Phenolics and Terpenoids Profiling in Diverse Loquat Fruit Varieties and Systematic Assessment of Their Mitigation of Alcohol-Induced Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction Method for Loquat Flesh and Peel Extracts
2.3. Identification and Quantification of Phenolics and Terpenoids in Loquat Fruits
2.4. Chemical Antioxidant Evaluation Methods
2.5. Cell Culture and Cell Viability Assay
2.6. Determination of Cellular ROS Content and Antioxidant Enzyme Activity
2.7. Animal Modeling Method
2.8. Animal Serum Index Detection Method
2.9. Animal Liver Index Detection Method
2.10. Real Time-PCR (RT-PCR)
2.11. Statistics
3. Results
3.1. Identification and Quantification of Loquat Peel and Flesh Extracts
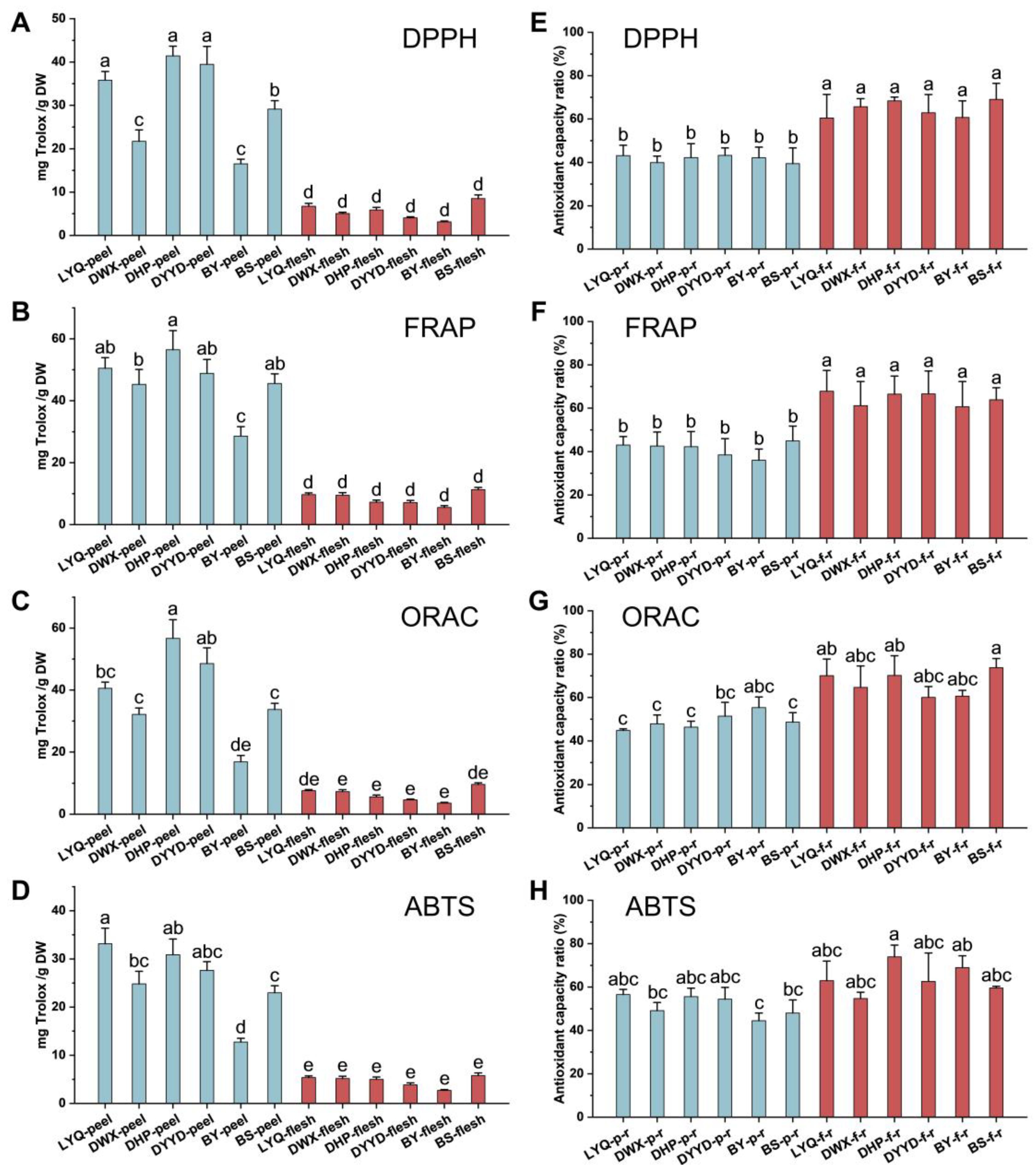
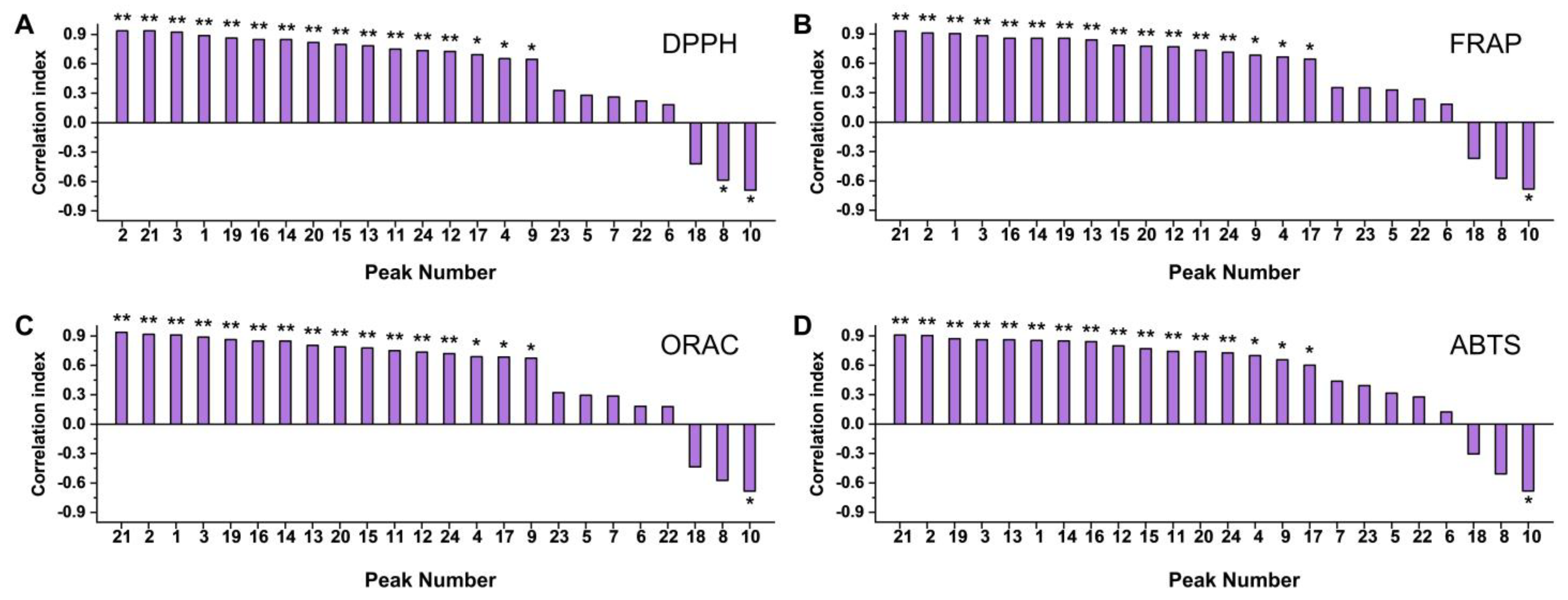
3.2. Chemical Antioxidant Evaluation of Loquat Fruit Extracts
3.3. In Vitro Antioxidant Evaluation of Loquat Fruit Extracts
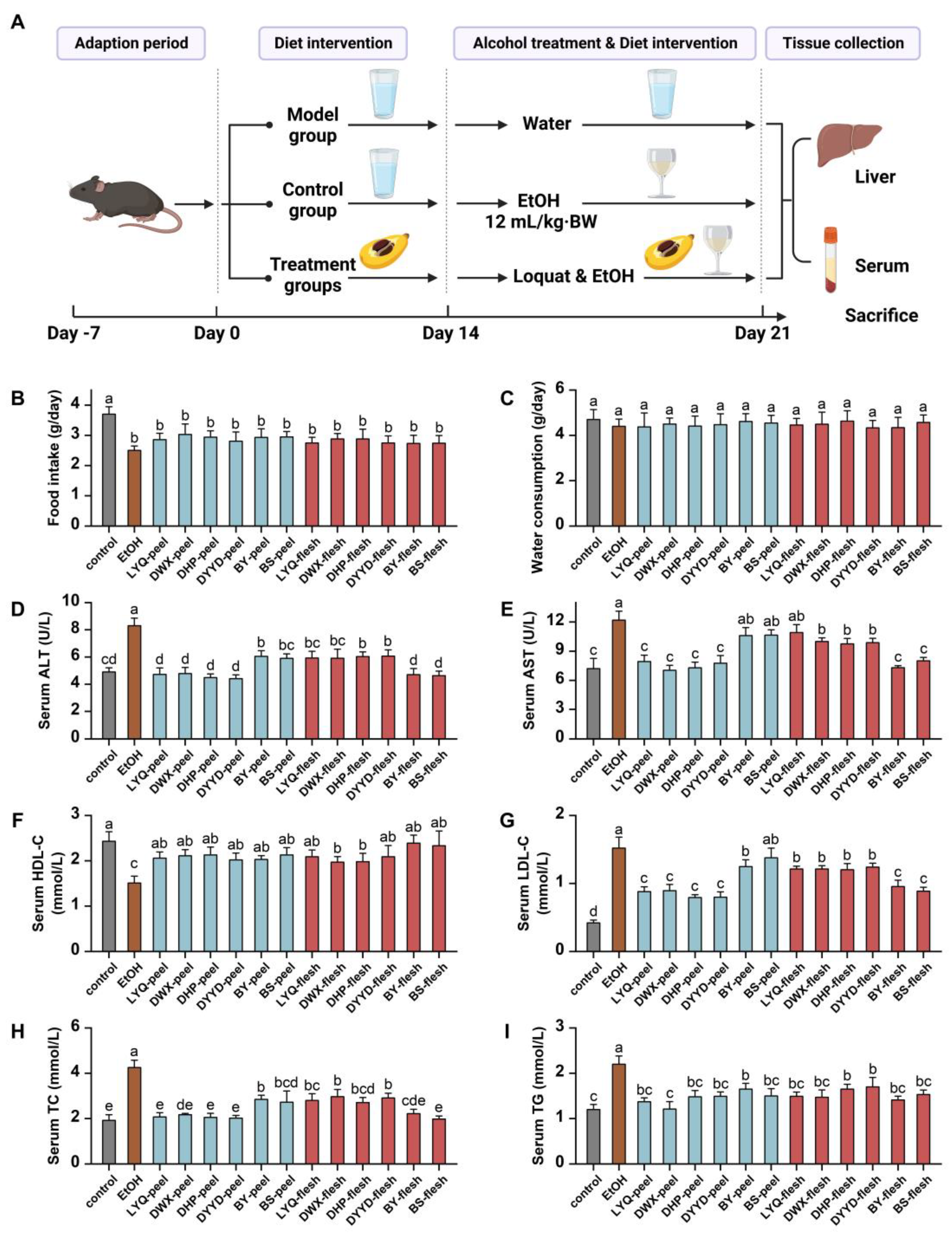
3.4. In Vivo Antioxidant Evaluation of Loquat Fruit Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, G.L.; Ma, J.J.; Sui, S.Y.; Wang, Y.N. Optimization of extraction of loquat flowers polyphenolics and its antioxidant and anti-polyphenol oxidase properties. Bioengineered 2020, 11, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Long, P.P.; Sun, Y.; Meng, Q.L.; Liu, X.X.; Cui, H.H.; Lv, Q.Y.; Zhang, L. The chemical profiling of loquat leaf extract by HPLC-DAD-ESI-MS and its effects on hyperlipidemia and hyperglycemia in rats induced by a high-fat and fructose diet. Food Funct. 2017, 8, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhang, W.N.; Xu, C.J.; Li, X. Biological activities of extracts from loquat (Eriobotrya japonica Lindl.): A review. Int. J. Mol. Sci. 2016, 17, 1983. [Google Scholar] [CrossRef]
- Ferreres, F.; Gomes, D.; Valentao, P.; Goncalves, R.; Pio, R.; Chagas, E.A.; Seabra, R.M.; Andrade, P.B. Improved loquat (Eriobotrya japonica Lindl.) cultivars: Variation of phenolics and antioxidative potential. Food Chem. 2009, 114, 1019–1027. [Google Scholar] [CrossRef]
- Zhang, L.; Saber, F.R.; Rocchetti, G.; Zengin, G.; Hashem, M.M.; Lucini, L. UHPLC-QTOF-MS based metabolomics and biological activities of different parts of Eriobotrya japonica. Food Res. Int. 2021, 143, 110242. [Google Scholar] [CrossRef]
