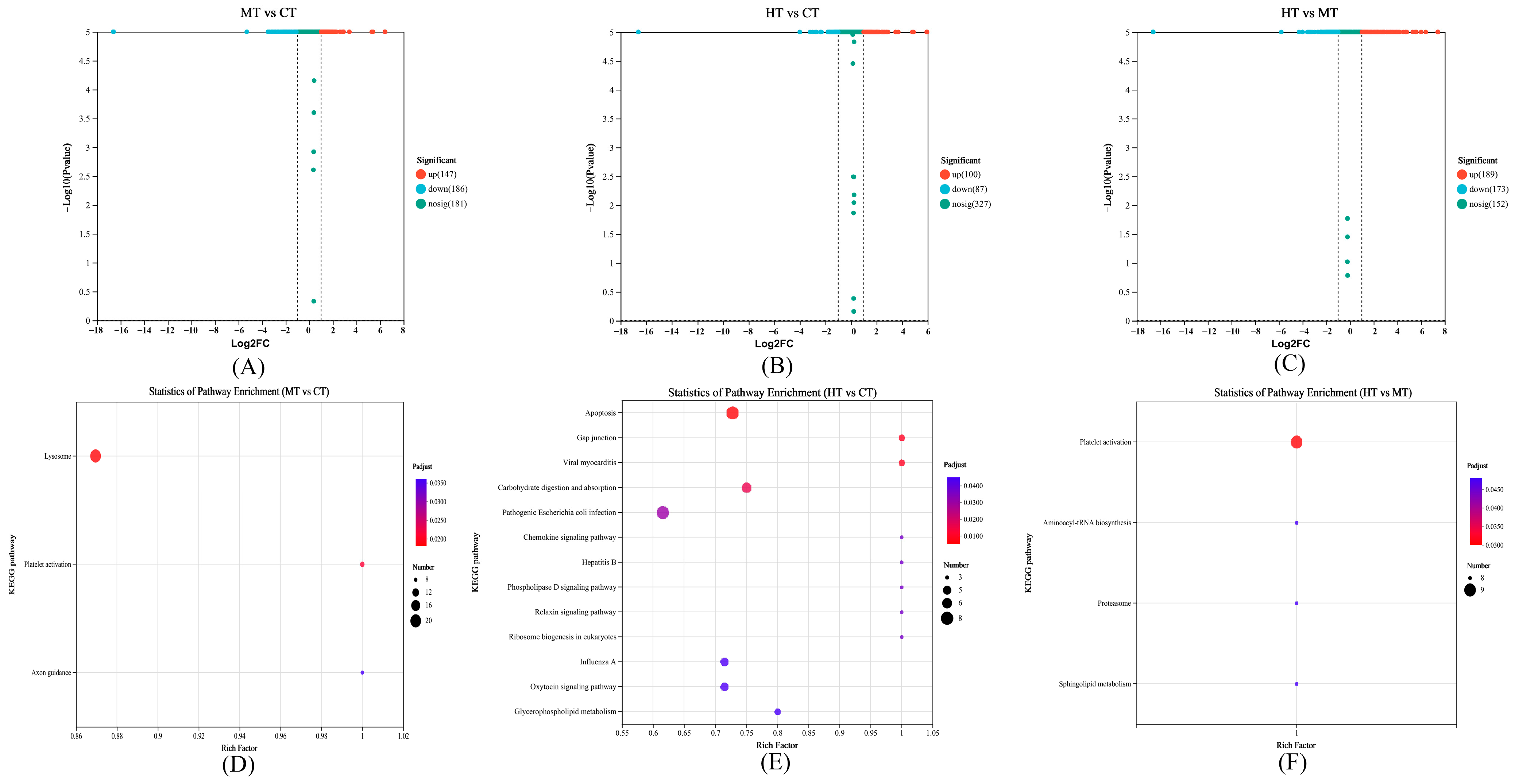
3.3. Comparison and Enrichment Analysis of DEPs
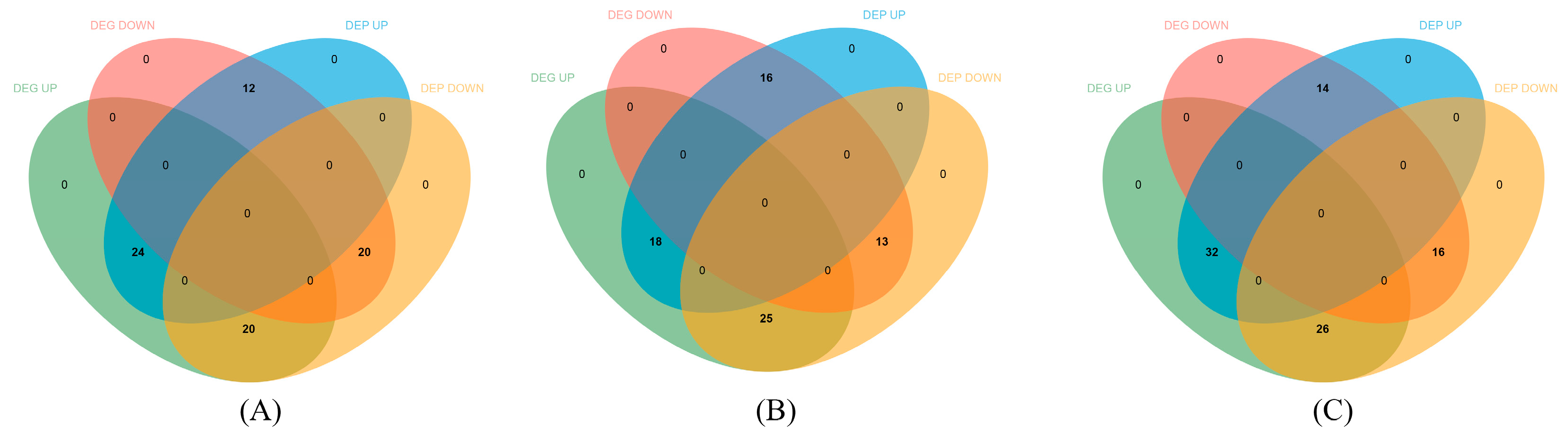
3.4. Integrative Analysis of the Transcriptome and Proteome
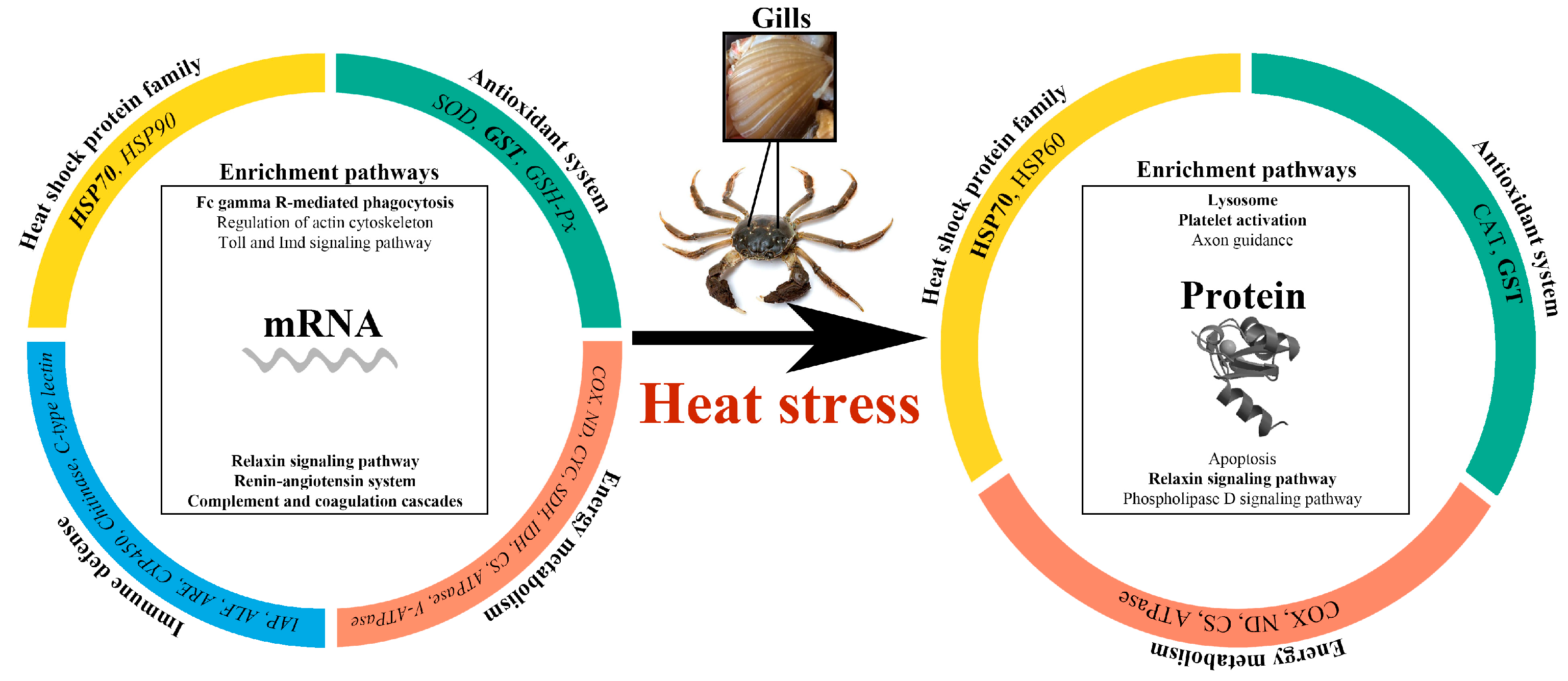
4. Discussion
4.1. The Differences of DEPs and DEGs in the Integrative Analysis
4.2. Co-Expression of HSP70 and GST at Gene and Protein Levels under Heat Stress
4.3. Effective Pathways Involved in Response to Heat Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Barbarossa, V.; Bosmans, J.; Wanders, N.; King, H.; Schipper, A.M. Threats of global warming to the world’s freshwater fishes. Nat. Commun. 2021, 12, 1701. [Google Scholar] [CrossRef]
- Ng’onga, M.; Kalaba, F.K.; Mwitwa, J.; Nyimbiri, B. The interactive effects of rainfall, temperature and water level on fish yield in Lake Bangweulu fishery, Zambia. J. Therm. Biol. 2019, 84, 45–52. [Google Scholar] [CrossRef]
- Roach, T.; Neuner, G.; Kranner, I.; Buchner, O. Heat acclimation under drought stress induces antioxidant enzyme activity in the alpine plant Primula minima. Antioxidants 2023, 12, 1093. [Google Scholar] [CrossRef]
- Sgobba, A.; Paradiso, A.; Dipierro, S.; De Gara, L.; De Pinto, M.C. Changes in antioxidants are critical in determining cell responses to short- and long-term heat stress. Physiol. Plant. 2015, 153, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, A.; Okomoda, V.T.; Iryani, M.T.M.; Andriani, Y.; Wahid, M.E.A.; Tan, M.P.; Danish-Daniel, M.; Wong, L.L.; Tengku-Muhammad, T.S.; Mok, W.J.; et al. Pandanus tectorius fruit extract promotes Hsp70 accumulation, immune-related genes expression and Vibrio parahaemolyticus tolerance in the white-leg shrimp Penaeus vannamei. Fish Shellfish Immunol. 2021, 109, 97–105. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I. A review on the involvement of heat shock proteins (extrinsic chaperones) in response to stress conditions in aquatic organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Staib, J.L.; Quindry, J.C.; French, J.P.; Criswell, D.S.; Powers, S.K. Increased temperature, not cardiac load, activates heat shock transcription factor 1 and heat shock protein 72 expression in the heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, 432–439. [Google Scholar] [CrossRef]
- Nitzan, T.; Kokou, F.; Doron-Faigenboim, A.; Slosman, T.; Biran, J.; Mizrahi, I.; Zak, T.; Benet, A.; Cnaani, A. Transcriptome analysis reveals common and differential response to low temperature exposure between tolerant and sensitive blue tilapia (Oreochromis aureus). Front. Genet. 2019, 10, 100. [Google Scholar] [CrossRef]
