Antioxidant Properties and Geroprotective Potential of Wheat Bran Extracts with Increased Content of Anthocyanins
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. High Performance Liquid Chromatography-Mass Spectrometry
2.4. Analysis of Anthocyanins
2.5. Evaluation of Antioxidant Activity
2.6. Drosophila Line and Maintenance Conditions
2.7. Lifespan Analysis and Treatment
2.8. Analysis of Stress Resistance
2.9. Analysis of Locomotor Activity
2.10. Statistical Analysis
3. Results
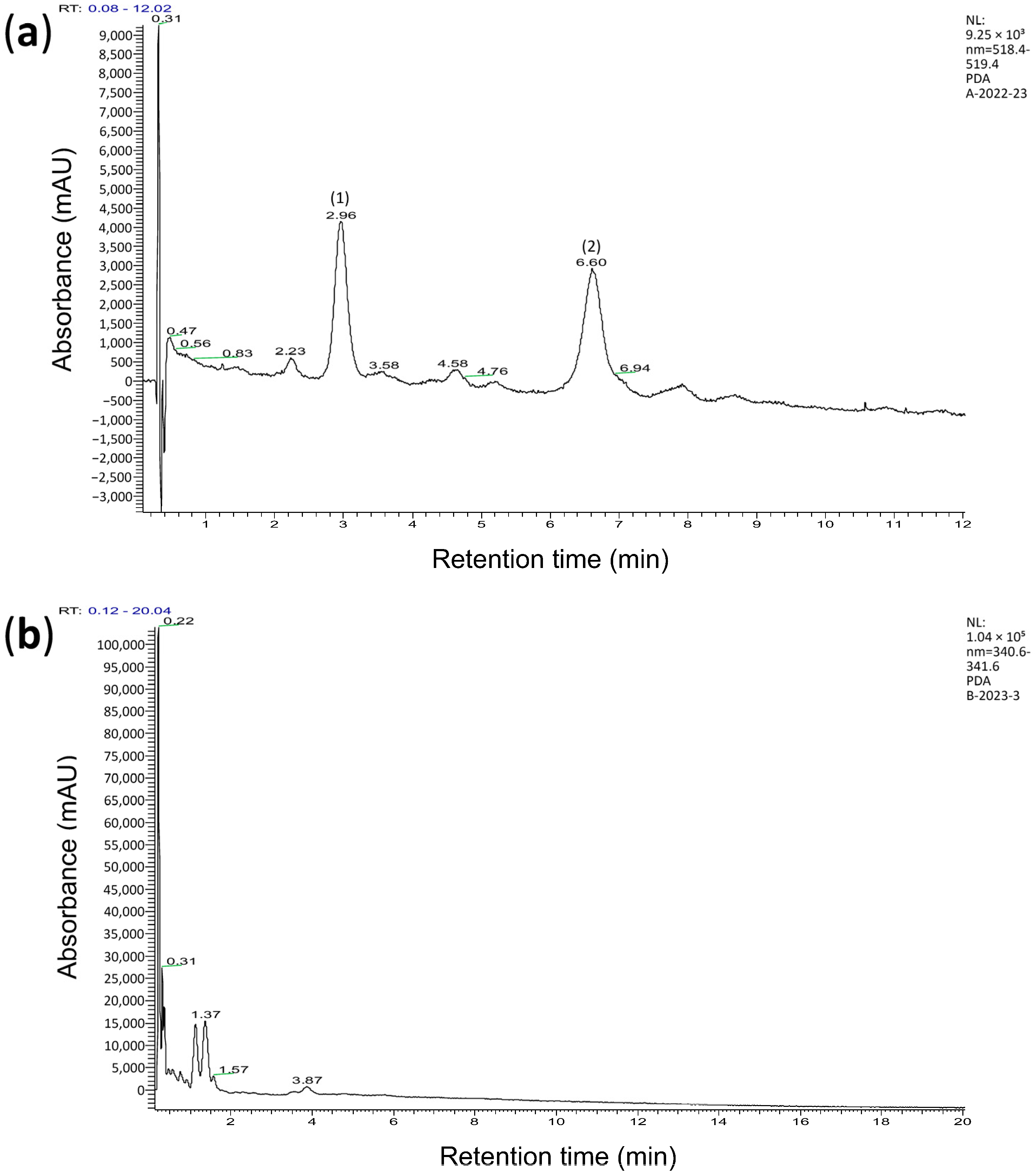
3.1. Chemical Analysis of Wheat Extracts
3.2. Antioxidant Activity of Extracts
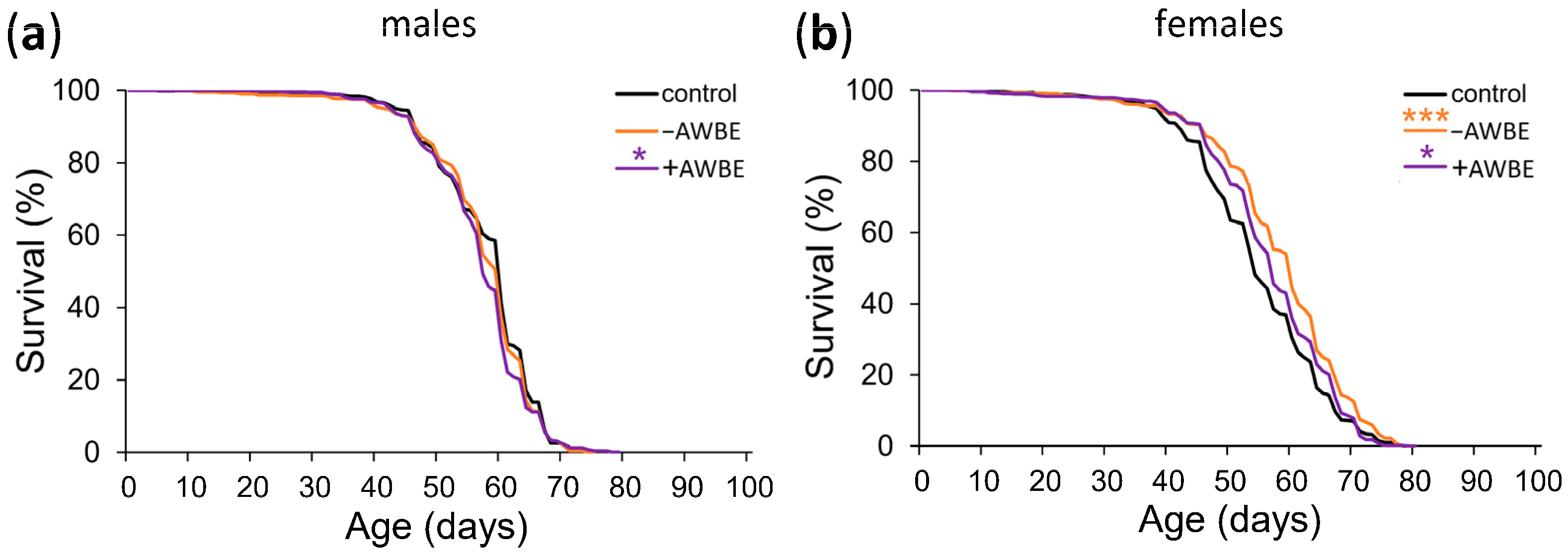
3.3. Effects of Bran Extracts on Drosophila Lifespan
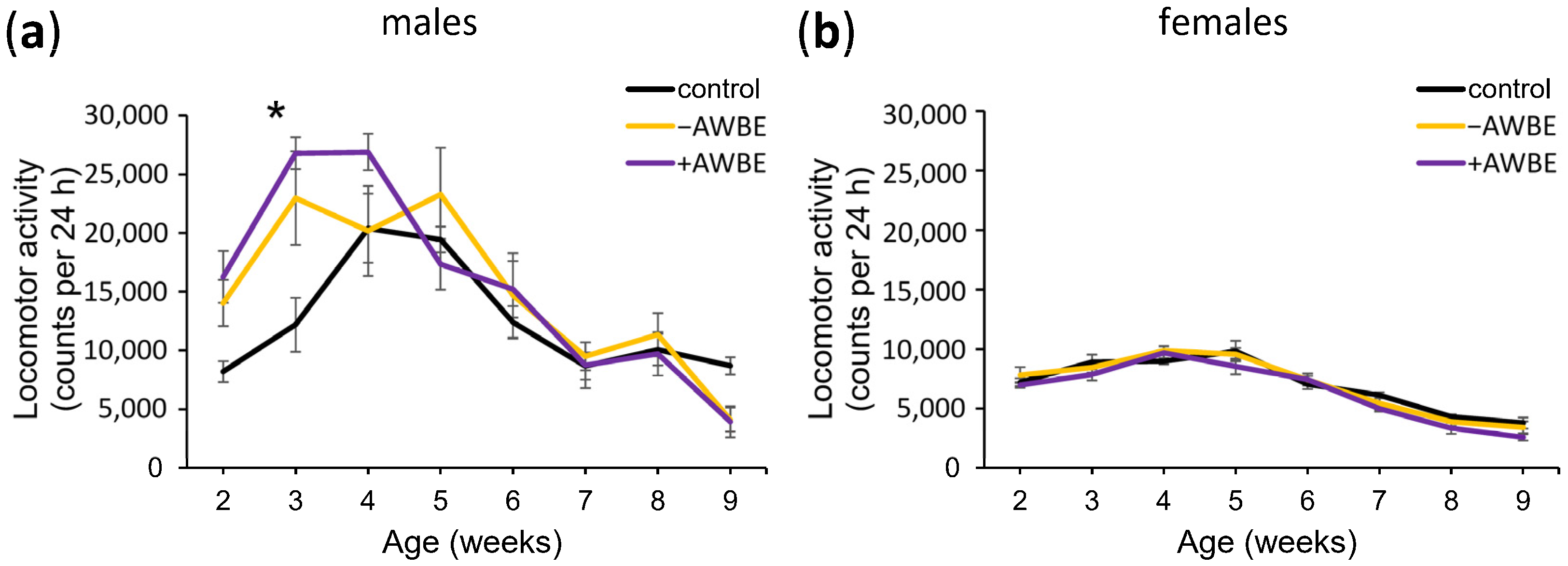
3.4. Age Dynamics of Locomotor Activity
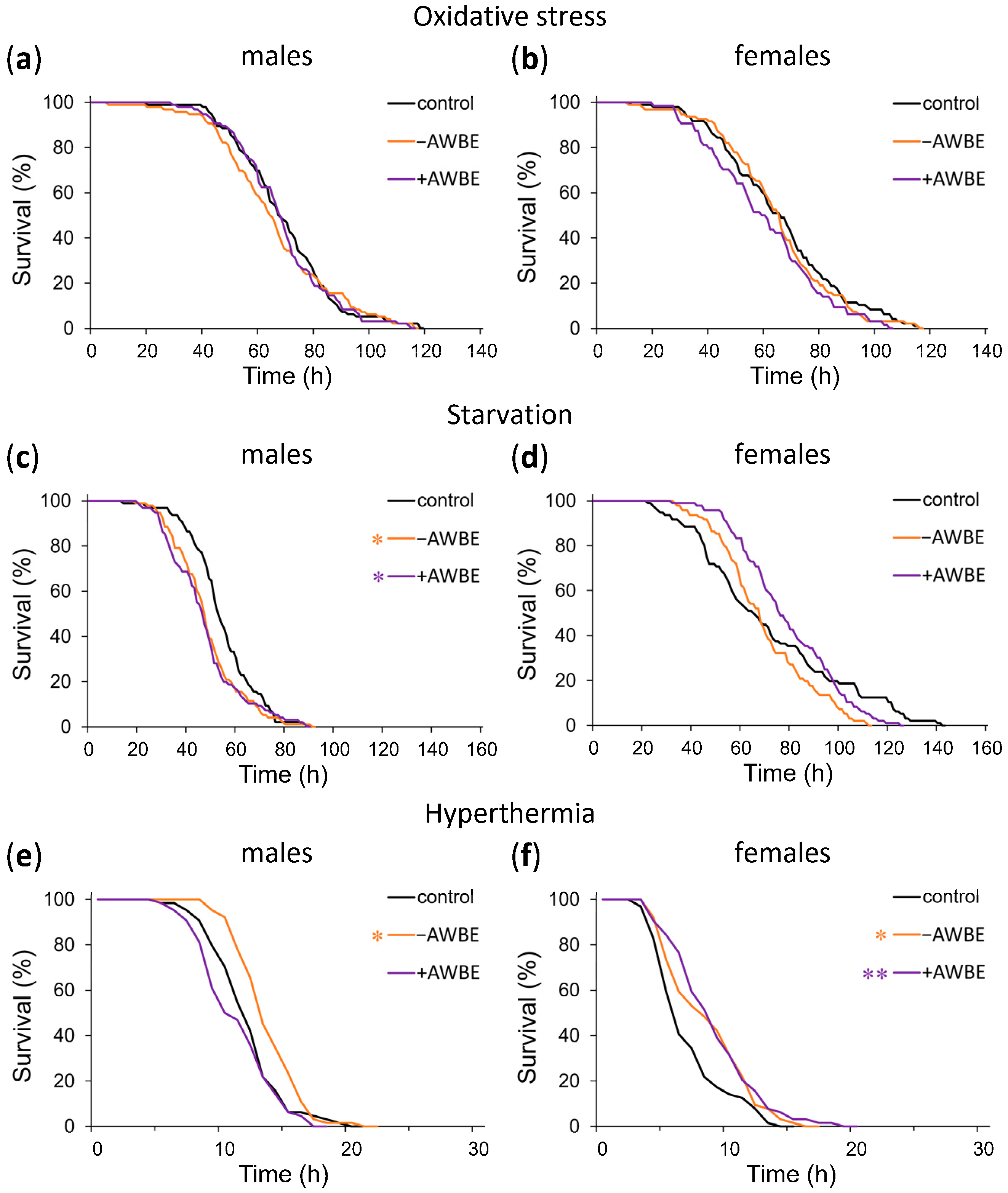
3.5. Resistance to Adverse Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P.; Anisimov, V.N.; Antonenko, Y.N.; Bakeeva, L.E.; Chernyak, B.V.; Erichev, V.P.; Filenko, O.F.; Kalinina, N.I.; Kapelko, V.I.; Kolosova, N.G.; et al. An attempt to prevent senescence: A mitochondrial approach. Biochim. Biophys. Acta 2009, 1787, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Roginsky, V.A.; Tashlitsky, V.N.; Skulachev, V.P. Chain-breaking antioxidant activity of reduced forms of mitochondria-targeted quinones, a novel type of geroprotectors. Aging 2009, 1, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, R.; Chassaing, S.; Isorez, G.; Kueny-Stotz, M.; Figueiredo, P. The Visible Flavonoids or Anthocyanins: From Research to Applications. In Recent Advances in Polyphenol Research; Santos-Buelga, C., Escribano-Bailon, M.T., Lattanzio, V., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; Volume 2, pp. 1–22. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Sarma, A.D.; Sreelakshmi, Y.; Sharma, R. Antioxidant ability of anthocyanins against ascorbic acid oxidation. Phytochemistry 1997, 45, 671–674. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Zhao, H.; Chen, G.; Jiang, Y.; Lyu, X.; Wu, T. Reduction of Aging-Induced Oxidative Stress and Activation of Autophagy by Bilberry Anthocyanin Supplementation via the AMPK-mTOR Signaling Pathway in Aged Female Rats. J. Agric. Food Chem. 2019, 67, 7832–7843. [Google Scholar] [CrossRef]
- Yoon, B.I.; Bae, W.J.; Choi, Y.S.; Kim, S.J.; Ha, U.S.; Hong, S.H.; Sohn, D.W.; Kim, S.W. Anti-inflammatory and Antimicrobial Effects of Anthocyanin Extracted from Black Soybean on Chronic Bacterial Prostatitis Rat Model. Chin. J. Integr. Med. 2018, 24, 621–626. [Google Scholar] [CrossRef]
- Wu, T.; Yu, Z.; Tang, Q.; Song, H.; Gao, Z.; Chen, W.; Zheng, X. Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct. 2013, 4, 1654–1661. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Z.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, M.A.; Shoeva, O.Y.; Tenditnik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders. Nutrients 2020, 12, 3877. [Google Scholar] [CrossRef] [PubMed]
