The Chemistry and the Anti-Inflammatory Activity of Polymethoxyflavonoids from Citrus Genus
Abstract
1. Introduction
| No. | Compound | Species | References |
|---|---|---|---|
| 1 | 2′,3′-Dimethoxyflavone | Brazilian orange essential oil | [17] |
| 2 | 2′,4′-Dimethoxyflavone | Brazilian orange essential oil | [17] |
| 3 | 3′,4′-Dimethoxyflavone | C. platymamma | [18] |
| 4 | 5,4′-Dimethoxyflavone | Brazilian orange essential oil | [17] |
| 5 | 5,7-Dimethoxyflavone (Chrysin dimethyl ether) | Brazilian orange essential oil | [17] |
| 6 | 7,4′-Dimethoxyflavone | Brazilian orange essential oil | [17] |
| 3′,4′-Dihydroxy-7,5′-dimethoxyflavone | C. reticulata; C. sinensis | [19] | |
| 7 | 5,6-Dihydroxy-7,4′-dimethoxyflavone (Ladanein) | Fructus Aurantii (C. aurantium dried) | [20] |
| 8 | 3,5,6-Trihydroxy-7,4′-dimethoxyflavone | C. medica | [21] |
| 9 | 5,7,4′-Trihydroxy-6,3′-dimethoxyflavone (Jaceosidin) | C. hassaku | [20] |
| 10 | 5,7,4′-Trihydroxy-6,8-dimethoxyflavone (Demethoxysudachitin) | C. sudachi | [22] |
| 3,5,7,4′-Tetrahydroxy-8,3′-dimethoxyflavone | C. unshiu | [23] | |
| 5,7,3′,4′-Tetrahydroxy-8,3′-dimethoxyflavone | C. sudachi | [24] | |
| 11 | 2′,3′,4′-Trimethoxyflavone | Brazilian orange essential oil | [17] |
| 12 | 3,5,7-Trimethoxyflavone (Galangin trimethyl ether) | Brazilian orange essential oil | [17] |
| 13 | 3′,4′,5′-Trimethoxyflavone | Brazilian orange essential oil | [17] |
| 14 | 5,7,4′-Trimethoxyflavone (Apigenin trimethyl ether) | C. rutaceae | [25] |
| 15 | 5,3′,4′-Trimethoxyflavone | Brazilian orange essential oil | [17] |
| 16 | 6,2′,3′-Trimethoxyflavone | Brazilian orange essential oil | [17] |
| 3-Hydroxy-5,7,8-trimethoxyflavone | C. reticulata; C. sinensis | [19] | |
| 17 | 5-Hydroxy-6,7,4′-trimethoxyflavone (Salvigenin) | C. sinensis | [20] |
| 5-Hydroxy-7,3′,4′-trimethoxyflavone | C. reticulata; C. sinensis | [19] | |
| 18 | 5-Hydroxy-7,8,4′-trimethoxyflavone (Isoscutellarein 7,8,4′-trimethyl ether) | C. reticulata | [26] |
| 19 | 8-Hydroxy-5,7,4′-trimethoxyflavone | C. reticulata Blanco | [27] |
| 4′-Hydroxy-5,6,7-trimethoxyflavone | C. aurantium | [28] | |
| 20 | 5,4′-Dihydroxy-6,7,8-trimethoxyflavone (Xanthomicrol) | C. reticulata | [29] |
| 21 | 3,5,6-Trihydroxy-7,3′,4′-trimethoxyflavone | C. medica | [21] |
| 22 | 5,7,4′-Trihydroxy-6,8,3′- trimethoxyflavone | C. sudachi | [24] |
| 23 | 3,5,7,4′-Tetrahydroxy-6,8,3′-trimethoxyflavone (Limocitrol) | C. limon | [30] |
| 24 | 3,6,7,4′-Tetramethoxyflavone | C. hallabong (hybrid) | [31] |
| 25 | 5,6,7,4′-Tetramethoxyflavone (Scutellarein tetramethyl ether) | C. sinensis; C. reticulata | [20] |
| 26 | 5,7,8,4′-Tetramethoxyflavone (6-Demethoxytangeretin) | C. reticulata Blanco cv. Ponkan | [20] |
| 27 | 5,7,3′,4′-Tetramethoxyflavone (Luteolin tetramethyl ether) | C. reticulata; C. sinensis | [19] |
| 28 | 6,7,8,4′-Tetramethoxyflavone | C. unshiu | [32] |
| 7,8,3′,4′-Tetramethoxyflavone | C. platymamma | [18] | |
| 29 | 7,3′,4′,5′-Tetramethoxyflavone | C. reticulata | [33] |
| 30 | 3-Hydroxy-5,6,7,4′-tetramethoxyflavone (Eupatorin-5-methylether) | C. sinensis | [34] |
| 5-Hydroxy-3,6,7,8-tetramethoxyflavone | C. reticulata; C. sinensis | [19] | |
| 31 | 5-Hydroxy-3,6,7,4′-tetramethoxyflavone (Penduletin 4′-methyl ether) | C. reticulata | [27] |
| 32 | 5-Hydroxy-3,7,8,4′-tetramethoxyflavone | C. albanicus; C. parviflorus | [35] |
| 33 | 5-Hydroxy-3,7,3′,4′-tetramethoxyflavone (Retusin) | C. miaray | [36] |
| 34 | 5-Hydroxy-6,7,8,4′-tetramethoxyflavone (Gardenin B) | Fructus aurantii (C. aurantium dried) | [37] |
| 35 | 5-Hydroxy-6,7,3′,4′-tetramethoxyflavone (5-Desmethylsinensetin) | C. aurantium | [28] |
| 5-Hydroxy-7,8,3′,4′-tetramethoxyflavone | C. reticulata; C. sinensis | [19] | |
| 36 | 6-Hydroxy-5,7,8,4′-tetramethoxyflavone | C. reticulata; C. sinensis | [19] |
| 37 | 7-Hydroxy-5,6,8,4′-tetramethoxyflavone | C. reticulata; C. sinensis | [19] |
| 7-Hydroxy-5,6,3′,4′-tetramethoxyflavone | C. reticulata | [38] | |
| 38 | 4′-Hydroxy-5,6,7,8-tetramethoxyflavone | C. reticulata | [26] |
| 39 | 5,7-Dihydroxy-6,8,3′,4′-tetramethoxyflavone (Hymenoxin) | C. medica L. | [21] |
| 5,8-Dihydroxy-3,7,3′,4′-tetramethoxyflavone | C. miaray | [36] | |
| 40 | 5,3′-Dihydroxy-3,7,4′,5′-tetramethoxyflavone | C. monspeliensis | [39] |
| 41 | 5,4′-Dihydroxy-6,7,8,3′-tetramethoxyflavone (8-Methoxycirsilineol) | C. reticulata | [29] |
| 7,4′-Dihydroxy-5,6,8,3′-tetramethoxyflavone | C. deliciosa | [40] | |
| 42 | 3,5,6,8,4′-Pentamethoxyflavone | C. reticulata | [41] |
| 43 | 3,5,7,3′,4′-Pentamethoxyflavone (Quercetin pentamethyl ether) | C. miaray | [20] |
| 3,6,7,8,4′-Pentamethoxyflavone | C. sinensis | [20] | |
| 44 | 5,6,7,8,4′-Pentamethoxyflavone (Tangeretin) | C. sinensis | [20] |
| 45 | 5,6,7,3′,4′-Pentamethoxyflavone (Sinensitin) | C. reticulata; C. sinensis | [19] |
| 46 | 5,7,8,3′,4′-Pentamethoxyflavone (Isosinensetin) | C. sinensis | [20] |
| 47 | 5,7,2′,3′,4′-Pentamethoxyflavone | C. reticulata Blanco; C. reticulata Chachi | [42] |
| 48 | 6,7,8,3′,4′-Pentamethoxyflavone (Demethylnobiletin) | C. reticulata Blanco cv. Ponkan | [20] |
| 49 | 3-Hydroxy-5,6,7,8,4′-pentamethoxyflavone (3-Demethylnobiletin) | C. sinensis Osbeck | [34] |
| 50 | 5-Hydroxy-3,6,7,8,4′-pentamethoxyflavone (5-Hydroxyauranetin) | C. aurantium | [43] |
| 51 | 5-Hydroxy-3,7,8,3′,4′-pentamethoxyflavone (Gossypetin pentamethylether) | C. sinensis Osbeck | [20] |
| 52 | 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone (5-Demethylnobiletin) | C. sinensis | [34] |
| 53 | 5-Hydroxy-6,7,3′,4′,5′-pentamethoxyflavone (Umuhengerin) | C. reticulata; C. sinensis | [19] |
| 54 | 7-Hydroxy-3,5,6,3′,4′-pentamethoxyflavone | C. reticulata | [44] |
| 55 | 7-Hydroxy-5,6,8,3′,4′-pentamethoxyflavone (7-Demethylnobiletin) | C. reticulata; C. sinensis | [19] |
| 56 | 8-Hydroxy-3,5,6,7,4′-pentamethoxyflavone | C. aurantifolia | [45] |
| 57 | 3′-Hydroxy-5,6,7,8,4′-pentamethoxyflavone (3-Hydroxytangeretin) | C. changshan-huyou | [46] |
| 58 | 4′-Hydroxy-5,6,7,8,3′-pentamethoxyflavone (4′-Demethylnobiletin) | C. reticulata; C. sinensis | [19] |
| 59 | 3,5,6,7,8,4′-Hexamethoxyflavone (3-Methoxytangeretin) | C. sinensis | [20] |
| 3,5,6,7,3′,4′-Hexamethoxyflavone | Commercially Citrus peels extract | [47] | |
| 3,5,6,8,3′,4′-Hexamethoxyflavone | C. hassaku | [20] | |
| 60 | 3,5,7,8,2′,5′-Hexamethoxyflavone | C. reticulata Blanco | [48] |
| 61 | 3,5,7,8,3′,4′-Hexamethoxyflavone (Gossypetin hexamethyl ether) | C. hassaku | [20] |
| 62 | 3,6,7,8,2′,5′-Hexamethoxyflavone | Citrus unshiu | [32] |
| 63 | 5,6,7,8,3′,4′-Hexamethoxyflavone (Nobiletin) | C. reticulata | [44] |
| 64 | 5,6,7,3′,4′,5′-Hexamethoxyflavone | C. reticulata; C. sinensis | [19] |
| 65 | 5,7,8,3′,4′,5′-Hexamethoxyflavone (Bannamurpanisin) | C. reticulata; C. sinensis | [19] |
| 66 | 3-Hydroxy-5,6,7,8,3′,4′-hexamethoxyflavone (Natsudaidain) | C. aurantium | [28] |
| 67 | 5-Hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone | C. kinokuni | [20] |
| 68 | 5-Hydroxy-6,7,8,3′,4′,5′-hexamethoxyflavone (Gardenin A) | C. reticulata; C. sinensis | [19] |
| 6-Hydroxy-3,5,7,8,3′,4′-hexamethoxyflavone | C. unshiu | [23] | |
| 69 | 7-Hydroxy-3,5,6,8,3′,4′-hexamethoxyflavone | C. reticulata | [44] |
| 70 | 8-Hydroxy-3,5,6,7,3′,4′-hexamethoxyflavone | C. aurantifolia | [45] |
| 8-Hydroxy-5,6,7,3′,4′,5′-hexamethoxyflavone | C. reticulata; C. sinensis | [19] | |
| 3,5,6,7,8,3′,4′-Heptamethoxyflavone | C. miaray | [36] | |
| 71 | 5,6,7,8,3′,4′,5′-Heptamethoxyflavone | Brazilian orange essential oil | [49] |
| No. | Compound | Species | References |
|---|---|---|---|
| 72 | 5,6,7,4′-Tetramethoxyflavanone | C. sinensis | [34] |
| 5-Hydroxy-3,6,7,4′-tetramethoxyflavanone | C. reticula Blanco | [50] | |
| 73 | 5,6,7,8,4′-Pentamethoxyflavanone | C. reticula Blanco | [50] |
| 74 | 5,6,7,3′,4′-Pentamethoxyflavanone | C. reticulata; C. sinensis | [19] |
| 75 | 5,7,8,3′,4′-Pentamethoxyflavanone | C. reticula Blanco | [27] |
| 6,7,8,3′,4′-Pentamethoxyflavanone | C. reticula Blanco | [50] | |
| 76 | 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavanone (5-Demethylcitromitine) | C. sinensis | [34] |
| 6-Hydroxy-5,7,8,3′,4′-pentamethoxyflavanone | C. jambhiri | [51] | |
| 77 | 5,6,7,8,3′,4′-Hexamethoxyflavanone (Citromitin) | C. miaray | [36] |
| No. | Compound | Species | References |
|---|---|---|---|
| 78 | 2′-Hydroxy-3,4,4′,5′,6′-pentamethoxychalcone | C. sinensis | [20] |
| 79 | 2′-Hydroxy-3,4,3′,4′,5′,6′-hexamethoxychalcone | C. sinensis | [34] |
2. Radical Scavenging Activity
3. Inhibition of Enzymatic Activity
4. Inhibition of Nitric Oxide (NO) Production and the Related Biochemical Effects In Vitro
5. Anti-Neuroinflammatory Activity of Citrus PMFs and HPMFs
6. Anti-Inflammatory Activity In Vivo of Citrus PMFs and HPMFs
7. Spectroscopical Data of Polymethoxy-Flavones, -Flavanones, and -Chalcones Isolated in Citrus Genus
8. Spectroscopical Data of Glycosylated Flavonoids Isolated in Citrus Genus
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scora, R.W. On the history and origin of Citrus. Bull. Torrey Bot. Club 1975, 102, 369–375. Available online: https://www.jstor.org/stable/248476 (accessed on 25 September 2022). [CrossRef]
- Jena, S.N.; Kumar, S.; Nair, N.K. Molecular phylogeny in Indian Citrus L. (Rutaceae) inferred through PCR-RFLP and trnL-trnF sequence data of chloroplast DNA. Sci. Hortic. 2009, 119, 403–416. [Google Scholar] [CrossRef]
- Kalita, B.; Roy, A.; Annamalai, A.; Ptv, L. A molecular perspective on the taxonomy and journey of Citrus domestication. Perspect. Plant Ecol. Evol. Syst. 2021, 53, 403–416. [Google Scholar] [CrossRef]
- Nicolosi, E.; Deng, Z.N.; Gentile, A.; La Malfa, S.; Continella, G.; Tribulato, E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor. Appl. Genet. 2000, 100, 1155–1166. [Google Scholar] [CrossRef]
- Abbas, F.; Fares, A. Best management practices in citrus production. Tree For. Sci. Biotechnol. 2009, 3, 1–11. [Google Scholar]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Food and Agricultue Organization of the United Nations (FAO). Citrus Fruit Fresh and Processed; Statistical Bulletin: Rome, Italy, 2020; Available online: https://www.fao.org/3/cb6492en/cb6492en.pdf (accessed on 2 December 2022).