- Simon, L.; Souza-Smith, F.M.; Molina, P.E. Alcohol-associated tissue injury: Current views on pathophysiological mechanisms. Annu. Rev. Physiol. 2022, 84, 87–112. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Dubey, K.K.; Singhal, R.S. Influence of food commodities on hangover based on alcohol dehydrogenase and aldehyde dehydrogenase activities. Curr. Res. Food Sci. 2019, 1, 8–16. [Google Scholar] [CrossRef]
- Lucey, M.R.; Im, G.Y.; Mellinger, J.L.; Szabo, G.; Crabb, D.W. Introducing the 2019 American Association for the Study of Liver Diseases Guidance on Alcohol-Associated Liver Disease. Liver Transpl. 2020, 26, 14–16. [Google Scholar] [CrossRef]
- GBD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. 2022, 62, 3833–3854. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.K.; Chachin, K.; Ueda, Y.; Imahori, Y.; Wang, C.Y. Metabolism of phenolic compounds during loquat fruit development. J. Agric. Food Chem. 2001, 49, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, B.; Nishitani, S.; Koeduka, T. Synthesis of deuterium-labeled cinnamic acids: Understanding the volatile benzenoid pathway in the flowers of the Japanese loquat Eriobotrya japonica. J. Label. Compd. Radiopharm. 2021, 64, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kobayashi, E.; Takamatsu, Y.; Li, S.H.; Hatano, T.; Sakagami, H.; Kusama, K.; Satoh, K.; Sugita, D.; Shimura, S.; et al. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem. Pharm. Bull. 2000, 48, 687–693. [Google Scholar] [CrossRef]
- Escudero-Lopez, B.; Calani, L.; Fernandez-Pachon, M.S.; Ortega, A.; Brighenti, F.; Crozier, A.; Del Rio, D. Absorption, metabolism, and excretion of fermented orange juice (poly)phenols in rats. Biofactors 2014, 40, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, L.; Shi, S.Y.; Cai, P.; Liang, X.J.; Zhang, S.H. Antioxidant capacity and phenolic compounds of Lonicerae macranthoides by HPLC-DAD-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2016, 124, 254–260. [Google Scholar] [CrossRef]
- Lucini, L.; Colla, G.; Moreno, M.B.M.; Bernardo, L.; Cardarelli, M.; Terzi, V.; Bonini, P.; Rouphael, Y. Inoculation of Rhizoglomus irregulare or Trichoderma atroviride differentially modulates metabolite profiling of wheat root exudates. Phytochemistry 2019, 157, 158–167. [Google Scholar] [CrossRef]
- Gudej, J.; Czapski, P. Components of the petroleum ether and chloroform extracts of Chrysosplenium alternifolium. Chem. Nat. Compd. 2009, 45, 717–719. [Google Scholar] [CrossRef]
- Lu, H.; Chen, J.; Li, W.L.; Ren, B.R.; Wu, J.L.; Zhang, H.Q. Hypoglycemic effect of the total flavonoid fraction from Folium Eriobotryae. Phytomedicine 2009, 16, 967–971. [Google Scholar] [CrossRef]
