Abstract
Iron plays an essential role in various physiological processes. A disruption in iron homeostasis can lead to severe consequences, including impaired neurodevelopment, neurodegenerative disorders, stroke, and cancer. Interestingly, the link between mental health disorders and iron homeostasis has not received significant attention. Therefore, our understanding of iron metabolism in the context of psychological diseases is incomplete. In this review, we aim to discuss the pathologies and potential mechanisms that relate to iron homeostasis in associated mental disorders. We propose the hypothesis that maintaining brain iron homeostasis can support neuronal physiological functions by impacting key enzymatic activities during neurotransmission, redox balance, and myelination. In conclusion, our review highlights the importance of investigating the relationship between trace element nutrition and the pathological process of mental disorders, focusing on iron. This nutritional perspective can offer valuable insights for the clinical treatment of mental disorders.
1. Introduction
Mental disorders, also referred to as mental health conditions, are afflictions that impact cognition, emotion, and behavioral regulation, significantly impeding both children’s learning abilities and adults’ functionality in their families, workplaces, and society at large [1]. These disorders typically manifest early in life and often exhibit a chronic recurring pattern. Due to their high occurrence, early onset, persistent nature, and associated impairments, mental disorders contribute significantly to the overall disease burden. While disability constitutes the primary burden stemming from these disorders, premature mortality, particularly resulting from suicide, should not be underestimated [2]. The etiology of mental disorders is multifaceted. A vast corpus of research has linked the origins of such disorders intimately to aspects of neuronal function, myelination, and neurotransmission. What began as a focus on abnormalities within distinct brain areas in early research has evolved into a comprehensive exploration of the deficiencies in neural systems and synaptic plasticity within neural regions relevant to mental health [3,4,5,6]. Over the past decade, a body of evidence has emerged suggesting that mental disorders are often accompanied by dysfunctional myelination changes, myelin sheath defects, and correlated alterations in white matter. This underlying pathology potentially compromises inter-neuronal information transfer, processing, and communication, which can feasibly culminate in behavioral, sensory–motor, affective, and cognitive symptoms manifesting in patients with mental disorders [7,8,9,10]. Numerous studies have additionally highlighted alterations in dopamine, serotonin, and glutamatergic neurotransmission in mental disorders, including, but not limited to, depression, cognitive disorders, and schizophrenia [11,12,13].
As a critical component in several proteins, iron is involved in numerous processes, such as oxygen transport, oxidative phosphorylation, myelin production, and the synthesis and metabolism of neurotransmitters [14]. We and others have demonstrated that altered iron metabolism leads to deleterious imbalances of homeostatic processes throughout the body. Iron overload will aggravate oxidative stress through Fenton chemistry, which can induce cell death and is related to several pathological processes, such as several neurodegenerative diseases [15,16,17]. Conversely, inadequate iron, either peripherally or centrally, also has profound effects on health. Iron deficiency is the most widespread single nutrient deficiency worldwide [18], and can lead to decreased immune function, stunted growth and impaired thermoregulation [19]. Iron deficiency anemia (IDA) in adolescents can even result in abnormal development of the central nervous system (CNS) [20,21].
At the cellular level, iron also plays an essential role as a cofactor for various enzymes in biological systems. Its unique properties, such as its ability to obtain and lose electrons, make it invaluable in many vital biochemical processes. The key role of iron as an enzyme cofactor is pivotal to many biological processes, ranging from energy production and oxygen transport to DNA synthesis and repair [22,23]. Additionally, this metal participates in the synthesis of various neurotransmitters including monoamines, functioning as a cofactor of aromatic amino acid hydroxylases (phenylalanine, tyrosine, tryptophan [Trp]), which is the limiting step in the synthesis of monoamines, whose levels can affect many mental disorders, including depression [24]. Iron is also essential for normal myelin production and maintenance. Dysregulated myelination can lead to psychiatric diseases, including schizophrenia, depression, and autism [25]. Thus, it is likely that iron plays an important role in the occurrence and processes surrounding mental disorders. In this review, we focus on the relationship between brain iron homeostasis and the pathogenesis of mental disorders, with an eye toward the potential molecular mechanisms connecting this essential metal to brain function.
2. Regulation of Brain Iron Homeostasis
2.1. Iron Uptake into the Brain
Outside the brain, the vast majority of circulating iron is derived from macrophages, with small, but essential, contributions from enterocytes and hepatocytes. The blood–brain barrier (BBB), a physical and metabolic barrier between the CNS and the blood circulation system [26,27], is the major route of iron entry into the brain from the periphery [27,28]. The BBB is composed of brain microvascular endothelial cells (BMVECs), including tight junctions, astrocytes (end-feet) and pericytes, all of which not only form the neurovascular unit that affords structural and inductive support, but also act as a controllable and selectable transport and metabolic system, playing an important role in protecting the brain from harmful, polar molecules, maintaining the physiological function of neurons [29,30,31].
The endothelial cells of BBB control iron uptake as an “entrance guard” [32,33]. Mathematical modeling has suggested that the endothelial cells can regulate both transferrin-bound iron (Tf-Fe) and the non-transferrin-bound iron (NTBI) entry into the CNS by controlling both the expression and internalization of transferrin receptor 1 (TfR1), and acidification of endocytic vesicles [34,35]. Overall, iron transport across the BBB involves two transmembrane steps: iron uptake into BMVECs at the luminal membrane (on the blood side), followed by iron efflux into the brain interstitium at the abluminal membrane (on the brain side) [27]. Various studies suggest that the Tf-TfR1 pathway is the primary route for iron transport across the luminal membrane of BMVECs [36,37,38]. To cross the abluminal membrane, iron export is mediated by ferroportin1/hephaestin (FPN1/HP) and/or FPN1/ceruloplasmin (CP). After iron efflux from the brain side of BMVECs through FPN1, the ferrous iron (Fe2+) transported by FPN1 is oxidized to ferric iron (Fe3+) by CP or HP. The Fe3+ can then be incorporated into apo-Tf circulating in the brain for iron uptake to neurons or glial cells [39,40]. Other pathways may also be involved in iron transport across the BBB, such as lactoferrin receptor/lactoferrin and Glycosylphosphatidylinositol-anchored (GPI-anchored) melanotransferrin/soluble melanotransferrin [41,42]. In addition to these pathways, Tf-Fe may also be transferred across the BBB through transcytosis [43,44]. However, the comprehensive regulatory mechanisms of iron entry across the BBB in vivo still require further elucidation. Cerebrospinal fluid can also be the iron entry route for the brain across the choroid plexus [45]. It is speculated that iron trafficking into the cerebrospinal fluid is similar to the route of iron across the BBB [37,46].
2.2. Iron Export from the Brain
After iron enters the brain, it is then released into the brain interstitial fluid. Brain cells, including neurons, astrocytes, oligodendrocytes and microglia, may take up Tf-Fe or NTBI via TfR1/divalent metal transporter 1 (DMT1) or DMT1 pathways, respectively; due to the presence of TfR1 and DMT1 on the membranes of these cells, they may also have additional, specific mechanisms of iron uptake [47]. It must be noted that different brain regions contain diverse iron contents [48,49], with relatively abundant levels in the basal ganglia region, subtantia nigra (SN), red nucleus and cerebellar dentate gyrus [50,51]. In contrast, iron is relatively low in the cerebral cortex, white matter and medulla oblongata [52,53].
Imported iron is usually used for neuronal and glial metabolism, with excess iron either stored in ferritin or released from the brain. Importantly, FPN1, also referred to as Slc40a1, MTP1, or Ireg1, is the sole protein responsible for cellular iron release in mammals [54]. FPN1 is widely expressed in brain cells, including those in the choroid plexus, ependyma, and the BBB [26,55]. Thus, it is not surprising that FPN1 plays a pivotal role in regulating brain iron homeostasis through mediating iron efflux from the BBB. We have reported that FPN1 ablation in neurons and astrocytes leads to iron deficiency in the cortex and hippocampus [26]. In addition, disrupted expression of FPN1 in BMVECs also shed light on the mechanism of iron delivery to the brain through the BBB [56]. Finally, we found that the formation of fear memory was impeded after neuronal FPN1 depletion [26]. Our results provide clues into the role played by brain iron in mental disorders, possibly identifying potential targets for the treatment of post-traumatic stress disorder (PTSD), which is characterized by abnormally persistent and distressing fear memory [26,57].
2.3. Regulation of Iron in the Brain
At the cellular level, the regulation of cellular iron levels is primarily controlled at the translational level by iron-responsive element (IRE)-binding proteins (IRP1 and IRP2), despite some control existing at the transcriptional level as well [58,59]. In the context of mental health, IRP2, particularly through its regulation of ferritin expression, has been significantly associated with schizophrenia. It has been observed that IRP2 is marginally overexpressed in the prefrontal cortex of individuals with schizophrenia, leading to lower levels of ferritin expression among patients with this condition [60]. Furthermore, mutations in other genes involved in controlling brain iron homeostasis, such as ferritin and CP, can also contribute to disorders of brain iron metabolism and impact myelin synthesis [61,62]. At the systemic level, hepcidin, a key regulator of iron, plays a vital role in modulating iron metabolism. Hepcidin combines with and subsequently degrades FPN1 to limit extracellular iron availability. Within the brain, the cells responsible for iron regulation (possibly astrocytes) sense the levels of extracellular iron and adjust hepcidin secretion accordingly to ensure appropriate iron transport at the BBB [56,63].
In summary, the BBB displays an important role in controlling brain iron levels because of its specialized anatomical and physiological functions in iron uptake from the circulatory system (Figure 1). Importantly, the brain needs a comparatively stricter regulation of iron metabolism for optimal cell functioning. Dysregulation of brain iron can lead to either local iron accumulation, resulting in neurodegenerative diseases, iron deficiency, or giving rise to brain development problems. Both iron accumulation and iron deficiency may cause psychiatric disorders [24]. Hence, it is crucial to comprehensively understand the mechanisms of iron transport across the BBB to evaluate and eventually affect brain iron in the field of mental health.
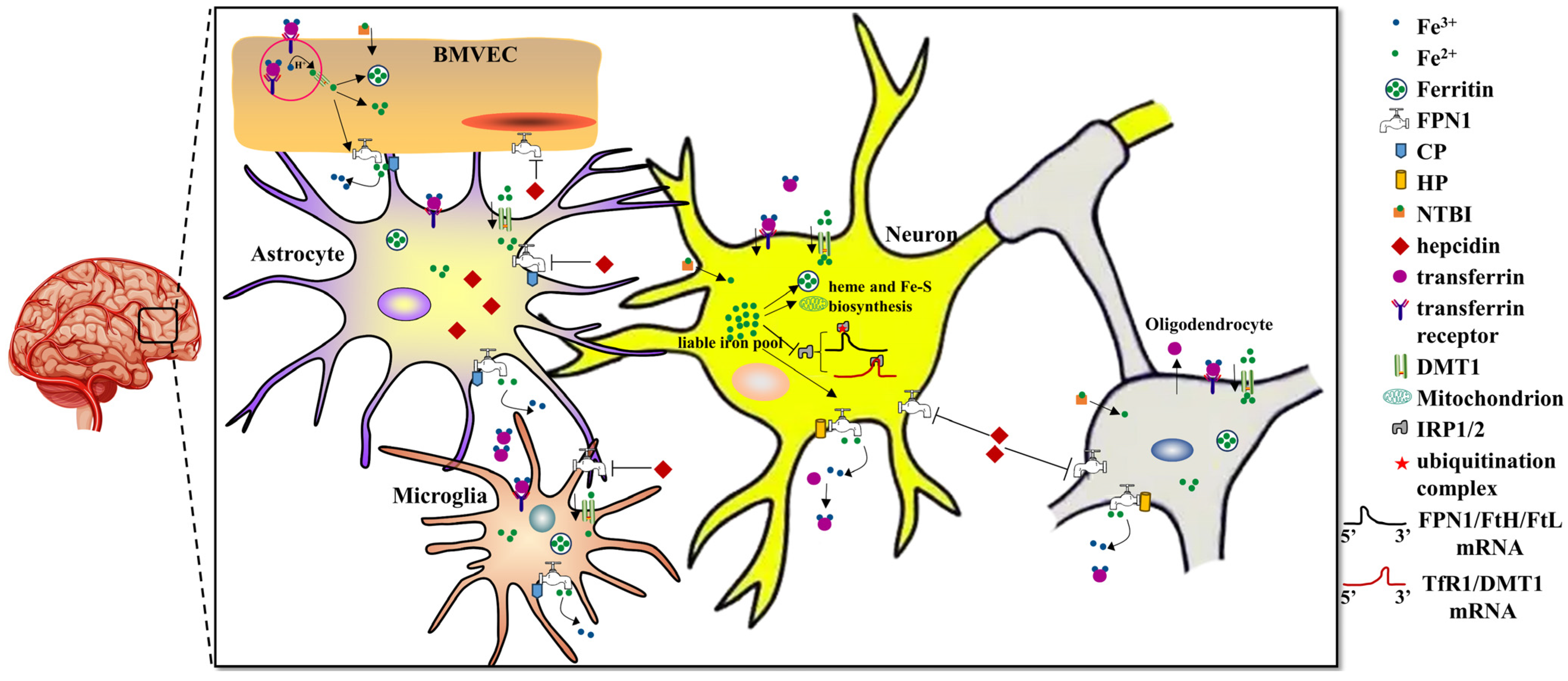
Figure 1.