- Lucia, C.; Laudicina, V.A.; Badalucco, L.; Galati, A.; Palazzolo, E.; Torregrossa, M.; Viviani, G.; Corsino, S.F. Challenges and opportunities for citrus wastewater management and valorisation: A review. J. Environ. Manag. 2022, 321, 115924. [Google Scholar] [CrossRef]
- Fontana, G. The Orange Peel: An Outstanding Source of Chemical Resources. Citrus—Res. Dev. Biotechnol. 2021, 207, 1–22. [Google Scholar]
- Sottile, F.; Del Signore, M.B.; Barone, E. Ornacitrus: Citrus plants (Citrus spp.) as ornamentals. Folia Hortic. 2019, 31, 239–251. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Tundis, R.; Loizzo, M.R. Reuse of food waste: The chemical composition and health properties of pomelo (Citrus maxima) cultivar essential oils. Molecules 2022, 27, 3273. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical compositions and antioxidant activities of essential oils, and their combinations, obtained from flavedo by-product of seven cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, F.; Lian, Y.; Xiao, H.; Zheng, J. Biosynthesis of citrus flavonoids and their health effects. Food Sci. Nutr. 2020, 60, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of bioactive flavonoids from Citrus fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Yi, L.; Ma, S.; Ren, D. Phytochemistry and bioactivity of Citrus flavonoids: A focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem. Rev. 2017, 16, 479–511. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Food Sci. Nutr. 2022, 62, 3833–3854. [Google Scholar] [CrossRef]
- Gaydou, E.M.; Berahia, T.; Wallet, J.C.; Bianchini, J.P. Gas chromatography of some polymethoxylated flavones and their determination in orange peel oils. J. Chromatogr. A 1991, 549, 440–445. [Google Scholar] [CrossRef]
- Kim, Y.D.; Ko, W.J.; Koh, K.S.; Jeon, Y.J.; Kim, S.H. Composition of flavonoids and antioxidative activity from juice of Jeju native Citrus fruits during maturation. Korean J. Nutr. 2009, 42, 278–290. [Google Scholar] [CrossRef]
- Xing, T.T.; Zhao, X.J.; Zhang, Y.D.; Li, Y.F. Fast separation and sensitive quantitation of polymethoxylated flavonoids in the peels of Citrus using UPLC-Q-TOF-MS. J. Agric. Food Chem. 2017, 65, 2615–2627. [Google Scholar] [CrossRef]
- Uckoo, R.M.; Jayaprakasha, G.K.; Patil, B.S. Chromatographic techniques for the separation of polymethoxyflavones from citrus. ACS Symp. Ser. 2012, 1093, 3–15. [Google Scholar] [CrossRef]
- He, H.; Ling, I. Chemical studies of a Chinese traditional drug fingered citron (Citrus medica L. var sarcodactylis (Noot.) Swingle. Acta Pharm. Sin. 1985, 20, 433–435. [Google Scholar]
- Tsukayama, M.; Sasaki, T.; Yamamoto, K.; Kawamura, Y.; Ichikawa, R. Microwave-Assisted extraction and methylation of useful flavones from waste peels of Citrus sudachi. Nippon. Shokuhin Kagaku Kogaku Kaishi 2010, 57, 427–433. [Google Scholar] [CrossRef]
- Chkhikvishvili, I.D.; Kurkin, V.A.; Pervykh, L.N. Flavonoids of the peel of the fruit of Citrus unshiu. Chem. Nat. Compd. 1994, 30, 765–766. [Google Scholar] [CrossRef]
- Nakasugi, T.; Nakashima, M.; Komai, K. Antimutagens in Citrus sudachi Hort. et Shirai. Nat. Med. 2000, 54, 26–28. [Google Scholar]
- Mizuno, M.; Iinuma, M.; Ohara, M.; Tanaka, T.; Iwamasa, M. Chemotaxonomy of the genus Citrus based on polymethoxyflavones. Chem. Pharm. Bull. 1991, 39, 945–949. [Google Scholar] [CrossRef]
- Iinuma, M.; Matsuura, S.; Kurogochi, K.; Tanaka, T. Studies on the constituents of useful plants. V. Multisubstituted flavones in the fruit peel of Citrus reticulata and their examination by Gas-Liquid Chromatography. Chem. Pharm. Bull. 1980, 28, 717–722. [Google Scholar] [CrossRef]
- Zhao, X.J.; Xing, T.T.; Li, Y.F.; Jiao, B.N. Analysis of phytochemical contributors to antioxidant capacity of the peel of Chinese mandarin and orange varieties. Int. J. Food Sci. Nutr. 2019, 70, 825–833. [Google Scholar] [CrossRef]
- Ding, Y.-Q.; Xiong, Y.; Zhou, B.; Deng, M.-Z.; Deng, K.-Z. Isolation and structural identification of flavonoids from Aurantii Fructus. Zhongguo Zhongyao Zazhi 2015, 40, 2352–2356. [Google Scholar] [CrossRef]
- Pinkas, J.; Lavie, D.; Chorin, M. Fungistatic constituents in citrus varieties resistant to the mal-secco disease. Phytochemistry 1968, 7, 169–174. [Google Scholar] [CrossRef]
- Gentili, B.; Horowitz, R.M. Flavonoids of Citrus—VII. Tetrahedron 1964, 20, 2313–2318. [Google Scholar] [CrossRef]
- Han, S.; Kim, H.M.; Lee, J.M.; Mok, S.Y.; Lee, S. Isolation and identification of polymethoxyflavones from the hybrid Citrus, hallabong. J. Agric. Food Chem. 2010, 58, 9488–9491. [Google Scholar] [CrossRef]
- Shin, H.J.; Nam, J.W.; Yoon, U.J.; Han, A.R.; Seo, E.K. Identification of three new flavonoids from the peels of Citrus unshiu. Helv. Chim. Acta 2012, 95, 240–245. [Google Scholar] [CrossRef]
- Rizzi, G.P.; Boeing, S.S. Mass spectral analysis of some naturally occurring polymethoxyflavones. J. Agric. Food Chem. 1984, 32, 551–555. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.Y.; Ho, C.T. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J. Agric. Food Chem. 2006, 54, 4176–4185. [Google Scholar] [CrossRef]
- Vogt, T.; Proksch, P.; Gülz, P.G.; Wollenweber, E. Rare 6- and 8-O-methylated epicuticular flavonols from two Cistus species. Phytochemistry 1987, 26, 1027–1030. [Google Scholar] [CrossRef]
- Uckoo, R.M.; Jayaprakasha, G.K.; Vikram, A.; Patil, B.S. Polymethoxyflavones isolated from the Peel of Miaray mandarin (Citrus miaray) have biofilm inhibitory activity in Vibrio harveyi. J. Agric. Food Chem. 2015, 63, 7180–7189. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, H.; Bao-Jin, Y.; Zhang, P.; Yan, Z. Study on the active constituents of fructus aurantii. J. Chin. Med. Mater. 2008, 32, 15–22. [Google Scholar]
- Mizuno, M.; Matoba, Y.; Tanaka, T.; Tachibana, H.; Iinuma, M.; Iwamasa, M. Two new flavones in Citrus reticulata. J. Nat. Prod. 1987, 50, 751–753. [Google Scholar] [CrossRef]
- Berti, G.; Livi, O.; Segnini, D.; Cavero, I. Determination of constitution and synthesis of a new flavone from Cistus monspeliensis L. Tetrahedron 1967, 23, 2295–2300. [Google Scholar] [CrossRef]
- Eldomiaty, M.; Abdelaal, M.; Elshafae, A. On the chemical, botanical, and chemotaxonomical evaluation of the genus Citrus. Part I. Thyroid. Off. J. Am. Thyroid. Assoc. 1997, 21, 469–470. [Google Scholar]
- Abdel Alim, M.A.; El-Hamouly, W.S.; Aboutabl, E.A.; Hetta, M.H. Polymethoxylated flavones from Citrus reticulata Blanco cuv. Baladi. Egypt. J. Pharm. Sci. 1998, 38, 71–78. [Google Scholar]
- Luo, Y.; Zeng, W.; Huang, K.-E.; Li, D.-X.; Chen, W.; Yu, X.-Q.; Ke, X.-H. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ as well as the Citrus reticulata ‘Chachi’ within different storage years using ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabol. J. Pharm. Biomed. Anal. 2019, 171, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Sarin, P.S.; Eshars, T.R. New components from Citrus aurantium. Tetrahedron 1960, 8, 64–66. [Google Scholar] [CrossRef]
- Chen, J.; Montanari, A.M.; Widmer, W.W. Two new polymethoxylated flavones, a class of compounds with potential anticancer activity, isolated from cold pressed dancy tangerine peel oil solids. J. Agric. Food Chem. 1997, 45, 364–368. [Google Scholar] [CrossRef]
- Johann, S.; Smânia, A.; Pizzolatti, M.G.; Schripsema, J.; Braz-Filho, R.; Branco, A. Complete 1H and 13C NMR assignments and antifungal activity of two 8-hydroxy flavonoids in mixture. An. Acad. Bras. Cienc. 2007, 79, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, W.; Wang, F.; Zhao, K.; Han, Y.; Xu, W.; Tang, L. Effects of Astragaloside IV on heart failure in rats. Chin. Med. 2009, 4, 6. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; Yang, S.; Wang, W.; Zhou, Y. Catalytic wet air oxidation of phenol with pelletized ruthenium catalysts. Appl. Catal. B Environ. 2008, 78, 30–37. [Google Scholar] [CrossRef]
- Zhong, W.-J.; Luo, Y.-J.; Li, J.; Wu, Y.-P.; Gao, Y.-J.; Luo, H.-J.; Yang, Y.-T.; Jiang, L. Polymethoxylated flavonoids from Citrus reticulata Blanco. Biochem. Syst. Ecol. 2016, 68, 11–14. [Google Scholar] [CrossRef]
- Shuji, K.; Fumiak, K. Polymethoxyflavones, their manufacture by extraction, and aqueous organic acid solutions containing them. JP, 2009051738 A, 3 December 2009. [Google Scholar]
- Zhao, X.J.; Xing, T.T.; Li, Y.F.; Jiao, B.N.; Jiang, D. Efficient analysis of phytochemical constituents in the peel of Chinese wild citrus Mangshanju (Citrus reticulata Blanco) by ultra high performance liquid chromatography–quadrupole time-of-flight-mass spectrometry. J. Sep. Sci. 2018, 41, 1947–1959. [Google Scholar] [CrossRef]
- Hamdan, D.; El-Readi, M.Z.; Tahrani, A.; Herrmann, F.; Kaufmann, D.; Farrag, N.; El-Shazly, A.; Wink, M. Chemical composition and biological activity of Citrus jambhiri Lush. Food Chem. 2011, 127, 394–403. [Google Scholar] [CrossRef]
- Li, S.; Pan, M.H.; Lo, C.Y.; Tan, D.; Wang, Y.; Shahidi, F.; Ho, C.T. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J. Funct. Foods 2009, 1, 2–12. [Google Scholar] [CrossRef]
- Lai, C.S.; Wu, J.C.; Ho, C.T.; Pan, M.H. Disease chemopreventive effects and molecular mechanisms of hydroxylated polymethoxyflavones. BioFactors 2015, 41, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Gao, W.; Zeng, S.L.; Li, P.; Liu, E.H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K.; Guthrie, N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr. Med. Chem. 2001, 8, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Owis, A.I. Citrus polymethoxyflavones: Biofunctional molecules of therapeutic interest. Stud. Nat. Prod. Chem. 2018, 59, 509–530. [Google Scholar] [CrossRef]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E.; Nabavi, S.F. Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in Alzheimer’s and Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2017, 16, 387–397. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Ghasemipour Afshar, E. Tangeretin: A mechanistic review of its pharmacological and therapeutic effects. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Guo, L.; Zhao, H.; Ho, C.T. Chemistry and bioactivity of nobiletin and its metabolites. J. Funct. Foods 2014, 6, 2–10. [Google Scholar] [CrossRef]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary Flavonoids: Molecular mechanisms of action as anti- inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef]
- Singh, A.P.; Kandpal, J.B.; Sharma, R.K.; Chitme, H. Nobiletin a biologically active phytoconstituent: Systematic review. J. Biol. Act. Prod. Nat. 2021, 11, 204–211. [Google Scholar] [CrossRef]
- Han Jie, L.; Jantan, I.; Yusoff, S.D.; Jalil, J.; Husain, K. Sinensetin: An insight on its pharmacological activities, mechanisms of action and toxicity. Front. Pharmacol. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Nageen, B.; Rasul, A.; Hussain, G.; Shah, M.A.; Anwar, H.; Syed, M.; Uddin, S.; Riaz, A.; Selamoglu, Z. Jaceosidin: A natural flavone with versatile pharmacological anf biological activities. Curr. Pharm. Des. 2021, 27, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.; Makhmoor, T.; Choudhary, M.I. Radical scavenging potential of compounds isolated from—Vitex agnus-castus. Turk. J. Chem. 2010, 34, 119–126. [Google Scholar] [CrossRef]
- García, B.F.; Torres, A.; Macías, F.A. Synergy and other interactions between polymethoxyflavones from citrus byproducts. Molecules 2015, 20, 20079–20106. [Google Scholar] [CrossRef]