- Slimestad, R.; Andersen, Ø.M.; Francis, G.W.; Marston, A.; Hostettmann, K. Syringetin 3-O-(6’’-acetyl)-β-glucopyranoside and other flavonols from needles of Norway spruce, Picea abies. Phytochemistry 1995, 40, 1537–1542. [Google Scholar] [CrossRef]
- Xu, H.X.; Li, X.Y.; Chen, J.W. Comparison of phenolic compound contents and antioxidant capacities of loquat (Eriobotrya japonica Lindl.) fruits. Food Sci. Biotechnol. 2014, 23, 2013–2020. [Google Scholar] [CrossRef]
- Zheng, M.Y.; Xia, Q.L.; Lu, S.M. Study on drying methods and their influences on effective components of loquat flower tea. LWT-Food Sci. Technol. 2015, 63, 14–20. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, T.L.; Yang, S.J.; Li, X.; Song, B.; Wang, Y.; Lin, Q.; Cao, J.P. Analysis of phenolic components and related biological activities of 35 apple (Malus pumila Mill.) cultivars. Molecules 2020, 25, 4253. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.; Goncalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus Persica): Phytochemicals and Health Benefits. Food Rev. Int. 2022, 38, 1703–1734. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.N. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Chen, J.B.; Zhao, C.N.; Liu, X.J.; Chen, Y.Y.; Liang, J.J.; Cao, J.P.; Wang, Y.; Sun, C.D. Advances in extraction and purification of citrus flavonoids. Food Front. 2023, 4, 750–781. [Google Scholar] [CrossRef]
- Cantin, C.M.; Moreno, M.A.; Gogorcena, Y. Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) Batsch] breeding progenies. J. Agric. Food Chem. 2009, 57, 4586–4592. [Google Scholar] [CrossRef]
- Maheshwari, S.; Kumar, V.; Bhadauria, G.; Mishra, A. Immunomodulatory potential of phytochemicals and other bioactive compounds of fruits: A review. Food Front. 2022, 3, 221–238. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.Y.; Zhao, F.L.; Hou, G.G.; Meng, Q.G. Food applications and potential health benefits of hawthorn. Foods 2022, 11, 2861. [Google Scholar] [CrossRef]
- Wu, D.F.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar] [PubMed]
- Patlevic, P.; Vaskova, J.; Svorc, P., Jr.; Vasko, L.; Svorc, P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr. Med. Res. 2016, 5, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.M.S.; Khan, A.S.; Singh, Z.; Ayyub, S. Postharvest biology and technology of loquat (Eriobotrya japonica Lindl.). Foods 2023, 12, 1329. [Google Scholar] [CrossRef] [PubMed]
