Effect of Ascosphaera apis Infestation on the Activities of Four Antioxidant Enzymes in Asian Honey Bee Larval Guts
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi and Bee Larvae
2.2. Purification of A. apis Spores
2.3. Experimental Inoculation and Survival Rate Calculation
2.4. Evaluation of the SOD Activity
2.5. Examination of CAT and GST Activities
2.6. Detection of PPO Activity
3. Results
3.1. Verification of A. cerana Larvae Infection by Inoculation with A. apis Spores
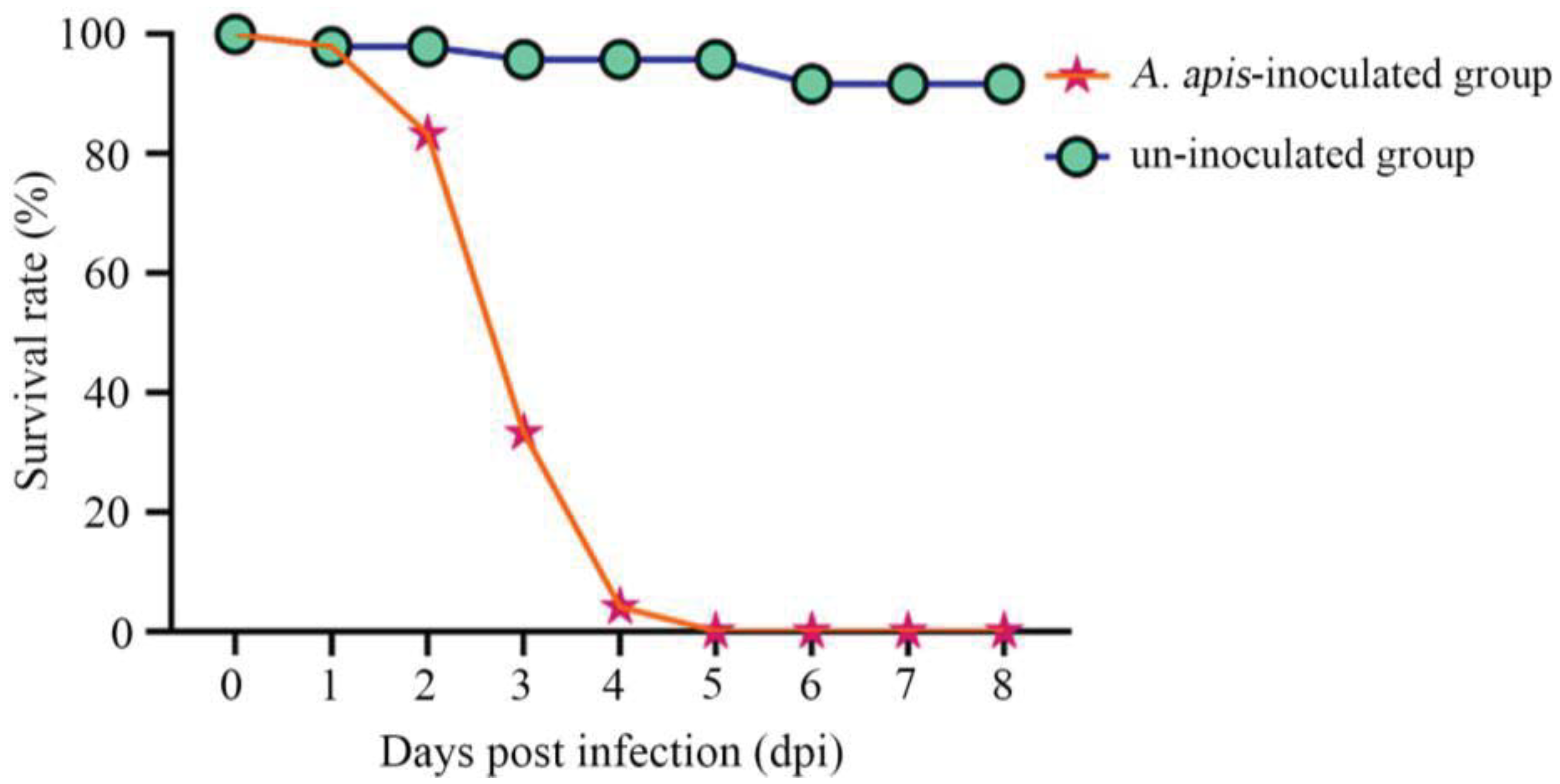
3.2. Survival Rate of A. cerana Larvae after A. apis Spores Infection
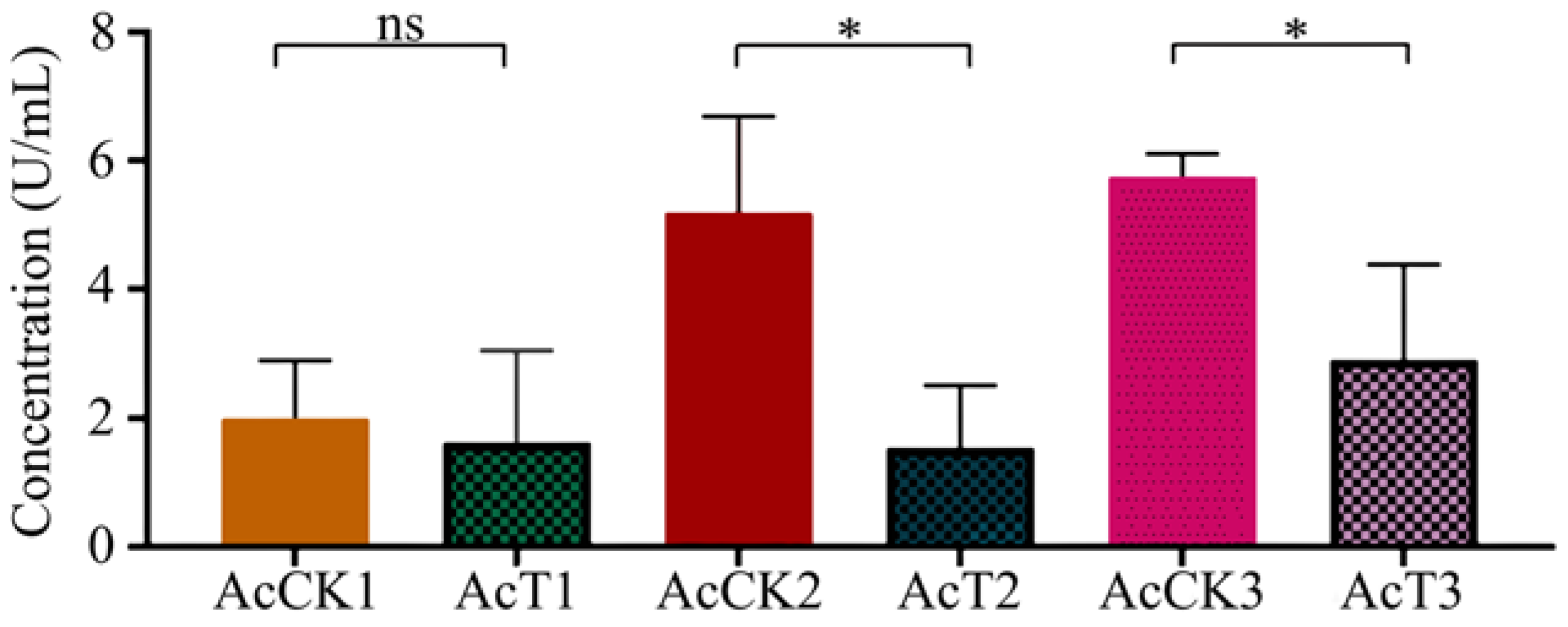
3.3. Effect of A. apis Infection on SOD Activity in A. cerana Larval Guts
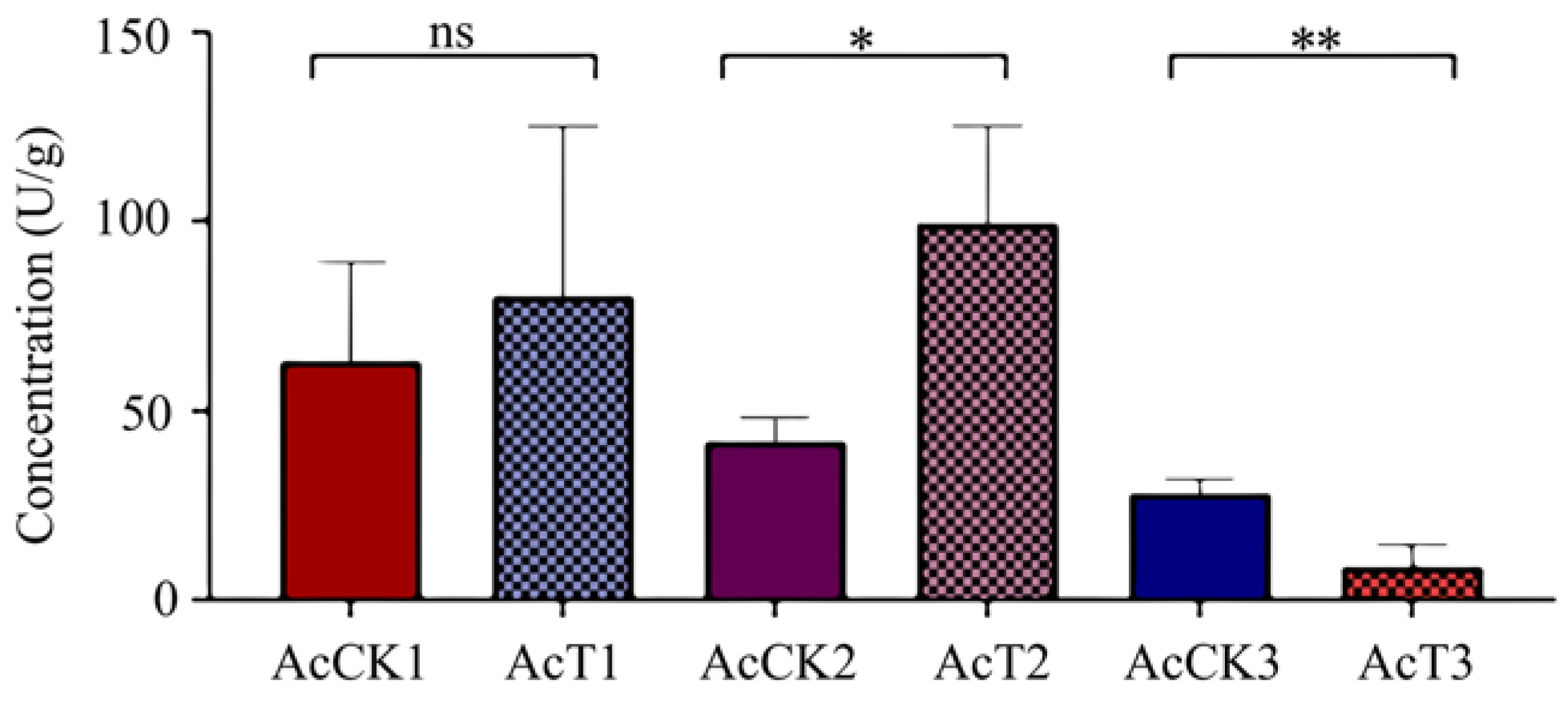
3.4. Effect of A. apis Infection on the CAT Activity in the A. cerana Larval Guts
3.5. Effect of A. apis Infection on GST Activity in A. cerana Larval Guts
3.6. Effect of A. apis Infection on PPO Activity in A. cerana Larval Guts
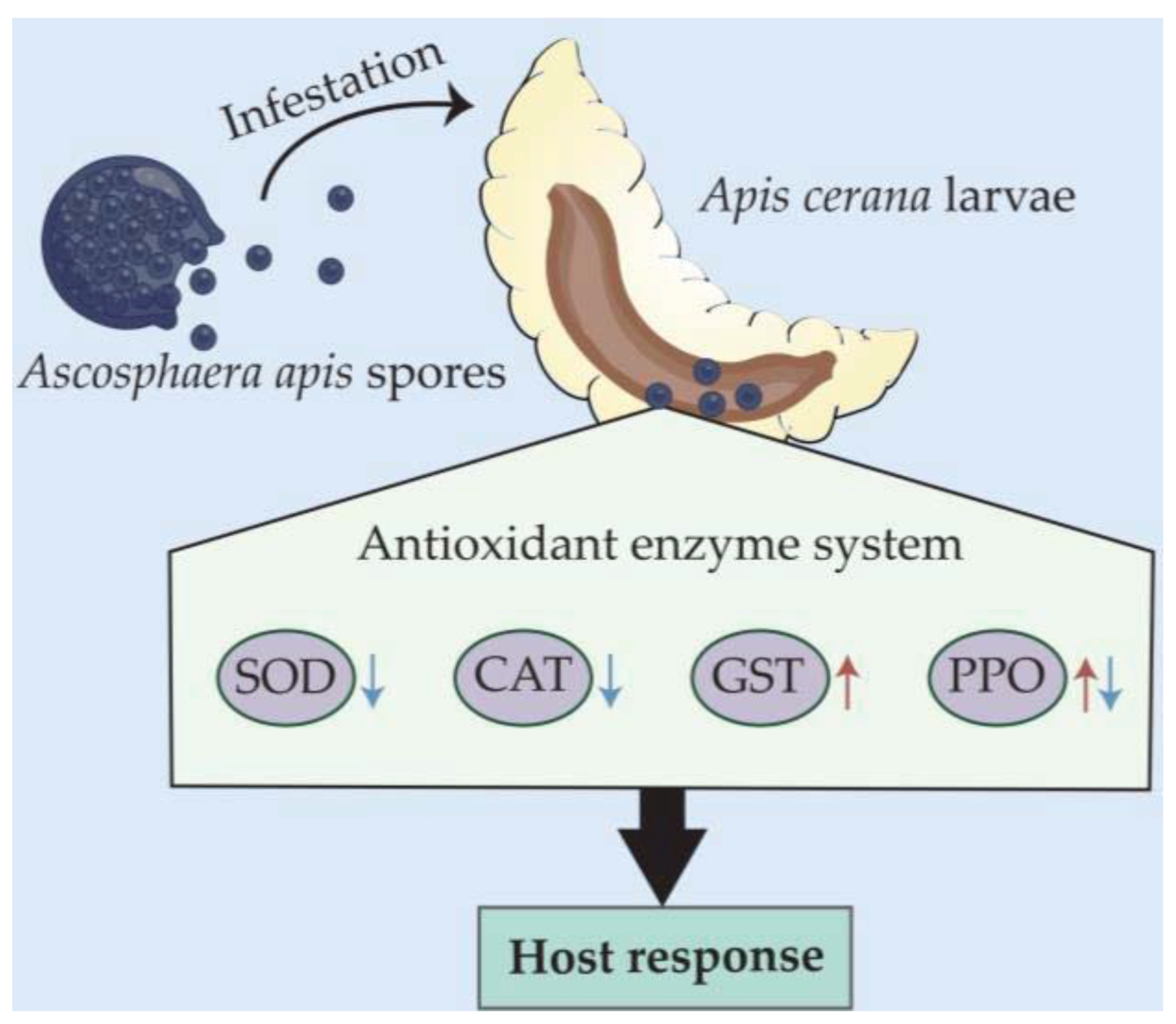
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallberg, A.; Bunikis, I.; Pettersson, O.V.; Mosbech, M.B.; Childers, A.K.; Evans, J.D. A hybrid de novo genome assembly of the honeybee, Apis mellifera, with chromosome-length scaffolds. BMC Genom. 2019, 20, 275. [Google Scholar] [CrossRef]
- Chen, D.; Guo, R.; Xu, X.; Xiong, C.; Liang, Q.; Zheng, Y.; Luo, Q.; Zhang, Z.; Huang, Z.; Kumar, D.; et al. Uncovering the immune responses of Apis mellifera ligustica larval gut to Ascosphaera apis infection utilizing transcriptome sequencing. Gene 2017, 621, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, J.; Fan, X.; Long, Q.; Chen, H.; Ye, Y.; Zhang, K.; Ren, Z.; Zhang, Y.; Niu, Q.; et al. CircRNA-regulated immune responses of asian honey bee workers to microsporidian infection. Front Genet. 2022, 13, 1013239. [Google Scholar] [CrossRef] [PubMed]