- Karioti, A.; Milošević-Ifantis, T.; Pachopos, N.; Niryiannaki, N.; Hadjipavlou-Litina, D.; Skaltsa, H. Antioxidant, anti-inflammatory potential and chemical constituents of Origanum dubium Boiss., growing wild in Cyprus. J. Enzym. Inhib. Med. Chem. 2015, 30, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Han, J.M.; Jin, Y.Y.; Baek, N.I.; Bang, M.H.; Chung, H.G.; Choi, M.S.; Lee, K.T.; Sok, D.E.; Jeong, T.S. In vitro antioxidant and anti-inflammatory activities of jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch. Pharm. Res. 2008, 31, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Tapia, A.; Rodriguez, J.; Theoduloz, C.; Lopez, S.; Feresin, G.E.; Schmeda-Hirschmann, G. Free radical scavengers and antioxidants from Baccharis grisebachii. J. Ethnopharmacol. 2004, 95, 155–161. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Kitahara, K.; Suganuma, T.; Hashimoto, F.; Tadera, K. Antioxidant and α-amylase inhibitory compounds from aerial parts of Varthemia iphionoides Boiss. Biosci. Biotechnol. Biochem. 2006, 70, 2178–2184. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.T.; Lee, S.H.; Jang, H.D.; Kim, Y.H. Anti-osteoporotic and antioxidant activities by rhizomes of Kaempferia parviflora Wall. Ex Baker. Nat. Prod. Sci. 2016, 22, 13–19. [Google Scholar] [CrossRef]
- Cheng, L.Y.; Liao, H.R.; Chen, L.C.; Wang, S.W.; Kuo, Y.H.; Chung, M.I.; Chen, J.J. Naphthofuranone derivatives and other constituents from Pachira aquatica with inhibitory activity on superoxide anion generation by neutrophils. Fitoterapia 2017, 117, 16–21. [Google Scholar] [CrossRef]
- Kanashiro, A.; Kabeya, L.M.; Polizello, A.C.M.; Lopes, N.P.; Lopes, J.L.C.; Lucisano-Valim, Y.M. Inhibitory activity of flavonoids from lychnophora sp. on generation of reactive oxygen species by neutrophils upon stimulation by immune complexes. Phyther. Res. 2004, 18, 61–65. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Lee, H.H.; Shin, J.S.; Yoo, H.S.; Park, J.S.; Son, S.H.; Kim, S.W.; Yu, J.; Lee, J.; Lee, K.T.; et al. Identification and structure activity relationship of novel flavone derivatives that inhibit the production of nitric oxide and PGE2 in LPS-induced RAW 264.7 cells. Bioorganic Med. Chem. Lett. 2017, 27, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Yang, Y.; Yu, M.; Han, Z.Z.; Wei, M.; Zhang, H.W.; Jia, H.M.; Zou, Z.M. Anti-inflammatory chemical constituents of: Flos Chrysanthemi Indici determined by UPLC-MS/MS integrated with network pharmacology. Food Funct. 2020, 11, 6340–6351. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, H.H.; Jeong, J.W.; Seo, M.J.; Kang, B.W.; Park, J.U.; Kim, K.S.; Cho, Y.S.; Seo, K.-I.; Kim, G.Y. Anti-inflammatory effects of 5-hydroxy-3,6,7,8,3′,4’- hexamethoxyflavone via NF-κB inactivation in lipopolysaccharide-stimulated RAW 264.7 macrophage. Mol. Med. Rep. 2014, 9, 1197–1203. [Google Scholar] [CrossRef]
- Maldonado-Rojas, W.; Olivero-Verbel, J. Potential interaction of natural dietary bioactive compounds with COX-2. J. Mol. Graph. Model. 2011, 30, 157–166. [Google Scholar] [CrossRef]
- Silva, D.H.S.; Zhang, Y.; Santos, L.A.; Bolzani, V.S.; Nair, M.G. Lipoperoxidation and cyclooxygenases 1 and 2 Inhibitory Compounds from Iryanthera juruensis. J. Agric. Food Chem. 2007, 55, 2569–2574. [Google Scholar] [CrossRef]
- Moscatelli, V.; Hnatyszyn, O.; Acevedo, C.; Megías, J.; Alcaraz, M.J.; Ferraro, G. Flavonoids from Artemisia copa with anti-inflammatory activity. Planta Med. 2006, 72, 72–74. [Google Scholar] [CrossRef]
- Jang, J.; Hyun, P.K.; Park, H. Structure and antiinflammatory activity relationships of wogonin derivatives. Arch. Pharm. Res. 2005, 28, 877–884. [Google Scholar] [CrossRef]
- Alcaraz, M.J.; Ferrandiz, M.L.; Villar, A. Flavonoid inhibition of soybean lipoxygenase. Pharmazie 1986, 41, 299–300. [Google Scholar]
- Chan, A.L.F.; Huang, H.L.; Chien, H.C.; Chen, C.M.; Lin, C.N.; Ko, W.C. Inhibitory effects of quercetin derivatives on phosphodiesterase isozymes and high-affinity [3H]-rolipram binding in guinea pig tissues. Investig. New Drugs 2008, 26, 417–424. [Google Scholar] [CrossRef]
- Sae-Wong, C.; Matsuda, H.; Tewtrakul, S.; Tansakul, P.; Nakamura, S.; Nomura, Y.; Yoshikawa, M. Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. J. Ethnopharmacol. 2011, 136, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, P.A.; Stephenson, K.K.; Wade, K.L.; Liu, H.; Fahey, J.W. Structure-activity analysis of flavonoids: Direct and indirect antioxidant, and antiinflammatory potencies and toxicities. Nutr. Cancer 2013, 65, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Drzewiecki, G.; Tatum, J. The effects of citrus flavonoids on human basophil and neutrophil function. Planta Med. 1987, 53, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Park, H.I.; Kim, H.-P. Inhibition of collagenase by anti-inflammatory synthetic flavones. J. Appl. Pharmacol. 2006, 14, 36–39. [Google Scholar]
- Jin, M.R.; Xu, H.; Duan, C.H.; Chou, G.X. Two new flavones from Salvia plebeia. Nat. Prod. Res. 2015, 29, 1315–1322. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Karalai, C.; Ponglimanont, C.; Cheenpracha, S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem. 2009, 115, 534–538. [Google Scholar] [CrossRef]
- Puripattanavong, J.; Tewtrakul, S. Anti-allergic and anti-inflammatory compounds from Aglaia andamanica leaves. Songklanakarin J. Sci. Technol. 2015, 37, 37–41. [Google Scholar]
- Bas, E.; Recio, M.C.; Giner, R.M.; Manez, S.; Lopez-Gines, C.; Gil-Benso, R.; Rios, J.L. Demethylnobiletin inhibits delayed-type hypersensitivity reactions, human lymphocyte proliferation and cytokine production. Br. J. Pharmacol. 2007, 152, 1272–1282. [Google Scholar] [CrossRef]
- Ramırez Apan, A.A.M.T.; Perez-Castorena, A.L.; Romo de Vivar, A. Anti-inflammatory constituents of Mortonia greggii Gray. Z. Naturforsch. 2004, 59, 237–243. [Google Scholar]
- Bai, N.; He, K.; Zhou, Z.; Lai, C.S.; Zhang, L.; Quan, Z.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids from Rabdosia rubescens exert anti-inflammatory and growth inhibitory effect against human leukemia HL-60 cells. Food Chem. 2010, 122, 831–835. [Google Scholar] [CrossRef]
- Pandith, H.; Zhang, X.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W.; Baek, S.J. Effect of Siam weed extract and its bioactive component scutellarein tetramethyl ether on anti-inflammatory activity through NF-κB pathway. J. Ethnopharmacol. 2013, 147, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wu, X.; Zheng, J.; Charoensinphon, N.; Dong, P.; Qiu, P.; Song, M.; Tang, Z.; Xiao, H. Anti-inflammatory effect of xanthomicrol, a major colonic metabolite of 5-demethyltangeretin. Food Funct. 2018, 9, 3104–3113. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sang, S.; Pan, M.H.; Lai, C.S.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorganic Med. Chem. Lett. 2007, 17, 5177–5181. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, M.; Rakariyatham, K.; Zheng, J.; Wang, M.; Xu, F.; Gao, Z.; Xiao, H. Inhibitory effects of 4′ -demethylnobiletin, a metabolite of nobiletin, on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mouse ears. J. Agric. Food Chem. 2015, 63, 10921–10927. [Google Scholar] [CrossRef]
- Nam, Y.; Choi, M.; Hwang, H.; Lee, M.G.; Kwon, B.M.; Lee, W.H.; Suk, K. Natural flavone jaceosidin is a neuroinflammation inhibitor. Phyther. Res. 2013, 27, 404–411. [Google Scholar] [CrossRef]
- Kang, C.H.; Kim, M.J.; Seo, M.J.; Choi, Y.H.; Jo, W.S.; Lee, K.T.; Jeong, Y.K.; Kim, G.Y. 5-Hydroxy-3,6,7,8,3’4’-hexamethoxyflavone inhibits nitric oxide production in lipopolysaccharide-stimulated BV2 microglia via NF-κB suppression and Nrf-2-dependent heme oxygenase-1 induction. Food Chem. Toxicol. 2013, 57, 119–125. [Google Scholar] [CrossRef]
- Soo, J.K.; Park, H.; Hyun, P.K. Inhibition of nitric oxide production from lipopolysaccharide-treated RAW 264.7 cells by synthetic flavones:structure-activity relationship and action mechanism. Arch. Pharm. Res. 2004, 27, 937–943. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.L.; Yu, K.Y.; Guo, L.; Bai-Zhong, C.; Li, P.; Liu, E.H. Polymethoxyflavones in peel of Citrus reticulata ‘Chachi’ and their biological activities. Food Chem. 2017, 234, 254–261. [Google Scholar] [CrossRef]
- Yuasa, K.; Tada, K.; Harita, G.; Fujimoto, T.; Tsukayama, M.; Tsuji, A. Sudachitin, a polymethoxyflavone from Citrus sudachi, suppresses lipopolysaccharide-induced inflammatory responses in mouse macrophage-like RAW264 cells. Biosci. Biotechnol. Biochem. 2012, 76, 598–600. [Google Scholar] [CrossRef]
- Wu, J.; Liu, K.; Shi, X. The anti-inflammatory activity of several flavonoids isolated from Murraya paniculata on murine macrophage cell line and gastric epithelial cell (GES-1). Pharm. Biol. 2016, 54, 868–881. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Yang, L.; Wei, J.; Huang, M.; Jiang, J.G. Bioactivity evaluations of ingredients extracted from the flowers of Citrus aurantium L. varamara Engl. Food Chem. 2012, 135, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Y.; Wang, H.Y.; Li, G.Y.; Du, X.W.; Zhang, X.T.; Han, Y.; Huang, J.; Li, X.X.; Wang, J.H. Constituents from Zhuyeqing Liquor and their inhibitory effects on nitric oxide production. Phytochem. Lett. 2014, 7, 150–155. [Google Scholar] [CrossRef]
- Guo, S.; Wu, X.; Zheng, J.; Smith, S.A.; Dong, P.; Xiao, H. Identification of 4′-demethyltangeretin as a Major urinary metabolite of tangeretin in mice and its anti-inflammatory activities. J. Agric. Food Chem. 2021, 69, 4381–4391. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.M.; Jo, Y.J.; Kim, J.E.; An, H.J.; Choi, Y.H.; Hyun, C.G.; Lee, N.H. Tetramethyl-O-scutellarin isolated from peels of immature shiranuhi fruit exhibits anti-inflammatory effects on LPS-induced RAW264.7 cells. Trop. J. Pharm. Res. 2017, 16, 2197–2205. [Google Scholar] [CrossRef]
- Jin, S.; Lee, M.Y. Kaempferia parviflora extract as a potential anti-acne agent with anti-inflammatory, sebostatic and anti-propionibacterium acnes activity. Int. J. Mol. Sci. 2018, 19, 3457. [Google Scholar] [CrossRef]
- Khan, M.S.; Halagowder, D.; Devaraj, S.N. Methylated chrysin induces co-ordinated attenuation of the canonical Wnt and NF-kB signaling pathway and upregulates apoptotic gene expression in the early hepatocarcinogenesis rat model. Chem. Biol. Interact. 2011, 193, 12–21. [Google Scholar] [CrossRef]
- Weng, Z.; Patel, A.B.; Panagiotidou, S.; Theoharides, T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015, 135, 1044–1052. [Google Scholar] [CrossRef]
- Patel, A.B.; Theoharides, T.C. Methoxyluteolin inhibits neuropeptide-stimulated proinflammatory mediator release via mTOR activation from human mast cells. J. Pharmacol. Exp. Ther. 2017, 361, 462–471. [Google Scholar] [CrossRef]
- Taracanova, A.; Tsilioni, I.; Conti, P.; Norwitz, E.R.; Leeman, S.E.; Theoharides, T.C. Substance P and IL-33 administered together stimulate a marked secretion of IL-1β from human mast cells, inhibited by methoxyluteolin. Proc. Natl. Acad. Sci. USA 2018, 115, E9381–E9390. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Zhang, G.; Xiao, H. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol. Nutr. Food Res. 2015, 59, 2383–2394. [Google Scholar] [CrossRef]
- Hu, Y.H.; Liu, J.; Li, H.; Tang, W.; Li, X.W.; Guo, Y.W. Chemical constituents from Citrus changshan-huyou and their anti-inflammatory activities. Chem. Biodivers. 2020, 17, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Horigome, S.; Yoshida, I.; Ito, S.; Inohana, S.; Fushimi, K.; Nagai, T.; Yamaguchi, A.; Fujita, K.; Satoyama, T.; Katsuda, S.; et al. Inhibitory effects of Kaempferia parviflora extract on monocyte adhesion and cellular reactive oxygen species production in human umbilical vein endothelial cells. Eur. J. Nutr. 2017, 56, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Vinh, L.B.; Jo, S.J.; Nguyen Viet, P.; Gao, D.; Cho, K.W.; Koh, E.J.; Park, S.S.; Kim, Y.R.; Kim, Y.H.; Yang, S.Y. The chemical constituents of ethanolic extract from Stauntonia hexaphylla leaves and their anti-inflammatory effects. Nat. Prod. Res. 2021, 35, 1852–1855. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, Y.; Zhou, Z.D.Z.; Zhu, P.; Luo, Y.L.L.Z.X. Bioassay-guided isolation of anti-inflammatory diterpenoids with highly oxygenated substituents from kidney tea (Clerodendranthus spicatus). J. Food Biochem. 2020, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leu, W.J.; Chen, J.C.; Guh, J.H. Extract from Plectranthus amboinicus inhibit maturation and release of interleukin 1β through inhibition of NF-κB nuclear translocation and NLRP3 inflammasome activation. Front. Pharmacol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Jie, X.; Liang, X.; Chen, Z.; Xie, P.; Pan, X.; Zhou, B.; Li, J. Sinensetin suppresses influenza a virus-triggered inflammation through inhibition of NF-κB and MAPKs signalings. BMC Complement. Med. Ther. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- During, A.; Larondelle, Y. The O-methylation of chrysin markedly improves its intestinal anti-inflammatory properties: Structure-activity relationships of flavones. Biochem. Pharmacol. 2013, 86, 1739–1746. [Google Scholar] [CrossRef]