- Pareek, S.; Benkeblia, N.; Janick, J.; Cao, S.F.; Yahia, E.M. Postharvest physiology and technology of loquat (Eriobotrya japonica Lindl.) fruit. J. Sci. Food Agric. 2014, 94, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Kotikova, Z.; Lachman, J.; Hejtmankova, A.; Hejtmankova, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT-Food Sci. Technol. 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sanchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid-flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, R.; Chen, J.; Cao, J.; Xiao, J.; Li, X.; Sun, C. Tangeretin maintains antioxidant activity by reducing CUL3 mediated NRF2 ubiquitination. Food Chem. 2021, 365, 130470. [Google Scholar] [CrossRef]
- Oliveira, A.K.S.; de Oliveira, E.S.A.M.; Pereira, R.O.; Santos, A.S.; Barbosa Junior, E.V.; Bezerra, M.T.; Barreto, R.S.S.; Quintans-Junior, L.J.; Quintans, J.S.S. Anti-obesity properties and mechanism of action of flavonoids: A review. Crit. Rev. Food Sci. 2022, 62, 7827–7848. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, Y.X.; Liang, Y.Z.; Ma, X.Y.; Qi, X.; Xiao, J.B.; Xue, W.M.; Luo, Y.N.; Yue, T.L. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. Food Front. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Sarıkaya, E.; Doğan, S. Glutathione peroxidase in health and diseases. In Glutathione System and Oxidative Stress in Health and Disease; IntechOpen: London, UK, 2020; Volume 49. [Google Scholar]
- Das, S.K.; Vasudevan, D. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.X.; Wong, C.W.; Feng, Y.B. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Cao, Q.; Yu, S.C.; Lv, X.W.; Jin, Y.; Zhang, L.; Zou, Y.H.; Ge, J.F. Anti-oxidative effect of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. leaf in chronic bronchitis rats. Life Sci. 2006, 78, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Huang, S.S.; Lin, S.S.; Amagaya, S.; Ho, H.Y.; Hou, W.C.; Shie, P.H.; Wu, J.B.; Huang, G.J. Anti-inflammatory activities of tormentic acid from suspension cells of Eriobotrya Japonica ex vivo and in vivo. Food Chem. 2011, 127, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Zheng, K.; Benede-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. The Lieber-DeCarli diet-A flagship model for experimental alcoholic liver disease. Alcohol. Clin. Exp. Res. 2018, 42, 1828–1840. [Google Scholar] [CrossRef] [PubMed]
- Brandon-Warner, E.; Schrum, L.W.; Schmidt, C.M.; McKillop, I.H. Rodent models of alcoholic liver disease: Of mice and men. Alcohol 2012, 46, 715–725. [Google Scholar] [CrossRef]