- Aronstein, K.A.; Murray, K.D. Chalkbrood disease in honey bees. J. Invertebr. Pathol. 2010, 103 (Suppl. 1), S20–S29. [Google Scholar] [CrossRef]
- Wang, X.R. The progress of study on the physiology and histopatology of insect midgut. J. Zhongkai Agrotech. Coll. 2000, 1, 58–68. (In Chinese) [Google Scholar]
- Bonnay, F.; Cohen-Berros, E.; Hoffmann, M.; Kim, S.Y.; Boulianne, G.L.; Hoffmann, J.A.; Matt, N.; Reichhart, J.M. Big bang gene modulates gut immune tolerance in Drosophila. Proc. Natl. Acad. Sci. USA 2013, 110, 2957–2962. [Google Scholar] [CrossRef]
- Sabová, L.; Sobeková, A.; Staroň, M.; Sabo, R.; Legáth, J.; Staroňová, D.; Lohajová, Ľ.; Javorský, P. Toxicity of oxalic acid and impact on some antioxidant enzymes on in vitro-reared honeybee larvae. Environ. Sci. Pollut. Res. Int. 2019, 26, 19763–19769. [Google Scholar] [CrossRef]
- Corona, M.; Robinson, G.E. Genes of the antioxidant system of the honey bee: Annotation and phylogeny. Insect. Mol. Biol. 2006, 15, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Wang, Z.H.; Nong, X.Q.; Cao, G.C.; Zhao, L.; Wang, G.J.; Zhang, Z.H. Effect of Metarhizium anisopiae on protective enzyme and detoxification enzyme in the midgut of Locusta migratoria manilensis. Chin. J. Bio. Control. 2015, 31, 876–881. (In Chinese) [Google Scholar]
- Li, Z.; Hou, M.; Qiu, Y.; Zhao, B.; Nie, H.; Su, S. Changes in antioxidant enzymes activity and metabolomic profiles in the guts of honey bee (Apis mellifera) larvae infected with Ascosphaera apis. Insects 2020, 11, 419. [Google Scholar] [CrossRef]