- Grotewiel, M.S.; Martin, I.; Bhandari, P.; Cook-Wiens, E. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 2005, 4, 372–397. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Partridge, L. Drosophila as a model for ageing. Biochim. Et. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 2707–2717. [Google Scholar] [CrossRef]
- He, Y.; Jasper, H. Studying aging in Drosophila. Methods 2014, 68, 129–133. [Google Scholar] [CrossRef]
- Lüersen, K.; Röder, T.; Rimbach, G. Drosophila melanogaster in nutrition research-the importance of standardizing experimental diets. Genes. Nutr. 2019, 14, 3. [Google Scholar] [CrossRef]
- Evangelakou, Z.; Manola, M.; Gumeni, S.; Trougakos, I.P. Nutrigenomics as a tool to study the impact of diet on aging and age-related diseases: The Drosophila approach. Genes. Nutr. 2019, 14, 12. [Google Scholar] [CrossRef]
- Promislow, D.E.L.; Flatt, T.; Bonduriansky, R. The Biology of Aging in Insects: From Drosophila to Other Insects and Back. Annu. Rev. Entomol. 2022, 67, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of ageing and role of dietary antioxidants. Biomed. Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.M.; Lei, L.; Liu, Y.; Wang, X.; Ma, K.Y.; Chen, Z.Y. Cranberry anthocyanin extract prolongs lifespan of fruit flies. Exp. Gerontol. 2015, 69, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Platonova, E.Y.; Zemskaya, N.V.; Shaposhnikov, M.V.; Golubev, D.A.; Kukuman, D.V.; Pakshina, N.R.; Ulyasheva, N.S.; Punegov, V.V.; Patov, S.A.; Moskalev, A. Geroprotective effects of ×Sorbaronia mitschurinii fruit extract on Drosophila melanogaster. J. Berry Res. 2022, 12, 73–92. [Google Scholar] [CrossRef]
- Golubev, D.; Zemskaya, N.; Shevchenko, O.; Shaposhnikov, M.; Kukuman, D.; Patov, S.; Punegov, V.; Moskalev, A. Honeysuckle extract (Lonicera pallasii L.) exerts antioxidant properties and extends the lifespan and healthspan of Drosophila melanogaster. Biogerontology 2022, 23, 215–235. [Google Scholar] [CrossRef]
- Platonova, E.Y.; Golubev, D.A.; Zemskaya, N.V.; Shevchenko, O.G.; Patov, S.A.; Shaposhnikov, M.V.; Moskalev, A.A. Antioxidant and Geroprotective Properties of the Extract of Mountain Ash (Sorbus aucuparia L.) Fruits. J. Mol. Biol. 2023, 57, 979–994. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Lander, H.M. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997, 11, 118–124. [Google Scholar] [CrossRef]
- Bókkon, I. Recognition of functional roles of free radicals. Curr. Neuropharmacol. 2012, 10, 287–288. [Google Scholar] [CrossRef][Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Sharma, A.; Yadav, M.; Tiwari, A.; Ali, U.; Krishania, M.; Bala, M.; Mridula, D.; Sharma, P.; Goudar, G.; Roy, J.K.; et al. A comparative study of colored wheat lines across laboratories for validation of their phytochemicals and antioxidant activity. J. Cereal Sci. 2023, 112, 103719. [Google Scholar] [CrossRef]