- Ongchai, S.; Chiranthanut, N.; Tangyuenyong, S.; Viriyakhasem, N.; Kongdang, P. Kaempferia parviflora extract alleviated rat arthritis, exerted chondroprotective properties in vitro, and reduced expression of genes associated with inflammatory arthritis. Molecules 2021, 26, 1527. [Google Scholar] [CrossRef]
- Lin, C.F.; Leu, Y.L.; Al-Suwayeh, S.A.; Ku, M.C.; Hwang, T.L.; Fang, J.Y. Anti-inflammatory activity and percutaneous absorption of quercetin and its polymethoxylated compound and glycosides: The relationships to chemical structures. Eur. J. Pharm. Sci. 2012, 47, 857–864. [Google Scholar] [CrossRef]
- Lai, C.S.; Li, S.; Chai, C.Y.; Lo, C.Y.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Inhibitory effect of citrus 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mice. Carcinogenesis 2007, 28, 2581–2588. [Google Scholar] [CrossRef]
- Kongdang, P.; Jaitham, R.; Thonghoi, S.; Kuensaen, C.; Pradit, W.; Ongchai, S. Ethanolic extract of Kaempferia parviflora interrupts the mechanisms-associated rheumatoid arthritis in SW982 culture model via p38/STAT1 and STAT3 pathways. Phytomedicine 2019, 59, 152755. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Chen, S.Y.; Ho, C.Y.; Yen, G.C. Citrus flavonoids suppress IL-5 and ROS through distinct pathways in PMA/ionomycin-induced EL-4 cells. Food Funct. 2020, 11, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateff, A.; Alarif, W.M.; Algandaby, M.M.; Alburae, N.A.; Abdel-Naim, A.B. Euryops arabicus displays anti-inflammatory activities in experimental models. J. Ethnopharmacol. 2020, 247, 112278. [Google Scholar] [CrossRef]
- Horigome, S.; Yoshida, I.; Tsuda, A.; Harada, T.; Yamaguchi, A.; Yamazaki, K.; Inohana, S.; Isagawa, S.; Kibune, N.; Satoyama, T. Identification and evaluation of anti-inflammatory compounds from Kaempferia parviflora. Biosci. Biotechnol. Biochem. 2014, 78, 851–860. [Google Scholar] [CrossRef]
- Matsui, T.; Ito, C.; Itoigawa, M.; Okada, T.; Furukawa, H. Effect of natsudaidain isolated from Citrus plants on TNFα and cyclooxygenase-2 expression in RBL-2H3 cells. J. Pharm. Pharmacol. 2008, 61, 109–114. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S. Effects of compounds from Kaempferia parviflora on nitric oxide, prostaglandin E2 and tumor necrosis factor-alpha productions in RAW264.7 macrophage cells. J. Ethnopharmacol. 2008, 120, 81–84. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, W.; Ji, S.; Cao, J.; Sun, C. Three polymethoxyflavones purified from ougan (Citrus reticulata Cv. suavissima) inhibited LPS-induced NO elevation in the neuroglia BV-2 cell line via the JAK2/STAT3 pathway. Nutrients 2019, 11, 791. [Google Scholar] [CrossRef]
- Adhikari-Devkota, A.; Kurauchi, Y.; Yamada, T.; Katsuki, H.; Watanabe, T.; Devkota, H.P. Anti-neuroinflammatory activities of extract and polymethoxyflavonoids from immature fruit peels of Citrus ‘Hebesu’. J. Food Biochem. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- Tran, T.V.A.; Malainer, C.; Schwaiger, S.; Atanasov, A.G.; Heiss, E.H.; Dirsch, V.M.; Stuppner, H. NF-κB inhibitors from Eurycoma Longifolia. J. Nat. Prod. 2014, 77, 483–488. [Google Scholar] [CrossRef]
- Yang, T.; Feng, C.; Wang, D.; Qu, Y.; Yang, Y.; Wang, Y.; Sun, Z. Neuroprotective and anti-inflammatory effect of tangeretin against cerebral ischemia-reperfusion injury in rats. Inflammation 2020, 43, 2332–2343. [Google Scholar] [CrossRef]
- Zhi, Z.; Tang, X.; Wang, Y.; Chen, R.; Ji, H. Sinensetin attenuates amyloid Beta 25-35-induced oxidative stress, inflammation, and apoptosis in SH-SY5Y cells through the TLR4/NF-κB signaling pathway. Neurochem. Res. 2021, 46, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jang, D.; Jeon, J.; Cho, C.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Hum, C.; Yu, P. Seomae mugwort and jaceosidin attenuate osteoarthritic cartilage damage by blocking IκB degradation in mice. J. Cell. Mol. Med. 2020, 46, 8126–8137. [Google Scholar] [CrossRef] [PubMed]
- Clavin, M.; Gorzalczany, S.; Macho, A.; Mu, E. Anti-inflammatory activity of flavonoids from Eupatorium arnottianum. J. Ethnopharmacol. 2007, 112, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Sirat, H.M.; Jamaludin, F.; Musa, N.; Ali, R.M.; Arbain, D.; Aboul-enein, H.Y. Antimicrobial and anti-inflammatory activities of Piper porphyrophyllum (Fam. Piperaceae). Arab. J. Chem. 2014, 7, 1031–1033. [Google Scholar] [CrossRef]
- Panthong, A.; Tassaneeyakul, W.; Kanjanapothi, D.; Tantiwac, P.; Reutrakul, V. Anti-inflammatory activity of 5, 7-dimethoxyflavone. Planta Med. 1989, 55, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Panthong, A.; Kanjanapothp, D.; Tuntiwachwutiikul, P.; Pancharoen, O.; Reutrakul, V. Antiinflammatory activity of flavonoids. Phytomedicine 1994, 1, 141–144. [Google Scholar] [CrossRef]
- Pelzer, L.E.; Guardia, T.; Juarez, O.; Guerreiro, E. Acute and chronic antiinflammatory effects of plant flavonoids. Il Farm. 1998, 53, 421–424. [Google Scholar] [CrossRef]
- Pandurangan, K.; Krishnappan, V. Anti-inflammatory effect of certain dimethoxy flavones. Inflammopharmacology 2015, 23, 307–317. [Google Scholar] [CrossRef]
- Reddy, G.B.S.; Udupa, A.I.; Shirwaikar, A.; Srinivasa Aithai, K.; Srinivasa, K.K. Comparison of the anti-inflammatory activity of certain methoxyflavonoids. Fitoterapia 1990, 61, 460–461. [Google Scholar]
- Chou, Y.; Lin, Y.; Lin, P.; Tung, Y.; Ho, C.; Pan, M. Dietary 5-demethylnobiletin modulates xenobiotic-metabolizing enzymes and ameliorates colon carcinogenesis in benzo-[a]-pyrene-induced mice. Food Chem. Toxicol. 2021, 155, 112380. [Google Scholar] [CrossRef]
- Chang, S.N.; Kim, S.H.; Dey, D.K.; Park, S.M.; Nasif, O.; Bajpai, V.K.; Kang, S.C.; Lee, J.; Park, J.G. 5-O-Demethylnobiletin alleviates CCl4 -induced acute liver injury by equilibrating ROS-mediated apoptosis and autophagy induction. Mol. Sci. 2021, 22, 1083. [Google Scholar] [CrossRef]
- Chen, B.; Luo, J.; Han, Y.; Du, H.; Liu, J.; He, W.; Zhu, J.; Xiao, J.; Wang, J.; Cao, Y. Dietary tangeretin alleviated dextran sulfate sodium-induced colitis in mice via inhibiting in fl ammatory response, restoring intestinal barrier function, and modulating gut microbiota. J. Agric. Food Chem. 2021, 69, 7663–7674. [Google Scholar] [CrossRef]
- Kang, M.; Kim, S.; Oh, S.Y.; Na, W.; Kang, Y. Tangeretin ameliorates glucose-induced podocyte injury through blocking epithelial to mesenchymal transition caused by oxidative stress and hypoxia. J. Mol. Sci. 2020, 21, 8577. [Google Scholar] [CrossRef]
- Ohyama, Y.; Ito, J.; Kitano, V.J.; Shimada, J.; Hakeda, Y. The polymethoxy flavonoid sudachitin suppresses inflammatory bone destruction by directly inhibiting osteoclastogenesis due to reduced ROS production and MAPK activation in osteoclast precursors. PLoS ONE 2018, 13, 1–17. [Google Scholar] [CrossRef]
- Min, S.W.; Kim, N.J.; Baek, N.I.; Kim, D.H. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J. Ethnopharmacol. 2009, 125, 497–500. [Google Scholar] [CrossRef]
- Schinella, G.R.; Giner, R.M.; Del Carmen Recio, M.; De Buschiazzo, P.M.; Ríos, J.L.; Máñez, S. Anti-inflammatory effects of South American Tanacetum vulgare. J. Pharm. Pharmacol. 1998, 50, 1069–1074. [Google Scholar] [CrossRef]
- Küpeli, E.; Şahin, F.P.; Yeşilada, E.; Çaliş, I.; Ezer, N. In vivo anti-inflammatory and antinociceptive activity evaluation of phenolic compounds from Sideritis stricta. Z. Naturforsch. Sect. C J. Biosci. 2007, 62, 519–525. [Google Scholar] [CrossRef]
- Manthey, J.A.; Bendele, P. Anti-inflammatory activity of an orange peel polymethoxylated flavone, 3′,4′,3,5,6,7,8-heptamethoxyflavone, in the rat carrageenan/paw edema and mouse lipopolysaccharide-challenge assays. J. Agric. Food Chem. 2008, 56, 9399–9403. [Google Scholar] [CrossRef]
- Apaza, T.L.; Serban, A.M.; Cabanillas, A.H.; Villacampa, A.; Rumbero, A. Flavonoids of Tripodanthus acutifolius inhibit TNF–α production in LPS–activated THP–1 and B16–F10 cells. J. Ethnopharmacol. 2019, 242, 112036. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Zhi, W.; Zhang, H.; He, Z.; Wang, Y.; Liu, F.; Niu, X.; Zhang, X. The gastroprotective effect of nobiletin against ethanol-induced acute gastric lesions in mice: Impact on oxidative stress and inflammation. Immunopharmacol. Immunotoxicol. 2017, 39, 354–363. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Rakariyatham, K.; Zheng, J.; Guo, S.; Tang, Z.; Zhou, S.; Xiao, H. Anti-inflammatory effects of 4’-demethylnobiletin, a major metabolite of nobiletin. J. Funct. Foods 2015, 19, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Bei, D.; An, G. Pharmacokinetics and tissue distribution of 5,7-dimethoxyflavone in mice following single dose oral administration. J. Pharm. Biomed. Anal. 2016, 119, 65–70. [Google Scholar] [CrossRef]
- Ji, H.Y.; Kim, S.Y.; Kim, D.K.; Jeong, J.H.; Lee, H.S. Effects of eupatilin and jaceosidin on cytochrome P450 enzyme activities in human liver microsomes. Molecules 2010, 15, 6466–6474. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Ji, H.Y.; Baek, N.I.; Jeong, T.S.; Lee, H.S. In Vitro metabolism of jaceosidin and characterization of cytochrome P450 and UDP-glucuronosyltransferase enzymes in human liver microsomes. Arch. Pharm. Res. 2010, 33, 1985–1996. [Google Scholar] [CrossRef]
- Wei, G.J.; Hwang, L.S.; Tsai, C.L. Absolute bioavailability, pharmacokinetics and excretion of 5,7,3’,4’-tetramethoxyflavone in rats. J. Funct. Foods 2014, 7, 136–141. [Google Scholar] [CrossRef]
- Lu, W.; Sheen, J.; Hwang, L.S.; Wei, G. Identification of 5,7,3′,4′-tetramethoxy flavone metabolites in rat urine by the isotope-labeling method and ultrahigh-performance liquid chromatography—Electrospray ionization—Mass spectrometry. J. Agric. Food Chem. 2012, 60, 8123–8128. [Google Scholar] [CrossRef]
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Sawamoto, A.; Nakajima, M.; Yoshimura, M.; Igase, M.; Fukuda, N.; Tamai, T.; Yoshida, T. Isolation and characterization of neuroprotective components from citrus peel and their application as functional food. Chem. Pharm. Bull. 2021, 69, 2–10. [Google Scholar] [CrossRef]
- Moon, B.H.; Lee, Y.; Ahn, J.H.; Lim, Y. Complete assignment of 1H and 13C NMR data of dihydroxyflavone derivatives. Magn. Reson. Chem. 2006, 44, 99–101. [Google Scholar] [CrossRef]
- Lee, S.; Moon, B.H.; Park, Y.; Lee, E.; Hong, S.; Lim, Y. Methyl substitution effects on 1H and 13C NMR data of methoxyflavones. Bull. Korean Chem. Soc. 2008, 29, 1793–1796. [Google Scholar] [CrossRef]
- Mayer, R. A β-hydroxychalcone from Leptospermum scoparium. Planta Med. 1993, 59, 269–271. [Google Scholar] [CrossRef]
- Das, B.; Chakravarty, A.K. Three flavone glycosides from Gelonium multiflorum. Phytochemistry 1993, 33, 493–496. [Google Scholar] [CrossRef]
- Horie, T.; Ohtsuru, Y.; Shibata, K.; Yamashita, K.; Tsukayama, M.; Kawamura, Y. 13C NMR spectral assignment of the A-ring of polyoxygenated flavones. Phytochemistry 1998, 47, 865–874. [Google Scholar] [CrossRef]
- Hajdú, Z.; Martins, A.; Orbán-Gyapai, O.; Forgo, P.; Jedlinszki, N.; Máthé, I.; Hohmann, J. Xanthine oxidase-inhibitory activity and antioxidant properties of the methanol extract and flavonoids of Artemisia asiatica. Rec. Nat. Prod. 2014, 8, 299–302. [Google Scholar]
