Correlation of Metabolic Syndrome with Redox Homeostasis Biomarkers: Evidence from High-Fat Diet Model in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet Composition
2.2. Body Weight Gain and Food Intake
2.3. Determination and Quantification of Metabolic Syndrome
2.3.1. Glucose Tolerance Test
2.3.2. Plasma Samples
2.3.3. Biochemical Analyses
2.3.4. Oxidative Stress Parameters
2.3.5. Plasma Antioxidant Status
2.4. Micro-Computed Tomography Scans
2.5. Histological Analyses
2.6. Statistical Analyses
3. Results
3.1. Effects of HFD Treatment on Body Weight and Food Intake
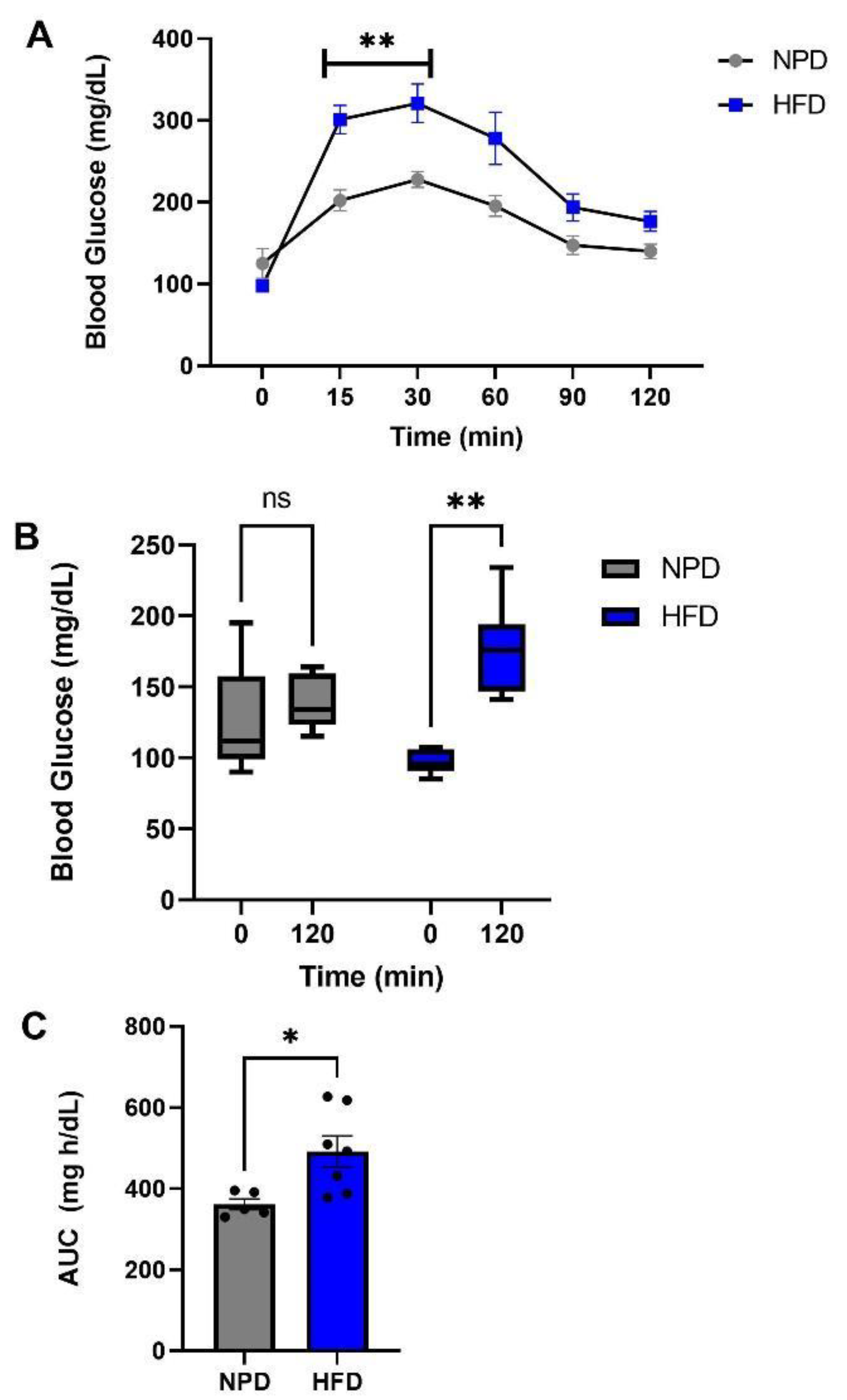
3.2. Effects of HFD Treatment on Glucose Tolerance
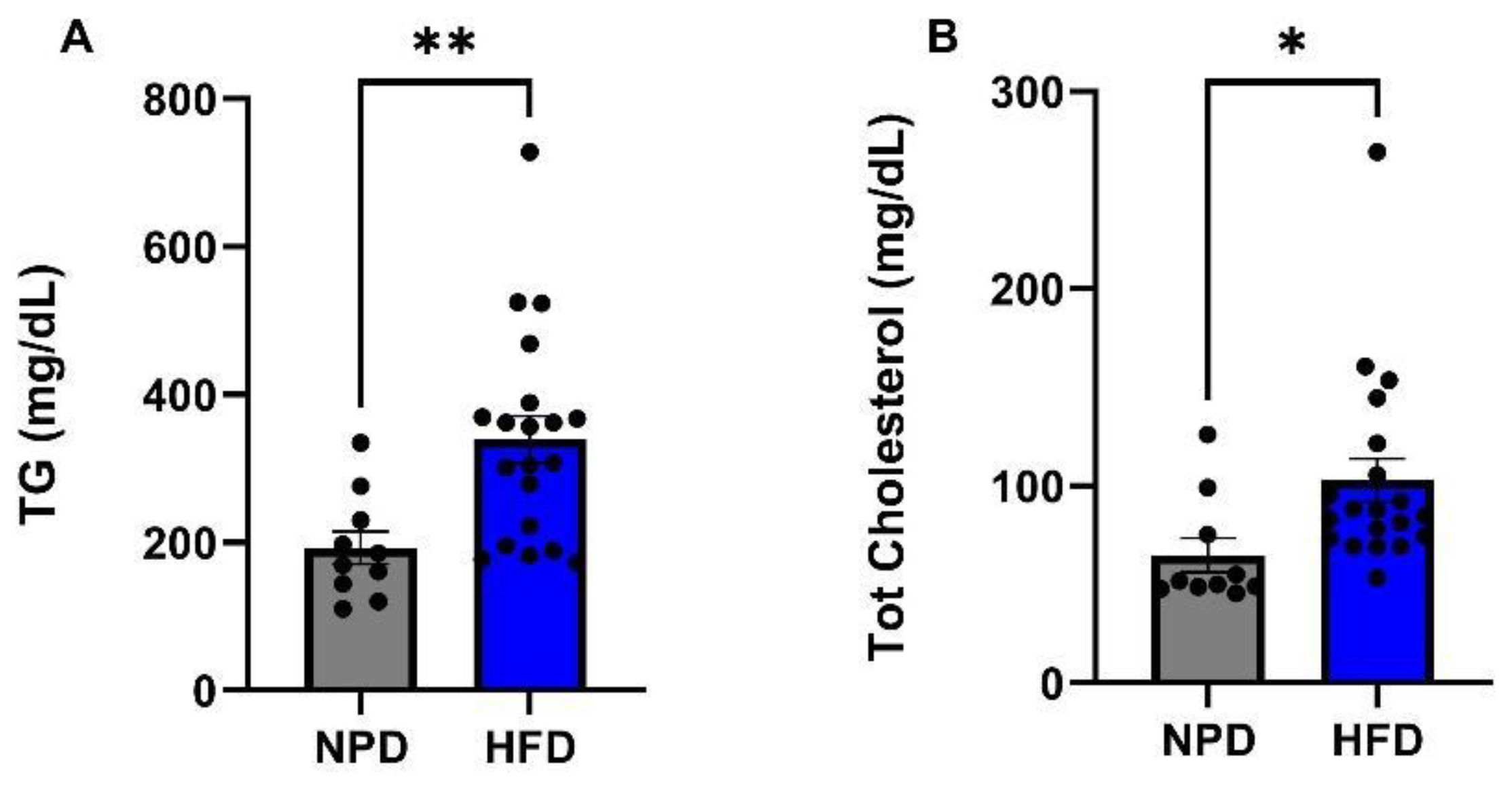
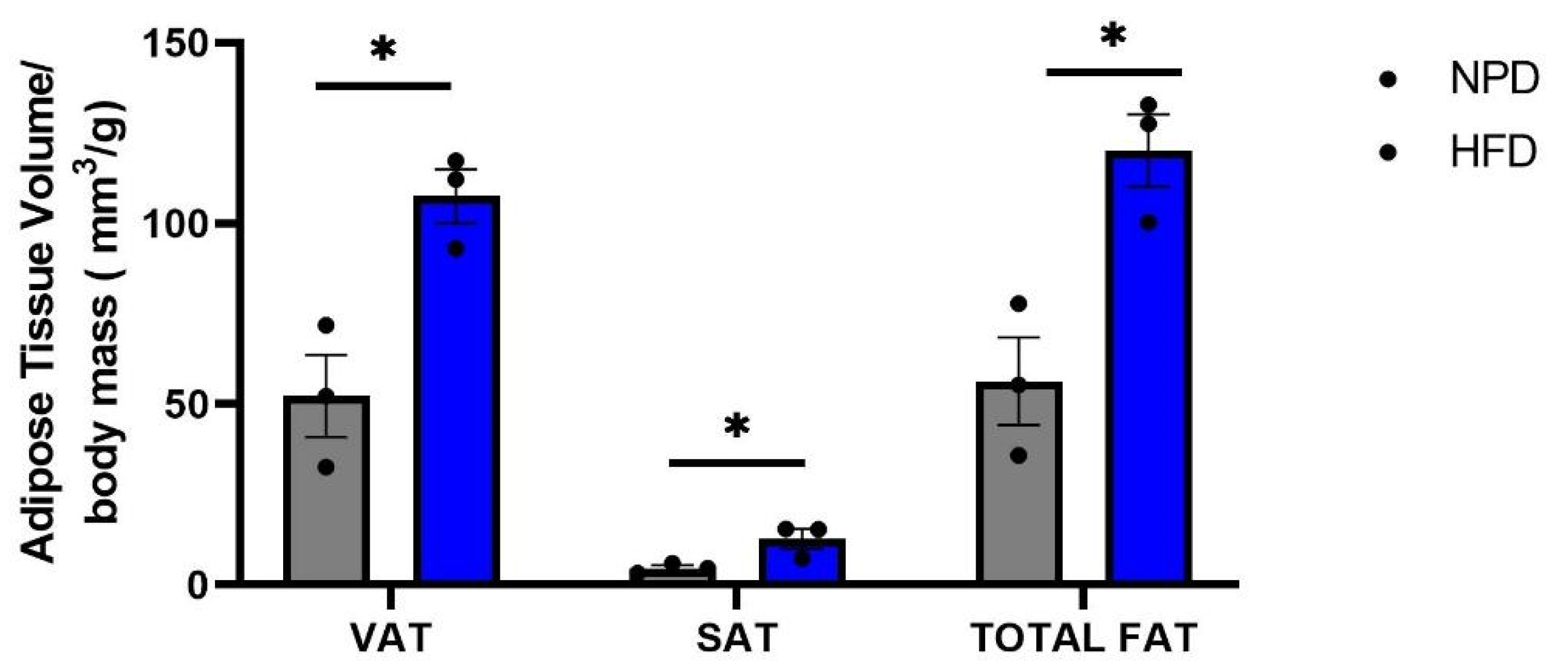
3.3. Effects of HFD Treatment on Lipid Profile and on the Distribution of Adipose Tissue
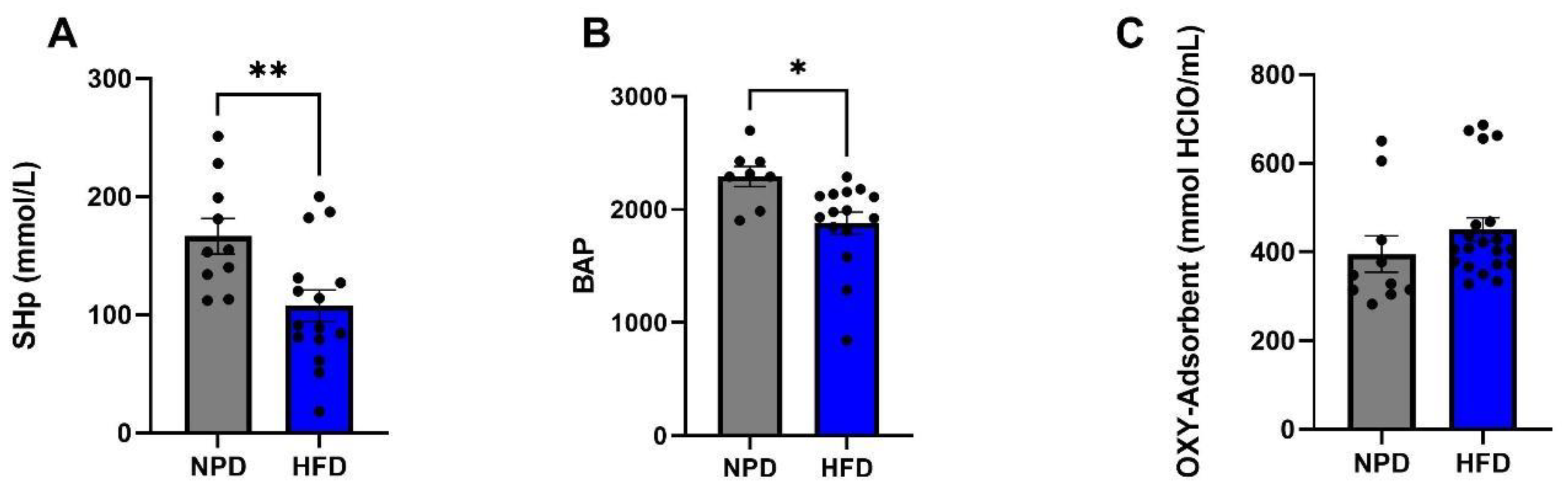
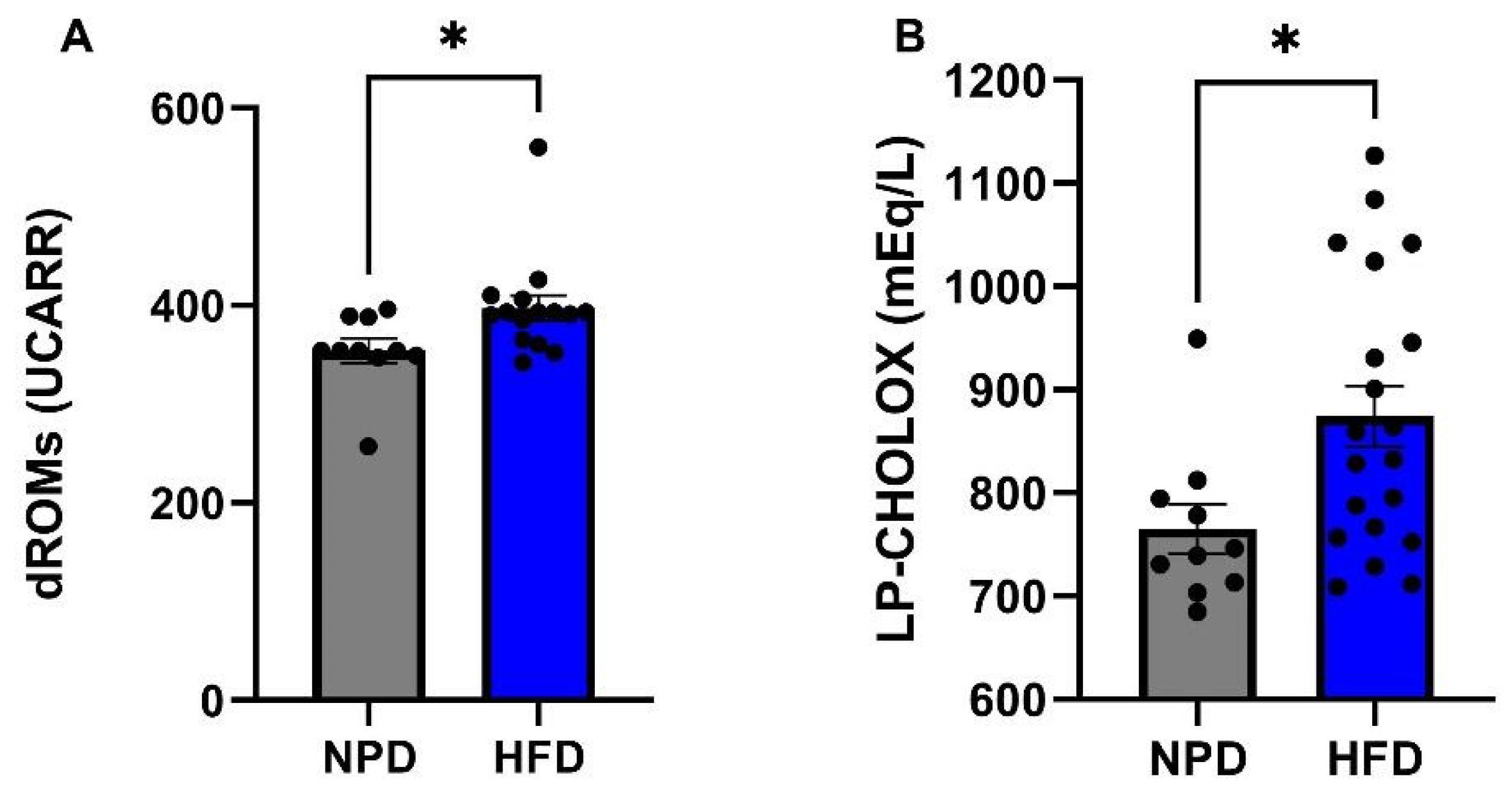
3.4. Effects of HFD on Plasma Redox Homeostasis Biomarkers
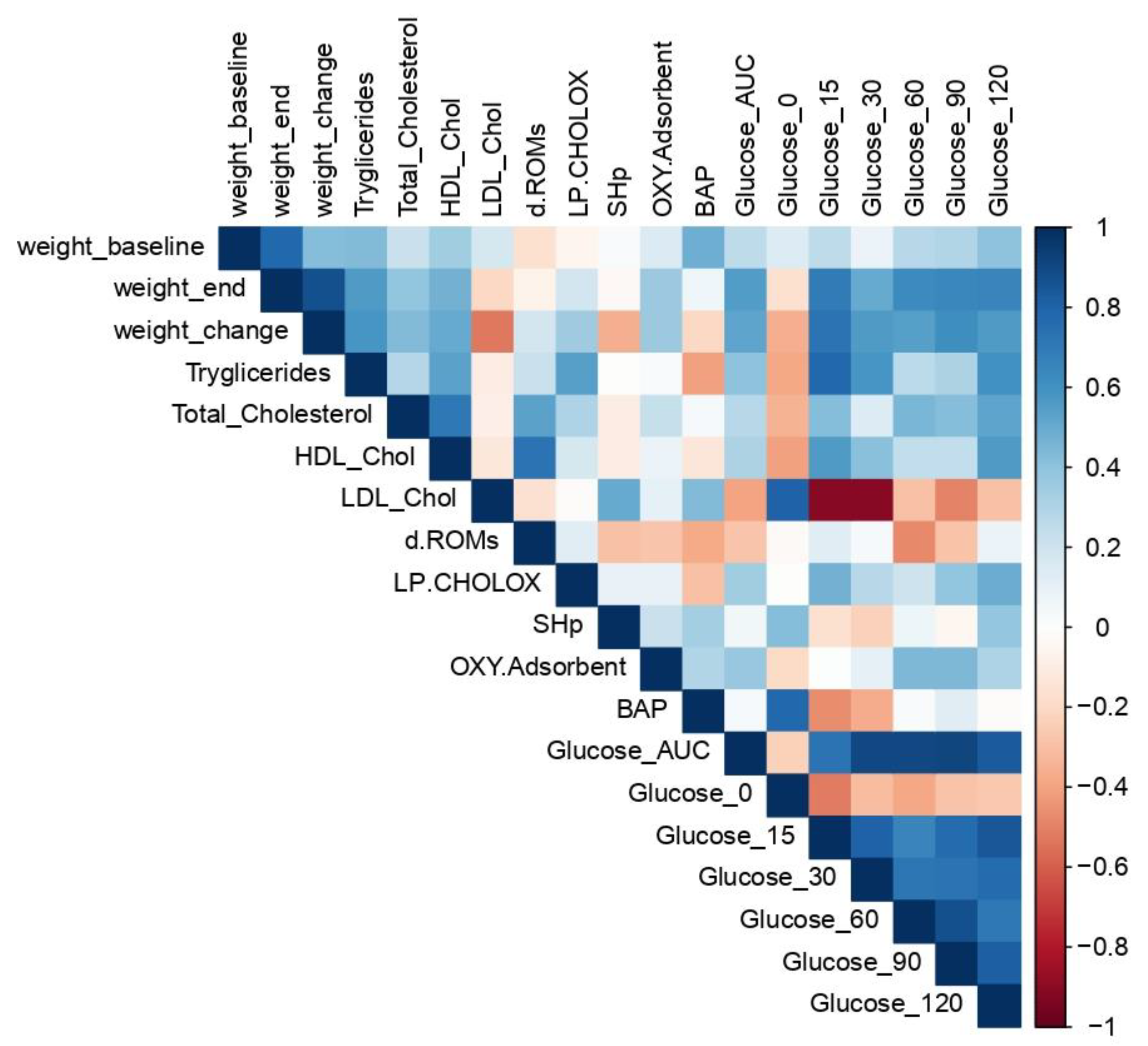
3.5. Spearman’s Correlation Matrix of MetS and Oxidative Stress Parameters
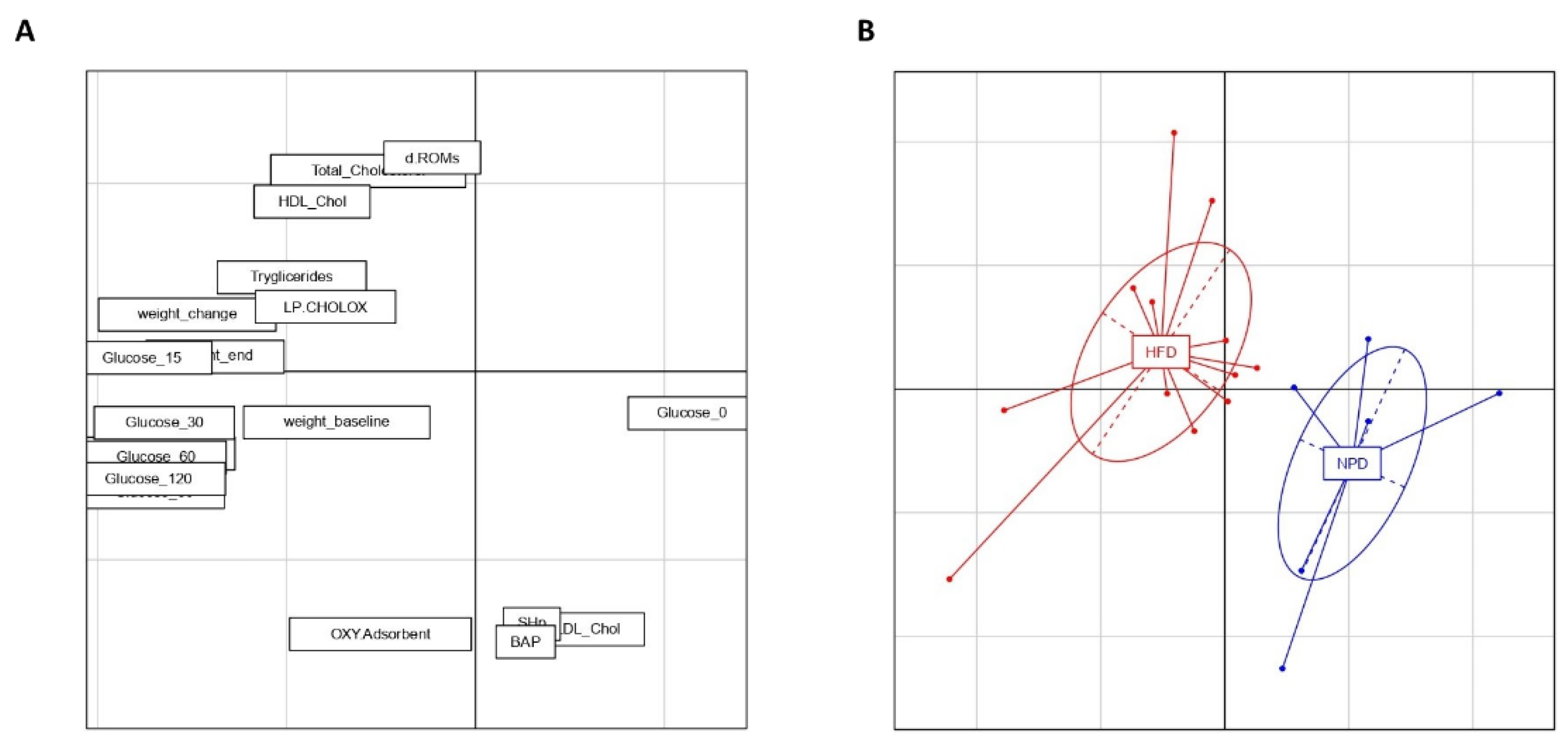
3.6. Principal Component Analysis
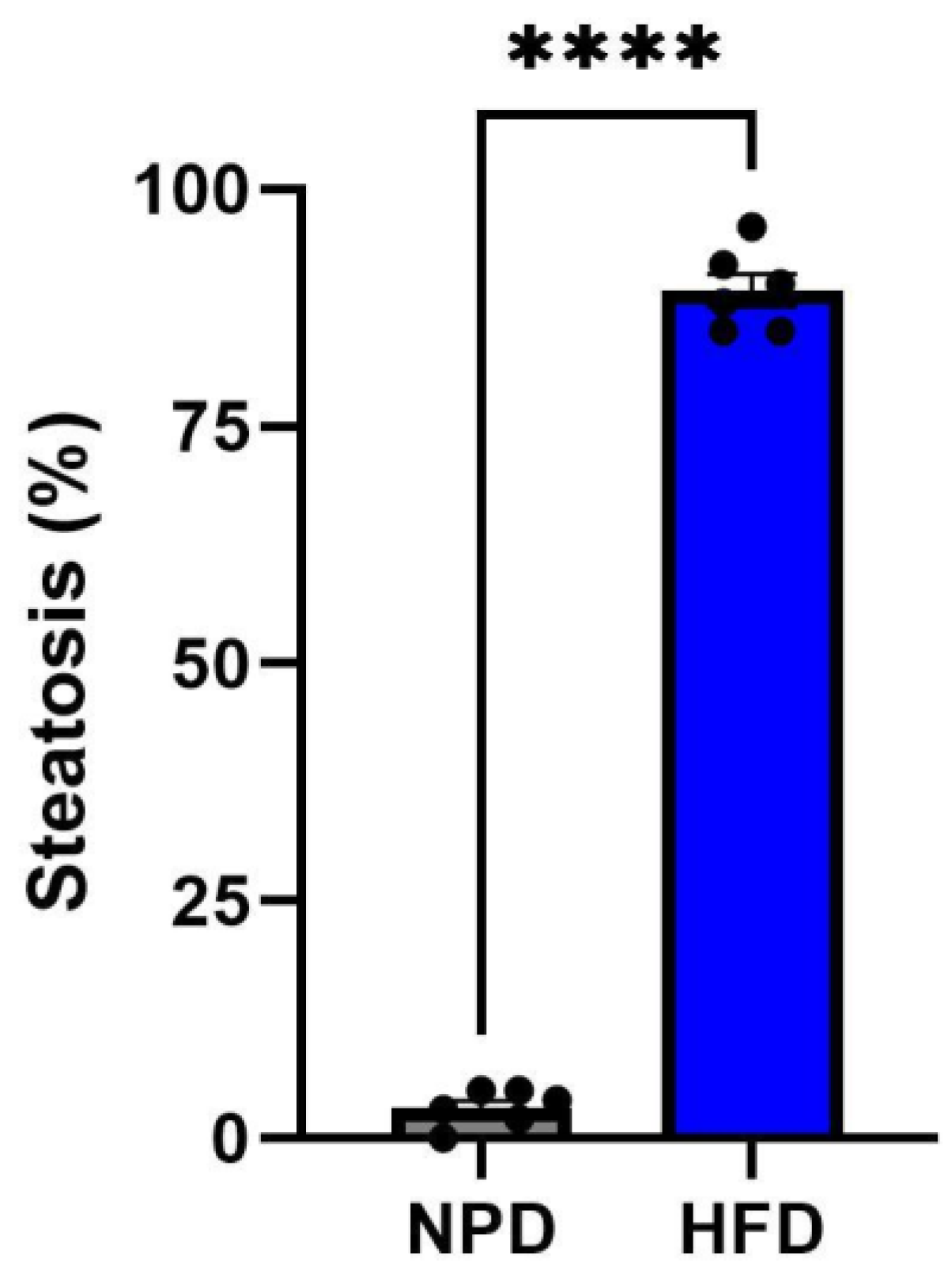
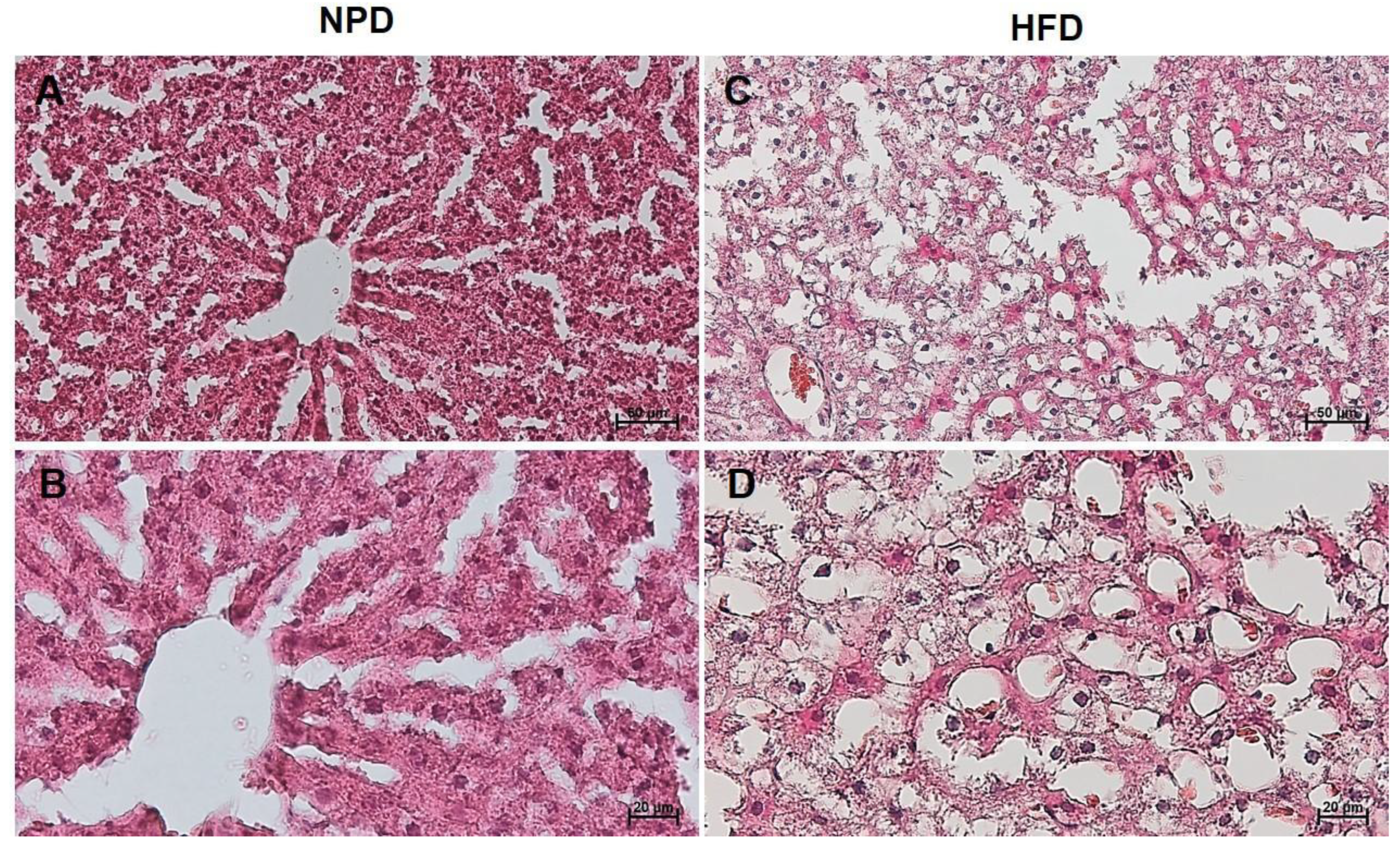
3.7. Effects of HFD on Hepatic Steatosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquilante, C.L.; Kosmiski, L.A.; Knutsen, S.D.; Zineh, I. Relationship between plasma resistin concentrations, inflammatory chemokines, and components of the metabolic syndrome in adults. Metabolism 2008, 57, 494–501. [Google Scholar] [CrossRef]
- Spahis, S.; Borys, J.-M.; Levy, E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid. Redox Signal. 2017, 26, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Aru, J.; Freeman, J.; Hudson, R.; Janssen, I. Abdominal adiposity and insulin resistance in obese men. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E657–E663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nakagawa, T.; Taniguchi, H.; Fujii, K.; Omatsu, T.; Nakajima, T.; Sarui, H.; Shimazaki, M.; et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2005, 143, 722–728. [Google Scholar] [CrossRef]