Systemic and cellular regulation of brain iron metabolism.
Brain iron uptake mainly occurs through the BBB via Tf-TfR1/DMT1 in the apical surface of BMVECs. FPN1 is transporter responsible for iron export from the BMVECs with the help of CP expressed from astrocytes. After iron influx into the brain, other brain cells, including astrocytes, neurons, oligodendrocytes and microglia, may, respectively, take up Tf-Fe or NTBI via the TfR1/DMT1 or DMT1 pathways. Iron in brain cells is used for electron transport biosynthesis of hemoproteins, Fe-S proteins and neurotransmitters. Excess iron will be either stored in ferritin or exported by FPN1 with oxidation of the extracellular metal by CP or HP. The other ferrous iron can be found in a soluble, chelatable state, which constitutes the labile iron pool. Hepcidin plays a key role in regulating brain iron homeostasis. Hepcidin that is mainly synthesized from astrocytes can sense extracellular iron levels and then bind to FPN1 and promote its degradation, ultimately decreasing cellular iron release. Cellular iron metabolism is primarily regulated by the IRE/IRP system. In iron deficient cells, IRPs bind to the 5′-IREs in ferritin and FPN1 mRNAs to repress transcription. In contrast, TFR1 and some DMT1 variants contain 3′ UTR IREs, which bind IRP2 under iron deficiency, stabilizing the mRNA, which guarantees the synthesis of iron importers.
3. Iron and Mental Disorders
Numerous mental disorders have been described, with signs and symptoms that vary widely between specific disorders [64]. Common disorders include depression/anxiety disorders, schizophrenia, fear disorders, including PTSD, and neurodevelopment disorders, including ADHD and autism spectrum disorder (ASD), which usually occurs early in development [65]. The stigma and discrimination associated with mental disorders can add to the suffering and disability, leading to social movements to increase awareness and challenge social exclusion [66]. In this section, we mainly discuss the relationship between brain iron metabolism and the pathology of mental disorders, including depression/anxiety disorders, schizophrenia, fear disorders (PTSD) and neurodevelopment disorders (ADHD and ASD).
3.1. Depression/Anxiety Disorders
Depression is one of the principal contributors to disability worldwide. This condition is present in all age groups and all socio-professional categories, with considerable morbidity and mortality, and affects one in seven people during their lifetime [67,68]. In psychiatric pathology, a characteristic depressive episode is referred to as a major depressive episode. The core features of depression are “depressed mood” and “loss of interest or pleasure in nearly all activities”, with symptoms of fatigue, sleep disturbance, anxiety, and neurocognitive and sexual dysfunction also worthy of monitoring [67]. Depression is also linked with numerous somatic and psychological comorbidities, such as smoking, chronic alcoholism and reduction in physical activity, which emphasizes its importance to public health. The main risk of depression is suicide. The risk of attempted suicide is increased in the event of a depressive episode. In depressed outpatients, a lack of improvement has been strongly associated with short-term attempts at suicide, particularly in patients with a history of previous attempted suicides [69,70].
Anxiety disorders, defined as the anticipation of future threat in the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition (DSM-V), comprise one of the major groups of disorders in psychiatry. Anxiety is distinguished from fear, the emotional response to real or perceived imminent threat [71]. Recent years have seen increased attention to providing a more sophisticated scientific approach to the possible etiology and pathology of anxiety disorders [72].
3.1.1. Iron Deficiency-Related Depression/Anxiety Disorders
The causes of depression include biological, psychological and psychosocial or environmental factors [73]. Of note, lower levels of circulating iron have been reported to be associated with depression across different age groups and genders [74]. Fatigue, lethargy and depression can all be symptoms of iron deficiency [75]. One study examining 2000 persons over 65 inferred that lower levels of serum ferritin, transferrin and hemoglobin were linked with depressive symptoms [76]. Numerous studies have linked symptoms of depression with decreased levels of serum ferritin in adults, including Japanese workers [77,78], female medical students [79] and female soldiers [80]. In adolescents aged 12 to 17, serum ferritin was inversely correlated with depressive symptom severity [81]. Children with severe, chronic iron deficiency in infancy have increased anxiety and/or depression along with social and attention problems [82]. In addition, consistent results have been observed in the context of maternal IDA patients, where impaired postpartum reasoning and emotional reactions, and even postpartum depression, can occur [83,84]. The fact that treatment with iron supplements can improve the key aspects of mood in a combined cohort, including symptoms of depression, validates the described correlations between iron levels and psychological conditions [80,83,84]. It is important to note that limited studies revealed the relevance of brain iron deficiency and depression/anxiety disorders. For instance, in a prospective and observational study, it was found that individuals with depression had a significantly higher prevalence of hypotransferrinemia < 2 g/L, which may indicate a novel form of brain functional iron deficiency [24].
Animal studies have also revealed the relevance of iron deficiency to depressed mood or depression. Two separate studies found that a low-iron diet can result in depressed mood in monkeys [85,86]. Although these studies did not identify the specific molecular mechanisms involved, they did discuss the association between iron and the synthesis of monoamine transmitters, as discussed in Section 4.1 of this review. Rodent studies linked depression and iron management guidelines as well. In postpartum rats, gestational iron supplementation ameliorated symptoms of depression. The authors also demonstrated that iron supplementation prevented neuronal loss and increased dendritic spine density in the rat brain. The overall findings that perinatal iron supplementation could reduce susceptibility to postpartum depression, while iron deficiency had the opposite effect, are consistent with human studies [87].
Growing evidence suggests that iron plays an important role in the neurological mechanisms underlying anxiety disorders. Individuals with anxiety disorders tend to be more iron-deficient than healthy individuals [81]. A longitudinal follow-up analysis showed that children who had been tested and treated for iron deficiency as infants have greater anxiety/depression and social discomfort. These patients also presented lower scores for general mental and motor functions, as well as differences in some cognitive processes, compared with controls [82]. Another clinical study shed light on the close relationship between low iron availability and anxiety disorders. A significantly higher risk of anxiety disorder, delayed development and intellectual disabilities was noted among children (below age 13) with IDA. Adolescents with IDA aged 13 to 18 exhibited an increased risk of unipolar depressive disorder, anxiety disorder, delayed development, and other emotional illness. These results suggest that iron deficiency, whether in childhood or adolescence, does indeed have a significant impact on psychiatric comorbidities [88].
In rodent models, rats fed an iron deficient diet showed more anxiety-like behavior than control rats, which is consistent with observations reported in human studies [89]. Another study revealed that prenatal iron deficiency can also lead to elevated anxiety behavior in neonatal rats, which seemed to be rescued by iron repletion; however, some aspects of altered exploratory behavior and growth persisted into adulthood [90]. Despite the fact that effects of maternal iron deficiency in rats may differ from humans, given the differences in the timing of brain growth between the species, the study’s results may have implications for humans [91]. An additional study using Cp-deficient mice showed that the knockout (KO) mice exhibited a heightened anxiety phenotype, without any impairment of memory acquisition or retention [92]. The authors also considered whether the reduced hippocampal iron in Cp-deficient mice may result in reduced levels of serotonin, norepinephrine, and decreased brain-derived neurotrophic factor (BDNF) expression, which may underlie the sensitive anxiety behavior in Cp-deficient mice.
3.1.2. Iron Overload-Related Depression/Anxiety Disorders
Compared with the relatively abundant research that links iron deficiency to depression, only a few studies have documented the influence of increased iron on depression and depressive-like behaviors. Treatment of a small group of psychiatric patients, who were found to have iron overload as manifested by abnormal ferritin, transferrin saturation index, or excessive urinary iron, with an iron chelator apparently led to clinical improvement [93]. A study in transfusion-dependent thalassemia children (TDT) aged 6 to 12 with iron overload showed that blood transfusions may be causally related to major depressive-like episodes in this population and that the effects are, at least in part, mediated by iron overload and the consequent immune-inflammatory response [94], this study is consistent with a previous report, which also reported the severity of depression in beta thalassemia patients [95]. In another study, researchers used quantitative susceptibility mapping (QSM) to examine brain iron concentration in depressed patients and evaluated whether iron was related to disease severity. The authors concluded that brain iron deposition may be associated with depression and may even be a biomarker for investigating the pathophysiological mechanism of depression [96]. Several similar findings are also found in late-life depressive patients on antidepressant medication and in patients with recurrent depression. It is reported that elevated iron concentration was found in several brain regions with the progress of depression [97,98].
In addition to the above clinical studies, an animal study in chronically stressed mice with depressive-like phenotypes demonstrated that iron overload exacerbated the depressive-like behaviors, and introduced motor and cognitive impairments that do not occur in mice exposed to chronic unpredictable mild stress (CUMS) alone [99,100]. Together, the above-mentioned studies indicate that increased iron uptake can exacerbate the pathophysiological evolution of depression and may suggest that excess iron uptake has to be considered as an additional risk factor for cognitive impairment in patients with depression.
There are few human studies focused on revealing the relevance of iron deposition and anxiety disorders. It is reported that children with thalassemia are also likely to have psychological problems like anxiety disorder or generalized anxiety disorder [101,102]. Furthermore, it has been observed that individuals with PD and anxiety exhibit increased iron accumulation in the fear circuit of the brain. This finding suggests that the excessive iron buildup in this region may potentially contribute to the onset and development of anxiety symptoms in individuals with PD [103]. Moreover, rodent studies have reported that iron overload in the brain appears to accelerate anxiety-like behavior and mood. Adult rats receiving daily intraperitoneal injections of iron [104,105] or being fed a carbonyl iron diet containing 20,000 ppm iron [106] both showed anxious responses and/or other behavioral impairments. After deletion of TfR2, which modulates systemic iron metabolism through the regulation of hepcidin, mice showed increased anxiety-like behaviors and brain iron availability. It is worth noting that these anxiety-related behaviors were iron dependent because they could be reverted by an iron deficient diet in Tfr2-KO mice [107]. An additional study reported that an axonal iron transport pathway from the ventral hippocampus (vHip) to the medial prefrontal cortex (mPFC) to the substantia nigra can modulate anxiety-related behaviors. Moreover, vHip-mPFC iron transport is essential for the anxiolytic effects of diazepam. These findings underscore the vital role of brain iron transport and identify it as a potential target for anxiety treatments [108]. Taken together, these findings support the hypothesis that imbalanced iron metabolism plays a pivotal role in modulating anxiety and emotional behaviors.
3.2. Schizophrenia
Schizophrenia is a severe mental disorder that can cause a combination of hallucinations, delusions, and severely disordered thinking and behavior that impairs daily functioning, and may be disabling. People with schizophrenia may perceive reality abnormally and often have persistent difficulties with cognitive skills such as memory, attention, and problem-solving. Worldwide, approximately 24 million people, or 1 in 300 people (0.32%), are affected by schizophrenia, and the rate is 1 in 222 people (0.45%) among adults. Unfortunately, stigma, discrimination, and violations of human rights in both mental health institutions and community settings against individuals with schizophrenia are common [109,110,111]. Research has not identified a single definitive cause of schizophrenia, but it is believed that an interaction between genes and environmental factors, including psychosocial factors, may contribute to the development of schizophrenia [112,113].
3.2.1. Iron Deficiency-Related Schizophrenia
Iron is demonstrated to affect the pathophysiology of schizophrenia through several mechanisms, which will be further summarized in the fourth section of this review. Iron deficiency and schizophrenia have been linked in multiple studies, suggesting that there may be a potential relationship between the two conditions. A recent narrative review systemically reviewed the current state of science regarding perinatal iron deficiency and the development and pathophysiology of schizophrenia. The authors proposed that perinatal iron deficiency may act as a potential early environmental insult in the developmental programming of schizophrenia, with evidence from animal, human, and epidemiological research [114]. A meta-analysis of 39 studies combined plasma and serum data, providing evidence of deficient iron in patients with schizophrenia [115]. Additionally, decreased iron concentration was found in gray matter nuclei in patients with first-episode schizophrenia, using QSM and effective transverse relaxation rate (R2*) maps [116]. Furthermore, iron supplementation has been shown to improve cognitive function and symptoms in patients with schizophrenia who were also iron deficient [117]. These findings suggests that there may be a potential clinical use for iron supplementation in the treatment of schizophrenia.
3.2.2. Iron Overload-Related Schizophrenia
Recent studies have suggested a potential relationship between iron overload and schizophrenia symptoms. Magnetic resonance brain neuroimaging research in schizophrenia patients has shown elevated brain iron levels in different brain regions, including the putamen and thalamus [118,119]. In addition, increased iron levels were also found in the prefrontal cortex of people with schizophrenia, as measured by inductively coupled plasma-mass spectrometry (ICP-MS) and Western Blots [60]. This emerging evidence suggests a potential association between iron overload and schizophrenia in individuals or in post-mortem human brain samples. However, the exact mechanisms through which iron overload or deficiency contributes to schizophrenia are not yet fully understood. It is believed that iron accumulation in certain brain regions may disrupt neurotransmitter systems or myelination involved in cognition and emotion regulation [120]. Additionally, high levels of iron may contribute to oxidative stress and inflammation, which have been implicated in the pathophysiology of schizophrenia [121,122].
