Bromelain Ameliorates Atherosclerosis by Activating the TFEB-Mediated Autophagy and Antioxidant Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mice
2.3. Histological Examination
2.4. Plasma Lipid Profile Analysis
2.5. Measurement of Inflammatory Cytokines
2.6. Western Blot Analysis
2.7. Immunohistochemistry
2.8. PET/CT Acquisition
2.9. Determination of Hepatic Lipids
2.10. Lipid Peroxidation Assay
2.11. Total Antioxidant Capacity Assay
2.12. Statistical Analysis
3. Results
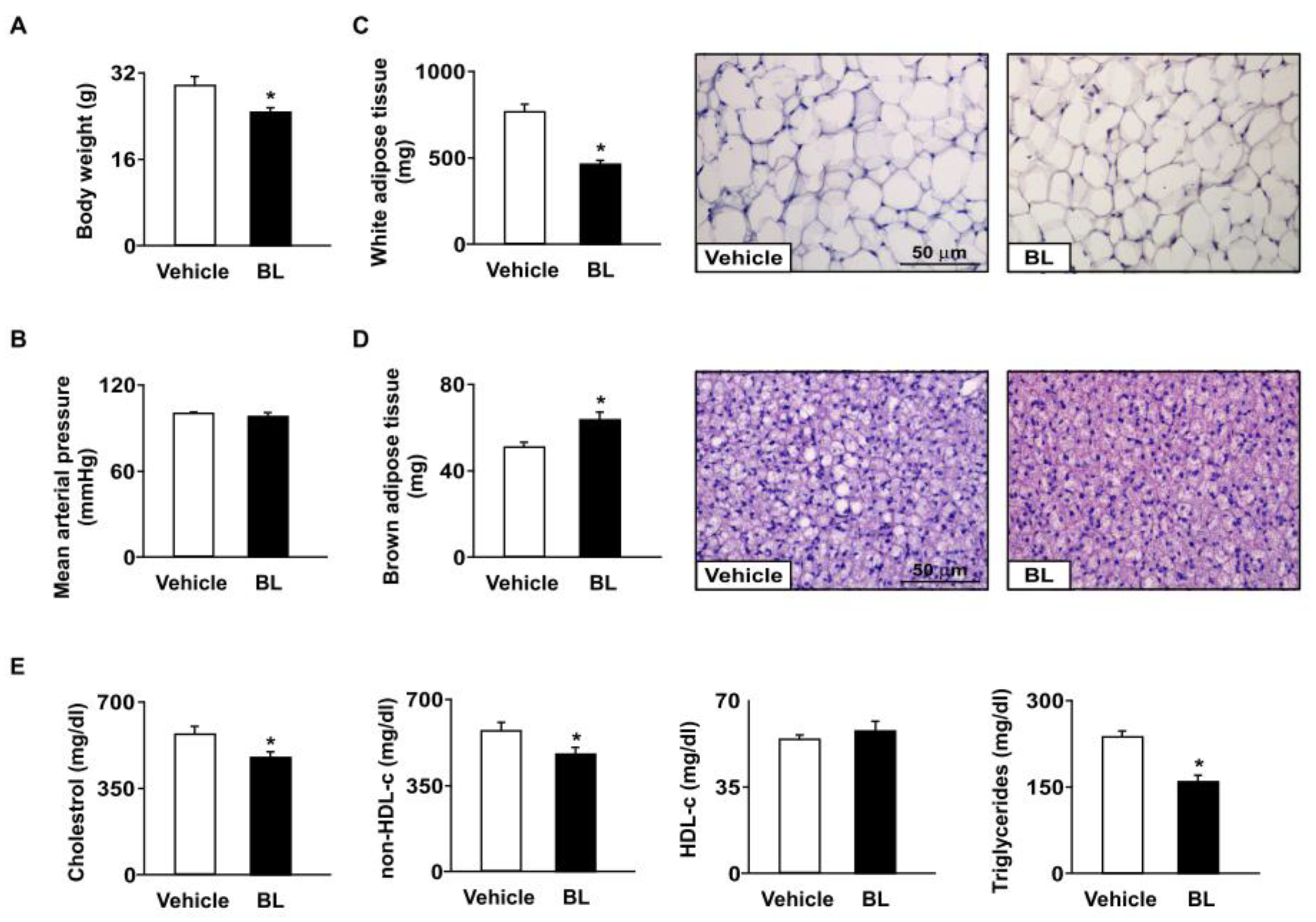
3.1. Effect of Bromelain on Body Weight, Fat Tissue Weight, and Hyperlipidemia in apoe−/− Mice
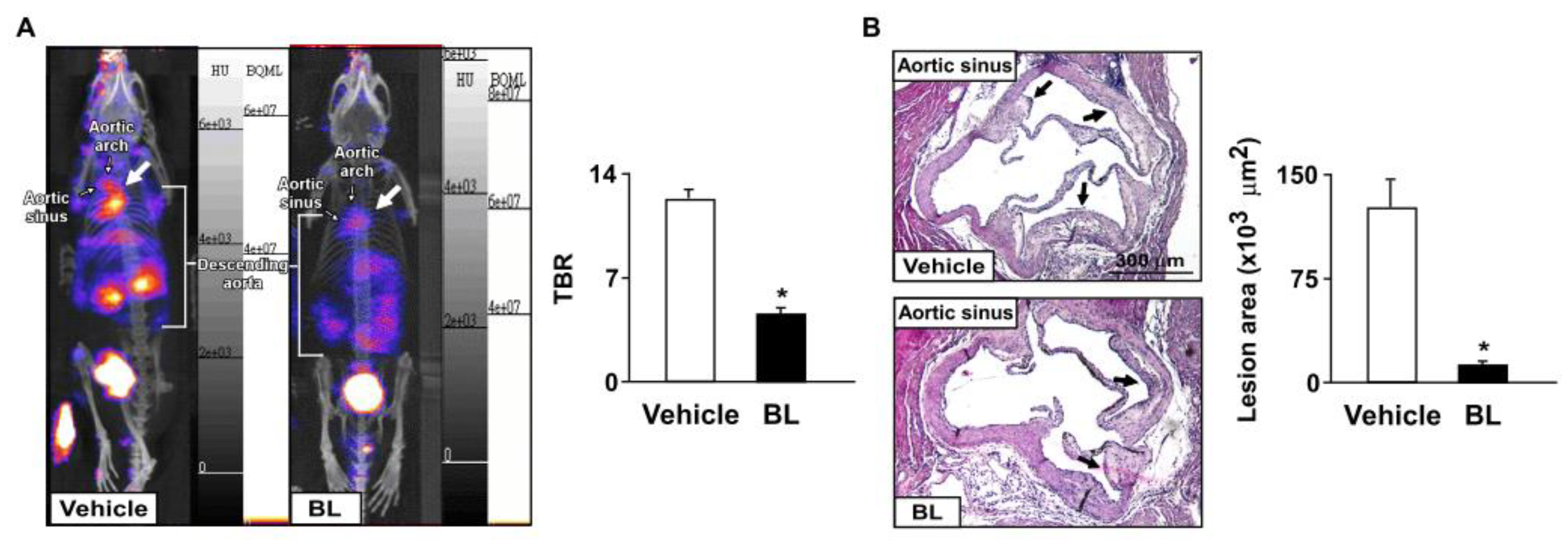
3.2. Bromelain Attenuates Aortic Inflammation and Results in the Retardation of Atherosclerosis in apoe−/− Mice
3.3. Effects of Bromelain on Hepatic Levels of Lipids in apoe−/− Mice
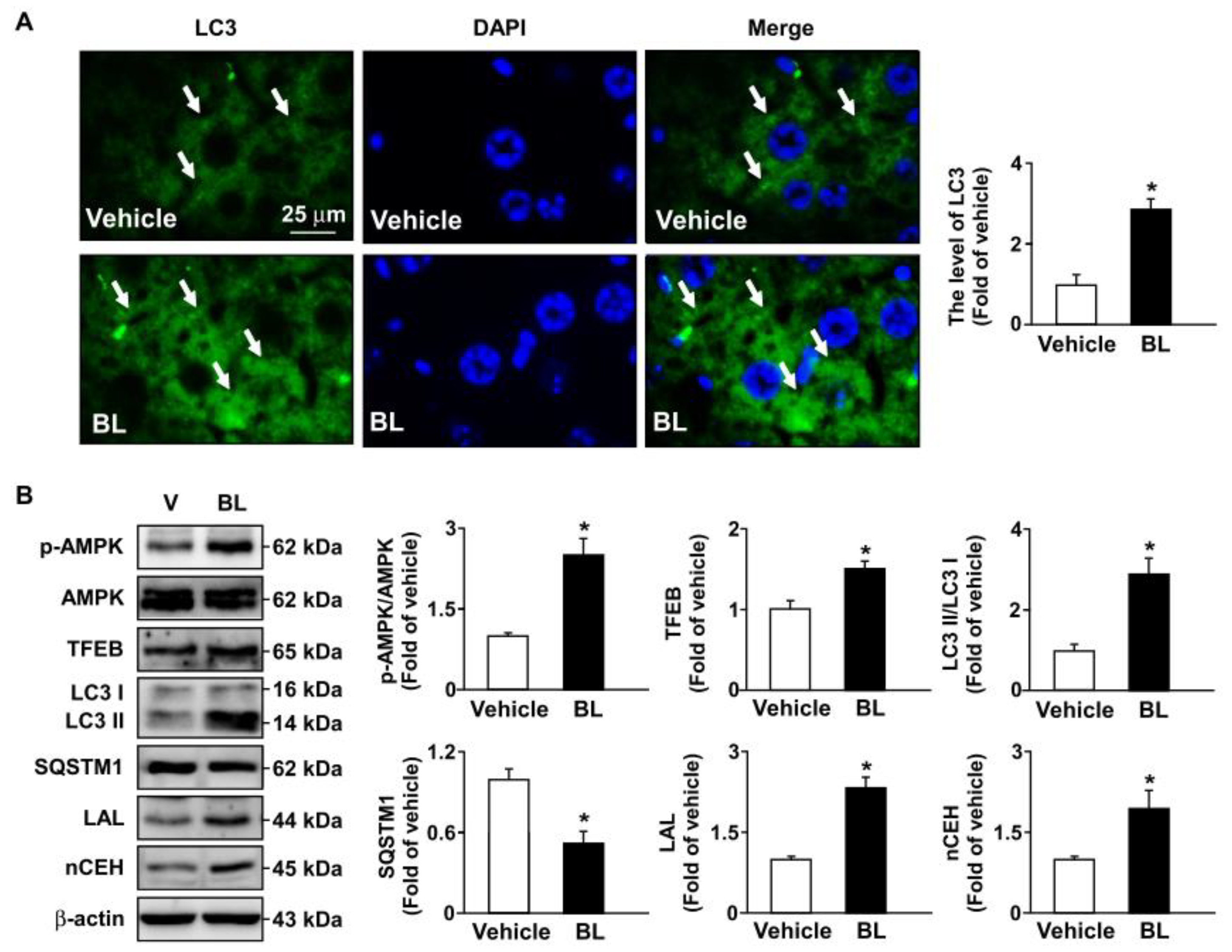
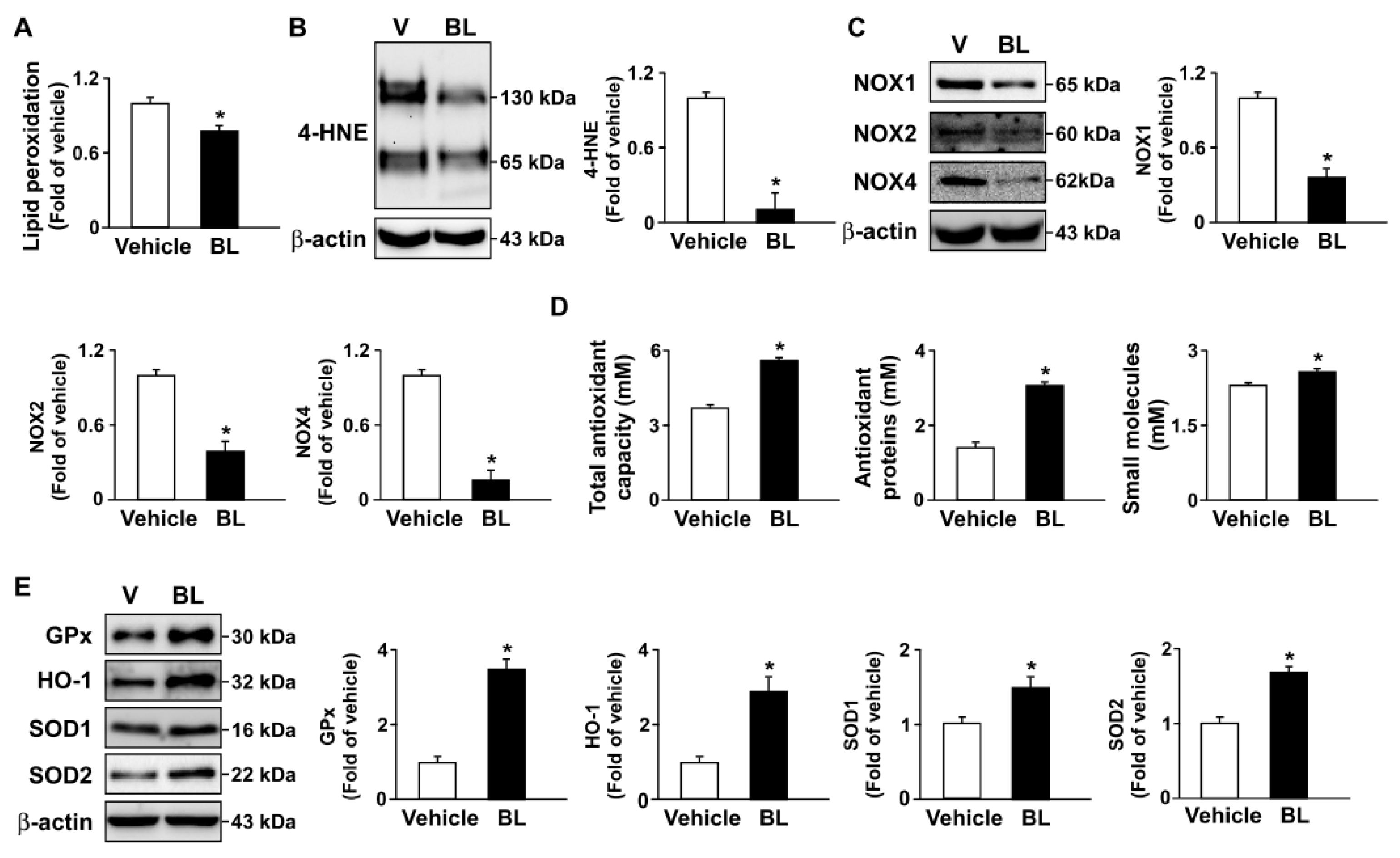
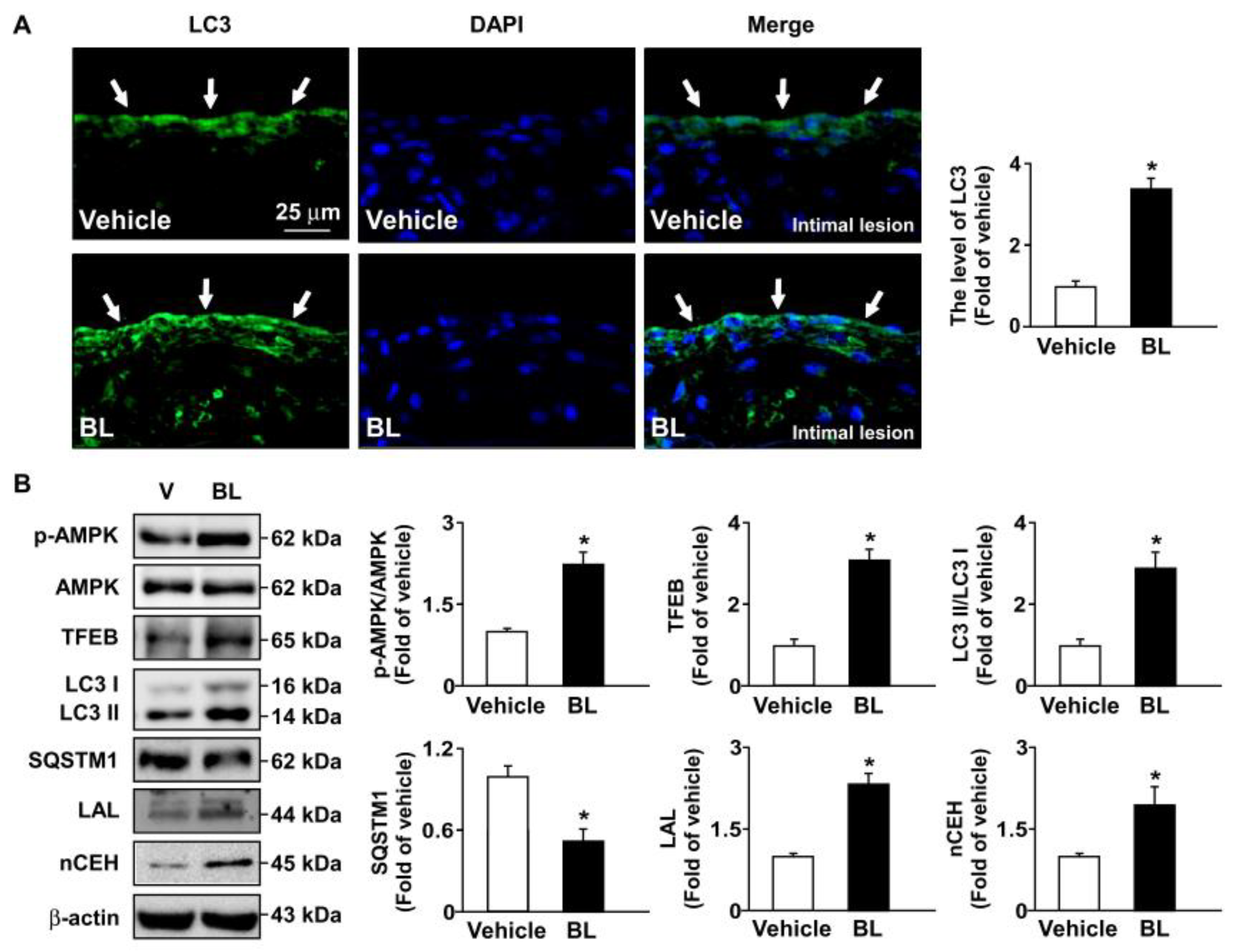
3.4. TFEB Activation Is Involved in Bromelain-Induced Activation of Autophagy Flux and Antioxidant Capacity
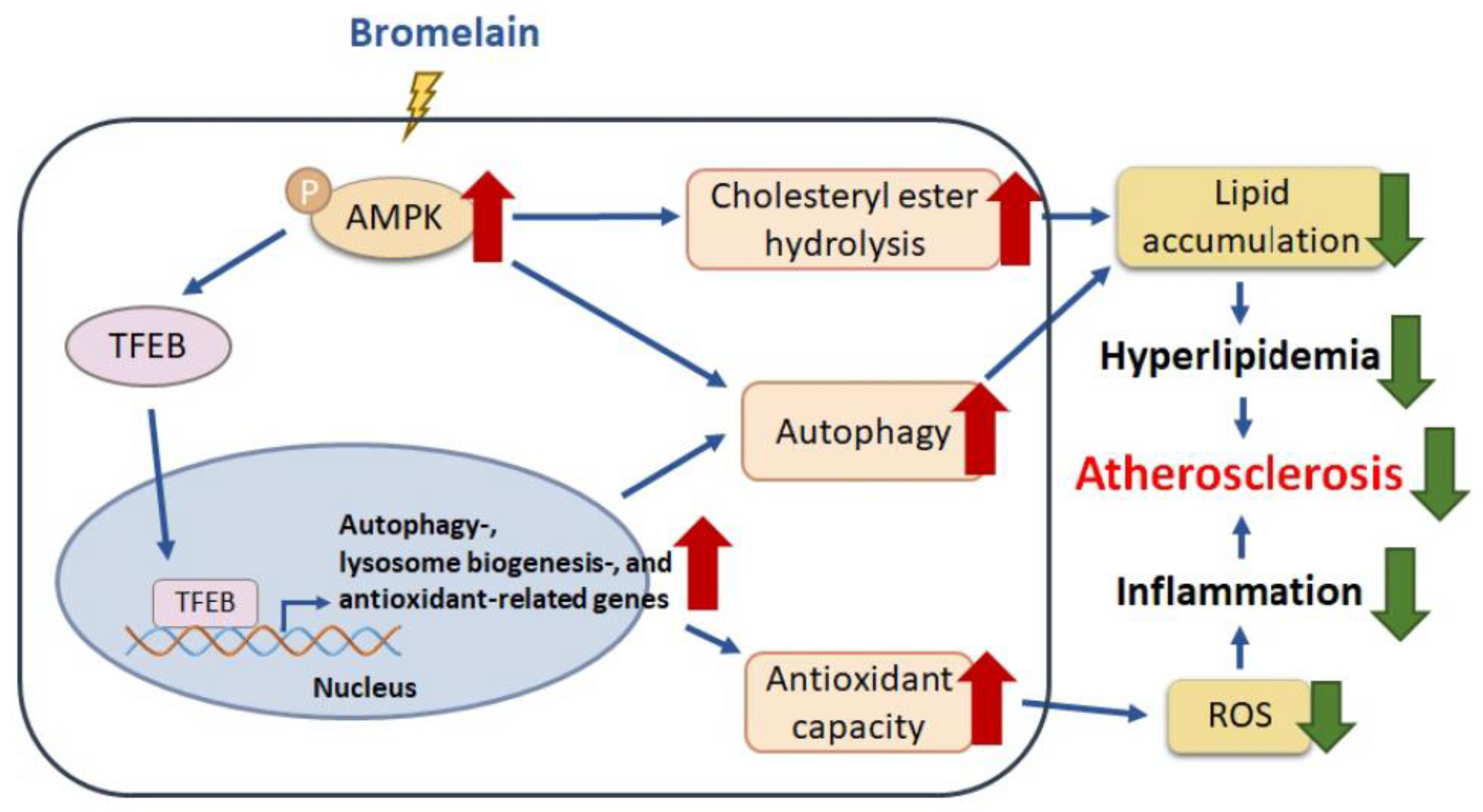
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ugwuodo, C.J.; Nwagu, T.N.T.; Ugwu, T.T.; Onwosi, C.O. Enhancement of the anti-inflammatory effect of bromelain by its immobilization on probiotic spore of bacillus cereus. Probiotics Antimicrob. Proteins 2021, 13, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.A.; Wang, S.H.; Chen, C.H.; Guo, B.C.; Huang, J.W.; Lee, T.S. New mechanisms of bromelain in alleviating non-alcoholic fatty liver disease-induced deregulation of blood coagulation. Nutrients 2022, 14, 2329. [Google Scholar] [CrossRef]
