Oxidative Stress and Antioxidants in Chronic Rhinosinusitis with Nasal Polyps
Abstract
1. Introduction
2. Oxidative Stress in Respiratory Diseases
3. CRS and Oxidative Stress
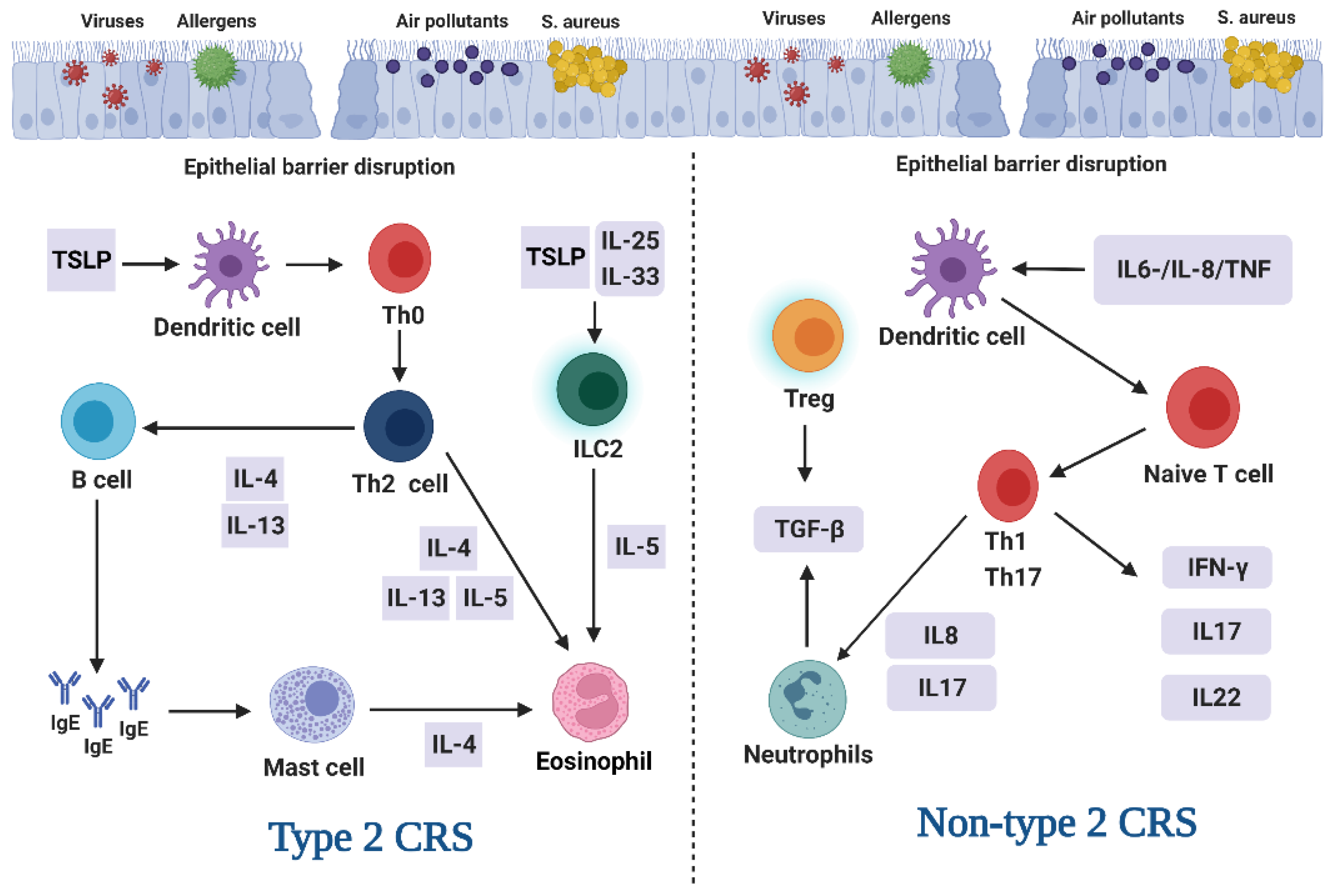
3.1. Endotype and Phenotype of CRS
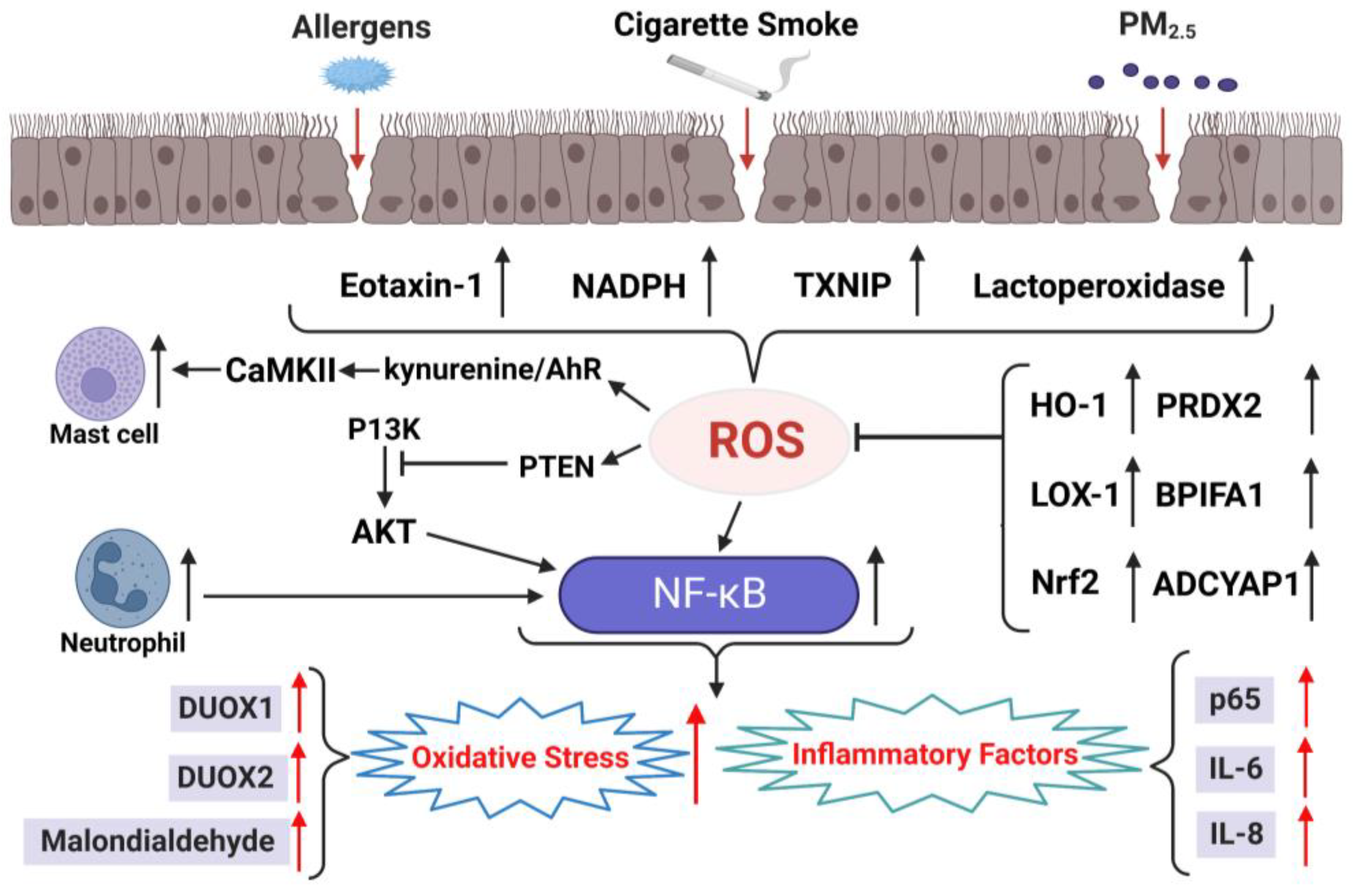
3.2. Oxidative Stress in Chronic Sinusitis with NPs
3.3. Therapeutic Antioxidants in Chronic Rhinosinusitis with NPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef]
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef]
- Tartaglia, L.A.; Storz, G.; Ames, B.N. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J. Mol. Biol. 1989, 210, 709–719. [Google Scholar] [CrossRef]
- Szewczyk-Golec, K.; Czuczejko, J.; Tylzanowski, P.; Lecka, J. Strategies for Modulating Oxidative Stress under Diverse Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2018, 2018, 3987941. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, C.; Li, K.; Adler, K.B.; Wang, X. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir. Med. 2008, 102, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Nadeem, A.; Al-Harbi, M.M.; Ansari, M.A.; AlSharari, S.D.; Bahashwan, S.A.; Attia, S.M.; Al-Hosaini, K.A.; Al Hoshani, A.R.; Ahmad, S.F. Airway oxidative stress causes vascular and hepatic inflammation via upregulation of IL-17A in a murine model of allergic asthma. Int. Immunopharmacol. 2016, 34, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Peden, D.B.; Brown, M.E.; Wade, Y.; Raphael, G.D.; Berkebile, C.; Kaliner, M.A. Human nasal glandular secretion of novel antioxidant activity: Cholinergic control. Am. Rev. Respir. Dis. 1991, 143, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Guo, Z.; Zhang, R.; Xu, J.; Dong, W.; Zhuang, G.; Deng, C. Airborne Fine Particulate Matter Induces Oxidative Stress and Inflammation in Human Nasal Epithelial Cells. Tohoku J. Exp. Med. 2016, 239, 117–125. [Google Scholar] [CrossRef]