- Shi, W.; Hu, R.; Wang, P.; Zhao, R.; Shen, H.; Li, H.; Wang, L.; Qiao, Y.; Jiang, G.; Cheng, J.; et al. Transcriptome analysis of acute high temperature-responsive genes and pathways in Palaemon gravieri. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 41, 100958. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.M.S.; Lilagan, C.A.I.; Guiao, J.E.B.; Romana-Eguia, M.R.R.; Lagman, M.C.A. Comparative transcriptome profiling of heat stress response of the mangrove crab Scylla serrata across sites of varying climate profiles. BMC Genom. 2021, 22, 580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Lei, G.; Zhou, H.W.; He, C.; Liao, J.L.; Huang, Y.J. Quantitative iTRAQ-based proteomic analysis of rice grains to assess high night temperature stress. Proteomics 2017, 17, 1600365. [Google Scholar] [CrossRef]
- Becker, D.; Reydelet, Y.; Lopez, J.A.; Jackson, C.; Colbourne, J.K.; Hawat, S.; Hippler, M.; Zeis, B.; Paul, R.J. The transcriptomic and proteomic responses of Daphnia pulex to changes in temperature and food supply comprise environment-specific and clone-specific elements. BMC Genom. 2018, 19, 376. [Google Scholar] [CrossRef]
- Huang, L.; Peng, L.; Yan, X. Multi-omics responses of red algae Pyropia haitanensis to intertidal desiccation during low tides. Algal. Res. 2021, 58, 102376. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, T.; He, Y.; Zhan, W.; Xie, Q.; Lou, B. Integration of transcriptome and proteome analyses reveals the regulation mechanisms of Larimichthys polyactis liver exposed to heat stress. Fish Shellfish Immunol. 2023, 135, 108704. [Google Scholar] [CrossRef]
- Feng, G.; Zhuang, P. Stock enhancement and genetic preservation of Chinese mitten crab (Eriocheir sinensis) in the Yangtze River estuary. In Aquaculture in China, Success Stories and Modern Trends, 2nd ed.; Gui, J., Tang, Q., Li, Z., Liu, J., De Silva, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 6, pp. 514–526. [Google Scholar]
- Herborg, L.M.; Rushton, S.; Clare, A.; Bentley, M.G. The invasion of the Chinese mitten crab (Eriocheir sinensis) in the United Kingdom and its comparison to continental Europe. Biol. Invasions 2005, 7, 959–968. [Google Scholar] [CrossRef]
- Sébert, P.; Simon, B.; Péqueux, A. Effects of hydrostatic pressure on energy metabolism and osmoregulation in crab and fish. Comp. Biochem. Physiol. Part A Physiol. 1997, 116, 281–290. [Google Scholar] [CrossRef]
- Chang, H.K.; Sang, G.H. The complete larval development of the mitten crab Eriocheir sinensis H. Milne Edwards, 1853 (Decapoda, Brachyura, Grapsidae) reared in the laboratory and a key to the known zoeae of the Varuninae. Crustaceana 1995, 68, 793–812. [Google Scholar] [CrossRef]
- Geng, Z. The Environment Adaptability and Conservation Strategy of Eriocheir sinensis in Estuarine Life Stages. Ph.D. Thesis, East China Normal University, Shanghai, China, 2018. (In Chinese). [Google Scholar]
- Zhang, L.S. Study on forecast of fishing season of Chinese mitten-handed crab (Eriocheir sinensis) seeds at the mouth of Yangtse River. Fish. Sci. Technol. Inf. 2002, 2, 56–60. (In Chinese) [Google Scholar]