- Gordeeva, E.; Shoeva, O.; Mursalimov, S.; Adonina, I.; Khlestkina, E. Fine Points of Marker-Assisted Pyramiding of Anthocyanin Biosynthesis Regulatory Genes for the Creation of Black-Grained Bread Wheat (Triticum aestivum L.) Lines. Agronomy 2022, 12, 2934. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Hou, H.; Ma, X.; Sun, S.; Wang, H.; Kong, L. Metabolomics and gene expression analysis reveal the accumulation patterns of phenylpropanoids and flavonoids in different colored-grain wheats (Triticum aestivum L.). Food Res. Int. 2020, 138, 109711. [Google Scholar] [CrossRef]
- Ma, D.; Wang, C.; Feng, J.; Xu, B. Wheat grain phenolics: A review on composition, bioactivity, and influencing factors. J. Sci. Food Agric. 2021, 101, 6167–6185. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Kaur, A.; Singh, B.; Simon, S.; Kaur, N.; Powell, M.; Sarker, M. Extraction and characterization of lipids and phenolic compounds from the brans of different wheat varieties. Food Hydrocoll. 2021, 117, 106734. [Google Scholar] [CrossRef]
- Parker, M.L.; Ng, A.; Waldron, K.W. The phenolic acid and polysaccharide composition of cell walls of bran layers of mature wheat (Triticum aestivum L. cv. Avalon) grains. J. Sci. Food Agric. 2005, 85, 2539–2547. [Google Scholar] [CrossRef]
- Kim, K.; Tsao, R.; Yang, R.; Cui, S. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Abdel-Aal, E.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef]
- Laddomada, B.; Caretto, S.; Mita, G. Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods. Molecules 2015, 20, 15666–15685. [Google Scholar] [CrossRef]
- Gamel, T.H.; Saeed, S.M.G.; Ali, R.; Abdel-Aal, E.M. Purple Wheat: Food Development, Anthocyanin Stability, and Potential Health Benefits. Foods 2023, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Khlestkina, E.K.; Usenko, N.I.; Gordeeva, E.I.; Stabrovskaya, O.I.; Sharfunova, I.B.; Otmakhova, Y.S. Evaluation of wheat products with high flavonoid content: Justification of importance of marker-assisted development and production of flavonoid-rich wheat cultivars. Vavilov. J. Genet. Breed. 2017, 21, 545–553. [Google Scholar] [CrossRef]
- Gamel, T.H.; Wright, A.J.; Pickard, M.; Abdel-Aal, E.S.M. Characterization of anthocyanin-containing purple wheat prototype products as functional foods with potential health benefits. Cereal Chem. 2019, 97, 34–38. [Google Scholar] [CrossRef]
- Gordeeva, E.I.; Shoeva, O.Y.; Khlestkina, E.K. Marker-assisted development of bread wheat near-isogenic lines carrying various combinations of purple pericarp (Pp) alleles. Euphytica 2014, 203, 469–476. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Celik, S.E.; Ozyürek, M.; Güçlü, K.; Apak, R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef]
- Boulebd, H.; Zine, Y.; Khodja, I.A.; Mermer, A.; Demir, A.; Debache, A. Synthesis and radical scavenging activity of new phenolic hydrazone/hydrazide derivatives: Experimental and theoretical studies. J. Mol. Struct. 2022, 1249, 131546. [Google Scholar] [CrossRef]
