Dietary Supplementation of Sophora flavescens Root Extract Improved the Growth Performance, Antioxidant Capacity, Innate Immunity, and Disease Resistance against Edwardsiella tarda Challenge in Turbot (Scophthalmus maximus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish and Growth Trial
2.3. Sample Collection
2.4. Chemical Analysis
2.5. Real-Time Quantitative PCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Plasma Antioxidant and Lipid Peroxidation Parameters
3.3. Plasma Immune Indexes
3.4. Antioxidant-Related Gene Expression
3.5. Inflammation-Related Gene Expression
3.6. Disease Resistance
4. Discussion
4.1. SFE improved the Growth Performance and Feed Utilization of Fish
4.2. SFE Enhanced the Antioxidant Capacity of Fish
4.3. SFE Enhanced the Innate Immunity and Disease Resistance of Fish
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Leung, T.L.F.; Bates, A.E. More rapid and severe disease outbreaks for aquaculture at the tropics: Implications for food security. J. Appl. Ecol. 2013, 50, 215–222. [Google Scholar] [CrossRef]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Q.; Zheng, L.; Zhou, J.L.; Zhao, H. Persistence and risk of antibiotic residues and antibiotic resistance genes in major mariculture sites in Southeast China. Sci. Total Environ. 2017, 580, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, H.; Wang, Y.; Xie, H.; Zhu, M.; Chen, J. Presence and environmental risk assessment of selected antibiotics in coastal water adjacent to mariculture areas in the Bohai Sea. Ecotoxicol. Environ. Saf. 2019, 177, 117–123. [Google Scholar] [CrossRef]
- Zhang, R.; Pei, J.; Zhang, R.; Wang, S.; Zeng, W.; Huang, D.; Wang, Y.; Zhang, Y.; Wang, Y.; Yu, K. Occurrence and distribution of antibiotics in mariculture farms, estuaries and the coast of the Beibu Gulf, China: Bioconcentration and diet safety of seafood. Ecotoxicol. Environ. Saf. 2018, 154, 27–35. [Google Scholar] [CrossRef]
- Stratev, D.; Zhelyazkov, G.; Noundou, X.S.; Krause, R.W.M. Beneficial effects of medicinal plants in fish diseases. Aquac. Int. 2017, 26, 289–308. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Mohammadzadeh, S.; Kalhor, N.; Yousefi, M.; Moghadam, M.S.; Naraballobh, W.; Ahmadifar, M.; Hoseinifar, S.H.; Van Doan, H. Cornelian cherry (Cornus mas L.) fruit extract improves growth performance, disease resistance, and serum immune-and antioxidant-related gene expression of common carp (Cyprinus carpio). Aquaculture 2022, 558, 738372. [Google Scholar]