- Jakubowska, M.; Normant, M. Effect of temperature on the physiology and bioenergetics of adults of the Chinese mitten crab Eriocheir sinensis: Considerations for a species invading cooler waters. Mar. Freshwater Behav. Physiol. 2011, 44, 171–183. [Google Scholar] [CrossRef]
- Lv, F. Effects of Environmental Factors on the Osmoregulation of Eriocheir sinensis. Master’s Thesis, Ocean University of China, Qingdao, China, 2002. (In Chinese). [Google Scholar]
- Burnett, L.E.; McMahon, B.R. Facilitation of CO2 excretion by carbonic anhydrase located on the surface of the basal membrane of crab gill epithelium. Resp. Physiol. 1985, 62, 348. [Google Scholar] [CrossRef]
- Henry, R.P.; Lucu, C.; Onken, H.; Weihrauch, D. Multiple functions of the crustacean gill: Osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front. Physiol. 2012, 3, 431. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, M.; Zhao, X.; Shi, Q. Transcriptome sequencing of the gill and barbel of Southern catfish (Silurus meridionalis) revealed immune responses and novel rhamnose-binding lectins (RBLs). Genomics 2018, 111, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, H.; Tang, L.; Lu, J.; Mu, C.; Wang, C. A proteomics of gills approach to understanding salinity adaptation of Scylla paramamosain. Gene 2018, 677, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Tang, D.; Bai, Y.; Luo, Y.; Wu, L.; Zhang, Y.; Wang, Z. Comparative transcriptome analysis of the gills of Procambarus clarkii provide novel insights into the response mechanism of ammonia stress tolerance. Mol. Biol. Rep. 2021, 48, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, T.; Liang, Z.; Fan, L.; Shen, Y.; Zhou, D. iTRAQ and PRM-based comparative proteomic profiling in gills of white shrimp Litopenaeus vannamei under copper stress. Chemosphere 2021, 263, 128270. [Google Scholar] [CrossRef]
- Zheng, J.; Cao, J.; Mao, Y.; Su, Y.; Wang, J. Comparative transcriptome analysis provides comprehensive insights into the heat stress response of Marsupenaeus japonicu. Aquaculture 2019, 502, 338–346. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Mu, H.; Li, J.; Zhang, Y.; Xu, F.; Xiang, Z.; Qian, P.; Qiu, J.; Yu, Z. Proteomic basis of stress responses in the gills of the pacific oyster Crassostrea gigas. J. Proteome Res. 2015, 14, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.A.; Burmester, E.M.; Cox, K.M.; Karch, K.R. Rapid proteomic responses to a near-lethal heat stress in the salt marsh mussel Geukensia demissa. J. Exp. Biol. 2016, 219, 2673–2686. [Google Scholar]
- Xu, Z.; Takizawa, F.; Parra, D.; Gómez, D.; Jørgensen, L.V.G.; LaPatra, S.E.; Sunyer, J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016, 7, 10728. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.P.; Wang, C.G.; Shi, L.L.; Guo, H.; Chan, S.M. Gill transcriptomes reveal involvement of cytoskeleton remodeling and immune defense in ammonia stress response in the banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 2017, 71, 319. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chen, X.; Qian, L.; Ai, T.; Xu, Q.; Xiang, W.; Hu, B.; Liu, X.; Wang, J.; Wang, C. Integrated transcriptomic and proteomic analysis of the physiological changes of the liver in domesticated Eurasian perch, Perca fluviatilis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 41, 100957. [Google Scholar] [CrossRef]