- Aragon, G.; Younossi, Z.M. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin. J. Med. 2010, 77, 195–204. [Google Scholar] [CrossRef]
- Hoiseth, G.; Hilberg, T.; Trydal, T.; Husa, A.; Vindenes, V.; Bogstrand, S.T. The alcohol marker phosphatidylethanol is closely related to AST, GGT, ferritin and HDL-C. Basic Clin. Pharmacol. 2022, 130, 182–190. [Google Scholar] [CrossRef]
- Whitfield, J.B.; Heath, A.C.; Madden, P.A.F.; Pergadia, M.L.; Montgomery, G.W.; Martin, N.G. Metabolic and biochemical effects of low-to-moderate alcohol consumption. Alcohol. Clin. Exp. Res. 2013, 37, 575–586. [Google Scholar] [CrossRef]
- Nishiwaki, M.; Ishikawa, T.; Ito, T.; Shige, H.; Tomiyasu, K.; Nakajima, K.; Kondo, K.; Hashimoto, H.; Saitoh, K.; Manabe, M.; et al. Effects of alcohol on lipoprotein lipase, hepatic lipase, cholesteryl ester transfer protein, and lecithin:cholesterol acyltransferase in high-density lipoprotein cholesterol elevation. Atherosclerosis 1994, 111, 99–109. [Google Scholar] [CrossRef]
- Tuma, D.J. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic. Biol. Med. 2002, 32, 303–308. [Google Scholar] [CrossRef]

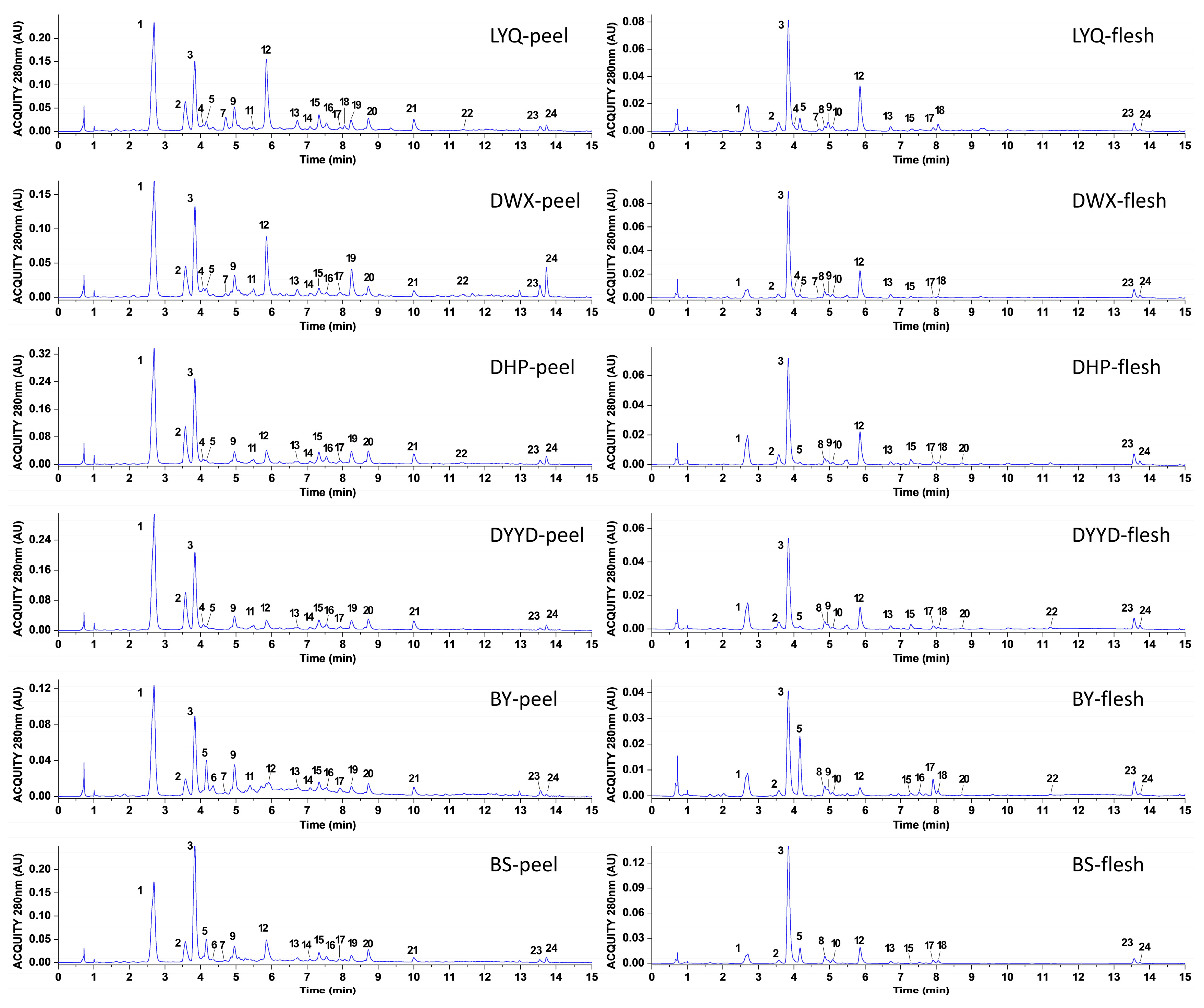



| Peak No. | TR (min) | (M − H) (m/z) | (M + H) (m/z) | Fragment Ions (m/z) | Formula | Tentative Compounds | References |
|---|---|---|---|---|---|---|---|
| 1 | 2.6840 | 355.1027 | 163.0399, 145.0290, 135.0447, 117.0346 | C16H18O9 | 5-caffeoylquinic acid | [4] | |
| 2 | 3.5737 | 337.0931 | 191.0549, 163.0395, 119.0504 | C16H18O8 | 3-p-coumaroylquinic acid | [4] | |
| 3 | 3.8384 | 353.0873 | 191.0556 | C16H18O9 | 3-caffeoylquinic acid | [4] | |
| 4 | 4.0717 | 353.0875 | 191.0552, 179.0337, 173.0446, 135.0447 | C16H18O9 | 4-O-caffeoylquinic acid | [12] | |
| 5 | 4.1621 | 225.0759 | 175.0392, 147.0449, 91.0566 | C11H12O5 | sinapic acid | [13] | |
| 6 | 4.3463 | 577.1331 | 407.0777, 289.0708, 125.0239 | C30H26O12 | procyanidin B2 | [14] | |
| 7 | 4.7120 | 355.1030 | 217.0500. 193.0500, 175.0402, 160.0165, 134.0373, 132.0218 | C16H20O9 | ferulic acid-4-O-glucoside | [15] | |
| 8 | 4.8687 | 353.0875 | 191.0550 | C16H18O9 | 1-O-caffeoylquinic acid | [16] | |
| 9 | 4.9564 | 181.0495 | 163.0394, 145.0299, 135.0444, 117.0348, 89.0409 | C9H8O4 | caffeic acid | [12] | |
| 10 | 5.0809 | 337.0927 | 191.0543, 173.0439, 163.0383, 119.0495, 93.0349 | C16H18O8 | 4-p-coumaroylquinic acid | [4] | |
| 11 | 5.4931 | 865.1978 | 713.1607, 695.1464, 577.1390, 407.0776, 289.0698, 125.0233 | C45H38O18 | procyanidin C1 | [14] | |
| 12 | 5.8568 | 367.1034 | 191.0552, 134.0367, 93.0356 | C17H20O9 | 5-feruloylquinic acid | [4] | |