- Uma, A.; Philominal, P.; Prabu, E.; Musthafa, M.S. Dietary Bougainvillea glabra leaf meal on growth, haemato-biochemical responses and disease resistance in Nile tilapia, Oreochromis niloticus against Enterococcus faecalis. Aquaculture 2022, 549, 737806. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Wei, Z.; Liu, Q.; Mai, H.; Liu, Y.; Liu, B.; Zhuang, Y.; Zou, D.; Zhang, W.; et al. Effects of dietary dandelion extract on the growth performance, serum biochemical parameters, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) at different feeding period. Fish Shellfish Immunol. 2022, 120, 280–286. [Google Scholar]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Zhu, F. A review on the application of herbal medicines in the disease control of aquatic animals. Aquaculture 2020, 526, 735422. [Google Scholar] [CrossRef]
- He, Q.; Xiao, S.; Zhang, C.; Zhang, Y.; Shi, H.; Zhang, H.; Lin, F.; Liu, X.; Yang, H.; Wang, Q.; et al. Modulation of the growth performance, biochemical parameters, and non-specific immune responses of the hybrid grouper (Epinephelus fuscoguttatus ♀ × E lanceolatus ♂) by two kinds of Chinese herb. Aquac. Rep. 2021, 19, 100604. [Google Scholar]
- Pu, H.; Li, X.; Du, Q.; Cui, H.; Xu, Y. Research Progress in the Application of Chinese Herbal Medicines in Aquaculture: A Review. Engineering 2017, 3, 731–737. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar]
- Ma, H.; Huang, Q.; Qu, W.; Li, L.; Wang, M.; Li, S.; Chu, F. In vivo and in vitro anti-inflammatory effects of Sophora flavescens residues. J. Ethnopharmacol. 2018, 224, 497–503. [Google Scholar] [CrossRef]
- Weng, Z.; Guo, S.; Qian, D.; Zhu, Z.; Zhang, S.; Li, A.; Lei, Z.; Duan, J. Sophora flavescens Seed as a promising high potential by-product: Phytochemical characterization and bioactivity evaluation. Ind. Crops Prod. 2017, 109, 19–26. [Google Scholar] [CrossRef]
- Wu, Y.R.; Gong, Q.F.; Fang, H.; Liang, W.W.; Chen, M.; He, R.J. Effect of Sophora flavescens on non-specific immune response of tilapia (GIFT Oreochromis niloticus) and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 2013, 34, 220–227. [Google Scholar] [CrossRef]
- Seo, J.-S.; Jeon, E.-J.; Kwon, M.-G.; Hwang, J.-Y.; Kim, J.-D.; Jung, S.-H.; Kim, N.-Y.; Jee, B.-Y.; Park, M.-A. Effect of Disease Resistance on Oral Administration of Lightyellow Sophora Extract in Olive flounder. J. Fish. Mar. Sci. Educ. 2015, 27, 1656–1664. [Google Scholar]
- Wen, L.M.; Feng, L.; Jiang, W.D.; Liu, Y.; Wu, P.; Zhao, J.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Thiamin deficiency induces impaired fish gill immune responses, tight junction protein expression and antioxidant capacity: Roles of the NF-kappaB, TOR, p38 MAPK and Nrf2 signaling molecules. Fish Shellfish Immunol. 2016, 51, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Tang, R.J.; Liu, Y.; Wu, P.