Further research is needed to understand the complex relationship between iron metabolism and schizophrenia. Identifying and treating iron overload or deficiency in these cases may help alleviate psychiatric manifestations, but more research is needed to elucidate the underlying mechanisms and explore potential therapeutic interventions.
3.3. Post-Traumatic Stress Disorder (PTSD)
Fear memory is universally present in humans and several animal groups. This memory is a self-protective reaction that evolved to enable adaptation to the environment, and, ultimately, survival. Although fear has a strong survival value, excessive fear stress response can surpass the needs of the individual to effectively avoid danger and acquire coping strategies, leading to the occurrence of pathological mental disorders, such as PTSD [123,124]. PTSD is a disabling neuropsychiatric disorder characterized by the intrusion of an event, avoidance of recall of an event, alterations in cognition and mood, and hyperarousal, as per the DSM-V. In PTSD, fear memory becomes recurring and may be recalled very intensely in the form of flashbacks in circumstances in which the sufferer recognizes elements or components of the traumatic experience, even in the absence of stimuli that resemble the conditional stimuli. Therefore, PTSD is viewed as a serious psychiatric condition in which the most prominent symptom is a widely generalized retrieval of fearsome and traumatic experiences [123].
3.3.1. Iron Deficiency in the Pathology of PTSD
Brain iron plays a vital role in the function of learning and memory. An increasing number of studies have found that aberrant iron metabolism is involved in PTSD pathology. A follow up in adolescents from a study in infants reported that iron deficiency at 12 or 18 months of age predicted greater adolescent behavioral issues compared with iron sufficiency; the iron deficient infants exhibited increases in adolescent-reported anxiety and social problems, and parent-reported social, PTSD and attention-deficit/hyperactivity disorder (ADHD) [125]. A study employing a trace heart rate-fear conditioning paradigm demonstrated that perinatal nutritional iron deficiency impaired hippocampus-dependent fear learning, which was not reversed by a subsequent normal iron diet, and appeared to persist into adulthood [126]. Investigation of gestational iron deficient rats revealed diminished fear conditioning in postnatal (P) rats on days P28 and P35. Serendipitously, this research also found that trace fear conditioning was enhanced by prior iron deficiency in the P56 group. This unanticipated finding in iron-repleted adults is consistent with the effects of developmental iron deficiency on inhibitory avoidance learning, but contrasts with the persistent deleterious long-term effects of a more severe iron deficiency protocol, suggesting that the degree and duration of iron deficiency can affect the possibility of recovery from its deleterious effects [127]. In our recent study, we investigated the affected contextual fear memory caused by brain iron deficiency in genetically modified mice. After specific deletion of FPN1 in neurons and astrocytes, the total iron contents in the cortex and hippocampus were dramatically decreased. The deficient brain iron, which was subsequently shown to be a consequence of decreased iron delivery through the BBB, ultimately depressed the contextual fear memory. The study highlights the major role played by FPN1 in brain iron homeostasis and identifies this pathway as a potential target for the treatment of PTSD [26].
3.3.2. Iron Overload in the Pathology of PTSD and Fear Memory
Iron overload may also function in fear memory and in the pathology of PTSD. In peripheral tissues, chronic psychological stress in mice led to significantly increased iron content in plasma and the duodenum, whereas the hepatic iron level was markedly decreased, providing insights into the mechanisms of iron metabolism during the stress disorder [128]. In a rat model of PTSD, stress exposure increased the level of total brain iron, and TfR1 and ferritin expression in the hippocampus, frontal cortex, and striatum, all of which are associated with fear memory treatment. The elevated iron in these cognition-related brain regions may be responsible for the neuronal injury, suggesting that iron may function in the pathology of PTSD [129]. In addition, the loss of FPN1 in neurons, which leads to brain iron accumulation, impairs fear memory by promoting ferroptosis in Alzheimer’s disease (AD) [130]. It is worthy to point out that, in a neonatal iron overload model in rats, permanently elevated brain iron also impaired inhibitory avoidance memory, an instrumental fear conditioning memory [131,132,133,134]. In another study, anesthesia-induced brain iron overload or iron deficiency also led to fear memory deficits with different mechanisms that warrant further elucidation [135,136].
3.4. Neurodevelopment Disorders
3.4.1. Brain Iron and ADHD
ADHD is a neurodevelopmental disorder characterized by excessive amounts of inattention, carelessness, hyperactivity (which can evolve into inner restlessness in adulthood), and impulsivity that are pervasive, debilitating and otherwise age-inappropriate [137]. Some individuals with ADHD also display difficulty regulating emotions and have executive dysfunction. ADHD is also associated with other mental disorders and substance abuse disorders, which can cause additional impairment [138,139].
Limited research has been conducted on the impact of brain iron overload on ADHD. Nevertheless, several studies have established a correlation between iron deficiency (ID), particularly serum iron deficiency, and ADHD [19,140,141]. To establish a potential correlation between iron deficiency in specific brain regions and ADHD symptoms, larger, longitudinal magnetic resonance imaging (MRI) studies were also conducted, offering a deeper understanding of the underlying mechanisms. In a pilot MRI study, children aged 8–14 years with ADHD exhibited significantly lower estimated brain iron levels in both the right and left thalamus compared to healthy controls. Furthermore, these children also demonstrated significantly lower levels of serum ferritin compared to the control group [142]. Similar findings have been reported in different brain regions, including the thalamus, using non-invasive MRI methods [143]. Another whole-brain analysis using QSM revealed iron deficiency in several brain regions in children with ADHD, such as bilateral striatums, anterior cingulum, olfactory gyrus, and right lingual gyri [140]. These studies suggest that iron deficiency may play a role in the pathophysiology of ADHD.
The long-term association found between infant iron deficiency and sluggish cognitive tempo and attention-deficit/hyperactive-impulsive behaviors suggest that the neurodevelopmental alterations that stem from postnatal iron deficiency may play an etiological role in the development of ADHD [144,145]. A recent, systematic review including a meta-analysis of three studies, in which brain iron content was assessed, revealed a statistically significant, lower thalamic iron concentration in children with ADHD than in healthy control subjects [146]. A double-blind, randomized, placebo-controlled trial investigating the effects of oral ferrous sulfate on ADHD revealed significant improvement in subjects who received iron supplementation therapy. The improvements were observed in the total score as well as the hyperactive/impulsive and inattentive subscales of the ADHD Rating Scale, a standardized assessment tool used to evaluate ADHD symptoms [147]. These findings suggest that iron supplementation (at a dosage of 80 mg/day) may be beneficial for children with low serum ferritin levels, indicating the need for further investigation through larger controlled trials. Importantly, iron therapy was well-tolerated and demonstrated comparable effectiveness to stimulant medications [147]. Additionally, a qualitative systematic review of nine randomized clinical trials examined the efficacy of iron-zinc supplementation in the treatment of ADHD in children and adolescents [148]. The review findings indicated that lower baseline levels of zinc and iron were associated with higher ADHD severity and poorer treatment outcomes. Dietary supplementation with zinc and iron showed improvements in ADHD symptom severity compared to the placebo control, although the effect sizes were small and specific to certain ADHD symptoms. This suggests that iron–zinc supplementation may be beneficial for specific subgroups of individuals with ADHD [148]. Taken together, these findings further support a potential association between reduced brain iron concentration and ADHD in this specific age group and highlight the importance of early intervention and treatment to prevent or minimize the impact of this condition on an individual’s cognitive and behavioral functioning. However, further research is required to confirm and expand upon these results.
3.4.2. Brain Iron and ASD
ASD is a range of neurodevelopmental conditions primarily characterized by significant difficulties in social interactions, differences in communication, and rigid and repetitive behavior. Unusual responses to sensory input, including high or low sensitivity, sensory discrimination, and sensory-based motor differences, are also highly prevalent [149].
Previous studies have raised concerns that the intake of the trace elements, iron and zinc, vitamins (B vitamins, vitamin D, vitamin K), and calcium may be deficient in up to one-third of children with ASD [150,151]; children with ASD are at risk for low serum ferritin, which has been associated with attention and sleep issues [152,153]. As with the other neuropsychiatric disorders discussed herein, the association between brain iron status and ASD is also unclear, i.e., whether a lower-than-normal level of serum iron may result in a lower-than-normal level of brain iron requires confirmation. A study measuring brain iron contents in various regions using QSM showed lower brain iron content in preschool children with autism than normal preschool children [154]. A population-based case–control study revealed that children with autism exhibited neurodevelopmental delays, and provided initial evidence for an association between increased maternal supplemental iron intake and reduced risk of ASD [155]. Also, abnormalities in brain iron may contribute to various psychiatric disorders, including autism with altered myelin-related molecular systems [62,153]. Based on the previous findings, Table 1 presents an overview of the current progress in clinical evidence regarding the relationship between brain iron (Fe) and the aforementioned mental disorders.

Table 1.
Summary of current clinical evidence for the relationship between brain iron (Fe) and mental disorders.
4. Potential Mechanisms Underlying the Influence of Iron on Mental Disorders
Numerous studies, from animal models to humans, have considered the effects of peripheral and central iron status on mental disorders. However, limited research has focused on revealing the potential molecular mechanisms of iron’s involvement in the pathogenesis or pathology of such diseases. Iron’s role in physiological functions includes, but is not limited to, energy metabolism, neurotransmission and myelination. These functions also have important roles in the development of mental disorders. For instance, altered dopaminergic and serotonergic neurotransmission have been described in depression, anxiety-related disorders, ADHD and ASD [156,157,158]. An accumulating body of evidence also implicates both energy and myelination, both of which are affected by iron status, in mental disorders [159]. While there may be additional mechanisms linking iron to brain function, since there is a relatively clear relationship between iron status and neurotransmission, oxidative stress and myelination, we will focus on these three mechanisms by exploring the literature linking iron to mental disorders (Figure 2).
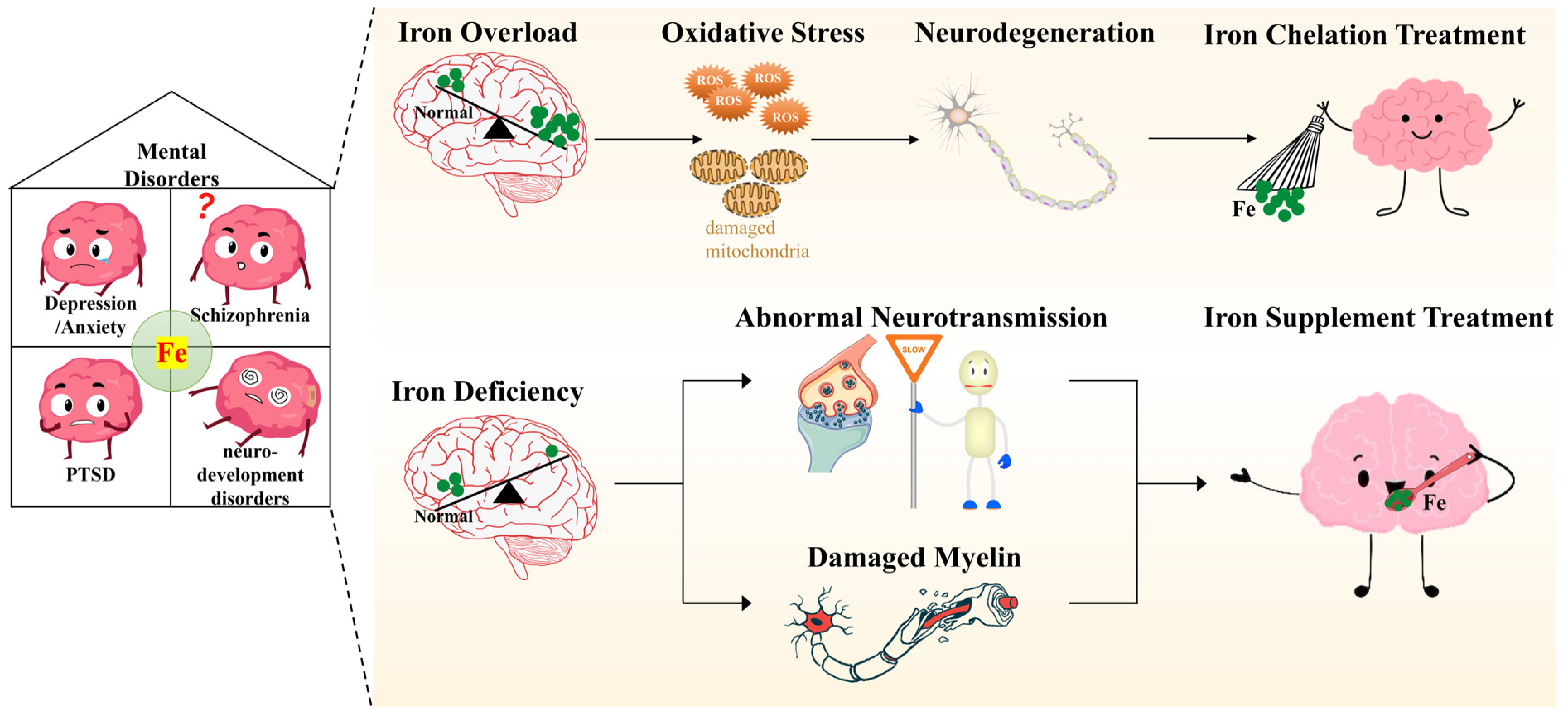
Figure 2.