- Mohamad, N.E.; Abu, N.; Yeap, S.K.; Alitheen, N.B. Bromelain enhances the snti-tumor effects of cisplatin on 4T1 breast tumor model in vivo. Integr. Cancer. Ther. 2019, 18, 1534735419880258. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Ehtram, A.; Chaturvedi, S.K.; Nusrat, S.; Khan, R.H. Amyloidogenic behavior of different intermediate state of stem bromelain: A biophysical insight. Int. J. Biol. Macromol. 2016, 91, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Chandanwale, A.; Langade, D.; Sonawane, D.; Gavai, P. A randomized, clinical trial to evaluate efficacy and tolerability of trypsin: Chymotrypsin as compared to serratiopeptidase and trypsin: Bromelain: Rutoside in wound management. Adv. Ther. 2017, 34, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Kaur, N.J.; Nanduri, R.; Dkhar, H.K.; Kumar, A.; Gupta, P. Inhibition of adipogenesis and induction of apoptosis and lipolysis by stem bromelain in 3T3-L1 adipocytes. PLoS ONE 2012, 7, e30831. [Google Scholar] [CrossRef]
- Hu, P.A.; Chen, C.H.; Guo, B.C.; Kou, Y.R.; Lee, T.S. Bromelain confers protection against the non-alcoholic fatty liver disease in male c57bl/6 mice. Nutrients 2020, 12, 1458. [Google Scholar] [CrossRef]
- Hu, P.A.; Hsu, M.C.; Chen, S.H.; Chen, C.H.; Kou, Y.R.; Huang, J.W.; Lee, T.S. Bromelain activates the AMP-activated protein kinase-autophagy pathway to alleviate hepatic lipid accumulation. J. Food Drug Anal. 2022, 30, 3. [Google Scholar] [CrossRef]
- Chen, C.H.; Shyue, S.K.; Hsu, C.P.; Lee, T.S. Atypical antipsychotic drug olanzapine deregulates hepatic lipid metabolism and aortic inflammation and aggravates atherosclerosis. Cell. Physiol. Biochem. 2018, 50, 1216–1229. [Google Scholar] [CrossRef]
- Chen, C.H.; Ho, S.N.; Hu, P.A.; Kou, Y.R.; Lee, T.S. Food preservative sorbic acid deregulates hepatic fatty acid metabolism. J. Food Drug Anal. 2020, 28, 206–216. [Google Scholar] [CrossRef]