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Impairment of gill structural integrity by manganese deficiency or excess related to induction of oxidative damage, apoptosis and dysfunction of the physical barrier as regulated by NF-kappaB, caspase and Nrf2 signaling in fish. Fish Shellfish Immunol. 2017, 70, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.D.; Feng, L.; Qu, B.; Wu, P.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Changes in integrity of the gill during histidine deficiency or excess due to depression of cellular anti-oxidative ability, induction of apoptosis, inflammation and impair of cell-cell tight junctions related to Nrf2, TOR and NF-kappaB signaling in fish. Fish Shellfish Immunol. 2016, 56, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, B.; Shan, F.; Liu, B.; Gu, Z.; Song, C.; Sun, C.; Zhou, Q. Effects of oxidized fish oil on digestive enzyme activity and antioxidant system in Macrobrachium rosenbergii post-larvae. Aquac. Rep. 2022, 23, 101062. [Google Scholar] [CrossRef]
- Gong, S.S.; Li, Y.X.; Zhang, M.T.; Du, J.; Ma, P.S.; Yao, W.X.; Zhou, R.; Niu, Y.; Sun, T.; Yu, J.Q. Neuroprotective Effect of Matrine in Mouse Model of Vincristine-Induced Neuropathic Pain. Neurochem. Res. 2016, 41, 3147–3159. [Google Scholar] [CrossRef]
- Zhang, H.F.; Shi, L.J.; Song, G.Y.; Cai, Z.G.; Wang, C.; An, R.J. Protective effects of matrine against progression of high-fructose diet-induced steatohepatitis by enhancing antioxidant and anti-inflammatory defences involving Nrf2 translocation. Food Chem. Toxicol. 2013, 55, 70–77. [Google Scholar] [CrossRef]
- Sun, P.; Sun, N.; Yin, W.; Sun, Y.; Fan, K.; Guo, J.; Khan, A.; He, Y.; Li, H. Matrine inhibits IL-1beta secretion in primary porcine alveolar macrophages through the MyD88/NF-kappaB pathway and NLRP3 inflammasome. Vet. Res. 2019, 50, 53. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, P.; Sun, H.; Hou, Y.; Zhang, Y.; Liu, H. Evaluation of extruded full-fat soybean as the substitution for fish meal in diets for juvenile Scophthalmus maximus based on growth performance, intestinal microbiota, and aquaculture water quality. Aquaculture 2023, 562, 738734. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Ye, H.; Zou, C.; Ye, C.; Wang, A. Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology and immune related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) fed high lipid diets. Fish Shellfish Immunol. 2018, 73, 234–244. [Google Scholar] [PubMed]