- Sparks, L.M.; Ukropcova, B.; Smith, J.; Pasarica, M.; Hymel, D.; Xie, H.; Bray, G.A.; Miles, J.M.; Smith, S.R. Relation of adipose tissue to metabolic flexibility. Diabetes Res. Clin. Pract. 2009, 83, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Lionetti, L.; Mollica, M.P.; Lombardi, A.; Cavaliere, G.; Gifuni, G.; Barletta, A. From chronic overnutrition to insulin resistance: The role of fat-storing capacity and inflammation. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 146–152. [Google Scholar] [CrossRef]
- Gambino, G.; Giglia, G.; Schiera, G.; Di Majo, D.; Epifanio, M.S.; La Grutta, S.; Lo Baido, R.; Ferraro, G.; Sardo, P. Haptic perception in extreme obesity: qEEG study focused on predictive coding and body schema. Brain Sci. 2020, 10, 908. [Google Scholar] [CrossRef]
- Giammanco, M.; Lantieri, L.; Leto, G.; Pescia, F.; Di Majo, D. Nutrition, obesity and hormones. J. Biol. Res. 2018, 91. [Google Scholar] [CrossRef]
- Di Majo, D.; Cacciabaudo, F.; Accardi, G.; Gambino, G.; Giglia, G.; Ferraro, G.; Candore, G.; Sardo, P. Ketogenic and modified Mediterranean diet as a tool to counteract neuroinflammation in multiple sclerosis: Nutritional suggestions. Nutrients 2022, 14, 2384. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Vera, G.; Astier, J.; Landrier, J.F.; Miguel, M. High fat/high glucose diet induces metabolic syndrome in an experimental rat model. Nutrients 2018, 10, 1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avtanski, D.; Pavlov, V.A.; Tracey, K.J.; Poretsky, L. Characterization of inflammation and insulin resistance in high-fat diet-induced male C57BL/6J mouse model of obesity. Anim. Model Exp. Med. 2019, 2, 252–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef] [Green Version]
- Giammanco, M.; Aiello, S.; Casuccio, A.; La Guardia, M.; Cicero, L.; Puleio, R.; Vazzana, I.; Tomasello, G.; Cassata, G.; Leto, G.; et al. Effects of 3,5-Diiodo-L-Thyronine on the liver of high fat diet fed rats. J. Biol. Res. 2016, 89. [Google Scholar] [CrossRef] [Green Version]
- Vatashchuk, M.V.; Bayliak, M.M.; Hurza, V.V.; Storey, K.B.; Lushchak, V.I. Metabolic syndrome: Lessons from rodent and models. BioMed Res. Int. 2022, 2022, 5850507. [Google Scholar] [CrossRef]