- Jia, F.X. Research on Mechanisms of Bactrocera dorsalis (Hendel) in Response to Thermal Stress. Ph.D. Thesis, Southwest University, Chongqing, China, 2012. (In Chinese). [Google Scholar]
- Zhao, G.S.; Wang, X.B. Advance in antitumor agents targeting glutathione-S-transferase. Curr. Med. Chem. 2006, 13, 1461–1471. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Belzunces, L.P.; Carvalho, G.A.; Brunet, J.L.; Badiou-Beneteau, A. Enzymatic biomarkers as tools to assess environmental quality: A case study of exposure of the honeybee Apis mellifera to insecticides. Environ. Toxicol. Chem. 2013, 32, 2117–2124. [Google Scholar] [CrossRef]
- Karthi, S.; Vaideki, K.; Shivakumar, M.S.; Ponsankar, A.; Thanigaivel, A.; Chellappandian, M.; Vasantha-Srinivasan, P.; Muthu-Pandian, C.K.; Hunter, W.B.; Senthil-Nathan, S. Effect of Aspergillus flavus on the mortality and activity of antioxidant enzymes of Spodoptera litura Fab. (Lepidoptera: Noctuidae) larvae. Pestic. Biochem. Physiol. 2018, 149, 54–60. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Lewis, E.E.; Tarasco, E. Activity changes of antioxidant and detoxifying enzymes in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae infected by the entomopathogenic nematode Heterorhabditis beicherriana (Rhabditida: Heterorhabditidae). Parasitol. Res. 2016, 115, 4485–4494. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Kang, Z.; Liu, H.; Ren, F.; Zhou, Y. The effects of quercetin combined with nucleopolyhedrovirus on the growth and immune response in the silkworm (Bombyx mori). Arch. Insect Biochem. Physiol. 