- Wang, Q.; Shen, J.; Yan, Z.; Xiang, X.; Mu, R.; Zhu, P.; Yao, Y.; Zhu, F.; Chen, K.; Chi, S.; et al. Dietary Glycyrrhiza uralensis extracts supplementation elevated growth performance, immune responses and disease resistance against Flavobacterium columnare in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2020, 97, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, Q.; Sun, C.; Liu, B.; Liu, X.; Zhou, Q.; Zheng, X.; Xu, P.; Liu, B. Effects of dietary tea tree oil on the growth, physiological and non-specific immunity response in the giant freshwater prawn (Macrobrachium rosenbergii) under high ammonia stress. Fish Shellfish Immunol. 2022, 120, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.V.; Hoseinifar, S.H.; Jaturasitha, S.; Dawood, M.A.O.; Harikrishnan, R. The effects of berberine powder supplementation on growth performance, skin mucus immune response, serum immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 2020, 520, 734927. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Tian, J.; Liu, M.; Wang, G. Dietary flavonoids from Allium mongolicum Regel promotes growth, improves immune, antioxidant status, immune-related signaling molecules and disease resistance in juvenile northern snakehead fish (Channa argus). Aquaculture 2019, 501, 473–481. [Google Scholar] [CrossRef]
- Liu, F.; Geng, C.; Qu, Y.; Cheng, B.; Zhang, Y.; Wang, A.; Zhang, J.H.; Liu, B.; Tian, H.; Yang, W.; et al. The feeding of dietary Codonopsis pilosula polysaccharide enhances the immune responses, the expression of immune-related genes and the growth performance of red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2020, 103, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Zhou, H.; Mai, K.; He, G. Dietary Astragalus polysaccharides ameliorates the growth performance, antioxidant capacity and immune responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 99, 603–608. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Schupp, T.; Allmendinger, H.; Bossuyt, B.T.A.; Hidding, B.; Tury, B.; West, R.J. Review of the Ecotoxicological Properties of the Methylenedianiline Substances. Rev. Environ. Contam. Toxicol. 2017, 241, 39–72. [Google Scholar]
- Zhao, P.; Zhou, R.; Li, H.N.; Yao, W.X.; Qiao, H.Q.; Wang, S.J.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Oxymatrine attenuated hypoxic-ischemic brain damage in neonatal rats via improving antioxidant enzyme activities and inhibiting cell death. Neurochem. Int. 2015, 89, 17–27. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Bahrami Kamangar, B.; Zarei, M.A. Effects of diets containing grape seed proanthocyanidin extract on the growth and oxidative capacity of common carp (Cyprinus carpio). Aquaculture 2021, 540, 736689. [Google Scholar] [CrossRef]