- Alli Shaik, A.; Wee, S.; Li, R.H.X.; Li, Z.; Carney, T.J.; Mathavan, S.; Gunaratne, J. Functional mapping of the zebrafish early embryo proteome and transcriptome. J. Proteome Res. 2014, 13, 5536–5550. [Google Scholar] [CrossRef]
- Lundberg, E.; Fagerberg, L.; Klevebring, D.; Matic, I.; Geiger, T.; Cox, J.; Älgenäs, C.; Lundeberg, J.; Mann, M.; Uhlen, M. Defining the transcriptome and proteome in three functionally different human cell lines. Mol. Syst. Biol. 2010, 6, 450. [Google Scholar] [CrossRef]
- Nakayama, K.I.; Nakayama, K. Ubiquitin ligases: Cell-cycle control and cancer. Nat. Rev. Cancer 2006, 6, 369–381. [Google Scholar] [CrossRef]
- Le Roch, K.G.; Johnson, J.R.; Florens, L.; Zhou, Y.; Santrosyan, A.; Grainger, M.; Yan, S.F.; Williamson, K.C.; Holder, A.A.; Carucci, D.J.; et al. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 2014, 14, 2308–2318. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Yuan, F.; Wang, B.; Chen, M. Integrative transcriptome and proteome analyses provide deep insights into the molecular mechanism of salt tolerance in Limonium bicolor. Plant Mol. Biol. 2022, 108, 127–143. [Google Scholar] [CrossRef]
- Ahmed, R.G. Heat stress induced histopathology and pathophysiology of the central nervous system. Int. J. Dev. Neurosci. 2005, 23, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Iryani, M.; Lv, A.; Sun, J.; Anirudhan, A.; Tan, M.P.; Danish-Daniel, M.; Wong, L.L.; Baruah, K.; Sorgeloos, P.; et al. Effects of heat shock protein 70 knockdown on the tolerance of the brine shrimp Artemia franciscana to aquaculture-related stressors: Implications for aquatic animal health and production. Aquaculture 2022, 550, 737872. [Google Scholar]
- Huang, W.J.; Xia, L.M.; Zhu, F.; Huang, B.; Zhou, C.; Zhu, H.F.; Wang, B.; Chen, B.; Lei, P.; Shen, G.X. Transcriptional upregulation of HSP70-2 by HIF-1 in cancer cells in response to hypoxia. Int. J. Cancer 2010, 124, 298–305. [Google Scholar] [CrossRef]
- Zhou, J.; Schmid, T.; Frank, R.; Brüne, B. PI3K/Akt is required for heat shock proteins to protect hypoxiainducible factor 1 α from pVHl-independent degradation. J. Biol. Chem. 2004, 279, 13506–13513. [Google Scholar] [CrossRef]
- Cottin, D.; Shillito, B.; Chertemps, T.; Tanguy, A.; Léger, N.; Ravaux, J. Identification of differentially expressed genes in the hydrothermal vent shrimp Rimicaris exoculata exposed to heat stress. Mar. Genom. 2010, 3, 71–78. [Google Scholar] [CrossRef]