2021, 108, e21839. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Jung, J.W.; Choi, B.S.; Jayakodi, M.; Lee, J.; Lim, J.; Yu, Y.; Choi, Y.S.; Lee, M.L.; Park, Y.; et al. Uncovering the novel characteristics of Asian honey bee, Apis cerana, by whole genome sequencing. BMC Genom. 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, H.; Du, Y.; Zhu, Z.; Wang, J.; Geng, S.; Xiong, C.; Zheng, Y.; Hou, C.; Diao, Q.; et al. Systematic identification of circular RNAs and corresponding regulatory networks unveil their potential roles in the midguts of eastern honeybee workers. Appl. Microbiol. Biotechnol. 2020, 104, 257–276. [Google Scholar] [CrossRef]
- Evison, S.E. Chalkbrood: Epidemiological perspectives from the host-parasite relationship. Curr. Opin. Insect Sci. 2015, 10, 65–70. [Google Scholar] [CrossRef]
- Tejerina, M.R.; Cabana, M.J.; Benitez-Ahrendts, M.R. Strains of Lactobacillus spp. reduce chalkbrood in Apis mellifera. J. Invertebr. Pathol. 2021, 178, 107521. [Google Scholar] [CrossRef]
- Ye, Y.; Fan, X.; Long, Q.; Wang, J.; Zhang, W.; Cai, Z.; Sun, M.; Gu, X.; Zou, P.; Chen, D.; et al. Comprehensive investigation and regulatory function of lncRNAs engaged in western honey bee larval immune response to Ascosphaera apis invasion. Front. Physiol. 2022, 13, 1082522. [Google Scholar] [CrossRef]
- Chen, D.F.; Guo, R.; Xiong, C.L.; Zheng, Y.Z.; Hou, C.H.; Fu, Z.M. Morphological and molecular identification of chalkbrood disease pathogen Ascosphaera apis in Apis cerana cerana. J. Apicult. Res. 2018, 57, 516–521. [Google Scholar] [CrossRef]