- Kong, Y.; Li, M.; Guo, G.; Yu, L.; Sun, L.; Yin, Z.; Li, R.; Chen, X.; Wang, G. Effects of dietary curcumin inhibit deltamethrin-induced oxidative stress, inflammation and cell apoptosis in Channa argus via Nrf2 and NF-κB signaling pathways. Aquaculture 2021, 540, 736744. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Jiang, J.; Wu, P.; Chen, G.; Jiang, W.; Li, S.; Tang, L.; Kuang, S.; Feng, L. Effects of dietary isoleucine on growth, the digestion and absorption capacity and gene expression in hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2012, 368, 117–128. [Google Scholar] [CrossRef]
- Copple, I.M.; Goldring, C.E.; Kitteringham, N.R.; Park, B.K. The Nrf2-Keap1 defence pathway: Role in protection against drug-induced toxicity. Toxicology 2008, 246, 24–33. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.-M.; Li, J.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef]
- Cheng, C.; Park, S.C.; Giri, S.S. Effect of Pandanus tectorius extract as food additive on oxidative stress, immune status, and disease resistance in Cyprinus carpio. Fish Shellfish Immunol. 2022, 120, 287–294. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.; Li, H.; Tang, Y.; Ge, X.; Liu, B.; Xu, P. Protective Effects of Emodin on Oxidized Fish Oil-Induced Metabolic Disorder and Oxidative Stress through Notch-Nrf2 Crosstalk in the Liver of Teleost Megalobrama amblycephala. Antioxidants 2022, 11, 1179. [Google Scholar] [CrossRef]
- Pacitti, D.; Wang, T.; Martin, S.A.; Sweetman, J.; Secombes, C.J. Insights into the fish thioredoxin system: Expression profile of thioredoxin and thioredoxin reductase in rainbow trout (Oncorhynchus mykiss) during infection and in vitro stimulation. Dev. Comp. Immunol. 2014, 42, 261–277. [Google Scholar] [CrossRef]
- Kugapreethan, R.; Umasuthan, N.; Wan, Q.; Thulasitha, W.S.; Kim, C.; Lee, J. Comparative analysis of two thioredoxin-like genes in black rockfish Sebastes schlegelii and their possible involvement in redox homeostasis and innate immune responses. Dev. Comp. Immunol. 2017, 67, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, S.; Sahoo, P. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Zhang, B.; Li, C.; Wang, X.; Zhou, H.; Mai, K.; He, G. The effects of dietary Eucommia ulmoides Oliver on growth, feed utilization, antioxidant activity and immune responses of turbot (Scophthalmus maximus L.). Aquac. Nutr. 2019, 25, 367–376. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Bols, N.C.; Brubacher, J.L.; Ganassin, R.C.; Lee, L.E. Ecotoxicology and innate immunity in fish. Dev. Comp. Immunol. 2001, 25, 853–873. [Google Scholar] [CrossRef] [PubMed]