Potential mechanisms underlying the pathological processes of mental health conditions and targeting brain iron metabolism in the treatment of related mental disorders.
Imbalanced brain iron metabolism, including iron overload and iron deficiency, is involved in the pathological processes of mental disorders, including depression, anxiety, schizophrenia, PTSD and neurodevelopment disorders. Brain iron overload, seen in depression, anxiety, schizophrenia and PTSD, results in elevated oxidative stress that can damage mitochondria, ultimately leading to neurodegeneration. Sequestering excess brain iron using exogenous iron chelators is becoming a viable strategy in the treatment of iron overload-related mental disorders, such as cognitive impairments, depression and anxiety. Moreover, brain iron deficiency is particularly relevant to the pathogenesis of neurodevelopment disorders, including ADHD and ASD, depression, anxiety, schizophrenia and PTSD. Insufficient brain iron results in abnormal neurotransmission and neurodevelopment due to both a lack of neurotransmitters and damaged myelin. Accordingly, iron supplementation may also be a successful approach in the clinic.
4.1. Iron and Neurotransmission
Mounting evidence supports an intimate connection between brain iron status, especially iron deficiency, and dysfunction in both myelination and neurotransmission, especially monoamine metabolism [160]. Monoamines, including serotonin, dopamine (DA) and noradrenaline (NA), have major roles in various aspects of emotional behaviors. Indeed, the balance of these three neurotransmitters (NTs) can determine various mental disorders [76,161].
Several studies have focused on how brain iron status affects the neurotransmission mediated by monoamines. Iron plays a pivotal role in the synthesis of serotonin, DA and NA, as the metal is an essential cofactor of aromatic amino acid hydroxylases (phenylalanine, tyrosine, tryptophan [Trp]). These enzymes can limit the synthesis of the above-mentioned monoamines. Neuronal iron deficiency decreased the availability of Trp in neurons, resulting in lower synaptic serotonin concentration. Dysregulation and diminished serotonin concentration then reduced the synaptic connectivity in the prefrontal cortex and hippocampus, ultimately triggering neuro-behavioral manifestations, neuropsychological disorders and depression [24]. A study in humans revealed that children and young adults with IDA in infancy show altered social-emotional behavior, specifically wariness and hesitance, a lack of positive affect, and diminished social engagement, among other behavioral abnormalities. Pharmacologic and neuroimaging studies implicate an altered mesolimbic dopamine pathway [162]. Studies employing iron-deficient rodent models have also demonstrated a robust relationship between behavioral abnormalities and altered dopamine metabolism in the brain [163,164]. It has also been documented that iron metabolism is involved in the dopaminergic pathway and neurotransmission. DA anabolism involves the iron-dependent phenylalanine and tyrosine hydroxylases (TyrH). Reduced activity of TyrH caused by iron deficiency, especially in the substantia nigra, can impair DA synthesis, which may then cause psychological and motor issues in adults [165,166,167].
Compared to the various studies on the consequences to DA affected by brain iron metabolism, few studies have examined serotonin and NA signaling. However, alterations in serotonin signaling may also be responsible for emotional behaviors since the neurotransmitter has an important role in mediating affective behaviors. A study in patients with Parkinson’s disease (PD) revealed an early, linear association between low serotonin and higher nigral iron, which was absent in controls, warranting further studies to investigate the relationship between brain iron status and serotonin-related emotional behaviors in the pathology of PD [168]. In animal studies, differing results have been observed. Brain iron deficiency can both up-regulate or decrease the levels of serotonin in different rat models [169,170]. Additionally, in other rat studies, diminished brain iron levels had no effect on serotonin levels or metabolism in newborns or adults, and brain serotonin in the iron-deficient rats did not differ from controls [171,172]. Extracellular NA concentrations are elevated under iron deficiency states, whereas tissue levels are unchanged compared with controls [173,174,175]. A diverse set of results from NA transporters (NAT) and α2 adrenergic receptor expression was observed in ID rats [176]. In CP deficient mice, increased anxiety is associated with elevated levels of plasma corticosterone and decreased levels of serotonin and NA, as well as brain-derived neurotrophic factor (BDNF) and its receptor [92]. Altered hippocampal BDNF signaling is linked to changes in serotonin and NA levels. Reduced BDNF and increased corticosterone are associated with increased anxiety [177,178]. These observations of monoamine homeostasis affected by brain iron status are not necessarily conflicting, but may hint that there is particular significance to the molecular mechanisms around the iron–monoamine relationship in emotional behaviors.
4.2. Iron and Oxidative Stress
Iron plays important roles in cell biology, including oxygen transport, storage, and delivery in the form of heme in hemoglobin and myoglobin. The heme cofactor is also a major component in numerous oxidase and oxygenase enzymes, and in electron transfer proteins (cytochromes) for mitochondrial electron transport [179,180]. Due to iron’s unique redox properties, coupled with the high-energy demand of the brain, brain iron must be strictly regulated. Iron can induce oxidative stress by catalyzing the formation of hydroxyl radicals, the most harmful cellular reactive oxygen species (ROS), through the Fenton reaction [181]. ROS can directly damage DNA, cause several direct oxidative modifications of amino acid residues, and promote the peroxidation of polyunsaturated fatty acids in membrane lipids, leading to alterations in and functional loss of membranes [16,182].
TDT patients with iron overload exhibit severe depression, which may be related to increased iron-induced oxidative stress and immune inflammation [94,183]. In a rat model of PTSD, increased iron levels in the hippocampus, frontal cortex and striatum were shown to lead to mitochondrial swelling and neuronal cell apoptosis through increased oxidative stress [129]. Another mouse study demonstrated that hippocampal iron overload, induced by the deletion of the iron trafficking protein lipocalin-2 (LCN2), led to elevated oxidative stress, thus interfering with neurogenesis in adult animals and ultimately abolishing hippocampal-dependent contextual fear discriminative task behavior. Of note, supplementation with the iron-chelating agent, deferoxamine, rescues oxidative stress in adult neural stem cells (NSCs), promotes cell cycle progression and improves contextual fear conditioning [184]. Additional studies have reached similar conclusions that brain iron overload may cause mood disorders through oxidative stress [185,186].
Excess iron-induced oxidative stress can also have an impact on monoamine function. Administration of a small amount of iron for only a few days promoted iron accumulation in the substantia nigra of the adult brain, where iron may generate cytotoxic free radicals. Such oxidative stress can impair dopaminergic signaling and monoamine function, leading to behavioral impairments [186,187,188]. M30, an inhibitor of monoamine oxidase A, can attenuate chronic intermittent hypoxia-induced oxidative stress, inflammation and serotonin deficiency by chelating excess iron, and may prevent the onset of depressive symptoms in obstructive sleep apnea patients [189]. In addition, ferroptosis, a newly recognized type of cell death caused by the accumulation of intracellular iron that promotes lipid peroxidation and then cell death, may also have an influence on mental health and related emotional disorders [130,190,191]. Iron-related oxidative stress also participates in the pathology of neurodegenerative diseases and the related cognitive dysfunction, such as in AD and PD [192]; however, the exact role of iron and oxidative stress in emotional disorders require further clarification.
4.3. Iron and Myelination
Various studies have reported perturbations of myelin and myelin-related systems in psychiatric disorders, with altered myelination during childhood and adolescence implicated in schizophrenia, autism and other conditions [193,194]. Altered myelination can affect synapse formation and its plasticity, conduction velocity and synchronic impulse trafficking between different brain regions. This may both influence normal mental performance and contribute to psychiatric symptoms in disorders involving myelin abnormalities [195]. As iron is directly required for myelination, it has been shown that poor brain myelination resulting from iron deficiency in early development has long-lasting effects on behavioral functions [196,197,198]. A few studies have examined the relationship between emotional disorders and interdependent abnormalities in brain iron metabolism and myelin, or the molecular systems involved. A study using a mouse model of increased brain iron levels demonstrated that brain iron accumulation affects myelin-related molecular systems implicated in neurodegeneration with brain iron accumulation (NBIA) with neuropsychiatric features [62]. These studies are indicative of a link between brain iron status and myelination, whose detailed mechanisms still require in-depth understanding.
5. Limitations of the Presented Studies
While the above review provides important insights into the association between iron deficiency and iron overload with mental disorders, we must also take note of the limitations of these studies. Firstly, most of the studies are based on observational data and cannot establish causality. Although there are some experimental studies, more randomized controlled trials are still needed to validate these findings. Secondly, different methods of behavioral measurements and the influence of other metals may also produce different behavioral outcomes [161]. There are variations in the psychological health assessment tools and iron indicators used in the studies, limiting the comparability of results. Thirdly, there are differences in the characteristics of the study samples, such as age, gender, and baseline iron levels, which may affect the interpretation and generalizability of the results. Additionally, although animal studies provide valuable information for exploring the relationship between iron and mental disorders, there are biological and physiological differences between humans and animals, so these findings need to be cautiously interpreted and extrapolated. Moreover, studies exploring the relationship between mental disorders and changes of endogenous brain iron metabolism are rare. Therefore, further research on patients with gene mutation that can affect the brain iron metabolism should be carried out to reveal its relevance. Fourthly, the occurrence and development of mental disorders is a complex process involving the interaction of multiple factors, such as genetics, environment, and lifestyle. Iron is just one factor, and other factors, like other trace elements, may also play important roles in the occurrence of mental disorders. Finally, the use of iron supplementation and iron chelation therapy holds great promise in the treatment of psychological disorders, but it also has its limitations. The action of medications often affects a wide range of areas in the body, while iron deficiency or iron overload typically occurs in specific regions of the brain in the pathophysiology of mental disorders. However, our research has found that liposome-based DFO (Deferoxamine) has shown promising results in the treatment of cerebral ischemia, which suggests that liposome-based iron chelators with their targeted nature provide a valuable avenue for research [199]. Therefore, the development of targeted therapeutic agents for the treatment of psychological disorders is a highly promising and necessary research direction. Further research needs to employ more rigorous study designs, control for potential confounding factors, and conduct larger-scale, multicenter studies to better understand the relationship between iron and mental disorders and provide more reliable evidence for prevention and treatment. We must improve our understanding of the pathologies associated with other psychiatric disorders as well to develop therapies to alleviate mental dysfunction.
6. Conclusions and Prospects
As a group, mental disorders have a generally high incidence, compared with other health conditions. According to the World Health Organization (WHO), 970 million people around the world live with a mental disorder, with anxiety and depressive disorders being the most common [200]. In 2020, the number of people living with anxiety and depressive disorders rose significantly as a result of the COVID-19 pandemic [64]. While effective prevention and treatment options exist, most people with mental disorders do not have access to effective care. Untreated mental health conditions can lead to severe personal consequences, such as self-harm and suicide, creating significant familial and societal burdens [201,202]. Individuals with mental disorders can also experience stigma, discrimination and a violation of human rights [203,204,205]. In view of this, uncovering effective prevention and treatment options has become a major aim in a multitude of research programs.
Iron has specific and unique roles in the brain because of the metal’s direct involvement in the CNS function, including myelin synthesis, neurotransmitter synthesis and metabolism [59]. Imbalanced brain iron metabolism, such as iron overload, can participate in and even aggravate the pathology of neurological diseases, such as AD, PD and stroke, through elevating oxidative stress that can destroy the cell membrane and induce cell death [16,206,207]. On the other hand, iron deficiency and brain iron deficiency are correlated with mental disorders through impairing neurodevelopment, largely in pathways related to neurotransmission and myelination. Moreover, in the treatment of diseases induced by imbalanced brain iron homeostasis, numerous studies have suggested that exogenous modulation of iron metabolism may be successful at preventing or treating neurological disorders. For instance, iron chelators have been reported to alleviate the symptoms of AD, PD and stroke, or show therapeutic promise in preclinical and clinical models of other neurological disorders [208,209,210]. In contrast, iron supplement has been suggested to relieve ID-related mental disorders [211,212]. Finally, several key molecules of brain iron metabolism, such as hepcidin and CP, have also been reported as potential therapeutic targets for AD and PD through their neuroprotective roles in the pathological processes of these diseases [16]. Expanding our understanding of brain iron metabolism is crucial to affecting underlying mechanisms in order to improve the strategies that help prevent and possibly cure mental disorders.
The mechanisms underlying the influence of iron on mental disorders are various and complicated. The effects of iron in psychological health are determined by numerous physiological/biological properties and spatial/temporal factors. These include neurotransmission, iron-related myelination and oxidative stress, among many others. Recently, the influence of ferroptosis on mental health and related emotional disorders as also been illustrated in several studies [130,190,191]. In addition, iron distribution in different brain regions, and the duration and timing of iron exposure (i.e., during different development periods), the route of exposure, animal species, sex, nutritional status and disease state all can contribute to the influence of iron on mental disorders.
Previous studies on iron homeostasis and mental health conditions revealed that targeting brain iron metabolism-related molecules, particularly those relating to iron supplementation, for drug development is expected to identify innovative ways to treat neuropsychological disorders [80,83]. In a previous, recent work, we reviewed the methods of iron supplementation and iron chelation, as well as the latest progresses in this area [16]. With the continuous developments in brain iron metabolism and mental disorders, the relationship between iron and the brain is becoming a rich area for translatable scientific exploration. Important connections between mental health conditions and other trace elements, such as copper and zinc, are also becoming apparent [213,214]. It is therefore expected that a novel discipline of trace element nutrition and mental disorders will emerge as a new direction for clinical therapy.