- Kotzé-Hörstmann, L.; Cois, A.; Johnson, R.; Mabasa, L.; Shabalala, S.; Van Jaarsveld, P.J.; Sadie-Van Gijsen, H. Characterization and comparison of the divergent metabolic consequences of high-sugar and high-fat diets in male Wistar rats. Front. Physiol. 2022, 13, 904366. [Google Scholar] [CrossRef]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Sun, Y.; Li, Y.; Ma, S.; Zhang, K.; Chen, A.; Lyu, Y.; Yu, R. Sodium butyrate protects against oxidative stress in high-fat-diet-induced obese rats by promoting GSK-3β/Nrf2 signaling pathway and mitochondrial function. J. Food Biochem. 2022, 46, e14334. [Google Scholar] [CrossRef]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Korac, A.; Jankovic, A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021, 42, 101887. [Google Scholar] [CrossRef] [PubMed]
- Carrier, A. Metabolic syndrome and oxidative stress: A complex relationship. Antioxid. Redox Signal. 2017, 26, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Chida, A.S.; Rahman, I. Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochem. Pharmacol. 2006, 71, 551–564. [Google Scholar] [CrossRef]
- Matteucci, E.; Giampietro, O. Thiol signalling network with an eye to diabetes. Molecules 2010, 15, 8890–8903. [Google Scholar] [CrossRef] [Green Version]
- Go, Y.-M.; Jones, D.P. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic. Biol. Med. 2011, 50, 495–509. [Google Scholar] [CrossRef] [Green Version]
- Bełtowski, J.; Wójcicka, G.; Górny, D.; Marciniak, A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J. Physiol. Pharmacol. 2000, 51, 883–896. [Google Scholar]
- Elmarakby, A.A.; Imig, J.D. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin. Sci. 2010, 118, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Smirne, C.; Croce, E.; Di Benedetto, D.; Cantaluppi, V.; Comi, C.; Sainaghi, P.P.; Minisini, R.; Grossini, E.; Pirisi, M. Oxidative stress in non-alcoholic fatty liver disease. Livers 2022, 2, 30–76. [Google Scholar] [CrossRef]
- Cheng, H.S.; Ton, S.H.; Phang, S.C.W.; Tan, J.B.L.; Abdul Kadir, K. Increased susceptibility of post-weaning rats on high-fat diet to metabolic syndrome. J. Advert. Res. 2017, 8, 743–752. [Google Scholar] [CrossRef]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. Metabolic phenotyping guidelines: Assessing glucose homeostasis in rodent models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Takeda, K.; Maeda, M.; Ogawa, W.; Sato, T.; Okada, S.; Ohnishi, Y.; Nakajima, H.; Kashiwagi, A. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol. Int. 2016, 7, 53–58. [Google Scholar] [CrossRef]