- Fast, M.D.; Johnson, S.C.; Jones, S.R. Differential expression of the pro-inflammatory cytokines IL-1beta-1, TNFalpha-1 and IL-8 in vaccinated pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon juveniles. Fish Shellfish Immunol. 2007, 22, 403–407. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Jiang, J.; Jiang, W.; Kuang, S.; Tang, L.; Tang, W.; Zhang, Y.A.; Zhou, X.; Feng, L. Effect of dietary arginine on the immune response and gene expression in head kidney and spleen following infection of Jian carp with Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 44, 195–202. [Google Scholar] [CrossRef]
- Giri, S.S.; Sukumaran, V.; Park, S.C. Effects of bioactive substance from turmeric on growth, skin mucosal immunity and antioxidant factors in common carp, Cyprinus carpio. Fish Shellfish Immunol. 2019, 92, 612–620. [Google Scholar] [CrossRef]
- Denis, F.; Archambault, D. Molecular cloning and characterization of beluga whale (Delphinapterus leucas) interleukin-1beta and tumor necrosis factor-alpha. Can. J. Vet. Res. 2001, 65, 233. [Google Scholar]
- Awad, E.; Austin, D.; Lyndon, A.; Awaad, A. Possible effect of hala extract (Pandanus tectorius) on immune status, anti-tumour and resistance to Yersinia ruckeri infection in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 87, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Balasundaram, C.; Thamizharasan, S.; Hoseinifar, S.H.; Abdel-Tawwab, M. Effect of diet enriched with Agaricus bisporus polysaccharides (ABPs) on antioxidant property, innate-adaptive immune response and pro-anti inflammatory genes expression in Ctenopharyngodon idella against Aeromonas hydrophila. Fish Shellfish Immunol. 2021, 114, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Cong, C.; Wang, L.; Li, X.; Li, J.; Yang, H.; Li, S.; Xu, Y. Control of Edwardsiella tarda infection in turbot Scophthalmus maximus (L.) using phage vB_EtaM_ET-ABTNL-9. Aquac. Res. 2022, 53, 3010–3024. [Google Scholar]
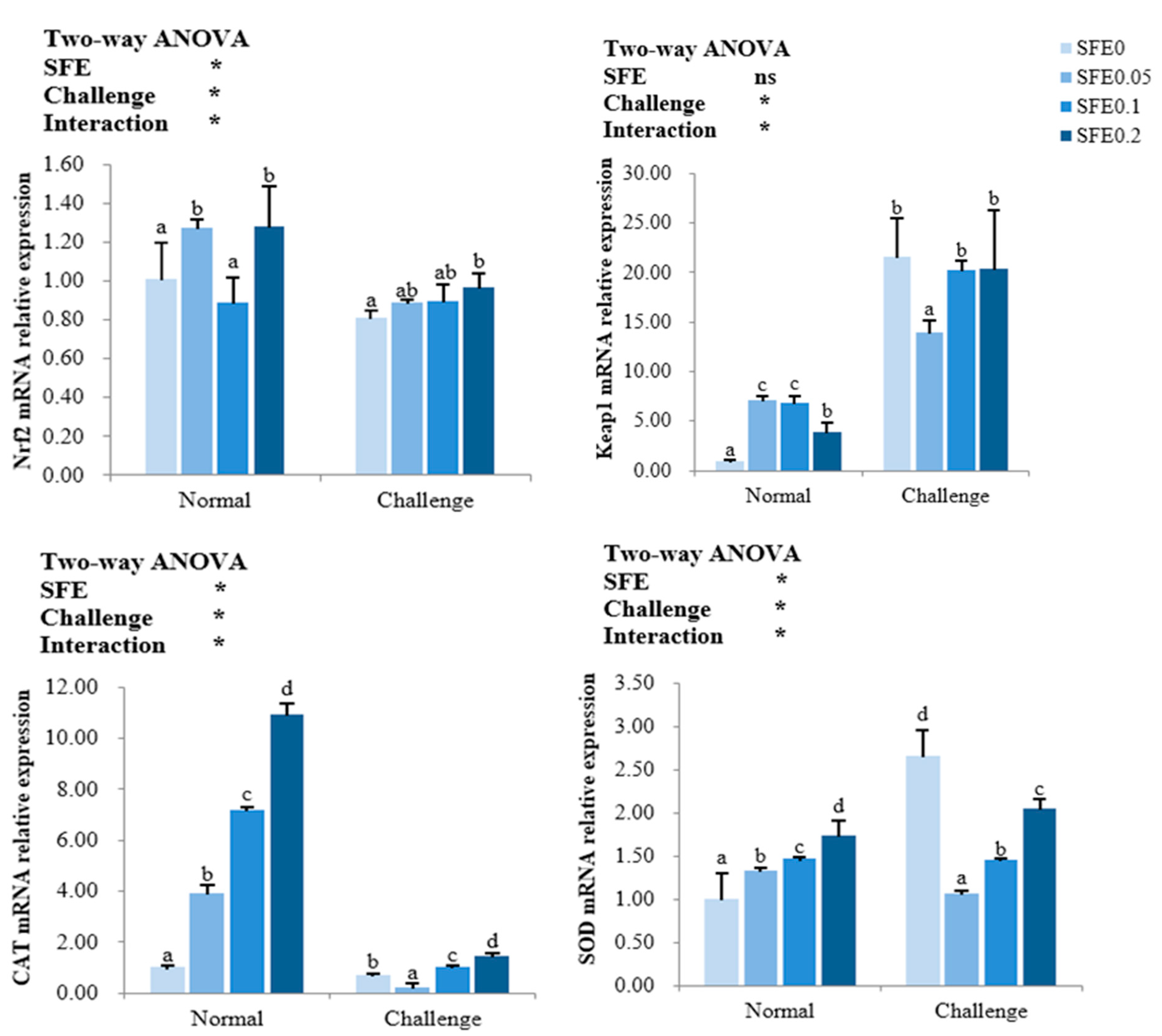
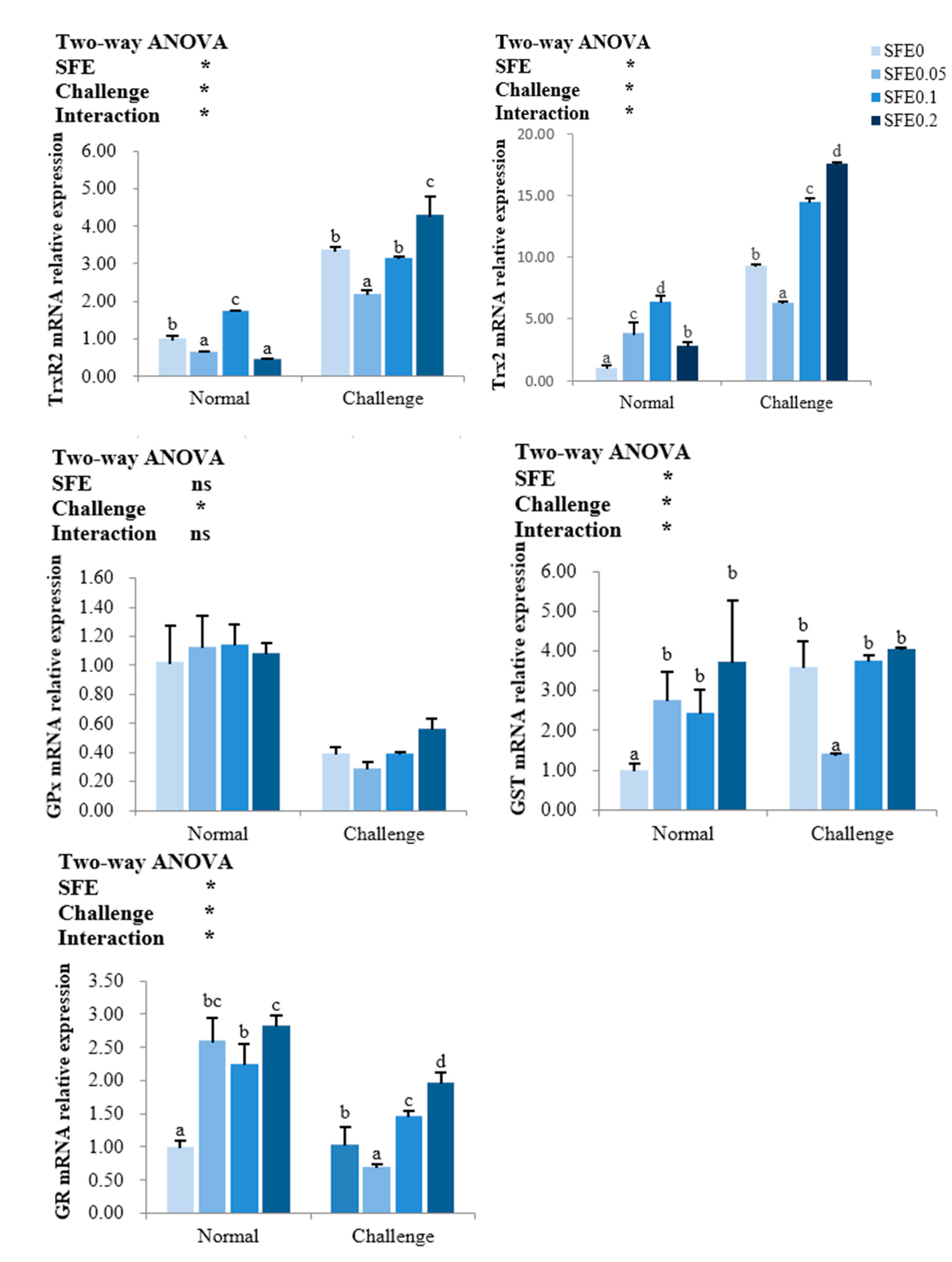
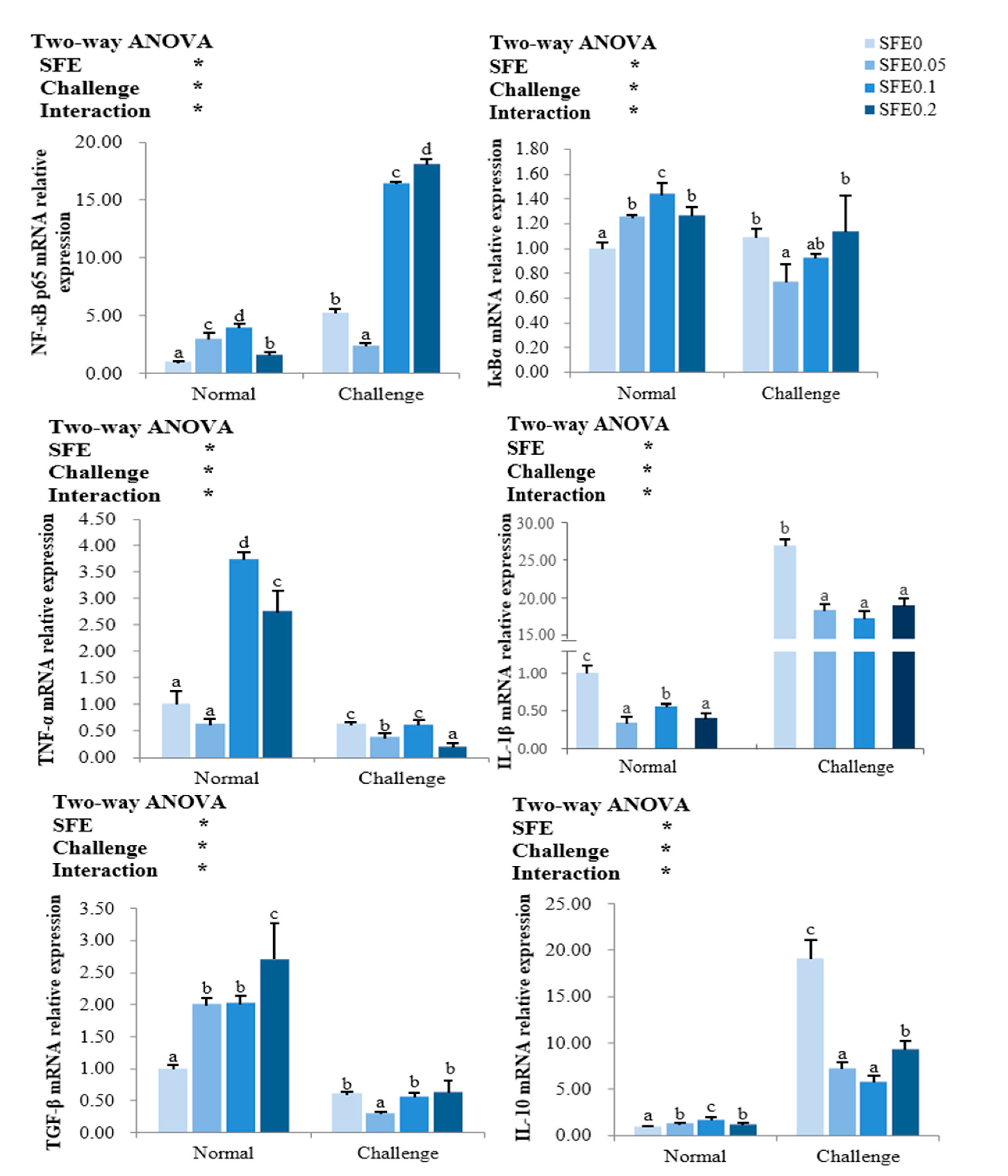
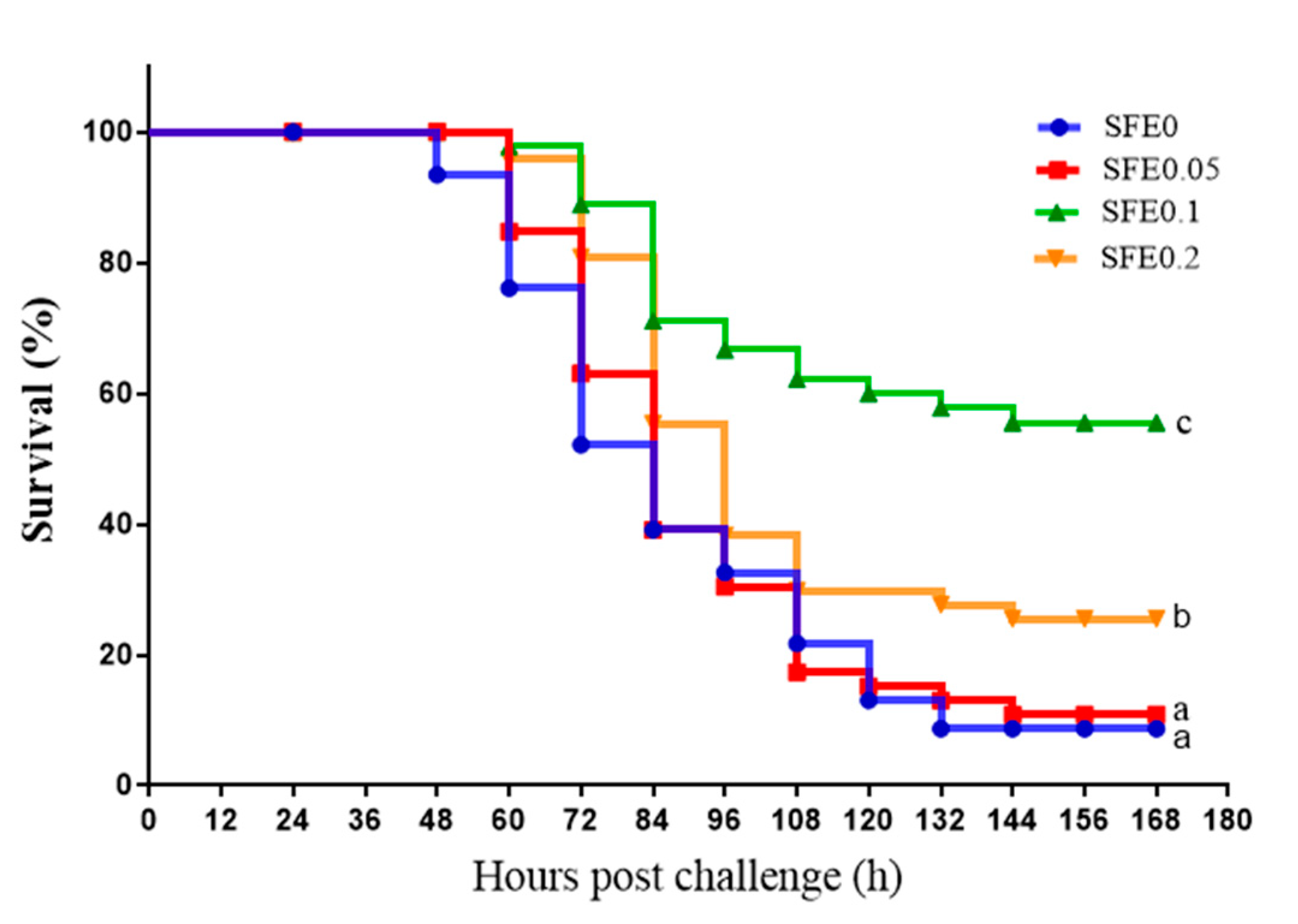
 : indicates a significant impact. The red arrow indicates the promoting effect, the blue arrow indicates the inhibitory effect, and the orange arrow indicates a positive effect on fish health).
: indicates a significant impact. The red arrow indicates the promoting effect, the blue arrow indicates the inhibitory effect, and the orange arrow indicates a positive effect on fish health).
 : indicates a significant impact. The red arrow indicates the promoting effect, the blue arrow indicates the inhibitory effect, and the orange arrow indicates a positive effect on fish health).
: indicates a significant impact. The red arrow indicates the promoting effect, the blue arrow indicates the inhibitory effect, and the orange arrow indicates a positive effect on fish health).