- Bovicelli, P.; D’Angelo, V.; Collalto, D.; Verzina, A.; D’Antona, N.; Lambusta, D. Efficient synthesis of polyoxygenated flavones from naturally occurring flavanones. J. Pharm. Pharmacol. 2007, 59, 1697–1701. [Google Scholar] [CrossRef]
- Wenkert, E.; Gottlieb, H.E. Carbon-13 nuclear magnetic resonance spectroscopy of flavonoid and isoflavonoid compounds. Phytochemistry 1977, 16, 1811–1816. [Google Scholar] [CrossRef]
- Chari, V.M.; Ilyas, M.; Wagner, H.; Neszmélyi, A.; Fa-Ching, C.; Li-Kuang, C.; Yu-Chin, L.; Yu-Meei, L. 13C-NMR spectroscopy of biflavanoids. Phytochemistry 1977, 16, 1273–1278. [Google Scholar] [CrossRef]
- Lee, J.I.; Son, H.S.; Park, H. An efficient synthesis of flavones from 2-hydroxybenzoic acids. Bull. Korean Chem. Soc. 2004, 25, 1945–1947. [Google Scholar] [CrossRef]
- Wollenweber, E.; Dörr, M.; Rivera, D.; Roitman, J.N. Externally accumulated flavonoids in three mediterranean ononis species. Z. Naturforsch. Sect. C J. Biosci. 2003, 58, 771–775. [Google Scholar] [CrossRef]
- Tominaga, H.; Horie, T. Studies of the selective o-alkylation and dealkylation of flavonoids. XV. A convenient synthesis of 3,5,6-trihydroxy-7-methoxyflavones and revised structures of two natural flavones. Bull. Chem. Soc. Jpn. 1993, 66, 2668–2675. [Google Scholar] [CrossRef]
- Facundo, V.A.; Morais, S.M.; Braz Filho, R. Constituintes químicos de Ottonia corcovadensis Miq. da floresta Amazônica: Atribuição dos deslocamentos químicos dos átomos de hidrogênio e carbono. Quim. Nova 2004, 27, 79–83. [Google Scholar] [CrossRef]
- Yoshida, M.; Fujino, Y.; Doi, T. Synthesis of γ-benzopyranone by TfOH-promoted regioselective cyclization of O-alkynoylphenols. Org. Lett. 2011, 13, 4526–4529. [Google Scholar] [CrossRef]
- Akkal, S.; Benayache, F.; Bentamene, A.; Medjroubi, K.; Seguin, E.; Tillequin, F. Flavonoid Aglycones from Centaurea napifolia. Chem. Nat. Compd. 2003, 39, 219–220. [Google Scholar] [CrossRef]
- Nawasreh, M.; Zarga, M.A.; Sabri, S.S. New anthranilic acid derivatives from Ononis natrix of Jordanian origin. Alex. J. Pharm. Sci. 2004, 18, 165–170. [Google Scholar]
- Datta, B.K.; Datta, S.K.; Rashid, M.A.; Nash, R.J.; Sarker, S.D. A sesquiterpene acid and flavonoids from Polygonum viscosum. Phytochemistry 2000, 54, 201–205. [Google Scholar] [CrossRef]
- Dong, H.; Gou, Y.L.; Cao, S.G.; Chen, S.X.; Sim, K.Y.; Goh, S.H.; Kini, R.M. Eicosenones and methylated flavonols from Amomum koenigii. Phytochemistry 1999, 50, 899–902. [Google Scholar] [CrossRef]
- Machida, K.; Osawa, K. On the flavonoid constituents from the peels of Citrus hassaku Hort. ex. Tanaka. Chem. Pharm. Bull. 1989, 37, 1092–1094. [Google Scholar] [CrossRef]
- Wallet, J.-C.; Gaydou, E.M. 13C NMR spectra of a series of 2’,3’,4’-trimetboxyíiavones. Reinvestigation of signal assignment in 13C NMR spectra of flavones. Ref. Data 1993, 31, 518–519. [Google Scholar]
- Rwangabo, P.C.; Claeys, M.; Pieters, L.; Corthout, J.; Vanden Berghe, D.A.; Vlietinck, A.J. Umuhengerin, a new antimicrobially active flavonoid from Lantana trifolia. J. Nat. Prod. 1988, 51, 966–968. [Google Scholar] [CrossRef]
- Sugiyama, S.; Umehara, K.; Kuroyanagi, M.; Ueno, A.; Taki, T. Studies on the differentiation inducers of myeloid leukemic cells from Citrus species. Chem. Pharm. Bull. 1993, 41, 714–719. [Google Scholar] [CrossRef]
- Okuno, Y.; Miyazawa, M. Biotransformation of nobiletin by Aspergillus niger and the antimutagenic activity of a metabolite, 4′-hydroxy-5,6,7,8,3′-pentamethoxyflavone. J. Nat. Prod. 2004, 67, 1876–1878. [Google Scholar] [CrossRef]
- Ferracin, R.J.; Silva, M.; Fernandes, J.B.; Vieira, P.C. Flavonoids from the fruits of Murraya paniculata. Phytochemistry 1998, 41, 393–396. [Google Scholar] [CrossRef]
- Sukari, M.A.; Azziz, S.S.S.A.; Rahmani, M.; Ali, A.M.; Aimi, N.; Kitajima, M. Polysubstituted flavonoids from the leaves of Murraya paniculata (Rutaceae). Nat. Prod. Sci. 2003, 9, 56–59. [Google Scholar]
- Wang, Q.; Wu, Z.; Liu, L.; Zou, L.; Luo, M. Synthesis of citrus bioactive polymethoxyflavonoids and flavonoid glucosides. Chin. J. Org. Chem. 2010, 30, 1682–1688. [Google Scholar]
- Chen, J.; Montanari, A.M. Isolation and identification of new polymethoxyflavonoids from Dancy tangerine leaves. J. Agric. Food Chem. 1998, 46, 1235–1238. [Google Scholar] [CrossRef]
- Baek, J.; Lee, H.L.; Kang, K.S.; Kim, K.H. Chemical constituents from the fruit of Citrus unshiu and their inhibitory effects on acetylcholinesterase. Maced. J. Chem. Chem. Eng. 2017, 36, 1–8. [Google Scholar] [CrossRef]
- Sawabe, A.; Matsubara, Y. Bioactive glycosides in citrus fruit peels. Stud. Plant Sci. 1999, 6, 261–274. [Google Scholar] [CrossRef]
- Horie, T.; Tsukayama, M.; Yamada, T.; Miura, I.; Nakayama, M. Three flavone glycosides from Citrus sudachi. Phytochemistry 1986, 25, 2621–2624. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The effect of natural antioxidants in the development of metabolic syndrome: Focus on bergamot polyphenolic fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef]
- Mollace, V.; Rosano, G.M.C.; Anker, S.D.; Coats, A.J.S.; Seferovic, P.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; et al. Pathophysiological basis for nutraceutical supplementation in heart failure: A comprehensive review. Nutrients 2021, 13, 257. [Google Scholar] [CrossRef]
- Nobile, V.; Pisati, M.; Cestone, E.; Insolia, V.; Zaccaria, V.; Malfa, G.A. Antioxidant efficacy of a standardized red orange (Citrus sinensis (L.) Osbeck) extract in elderly subjects: A randomized, double blind, controlled study. Nutrients 2022, 14, 4235. [Google Scholar] [CrossRef]
- PubChem. Nobiletin. 2005. Available online: https://pubchem.ncbi.nlm.nih.gov/#query=nobiletin (accessed on 14 December 2022).
- European Chemicals Agency. Summary of Classification and Labelling. 2021. Available online: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/236961 (accessed on 13 December 2022).





| Compound | Type of Test | Result (μM) | References |
|---|---|---|---|
| 30 | DPPH (% inhib.) | 24.27 at 1000 μM | [64] |
| 35 | 11.44 at 25 μM | [65] | |
| 26, 44, 45, 48, 52, 63, 71 | 2–6 at 25 μM | ||
| 20 | 3.5 at 100 μM | [66] | |
| 9 | 20 at 100 μM | [67] | |
| 10 | 25 at 300 μM | [68] | |
| 34 | 0 at 300 μM | ||
| 59 | 10 at 500 μM | [69] | |
| 43 | DPPH (IC50 mg/mL) | 0.06 | [70] |
| 67 | 0.736 | [51] | |
| 52 | 0.752 | ||
| 63 | 3.56 | ||
| 9 | 1.15 | [68] | |
| 9 | Inhibition % of lipoperoxidation in erythrocytes | 71 at 300 μM | [68] |
| 10 | 64 at 300 μM | ||
| 34 | 28 at 300 μM | ||
| 5 | Reducing capacity Copper(I) ions μM (CUPRAC) | 0.08 at 10 μM | [71] |
| 12 | 0.29 at 1 μM | ||
| 14 | 0.17 at 10 μM | ||
| 33 | 1.05 at 10 μM | ||
| 43 | 0 at 10 μM | ||
| 5 | Peroxyl radical-scavenging capacity (ORAC) µM of Trolox equivalent | 1.26 at 10 μM | |
| 12 | 1.11 at 10 μM | ||
| 14 | 1.51 at 10 μM | ||
| 33 | 2.66 at 10 μM | ||
| 43 | 0.79 at 10 μM | ||
| 10 | Superoxide anion scavenging (%) | 60 at 150 μM | [68] |
| 34 | 29 at 150 μM | ||
| 31 | Superoxide anion scavenging (IC50) | >100 μM | [72] |
| 50 | 28.8 μM | ||
| 9 | % LDL-oxidation inhibition (TBARS) | 65 at 20 μM | [67] |
| Compound | Test Type | Result | References |
|---|---|---|---|
| 5 | IC50 (μM) on RAW264.7 LPS-activated cells | 7.43 | [74] |
| 27 | 3.13 | ||
| 9 | PGE2 production in RAW264.7 LPS-activated cells | 290 pg/mL at 20 μM | [75] |
| 67 | 1500 pg/mL at 40 μM | [76] | |
| 43 | IC50 (μM) on human platelets | 200 | [70] |
| Compound | Type of Test | Result | References |
|---|---|---|---|
| 6 | COX-1 inhibition (%) | 63 at 350 μM | [78] |
| 9 | 35 at 10 μM | [79] | |
| 6 | COX-2 inhibition (%) | 30 at 350 μM | [78] |
| 46 | 12 at 10 μM | [80] | |
| 65 | 65 at 10 μM | ||
| 5 | TRAP activity in RANKL-induced osteoclastic RAW 264.7 cells expressed as % respect to control (Trolox) | 200 | [71] |
| 12 | 290 | ||
| 14 | 240 | ||
| 33 | 400 | ||
| 43 | 200 | ||
| 10 | Xanthine oxidase activity inhibition (%) | 28 at 150 μM | [68] |
| 34 | 0 at 150 μM | ||
| 52 | Lipoxygenase activity inhibition (%) | 69.0 at 10 μM | [81] |
| 33 43 | Phosphodiesterase 1 (IC50, μM) | >100 | [82] |
| 33 43 | Phosphodiesterase 2 (IC50, μM) | 9.2 | |
| 33 43 | Phosphodiesterase 3 (IC50, μM) | >100 | |
| 33 43 | Phosphodiesterase 4 (IC50, μM) | 7.8 | |
| 33 43 | Phosphodiesterase 5 (IC50, μM) | 22.8 | |
| 14 | AKT1 (PKB) inhib. % with ATP 75 μM | 7 at 10 μM | [83] |
| BTK inhib. % with ATP 36 μM | 7 at 10 μM | ||
| CHUK (IKKα). Inhib. % with ATP 9 μM | 15 at 10 μM | ||
| IGF1R Inhib. % with ATP 9 μM | 7 at 10 μM | ||
| IKBKB (IKKβ) Inhib. % with ATP 5 μM | 11 at 100 μM | ||
| IKBKE (IKKe) Inhib. % with ATP 16 μM | 2 at 10 μM | ||
| IRAK4 Inhib. % with ATP 34 μM | 3 at 10 μM | ||
| JAK1 Inhib. % with ATP 87 μM | −10 at 10 μM | ||
| JAK2 Inhib. % with ATP 31 μM | −7 at 10 μM | ||
| MAP4K5 (KHS1) Inhib. % with ATP 55 μM | 5 at 10 μM | ||
| MAPK1 (ERK2) Inhib. % with ATP 100 μM | −5 at 100 μM | ||
| MAPK3 (ERK1) Inhib. % with ATP 45 μM | 3 at 10 μM | ||
| MAPK8 (JNK1) Inhib. % with ATP 100 μM | −5 at 10 μM | ||
| MAPK9 (JNK2) Inhib. % with ATP 100 μM | 5 at 10 μM | ||
| MAPK10 (JNK3) Inhib. % with ATP 100 μM | 3 at 10 μM | ||
| MAPKAPK2 Inhib. % with ATP 3 μM | 2 at 10 μM | ||
| NEK1 Inhib. % with ATP 119 μM | 5 at 10 μM | ||
| NEK2 Inhib. % with ATP 150 μM | 1 at 10 μM | ||
| PDK 1 Direct Inhib. % with ATP 27 μM | 7 at 10 μM | ||
| PRKACA (PKA) Inhib. % with ATP 4 μM | 1 at 10 μM | ||
| SYK Inhib. % with ATP 25 μM | 35 at 10 μM | ||
| TBK1 Inhib. % with ATP 31 μM | 17 at 10 μM | ||
| ZAP70 Inhib. % with ATP 2 μM | 3 at 10 μM | ||
| 3 | NAD(P)H:quinoneoxidoreductase 1 (NQO1): = Concentration required to double the specific activity (μM) | 18.0 | [84] |
| 4 | 0.085 | ||
| 5 | 0.2 | ||
| 27 | 12.5 | ||
| 4 | IC50 inhib. of inducible NO-syntase on RAW264.7 LPS-activated cells (μM) | 5 | [84] |
| 5 | 2.5 |
| Compound | Test Type | Result | References |
|---|---|---|---|
| 5 | IC50 (μM) on RAW264.7 LPS-activated cells | 33.2 | [74] |
| 5.1 | [83] | ||
| 9 | 14.3 | [87] | |
| 12 | 88.5 | [88] | |
| 60 | [83] | ||
| 14 | 4.6 | ||
| 27 | 8.7 | ||
| 32.5 | [74] | ||
| 33 | >100 | [89] | |
| 16.1 | [88] | ||
| 66 | [83] | ||
| 43 | 96 | ||
| 52 | 7.53 | [90] | |
| 33 | % Inhibition on murine Mϕ LPS-activated | 14.2 at 100 μM | [91] |
| 41 | 8.96 at 50 μM | [92] | |
| 41 | Nitrite production (μM) in Raw 246.7 LPS-activated cells | 19 at 50 μM | [92] |
| 25 | 3.5 at 50 μM | [93] | |
| 20 | 3 at 30 μM | [94] | |
| 34 | 11 at 2 μM | ||
| 57 | 27 at 30 μM | [95] | |
| 58 | 9 at 30 μM | ||
| <2 at 30 μM | [96] | ||
| 63 | 45 at 30 μM | [95] | |
| 67 | 8 at 40 μM | [76] | |
| 9 | 19 at 20 μM | [67] | |
| 21 at 20 μM | [75] | ||
| 6 at 10 μM | [79] | ||
| 4 at 50 μM | [97] | ||
| 9 | Nitrite production (μM) in primary microglia | 1 at 50 μM | [97] |
| Nitrite production (μM) in HAPI microglial cells | 3 at 50 μM | ||
| Nitrite production (μM) in primary astrocytes | 20 at 50 μM | ||
| 9 | Nitrite production (μM) in BV2 microglia | 12 at 30 μM | [97] |
| 67 | 5 at 30 μM | [98] | |
| 1 | % Inhibition on RAW264.7 LPS-activated cells | 23 at 50 μM | [99] |
| 2 | 0 at 50 μM | ||
| 74 | 40 at 50 μM | ||
| 49 at 200 μM | [100] | ||
| 9 | 72.5 at 50 μM | [87] | |
| 11 | 33 at 50 μM | [99] | |
| 13 | 32 at50 μM | ||
| 22 | 50 at 30 μM | [101] | |
| 27 | 70 at 3 μM | [102] | |
| 35 | 90 at 30 μM | [103] | |
| 44 | 73 at 200 μM | [100] | |
| 42 at 50 μM | [104] | ||
| 45 | 75 at 200 μM | [100] | |
| 52 | 48 at 200 μM | ||
| 55 at 3 μM | [102] | ||
| 40 at 50 μM | [104] | ||
| 55 | 27.9 at 50 μM | ||
| 63 | 47 at 200 μM | [100] | |
| 42 at 50 μM | [104] | ||
| 71 | 49 at 200 μM | [100] | |
| 38 | 95 at 50 μM | [105] | |
| 25 | 50 at 50 μM | [106] |
| Compound | Test Type | Result * | References |
|---|---|---|---|
| 14 | IC50 (μM) on RAW264.7 LPS-activated cells | 206 | [83] |
| 27 | 292 | ||
| 25 | % production in RAW264.7 LPS-activated cells | 65 at 100 μM | [106] |
| 22 | 48 at 30 μM | [101] |
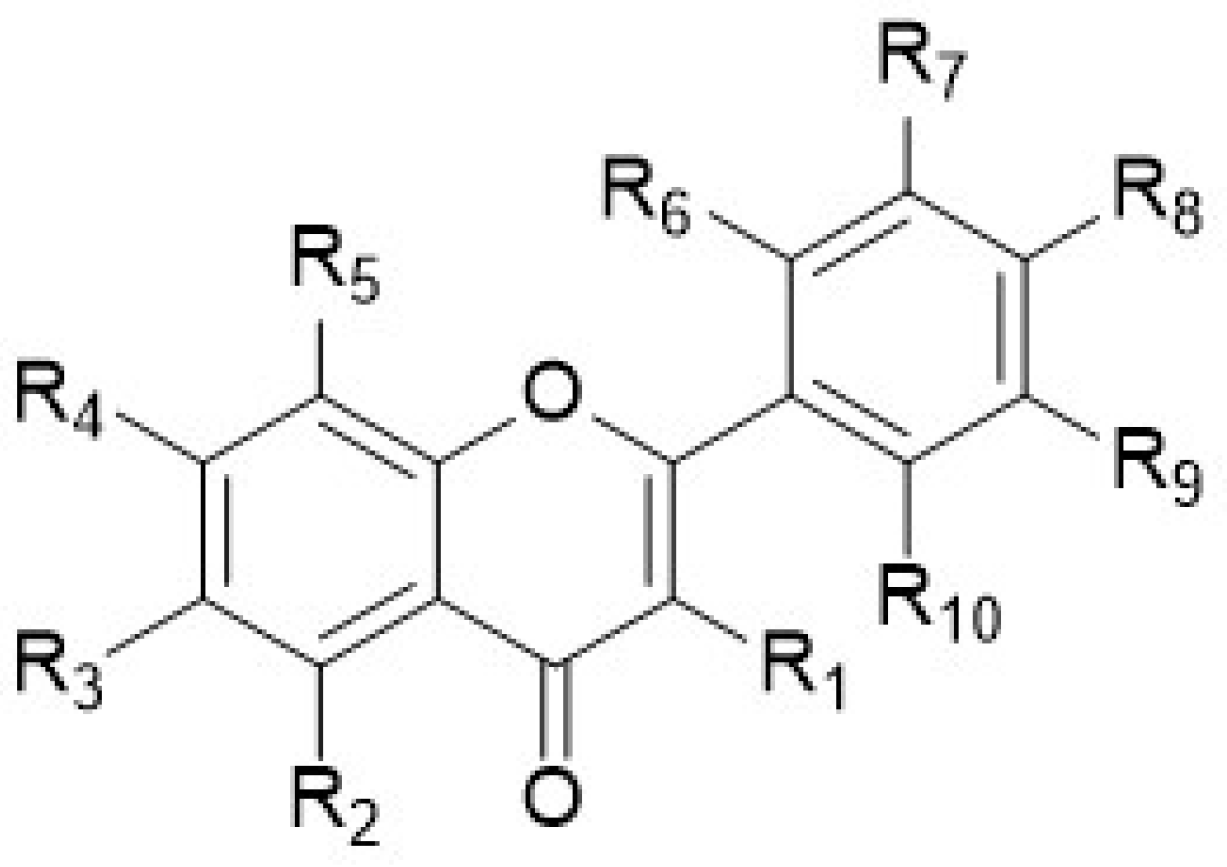
| No. | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2′,3′-Dimethoxyflavone | H | H | H | H | H | OMe | OMe | H | H | H |