Author Contributions
Q.W. wrote Section 1, Section 2, Section 3, Section 4 and Section 5, Q.R. wrote Section 2 (Regulation of brain iron homeostasis), J.M. wrote Section 4.1 (Iron and Neurotransmission), Y.-Z.C. and W.-J.G. wrote Section 1 (Introduction) and Section 5 and Section 6 (Limitations of Presented Studies, Conclusion and prospects). W.-J.G. and Y.-Z.C. conceived the review and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Hebei Province (Grant Number: C2023423008), the Science Research Project of Hebei Education Department (Grant Number: BJK2023095), and the Basic research program for young teachers of Hebei University of Chinese Medicine (Grant Number: YQ2018007).
Data Availability Statement
All data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Solmi, M.; Soardo, L.; Kaur, S.; Azis, M.; Cabras, A.; Censori, M.; Fausti, L.; Besana, F.; Salazar de Pablo, G.; Fusar-Poli, P. Meta-analytic prevalence of comorbid mental disorders in individuals at clinical high risk of psychosis: The case for transdiagnostic assessment. Mol. Psychiatry 2023, 28, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.; Chisholm, D.; Kessler, R.; Patel, V.; Whiteford, H. Mental Disorders. Disease Control Priorities Related to Mental, Neurological, Developmental and Substance Abuse Disorders; World Health Organization: Geneva, Switzerland, 2006; pp. 1–20. [Google Scholar]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology 2023, 48, 113–120. [Google Scholar] [CrossRef]
- Craske, M.G.; Herzallah, M.M.; Nusslock, R.; Patel, V. From neural circuits to communities: An integrative multidisciplinary roadmap for global mental health. Nat. Ment. Health 2023, 1, 12–24. [Google Scholar] [CrossRef]
- Tong, Q.; Cui, X.; Xu, H.; Zhang, X.; Hu, S.; Huang, F.; Xiao, L. D1 receptor-expressing neurons in ventral tegmental area alleviate mouse anxiety-like behaviors via glutamatergic projection to lateral septum. Mol. Psychiatry 2023, 28, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pan, X.; Zhou, Y.; Wu, Z.; Ren, K.; Liu, H.; Huang, C.; Yu, Y.; He, T.; Zhang, X.; et al. Lateral septum-lateral hypothalamus circuit dysfunction in comorbid pain and anxiety. Mol. Psychiatry 2023, 28, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.M.; Barraza, M.; Hu, K.Y.; Frias, S.J.; Long, K.L.; Kaufer, D. Juvenile exposure to acute traumatic stress leads to long-lasting alterations in grey matter myelination in adult female but not male rats. Neurobiol. Stress 2021, 14, 100319. [Google Scholar] [CrossRef]
- Boda, E. Myelin and oligodendrocyte lineage cell dysfunctions: New players in the etiology and treatment of depression and stress-related disorders. Eur. J. Neurosci. 2021, 53, 281–297. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Dong, D.; Zhou, F.; Li, Z.; Zhao, L.; Long, Z. Stress and the brain: Emotional support mediates the association between myelination in the right supramarginal gyrus and perceived chronic stress. Neurobiol. Stress 2023, 22, 100511. [Google Scholar] [CrossRef]
- Zlomuzica, A.; Plank, L.; Kodzaga, I.; Dere, E. A fatal alliance: Glial connexins, myelin pathology and mental disorders. J. Psychiatr. Res. 2023, 159, 97–115. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dey, A.; Gopalakrishnan, A.V.; Swati, K.; Ojha, S.; Prakash, A.; Kumar, D.; Ambasta, R.K.; Jha, N.K.; Jha, S.K.; et al. Glutamatergic neurotransmission: A potential pharmacotherapeutic target for the treatment of cognitive disorders. Ageing Res. Rev. 2023, 85, 101838. [Google Scholar] [CrossRef]
- Ochi, T.; Vyalova, N.M.; Losenkov, I.S.; Paderina, D.Z.; Pozhidaev, I.V.; Loonen, A.J.; Simutkin, G.G.; Bokhan, N.A.; Wilffert, B.; Ivanova, S.A. Polymorphisms in the adrenergic neurotransmission pathway impact antidepressant response in depressed patients. Neurosci. Appl. 2023, 2, 101016. [Google Scholar] [CrossRef]
- Wang, F.; Yang, X.; Ren, Z.; Chen, C.; Liu, C. Alternative splicing in mouse brains affected by psychological stress is enriched in the signaling, neural transmission and blood-brain barrier pathways. Mol. Psychiatry, 2023; online ahead of print. [Google Scholar]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Senussi, N.H.; Fertrin, K.Y.; Kowdley, K.V. Iron overload disorders. Hepatol. Commun. 2022, 6, 1842–1854. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; You, L.; Zhang, J.; Chang, Y.Z.; Yu, P. Brain Iron Metabolism, Redox Balance and Neurological Diseases. Antioxidants 2023, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.D.; Li, H.; Kang, S.; Cui, Y.G.; Zheng, H.; Wang, P.; Han, K.; Yu, P.; Chang, Y.Z. The divergent effects of astrocyte ceruloplasmin on learning and memory function in young and old mice. Cell Death Dis. 2022, 13, 1006. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lin, S.; He, X.; Sun, N. Iron delivery systems for controlled release of iron and enhancement of iron absorption and bioavailability. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Murray-Kolb, L.E. Iron and brain functions. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 703–707. [Google Scholar] [CrossRef]
- Hua, M.; Shi, D.; Xu, W.; Zhu, L.; Hao, X.; Zhu, B.; Shu, Q.; Lozoff, B.; Geng, F.; Shao, J. Differentiation between fetal and postnatal iron deficiency in altering brain substrates of cognitive control in pre-adolescence. BMC Med. 2023, 21, 167. [Google Scholar] [CrossRef]
- Andrade, C. Antidepressant Prescription in Pregnancy: The Importance of Prenatal Maternal Anemia as a Potential Confound in Studies on Neurodevelopmental and Other Outcomes. J. Clin. Psychiatry 2020, 81, 20f13347. [Google Scholar] [CrossRef]
- Vigani, G.; Murgia, I. Iron-requiring enzymes in the spotlight of oxygen. Trends Plant Sci. 2018, 23, 874–882. [Google Scholar] [CrossRef]
- Todorich, B.; Pasquini, J.M.; Garcia, C.I.; Paez, P.M.; Connor, J.R. Oligodendrocytes and myelination: The role of iron. Glia 2009, 57, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Berthou, C.; Iliou, J.P.; Barba, D. Iron, neuro-bioavailability and depression. eJHaem 2022, 3, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A.; Ehrenreich, H. Myelination and oligodendrocyte functions in psychiatric diseases. JAMA Psychiatry 2014, 71, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hao, Q.; Li, H.; Wang, B.; Wang, P.; Jin, X.; Yu, P.; Gao, G.; Chang, Y.Z. Brain iron deficiency and affected contextual fear memory in mice with conditional Ferroportin1 ablation in the brain. FASEB J. 2021, 35, e21174. [Google Scholar] [CrossRef]
- Qian, Z.M.; Ke, Y. Brain iron transport. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1672–1684. [Google Scholar] [CrossRef]
- Baringer, S.L.; Simpson, I.A.; Connor, J.R. Brain iron acquisition: An overview of homeostatic regulation and disease dysregulation. J. Neurochem. 2023, 165, 625–642. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- McCarthy, R.C.; Kosman, D.J. Mechanistic analysis of iron accumulation by endothelial cells of the BBB. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2012, 25, 665–675. [Google Scholar] [CrossRef]
- Duck, K.A.; Simpson, I.A.; Connor, J.R. Regulatory mechanisms for iron transport across the blood-brain barrier. Biochem. Biophys. Res. Commun. 2017, 494, 70–75. [Google Scholar] [CrossRef]
- Rudisill, S.S.; Martin, B.R.; Mankowski, K.M.; Tessier, C.R. Iron Deficiency Reduces Synapse Formation in the Drosophila Clock Circuit. Biol. Trace Elem. Res. 2019, 189, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Ponnuru, P.; Klinger, M.E.; Myers, R.L.; Devraj, K.; Coe, C.L.; Lubach, G.R.; Carruthers, A.; Connor, J.R. A novel model for brain iron uptake: Introducing the concept of regulation. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2015, 35, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.I.; Liu, J.; Dutta, P. Iron transport kinetics through blood-brain barrier endothelial cells. Biochim. Biophys. Acta. Gen. Subj. 2018, 1862, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.W. Transport of iron in the blood-brain-cerebrospinal fluid system. J. Neurochem. 1997, 69, 443–454. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. Transferrin and transferrin receptor function in brain barrier systems. Cell. Mol. Neurobiol. 2000, 20, 77–95. [Google Scholar] [CrossRef]
- McCarthy, R.C.; Kosman, D.J. Ferroportin and exocytoplasmic ferroxidase activity are required for brain microvascular endothelial cell iron efflux. J. Biol. Chem. 2013, 288, 17932–17940. [Google Scholar] [CrossRef]
- McCarthy, R.C.; Kosman, D.J. Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS ONE 2014, 9, e89003. [Google Scholar] [CrossRef]
- Faucheux, B.A.; Nillesse, N.; Damier, P.; Spik, G.; Mouatt-Prigent, A.; Pierce, A.; Leveugle, B.; Kubis, N.; Hauw, J.J.; Agid, Y.; et al. Expression of lactoferrin receptors is increased in the mesencephalon of patients with Parkinson disease. Proc. Natl. Acad. Sci. USA 1995, 92, 9603–9607. [Google Scholar] [CrossRef]
- Moroo, I.; Ujiie, M.; Walker, B.L.; Tiong, J.W.; Vitalis, T.Z.; Karkan, D.; Gabathuler, R.; Moise, A.R.; Jefferies, W.A. Identification of a novel route of iron transcytosis across the mammalian blood-brain barrier. Microcirculation 2003, 10, 457–462. [Google Scholar] [CrossRef]
- Malecki, E.A.; Devenyi, A.G.; Beard, J.L.; Connor, J.R. Existing and emerging mechanisms for transport of iron and manganese to the brain. J. Neurosci. Res. 1999, 56, 113–122. [Google Scholar] [CrossRef]
- Fishman, J.B.; Rubin, J.B.; Handrahan, J.V.; Connor, J.R.; Fine, R.E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res. 1987, 18, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Chang, Y.Z.; Zhu, L.; Yang, L.; Du, J.R.; Ho, K.P.; Wang, Q.; Li, L.Z.; Wang, C.Y.; Ge, X.; et al. Development and iron-dependent expression of hephaestin in different brain regions of rats. J. Cell. Biochem. 2007, 102, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.C.; Kosman, D.J. Mechanisms and regulation of iron trafficking across the capillary endothelial cells of the blood-brain barrier. Front. Mol. Neurosci. 2015, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Johnsen, K.B.; Moos, T. Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell. Mol. Life Sci. CMLS 2014, 71, 1607–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, R.; Liu, Q.; Feng, J.; Lao, G.; Zhang, M.; Li, J.; Zhang, Y.; Wei, H. APART-QSM: An improved sub-voxel quantitative susceptibility mapping for susceptibility source separation using an iterative data fitting method. Neuroimage 2023, 274, 120148. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Chang, Y.Z. Brain Iron Metabolism and Regulation. Adv. Exp. Med. Biol. 2019, 1173, 33–44. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Qi, L.; Fu, Y.; Sun, D.; Chen, S.; Xu, W.; Liu, C.; Zhou, X.; He, G. Distribution pattern of iron deposition in the basal ganglia of different motor subtypes of Parkinson’s disease. Neurosci. Lett. 2023, 807, 137249. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, C.; Tong, R.; Chen, L.; Chen, M.; Gillen, K.M.; Li, G.; Ma, C.; Wang, Y.; Wu, X.; et al. Relationship Between Iron Distribution in Deep Gray Matter Nuclei Measured by Quantitative Susceptibility Mapping and Motor Outcome After Deep Brain Stimulation in Patients With Parkinson’s Disease. J. Magn. Reson. Imaging 2023, 58, 581–590. [Google Scholar] [CrossRef]
- Hosseinpour Mashkani, S.M.; Bishop, D.P.; Raoufi-Rad, N.; Adlard, P.A.; Shimoni, O.; Golzan, S.M. Distribution of Copper, Iron, and Zinc in the Retina, Hippocampus, and Cortex of the Transgenic APP/PS1 Mouse Model of Alzheimer’s Disease. Cells 2023, 12, 1144. [Google Scholar] [CrossRef]
- Juan, S.M.A.; Daglas, M.; Gunn, A.P.; Lago, L.; Adlard, P.A. Characterization of the spatial distribution of metals and profile of metalloprotein complexes in a mouse model of repetitive mild traumatic brain injury. Metallomics 2022, 14, mfac092. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Cellular iron: Ferroportin is the only way out. Cell Metab. 2005, 1, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Leenders, A.G.; Cooperman, S.; Meyron-Holtz, E.; Smith, S.; Land, W.; Tsai, R.Y.; Berger, U.V.; Sheng, Z.H.; Rouault, T.A. Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain Res. 2004, 1001, 108–117. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Yu, P.P.; Dong, T.; Guo, W.; Chang, S.; Zheng, B.; Ci, Y.; Wang, F.; Yu, P.; Gao, G.; et al. Astrocyte-derived hepcidin controls iron traffic at the blood-brain-barrier via regulating ferroportin 1 of microvascular endothelial cells. Cell Death Dis. 2022, 13, 667. [Google Scholar] [CrossRef]
- Raut, S.B.; Marathe, P.A.; van Eijk, L.; Eri, R.; Ravindran, M.; Benedek, D.M.; Ursano, R.J.; Canales, J.J.; Johnson, L.R. Diverse therapeutic developments for post-traumatic stress disorder (PTSD) indicate common mechanisms of memory modulation. Pharmacol. Ther. 2022, 239, 108195. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Galy, B.; Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Lotan, A.; Luza, S.; Opazo, C.M.; Ayton, S.; Lane, D.J.R.; Mancuso, S.; Pereira, A.; Sundram, S.; Weickert, C.S.; Bousman, C.; et al. Perturbed iron biology in the prefrontal cortex of people with schizophrenia. Mol. Psychiatry 2023, 28, 2058–2070. [Google Scholar] [CrossRef]
- Peng, Y.; Chang, X.; Lang, M. Iron Homeostasis Disorder and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12442. [Google Scholar] [CrossRef]
- Heidari, M.; Johnstone, D.M.; Bassett, B.; Graham, R.M.; Chua, A.C.G.; House, M.J.; Collingwood, J.F.; Bettencourt, C.; Houlden, H.; Ryten, M.; et al. Brain iron accumulation affects myelin-related molecular systems implicated in a rare neurogenetic disease family with neuropsychiatric features. Mol. Psychiatry 2016, 21, 1599–1607. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Meng, D.; Zhang, D.; Wang, B.; Xie, J.; Wang, J. 6-Hydroxydopamine Induces Abnormal Iron Sequestration in BV2 Microglia by Activating Iron Regulatory Protein 1 and Inhibiting Hepcidin Release. Biomolecules 2022, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 14 May 2022).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Wikipedia. Mental Disorder. Available online: https://en.wikipedia.org/wiki/Mental_disorder#cite_note-WHO2018-10 (accessed on 14 May 2022).