- Liu, Z.M.; Zhu, X.L.; Lu, J.; Cai, W.J.; Ye, Y.P.; Lv, Y.P. Effect of high temperature stress on heat shock protein expression and antioxidant enzyme activity of two morphs of the mud crab Scylla paramamosain. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 223, 10–17. [Google Scholar] [CrossRef]
- Rahi, M.L.; Mahmud, S.; Dilruba, K.J.; Sabbir, W.; Aziz, D.; Hurwood, D.A. Temperature induced changes in physiological traits and expression of selected candidate genes in black tiger shrimp (Penaeus monodon) larvae. Aquacult. Rep. 2021, 19, 100620. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, E.; Li, C.; Pan, C.; Zhao, X.; Wang, Y.; Ling, Q. Effects of heat stress on histopathology, antioxidant enzymes, and transcriptomic profiles in gills of pikeperch Sander lucioperca. Aquaculture 2021, 534, 736277. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Li, Y.; Li, D.; Gong, Q.; Huang, X.; Wu, J.; Huang, A.; Kong, F.; Han, X.; et al. The multilevel responses of Acipenser baerii and its hybrids (A. baerii ♀ × A. schrenckii ♂) to chronic heat stress. Aquaculture 2021, 541, 736773. [Google Scholar] [CrossRef]
- Burmester, T. Origin and evolution of arthropod hemocyanins and related proteins. J. Comp. Physiol. B 2002, 172, 95–107. [Google Scholar] [PubMed]
- Burmester, T. Molecular evolution of the arthropod hemecyanin superfamily. Mol. Biol. Evol. 2001, 18, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Z.; Ong, F.S.; Bernstein, E.A.; Janjulia, T.; Blackwell, W.L.B.; Shah, K.H.; Taylor, B.L.; Gonzalez-Villalobos, R.A.; Fuchs, S.; Bernstein, K.E. Nontraditional roles of angiotensin-converting enzyme. Hypertension 2012, 59, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.S.; Wong, T.P.; Lam, S.Y.; Chan, H.C.; Wong, P.Y.D. Testicular hormonal regulation of the renin-angiotensin system in the rat epididymis. Life Sci. 2000, 66, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yan, R.; Zhou, K.; Li, X.; Zhang, Y.; Liu, C.; Jiang, M.; Ye, H.; Meng, X.; Pang, N.; et al. Akt-mediated platelet apoptosis and its therapeutic implications in immune thrombocytopenia. Proc. Natl. Acad. Sci. USA 2018, 115, 10682–10691. [Google Scholar] [CrossRef]
- Isenberg, W.M.; Mcever, R.P.; Phillips, D.R.; Shuman, M.A.; Bainton, D.F. The platelet fibrinogen receptor: An immunogold-surface replica study of agonist-induced ligand binding and receptor clustering. J. Cell Biol. 1987, 104, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Hyrsl, P.; Dobes, P.; Wang, Z.; Hauling, T.; Wilhelmsson, C.; Theopold, U. Clotting factors and eicosanoids protect against nematode infections. J. Innate Immun. 2010, 3, 65–70. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, Z.; Shi, H.; Wang, J.; Huang, J.; Li, Y.; Li, J.; Wang, Y. Label-free quantification of protein expression in the rainbow trout (Oncorhynchus mykiss) in response to short-term exposure to heat stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Rebl, A.; Verleih, M.; Köbis, J.M.; Kühn, C.; Wimmers, K.; Köllner, B.; Goldammer, T. Transcriptome profiling of gill tissue in regionally bred and globally farmed rainbow trout strains reveals different strategies for coping with thermal stress. Mar. Biotechnol. 