| 13 | 6.7237 | 611.1605 | 303.0507, 287.0557 | C27H30O16 | quercetin-3-O-neohesperidoside | [4] | |
| 14 | 7.0836 | 595.1289 | 301.0351, 300.0275, 271.0234 | C26H28O16 | quercetin-3-O-sambubioside | [4] | |
| 15 | 7.3362 | 465.1026 | 303.0500 | C21H20O12 | quercetin-3-O-galactoside | [4] | |
| 16 | 7.5455 | 594.1531 | 284.0330, 255.0291, 227.0341 | C27H30O15 | kaempferol-3-O-neohesperidoside | [4] | |
| 17 | 7.9160 | 565.1557 | 341.0878, 327.0721, 223.0599, 197.0446, 183.0288 | C26H30O14 | 3,6,7,2′,4′-pentamethylquercetagetin 3′-O-glucoside | [17] | |
| 18 | 8.0549 | 535.1444 | 311.0770, 297.0603, 223.0601, 208.0360, 167.0339 | C25H28O13 | chrysosplenoside B | [18] | |
| 19 | 8.2381 | 447.0921 | 285.0397, 284.0326, 255.0296, 227.0344 | C21H20O11 | kaempferol-3-O-glucoside | [19] | |
| 20 | 8.7253 | 447.0920 | 301.0349, 271.0239, 151.0028 | C21H20O11 | quercetin-3-O-rhamnoside | [4] | |
| 21 | 10.0067 | 431.0976 | 285.0400, 255.0297, 227.0343 | C21H20O10 | kaempferol-3-O-rhamnoside | [4] | |
| 22 | 11.3885 | 489.1024 | 301.0340, 300.0267, 271.0215 | C23H22O12 | kaempferol-3-O-(6″-acetyl)glucoside | [20] | |
| 23 | 13.5589 | 455.3489 | 408.3362, 407.3301 | C30H48O3 | oleanolic acid | [2] | |
| 24 | 13.7329 | 457.3675 | 397.2401, 355.1902, 333.1325, 317.1980, 275.1283 | C30H48O3 | ursolic acid | [2] |
| Peak No. | Tentative Compounds | LYQ-Peel | LYQ-Flesh | DWX-Peel | DWX-Flesh | DHP-Peel | DHP-Flesh | DYYD-Peel | DYYD-Flesh | BY-Peel | BY-Flesh | BS-Peel | BS-Flesh |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5-caffeoylquinic acid | 4.62 ± 0.32 c | 0.40 ± 0.00 f | 3.59 ± 0.14 d | 0.13 ± 0.03 f | 7.10 ± 0.01 a | 0.40 ± 0.02 f | 6.44 ± 0.07 b | 0.35 ± 0.00 f | 2.73 ± 0.01 e | 0.22 ± 0.00 f | 3.74 ± 0.03 d | 0.26 ± 0.01 f |
| 2 | 3-p-coumaroylquinic acid | 1.18 ± 0.11 c | 0.12 ± 0.00 f | 0.88 ± 0.04 d | 0.06 ± 0.00 f | 2.01 ± 0.00 a | 0.11 ± 0.01 f | 1.83 ± 0.00 b | 0.08 ± 0.00 f | 0.35 ± 0.00 e | 0.03 ± 0.01 f | 0.84 ± 0.01 d | 0.06 ± 0.00 f |
| 3 | 3-caffeoylquinic acid | 2.31 ± 0.16 c | 1.38 ± 0.02 d | 2.20 ± 0.08 c | 1.45 ± 0.04 d | 4.15 ± 0.02 a | 1.12 ± 0.06 e | 3.43 ± 0.03 b | 0.90 ± 0.01 ef | 1.44 ± 0.01 d | 0.70 ± 0.00 f | 4.28 ± 0.04 a | 2.32 ± 0.07 c |
| 4 | 4-O-caffeoylquinic acid | 0.18 ± 0.01 b | 0.01 ± 0.00 d | 0.17 ± 0.01 b | 0.10 ± 0.00 c | 0.20 ± 0.00 a | n.d. | 0.21 ± 0.00 a | n.d. | n.d. | n.d. | n.d. | n.d. |