- Guo, R.; Chen, D.; Diao, Q.; Xiong, C.; Zheng, Y.; Hou, C. Transcriptomic investigation of immune responses of the Apis cerana cerana larval gut infected by Ascosphaera apis. J. Invertebr. Pathol. 2019, 166, 107210. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.L.; Du, Y.; Feng, R.R.; Jiang, H.B.; Shi, X.Y.; Wang, H.P.; Fan, X.X.; Wang, J.; Zhu, Z.W.; Fan, Y.C.; et al. Differential expression pattern and regulation network of microRNAs in Ascosphaera apis invading Apis cerana cerana 6-day-old larvae. Acta Microbiol. Sin. 2020, 60, 992–1009. (In Chinese) [Google Scholar]
- Du, Y.; Feng, R.R.; Wang, J.; Zhu, Z.W.; Zhang, W.D.; Yu, K.J.; Long, Q.; Cai, Z.B.; Xie, Y.L.; Xiong, C.L.; et al. Long non-coding RNA response of 6-day-old Apis cerana cerana larvae to Ascosphaera apis infection. Acta Microbiol. Sin. 2021, 61, 1338–1358. (In Chinese) [Google Scholar]
- Chen, H.Z.; Fu, Z.M.; Wang, J.; Zhu, Z.W.; Fan, X.X.; Jiang, H.B.; Fan, Y.C.; Zhou, D.D.; Li, W.D.; Xiong, C.L.; et al. Circular RNA response of Apis cerana cerana 6-day-old larvae to Ascosphaera apis stress. Acta Microbiol. Sin. 2020, 60, 2292–2310. (In Chinese) [Google Scholar]
- Guo, R.; Chen, D.F.; Huang, Z.J.; Liang, Q.; Xiong, C.L.; Xu, X.J.; Zheng, Y.Z.; Zhang, Z.N.; Xie, Y.L.; Tong, X.Y.; et al. Transcriptome analysis of Ascosphaera apis stressing larval gut of Apis cerana cerana. Acta Microbiol. Sin. 2017, 57, 1865–1878. (In Chinese) [Google Scholar]
- Guo, R.; Chen, D.; Chen, H.; Fu, Z.; Xiong, C.; Hou, C.; Zheng, Y.; Guo, Y.; Wang, H.; Du, Y.; et al. Systematic investigation of circular RNAs in Ascosphaera apis, a fungal pathogen of honeybee larvae. Gene 2018, 678, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, D.; Xiong, C.; Hou, C.; Zheng, Y.; Fu, Z.; Diao, Q.; Zhang, L.; Wang, H.; Hou, Z.; et al. Identification of long non-coding RNAs in the chalkbrood disease pathogen Ascospheara apis. J. Invertebr. Pathol. 