2013, 15, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.J.; Kim, A.; Kim, N.; Lee, Y.; Kim, D.H. Multi-omics analysis provides novel insight into immuno-physiological pathways and development of thermal resistance in rainbow trout exposed to acute thermal stress. Int. J. Mol. Sci. 2020, 21, 9198. [Google Scholar] [CrossRef]
- Fibach, E.; Dana, M. Oxidative stress in paroxysmal nocturnal hemoglobinuria and other conditions of complement-mediated hemolysis. Free Radic. Biol. Med. 2015, 88, 63–69. [Google Scholar] [CrossRef]
- Luo, L.; Huang, J.H.; Liu, D.L.; Jiang, S.G.; Zhou, F.L.; Jiang, S.; Yang, Q.B.; Li, Y.D.; Li, T.; Tan, L.Q.; et al. Transcriptome reveals the important role of metabolic imbalances, immune disorders and apoptosis in the treatment of Procambarus clarkii at super high temperature. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100781. [Google Scholar] [CrossRef]
- Helip-Wooley, A.; Thoene, J.G. Sucrose-induced vacuolation results in increased expression of cholesterol biosynthesis and lysosomal genes. Exp. Cell Res. 2004, 292, 89–100. [Google Scholar] [CrossRef]
- Bi, W.J.; Li, D.X.; Xu, Y.H.; Xu, S.; Li, J.; Zhao, X.F.; Wang, J.X. Scavenger receptor B protects shrimp from bacteria by enhancing phagocytosis and regulating expression of antimicrobial peptides. Dev. Comp. Immunol. 2015, 51, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, T. Function of FREP and SRB-Croquemort on Immune Response in Litopenaeus vannamei. Master’s Thesis, Northwest A&F University, Xianyang, China, 2016. (In Chinese). [Google Scholar]
- Wu, Y.M. Phagocytosis Function Research of Class B Scavenger Receptor (EsSR-B1) in Hemocytes of Chinese Mitten Crab (Eriocheir sinensis). Master’s Thesis, East China Normal University, Shanghai, China, 2018. (In Chinese). [Google Scholar]
- Zhong, A.H.; Wang, Y.B.; Bi, L.F.; Xu, H.L. The transcriptomic analysis of in vitro hemocytes of blood clam (Tegillarca granosa) challenged with Vibrio alginolyticus. Oceanol. Limnol. Sin. 2023, 54, 286–295. (In Chinese) [Google Scholar]
- Perna, A.M.; Masini, E.; Nistri, S.; Briganti, V.; Chiappini, L.; Stefano, P.; Bigazzi, M.; Pieroni, C.; Sacchi, T.B.; Bani, D. Novel drug development opportunity for relaxin in acute myocardial infarction: Evidences from as wine model. FASEB J. 2005, 19, 1525–1527. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, B.; Zhao, L.; Amanguli, R.; Zhao, B.; Jiang, T.; Zhang, C.; Li, Z.; Duan, M.; Gao, X. Serelaxin protects against hypoxia/reoxygenation-induced mouse cardiac microvascular endothelialcell injury by inhibiting oxidative stress responses. Chin. J. Tissue Eng. Res. 2023, 27, 3621–3627. (In Chinese) [Google Scholar]