- Kennedy, D.W. Pathogenesis of chronic rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 2004, 193, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Bhattacharyya, N.; Desrosiers, M.; Khan, A.H. Burden of Disease in Chronic Rhinosinusitis with Nasal Polyps. J. Asthma Allergy 2021, 14, 127–134. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.; Bachert, C.; Mullol, J.; Bjermer, L.; Bousquet, J.; Canonica, G.W.; Deneyer, L.; Desrosiers, M.; Diamant, Z.; et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy 2019, 74, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Cho, S.H. Emerging Endotypes of Chronic Rhinosinusitis and Its Application to Precision Medicine. Allergy Asthma Immunol. Res. 2017, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Han, M.; Kim, T.H. Therapeutic Strategies of Biologics in Chronic Rhinosinusitis: Current Options and Future Targets. Int. J. Mol. Sci. 2022, 23, 5523. [Google Scholar] [CrossRef]
- Kao, S.S.; Bassiouni, A.; Ramezanpour, M.; Finnie, J.; Chegeni, N.; Colella, A.D.; Chataway, T.K.; Wormald, P.J.; Vreugde, S.; Psaltis, A.J. Proteomic analysis of nasal mucus samples of healthy patients and patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2021, 147, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Fu, Z.; Wang, H.; Feng, J.; Wei, J.; Guo, J. Peroxiredoxin 2 is upregulated in colorectal cancer and contributes to colorectal cancer cells’ survival by protecting cells from oxidative stress. Mol. Cell. Biochem. 2014, 387, 261–270. [Google Scholar] [CrossRef]
- Britto, C.J.; Cohn, L. Bactericidal/Permeability-increasing protein fold-containing family member A1 in airway host protection and respiratory disease. Am. J. Respir. Cell Mol. Biol. 2015, 52, 525–534. [Google Scholar] [CrossRef]
- Yim, S.K.; Kim, K.M.; Lee, C.H.; Song, E.K.; Lee, S.O.; Kim, S.W.; Kim, I.H.; Kim, S.H.; Seo, S.Y.; Lee, S.T. The Superoxide Dismutase Mimetic M40403, Improves 5-Fluorouracil-induced Small Intestinal Mucositis in a Mouse Model. In Vivo 2021, 35, 1485–1497. [Google Scholar] [CrossRef]