- Rader, D.J.; Hoeg, J.M.; Brewer, H.B., Jr. Quantitation of plasma apolipoproteins in the primary and secondary prevention of coronary artery disease. Ann. Intern. Med. 1994, 120, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- März, W.; Kleber, M.E.; Scharnagl, H.; Speer, T.; Zewinger, S.; Ritsch, A.; Parhofer, K.G.; von Eckardstein, A.; Landmesser, U.; Laufs, U. HDL cholesterol: Reappraisal of its clinical relevance. Clin. Res. Cardiol. 2017, 106, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Arida, A.; Protogerou, A.D.; Kitas, G.D.; Sfikakis, P.P. Systemic inflammatory response and atherosclerosis: The paradigm of chronic inflammatory rheumatic diseases. Int. J. Mol. Sci. 2018, 19, 1890. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Moroni, F.; Norata, G.D.; Magnoni, M.; Camici, P.G. Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediators Inflamm. 2015, 2015, 718329. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Ching, L.C.; Kou, Y.R.; Shyue, S.K.; Su, K.H.; Wei, J.; Cheng, L.C.; Yu, Y.B.; Pan, C.C.; Lee, T.S. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1. Cardiovasc. Res. 2011, 91, 492–501. [Google Scholar] [CrossRef]
- Poredos, P. Endothelial dysfunction in the pathogenesis of atherosclerosis. Clin. Appl. Thromb. Hemost. 2001, 7, 276–280. [Google Scholar] [CrossRef]
- Zhao, J.F.; Chen, H.Y.; Wei, J.; Jim Leu, S.J.; Lee, T.S. CCN family member 1 deregulates cholesterol metabolism and aggravates atherosclerosis. Acta Physiol. 2019, 225, e13209. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, T.S.; Chen, C.C.; Chang, C.A.; Lin, Y.J.; Hsu, Y.P.; Ho, L.T. Endothelin-1 exacerbates lipid accumulation by increasing the protein degradation of the ATP-binding cassette transporter G1 in macrophages. J. Cell. Physiol. 2011, 226, 2198–2205. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yao, R.Q.; Li, Y.X.; Zhao, P.Y.; Ren, C.; Du, X.H.; Yao, Y.M. The role and regulatory mechanism of transcription factor EB in health and diseases. Front. Cell Dev. Biol. 2021, 9, 667750. [Google Scholar] [CrossRef]