- Balestrieri, M.L.; De Prisco, R.; Nicolaus, B.; Pari, P.; Moriello, V.S.; Strazzullo, G.; Iorio, E.L.; Servillo, L.; Balestrieri, C. Lycopene in association with alpha-tocopherol or tomato lipophilic extracts enhances acyl-platelet-activating factor biosynthesis in endothelial cells during oxidative stress. Free Radic. Biol. Med. 2004, 36, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- La Russa, D.; Marrone, A.; Mandalà, M.; Macirella, R.; Pellegrino, D. Antioxidant/anti-inflammatory effects of caloric restriction in an aged and obese rat model: The role of adiponectin. Biomedicines 2020, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, D.; La Russa, D.; Marrone, A. Oxidative imbalance and kidney damage: New study perspectives from animal models to hospitalized patients. Antioxidants 2019, 8, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boakye, D.; Jansen, L.; Schöttker, B.; Jansen, E.H.J.M.; Schneider, M.; Halama, N.; Gào, X.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Blood markers of oxidative stress are strongly associated with poorer prognosis in colorectal cancer patients. Int. J. Cancer 2020, 147, 2373–2386. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Hashimoto, J.; Suzuki, T.; Satoh, A. The effects of exercise load during development on oxidative stress levels and antioxidant potential in adulthood. Free Radic. Res. 2017, 51, 179–186. [Google Scholar] [CrossRef]
- Macri, A.; Scanarotti, C.; Bassi, A.M.; Giuffrida, S.; Sangalli, G.; Traverso, C.E.; Iester, M. Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0.15 % and vitamin B12 eye drops. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 425–430. [Google Scholar] [CrossRef]
- Mancini, S.; Mariani, F.; Sena, P.; Benincasa, M.; Roncucci, L. Myeloperoxidase expression in human colonic mucosa is related to systemic oxidative balance in healthy subjects. Redox Rep. 2017, 22, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Carratelli, M.; Porcaro, L.; Ruscica, M.; De Simone, E.; Bertelli, A.A.; Corsi, M.M. Reactive oxygen metabolites and prooxidant status in children with Down’s syndrome. Int. J. Clin. Pharmacol. Res. 2001, 21, 79–84. [Google Scholar]