| 5 | sinapic acid | 0.34 ± 0.04 c | 0.13 ± 0.00 ef | 0.17 ± 0.01 e | 0.04 ± 0.00 g | 0.15 ± 0.00 e | 0.02 ± 0.00 g | 0.09 ± 0.02 f | 0.03 ± 0.00 g | 0.51 ± 0.00 b | 0.31 ± 0.00 c | 0.67 ± 0.01 a | 0.24 ± 0.01 d |
| 6 | procyanidin B2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.20 ± 0.00 a | n.d. | 0.12 ± 0.00 b | n.d. |
| 7 | ferulic acid-4-O-glucoside | 0.39 ± 0.02 a | 0.01 ± 0.01 b | 0.06 ± 0.00 b | 0.01 ± 0.00 b | n.d. | n.d. | n.d. | n.d. | 0.04 ± 0.00 b | n.d. | 0.07 ± 0.04 b | n.d. |
| 8 | 1-O-caffeoylquinic acid | n.d. | 0.04 ± 0.00 d | 0.47 ± 0.02 a | 0.07 ± 0.00 c | n.d. | 0.05 ± 0.00 cd | n.d. | 0.06 ± 0.00 cd | n.d. | 0.06 ± 0.00 cd | n.d. | 0.12 ± 0.00 b |
| 9 | caffeic acid | 0.76 ± 0.01 a | 0.10 ± 0.00 e | n.d. | 0.04 ± 0.00 c | 0.53 ± 0.00 f | 0.03 ± 0.01 d | 0.55 ± 0.01 f | 0.03 ± 0.01 f | 0.55 ± 0.00 f | 0.04 ± 0.00 b | 0.50 ± 0.00 bc | n.d. |
| 10 | 4-p-coumaroylquinic acid | n.d. | 0.05 ± 0.00 b | n.d. | 0.05 ± 0.00 b | n.d. | 0.03 ± 0.00 c | n.d. | 0.01 ± 0.00 d | n.d. | 0.02 ± 0.00 cd | n.d. | 0.07 ± 0.00 a |
| 11 | procyanidin C1 | 0.09 ± 0.02 b | n.d. | 0.12 ± 0.01 ab | n.d. | 0.15 ± 0.00 a | n.d. | 0.13 ± 0.00 a | n.d. | 0.15 ± 0.00 a | n.d. | n.d. | n.d. |
| 12 | 5-feruloylquinic acid | 2.43 ± 0.17 a | 0.52 ± 0.00 e | 1.41 ± 0.06 b | 0.36 ± 0.01 efg | 0.77 ± 0.00 d | 0.33 ± 0.02 fg | 0.51 ± 0.00 ef | 0.21 ± 0.00 gh | 0.24 ± 0.00 gh | 0.06 ± 0.00 h | 0.99 ± 0.01 c | 0.29 ± 0.01 g |
| 13 | quercetin-3-O-neohesperidoside | 0.28 ± 0.07 a | 0.05 ± 0.00 de | 0.15 ± 0.01 bc | 0.05 ± 0.00 de | 0.09 ± 0.00 cde | 0.03 ± 0.00 e | 0.07 ± 0.00 cde | 0.03 ± 0.00 e | 0.12 ± 0.00 bcd | n.d. | 0.18 ± 0.02 b | 0.04 ± 0.00 e |
| 14 | quercetin-3-O-sambubioside | 0.08 ± 0.01 cd | n.d. | 0.07 ± 0.00 d | n.d. | 0.11 ± 0.00 a | n.d. | 0.09 ± 0.00 bc | n.d. | 0.09 ± 0.00 ab | n.d. | 0.07 ± 0.00 cd | n.d. |
| 15 | quercetin-3-O-galactoside | 0.45 ± 0.05 b | 0.01 ± 0.00 e | 0.21 ± 0.01 d | 0.01 ± 0.00 e | 0.60 ± 0.00 a | 0.05 ± 0.00 e | 0.46 ± 0.01 b | 0.04 ± 0.00 e | 0.21 ± 0.00 d | 0.02 ± 0.00 e | 0.33 ± 0.00 c | 0.01 ± 0.00 e |
| 16 | kaempferol-3-O-neohesperidoside | 0.26 ± 0.01 b | n.d. | 0.07 ± 0.00 d | n.d. | 0.39 ± 0.00 a | n.d. | 0.25 ± 0.02 b | n.d. | 0.08 ± 0.00 d | 0.02 ± 0.00 e | 0.21 ± 0.00 c | n.d. |
| 17 | 3,6,7,2′,4′-pentamethylquercetagetin 3′-O-glucoside | 0.04 ± 0.01 d | 0.03 ± 0.00 e | 0.10 ± 0.00 c | 0.01 ± 0.00 f | 0.17 ± 0.00 a | 0.02 ± 0.00 f | 0.14 ± 0.00 b | 0.02 ± 0.00 ef | 0.09 ± 0.00 c | 0.09 ± 0.00 c | 0.10 ± 0.00 c | 0.05 ± 0.00 d |
| 18 | chrysosplenoside B | 0.11 ± 0.02 a | 0.06 ± 0.00 b | n.d. | 0.01 ± 0.00 d | n.d. | 0.03 ± 0.00 cd | n.d. | 0.02 ± 0.00 d | n.d. | 0.02 ± 0.01 cd | n.d. | 0.05 ± 0.00 bc |