- Bereswill, S.; Escher, U.; Grunau, A.; Kühl, A.A.; Dunay, I.R.; Tamas, A.; Reglodi, D.; Heimesaat, M.M. Pituitary Adenylate Cyclase-Activating Polypeptide-A Neuropeptide as Novel Treatment Option for Subacute Ileitis in Mice Harboring a Human Gut Microbiota. Front. Immunol. 2019, 10, 554. [Google Scholar] [CrossRef]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef]
- Testa, D.; Marcuccio, G.; Panin, G.; Bianco, A.; Tafuri, D.; Thyrion, F.Z.; Nunziata, M.; Piombino, P.; Guerra, G.; Motta, G. Nasal mucosa healing after endoscopic sinus surgery in chronic rhinosinusitis of elderly patients: Role of topic alpha-tocopherol acetate. Aging Clin. Exp. Res. 2017, 29, 191–195. [Google Scholar] [CrossRef]
- Arbex, M.A.; Santos Ude, P.; Martins, L.C.; Saldiva, P.H.; Pereira, L.A.; Braga, A.L. Air pollution and the respiratory system. J. Bras. Pneumol. 2012, 38, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Corsico, A.; Solidoro, P.; Braido, F.; Di Marco, F.; Scichilone, N. Oxidative stress and respiratory system: Pharmacological and clinical reappraisal of N-acetylcysteine. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F. Oxidative stress in airway diseases. Ann. Am. Thorac. Soc. 2013, 10, S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Voynow, J.A.; Ghio, A.J. COPD: Balancing oxidants and antioxidants. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 261–276. [Google Scholar] [CrossRef]
- Mehta, P.; Bothiraja, C.; Mahadik, K.; Kadam, S.; Pawar, A. Phytoconstituent based dry powder inhalers as biomedicine for the management of pulmonary diseases. Biomed. Pharmacother. 2018, 108, 828–837. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef]