| 2 | 2′,4′-Dimethoxyflavone | H | H | H | H | H | OMe | H | OMe | H | H |
| 3 | 3′,4′-Dimethoxyflavone | H | H | H | H | H | H | OMe | OMe | H | H |
| 4 | 5,4′-Dimethoxyflavone | H | OMe | H | H | H | H | H | OMe | H | H |
| 5 | 5,7-Dimethoxyflavone (Chrysin dimethyl ether) | H | OMe | H | OMe | H | H | H | H | H | H |
| 6 | 7,4′-Dimethoxyflavone | H | H | H | OMe | H | H | H | OMe | H | H |
| 7 | 5,6-Dihydroxy-7,4′-dimethoxyflavone (Ladanein) | H | OH | OH | OMe | H | H | H | OMe | H | H |
| 8 | 3,5,6-Trihydroxy-7,4′-dimethoxyflavone | OH | OH | OH | OMe | H | H | H | OMe | H | H |
| 9 | 5,7,4′-Trihydroxy-6,3′-dimethoxyflavone (Jaceosidin) | H | OH | OMe | OH | H | H | OMe | OH | H | H |
| 10 | 5,7,4′-Trihydroxy-6,8-dimethoxyflavone (Demethoxysudachitin) | H | OH | OMe | OH | OMe | H | H | OMe | H | H |
| 11 | 2′,3′,4′-Trimethoxyflavone | H | H | H | H | H | OMe | OMe | OMe | H | H |
| 12 | 3,5,7-Trimethoxyflavone (Galangin trimethyl ether) | OMe | OMe | H | OMe | H | H | H | H | H | H |
| 13 | 3′,4′,5′-Trimethoxyflavone | H | H | H | H | H | H | OMe | OMe | OMe | H |
| 14 | 5,7,4′-Trimethoxyflavone (Apigenin trimethyl ether) | H | OMe | H | OMe | H | H | H | OMe | H | H |
| 15 | 5,3′,4′-Trimethoxyflavone | H | OMe | H | H | H | H | OMe | OMe | H | H |
| 16 | 6,2′,3′-Trimethoxyflavone | H | H | OMe | H | H | OMe | OMe | H | H | H |
| 17 | 5-Hydroxy-6,7,4′-trimethoxyflavone (Salvigenin) | H | OH | OMe | OMe | H | H | H | OMe | H | H |
| 18 | 5-Hydroxy-7,8,4′-trimethoxyflavone (Isoscutellarein 7,8,4′-trimethyl ether) | H | OH | H | OMe | OMe | H | H | OMe | H | H |
| 19 | 8-Hydroxy-5,7,4′-trimethoxyflavone | H | OMe | H | OMe | OH | H | H | OMe | H | H |
| 20 | 5,4′-Dihydroxy-6,7,8-trimethoxyflavone (Xanthomicrol) | H | OH | OMe | OMe | OMe | H | H | OH | H | H |
| 21 | 3,5,6-Trihydroxy-7,3′,4′-trimethoxyflavone | OH | OH | OH | OMe | H | H | OMe | OMe | H | H |
| 22 | 5,7,4′-Trihydroxy-6,8,3′- trimethoxyflavone | H | OH | OMe | OH | OMe | H | OMe | OMe | H | H |
| 23 | 3,5,7,4′-Tetrahydroxy-6,8,3′-trimethoxyflavone (Limocitrol) | OH | OH | OMe | OH | OMe | H | OMe | OH | H | H |
| 24 | 3,6,7,4′-Tetramethoxyflavone | OMe | H | OMe | OMe | H | H | H | OMe | H | H |
| 25 | 5,6,7,4′-Tetramethoxyflavone (Scutellarein tetramethyl ether) | H | OMe | OMe | OMe | H | H | H | OMe | H | H |
| 26 | 5,7,8,4′-Tetramethoxyflavone (6-Demethoxytangeretin) | H | OMe | H | OMe | OMe | H | H | OMe | H | H |
| 27 | 5,7,3′,4′-Tetramethoxyflavone (Luteolin tetramethyl ether) | H | OMe | H | OMe | H | H | OMe | OMe | H | H |
| 28 | 6,7,8,4′-Tetramethoxyflavone | H | H | OMe | OMe | OMe | H | H | OMe | H | H |
| 29 | 7,3′,4′,5′-Tetramethoxyflavone | H | H | H | OMe | H | H | OMe | OMe | OMe | H |
| 30 | 3-Hydroxy-5,6,7,4′-tetramethoxyflavone (Eupatorin-5-methylether) | OH | OMe | OMe | OMe | H | H | H | OMe | H | H |
| 31 | 5-Hydroxy-3,6,7,4′-tetramethoxyflavone (Penduletin 4′-methyl ether) | OMe | OH | OMe | OMe | H | H | H | OMe | H | H |
| 32 | 5-Hydroxy-3,7,8,4′-tetramethoxyflavone | OMe | OH | H | OMe | OMe | H | H | OMe | H | H |
| 33 | 5-Hydroxy-3,7,3′,4′-tetramethoxyflavone (Retusin) | OMe | OH | H | OMe | H | H | OMe | OMe | H | H |
| 34 | 5-Hydroxy-6,7,8,4′-tetramethoxyflavone (Gardenin B) | H | OH | OMe | OMe | OMe | H | H | OMe | H | H |
| 35 | 5-Hydroxy-6,7,3′,4′-tetramethoxyflavone (5-Desmethylsinensetin) | H | OH | OMe | OMe | H | H | OMe | OMe | H | H |
| 36 | 6-Hydroxy-5,7,8,4′-tetramethoxyflavone | H | OMe | OH | OMe | OMe | H | H | OMe | H | H |
| 37 | 7-Hydroxy-5,6,8,4′-tetramethoxyflavone | H | OMe | OMe | OH | OMe | H | H | OMe | H | H |
| 38 | 4′-Hydroxy-5,6,7,8-tetramethoxyflavone | H | OMe | OMe | OMe | OMe | H | H | OH | H | H |
| 39 | 5,7-Dihydroxy-6,8,3′,4′-tetramethoxyflavone (Hymenoxin) | H | OH | OMe | OH | OMe | H | OMe | OMe | H | H |
| 40 | 5,3′-Dihydroxy-3,7,4′,5′-tetramethoxyflavone | OMe | OH | H | OMe | H | H | OH | OMe | OMe | H |
| 41 | 5,4′-Dihydroxy-6,7,8,3′-tetramethoxyflavone (8-Methoxycirsilineol) | H | OH | OMe | OMe | OMe | H | OMe | OH | H | H |
| 42 | 3,5,6,8,4′-Pentamethoxyflavone | OMe | OMe | OMe | H | OMe | H | H | OMe | H | H |
| 43 | 3,5,7,3′,4′-Pentamethoxyflavone (Quercetin pentamethyl ether) | OMe | OMe | H | OMe | H | H | OMe | OMe | H | H |
| 44 | 5,6,7,8,4′-Pentamethoxyflavone (Tangeretin) | H | OMe | OMe | OMe | OMe | H | H | OMe | H | H |
| 45 | 5,6,7,3′,4′-Pentamethoxyflavone (Sinensitin) | H | OMe | OMe | OMe | H | H | OMe | OMe | H | H |
| 46 | 5,7,8,3′,4′-Pentamethoxyflavone (Isosinensetin) | H | OMe | H | OMe | OMe | H | OMe | OMe | H | H |
| 47 | 5,7,2′,3′,4′-Pentamethoxyflavone | H | OMe | H | OMe | H | OMe | OMe | OMe | H | H |
| 48 | 6,7,8,3′,4′-Pentamethoxyflavone (Demethylnobiletin) | H | H | OMe | OMe | OMe | H | OMe | OMe | H | H |
| 49 | 3-Hydroxy-5,6,7,8,4′-pentamethoxyflavone (3-Demethylnobiletin) | OH | OMe | OMe | OMe | OMe | H | H | OMe | H | H |
| 50 | 5-Hydroxy-3,6,7,8,4′-Pentamethoxyflavone (5-Hydroxyauranetin) | OMe | OH | OMe | OMe | OMe | H | H | OMe | H | H |
| 51 | 5-Hydroxy-3,7,8,3′,4′-pentamethoxyflavone (Gossypetin pentamethylether) | OMe | OH | H | OMe | OMe | H | OMe | OMe | H | H |
| 52 | 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone (5-Demethylnobiletin) | H | OH | OMe | OMe | OMe | H | OMe | OMe | OH | H |
| 53 | 5-Hydroxy-6,7,3′,4′,5′-pentamethoxyflavone (Umuhengerin) | H | OH | OMe | OMe | H | H | OMe | OMe | OMe | H |
| 54 | 7-Hydroxy-3,5,6,3′,4′-pentamethoxyflavone | OMe | OMe | OMe | OH | H | H | OMe | OMe | H | H |
| 55 | 7-Hydroxy-5,6,8,3′,4′-pentamethoxyflavone (7-Demethylnobiletin) | H | OMe | OMe | OH | OMe | H | OMe | OMe | H | H |
| 56 | 8-Hydroxy-3,5,6,7,4′-pentamethoxyflavone | OMe | OMe | OMe | OMe | OH | H | H | OMe | H | H |
| 57 | 3′-Hydroxy-5,6,7,8,4′-pentamethoxyflavone (3-Hydroxytangeretin) | H | OMe | OMe | OMe | OMe | H | OH | OMe | H | H |
| 58 | 4′-Hydroxy-5,6,7,8,3′-pentamethoxyflavone (4′-Demethylnobiletin) | H | OMe | OMe | OMe | OMe | H | OMe | OH | H | H |
| 59 | 3,5,6,7,8,4′-Hexamethoxyflavone (3-Methoxytangeretin) | OMe | OMe | OMe | OMe | OMe | H | H | OMe | H | H |
| 60 | 3,5,7,8,2′,5′-Hexamethoxyflavone | OMe | OMe | H | OMe | OMe | OMe | H | H | OMe | H |
| 61 | 3,5,7,8,3′,4′-Hexamethoxyflavone (Gossypetin hexamethyl ether) | OMe | OMe | H | OMe | OMe | H | OMe | OMe | H | H |
| 62 | 3,6,7,8,2′,5′-Hexamethoxyflavone | OMe | H | OMe | OMe | OMe | OMe | H | H | OMe | H |
| 63 | 5,6,7,8,3′,4′-Hexamethoxyflavone (Nobiletin) | H | OMe | OMe | OMe | OMe | H | OMe | OMe | H | H |
| 64 | 5,6,7,3′,4′,5′-Hexamethoxyflavone | H | OMe | OMe | OMe | H | H | OMe | OMe | OMe | H |
| 65 | 5,7,8,3′,4′,5′-Hexamethoxyflavone (Bannamurpanisin) | H | OMe | H | OMe | OMe | H | OMe | OMe | OMe | H |
| 66 | 3-Hydroxy-5,6,7,8,3′,4′-hexamethoxyflavone (Natsudaidain) | OH | OMe | OMe | OMe | OMe | H | OMe | OMe | H | H |
| 67 | 5-Hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone | OMe | OH | OMe | OMe | OMe | H | OMe | OMe | H | H |
| 68 | 5-Hydroxy-6,7,8,3′,4′,5′-hexamethoxyflavone (Gardenin A) | H | OH | OMe | OMe | OMe | H | OMe | OMe | OMe | H |
| 69 | 7-Hydroxy-3,5,6,8,3′,4′-hexamethoxyflavone | OMe | OMe | OMe | OH | OMe | H | OMe | OMe | H | H |
| 70 | 8-Hydroxy-3,5,6,7,3′,4′-hexamethoxyflavone | OMe | OMe | OMe | OMe | OH | H | OMe | OMe | H | H |
| 71 | 3,5,6,7,8,3′,4′-Heptamethoxyflavone | OMe | OMe | OMe | OMe | OMe | H | OMe | OMe | H | H |
| No. | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C1′ | C2′ | C3′ | C4′ | C5′ | C6′ | Solvent | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 161.7 | - | 177.6 | 125.4 | 124.5 | 134.3 | 118.3 | 155.9 | 123.0 | 111.2 | 147.1 | 153.0 | 111.2 | 120.5 | 116.0 | DMSO-d6 | [26] |
| 2 | 160.6 | 110.2 | 177.1 | 124.7 | 125.2 | 134.1 | 118.4 | 155.8 | 123.1 | 112.3 | 159.5 | 99.1 | 163.2 | 106.3 | 130.4 | DMSO-d6 | [160] |
| 3 | 162.7 | 105.7 | 177.0 | 124.7 | 125.3 | 134.0 | 118.5 | 155.6 | 123.3 | 123.3 | 109.4 | 149.0 | 151.9 | 111.7 | 119.9 | DMSO-d6 | [160] |
| 4 | 160.1 | 106.9 | 176.4 | 159.0 | 107.2 | 134.1 | 109.9 | 157.5 | 113.7 | 122.9 | 127.8 | 114.5 | 161.9 | 114.5 | 127.8 | DMSO-d6 | [161] |
| 5 | 163.9 | 109.0 | 177.4 | 160.5 | 92.8 | 160.8 | 96.1 | 159.8 | 109.3 | 131.5 | 125.9 | 128.8 | 131.1 | 128.8 | 125.9 | CDCl3 | [162] |
| 6 | 162.3 | 105.2 | 176.3 | 126.1 | 114.5 | 163.7 | 100.9 | 157.4 | 117.1 | 123.3 | 128.0 | 114.5 | 162.0 | 114.5 | 128.0 | DMSO-d6 | [161] |
| 7 | 163.1 | 102.9 | 182.0 | 146.1 | 129.9 | 154.2 | 90.9 | 149.5 | 105.0 | 122.8 | 127.9 | 114.3 | 162.0 | 114.3 | 127.9 | DMSO-d6 | [163] |
| 8 | 146.4 | 135.9 | 176.0 | 144.9 | 129.2 | 154.6 | 90.7 | 148.8 | 104.3 | 123.3 | 129.2 | 113.9 | 160.4 | 113.9 | 129.2 | DMSO-d6 | [164] |
| 9 | 163.7 | 102.7 | 182.1 | 152.7 | 131.3 | 157.3 | 94.3 | 152.4 | 104.0 | 121.5 | 110.1 | 150.7 | 148.0 | 115.7 | 120.3 | DMSO-d6 | [165] |
| 10 | 165.0 | 103.6 | 183.7 | 149.8 | 132.4 | 151.3 | 117.0 | 146.7 | 104.6 | 123.4 | 129.2 | 116.5 | 162.0 | 116.5 | 129.2 | Acetone-d6 | [166] |
| 11 | 160.9 | 110.0 | 176.6 | 124.4 | 124.0 | 133.8 | 118.0 | 155.9 | 122.8 | 108.0 | 152.0 | 141.9 | 155.6 | 108.0 | 118.0 | DMSO-d6 | [26] |
| 12 | 152.0 | 141.4 | 173.6 | 160.5 | 95.5 | 163.5 | 92.1 | 158.4 | 109.1 | 130.5 | 127.7 | 128.0 | 129.0 | 128.0 | 127.7 | CDCl3 | [167] |
| 13 | 162.0 | 106.3 | 176.6 | 124.9 | 124.3 | 133.5 | 118.0 | 155.2 | 123.0 | 126.0 | 104.1 | 152.9 | 140.8 | 152.9 | 104.1 | DMSO-d6 | [26] |
| 14 | 160.4 | 106.8 | 176.0 | 160.0 | 93.3 | 163.7 | 96.2 | 159.3 | 108.5 | 123.2 | 127.6 | 114.4 | 161.9 | 114.4 | 127.6 | DMSO-d6 | [168] |
| 15 | 161.0 | 106.4 | 178.3 | 159.7 | 111.1 | 133.6 | 108.6 | 158.2 | 114.5 | 123.9 | 110.1 | 149.2 | 151.8 | 108.0 | 119.7 | CDCl3 | [169] |
| 16 | 161.3 | 110.4 | 176.6 | 104.6 | 156.6 | 123.3 | 120.1 | 150.6 | 123.8 | 126.0 | 147.1 | 153.0 | 115.8 | 124.5 | 120.6 | DMSO-d6 | [161] |
| 17 | 163.6 | 103.3 | 182.3 | 152.0 | 131.9 | 158.7 | 91.6 | 152.7 | 105.1 | 122.7 | 128.3 | 114.6 | 162.4 | 114.6 | 128.3 | DMSO-d6 | [34] |
| 18 | 163.9 | 103.8 | 182.7 | 157.5 | 95.7 | 158.5 | 128.9 | 149.4 | 104.8 | 123.6 | 128.1 | 114.6 | 162.7 | 114.6 | 128.1 | CDCl3 | [164] |
| 19 | 159.6 | 105.9 | 176.2 | 151.8 | 94.0 | 152.0 | 127.9 | 146.8 | 108.0 | 123.2 | 127.8 | 114.3 | 161.7 | 114.3 | 127.8 | DMSO-d6 | [164] |
| 20 | 164.1 | 102.6 | 182.5 | 145.5 | 135.6 | 152.4 | 132.6 | 145.1 | 106.1 | 121.0 | 128.4 | 116.1 | 161.4 | 116.1 | 128.4 | DMSO-d6 | [170] |
| 21 | 146.3 | 136.1 | 176.0 | 144.9 | 129.2 | 154.6 | 90.8 | 148.7 | 104.3 | 123.4 | 110.7 | 121.4 | 150.3 | 148.3 | 111.4 | DMSO-d6 | [171] |
| 22 | 163.1 | 102.7 | 182.0 | 148.1 | 131.3 | 150.5 | 127.7 | 145.1 | 115.7 | 121.4 | 109.7 | 119.9 | 150.5 | 115.7 | 147.8 | DMSO-d6 | [164] |
| 23 | 146.5 | 135.6 | 176.2 | 147.2 | 130.9 | 150.5 | 127.6 | 144.3 | 102.3 | 122.1 | 111.2 | 148.8 | 147.3 | 115.6 | 121.6 | DMSO-d6 | [164] |
| 24 | 160.3 | 106.1 | 175.6 | 97.3 | 139.8 | 161.8 | 157.4 | 153.9 | 114.5 | 123.0 | 127.8 | 114.5 | 151.6 | 114.5 | 127.8 | DMSO-d6 | [31] |
| 25 | 160.3 | 106.1 | 175.6 | 151.6 | 139.7 | 157.4 | 97.3 | 153.9 | 112.0 | 123.0 | 127.8 | 114.5 | 161.9 | 114.5 | 127.8 | DMSO-d6 | [161] |
| 26 | 159.4 | 106.2 | 175.8 | 155.6 | 93.5 | 156.2 | 129.8 | 150.9 | 107.8 | 123.1 | 127.5 | 114.5 | 161.7 | 114.5 | 127.5 | DMSO-d6 | [164] |
| 27 | 160.4 | 107.5 | 177.4 | 160.6 | 96.0 | 164.8 | 92.8 | 160.0 | 108.9 | 123.7 | 108.3 | 149.0 | 151.6 | - | 119.3 | CDCl3 | [172] |
| 28 | 161.2 | 107.1 | 177.2 | 96.2 | 157.6 | 140.3 | 152.6 | 154.5 | 112.9 | 123.9 | 127.7 | 114.4 | 162.1 | 114.4 | 127.7 | CDCl3 | [32] |
| 29 | 162.9 | 107.3 | 177.7 | 127.1 | 114.4 | 164.2 | 100.5 | 157.9 | 117.8 | 127.0 | 103.7 | 153.5 | 141.4 | 153.5 | 103.7 | CDCl3 | [173] |
| 30 | 142.6 | 137.6 | 171.0 | 151.0 | 139.3 | 157.5 | 96.9 | 153.0 | 110.0 | 123.5 | 128.8 | 114.0 | 160.1 | 114.0 | 128.8 | DMSO-d6 | [34] |
| 31 | 155.4 | 137.9 | 178.2 | 151.6 | 131.6 | 158.6 | 91.4 | 151.8 | 105.6 | 122.0 | 130.0 | 114.2 | 161.4 | 114.2 | 130.0 | DMSO-d6 | [164] |