- Vassalle, C.; Masini, S.; Carpeggiani, C.; L’Abbate, A.; Boni, C.; Zucchelli, G.C. In vivo total antioxidant capacity: Comparison of two different analytical methods. Clin. Chem. Lab. Med. 2004, 42, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Dupoué, A.; Blaimont, P.; Rozen-Rechels, D.; Richard, M.; Meylan, S.; Clobert, J.; Miles, D.B.; Martin, R.; Decencière, B.; Agostini, S.; et al. Water availability and temperature induce changes in oxidative status during pregnancy in a viviparous lizard. Funct. Ecol. 2020, 34, 475–485. [Google Scholar] [CrossRef]
- Candellone, A.; Gianella, P.; Ceccarelli, L.; Raviri, G.; Badino, P.; Roncone, S.; Kooistra, H.S.; Meineri, G. Redox unbalance in the hyperthyroid cat: A comparison with healthy and non-thyroidal diseased cats. BMC Vet. Res. 2019, 15, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, E.; Ruskovska, T. Serum biomarkers of (anti)oxidant status for epidemiological studies. Int. J. Mol. Sci. 2015, 16, 27378–27390. [Google Scholar] [CrossRef]
- Donato, A.J.; Henson, G.D.; Hart, C.R.; Layec, G.; Trinity, J.D.; Bramwell, R.C.; Enz, R.A.; Morgan, R.G.; Reihl, K.D.; Hazra, S.; et al. The impact of ageing on adipose structure, function and vasculature in the B6D2F1 mouse: Evidence of significant multisystem dysfunction. J. Physiol. 2014, 592, 4083–4096. [Google Scholar] [CrossRef]
- Auberval, N.; Dal, S.; Bietiger, W.; Pinget, M.; Jeandidier, N.; Maillard-Pedracini, E.; Schini-Kerth, V.; Sigrist, S. Metabolic and oxidative stress markers in wistar rats after 2 months on a high-fat diet. Diabetol. Metab. Syndr. 2014, 6, 130. [Google Scholar] [CrossRef] [Green Version]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Ghadge, A.A.; Khaire, A.A. Leptin as a predictive marker for metabolic syndrome. Cytokine 2019, 121, 154735. [Google Scholar] [CrossRef]
- Cavaliere, G.; Viggiano, E.; Trinchese, G.; De Filippo, C.; Messina, A.; Monda, V.; Valenzano, A.; Cincione, R.I.; Zammit, C.; Cimmino, F.; et al. Long feeding high-fat diet induces hypothalamic oxidative stress and inflammation, and prolonged hypothalamic AMPK activation in rat animal model. Front. Physiol. 2018, 9, 818. [Google Scholar] [CrossRef] [Green Version]