- Bathgate, R.A.D.; Halls, M.L.; van der Westhuizen, E.T.; Callander, G.E.; Kocan, M.; Summers, R.J. Relaxin family peptides and their receptors. Physiol. Rev. 2013, 93, 405. [Google Scholar] [CrossRef] [PubMed]
- Nistri, S.; Fiorillo, C.; Becatti, M.; Dani, D. Human relaxin-2 (serelaxin) attenuates oxidative stress in cardiac muscle cells exposed in vitro to hypoxia-reoxygenation. Evidence for the involvement of reduced glutathione up-regulation. Antioxidants 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]

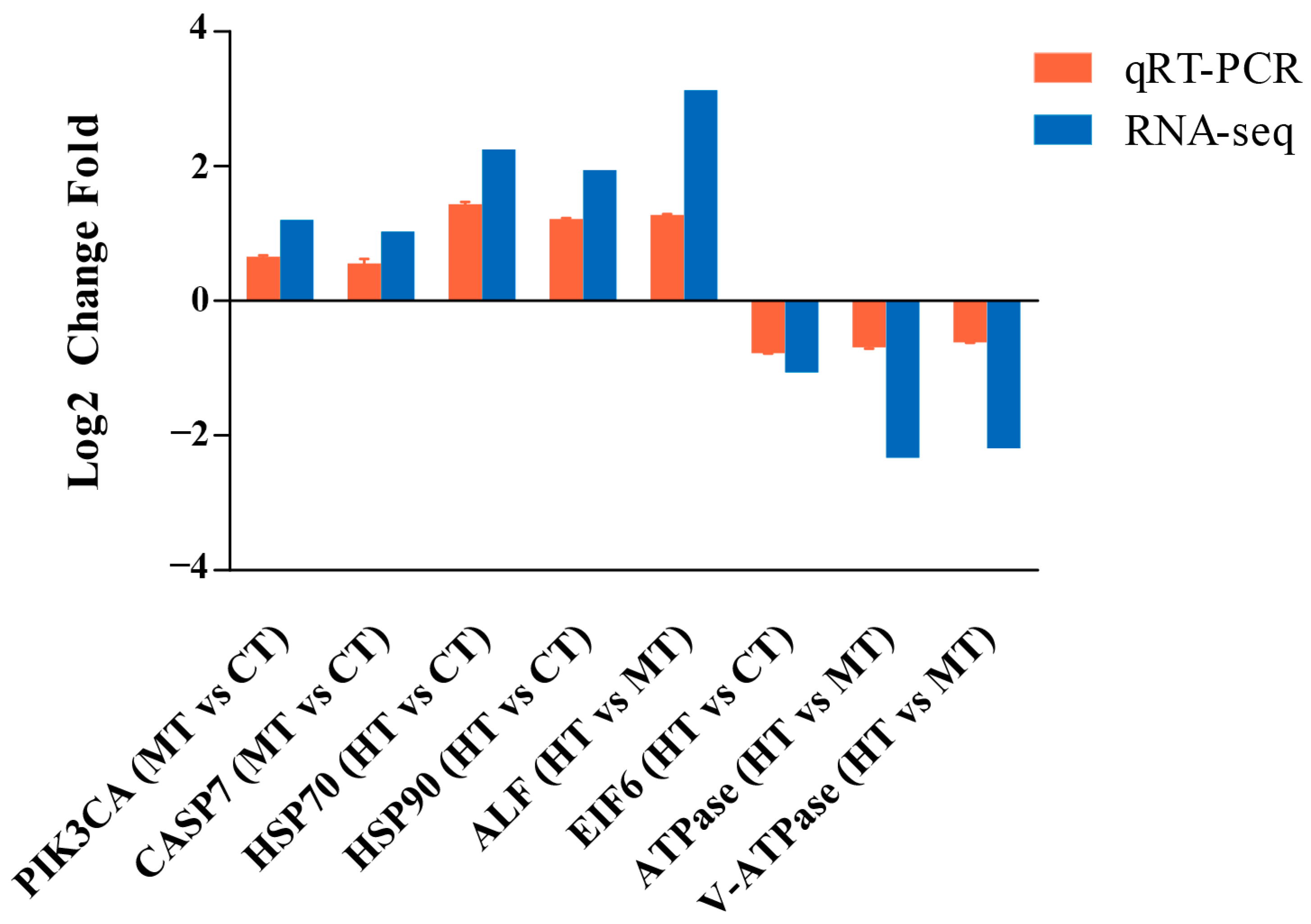
| Gene Name | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| β-actin | GGCATCCACGAGACCACTT | CTCCTGCTTGCTGATCCACAT |
| PIK3CA | GCTCAGCATAGGCATAAT | AGGAGATACGCCAAATAG |
| CASP7 | GATGCTGGAGGACAGGGAT | TAGCGATGGTTGAAGATG |
| HSP70 | CCAGCGTCAAGCAACCAA | TCAACTTCACGGCGGAGC |
| HSP90 | AGGTCGTCCGAGTCCACA | GTCGCTGCTCTTCATTCC |
| EIF6 | ATCCACGACACCCTACAAG | AGCCCAGTCATTCACTAACA |
| ATPase | CGCCCATCCTCAACTCCT | CTCCACCAAACAGACCAATC |
| V-ATPase | CCAGAACCAGCGTTACCA | CTCCTCTACCCTCAGCATC |
| ALF | TAGTAGTTCCTGGCTGTTCCC | CCTCTATCGGCTGGTTCTG |
| Functional Group/Gene | Description | Regulation | MT vs. CT | HT vs. CT | HT vs. MT |
|---|---|---|---|---|---|
| Heat shock protein family | |||||
| HSP 70 | Heat shock protein 70 | Up | X | X | X |
| HSP 90 | Heat shock protein 90 | Up | X | X | X |
| Antioxidant system | |||||
| SOD | Copper/zinc superoxide dismutase | Up | X | X | X |
| GST | Glutathione S-transferase | Up | X | X | X |
| GSH-Px | Glutathione peroxidase 3 | Up | X | ||
| Energy metabolism | |||||
| COX | Cytochrome c oxidase subunit | Up | X | ||
| Down | X | X | |||
| ND | NADH dehydrogenase subunit | Up | X | ||
| Down | X | X | |||
| CYC | Cytochrome c | Down | X | X | |
| SDH | Succinate dehydrogenase | Down | X | X | |
| IDH | Isocitrate dehydrogenase | Down | X | ||
| CS | Citrate synthase | Down | X | ||
| ATPase | ATP synthase | Down | X | X | |
| V-ATPase | V-type proton ATPase | Down | X | X | |
| Immune defense | |||||
| CTL | LDLa domain-containing C-type lectin | Up | X | X | X |
| CYP450 | Cytochrome P450 3A11 | Up | X | X | X |
| IAP | Inhibitor of apoptosis protein | Up | X | X | X |
| Chitinase | Chitinase | Up | X | X | X |
| ARE | Apoptosis-resistant E3 ubiquitin protein | Up | X | X | X |
| CASP3 | Caspase 3 | Up | X | X | |
| CASP7 | Caspase 7 | Up | X | ||
| CASP10 | Caspase 10 | Up | X | ||
| ALF | Anti-lipopolysaccharide factor | Up | X | X |
| Functional Pathway/Gene | Description | Regulation | MT vs. CT | HT vs. CT |
|---|---|---|---|---|
| Fc gamma R-mediated phagocytosis | ||||
| PTPRC | Receptor-type tyrosine-protein phosphatase | Up | X | |
| PIK3CA | Phosphoinositide-3 kinase | Up | X | |
| RAC1 | Rho-related protein | Up | X | |
| ECM–receptor interaction | ||||
| Integrin α | Integrin alpha | Up | X | |
| Integrin β | Integrin beta | Up | X | |
| Regulation of actin cytoskeleton | ||||
| Actin | Actin | Up | X | |
| Integrin α | Integrin alpha | Up | X | |