- Chawla, R.; Arora, R.; Kumar, R.; Sharma, A.; Prasad, J.; Singh, S.; Sagar, R.; Chaudhary, P.; Shukla, S.; Kaur, G.; et al. Antioxidant activity of fractionated extracts of rhizomes of high-altitude Podophyllum hexandrum: Role in radiation protection. Mol. Cell Biochem. 2005, 273, 193–208. [Google Scholar] [CrossRef]
- Kim, J. Preliminary evaluation for comparative antioxidant activity in the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel using chemical and biochemical in vitro assays. Food Nutr. Sci. 2013, 4, 177–188. [Google Scholar] [CrossRef]
- Acker, C.I.; Brandão, R.; Rosário, A.R.; Nogueira, C.W. Antioxidant effect of alkynylselenoalcohol compounds on liver and brain of rats in vitro. Environ. Toxicol. Pharmacol. 2009, 28, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, S.T.; Prestes, A.S.; Ogunmoyole, T.; Salman, S.M.; Schwab, R.S.; Brender, C.R.; Dornelles, L.; Rocha, J.B.; Soares, F.A. Evaluation of in vitro antioxidant effect of new mono and diselenides. Toxicol. Vitr. 2013, 27, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, T.; Matsushita, S. Coloring conditions of thiobarbituric acid test for detecting lipid hydroperoxides. Lipids 1980, 15, 137–140. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzym. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- van den Berg, J.J.; Op den Kamp, J.A.; Lubin, B.H.; Roelofsen, B.; Kuypers, F.A. Kinetics and site specificity of hydroperoxide-induced oxidative damage in red blood cells. Free Radic. Biol. Med. 1992, 12, 487–498. [Google Scholar] [CrossRef]
- Ko, F.N.; Hsiao, G.; Kuo, Y.H. Protection of oxidative hemolysis by demethyldiisoeugenol in normal and beta-thalassemic red blood cells. Free Radic. Biol. Med. 1997, 22, 215–222. [Google Scholar] [CrossRef]
- Takebayashi, J.; Chen, J.; Tai, A. A method for evaluation of antioxidant activity based on inhibition of free radical-induced erythrocyte hemolysis. Methods Mol. Biol. 2010, 594, 287–296. [Google Scholar] [CrossRef]
- Jones, M.A.; Grotewiel, M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp. Gerontol. 2011, 46, 320–325. [Google Scholar] [CrossRef]
- Le Bourg, E. The rate of living theory. Spontaneous locomotor activity, aging and longevity in Drosophila melanogaster. Exp. Gerontol. 1987, 22, 359–369. [Google Scholar] [CrossRef]
- Harrington, D.P.; Fleming, T.R. A Class of Rank Test Procedures for Censored Survival Data. Biometrika 1982, 69, 553–566. [Google Scholar] [CrossRef]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.S.; Lee, S.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Nikonova, N.N.; Hurshkainen, T.V.; Shevchenko, O.G.; Kuchin, A.V. “Green technology” processing of pine (Pinus sylvestris L.) and larch (Larix sibirica Ledeb.) wood greenery to produce bioactive extracts. Holzforschung 2022, 76, 276–284. [Google Scholar] [CrossRef]