- Lu, H.; Sun, J.; Hamblin, M.H.; Chen, Y.E.; Fan, Y. Transcription factor EB regulates cardiovascular homeostasis. EBioMedicine 2021, 63, 103207. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Liu, Z.; Mao, C.; Ma, Z.; Li, W.; Yu, F.; Wang, Y.; Huang, Y.; Zhang, W.; et al. Targeting macrophage TFEB-14-3-3 epsilon Interface by naringenin inhibits abdominal aortic aneurysm. Cell Discov. 2022, 8, 21. [Google Scholar] [CrossRef]
- Chen, D.; Yu, W.; Zhong, C.; Hong, Q.; Huang, G.; Que, D.; Wang, Y.; Yang, Y.; Rui, B.; Zhuang, Z.; et al. Elabela ameliorates doxorubicin-induced cardiotoxicity by promoting autophagic flux through TFEB pathway. Pharmacol. Res. 2022, 178, 106186. [Google Scholar] [CrossRef]
- Li, X.; Zhu, R.; Jiang, H.; Yin, Q.; Gu, J.; Chen, J.; Ji, X.; Wu, X.; Fu, H.; Wang, H.; et al. Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB-P300-BRD4 axis. Acta Pharm. Sin. B 2022, 12, 2280–2299. [Google Scholar] [CrossRef]
- Haas, M.J.; Feng, V.; Gonzales, K.; Bikkina, P.; Angelica Landicho, M.; Mooradian, A.D. Transcription factor EB protects against endoplasmic reticulum stress in human coronary artery endothelial cells. Eur. J. Pharmacol. 2022, 933, 175274. [Google Scholar] [CrossRef]
- Yang, Y.P.; Ren, Y.G.; Cai, B.Q.; Huang, D.D. Homocysteine suppresses autophagy through AMPK-mTOR-TFEB signaling in human THP-1 macrophages. J. Cardiovasc. Pharmacol. 2022, 79, 730–738. [Google Scholar] [CrossRef]
- Hsia, C.C.; Yeh, C.H.; Chen, C.T.; Peng, C.L. Imaging the cytokine receptor CXCR4 in atherosclerotic plaques with [68Ga]-APD: A novel agent on computer simulation approach. J. Clin. Cell. Immunol. 2022, 13, 1000663. [Google Scholar] [CrossRef]