- Viggiano, E.; Mollica, M.P.; Lionetti, L.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Chieffi, S.; Gaita, M.; Barletta, A.; De Luca, B.; et al. Effects of an high-fat diet enriched in lard or in fish oil on the hypothalamic amp-activated protein kinase and inflammatory mediators. Front. Cell. Neurosci. 2016, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.B. Leptin—Much more than a satiety signal. Annu. Rev. Nutr. 2000, 20, 45–75. [Google Scholar] [CrossRef]
- Leshan, R.L.; Björnholm, M.; Münzberg, H.; Myers, M.G.J. Leptin receptor signaling and action in the central nervous system. Obesity 2006, 14 (Suppl. S5), 208S–212S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, C.; Lama, A.; Pirozzi, C.; Cavaliere, G.; Trinchese, G.; Di Guida, F.; Nitrato Izzo, A.; Cimmino, F.; Paciello, O.; De Biase, D.; et al. Palmitoylethanolamide counteracts hepatic metabolic inflexibility modulating mitochondrial function and efficiency in diet-induced obese mice. FASEB J. 2020, 34, 350–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchese, G.; Cavaliere, G.; Cimmino, F.; Catapano, A.; Carta, G.; Pirozzi, C.; Murru, E.; Lama, A.; Meli, R.; Bergamo, P.; et al. Decreased metabolic flexibility in skeletal muscle of rat fed with a high-fat diet is recovered by individual CLA isomer supplementation via converging protective mechanisms. Cells 2020, 9, 823. [Google Scholar] [CrossRef] [Green Version]
- Agan, V.; Celik, H.; Eren, M.A.; Agan, F.Z.; Erel, O.; Neselioglu, S.; Koyuncu, I.; Gonel, A. An investigation of oxidative stress and thiol/disulphide homeostasis in Graves’ disease. Medicina 2019, 55, 275. [Google Scholar] [CrossRef] [Green Version]
- Rojkind, M.; Domínguez-Rosales, J.A.; Nieto, N.; Greenwel, P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell. Mol. Life Sci. 2002, 59, 1872–1891. [Google Scholar] [CrossRef]
- Ulla, A.; Alam, M.A.; Sikder, B.; Sumi, F.A.; Rahman, M.M.; Habib, Z.F.; Mohammed, M.K.; Subhan, N.; Hossain, H.; Reza, H.M. Supplementation of Syzygium cumini seed powder prevented obesity, glucose intolerance, hyperlipidemia and oxidative stress in high carbohydrate high fat diet induced obese rats. BMC Complement. Altern. Med. 2017, 17, 289. [Google Scholar] [CrossRef] [Green Version]