| Ingredients | SFE 0 | SFE 0.05 | SFE 0.1 | SFE 0.2 |
|---|---|---|---|---|
| White fish meal | 40.00 | 40.00 | 40.00 | 40.00 |
| Chicken meal | 17.50 | 17.50 | 17.50 | 17.50 |
| Dried squid liver meal | 5.00 | 5.00 | 5.00 | 5.00 |
| Wheat gluten | 5.00 | 5.00 | 5.00 | 5.00 |
| Yeast powder | 4.00 | 4.00 | 4.00 | 4.00 |
| α-starch | 18.00 | 17.95 | 17.90 | 17.80 |
| Fish oil | 4.00 | 4.00 | 4.00 | 4.00 |
| Sophora flavescens root extract | 0.00 | 0.05 | 0.10 | 0.20 |
| CaHPO4 | 3.00 | 3.00 | 3.00 | 3.00 |
| Zeolite powder | 1.30 | 1.30 | 1.30 | 1.30 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.20 |
| Premix | 2.00 | 2.00 | 2.00 | 2.00 |
| Nutrient composition | ||||
| Crude protein | 48.61 | 48.54 | 48.52 | 48.58 |
| Crude lipid | 10.53 | 10.54 | 10.56 | 10.51 |
| Crude ash | 16.58 | 16.60 | 16.62 | 16.68 |
| Gross energy (MJ/kg) | 19.54 | 19.59 | 19.60 | 19.56 |
| Target Gene | Primer Sequence | Annealing Temperature (°C) | Product Size (bp) | Accession Number |
|---|---|---|---|---|
| NF-κB p65 | F:ACTCACCCAGCCATCAAG R:AAGCAAAGCCGAACTGAA | 58 | 314 | XM_035627239.1 |
| IκBα | F:AGAAAGCAGGAAATCAACTAAG R:TTGCGACTGACGATAAGG | 58 | 205 | XM_035649251.1 |
| TNF-α | F:TGGAGATGGGTCTTGAGG R:TGGCATTGCTGCTGATTT | 55 | 249 | XM_035629860.2 |
| IL-1β | F:ATGGTGCGATTTCTGTTCTAC R:TTCCACTTTGGGTCGTCTT | 50 | 204 | XM_035640817.2 |
| TGF-β | F:TCAGCATTCCAGATGTAGGTG R:GGAGAGTGGCTTCAGTTTTTC | 54 | 312 | XM_035623668.2 |
| IL-10 | F:CCACGCCATGAACAGCATCCT R:ACATCGGACTTGAGCTCGTCGAA | 60 | 141 | XM_035632547.1 |
| Nrf2 | F:GGCAAGAACAAAGTGGCT R:GCAGACGCTCTTTCTCATC | 59 | 106 | XM_035651303.1 |
| Keap1 | F:TTGCCGAGCAGATTGGTT R:AGCGGACAGCCTGGAGTA | 58 | 249 | XM_035636756.1 |
| Trx2 | F:GGCTCACAGGCTGCTCGTA R:GGGCAGTTCGCTGTTGAT | 60 | 253 | XM_035616077.1 |
| TrxR2 | F:GAGGCTGTTCACAACCACG R:CCACCTGAGGCGATTACG | 60 | 182 | XM_035640386.1 |
| GPx | F:CCCTGATGACTGACCCAAAG R:GCACAAGGCTGAGGAGTTTC | 57 | 174 | XM_035632618.1 |
| GR | F:GTCTCCTCTGGGCTATTGG R:CGTGATACATCGGAGTGAAA | 55 | 375 | XM_035650249 |
| GST | F:TGGATTACTTCACTGGACCTT R:TTTACCTATGAGTCGTCGTTC | 53 | 272 | XM_035636617 |
| SOD | F:AAACAATCTGCCAAACCTCTG R:CAGGAGAACAGTAAAGCATGG | 58 | 165 | MG253620.1 |
| CAT | F:TCCCGTCCTTCATTCACT R:AATAGCATAATCTGGGTTGGT | 57 | 205 | MG253621.1 |
| RPS4 | F:CAACATCTTCGTCATCGGCAAGG R:ATTGAACCAGCCTCAGTGTTTAGC | 60 | 143 | XM_035608277.1 |
| Indices | SFE0 | SFE0.05 | SFE0.1 | SFE0.2 | p Value |
|---|---|---|---|---|---|
| IBW (g) | 8.40 ± 0.10 | 8.40 ± 0.00 | 8.37 ± 0.06 | 8.37 ± 0.12 | 0.916 |
| FBW (g) | 25.31 ± 1.03 a | 26.45 ± 0.42 ab | 30.00 ± 0.90 c | 27.67 ± 0.87 b | <0.001 |
| FR (%/d) | 1.38 ± 0.06 | 1.39 ± 0.04 | 1.36 ± 0.04 | 1.32 ± 0.04 | 0.310 |
| WGR (%) | 201.41 ± 15.74 a | 214.83 ± 5.01 ab | 258.60 ± 9.26 c | 230.65 ± 7.83 b | <0.001 |
| SGR (%/d) | 1.97 ± 0.09 a | 2.05 ± 0.03 ab | 2.28 ± 0.05 c | 2.14 ± 0.04 b | 0.001 |
| FCR | 0.77 ± 0.02 b | 0.75 ± 0.03 b | 0.68 ± 0.01 a | 0.69 ± 0.03 a | 0.002 |
| PER | 2.66 ± 0.09 a | 2.73 ± 0.09 a | 3.00 ± 0.06 b | 2.99 ± 0.11 b | 0.003 |
| SR (%) | 99.33 ± 1.15 | 99.33 ± 1.15 | 98.67 ± 1.15 | 100.00 ± 0.00 | 0.487 |
| CF (%) | 3.15 ± 0.23 a | 3.19 ± 0.24 a | 3.41 ± 0.23 b | 3.21 ± 0.21 a | 0.040 |
| VSI (%) | 6.83 ± 0.52 | 6.78 ± 0.31 | 6.74 ± 0.44 | 6.67 ± 0.31 | 0.814 |
| HSI (%) | 2.55 ± 0.43 | 2.66 ± 0.50 | 2.64 ± 0.49 | 2.42 ± 0.43 | 0.593 |
| Index | Group | Two-Way ANOVA (p Value) | ||||||