| 19 | kaempferol-3-O-glucoside | 0.31 ± 0.03 c | n.d. | 0.63 ± 0.02 a | n.d. | 0.62 ± 0.00 a | n.d. | 0.42 ± 0.01 b | n.d. | 0.13 ± 0.00 e | n.d. | 0.23 ± 0.00 d | n.d. |
| 20 | quercetin-3-O-rhamnoside | 0.35 ± 0.03 c | n.d. | 0.21 ± 0.01 d | n.d. | 0.60 ± 0.00 a | 0.02 ± 0.00 e | 0.44 ± 0.01 b | 0.01 ± 0.00 e | 0.18 ± 0.00 d | 0.01 ± 0.00 e | 0.40 ± 0.01 b | n.d. |
| 21 | kaempferol-3-O-rhamnoside | 0.37 ± 0.02 b | n.d. | 0.14 ± 0.01 c | n.d. | 0.51 ± 0.00 a | n.d. | 0.39 ± 0.00 b | n.d. | 0.15 ± 0.00 c | n.d. | 0.17 ± 0.00 c | n.d. |
| 22 | kaempferol-3-O-(6″-acetyl)glucoside | 0.05 ± 0.00 a | n.d. | 0.04 ± 0.00 a | n.d. | 0.04 ± 0.01 a | 0.02 ± 0.00 b | n.d. | 0.01 ± 0.00 b | n.d. | n.d.b | n.d. | n.d. |
| 23 | oleanolic acid | 0.14 ± 0.01 b | 0.06 ± 0.01 c | 0.22 ± 0.01 a | 0.08 ± 0.01 c | 0.16 ± 0.00 b | 0.09 ± 0.01 c | 0.09 ± 0.00 c | 0.08 ± 0.00 c | 0.08 ± 0.00 c | 0.07 ± 0.01 c | 0.08 ± 0.00 c | 0.07 ± 0.01 c |
| 24 | ursolic acid | 0.14 ± 0.00 c | 0.01 ± 0.00 d | 0.43 ± 0.02 a | 0.02 ± 0.00 d | 0.24 ± 0.00 b | 0.03 ± 0.00 d | 0.14 ± 0.00 c | 0.03 ± 0.00 d | 0.02 ± 0.00 d | 0.01 ± 0.00 d | 0.12 ± 0.00 c | 0.01 ± 0.00 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.-J.; Chen, Y.-Y.; Wu, M.-X.; Yang, H.; Cao, J.-P.; Sun, C.-D.; Wang, Y. Phenolics and Terpenoids Profiling in Diverse Loquat Fruit Varieties and Systematic Assessment of Their Mitigation of Alcohol-Induced Oxidative Stress. Antioxidants 2023, 12, 1795. https://doi.org/10.3390/antiox12101795
Yan Q-J, Chen Y-Y, Wu M-X, Yang H, Cao J-P, Sun C-D, Wang Y. Phenolics and Terpenoids Profiling in Diverse Loquat Fruit Varieties and Systematic Assessment of Their Mitigation of Alcohol-Induced Oxidative Stress. Antioxidants. 2023; 12(10):1795. https://doi.org/10.3390/antiox12101795
Chicago/Turabian StyleYan, Qun-Jiao, Yun-Yi Chen, Man-Xi Wu, Han Yang, Jin-Ping Cao, Chong-De Sun, and Yue Wang. 2023. "Phenolics and Terpenoids Profiling in Diverse Loquat Fruit Varieties and Systematic Assessment of Their Mitigation of Alcohol-Induced Oxidative Stress" Antioxidants 12, no. 10: 1795. https://doi.org/10.3390/antiox12101795
APA StyleYan, Q.-J., Chen, Y.-Y., Wu, M.-X., Yang, H., Cao, J.-P., Sun, C.-D., & Wang, Y. (2023). Phenolics and Terpenoids Profiling in Diverse Loquat Fruit Varieties and Systematic Assessment of Their Mitigation of Alcohol-Induced Oxidative Stress. Antioxidants, 12(10), 1795. https://doi.org/10.3390/antiox12101795