2018, 156, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Du, Y.; Zhou, N.H.; Liu, S.Y.; Xiong, C.L.; Zhen, Y.Z.; Fu, Z.M.; Xu, G.J.; Wang, H.P.; Geng, S.H.; et al. Comprehensive analysis of differentially expressed microRNAs and their target genes in the larval gut of Apis mellifera ligustica during the late stage of Ascosphaera apis stress. Acta Entomol. Sin. 2019, 62, 49–60. (In Chinese) [Google Scholar]
- Chen, H.Z.; Zhu, Z.W.; Jiang, H.B.; Wang, J.; Fan, Y.C.; Fan, X.X.; Wan, J.Q.; Lu, J.X.; Xiong, C.L.; Zhen, Y.Z.; et al. Comparative analysis of microRNAs and corresponding target mRNAs in Ascosphaera apis mycelium and spore. Sci. Agric. Sin. 2020, 53, 3606–3619. (In Chinese) [Google Scholar]
- Feng, R.R.; Fu, Z.M.; Du, Y.; Zhang, W.D.; Fan, X.X.; Wang, H.P.; Wan, J.Q.; Zhou, Z.Y.; Kang, Y.X.; Chen, D.F.; et al. Identification and analysis of micro RNAs in the larval gut of Apis cerana cerana. Sci. Agric. Sin. 2022, 55, 208–218. (In Chinese) [Google Scholar]
- Li, B.; Ding, Y.; Tang, X.; Wang, G.; Wu, S.; Li, X.; Huang, X.; Qu, T.; Chen, J.; Tang, X. Effect of L-arginine on maintaining storage quality of the white button mushroom (Agaricus bisporus). Food Bioprocess Technol. 2019, 12, 563–574. [Google Scholar] [CrossRef]
- Li, J.H.; Zheng, Z.Y.; Chen, D.F.; Liang, Q. Factors influencing Ascosphaera apis infection on honeybee larvae and observation on the infection process. Acta Entomol. Sin. 2012, 55, 790–797. (In Chinese) [Google Scholar]