- Kennedy, S.H. Core symptoms of major depressive disorder: Relevance to diagnosis and treatment. Dialogues Clin. Neurosci. 2008, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Briley, P.M.; Webster, L.; Boutry, C.; Cottam, W.J.; Auer, D.P.; Liddle, P.F.; Morriss, R. Resting-state functional connectivity correlates of anxiety co-morbidity in major depressive disorder. Neurosci. Biobehav. Rev. 2022, 138, 104701. [Google Scholar] [CrossRef] [PubMed]
- Hawton, K.; Casanas, I.C.C.; Haw, C.; Saunders, K. Risk factors for suicide in individuals with depression: A systematic review. J. Affect. Disord. 2013, 147, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Porras-Segovia, A.; Nobile, B.; Olie, E.; Gourguechon-Buot, E.; Garcia, E.B.; Gorwood, P.; Abascal-Peiro, S.; Courtet, P. Factors associated with transitioning from suicidal ideation to suicide attempt in the short-term: Two large cohorts of depressed outpatients. J. Affect Disord. 2023, 335, 155–165. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Anxiety Disorders: DSM-5® Selections; American Psychiatric Pub: Washington, DC, USA, 2015. [Google Scholar]
- Crocq, M.A. A history of anxiety: From Hippocrates to DSM. Dialogues Clin. Neurosci. 2015, 17, 319–325. [Google Scholar] [CrossRef]
- Hammen, C. Risk Factors for Depression: An Autobiographical Review. Annu. Rev. Clin. Psychol. 2018, 14, 1–28. [Google Scholar] [CrossRef]
- Shah, H.E.; Bhawnani, N.; Ethirajulu, A.; Alkasabera, A.; Onyali, C.B.; Anim-Koranteng, C.; Mostafa, J.A. Iron Deficiency-Induced Changes in the Hippocampus, Corpus Striatum, and Monoamines Levels That Lead to Anxiety, Depression, Sleep Disorders, and Psychotic Disorders. Cureus 2021, 13, e18138. [Google Scholar] [CrossRef]
- Verdon, F.; Burnand, B.; Stubi, C.L.; Bonard, C.; Graff, M.; Michaud, A.; Bischoff, T.; de Vevey, M.; Studer, J.P.; Herzig, L.; et al. Iron supplementation for unexplained fatigue in non-anaemic women: Double blind randomised placebo controlled trial. BMJ 2003, 326, 1124. [Google Scholar] [CrossRef]
- Stewart, R.; Hirani, V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom. Med. 2012, 74, 208–213. [Google Scholar] [CrossRef]
- Yi, S.; Nanri, A.; Poudel-Tandukar, K.; Nonaka, D.; Matsushita, Y.; Hori, A.; Mizoue, T. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011, 189, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Shalahuddin Qusar, M.M.A.; Islam, M.S.; Kabir, M.H.; Mustafizur Rahman, G.K.M.; Hasnat, A. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: A case-control study. BMC Psychiatry 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Vahdat Shariatpanaahi, M.; Vahdat Shariatpanaahi, Z.; Moshtaaghi, M.; Shahbaazi, S.H.; Abadi, A. The relationship between depression and serum ferritin level. Eur. J. Clin. Nutr. 2007, 61, 532–535. [Google Scholar] [CrossRef]
- McClung, J.P.; Karl, J.P.; Cable, S.J.; Williams, K.W.; Nindl, B.C.; Young, A.J.; Lieberman, H.R. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: Effects on iron status, physical performance, and mood. Am. J. Clin. Nutr. 2009, 90, 124–131. [Google Scholar] [CrossRef]
- Abbas, M.; Gandy, K.; Salas, R.; Devaraj, S.; Calarge, C.A. Iron deficiency and internalizing symptom severity in unmedicated adolescents: A pilot study. Psychol. Med. 2021, 53, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Jimenez, E.; Hagen, J.; Mollen, E.; Wolf, A.W. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics 2000, 105, E51. [Google Scholar] [CrossRef]
- Beard, J.L.; Hendricks, M.K.; Perez, E.M.; Murray-Kolb, L.E.; Berg, A.; Vernon-Feagans, L.; Irlam, J.; Isaacs, W.; Sive, A.; Tomlinson, M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J. Nutr. 2005, 135, 267–272. [Google Scholar] [CrossRef]
- Chang, S.; Wang, L.; Wang, Y.; Brouwer, I.D.; Kok, F.J.; Lozoff, B.; Chen, C. Iron-deficiency anemia in infancy and social emotional development in preschool-aged Chinese children. Pediatrics 2011, 127, e927–e933. [Google Scholar] [CrossRef]
- Coe, C.L.; Lubach, G.R.; Bianco, L.; Beard, J.L. A history of iron deficiency anemia during infancy alters brain monoamine activity later in juvenile monkeys. Dev. Psychobiol. 2009, 51, 301–309. [Google Scholar] [CrossRef]
- Golub, M.S.; Hogrefe, C.E.; Widaman, K.F.; Capitanio, J.P. Iron deficiency anemia and affective response in rhesus monkey infants. Dev. Psychobiol. 2009, 51, 47–59. [Google Scholar] [CrossRef]
- Kukuia, K.K.E.; Torbi, J.; Amoateng, P.; Adutwum-Ofosu, K.K.; Koomson, A.E.; Appiah, F.; Tagoe, T.A.; Mensah, J.A.; Ameyaw, E.O.; Adi-Dako, O.; et al. Gestational iron supplementation reverses depressive-like behavior in post-partum Sprague Dawley rats: Evidence from behavioral and neurohistological studies. IBRO Neurosci. Rep. 2022, 12, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Su, T.P.; Chen, Y.S.; Hsu, J.W.; Huang, K.L.; Chang, W.H.; Chen, T.J.; Bai, Y.M. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: A nationwide population-based study. BMC Psychiatry 2013, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L.; Erikson, K.M.; Jones, B.C. Neurobehavioral analysis of developmental iron deficiency in rats. Behav. Brain Res. 2002, 134, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Eseh, R.; Zimmerberg, B. Age-dependent effects of gestational and lactational iron deficiency on anxiety behavior in rats. Behav. Brain Res. 2005, 164, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Dobbing, J.; Sands, J. Maternal nutrition and neurological development. Ontogenesis of the Brain. Prague Univ. Karlov. 1974, 2, 167–172. [Google Scholar]
- Texel, S.J.; Camandola, S.; Ladenheim, B.; Rothman, S.M.; Mughal, M.R.; Unger, E.L.; Cadet, J.L.; Mattson, M.P. Ceruloplasmin deficiency results in an anxiety phenotype involving deficits in hippocampal iron, serotonin, and BDNF. J. Neurochem. 2012, 120, 125–134. [Google Scholar] [CrossRef]
- Cutler, P. Iron overload and psychiatric illness. Canadian journal of psychiatry. Rev. Can. Psychiatr. 1994, 39, 8–11. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Najm, A.H.; Al-Dujaili, A.H.; Maes, M. Major Depression in Children with Transfusion-Dependent Thalassemia Is Strongly Associated with the Combined Effects of Blood Transfusion Rate, Iron Overload, and Increased Pro-inflammatory Cytokines. Neurotox. Res. 2020, 38, 228–241. [Google Scholar] [CrossRef]
- Pattanashetti, M.; Mugali, J.; Pattanashetty, N.; Patil, S. A study of severity of depression in thalassemia patients. Int. J. Indian Psychol. 2017, 4, e05. [Google Scholar]
- Yao, S.; Zhong, Y.; Xu, Y.; Qin, J.; Zhang, N.; Zhu, X.; Li, Y. Quantitative Susceptibility Mapping Reveals an Association between Brain Iron Load and Depression Severity. Front. Hum. Neurosci. 2017, 11, 442. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Li, Y.; Li, Y.; Gong, H.; Li, J.; Zhang, Y.; Zhang, C.; Yan, F.; Sun, B.; et al. Alterations in brain iron deposition with progression of late-life depression measured by magnetic resonance imaging (MRI)-based quantitative susceptibility mapping. Quant Imaging Med. Surg. 2022, 12, 3873–3888. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xie, Y.; Zhu, X.; Chen, L.; Li, F.; Feng, G.; Li, L. Quantitative susceptibility mapping of brain iron deposition in patients with recurrent depression. Psychiatry Investig. 2022, 19, 668. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Lu, Y.; Li, Z.; Li, S.; Chen, B.; Zhang, M.; Ji, M.; Gong, W.; Xia, M.; Verkhratsky, A.; et al. Iron Aggravates the Depressive Phenotype of Stressed Mice by Compromising the Glymphatic System. Neurosci. Bull. 2020, 36, 1542–1546. [Google Scholar] [CrossRef]
- Zhang, M.J.; Song, M.L.; Zhang, Y.; Yang, X.M.; Lin, H.S.; Chen, W.C.; Zhong, X.D.; He, C.Y.; Li, T.; Liu, Y.; et al. SNS alleviates depression-like behaviors in CUMS mice by regluating dendritic spines via NCOA4-mediated ferritinophagy. J. Ethnopharmacol. 2023, 312, 116360. [Google Scholar] [CrossRef]
- Weatherall, D.J.; Clegg, J.B. Inherited haemoglobin disorders: An increasing global health problem. Bull. World Health Organ. 2001, 79, 704–712. [Google Scholar]
- Sahu, S.; Agrawal, A.; Shrivastava, J.; Tonk, S. Psychiatric disorders and caregiver burden in children with transfusion dependent beta-thalassaemia and their caregivers. World J. Clin. Pediatr. 2023, 12, 125–132. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, L.; Mao, H.; Chen, K.; Shi, Y.; Meng, X.; Wang, F.; Hu, X.; Fang, X. The impact of iron deposition on the fear circuit of the brain in patients with Parkinson’s disease and anxiety. Front. Aging Neurosci. 2023, 15, 1116516. [Google Scholar] [CrossRef]
- Maaroufi, K.; Ammari, M.; Jeljeli, M.; Roy, V.; Sakly, M.; Abdelmelek, H. Impairment of emotional behavior and spatial learning in adult Wistar rats by ferrous sulfate. Physiol. Behav. 2009, 96, 343–349. [Google Scholar] [CrossRef]
- Chtourou, Y.; Slima, A.B.; Gdoura, R.; Fetoui, H. Naringenin Mitigates Iron-Induced Anxiety-Like Behavioral Impairment, Mitochondrial Dysfunctions, Ectonucleotidases and Acetylcholinesterase Alteration Activities in Rat Hippocampus. Neurochem. Res. 2015, 40, 1563–1575. [Google Scholar] [CrossRef]
- Sobotka, T.J.; Whittaker, P.; Sobotka, J.M.; Brodie, R.E.; Quander, D.Y.; Robl, M.; Bryant, M.; Barton, C.N. Neurobehavioral dysfunctions associated with dietary iron overload. Physiol. Behav. 1996, 59, 213–219. [Google Scholar] [CrossRef]
- Pellegrino, R.M.; Boda, E.; Montarolo, F.; Boero, M.; Mezzanotte, M.; Saglio, G.; Buffo, A.; Roetto, A. Transferrin Receptor 2 Dependent Alterations of Brain Iron Metabolism Affect Anxiety Circuits in the Mouse. Sci. Rep. 2016, 6, 30725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zeng, Y.-N.; Yang, P.; Jin, L.-Q.; Xiong, W.-C.; Zhu, M.-Z.; Zhang, J.-Z.; He, X.; Zhu, X.-H. Axonal iron transport in the brain modulates anxiety-related behaviors. Nat. Chem. Biol. 2019, 15, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Schizophrenia. Available online: https://www.who.int/news-room/fact-sheets/detail/schizophrenia?gclid=Cj0KCQjwhfipBhCqARIsAH9msbnDoRWwwUtm7lZABoRqPR8PXu1VEnpjjohXP_hBixH0RcIpJywe2hMaAgEQEALw_wcB (accessed on 10 January 2023).