| Integrin β | Integrin beta | Up | X | |
| PIK3CA | Phosphoinositide-3 kinase | Up | X | |
| RAC1 | Rho-related protein | Up | X | |
| Toll and Imd signaling pathway | ||||
| Duox | Dual oxidase | Up | X | |
| IAP | Inhibitor of apoptosis protein | Up | X | |
| Relaxin signaling pathway | ||||
| RXFP1 | Relaxin family peptide receptor 1 | Up | X | |
| RXFP2 | Relaxin receptor 2 | Up | X | |
| GNAO | Guanine nucleotide-binding protein G(o) subunit alpha isoform X2 | Up | X | |
| NOS1 | Nitric oxide synthase | Up | X | |
| PIK3CA | Phosphoinositide-3 kinase | Up | X | |
| AC | Adenylate cyclase | Up | X | |
| Relish | Relish | Up | X | |
| PKC | Protein kinase C | Up | X | |
| PLC | 1-Phosphatidylinositol 4,5-bisphosphate phosphodiesterase | Up | X | |
| CREB | cAMP-Responsive element binding protein | Up | X | |
| VEGF | Vascular endothelial growth factor | Up | X | |
| JUN | Transcription factor AP-1 | Up | X | |
| PKA | cAMP-Dependent protein kinase catalytic | Up | X | |
| Renin–angiotensin system | ||||
| NAP | Aminopeptidase N-like | Up | X | |
| ACE | Angiotensin-converting enzyme | Up | X |
| Functional Group/Gene | Description | Regulation | MT vs. CT | HT vs. CT | HT vs. MT |
|---|---|---|---|---|---|
| Heat shock protein family | |||||
| HSP 70 | Heat shock protein 70 | Up | X | X | X |
| HSP 60 | Heat shock protein 60 | Up | X | ||
| Antioxidant system | |||||
| CAT | Catalase | Up | X | X | |
| Down | X | ||||
| GST | Glutathione S-transferase | Up | X | X | |
| Down | X | ||||
| Energy metabolism | |||||
| COX | Cytochrome c oxidase | Up | X | X | X |
| ND | NADH dehydrogenase | Up | X | X | |
| Down | X | ||||
| CS | Citrate synthase | Up | X | X | |
| Down | X | ||||
| ATPase | ATP synthase | Up | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Feng, G.; Zhao, F.; Huang, X.; Wang, M.; Wang, H. Integration of Transcriptomics and Proteomics Analysis Reveals the Molecular Mechanism of Eriocheir sinensis Gills Exposed to Heat Stress. Antioxidants 2023, 12, 2020. https://doi.org/10.3390/antiox12122020
Shen C, Feng G, Zhao F, Huang X, Wang M, Wang H. Integration of Transcriptomics and Proteomics Analysis Reveals the Molecular Mechanism of Eriocheir sinensis Gills Exposed to Heat Stress. Antioxidants. 2023; 12(12):2020. https://doi.org/10.3390/antiox12122020
Chicago/Turabian StyleShen, Chenchen, Guangpeng Feng, Feng Zhao, Xiaorong Huang, Min Wang, and Haihua Wang. 2023. "Integration of Transcriptomics and Proteomics Analysis Reveals the Molecular Mechanism of Eriocheir sinensis Gills Exposed to Heat Stress" Antioxidants 12, no. 12: 2020. https://doi.org/10.3390/antiox12122020
APA StyleShen, C., Feng, G., Zhao, F., Huang, X., Wang, M., & Wang, H. (2023). Integration of Transcriptomics and Proteomics Analysis Reveals the Molecular Mechanism of Eriocheir sinensis Gills Exposed to Heat Stress. Antioxidants, 12(12), 2020. https://doi.org/10.3390/antiox12122020