- Pasho, D.J.M.; Applegate, J.R., Jr.; Hopkins, D.I. Diseases and Pests of Honey Bees (Apis Mellifera). Vet. Clin. N. Am. Food Anim. Pract. 2021, 37, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Chen, J.M.; Shu, J.P.; Li, F.; Zhong, H.Y.; Wu, H. Physiological effects of Metarhiziym flavoviride on Brown Planthopper (Nilaparvata lugens). Chin. J. Bio. Control. 2018, 34, 701–707. (In Chinese) [Google Scholar]
- Ding, S.Y.; Li, H.Y.; Li, X.F.; Zhang, Z.Y. Effects of two kinds of transgenic poplar on protective enzymes system in the midgut of larvae of American white moth. J. For Res. 2001, 12, 119–122. [Google Scholar] [CrossRef]
- Yan, A.H.; Ren, X.Y.; Li, N.; Wang, Z.G. Changes of three kinds of detoxification enzymes activities in Anoplophora glabripennis lnfected by Entomo pathogenetic nematodes. J. Hebei Agric. Sci. 2012, 16, 78–80+84. (In Chinese) [Google Scholar]
- Huang, F.; Zhang, J.M.; Li, W.D.; Lv, Y.B. Effect of parasitism Diadegma semiclausum on the detoxification enzyme system of the larvae of Plutella xylostell. J. Zhejiang Agric. Sci. 2021, 5, 700–703. (In Chinese) [Google Scholar]
- Amparyup, P.; Wiriyaukaradecha, K.; Charoensapsri, W.; Tassanakajon, A. A clip domain serine proteinase plays a role in antibacterial defense but is not required for prophenoloxidase activation in shrimp. Dev. Comp. Immunol. 2010, 34, 168–176. [Google Scholar] [CrossRef]
- Jiang, J.W.; Cong, Y.; Dong, Y.; Zhou, Z.C. The variation of immune-related enzyme activities in the coelomic fluid of sea cucumber (Apostichopus japonicus) after challenge with different bacteria. Chin. J. Zool. 2015, 50, 947–956. (In Chinese) [Google Scholar]