- Hyafil, F.; Pelisek, J.; Laitinen, I.; Schottelius, M.; Mohring, M.; Döring, Y.; van der Vorst, E.P.; Kallmayer, M.; Steiger, K.; Poschenrieder, A.; et al. Imaging the cytokine receptor CXCR4 in atherosclerotic plaques with the radiotracer 68Ga-pentixafor for PET. J. Nucl. Med. 2017, 58, 499–506. [Google Scholar] [CrossRef]
- Derlin, T.; Sedding, D.G.; Dutzmann, J.; Haghikia, A.; König, T.; Napp, L.C.; Schütze, C.; Owsianski-Hille, N.; Wester, H.J.; Kropf, S.; et al. Imaging of chemokine receptor CXCR4 expression in culprit and nonculprit coronary atherosclerotic plaque using motion-corrected [68Ga]pentixafor PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1934–1944. [Google Scholar] [CrossRef]
- Lapa, C.; Schreder, M.; Schirbel, A.; Samnick, S.; Kortüm, K.M.; Herrmann, K.; Kropf, S.; Einsele, H.; Buck, A.K.; Wester, H.J.; et al. [68Ga] Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma-Comparison to [18F] FDG and laboratory values. Theranostics 2017, 7, 205–212. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar]
- Hsu, M.C.; Guo, B.C.; Chen, C.H.; Hu, P.A.; Lee, T.S. Apigenin ameliorates hepatic lipid accumulation by activating the autophagy-mitochondria pathway. J. Food Drug Anal. 2021, 29, 240–254. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Castell, J.V.; Friedrich, G.; Kuhn, C.S.; Poppe, G.E. Intestinal absorption of undegraded proteins in men: Presence of bromelain in plasma after oral intake. Am. J. Physiol. 1997, 273, G139–G146. [Google Scholar] [CrossRef]
- Sánchez-Martín, L.; Estecha, A.; Samaniego, R.; Sánchez-Ramón, S.; Vega, M.Á.; Sánchez-Mateos, P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 2011, 117, 88–97. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-induced macrophage polarization in inflammatory conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Gao, J.H.; He, L.H.; Yu, X.H.; Zhao, Z.W.; Wang, G.; Zou, J.; Wen, F.J.; Zhou, L.; Wan, X.J.; Zhang, D.W.; et al. CXCL12 promotes atherosclerosis by downregulating ABCA1 expression via the CXCR4/GSK3β/β-cateninT120/TCF21 pathway. J. Lipid Res. 2019, 60, 2020–2033. [Google Scholar] [CrossRef]