- Maciejczyk, M.; Żebrowska, E.; Zalewska, A.; Chabowski, A. Redox balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet-induced insulin resistance. Oxid. Med. Cell. Longev. 2018, 2018, 6940515. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Baker, S.S.; Baker, R.D.; Zhu, L. Antioxidant mechanisms in nonalcoholic fatty liver disease. Curr. Drug Targets 2015, 16, 1301–1314. [Google Scholar] [CrossRef]
- Vergara, D.; Casadei-Gardini, A.; Giudetti, A.M. Oxidative molecular mechanisms underlying liver diseases: From systems biology to the personalized medicine. Oxid. Med. Cell. Longev. 2019, 2019, 7864316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polimeni, L.; Del Ben, M.; Baratta, F.; Perri, L.; Albanese, F.; Pastori, D.; Violi, F.; Angelico, F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J. Hepatol. 2015, 7, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Van Guilder, G.P.; Hoetzer, G.L.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Metabolic syndrome and endothelial fibrinolytic capacity in obese adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R39–R44. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambino, G.; Gallo, D.; Covelo, A.; Ferraro, G.; Sardo, P.; Giglia, G. TRPV1 channels in nitric oxide-mediated signalling: Insight on excitatory transmission in rat CA1 pyramidal neurons. Free Radic. Biol. Med. 2022, 191, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Rizzo, V.; Giglia, G.; Ferraro, G.; Sardo, P. Microtubule dynamics and neuronal excitability: Advances on cytoskeletal components implicated in epileptic phenomena. Cell. Mol. Neurobiol. 2022, 42, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Rizzo, V.; Giglia, G.; Ferraro, G.; Sardo, P. Cannabinoids, TRPV and nitric oxide: The three ring circus of neuronal excitability. Brain Struct. Funct. 2020, 225, 1–15. [Google Scholar] [CrossRef]
- Palermo, A.; Giglia, G.; Vigneri, S.; Cosentino, G.; Fierro, B.; Brighina, F. does habituation depend on cortical inhibition? Results of an rTMS study in healthy subjects. Exp. Brain Res. 2011, 212, 101–107. [Google Scholar] [CrossRef]