- Li, H.P.; Huang, D.Z.; Su, X.Y.; Huang, Q.X. Change of activity of phenoloxodease and hemocytes in Apriona germari larvae infected by Beauveria bassiana. Sci. Sivae. Sin. 2009, 45, 175–178. (In Chinese) [Google Scholar]
- Wertheim, B.; Kraaijeveld, A.R.; Schuster, E.; Blanc, E.; Hopkins, M.; Pletcher, S.D.; Strand, M.R.; Partridge, L.; Godfray, H.C. Genome-wide gene expression in response to parasitoid attack in Drosophila. Genome Biol. 2005, 6, R94. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Fu, Z.; Fan, X.; Wang, Z.; Wang, S.; Guo, S.; Gao, X.; Zhao, H.; Jing, X.; Zou, P.; et al. Effect of Ascosphaera apis Infestation on the Activities of Four Antioxidant Enzymes in Asian Honey Bee Larval Guts. Antioxidants 2023, 12, 206. https://doi.org/10.3390/antiox12010206
Zhang K, Fu Z, Fan X, Wang Z, Wang S, Guo S, Gao X, Zhao H, Jing X, Zou P, et al. Effect of Ascosphaera apis Infestation on the Activities of Four Antioxidant Enzymes in Asian Honey Bee Larval Guts. Antioxidants. 2023; 12(1):206. https://doi.org/10.3390/antiox12010206
Chicago/Turabian StyleZhang, Kaiyao, Zhongmin Fu, Xiaoxue Fan, Zixin Wang, Siyi Wang, Sijia Guo, Xuze Gao, Haodong Zhao, Xin Jing, Peiyuan Zou, and et al. 2023. "Effect of Ascosphaera apis Infestation on the Activities of Four Antioxidant Enzymes in Asian Honey Bee Larval Guts" Antioxidants 12, no. 1: 206. https://doi.org/10.3390/antiox12010206
APA StyleZhang, K., Fu, Z., Fan, X., Wang, Z., Wang, S., Guo, S., Gao, X., Zhao, H., Jing, X., Zou, P., Li, Q., Chen, M., Chen, D., & Guo, R. (2023). Effect of Ascosphaera apis Infestation on the Activities of Four Antioxidant Enzymes in Asian Honey Bee Larval Guts. Antioxidants, 12(1), 206. https://doi.org/10.3390/antiox12010206