- Li, L.; Du, Z.; Rong, B.; Zhao, D.; Wang, A.; Xu, Y.; Zhang, H.; Bai, X.; Zhong, J. Foam cells promote atherosclerosis progression by releasing CXCL12. Biosci. Rep. 2020, 40, BSR20193267. [Google Scholar] [CrossRef]
- Bot, I.; Daissormont, I.T.; Zernecke, A.; van Puijvelde, G.H.; Kramp, B.; de Jager, S.C.; Sluimer, J.C.; Manca, M.; Hérias, V.; Westra, M.M.; et al. CXCR4 blockade induces atherosclerosis by affecting neutrophil function. J. Mol. Cell. Cardiol. 2014, 74, 44–52. [Google Scholar] [CrossRef]
- Döring, Y.; Noels, H.; van der Vorst, E.P.C.; Neideck, C.; Egea, V.; Drechsler, M.; Mandl, M.; Pawig, L.; Jansen, Y.; Schröder, K.; et al. Vascular CXCR4 limits atherosclerosis by maintaining arterial integrity: Evidence from mouse and human studies. Circulation 2017, 136, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The role of cytokines in the development of atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; Pawig, L.; Weber, C.; Noels, H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front. Physiol. 2014, 5, 212. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Tran-Gia, J.; Kemmer, L.; Zhang, X.; Schirbel, A.; Werner, R.A.; Buck, A.K.; Wester, H.J.; Hacker, M.; Lapa, C.; et al. Imaging inflammation in atherosclerosis with CXCR4-directed 68Ga-pentixafor PET/CT: Correlation with 18F-FDG PET/CT. J. Nucl. Med. 2020, 61, 751–756. [Google Scholar] [CrossRef]
- Yan, S. Role of TFEB in autophagy and the pathogenesis of liver diseases. Biomolecules 2022, 12, 672. [Google Scholar] [CrossRef]