- Navarro, C.; Salazar, J.; Díaz, M.P.; Chacin, M.; Santeliz, R.; Vera, I.; Parra, H.; Bernal, M.C.; Castro, A.; Escalona, D.; et al. Intrinsic and environmental basis of aging: A narrative review. Heliyon 2023, 9, e18239. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Fusco, D.; Colloca, G.; Lo Monaco, M.R.; Cesari, M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2007, 2, 377–387. [Google Scholar]
- Abozed, S.S.; El-kalyoubi, M.; Abdelrashid, A.; Salama, M.F. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann. Agric. Sci. 2014, 59, 63–67. [Google Scholar] [CrossRef]
- Higuchi, M. Antioxidant Properties of Wheat Bran against Oxidative Stress. In Wheat and Rice in Disease Prevention and Health; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 181–199. [Google Scholar] [CrossRef]
- Chen, X.; Tang, W.; Li, X.; Zhuang, K.; Lyu, Q.; Ding, W. Effect of extrusion on phenolics from Jizi439 black wheat bran: The profile, structure, and bioactivities. LWT 2023, 177, 114369. [Google Scholar] [CrossRef]
- Vaher, M.; Matso, K.; Levandi, T.; Helmja, K.; Kaljurand, M. Phenolic compounds and the antioxidant activity of the bran, flour and whole grain of different wheat varieties. Procedia Chem. 2010, 2, 76–82. [Google Scholar] [CrossRef]
- Bauer, J.L.; Harbaum-Piayda, B.; Stöckmann, H.; Schwarz, K. Antioxidant activities of corn fiber and wheat bran and derived extracts. LWT-Food Sci. Technol. 2013, 50, 132–138. [Google Scholar] [CrossRef]
- Huang, W.; Tian, F.; Wang, H.; Wu, S.; Jin, W.; Shen, W.; Hu, Z.; Cai, Q.; Liu, G. Comparative assessment of extraction, composition, and in vitro antioxidative properties of wheat bran polyphenols. LWT 2023, 180, 114706. [Google Scholar] [CrossRef]
- Gunenc, A.; HadiNezhad, M.; Farah, I.; Hashem, A.; Hosseinian, F. Impact of supercritical CO2 and traditional solvent extraction systems on the extractability of alkylresorcinols, phenolic profile and their antioxidant activity in wheat bran. J. Funct. Foods 2015, 12, 109–119. [Google Scholar] [CrossRef]
- Pazo-Cepeda, M.V.; Aspromonte, S.G.; Alonso, E. Extraction of ferulic acid and feruloylated arabinoxylo-oligosaccharides from wheat bran using pressurized hot water. Food Biosci. 2021, 44, 101374. [Google Scholar] [CrossRef]
- Morgounov, A.; Karaduman, Y.; Akin, B.; Aydogan, S.; Baenziger, P.S.; Bhatta, M.; Chudinov, V.; Dreisigacker, S.; Govindan, V.; Güler, S.; et al. Yield and Quality in Purple-Grained Wheat Isogenic Lines. Agronomy 2020, 10, 86. [Google Scholar] [CrossRef]
- Shamanin, V.P.; Tekin-Cakmak, Z.H.; Gordeeva, E.I.; Karasu, S.; Pototskaya, I.; Chursin, A.S.; Pozherukova, V.E.; Ozulku, G.; Morgounov, A.I.; Sagdic, O.; et al. Antioxidant Capacity and Profiles of Phenolic Acids in Various Genotypes of Purple Wheat. Foods 2022, 11, 2515. [Google Scholar] [CrossRef]
- Pandey, M.; Bansal, S.; Chawla, G. Evaluation of lifespan promoting effects of biofortified wheat in Drosophila melanogaster. Exp. Gerontol. 2022, 160, 111697. [Google Scholar] [CrossRef]