| 32 | 155.4 | 137.8 | 178.3 | 156.3 | 95.7 | 158.2 | 128.3 | 147.8 | 104.5 | 122.2 | 129.8 | 114.3 | 161.4 | 114.3 | 129.8 | DMSO-d6 | [164] |
| 33 | 155.5 | 138.3 | 178.1 | 156.4 | 97.9 | 165.2 | 92.5 | 156.4 | 105.3 | 122.1 | 111.6 | 148.5 | 151.4 | 111.3 | 122.1 | DMSO-d6 | [34] |
| 34 | 163.9 | 103.4 | 182.7 | 148.7 | 136.0 | 152.6 | 132.8 | 145.4 | 106.3 | 122.8 | 128.4 | 114.9 | 162.7 | 114.9 | 128.4 | DMSO-d6 | [34] |
| 35 | 163.0 | 104.5 | 182.5 | 153.8 | 133.0 | 159.0 | 91.0 | 155.0 | 105.5 | 120.1 | 112.2 | 152.4 | 149.5 | 110.0 | 123.9 | CDCl3 | [174] |
| 36 | 160.1 | 105.7 | 175.9 | 141.2 | 140.7 | 146.3 | 137.8 | 143.6 | 114.1 | 123.2 | 127.6 | 114.5 | 161.8 | 114.5 | 127.6 | DMSO-d6 | [164] |
| 37 | 159.8 | 105.8 | 175.7 | 147.3 | 139.4 | 149.1 | 132.5 | 147.5 | 110.6 | 123.2 | 127.6 | 114.6 | 161.8 | 114.6 | 127.6 | DMSO-d6 | [164] |
| 38 | 160.7 | 105.3 | 175.6 | 147.4 | 143.4 | 150.8 | 137.7 | 147.0 | 114.2 | 121.3 | 127.8 | 115.9 | 160.7 | 115.9 | 127.8 | DMSO-d6 | [164] |
| 39 | 163.7 | 104.0 | 182.9 | 148.4 | 130.9 | 148.9 | 127.3 | 145.8 | 104.6 | 123.7 | 108.8 | 149.4 | 152.4 | 111.3 | 120.1 | CDCl3 | [175] |
| 40 | 155.3 | 139.7 | 178.8 | 162.0 | 98.0 | 165.6 | 92.2 | 156.8 | 106.1 | 126.0 | 108.6 | 149.2 | 137.8 | 152.0 | 105.1 | CDCl3 | [176] |
| 41 | 164.0 | 102.9 | 182.5 | 148.4 | 135.8 | 152.4 | 132.5 | 145.1 | 106.1 | 121.3 | 110.0 | 148.0 | 151.0 | 115.9 | 120.3 | DMSO-d6 | [170] |
| 42 | 152.5 | 139.4 | 172.9 | 138.4 | 148.8 | 103.4 | 139.9 | 145.0 | 118.9 | 122.6 | 129.6 | 114.2 | 161.0 | 114.2 | 129.6 | DMSO-d6 | [164] |
| 43 | 152.6 | 141.2 | 174.0 | 158.8 | 95.8 | 163.9 | 92.5 | 161.1 | 109.5 | 123.4 | 111.3 | 148.7 | 150.9 | 110.8 | 121.6 | CDCl3 | [177] |
| 44 | 160.4 | 106.1 | 175.8 | 147.6 | 143.6 | 162.1 | 137.8 | 147.6 | 114.7 | 123.1 | 127.8 | 114.3 | 151.0 | 114.3 | 127.8 | DMSO-d6 | [31] |
| 45 | 159.6 | 106.7 | 176.1 | 151.7 | 131.8 | 156.5 | 94.2 | 155.8 | 111.1 | 123.4 | 108.9 | 149.1 | 151.2 | 112.3 | 119.3 | DMSO-d6 | [31] |
| 46 | 160.5 | 107.2 | 177.9 | 152.0 | 92.6 | 156.3 | 130.7 | 156.3 | 109.1 | 124.1 | 108.7 | 149.3 | 151.8 | 111.0 | 119.7 | CDCl3 | [178] |
| 47 | 160.3 | 111.5 | 175.7 | 158.1 | 96.1 | 163.7 | 93.2 | 159.8 | 108.2 | 117.8 | 152.1 | 142.2 | 155.8 | 108.2 | 123.9 | DMSO-d6 | [179] |
| 48 | 160.3 | 106.4 | 175.7 | 97.3 | 139.7 | 157.4 | 151.5 | 153.9 | 111.7 | 123.2 | 109.2 | 149.0 | 151.7 | 112.0 | 119.4 | DMSO-d6 | [31] |
| 49 | 142.9 | 137.8 | 171.2 | 147.0 | 143.1 | 150.7 | 137.4 | 146.2 | 112.3 | 123.5 | 128.8 | 114.2 | 160.3 | 114.2 | 128.8 | DMSO-d6 | [34] |
| 50 | 155.7 | 137.8 | 178.6 | 148.1 | 135.4 | 152.3 | 132.5 | 144.4 | 106.7 | 122.1 | 129.9 | 114.4 | 161.5 | 114.4 | 129.9 | DMSO-d6 | [164] |
| 51 | 155.2 | 138.0 | 178.3 | 156.4 | 95.8 | 158.2 | 128.8 | 147.8 | 104.5 | 122.2 | 111.7 | 148.4 | 151.3 | 110.9 | 121.9 | DMSO-d6 | [34] |
| 52 | 163.5 | 103.5 | 182.5 | 148.5 | 135.8 | 152.4 | 132.5 | 145.2 | 106.2 | 122.6 | 111.7 | 148.9 | 152.3 | 109.0 | 119.9 | DMSO-d6 | [34] |
| 53 | 163.8 | 105.4 | 182.6 | 153.2 | 132.9 | 158.9 | 90.7 | 153.1 | 106.3 | 126.5 | 103.9 | 153.7 | 141.6 | 153.7 | 103.9 | CDCl3 | [180] |
| 54 | 151.8 | 140.0 | 171.4 | 137.4 | 142.8 | 158.2 | 96.1 | 153.7 | 109.8 | 123.8 | 110.6 | 148.9 | 150.5 | 111.0 | 120.8 | CDCl3 | [44] |
| 55 | 161.4 | 106.7 | 177.3 | 145.1 | 140.1 | 140.3 | 138.1 | 145.6 | 114.2 | 124.2 | 108.7 | 149.4 | 152.0 | 111.3 | 119.7 | CDCl3 | [181] |
| 56 | 143.2 | 137.9 | 171.8 | 147.5 | 143.5 | 151.5 | 137.2 | 146.9 | 111.7 | 123.6 | 129.0 | 144.1 | 160.9 | 114.1 | 129.0 | CDCl3 | [45] |
| 57 | 142.8 | 137.7 | 171.2 | 146.9 | 143.0 | 150.7 | 137.4 | 146.1 | 112.2 | 123.5 | 128.7 | 114.1 | 160.2 | 114.1 | 128.7 | DMSO-d6 | [164] |
| 58 | 161.2 | 106.6 | 177.4 | 148.9 | 106.6 | 151.4 | 138.0 | 146.9 | 114.8 | 123.5 | 108.2 | 147.7 | 148.4 | 115.0 | 120.2 | CDCl3 | [182] |
| 59 | 152.5 | 139.8 | 172.2 | 147.2 | 143.2 | 150.8 | 137.4 | 146.2 | 114.4 | 122.5 | 129.5 | 114.3 | 161.0 | 114.3 | 129.5 | DMSO-d6 | [164] |
| 60 | 152.3 | 140.9 | 174.3 | 92.3 | 156.3 | 156.4 | 152.5 | 151.0 | 109.4 | 123.6 | 150.9 | 111.0 | 121.8 | 148.7 | 152.3 | CDCl3 | [32] |
| 61 | 150.8 | 140.8 | 174.2 | 152.2 | 92.4 | 156.4 | 130.4 | 156.3 | 109.4 | 123.6 | 110.9 | 148.7 | 150.9 | 111.0 | 121.8 | CDCl3 | [178] |
| 62 | 153.3 | 140.8 | 173.7 | 96.0 | 157.7 | 140.2 | 152.4 | 153.6 | 113.1 | 123.4 | 151.0 | 110.9 | 121.7 | 148.7 | 111.4 | CDCl3 | [32] |
| 63 | 160.2 | 106.3 | 175.8 | 143.5 | 137.6 | 150.9 | 147.5 | 147.1 | 114.3 | 123.1 | 108.9 | 149.0 | 151.8 | 111.9 | 119.3 | DMSO-d6 | [31] |
| 64 | 161.0 | 108.3 | 177.2 | 154.5 | 140.4 | 157.8 | 96.3 | 152.6 | 112.9 | 126.9 | 103.4 | 153.6 | 140.9 | 153.6 | 103.4 | CDCl3 | [183] |
| 65 | 160.2 | 107.8 | 177.8 | 151.9 | 92.3 | 156.6 | 130.5 | 156.3 | 108.7 | 126.6 | 103.1 | 153.4 | 140.7 | 153.4 | 103.1 | CDCl3 | [183] |
| 66 | 146.9 | 143.1 | 172.0 | 143.6 | 137.9 | 150.6 | 137.5 | 147.7 | 111.8 | 123.9 | 111.2 | 149.0 | 151.7 | 110.3 | 121.1 | CDCl3 | [181] |
| 67 | 155.6 | 138.1 | 178.7 | 148.5 | 135.5 | 152.5 | 132.4 | 144.4 | 106.8 | 122.1 | 110.9 | 148.1 | 151.4 | 111.8 | 122.0 | DMSO-d6 | [34] |
| 68 | 163.6 | 104.8 | 183.0 | 149.5 | 136.6 | 153.1 | 132.9 | 145.8 | 107.0 | 126.3 | 103.6 | 153.6 | 141.5 | 153.6 | 103.6 | CDCl3 | [184] |
| 69 | 150.7 | 142.9 | 171.8 | 143.6 | 147.7 | 146.9 | 137.4 | 146.9 | 111.7 | 123.9 | 110.6 | 149.0 | 151.7 | 111.3 | 121.1 | CDCl3 | [44] |
| 70 | 142.8 | 137.8 | 171.8 | 147.6 | 143.5 | 151.6 | 137.4 | 146.8 | 111.7 | 123.8 | 110.3 | 148.9 | 150.5 | 111.1 | 121.0 | CDCl3 | [45] |
| 71 | 150.9 | 140.0 | 172.3 | 143.1 | 137.4 | 151.9 | 138.9 | 147.9 | 114.4 | 122.5 | 110.0 | 148.5 | 154.1 | 111.7 | 121.5 | DMSO-d6 | [31] |
| No. | OMe-3 | OMe-5 | OMe-6 | OMe-7 | OMe-8 | OMe-2′ | OMe-3′ | OMe-4′ | OMe-5′ | OMe-6′ | Solvent | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | - | - | - | - | - | 60.2 | 55.9 | - | - | - | DMSO-d6 | [26] |
| 2 | - | - | - | - | - | 56.1 | - | 55.7 | - | - | DMSO-d6 | [160] |
| 3 | - | - | - | - | - | - | 55.8 | 55.7 | - | - | DMSO-d6 | [160] |
| 4 | - | 56.1 | - | - | - | - | - | 55.5 | - | - | DMSO-d6 | [161] |
| 5 | - | 56.5 | - | 55.8 | - | - | - | - | - | - | CDCl3 | [162] |
| 6 | - | - | - | 55.5 | - | - | - | 56.0 | - | - | DMSO-d6 | [161] |
| 7 | - | - | - | 56.1 | - | - | - | 55.4 | - | - | DMSO-d6 | [163] |
| 8 | - | - | - | 56.2 | - | - | - | 55.3 | - | - | DMSO-d6 | [164] |
| 9 | - | - | 59.9 | - | - | - | 55.9 | - | - | - | DMSO-d6 | [165] |
| 10 | - | - | 60.8 | - | 61.8 | - | - | - | - | - | Acetone-d6 | [166] |
| 11 | - | - | - | - | - | 60.9 | 60.2 | 55.8 | - | - | DMSO-d6 | [26] |
| 12 | 59.7 | 55.4 | - | 56.0 | - | - | - | - | - | - | CDCl3 | [167] |
| 13 | - | - | - | - | - | - | 56.1 | 59.4 | - | 56.1 | DMSO-d6 | [26] |
| 14 | - | 56.1 | - | 55.9 | - | - | - | 55.4 | - | - | DMSO-d6 | [168] |
| 15 | - | 56.5 | - | - | - | - | 56.1 | 56.1 | - | - | CDCl3 | [169] |
| 16 | - | - | 55.7 | - | - | 60.6 | 56.0 | - | - | - | DMSO-d6 | [161] |
| 17 | - | - | 60.6 | 56.5 | - | - | - | 55.6 | - | - | DMSO-d6 | [34] |
| 18 | - | - | - | 56.3 | 61.7 | - | - | 55.5 | - | - | CDCl3 | [164] |
| 19 | - | 56.3 | - | 56.2 | - | - | - | 55.4 | - | DMSO-d6 | [164] | |
| 20 | - | - | 60.5 | 61.8 | 61.6 | - | - | - | - | - | DMSO-d6 | [170] |
| 21 | - | - | - | 56.5 | - | - | 55.6 | 55.6 | - | - | DMSO-d6 | [171] |
| 22 | - | - | 61.0 | - | 59.8 | - | - | 56.0 | - | - | DMSO-d6 | [164] |
| 23 | - | - | - | - | - | - | 55.5 | - | - | - | DMSO-d6 | [164] |
| 24 | 61.0 | - | 55.5 | 61.8 | - | - | - | 56.4 | - | - | DMSO-d6 | [31] |
| 25 | - | 61.8 | 61.0 | 56.4 | - | - | - | 55.5 | - | - | DMSO-d6 | [161] |
| 26 | - | 56.3 | - | 56.1 | 60.9 | - | - | 55.4 | - | - | DMSO-d6 | [164] |
| 27 | - | 56.0 | - | 55.7 | - | - | 55.7 | 56.2 | - | - | CDCl3 | [172] |
| 28 | - | - | 56.3 | 61.6 | 62.2 | - | - | 55.5 | - | - | CDCl3 | [32] |
| 29 | - | - | - | 55.8 | - | - | 56.3 | 61.0 | 56.3 | - | CDCl3 | [173] |
| 30 | - | 61.9 | 61.1 | 56.5 | - | - | - | 55.3 | - | - | DMSO-d6 | [34] |
| 31 | 59.7 | - | 60.0 | 56.4 | - | - | - | 55.4 | - | - | DMSO-d6 | [164] |
| 32 | 59.7 | - | - | 56.5 | 61.0 | - | - | 55.4 | - | - | DMSO-d6 | [164] |
| 33 | 59.8 | - | - | 56.2 | - | - | 55.7 | 55.7 | - | - | DMSO-d6 | [34] |
| 34 | - | - | 60.7 | 62.0 | 61.6 | - | - | 55.7 | - | - | DMSO-d6 | [34] |
| 35 | - | - | 61.0 | 58.5 | - | - | 56.2 | 56.1 | - | - | CDCl3 | [174] |
| 36 | - | 61.7 | - | 61.4 | 60.8 | - | - | 55.4 | - | - | DMSO-d6 | [164] |
| 37 | - | 61.7 | 61.1 | - | 61.1 | - | - | 55.4 | - | - | DMSO-d6 | [164] |
| 38 | - | 61.3 | 61.4 | 61.7 | 61.8 | - | - | - | - | - | DMSO-d6 | [164] |
| 39 | - | - | 61.0 | - | 61.8 | - | 56.1 | 56.0 | - | - | CDCl3 | [175] |
| 40 | 60.3 | - | - | 56.1 | - | - | - | 61.1 | 55.8 | - | CDCl3 | [176] |
| 41 | - | - | 60.5 | 61.8 | 61.4 | - | 55.8 | - | - | - | DMSO-d6 | [170] |
| 42 | 59.2 | 56.9 | 56.7 | - | 61.0 | - | - | 55.3 | - | - | DMSO-d6 | [164] |
| 43 | 59.9 | 55.8 | - | 55.9 | - | - | 56.1 | 56.4 | - | - | CDCl3 | [177] |
| 44 | - | 62.0 | 55.6 | 61.9 | 61.5 | - | - | 61.6 | - | - | DMSO-d6 | [31] |
| 45 | - | 61.1 | 55.8 | 56.5 | - | - | 56.4 | 55.9 | - | - | DMSO-d6 | [31] |
| 46 | - | 56.5 | - | 56.3 | 61.5 | - | 56.1 | 56.0 | - | - | CDCl3 | [178] |
| 47 | - | 56.0 | - | 56.1 | - | 61.0 | 60.5 | 56.1 | - | - | DMSO-d6 | [179] |
| 48 | - | - | 55.7 | 61.8 | 55.9 | - | 61.0 | 56.4 | - | - | DMSO-d6 | [31] |
| 49 | - | 61.8 | 61.4 | 61.9 | 61.5 | - | - | 55.3 | - | - | DMSO-d6 | [34] |
| 50 | 59.7 | - | 60.5 | 61.8 | 61.4 | - | - | 55.4 | - | - | DMSO-d6 | [164] |
| 51 | 59.7 | - | - | 56.5 | 61.0 | - | 55.3 | 55.6 | - | - | DMSO-d6 | [34] |
| 52 | - | - | 60.5 | 61.7 | 61.4 | - | 55.7 | 55.6 | - | - | DMSO-d6 | [34] |
| 53 | - | - | 60.8 | 56.4 | - | - | 56.5 | 60.8 | 56.5 | - | CDCl3 | [180] |
| 54 | 62.3 | 61.6 | 56.4 | - | - | - | 56.1 | 56.0 | - | - | CDCl3 | [44] |
| 55 | - | 62.8 | 62.1 | - | 61.5 | - | 56.5 | 56.0 | - | - | CDCl3 | [181] |
| 56 | 61.9 | 62.3 | 61.8 | 61.6 | - | - | - | 55.3 | - | - | CDCl3 | [45] |
| 57 | - | 61.7 | 61.3 | 61.9 | 61.5 | - | - | 55.3 | - | - | DMSO-d6 | [164] |
| 58 | - | 61.8 | 61.9 | 61.6 | 62.2 | - | 56.0 | - | - | - | CDCl3 | [182] |
| 59 | 59.2 | 61.7 | 61.3 | 61.8 | 61.5 | - | - | 55.3 | - | - | DMSO-d6 | [164] |
| 60 | 59.9 | 56.4 | - | 56.6 | 61.5 | 56.0 | - | - | 55.9 | - | CDCl3 | [32] |
| 61 | 61.4 | 56.5 | - | 56.4 | 59.9 | - | 56.0 | 55.9 | - | - | CDCl3 | [178] |
| 62 | 60.0 | - | 56.3 | 61.6 | 62.2 | 56.1 | - | - | 56.0 | - | CDCl3 | [32] |
| 63 | - | 61.9 | 55.6 | 61.8 | 55.7 | - | 61.5 | 61.4 | - | - | DMSO-d6 | [31] |
| 64 | - | 61.1 | 61.6 | 62.2 | - | - | 56.4 | 56.4 | 56.4 | - | CDCl3 | [183] |
| 65 | - | 61.0 | - | 56.5 | 61.4 | - | 55.9 | 56.1 | 55.9 | - | CDCl3 | [183] |
| 66 | - | 62.4 | 62.0 | 61.9 | 61.8 | - | 56.1 | 55.8 | - | - | CDCl3 | [181] |
| 67 | 59.7 | - | 60.6 | 61.8 | 61.5 | - | 55.4 | 55.7 | - | - | DMSO-d6 | [34] |
| 68 | - | - | 62.0 | 61.1 | 61.7 | - | 56.3 | 61.1 | 56.3 | - | CDCl3 | [184] |
| 69 | 61.6 | 62.3 | 61.8 | - | 61.7 | - | 56.0 | 55.9 | - | - | CDCl3 | [44] |
| 70 | 61.9 | 62.3 | 61.8 | 61.6 | - | - | 55.9 | 55.8 | - | - | CDCl3 | [45] |
| 71 | 61.5 | 63.7 | 59.3 | 61.6 | 61.4 | - | 56.3 | 55.4 | - | - | DMSO-d6 | [31] |
| No. | Name | R1 | R2 | R3 | R4 | R5 | R6 | References |
|---|---|---|---|---|---|---|---|---|
| 72 | 5,6,7,4′-Tetramethoxyflavanone | OMe | OMe | OMe | H | H | OMe | [34] |
| 73 | 5,6,7,8,4′-Pentamethoxyflavanone | OMe | OMe | OMe | OMe | H | OMe | [185] |
| 74 | 5,6,7,3′,4′-Pentamethoxyflavanone | OMe | OMe | OMe | H | OMe | OMe | [100] |
| 75 | 5,7,8,3′,4′-Pentamethoxyflavanone | OMe | H | OMe | OMe | OMe | OMe | [186] |
| 76 | 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavanone (5-Demethylcitromitine) | OH | OMe | OMe | OMe | OMe | OMe | [34] |
| 77 | 5,6,7,8,3′,4′-Hexamethoxyflavanone (Citromitin) | OMe | OMe | OMe | OMe | OMe | OMe | [186] |