- Van Os, J.; Kenis, G.; Rutten, B.P. The environment and schizophrenia. Nature 2010, 468, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Pickard, B. Progress in defining the biological causes of schizophrenia. Expert Rev. Mol. Med. 2011, 13, e25. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.C. Understanding the causes of schizophrenia. N. Engl. J. Med. 1999, 340, 645–647. [Google Scholar] [CrossRef]
- Maxwell, A.M.; Rao, R.B. Perinatal iron deficiency as an early risk factor for schizophrenia. Nutr. Neurosci. 2022, 25, 2218–2227. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Mahmoudi, M.; Shahrokhi, S.; Mojarrad, M.; Dastmardi, M.; Mirbeyk, M.; Rezaei, N. Trace elements in schizophrenia: A systematic review and meta-analysis of 39 studies (N = 5151 participants). Nutr. Rev. 2020, 78, 278–303. [Google Scholar] [CrossRef]
- Xu, M.; Guo, Y.; Cheng, J.; Xue, K.; Yang, M.; Song, X.; Feng, Y.; Cheng, J. Brain iron assessment in patients with First-episode schizophrenia using quantitative susceptibility mapping. NeuroImage Clin. 2021, 31, 102736. [Google Scholar] [CrossRef]
- McGrath, J.; Brown, A.; St Clair, D. Prevention and schizophrenia—The role of dietary factors. Schizophr. Bull. 2011, 37, 272–283. [Google Scholar] [CrossRef]
- Sonnenschein, S.F.; Parr, A.C.; Larsen, B.; Calabro, F.J.; Foran, W.; Eack, S.M.; Luna, B.; Sarpal, D.K. Subcortical brain iron deposition in individuals with schizophrenia. J. Psychiatr. Res. 2022, 151, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Ravanfar, P.; Syeda, W.T.; Jayaram, M.; Rushmore, R.J.; Moffat, B.; Lin, A.P.; Lyall, A.E.; Merritt, A.H.; Yaghmaie, N.; Laskaris, L. In Vivo 7-Tesla MRI investigation of brain iron and its metabolic correlates in chronic schizophrenia. Schizophrenia 2022, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Lange, K.W.; Kruzik, P.; Rausch, W.-D.; Gabriel, E.; Jellinger, K.; Riederer, P. Iron, copper, zinc, magnesium, and calcium in postmortem brain tissue from schizophrenic patients. Biol. Psychiatry 1994, 36, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Bitanihirwe, B.K.; Woo, T.-U.W. Oxidative stress in schizophrenia: An integrated approach. Neurosci. Biobehav. Rev. 2011, 35, 878–893. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Miller, B.J. Inflammation and schizophrenia. Schizophr. Bull. 2013, 39, 1174–1179. [Google Scholar] [CrossRef]
- Izquierdo, I.; Furini, C.R.; Myskiw, J.C. Fear Memory. Physiol. Rev. 2016, 96, 695–750. [Google Scholar] [CrossRef]
- Oota-Ishigaki, A.; Takao, K.; Yamada, D.; Sekiguchi, M.; Itoh, M.; Koshidata, Y.; Abe, M.; Natsume, R.; Kaneko, M.; Adachi, T.; et al. Prolonged contextual fear memory in AMPA receptor palmitoylation-deficient mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2022, 47, 2150–2159. [Google Scholar] [CrossRef]
- Doom, J.R.; Richards, B.; Caballero, G.; Delva, J.; Gahagan, S.; Lozoff, B. Infant Iron Deficiency and Iron Supplementation Predict Adolescent Internalizing, Externalizing, and Social Problems. J. Pediatr. 2018, 195, 199–205.e2. [Google Scholar] [CrossRef]
- McEchron, M.D.; Cheng, A.Y.; Liu, H.; Connor, J.R.; Gilmartin, M.R. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr. Neurosci. 2005, 8, 195–206. [Google Scholar] [CrossRef]
- Gewirtz, J.C.; Hamilton, K.L.; Babu, M.A.; Wobken, J.D.; Georgieff, M.K. Effects of gestational iron deficiency on fear conditioning in juvenile and adult rats. Brain Res. 2008, 1237, 195–203. [Google Scholar] [CrossRef][Green Version]
- Guo, S.; Dong, Y.; Cheng, X.; Chen, Z.; Ni, Y.; Zhao, R.; Ma, W. Chronic Psychological Stress Disrupts Iron Metabolism and Enhances Hepatic Mitochondrial Function in Mice. Biol. Trace Elem. Res. 2023, 201, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yu, Z.; Zhang, Y.; Huang, X.; Hou, J.; Zhao, Y.; Luo, W.; Chen, L.; Ou, L.; Li, H.; et al. Iron-induced neuronal damage in a rat model of post-traumatic stress disorder. Neuroscience 2016, 330, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.D.; Pang, P.; Zhou, X.T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.Z.; et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562. [Google Scholar] [CrossRef] [PubMed]
- Schroder, N.; Fredriksson, A.; Vianna, M.R.; Roesler, R.; Izquierdo, I.; Archer, T. Memory deficits in adult rats following postnatal iron administration. Behav. Brain Res. 2001, 124, 77–85. [Google Scholar] [CrossRef]
- Silva, P.F.; Garcia, V.A.; Dornelles Ada, S.; Silva, V.K.; Maurmann, N.; Portal, B.C.; Ferreira, R.D.; Piazza, F.C.; Roesler, R.; Schroder, N. Memory impairment induced by brain iron overload is accompanied by reduced H3K9 acetylation and ameliorated by sodium butyrate. Neuroscience 2012, 200, 42–49. [Google Scholar] [CrossRef]
- Zhong, T.; Qing, Q.J.; Yang, Y.; Zou, W.Y.; Ye, Z.; Yan, J.Q.; Guo, Q.L. Repression of contexual fear memory induced by isoflurane is accompanied by reduction in histone acetylation and rescued by sodium butyrate. Br. J. Anaesth. 2014, 113, 634–643. [Google Scholar] [CrossRef]
- Uberti, V.H.; de Freitas, B.S.; Molz, P.; Bromberg, E.; Schroder, N. Iron Overload Impairs Autophagy: Effects of Rapamycin in Ameliorating Iron-Related Memory Deficits. Mol. Neurobiol. 2020, 57, 1044–1054. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.J.; Cao, Y.; Li, H.; Zhao, H.; Yang, S.; Li, K. Iron overload contributes to general anaesthesia-induced neurotoxicity and cognitive deficits. J. Neuroinflamm. 2020, 17, 110. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, B.; Xie, J.; Ma, Z.; Thirupathi, A.; Yu, P.; Gao, G.; Zhou, J.; Zhou, C.; Xu, H.; et al. Sevoflurane anesthesia during pregnancy in mice induces cognitive impairment in the offspring by causing iron deficiency and inhibiting myelinogenesis. Neurochem. Int. 2020, 135, 104693. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, G.; Ran, Q.; Nie, L.; Liu, X.; Pan, Z.; He, L. Quantitative susceptibility mapping shows lower brain iron content in children with attention-deficit hyperactivity disorder. Hum. Brain Mapp. 2022, 43, 2495–2502. [Google Scholar] [CrossRef]
- Craig, S.G.; Bondi, B.C.; O’Donnell, K.A.; Pepler, D.J.; Weiss, M.D. ADHD and Exposure to Maltreatment in Children and Youth: A Systematic Review of the Past 10 Years. Curr. Psychiatry Rep. 2020, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Hoogman, M.; Stolte, M.; Baas, M.; Kroesbergen, E. Creativity and ADHD: A review of behavioral studies, the effect of psychostimulants and neural underpinnings. Neurosci. Biobehav. Rev. 2020, 119, 66–85. [Google Scholar] [CrossRef]
- Chen, Y.; Su, S.; Dai, Y.; Zou, M.; Lin, L.; Qian, L.; Zhou, Q.; Zhang, H.; Liu, M.; Zhao, J.; et al. Quantitative susceptibility mapping reveals brain iron deficiency in children with attention-deficit/hyperactivity disorder: A whole-brain analysis. Eur. Radiol. 2022, 32, 3726–3733. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, Y.; Gao, W.; Lin, N.; Li, R.; Zhao, Z. Blood Levels of Trace Elements in Children with Attention-Deficit Hyperactivity Disorder: Results from a Case-Control Study. Biol. Trace Elem. Res. 2019, 187, 376–382. [Google Scholar] [CrossRef]
- Cortese, S.; Azoulay, R.; Castellanos, F.X.; Chalard, F.; Lecendreux, M.; Chechin, D.; Delorme, R.; Sebag, G.; Sbarbati, A.; Mouren, M.-C.; et al. Brain iron levels in attention-deficit/hyperactivity disorder: A pilot MRI study. World J. Biol. Psychiatry 2012, 13, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Adisetiyo, V.; Jensen, J.H.; Tabesh, A.; Deardorff, R.L.; Fieremans, E.; Di Martino, A.; Gray, K.M.; Castellanos, F.X.; Helpern, J.A. Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: A noninvasive biomarker that responds to psychostimulant treatment? Radiology 2014, 272, 524–532. [Google Scholar] [CrossRef] [PubMed]
- East, P.L.; Doom, J.R.; Blanco, E.; Burrows, R.; Lozoff, B.; Gahagan, S. Iron Deficiency in Infancy and Sluggish Cognitive Tempo and ADHD Symptoms in Childhood and Adolescence. J. Clin. Child. Adolesc. Psychol. 2023, 52, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Fiani, D.; Engler, S.; Fields, S.; Calarge, C.A. Iron Deficiency in Attention-Deficit Hyperactivity Disorder, Autism Spectrum Disorder, Internalizing and Externalizing Disorders, and Movement Disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2023, 32, 451–467. [Google Scholar] [CrossRef]
- Degremont, A.; Jain, R.; Philippou, E.; Latunde-Dada, G.O. Brain iron concentrations in the pathophysiology of children with attention deficit/hyperactivity disorder: A systematic review. Nutr. Rev. 2020, 79, 615–626. [Google Scholar] [CrossRef]
- Konofal, E.; Lecendreux, M.; Deron, J.; Marchand, M.; Cortese, S.; Zaïm, M.; Mouren, M.C.; Arnulf, I. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatr. Neurol. 2008, 38, 20–26. [Google Scholar] [CrossRef]
- Granero, R.; Pardo-Garrido, A.; Carpio-Toro, I.L.; Ramírez-Coronel, A.A.; Martínez-Suárez, P.C.; Reivan-Ortiz, G.G. The role of iron and zinc in the treatment of adhd among children and adolescents: A systematic review of randomized clinical trials. Nutrients 2021, 13, 4059. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Primers 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Dosman, C.F.; Brian, J.A.; Drmic, I.E.; Senthilselvan, A.; Harford, M.M.; Smith, R.W.; Sharieff, W.; Zlotkin, S.H.; Moldofsky, H.; Roberts, S.W. Children with autism: Effect of iron supplementation on sleep and ferritin. Pediatr. Neurol. 2007, 36, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Herndon, A.C.; DiGuiseppi, C.; Johnson, S.L.; Leiferman, J.; Reynolds, A. Does nutritional intake differ between children with autism spectrum disorders and children with typical development? J. Autism Dev. Disord. 2009, 39, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Millichap, J.G.; Yee, M.M.; Davidson, S.I. Serum ferritin in children with attention-deficit hyperactivity disorder. Pediatr. Neurol. 2006, 34, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Krebs, N.F.; Stewart, P.A.; Austin, H.; Johnson, S.L.; Withrow, N.; Molloy, C.; James, S.J.; Johnson, C.; Clemons, T.; et al. Iron status in children with autism spectrum disorder. Pediatrics 2012, 130 (Suppl. S2), S154–S159. [Google Scholar] [CrossRef]
- Tang, S.; Xu, Y.; Liu, X.; Chen, Z.; Zhou, Y.; Nie, L.; He, L. Quantitative susceptibility mapping shows lower brain iron content in children with autism. Eur. Radiol. 2021, 31, 2073–2083. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Tancredi, D.J.; Krakowiak, P.; Hansen, R.L.; Ozonoff, S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am. J. Epidemiol. 2014, 180, 890–900. [Google Scholar] [CrossRef]
- Leo, D.; Sorrentino, E.; Volpicelli, F.; Eyman, M.; Greco, D.; Viggiano, D.; di Porzio, U.; Perrone-Capano, C. Altered midbrain dopaminergic neurotransmission during development in an animal model of ADHD. Neurosci. Biobehav. Rev. 2003, 27, 661–669. [Google Scholar] [CrossRef]
- Squillace, M.; Dodero, L.; Federici, M.; Migliarini, S.; Errico, F.; Napolitano, F.; Krashia, P.; Di Maio, A.; Galbusera, A.; Bifone, A.; et al. Dysfunctional dopaminergic neurotransmission in asocial BTBR mice. Transl. Psychiatry 2014, 4, e427. [Google Scholar] [CrossRef]