- Chen, W.; Muller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemical profiles and antioxidant activity of wheat varieties. J. Agric. Food Chem. 2003, 51, 7825–7834. [Google Scholar] [CrossRef]
- Lushchak, O.; Strilbytska, O.; Storey, K.B. Gender-specific effects of pro-longevity interventions in Drosophila. Mech. Ageing Dev. 2023, 209, 111754. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, E. Patterns of movement and ageing in Drosophila melanogaster. Arch. Gerontol. Geriatr. 1983, 2, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Smith, N.; Saunders, D.; Ranjit, R.; Kneis, P.; Towner, R.A.; Van Remmen, H. Using MRI to measure in vivo free radical production and perfusion dynamics in a mouse model of elevated oxidative stress and neurogenic atrophy. Redox Biol. 2019, 26, 101308. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. The roles of free radicals in amyotrophic lateral sclerosis. J. Mol. Neurosci. 1996, 7, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.; Zhang, X.; Cheng, J.; Liu, D.; Yan, Y.; Wang, H. Transcriptomic analysis of the life-extending effect exerted by black rice anthocyanin extract in D. melanogaster through regulation of aging pathways. Exp. Gerontol. 2019, 119, 33–39. [Google Scholar] [CrossRef]
- Kishimoto, S.; Uno, M.; Nishida, E. Molecular mechanisms regulating lifespan and environmental stress responses. Inflamm. Regen. 2018, 38, 22. [Google Scholar] [CrossRef]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef]
- Bubliy, O.A.; Loeschcke, V. Correlated responses to selection for stress resistance and longevity in a laboratory population of Drosophila melanogaster. J. Evol. Biol. 2005, 18, 789–803. [Google Scholar] [CrossRef]
- Belyi, A.A.; Alekseev, A.A.; Fedintsev, A.Y.; Balybin, S.N.; Proshkina, E.N.; Shaposhnikov, M.V.; Moskalev, A.A. The Resistance of Drosophila melanogaster to Oxidative, Genotoxic, Proteotoxic, Osmotic Stress, Infection, and Starvation Depends on Age According to the Stress Factor. Antioxidants 2020, 9, 1239. [Google Scholar] [CrossRef]
- Deepashree, S.; Shivanandappa, T.; Ramesh, S.R. Life History Traits of an Extended Longevity Phenotype of Drosophila melanogaster. Curr. Aging Sci. 2017, 10, 224–238. [Google Scholar] [CrossRef]
- Liu, J.K. Antiaging agents: Safe interventions to slow aging and healthy life span extension. Nat. Prod. Bioprospect 2022, 12, 18. [Google Scholar] [CrossRef]
- Garcia, A.M.; Calder, R.B.; Dolle, M.E.; Lundell, M.; Kapahi, P.; Vijg, J. Age- and temperature-dependent somatic mutation accumulation in Drosophila melanogaster. PLoS Genet. 2010, 6, e1000950. [Google Scholar] [CrossRef] [PubMed]
- Zatsepina, O.G.; Przhiboro, A.A.; Yushenova, I.A.; Shilova, V.; Zelentsova, E.S.; Shostak, N.G.; Evgen’ev, M.B.; Garbuz, D.G. A Drosophila heat shock response represents an exception rather than a rule amongst Diptera species. Insect Mol. Biol. 2016, 25, 431–449. [Google Scholar] [CrossRef]