| Compound | 72 | 73 | 74 | 75 | 76 | 77 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | DMSO-d6 | CDCl3 | CDCl3 | CDCl3 | DMSO-d6 | CDCl3 | CDCl3 | CDCl3 | ||||
| Position | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) |
| 2 | 79.0 | 5.34 (dd, 13.5, 3.0) | - | 5.40 (dd, 13.2, 2.8) | 79.4 | 5.34 (dd, 13.4, 2.7) | 79.0 | 5.35 (dd, 13.0, 3.0) | 78.7 | 5.40 (dd, 13.0, 3.0) | 78.0 | 5.40 (dd, 13.0, 3.0) |
| 3 | 45.3 | 3.02 (dd, 16.5, 13.5) 2.75 (dd, 16.5, 3.0) | - | 3.03 (dd, 16.8, 13.2) 2.88 (dd, 16.8, 2.8) | 45.5 | 3.03 (dd, 16.7, 13.4) 2.76 (dd, 16.7, 2.7) | 45.6 | 3.02 (dd, 17.5, 13.0) 2.75 (dd, 17.5, 3.0) | 42.1 | 3.09 (dd, 17.0, 13.0) 2.91 (dd, 17.0, 3.0) | 45.6 | 3.05 (dd, 17.5, 13.0) 2.84 (dd, 17.5, 3.0) |
| 4 | 189.3 | - | - | - | 189.4 | - | 189.2 | - | 198.2 | - | 190.2 | - |
| 5 | 156.7 | - | - | - | 154.2 | - | 156.2 | - | 150.8 | - | 151.2 | - |
| 6 | 137.5 | - | - | - | 137.5 | - | 89.5 | - | 133.4 | - | 139.5 | - |
| 7 | 160.1 | - | - | - | 159.4 | - | 157.7 | - | 154.9 | - | 154.7 | - |
| 8 | 97.5 | 6.31 (s) | - | - | 96.4 | - | 132.0 | - | 132.8 | - | 141.6 | - |
| 9 | 160.0 | - | - | - | 159.7 | - | 156.8 | - | 148.7 | - | 150.5 | - |
| 10 | 109.4 | - | - | - | 109.1 | - | 107.5 | - | 103.9 | - | 112.0 | - |
| 1′ | 131.5 | - | - | - | 131.1 | - | 131.6 | - | 130.9 | - | 131.4 | - |
| 2′ | 1128.8 | 7.39 (d, 9.0) | - | 7.01 (m) | 109.4 | 6.99 (d, 1.8) | 114.7 | 7.02 (d, 2.5) | 110.5 | 7.00 (d, 2.5) | 114.3 | 7.02 (d, 2.5) |
| 3′ | 114.5 | 6.95 (d, 9.0) | - | - | 149.2 | - | 147.0 | - | 149.1 | - | 146.7 | - |
| 4′ | 154.1 | - | - | - | 149.4 | - | 148.2 | - | 150.4 | - | 148.1 | - |
| 5′ | 114.5 | 6.95 (d, 9.0) | - | 6.90 (d, 8.8) | 111.2 | 6.90 (d, 8.4) | 112.1 | 6.90 (d, 9.0) | 111.6 | 6.90 (d, 9.0) | 112.1 | 6.90 (d, 9.0) |
| 6′ | 128.8 | 7.39 (d, 9.0) | - | 7.01 (m) | 118.8 | 7.00 (dd, 8.4, 1.8) | 118.0 | 7.00 (dd, 9.0, 2.5) | 119.1 | 6.99 (dd, 9.0, 2.5) | 118.0 | 7.00 (dd, 9.0, 2.5) |
| OMe-5 | 61.9 | 3.94 (s) | - | 3.90 (s) | 61.3 | 3.91 (s) | 56.5 | 3.92 (s) | - | - | 62.0 | 3.90 |
| OMe-6 | 61.5 | 3.83 (s) | - | 4.06 (s) | 56.1 | 3.91 (s) | - | - | 60.6 | 4.09 (s) | 61.7 | 4.05 (s) |
| OMe-7 | 56.9 | 3.87 (s) | - | 3.90 (s) | 61.6 | 3.82 (s) | 61.4 | 3.82 (s) | 61.2 | 3.80 (s) | 61.8 | 3.82 (s) |
| OMe-8 | - | - | - | 3.86 (s) | - | - | 56.5 | 3.95 (s) | 61.0 | 3.86 (s) | 56.1 | 3.88 (s) |
| OMe-3′ | - | - | - | - | 55.9 | 3.91 (s) | 56.1 | 3.87 (s) | 55.6 | 3.91 (s) | 60.8 | 3.89 (s) |
| OMe-4′ | 55.9 | 3.83 (s) | - | 3.86 (s) | 55.9 | 3.91 (s) | 56.0 | 3.90 (s) | 55.6 | 3.91 (s) | 56.0 | 3.85 (s) |
| No. | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|---|
| 78 | 2′-Hydroxy-3,4,4′,5′,6′- pentamethoxychalcone | OH | H | OMe | OMe | OMe | OMe | OMe |
| 79 | 2′-Hydroxy-3,4,3′,4′,5′,6′-hexamethoxychalcone | OH | OMe | OMe | OMe | OMe | OMe | OMe |
| Compounds | 1H-NMR (CDCl3) | 13C-NMR (DMSO-d6) | References |
|---|---|---|---|
| 78 | 3.71 (s, 3H), 3.82 (s, 3H), 3.83 (s, 3H), 3.85 (s, 6H), 6.38 (s, 1H, H-3′), 7.02 (d, J = 8 Hz, 1H), 7.29 (dd, J = 8 Hz, 2 Hz, 1H), 7.32 (d, J = 2 Hz, 1H), 7.45 (d, J = 16 Hz, 1H), 7.57 (d, J = 16 Hz, 1H), 12.38 (s, 1H, 2′-OH) | 55.5, 55.6, 56.0, 60.6, 61.5, 96.4, 110.6, 110.8, 111.7, 122.8, 125.0, 127.4, 134.6, 143.9, 149.0, 151.2, 153.0, 157.5, 157.8, 192.6 | [34] |
| 79 | 3.74 (s, 3H), 3.75 (s, 3H), 3.77 (s, 3H), 3.80 (s, 3H), 3.81 (s, 3H), 3.93 (s, 3H), 7.00 (d, J = 9 Hz, 1H,), 7.16 (d, J = 16 Hz, 1H), 7.27 (dd, J = 9 Hz, 3 Hz, 1H), 7.34 (d, J = 3 Hz, 1H), 7.40 (d, J = 16 Hz, 1H), 10.04 (s, 1H, 2′-OH) | 55.6, 60.8, 60.9, 61.0, 61.5, 110.7, 111.6, 116.7, 123.2, 125.9, 127.2, 137.2, 138.6, 144.8, 145.7, 147.1, 148.9, 149.0, 151.2, 192.9 | [34] |
| No. | 80 | 81 | 82 | 83 | ||||
|---|---|---|---|---|---|---|---|---|
| Ref. | [181] | [181] | [187] | [187] | ||||
| Solvent | DMSO-d6 | DMSO-d6 | CD3OD-d4 | DMSO-d6 | ||||
| Position | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) |
| 2 | 151.0 | - | 151.0 | - | 157.6 | - | 156.3 | - |
| 3 | 146.2 | - | 146.2 | - | 134.4 | - | 133.2 | - |
| 4 | 172.2 | - | 172.2 | - | 178.5 | - | 177.7 | - |
| 5 | 143.5 | - | 143.5 | - | 157.1 | - | 156.3 | - |
| 6 | 137.4 | - | 137.4 | - | 99.3 | 6.29 (brs) | 95.7 | 6.57 (s) |
| 7 | 151.0 | - | 151.0 | - | 157.9 | - | 158.0 | - |
| 8 | 135.5 | - | 135.5 | - | 128.2 | - | 128.3 | - |
| 9 | 148.1 | - | 148.1 | - | 149.4 | - | 148.7 | - |
| 10 | 114.2 | - | 114.2 | - | 104.7 | - | 104.2 | - |
| 1′ | 122.7 | - | 122.7 | - | 122.1 | - | 121.2 | - |
| 2′ | 111.6 | 7.82 (d, 2.0) | 111.5 | 7.83 (d, 2.0) | 113.3 | 7.97 (brs) | 115.2 | 7.63 (brs) |
| 3′ | 147.3 | - | 147.3 | - | 147.5 | - | 144.9 | - |
| 4′ | 153.6 | - | 153.6 | - | 150.1 | - | 147.7 | - |
| 5′ | 112.5 | 7.13 (d, 9.0) | 112.5 | 7.12 (d, 9.0) | 115.2 | 6.94 (d, 8.0) | 116.0 | 6.86 (d, 8.0) |
| 6′ | 121.8 | 7.71 (dd, 9.0, 2.0) | 121.7 | 7.70 (dd, 9.0, 2.0) | 123.1 | 7.73 (brd, 8.0) | 121.7 | 7.65 (dd, 2.0, 8.0) |
| OMe | 61.9, 61.8, 61.5, 61.4, 55.7, 55.6 | 4.03 (s) 3.95 (s) 2× 3.86 (s) 3.84 (s) 3.82 (s) | 61.9, 61.7, 61.5, 61.4, 55.7, 55.6 | 4.02 (s) 3.95 (s) 2× 3.85 (s) 3.84 (s) 3.82 (s) | 61.2 (8-OMe) 55.8 (3′-OMe) | 3.94 (s, 3H, 8-OMe) 3.98 (s, 3H, 3′-OMe) | 61.1 (8-OMe) 56.5 (3′-OMe) | 3.81 (s, 3H, 8-OMe) 3.90 (s, 3H, 3′-OMe) |
| 1″ | 101.0 | - | 101.0 | - | 103.0 | 5.36 (d, 7.5) | 100.9 | 5. 46 (d, 7.0) |
| 2″ | 74.3 | - | 74.6 | - | 74.9 | 3.53 (m) | 74.0 | 3.10-3.40 (m) |
| 3″ | 74.3 | - | 74.3 | - | 77.0 | 3.39 (m) | 76.4 | 3.10-3.40 (m) |
| 4″ | 70.1 | - | 70.2 | - | 70.8 | 3.33 (m) | 69.9 | 3.10-3.40 (m) |
| 5″ | 76.4 | - | 76.4 | - | 75.0 | 3.50 (m) | 77.5 | 3.08 (m) |
| 6″ | 62.8 | - | 63.3 | - | 63.6 | 4.14 (dd, 1H, 3.0, 11.0) 4.25 (dd, 1H, 1.0, 11.0) | 60.9 | 3.40 (m, 1H) 3.57 (brd, 1H, 11.5) |
| 1‴ | 170.3 | - | 170.7 | - | 171.3 | - | - | - |
| 2‴ | 47.0 | 2.14 (d, 1H, 13.0 Hz) 2.09 (d, 1H, 13.0 Hz) | 45.3 | 2.39 (2H, m) | 45.2 | 2.52 (2H, m) | - | - |
| 3‴ | 68.5 | - | 68.7 | - | 69.7 | - | - | - |
| 4‴ | 46.7 | 1.86 (d, 1H, 15.0 Hz) 2.06 (d, 1H, 15.0 Hz) | 45.0 | 2.28 (2H, m) | 45.1 | 2.52 (2H, m) | - | - |
| 5‴ | 175.7 | - | 170.0 | - | 171.5 | - | - | - |
| 6‴ | 27.9 | 0.87 (s, 3H) | 27.1 | 1.02 (s, 3H) | 26.9 | 1.23 (s, 3H) | - | - |
| 5‴−OMe | - | - | 50.8 | 3.48 (s) | - | - | - | - |
| No. | 84 | 85 | 86 | 87 | ||||
|---|---|---|---|---|---|---|---|---|
| Ref. | [188] | [188] | [188] | [189] | ||||
| Solvent | CD3OD-d4 | CD3OD-d4 | DMSO-d6 | Pyridine-d5 | ||||
| Position | δH (J in Hz) | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) |
| 2 | - | - | 156.8 | - | 163.8 | - | 165.0 | - |
| 3 | - | - | 135.5 | - | 104.7 | 6.99 (s) | 103.9 | 7.02 (s) |
| 4 | - | - | 170.1 | - | 183.8 | - | 183.5 | - |
| 5 | - | - | 147.1 | - | 150.0 | - | 149.2 | - |
| 6 | - | - | 133.0 | - | 133.2 | - | 137.4 | - |
| 7 | - | - | 150.1 | - | 152.8 | - | 150.0 | - |
| 8 | - | - | 131.3 | - | 129.4 | - | 134.1 | - |
| 9 | - | - | 144.3 | - | 146.6 | - | 146.3 | - |
| 10 | - | - | 106.5 | - | 104.4 | - | 108.1 | - |
| 1′ | - | - | 120.6 | - | 126.1 | - | 122.6 | - |
| 2′ | 7.87 (d, 2.0) | 7.98 (d, 2.0) | 113.0 | 7.84 (d, 2.0) | 111.1 | 7.61 (d, 2.2) | 110.6 | 7.67 (d, 2.2) |
| 3′ | - | - | 152.4 | - | 150.6 | - | 149.6 | - |
| 4′ | - | - | 148.2 | - | 151.1 | - | 152.8 | - |
| 5′ | 6.96 (d, 9.0) | 6.93 (d, 8.0) | 115.5 | 6.97 (d, 8.0) | 117.0 | 7.68 (d, 8.6) | 117.3 | 7.30 (d, 8.2) |
| 6′ | 7.85 (dd, 9.0, 2.0) | 7.70 (dd, 8.0, 2.0) | 122.5 | 7.64 (dd, 8.0, 2.0) | 120.7 | 7.87 (dd, 8.6, 2.2) | 121.6 | 7.77 (dd, 8.2, 2.2) |
| −OMe | 4.08 (s) 3.97 (s) 2x 3.92 (s) 3.90 (s) 3.89 (s) | 4.04 (s) 3.91 (s) 3.87 (s) 3.83 (s) | 61.7 61.4 60.5 55.6 | 4.02 (s) 3.91 (s) 3.87 (s) 3.83 (s) | 61.6 60.6 56.3 | 4.09 (s) 4.00 (s) | 62.4 61.2 56.2 | 4.24 (s) 4.21 (s) |
| 1″ | 4.88 (d, 7.0) | 5.46 (d, 7.0) | 100.9 | 5.45 (d, 8.0) | 102.3 | 5.73 (d, 7.3) | 104.5 | 6.15 (d, 7.3) |
| 2″ | - | - | 74.2 | 2.18-2.33 (m, 2H) | 74.7 | - | 75.7 | - |
| 3″ | - | - | 76.2 | 78.3 | - | 78.2 | - | |
| 4″ | - | - | 70.0 | 2.18-2.33 (m, 2H) | 71.5 | - | 71.4 | - |
| 5″ | - | - | 74.2 | 75.7 | - | 75.9 | - | |
| 6″ | - | - | 63.0 | 1.01 (s, 2H) | 64.7 | 5.02 (dd, 12.0, 2.2) 4.74 (dd, 12.0, 6.5) | 64.4 | 4.99 (dd, 12.0, 1.0) 4.81 (dd, 12.0, 5.2) |
| 1‴ | - | - | 173.7 | - | ||||
| 2‴ | - | - | 45.3 | - | ||||
| 3‴ | - | - | 68.6 | - | ||||
| 4‴ | - | - | 45.6 | - | ||||
| 5‴ | - | - | 177.6 | - | ||||
| 6‴ | - | - | 27.0 | - | ||||
| COO- | - | - | - | - | 171.6 | - | 171.6 | - |
| - | - | - | - | δC | δH (J in Hz) | |||
| −CH2− | - | - | - | - | 46.3 | 2.88 (d, 14.6) 2.99 (d, 14.6) | ||
| −CH2− | - | - | - | - | 46.5 | 2.97 (d, 14.5) 3.04 (d, 14.5) | ||
| −COH | - | - | - | - | 70.1 | - | ||
| Me | - | - | - | - | 28.0 | 1.57 (s) | ||
| No. | 88 | 89 | 90 | 91 | ||||
|---|---|---|---|---|---|---|---|---|
| Ref. | [189] | [189] | [189] | [189] | ||||
| Solvent | Pyridine-d5 | Pyridine-d5 | Pyridine-d5 | Pyridine-d5 | ||||
| Position | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) |
| 2 | 163.8 | - | 163.8 | - | 165.3 | - | 164.8 | - |
| 3 | 104.6 | 6.98 (s) | 104.8 | 6.99 (s) | 104.1 | 7.01 (s) | 103.9 | 6.99 (s) |
| 4 | 183.3 | - | 183.3 | - | 183.7 | - | 183.4 | - |
| 5 | 150.0 | - | 150.0 | - | 150.1 | - | 149.2 | - |
| 6 | 133.2 | - | 133.2 | - | 137.6 | - | 137.3 | 6.57 (s) |
| 7 | 152.8 | - | 152.8 | - | 150.1 | - | 150.0 | - |
| 8 | 129.4 | - | 129.4 | - | 134.2 | - | 133.9 | - |
| 9 | 146.6 | - | 146.6 | - | 146.4 | - | 146.2 | - |
| 10 | 104.5 | - | 104.4 | - | 108.2 | - | 107.8 | - |
| 1′ | 125.8 | - | 126.1 | - | 122.8 | - | 122.6 | - |
| 2′ | 111.2 | 7.64 (d, 1.4) | 111.2 | 7.61 (d, 2.0) | 110.9 | 7.69 (d, 2.0) | 110.6 | 7.67 (d, 2.2) |
| 3′ | 150.5 | - | 150.7 | - | 150.2 | - | 150.1 | - |
| 4′ | 151.3 | - | 151.2 | - | 152.9 | - | 152.9 | - |
| 5′ | 116.7 | 7.68 (d, 8.5) | 117.1 | 7.74 (d, 8.4) | 117.4 | 7.31 (d, 8.4) | 117.3 | 7.32 (d, 8.6) |
| 6′ | 120.6 | 7.71 (dd, 8.5, 1.4) | 120.7 | 7.91 (dd, 8.4, 2.0) | 121.7 | 7.77 (dd, 8.4, 2.0) | 121.5 | 7.78 (dd, 2.2, 8.5) |
| OMe | 61.5 60.5 56.4 | 4.03 (s) 4.00 (s) | 61.6 60.6 56.4 | 4.10 (s) 4.00 (s) | 62.4 61.3 56.4 | 4.28 (s) 4.23 (s) | 62.2 61.1 56.2 | 4.21 (s) 4.19 (s) |
| 1″ | 102.2 | 5.83 (d, 7.1) | 102.8 | 5.74 (d, 6.8) | 104.6 | 6.15 (d, 7.2) | 104.5 | 6.28 (d, 7.0) |
| 2″ | 74.9 | - | 74.8 | - | 75.7 | - | 75.9 | - |
| 3″ | 78.6 | - | 78.5 | - | 78.4 | - | 78.2 | - |
| 4″ | 71.5 | - | 71.6 | - | 71.5 | - | 71.7 | - |
| 5″ | 79.1 | - | 75.8 | - | 76.0 | - | 79.2 | - |
| 6″ | 62.6 | 4.59 (dd, 12.1, 2.1) | 64.8 | 5.14 (dd, 12.1, 1.6) 4.77 (dd, 12.1, 6.8) | 64.5 | 4.99 (d, 11.6) 4.80 (dd, 11.6, 5.6) | 62.7 | 4.54 (dd, 11.8, 2.6) |
| COO- | - | - | 171.7 | - | 171.8 | - | - | - |
| COOH | - | - | 174.7 | - | 174.8 | - | - | - |
| −CH2− | - | - | 46.4 | 3.13 (d, 14.1) 3.32 (d, 14.1) | 46.5 | 2,99 (d, 14.5) 3.08 (d, 14.5) | - | - |
| −CH2− | - | - | 46.7 | 3.14 (d, 15.3) 3.20 (d, 15.3) | 46.5 | 3.01 (d, 15.0) 3.07 (d, 15.0) | - | - |
| −COH | - | - | 70.1 | - | 70.1 | - | - | - |
| Me | - | - | 28.3 | 1.74 (s) | 28.1 | 1.63 (s) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, G.; Bruno, M.; Sottile, F.; Badalamenti, N. The Chemistry and the Anti-Inflammatory Activity of Polymethoxyflavonoids from Citrus Genus. Antioxidants 2023, 12, 23. https://doi.org/10.3390/antiox12010023
Fontana G, Bruno M, Sottile F, Badalamenti N. The Chemistry and the Anti-Inflammatory Activity of Polymethoxyflavonoids from Citrus Genus. Antioxidants. 2023; 12(1):23. https://doi.org/10.3390/antiox12010023
Chicago/Turabian StyleFontana, Gianfranco, Maurizio Bruno, Francesco Sottile, and Natale Badalamenti. 2023. "The Chemistry and the Anti-Inflammatory Activity of Polymethoxyflavonoids from Citrus Genus" Antioxidants 12, no. 1: 23. https://doi.org/10.3390/antiox12010023
APA StyleFontana, G., Bruno, M., Sottile, F., & Badalamenti, N. (2023). The Chemistry and the Anti-Inflammatory Activity of Polymethoxyflavonoids from Citrus Genus. Antioxidants, 12(1), 23. https://doi.org/10.3390/antiox12010023