- Beard, J. Iron deficiency alters brain development and functioning. J. Nutr. 2003, 133, 1468S–1472S. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L.; Connor, J.R. Iron status and neural functioning. Annu. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wessling-Resnick, M. Iron and mechanisms of emotional behavior. J. Nutr. Biochem. 2014, 25, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J. Nutr. 2011, 141, 740S–746S. [Google Scholar] [CrossRef]
- Felt, B.T.; Beard, J.L.; Schallert, T.; Shao, J.; Aldridge, J.W.; Connor, J.R.; Georgieff, M.K.; Lozoff, B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav. Brain Res. 2006, 171, 261–270. [Google Scholar] [CrossRef]
- Beard, J.L.; Felt, B.; Schallert, T.; Burhans, M.; Connor, J.R.; Georgieff, M.K. Moderate iron deficiency in infancy: Biology and behavior in young rats. Behav. Brain Res. 2006, 170, 224–232. [Google Scholar] [CrossRef]
- Frantom, P.A.; Seravalli, J.; Ragsdale, S.W.; Fitzpatrick, P.F. Reduction and oxidation of the active site iron in tyrosine hydroxylase: Kinetics and specificity. Biochemistry 2006, 45, 2372–2379. [Google Scholar] [CrossRef]
- Sarchiapone, M.; Carli, V.; Camardese, G.; Cuomo, C.; Di Giuda, D.; Calcagni, M.L.; Focacci, C.; De Risio, S. Dopamine transporter binding in depressed patients with anhedonia. Psychiatry Res. 2006, 147, 243–248. [Google Scholar] [CrossRef]
- Unger, E.L.; Wiesinger, J.A.; Hao, L.; Beard, J.L. Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J. Nutr. 2008, 138, 2487–2494. [Google Scholar] [CrossRef]
- Jellen, L.C.; Lewis, M.M.; Du, G.; Wang, X.; Galvis, M.L.E.; Krzyzanowski, S.; Capan, C.D.; Snyder, A.M.; Connor, J.R.; Kong, L.; et al. Low plasma serotonin linked to higher nigral iron in Parkinson’s disease. Sci. Rep. 2021, 11, 24384. [Google Scholar] [CrossRef] [PubMed]
- Mackler, B.; Person, R.; Miller, L.R.; Inamdar, A.R.; Finch, C.A. Iron deficiency in the rat: Biochemical studies of brain metabolism. Pediatr. Res. 1978, 12, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Agarwal, K.N.; Chansuria, J.P.; Taneja, V. Effect of latent iron deficiency on 5-hydroxytryptamine metabolism in rat brain. J. Neurochem. 1989, 52, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Ben-Shachar, D. Minimal brain damage induced by early iron deficiency: Modified dopaminergic neurotransmission. Isr. J. Med. Sci. 1987, 23, 19–25. [Google Scholar]
- Li, Y.; Kim, J.; Buckett, P.D.; Bohlke, M.; Maher, T.J.; Wessling-Resnick, M. Severe postnatal iron deficiency alters emotional behavior and dopamine levels in the prefrontal cortex of young male rats. J. Nutr. 2011, 141, 2133–2138. [Google Scholar] [CrossRef]
- Kwik-Uribe, C.L.; Golub, M.S.; Keen, C.L. Chronic marginal iron intakes during early development in mice alter brain iron concentrations and behavior despite postnatal iron supplementation. J. Nutr. 2000, 130, 2040–2048. [Google Scholar] [CrossRef]
- Youdim, M.B.; Green, A.R.; Bloomfield, M.R.; Mitchell, B.D.; Heal, D.J.; Grahame-Smith, D.G. The effects of iron deficiency on brain biogenic monoamine biochemistry and function in rats. Neuropharmacology 1980, 19, 259–267. [Google Scholar] [CrossRef]
- Youdim, M.B.; Ben-Shachar, D.; Yehuda, S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am. J. Clin. Nutr. 1989, 50, 607–615; discussion 615–607. [Google Scholar] [CrossRef]
- Anderson, J.G.; Fordahl, S.C.; Cooney, P.T.; Weaver, T.L.; Colyer, C.L.; Erikson, K.M. Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure. Brain Res. 2009, 1281, 1–14. [Google Scholar] [CrossRef]
- Kikusui, T.; Nakamura, K.; Kakuma, Y.; Mori, Y. Early weaning augments neuroendocrine stress responses in mice. Behav. Brain Res. 2006, 175, 96–103. [Google Scholar] [CrossRef]
- Kikusui, T.; Ichikawa, S.; Mori, Y. Maternal deprivation by early weaning increases corticosterone and decreases hippocampal BDNF and neurogenesis in mice. Psychoneuroendocrinology 2009, 34, 762–772. [Google Scholar] [CrossRef]
- Wu, L.; Xian, X.; Tan, Z.; Dong, F.; Xu, G.; Zhang, M.; Zhang, F. The Role of Iron Metabolism, Lipid Metabolism, and Redox Homeostasis in Alzheimer’s Disease: From the Perspective of Ferroptosis. Mol. Neurobiol. 2023, 60, 2832–2850. [Google Scholar] [CrossRef] [PubMed]
- Mundra, S.; Tits, J.; Wieland, E.; Angst, U.M. Aerobic and anaerobic oxidation of ferrous ions in near-neutral solutions. Chemosphere 2023, 335, 138955. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Zhou, G.N.; Ding, R.R.; Li, Q.; Zhao, H.Q.; Mu, Y. Ferrous ion enhanced Fenton-like degradation of emerging contaminants by sulfidated nanosized zero-valent iron with pH insensitivity. J. Hazard. Mater. 2023, 459, 132229. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, R. Iron Homeostasis, Oxidative Stress, and DNA Damage. Free. Radic. Biol. Med. 1997, 23, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Najm, A.H.; Moustafa, S.R.; Maes, M. Construction of an exposure-pathway-phenotype in children with depression due to transfusion-dependent thalassemia: Results of (un)supervised machine learning. J. Affect. Disord. 2021, 282, 644–655. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Santos, T.; Sampaio-Marques, B.; Novais, A.; Mesquita, S.D.; Ludovico, P.; Bernardino, L.; Correia-Neves, M.; Sousa, N.; Palha, J.A.; et al. Lipocalin-2 regulates adult neurogenesis and contextual discriminative behaviours. Mol. Psychiatry 2018, 23, 1031–1039. [Google Scholar] [CrossRef]
- Han, M.; Chang, J.; Kim, J. Loss of divalent metal transporter 1 function promotes brain copper accumulation and increases impulsivity. J. Neurochem. 2016, 138, 918–928. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant effects of curcumin-coated iron oxide nanoparticles in a rat model of depression. Eur. J. Pharmacol. 2021, 908, 174384. [Google Scholar] [CrossRef]
- Beard, J.L.; Connor, J.D.; Jones, B.C. Brain iron: Location and function. Prog. Food Nutr. Sci. 1993, 17, 183–221. [Google Scholar]
- Olanow, C.W. An introduction to the free radical hypothesis in Parkinson’s disease. Ann. Neurol. 1992, 32, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Li, J.J.; Tipoe, G.L.; Youdim, M.B.H.; Fung, M.L. Monoamine oxidase A upregulated by chronic intermittent hypoxia activates indoleamine 2,3-dioxygenase and neurodegeneration. PLoS ONE 2017, 12, e0177940. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Ferroptosis Mechanisms Involved in Hippocampal-Related Diseases. Int. J. Mol. Sci. 2021, 22, 9902. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xin, Y.; Zhang, J.; Yang, Z.; Liu, C. Hydrogen sulfide alleviates the anxiety-like and depressive-like behaviors of type 1 diabetic mice via inhibiting inflammation and ferroptosis. Life Sci. 2021, 278, 119551. [Google Scholar] [CrossRef] [PubMed]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Munoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Hakak, Y.; Walker, J.R.; Li, C.; Wong, W.H.; Davis, K.L.; Buxbaum, J.D.; Haroutunian, V.; Fienberg, A.A. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 4746–4751. [Google Scholar] [CrossRef]
- Regenold, W.T.; Phatak, P.; Marano, C.M.; Gearhart, L.; Viens, C.H.; Hisley, K.C. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res. 2007, 151, 179–188. [Google Scholar] [CrossRef]
- Pajevic, S.; Basser, P.J.; Fields, R.D. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 2014, 276, 135–147. [Google Scholar] [CrossRef]
- Connor, J.R.; Menzies, S.L. Relationship of iron to oligodendrocytes and myelination. Glia 1996, 17, 83–93. [Google Scholar] [CrossRef]
- Yu, G.S.; Steinkirchner, T.M.; Rao, G.A.; Larkin, E.C. Effect of prenatal iron deficiency on myelination in rat pups. Am. J. Pathol. 1986, 125, 620–624. [Google Scholar]
- Schmitt, A.; Simons, M.; Cantuti-Castelvetri, L.; Falkai, P. A new role for oligodendrocytes and myelination in schizophrenia and affective disorders? Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Jin, X.; Han, K.; Kang, S.; Tian, S.; Lv, X.; Feng, M.; Zheng, H.; Zuo, Y.; Xu, G. Iron promotes neurological function recovery in mice with ischemic stroke through endogenous repair mechanisms. Free. Radic. Biol. Med. 2022, 182, 59–72. [Google Scholar] [CrossRef]
- The Institute for Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 14 May 2022).
- Charlson, F.; van Ommeren, M.; Flaxman, A.; Cornett, J.; Whiteford, H.; Saxena, S. New WHO prevalence estimates of mental disorders in conflict settings: A systematic review and meta-analysis. Lancet 2019, 394, 240–248. [Google Scholar] [CrossRef]
- Moitra, M.; Santomauro, D.; Collins, P.Y.; Vos, T.; Whiteford, H.; Saxena, S.; Ferrari, A.J. The global gap in treatment coverage for major depressive disorder in 84 countries from 2000-2019: A systematic review and Bayesian meta-regression analysis. PLoS Med. 2022, 19, e1003901. [Google Scholar] [CrossRef] [PubMed]
- Farwin, A.; Low, A.; Howard, N.; Yi, H. “My young life, finished already?”: A qualitative study of embedded social stressors and their effects on mental health of low-wage male migrant workers in Singapore. Glob. Health 2023, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.H.; Tran, K.T.; Visoki, E.; Argabright, S.; DiDomenico, G.E.; Saiegh, E.; Hoffman, K.W.; Erez, G.; Barzilay, R. The Role of Individual Discrimination and Structural Stigma in the Mental Health of Sexual Minority Youth. J. Am. Acad. Child Adolesc. Psychiatry 2023. ahead of print. [Google Scholar] [CrossRef]
- Baron, S.; Cuervo, I.; Shah, D.; Gonzalez, A.; Harari, H.; Flores, D. COVID-19 Infections, Pandemic-Related Social and Economic Impacts, and Changes to Mental and Self-Rated Health Among Latinx Immigrant Housecleaners in New York City: The Safe and Just Cleaners Study. Am. J. Public Health 2023, 113, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ming Qian, Z. Iron misregulation in the brain: A primary cause of neurodegenerative disorders. Lancet Neurol. 2003, 2, 246–253. [Google Scholar] [CrossRef]
- Oshiro, S.; Morioka, M.S.; Kikuchi, M. Dysregulation of iron metabolism in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Adv. Pharmacol. Sci. 2011, 2011, 378278. [Google Scholar] [CrossRef]
- Crapper McLachlan, D.R.; Dalton, A.J.; Kruck, T.P.; Bell, M.Y.; Smith, W.L.; Kalow, W.; Andrews, D.F. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991, 337, 1304–1308. [Google Scholar] [CrossRef]
- Zeng, X.; An, H.; Yu, F.; Wang, K.; Zheng, L.; Zhou, W.; Bao, Y.; Yang, J.; Shen, N.; Huang, D. Benefits of Iron Chelators in the Treatment of Parkinson’s Disease. Neurochem. Res. 2021, 46, 1239–1251. [Google Scholar] [CrossRef]
- Palmer, C.; Roberts, R.L.; Bero, C. Deferoxamine posttreatment reduces ischemic brain injury in neonatal rats. Stroke 1994, 25, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Konofal, E.; Cortese, S.; Lecendreux, M.; Arnulf, I.; Mouren, M.C. Effectiveness of iron supplementation in a young child with attention-deficit/hyperactivity disorder. Pediatrics 2005, 116, e732–e734. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Tran, T.; Kriitmaa, K.; Rosenthal, D.; Tran, T. Common perinatal mental disorders in northern Viet Nam: Community prevalence and health care use. Bull. World Health Organ. 2010, 88, 737–745. [Google Scholar] [CrossRef]
- Liu, X.; Lin, C.; Wang, S.; Yu, X.; Jia, Y.; Chen, J. Effects of High Levels of Copper on the Depression-Related Memory Disorders. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Totten, M.S.; Davenport, T.S.; Edwards, L.F.; Howell, J.M. Trace Minerals and Anxiety: A Review of Zinc, Copper, Iron, and Selenium. Dietetics 2023, 2, 83–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).