- Gambardella, G.; Staiano, L.; Moretti, M.N.; De Cegli, R.; Fagnocchi, L.; Di Tullio, G.; Polletti, S.; Braccia, C.; Armirotti, A.; Zippo, A.; et al. GADD34 is a modulator of autophagy during starvation. Sci. Adv. 2020, 6, eabb0205. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, X.; Zhai, Y.; Liu, H.; Guan, L.; Qiao, Y.; Jiang, J.; Peng, L. Phillygenin ameliorates nonalcoholic fatty liver disease via TFEB-mediated lysosome biogenesis and lipophagy. Phytomedicine 2022, 103, 154235. [Google Scholar] [CrossRef]
- Chun, Y.S.; Kim, M.Y.; Lee, S.Y.; Kim, M.J.; Hong, T.J.; Jeon, J.K.; Ganbat, D.; Kim, H.T.; Kim, S.S.; Kam, T.I.; et al. MEK1/2 inhibition rescues neurodegeneration by TFEB-mediated activation of autophagic lysosomal function in a model of Alzheimer’s Disease. Mol. Psychiatry 2022, 27, 4770–4780. [Google Scholar] [CrossRef]
- Yoo, J.; Jeong, I.K.; Ahn, K.J.; Chung, H.Y.; Hwang, Y.C. Fenofibrate, a PPARα agonist, reduces hepatic fat accumulation through the upregulation of TFEB-mediated lipophagy. Metabolism 2021, 120, 154798. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Vendrov, A.E.; Sumida, A.; Canugovi, C.; Lozhkin, A.; Hayami, T.; Madamanchi, N.R.; Runge, M.S. NOXA1-dependent NADPH oxidase regulates redox signaling and phenotype of vascular smooth muscle cell during atherogenesis. Redox Biol. 2019, 21, 101063. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, E.; Gray, S.P.; Chew, P.; Koulis, C.; Ziegler, A.; Szyndralewiez, C.; Touyz, R.M.; Schmidt, H.H.; Cooper, M.E.; Slattery, R.; et al. Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe−/− mice. Diabetologia 2014, 57, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Zhao, J.F.; Hsu, C.P.; Kou, Y.R.; Lu, T.M.; Lee, T.S. The detrimental effect of asymmetric dimethylarginine on cholesterol efflux of macrophage foam cells: Role of the NOX/ROS signaling. Free Radic. Biol. Med. 2019, 143, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Leu, S.J.; Hsu, C.P.; Pan, C.C.; Shyue, S.K.; Lee, T.S. Atypical antipsychotic drugs deregulate the cholesterol metabolism of macrophage-foam cells by activating NOX-ROS-PPARγ-CD36 signaling pathway. Metabolism 2021, 123, 154847. [Google Scholar] [CrossRef]