- Murakami, A.; Nesumi, A.; Maeda-Yamamoto, M.; Yamaguchi, H.; Yashima, K.; Miura, M.; Nakano, T.; Nekoshima, K. Anthocyanin-rich tea Sunrouge upregulates expressions of heat shock proteins in the gastrointestinal tract of ICR mice: A comparison with the conventional tea cultivar Yabukita. J. Food Drug Anal. 2015, 23, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rion, S.; Kawecki, T.J. Evolutionary biology of starvation resistance: What we have learned from Drosophila. J. Evol. Biol. 2007, 20, 1655–1664. [Google Scholar] [CrossRef]
| Variant | RSA (DPPH), % | RSA (ABTS), % | TBA-RS *, nmol/mL | |
|---|---|---|---|---|
| Brain Substrate | Testes Substrate | |||
| Control | – | – | 55.0 ± 0.5 | 16.6 ± 0.3 |
| Intact | – | – | 36.6 ± 0.3 | 9.9 ± 0.1 |
| Extract concentration is 0.5 mg/mL | ||||
| −AWBE | 12.5 ± 0.1 | 57.3 ± 0.3 | 5.0 ± 0.2 | 8.7 ± 0.3 |
| +AWBE | 24.3 ± 0.5 * | 82.4 ± 0.3 * | 5.2 ± 0.2 | 9.4 ± 0.3 |
| Trolox | 96.0 ± 0.0 | 99.4 ± 0.0 | 4.4 ± 0.0 | 4.9 ± 0.1 |
| Extract concentration is 0.05 mg/mL | ||||
| −AWBE | 3.2 ± 0.3 | 30.9 ± 0.3 | 6.8 ± 0.1 | – |
| +AWBE | 4.3 ± 0.1 | 47.3 ± 0,1 | 6.2 ± 0.4 | – |
| Trolox | 96.7 ± 0.0 | 99.4 ± 0.0 | 4.5 ± 0.0 | – |
| Sample | Hemolysis, % | ||||
|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | |
| H2O2 | |||||
| Control | 18.7 ± 0.7 | 32.3 ± 0.8 | 39.2 ± 0.5 | 45.9 ± 0.6 | 50.5 ± 0.6 |
| −AWBE | 11.2 ± 0.4 | 22.4 ± 0.8 | 32.4 ± 0.9 | 40.7 ± 0.7 | 45.7 ± 0.5 |
| +AWBE | 11.8 ± 0.6 | 23.6 ± 0.6 | 34.6 ± 0.3 | 41.7 ± 0.4 | 45.6 ± 0.3 |
| Trolox | 5.5 ± 0.1 | 8.8 ± 0.2 | 9.3 ± 0.2 | 9.3 ± 0.2 | 9.3 ± 0.2 |
| AAPH | |||||
| Control | 1.7 ± 0.2 | 9.8 ± 1.0 | 55.6 ± 1.8 | 77.5 ± 0.4 | 84.9 ± 0.5 |
| −AWBE | 3.5 ± 0.1 | 4.7 ± 0.2 | 22.7 ± 2.2 | 72.1 ± 0.5 | 85.4 ± 0.3 |
| +AWBE | 3.1 ± 0.3 | 4.5 ± 0.3 | 10.4 ± 2.1 ** | 52.4 ± 1.6 ** | 79.6 ± 1.1 ** |
| Trolox | 2.7 ± 0.1 | 3.7 ± 0.1 | 4.5 ± 0.1 | 5.2 ± 0.3 | 6.3 ± 0.3 |
| Variant | Sex | M | 90% | LR, p | N | ||||
|---|---|---|---|---|---|---|---|---|---|
| M, Days | dM, % (CTR) | dM, % (ACN) | 90%, Days | d90%, % (CTR) | d90%, % (ACN) | ||||
| Control | ♂ | 60 | n/a | n/a | 67 | n/a | n/a | n/a | 601 |
| −AWBE | ♂ | 60 | 0 | n/a | 67 | 0 | n/a | >0.05 | 585 |
| +AWBE | ♂ | 57 | −5 *** | −5 | 67 | 0 | 0 | <0.05 | 573 |
| Control | ♀ | 54 | n/a | n/a | 67 | n/a | n/a | n/a | 586 |
| −AWBE | ♀ | 60 | +11 *** | n/a | 71 | +6 ** | n/a | <0.001 | 577 |
| +AWBE | ♀ | 57 | +6 *** | −5 *** | 68 | +1 | −4 *** | <0.05 | 592 |
| Source of Variation | SS | DF | MS | F | p |
|---|---|---|---|---|---|
| males | |||||
| Age | 3.93 × 109 | 7 | 5.62 × 108 | 22.638 | 7.9 × 10−18 |
| Treatment | 2.16 × 108 | 2 | 1.08 × 108 | 4.358 | 0.0154 |
| Age × Treatment | 8.7 × 108 | 14 | 62,150,972 | 2.505 | 0.0044 |
| Error | 2.38 × 109 | 96 | 24,809,477 | ||
| females | |||||
| Age | 5.94 × 108 | 7 | 84,872,011 | 76.314 | 2.1 × 10−36 |
| Treatment | 9,096,658 | 2 | 4,548,329 | 4.09 | 0.0197 |
| Age × Treatment | 12,545,984 | 14 | 896,141.8 | 0.806 | 0.6609 |