|---|---|---|---|---|---|---|---|---|
| SFE0 | SFE0.05 | SFE0.1 | SFE0.2 | SFE | Challenge | SFE × Challenge | ||
| SOD(U/mL) | Normal | 59.58 ± 1.94 a | 60.13 ± 1.95 a | 67.63 ± 1.32 c | 63.97 ± 3.62 b | <0.001 | <0.001 | 0.002 |
| Challenge | 73.69 ± 7.01 ab | 77.79 ± 1.21 bc | 80.14 ± 2.30 c | 72.50 ± 1.24 a | ||||
| CAT(U/mL) | Normal | 5.13 ± 1.16 a | 6.07 ± 1.45 ab | 7.14 ± 1.31 b | 6.55 ± 1.07 b | <0.001 | <0.001 | <0.001 |
| Challenge | 10.96 ± 4.19 a | 30.56 ± 11.73 b | 14.32 ± 5.17 a | 17.29 ± 4.05 a | ||||
| GPx (U/mL) | Normal | 30.76 ± 6.21 a | 42.72 ± 6.43 b | 45.90 ± 12.94b | 41.18 ± 11.88 b | <0.001 | <0.001 | <0.001 |
| Challenge | 240.67 ± 20.00 a | 331.33 ± 11.60 b | 243.33 ± 11.18 a | 247.33 ± 27.05 a | ||||
| T-AOC (mM) | Normal | 0.93 ± 0.08 a | 0.98 ± 0.08 ab | 1.06 ± 0.05 c | 1.00 ± 0.05 b | <0.001 | 0.022 | 0.036 |
| Challenge | 0.87 ± 0.01 a | 0.92 ± 0.02 b | 1.05 ± 0.05 c | 1.03 ± 0.03 c | ||||
| GST (U/mL) | Normal | 17.82 ± 5.14 a | 24.43 ± 1.66 a | 40.96 ± 16.82 b | 17.96 ± 2.75 a | <0.001 | 0.001 | 0.104 |
| Challenge | 63.06 ± 5.88 a | 51.53 ± 21.79 a | 95.29 ± 3.55 b | 46.59 ± 17.92 a | ||||
| GR (U/L) | Normal | 30.55 ± 7.01 | 35.37 ± 9.78 | 20.90 ± 12.56 | 28.62 ± 13.90 | 0.347 | 0.012 | 0.651 |
| Challenge | 21.57 ± 6.71 | 20.19 ± 10.28 | 17.68 ± 8.57 | 19.65 ± 8.12 | ||||
| MDA (nmol/mL) | Normal | 30.38 ± 1.32 c | 23.96 ± 1.49 b | 19.68 ± 0.88 a | 19.68 ± 0.74 a | <0.001 | <0.001 | <0.001 |
| Challenge | 32.37 ± 6.73 b | 26.31 ± 2.71 a | 25.43 ± 3.84 a | 34.89 ± 4.40 b | ||||
| Index | SFE0 | SFE0.05 | SFE0.1 | SFE0.2 | p Value |
|---|---|---|---|---|---|
| LZM (μg/mL) | 3.89 ± 0.26 a | 4.00 ± 0.25 b | 4.17 ± 0.21 c | 4.02 ± 0.29 b | 0.027 |
| Complement C3 (g/L) | 3.70 ± 0.09 | 3.70 ± 0.07 | 3.71 ± 0.09 | 3.69 ± 0.08 | 0.978 |
| IgM (μg/mL) | 54.60 ± 7.79 | 63.81 ± 10.88 | 64.25 ± 11.33 | 61.65 ± 16.91 | 0.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Gao, X.; Shi, X.; Dong, N.; Yue, T.; Zhang, P.; Liu, H. Dietary Supplementation of Sophora flavescens Root Extract Improved the Growth Performance, Antioxidant Capacity, Innate Immunity, and Disease Resistance against Edwardsiella tarda Challenge in Turbot (Scophthalmus maximus). Antioxidants 2023, 12, 69. https://doi.org/10.3390/antiox12010069
Hou Y, Gao X, Shi X, Dong N, Yue T, Zhang P, Liu H. Dietary Supplementation of Sophora flavescens Root Extract Improved the Growth Performance, Antioxidant Capacity, Innate Immunity, and Disease Resistance against Edwardsiella tarda Challenge in Turbot (Scophthalmus maximus). Antioxidants. 2023; 12(1):69. https://doi.org/10.3390/antiox12010069
Chicago/Turabian StyleHou, Yuqing, Xuezheng Gao, Xueying Shi, Na Dong, Tongtong Yue, Peiyu Zhang, and Haiyan Liu. 2023. "Dietary Supplementation of Sophora flavescens Root Extract Improved the Growth Performance, Antioxidant Capacity, Innate Immunity, and Disease Resistance against Edwardsiella tarda Challenge in Turbot (Scophthalmus maximus)" Antioxidants 12, no. 1: 69. https://doi.org/10.3390/antiox12010069
APA StyleHou, Y., Gao, X., Shi, X., Dong, N., Yue, T., Zhang, P., & Liu, H. (2023). Dietary Supplementation of Sophora flavescens Root Extract Improved the Growth Performance, Antioxidant Capacity, Innate Immunity, and Disease Resistance against Edwardsiella tarda Challenge in Turbot (Scophthalmus maximus). Antioxidants, 12(1), 69. https://doi.org/10.3390/antiox12010069