- Signorelli, P.; Pivari, F.; Barcella, M.; Merelli, I.; Zulueta, A.; Dei Cas, M.; Rosso, L.; Ghidoni, R.; Caretti, A.; Paroni, R.; et al. Myriocin modulates the altered lipid metabolism and storage in cystic fibrosis. Cell. Signal. 2021, 81, 109928. [Google Scholar] [CrossRef]
- Lu, H.; Fan, Y.; Qiao, C.; Liang, W.; Hu, W.; Zhu, T.; Zhang, J.; Chen, Y.E. TFEB inhibits endothelial cell inflammation and reduces atherosclerosis. Sci. Signal. 2017, 10, eaah4214. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Hsia, C.-C.; Hu, P.-A.; Yeh, C.-H.; Chen, C.-T.; Peng, C.-L.; Wang, C.-H.; Lee, T.-S. Bromelain Ameliorates Atherosclerosis by Activating the TFEB-Mediated Autophagy and Antioxidant Pathways. Antioxidants 2023, 12, 72. https://doi.org/10.3390/antiox12010072
Chen C-H, Hsia C-C, Hu P-A, Yeh C-H, Chen C-T, Peng C-L, Wang C-H, Lee T-S. Bromelain Ameliorates Atherosclerosis by Activating the TFEB-Mediated Autophagy and Antioxidant Pathways. Antioxidants. 2023; 12(1):72. https://doi.org/10.3390/antiox12010072
Chicago/Turabian StyleChen, Chia-Hui, Chien-Chung Hsia, Po-An Hu, Chung-Hsin Yeh, Chun-Tang Chen, Cheng-Liang Peng, Chih-Hsien Wang, and Tzong-Shyuan Lee. 2023. "Bromelain Ameliorates Atherosclerosis by Activating the TFEB-Mediated Autophagy and Antioxidant Pathways" Antioxidants 12, no. 1: 72. https://doi.org/10.3390/antiox12010072
APA StyleChen, C.-H., Hsia, C.-C., Hu, P.-A., Yeh, C.-H., Chen, C.-T., Peng, C.-L., Wang, C.-H., & Lee, T.-S. (2023). Bromelain Ameliorates Atherosclerosis by Activating the TFEB-Mediated Autophagy and Antioxidant Pathways. Antioxidants, 12(1), 72. https://doi.org/10.3390/antiox12010072