| Error | 1.07 × 108 | 96 | 1,112,145 | ||
| Variant | Sex | M | 90% | LR, p | N | ||||
|---|---|---|---|---|---|---|---|---|---|
| M, h | dM, % (CTR) | dM, % (ACN) | 90%, h | d90%, % (CTR) | d90%, % (ACN) | ||||
| Oxidative stress | |||||||||
| Control | ♂ | 67 | n/a | n/a | 89 | n/a | n/a | n/a | 96 |
| −AWBE | ♂ | 64 | −4 | n/a | 93 | +4 | n/a | >0.05 | 96 |
| +AWBE | ♂ | 69 | +3 | +8 | 91 | +2 | −2 | >0.05 | 96 |
| Control | ♀ | 66 | n/a | n/a | 97 | n/a | n/a | n/a | 96 |
| −AWBE | ♀ | 66 | 0 | n/a | 90 | −7 | n/a | >0.05 | 95 |
| +AWBE | ♀ | 59 | −11 | −11 | 85 | −12 | −6 | >0.05 | 64 |
| Starvation | |||||||||
| Control | ♂ | 53 | n/a | n/a | 72 | n/a | n/a | n/a | 96 |
| −AWBE | ♂ | 48 | −9 ** | n/a | 68 | −6 | n/a | <0.05 | 96 |
| +AWBE | ♂ | 47 | −11 *** | −2 | 69 | −4 | +1 | <0.05 | 96 |
| Control | ♀ | 65 | n/a | n/a | 122 | n/a | n/a | n/a | 96 |
| −AWBE | ♀ | 68 | +5 | n/a | 99 | −19 ** | n/a | >0.05 | 96 |
| +AWBE | ♀ | 76 | +17 | +12 ** | 106 | −13 | +7 | >0.05 | 96 |
| Hyperthermia | |||||||||
| Control | ♂ | 12 | n/a | n/a | 15 | n/a | n/a | n/a | 64 |
| −AWBE | ♂ | 13 | +8 * | n/a | 17 | +13 | n/a | <0.05 | 64 |
| +AWBE | ♂ | 10 | −17 | −23 * | 15 | 0 | −12 | >0.05 | 64 |
| Control | ♀ | 6 | n/a | n/a | 12 | n/a | n/a | n/a | 64 |
| −AWBE | ♀ | 8 | +33 * | n/a | 12 | 0 | n/a | <0.05 | 64 |
| +AWBE | ♀ | 9 | +50 * | +13 | 13 | +8 | +8 | <0.01 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, D.V.; Shevchenko, O.G.; Golubev, D.A.; Platonova, E.Y.; Zemskaya, N.V.; Shoeva, O.Y.; Gordeeva, E.I.; Patov, S.A.; Shaposhnikov, M.V.; Khlestkina, E.K.; et al. Antioxidant Properties and Geroprotective Potential of Wheat Bran Extracts with Increased Content of Anthocyanins. Antioxidants 2023, 12, 2010. https://doi.org/10.3390/antiox12112010
Mikhailova DV, Shevchenko OG, Golubev DA, Platonova EY, Zemskaya NV, Shoeva OY, Gordeeva EI, Patov SA, Shaposhnikov MV, Khlestkina EK, et al. Antioxidant Properties and Geroprotective Potential of Wheat Bran Extracts with Increased Content of Anthocyanins. Antioxidants. 2023; 12(11):2010. https://doi.org/10.3390/antiox12112010
Chicago/Turabian StyleMikhailova, Daria V., Oksana G. Shevchenko, Denis A. Golubev, Elena Y. Platonova, Nadezhda V. Zemskaya, Olesya Yu. Shoeva, Elena I. Gordeeva, Sergey A. Patov, Mikhail V. Shaposhnikov, Elena K. Khlestkina, and et al. 2023. "Antioxidant Properties and Geroprotective Potential of Wheat Bran Extracts with Increased Content of Anthocyanins" Antioxidants 12, no. 11: 2010. https://doi.org/10.3390/antiox12112010
APA StyleMikhailova, D. V., Shevchenko, O. G., Golubev, D. A., Platonova, E. Y., Zemskaya, N. V., Shoeva, O. Y., Gordeeva, E. I., Patov, S. A., Shaposhnikov, M. V., Khlestkina, E. K., & Moskalev, A. (2023). Antioxidant Properties and Geroprotective Potential of Wheat Bran Extracts with Increased Content of Anthocyanins. Antioxidants, 12(11), 2010. https://doi.org/10.3390/antiox12112010