- Leonardi, B.F.; Gosmann, G.; Zimmer, A.R. Modeling diet-induced metabolic syndrome in rodents. Mol. Nutr. Food Res. 2020, 64, e2000249. [Google Scholar] [CrossRef]

| Pellet HFD (PF4215) | NPD (PF1609) | |
|---|---|---|
| Energy (Kcal/Kg) | 5500–6000 | 3947 |
| Fat Total (g/100 g) | 60 | 3.50 |
| SFA (g/60 g) | 30 | 0.7 |
| MUFA (g/60 g) | 23 | 0.8 |
| PUFA (g/60 g) | 7 | 2 |
| Crude Protein (g/100 g) | 23 | 22 |
| Carbohydrates (Starch g/100 g) | 38 | 35.18 |
| Sugar (g/100 g) | - | 5.66 |
| Fibre (g/100 g) | 5 | 4.5 |
| Ash (g/100 g) | 5.50 | 7.5 |
| Vitamin A (IU) | 8400 | 19.533 |
| Vitamin D3 (IU) | 2100 | 1260 |
| Basal Biochemical Parameters (T0) | Mean ± S.D. |
|---|---|
| Glucose (mg/dL) | 180.51 ± 38.71 |
| Triglycerides | 166.94 ± 41.83 |
| Tot Cholesterol | 66.67 ± 7.86 |
| HDL Cholesterol | 36.93 ± 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Majo, D.; Sardo, P.; Giglia, G.; Di Liberto, V.; Zummo, F.P.; Zizzo, M.G.; Caldara, G.F.; Rappa, F.; Intili, G.; van Dijk, R.M.; et al. Correlation of Metabolic Syndrome with Redox Homeostasis Biomarkers: Evidence from High-Fat Diet Model in Wistar Rats. Antioxidants 2023, 12, 89. https://doi.org/10.3390/antiox12010089
Di Majo D, Sardo P, Giglia G, Di Liberto V, Zummo FP, Zizzo MG, Caldara GF, Rappa F, Intili G, van Dijk RM, et al. Correlation of Metabolic Syndrome with Redox Homeostasis Biomarkers: Evidence from High-Fat Diet Model in Wistar Rats. Antioxidants. 2023; 12(1):89. https://doi.org/10.3390/antiox12010089
Chicago/Turabian StyleDi Majo, Danila, Pierangelo Sardo, Giuseppe Giglia, Valentina Di Liberto, Francesco Paolo Zummo, Maria Grazia Zizzo, Gaetano Felice Caldara, Francesca Rappa, Giorgia Intili, Roelof Maarten van Dijk, and et al. 2023. "Correlation of Metabolic Syndrome with Redox Homeostasis Biomarkers: Evidence from High-Fat Diet Model in Wistar Rats" Antioxidants 12, no. 1: 89. https://doi.org/10.3390/antiox12010089
APA StyleDi Majo, D., Sardo, P., Giglia, G., Di Liberto, V., Zummo, F. P., Zizzo, M. G., Caldara, G. F., Rappa, F., Intili, G., van Dijk, R. M., Gallo, D., Ferraro, G., & Gambino, G. (2023). Correlation of Metabolic Syndrome with Redox Homeostasis Biomarkers: Evidence from High-Fat Diet Model in Wistar Rats. Antioxidants, 12(1), 89. https://doi.org/10.3390/antiox12010089