- Ciencewicki, J.; Trivedi, S.; Kleeberger, S.R. Oxidants and the pathogenesis of lung diseases. J. Allergy Clin. Immunol. 2008, 122, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Rajendrasozhan, S.; Yao, H.; Chung, S.; Sundar, I.K.; Huyck, H.L.; Pryhuber, G.S.; Kinnula, V.L.; Rahman, I. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J. Immunol. 2011, 187, 987–998. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- Dozor, A.J. The role of oxidative stress in the pathogenesis and treatment of asthma. Ann. N. Y. Acad. Sci. 2010, 1203, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.; Bhathena, P.R.; Dweik, R.A.; Kavuru, M.; Erzurum, S.C. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet 2000, 355, 624. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Comhair, S.A.; Gaston, B.; Thunnissen, F.B.; Farver, C.; Thomassen, M.J.; Kavuru, M.; Hammel, J.; Abu-Soud, H.M.; Erzurum, S.C. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc. Natl. Acad. Sci. USA 2001, 98, 2622–2627. [Google Scholar] [CrossRef]
- Kleniewska, P.; Pawliczak, R. The participation of oxidative stress in the pathogenesis of bronchial asthma. Biomed. Pharmacother. 2017, 94, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Cepelak, I.; Dodig, S. Exhaled breath condensate: A new method for lung disease diagnosis. Clin. Chem. Lab. Med. 2007, 45, 945–952. [Google Scholar] [CrossRef]
- Araneda, O.F.; Carbonell, T.; Tuesta, M. Update on the Mechanisms of Pulmonary Inflammation and Oxidative Imbalance Induced by Exercise. Oxid. Med. Cell. Longev. 2016, 2016, 4868536. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, F.M.; Thomas, P.S.; Bourke, J.E.; Matheson, M.C.; Dharmage, S.C.; Lowe, A.J. Relationships between adult asthma and oxidative stress markers and pH in exhaled breath condensate: A systematic review. Allergy 2016, 71, 741–757. [Google Scholar] [CrossRef]
- Thomas, P.S.; Lowe, A.J.; Samarasinghe, P.; Lodge, C.J.; Huang, Y.; Abramson, M.J.; Dharmage, S.C.; Jaffe, A. Exhaled breath condensate in pediatric asthma: Promising new advance or pouring cold water on a lot of hot air? a systematic review. Pediatr. Pulmonol. 2013, 48, 419–442. [Google Scholar] [CrossRef]
- Peterson, J.D.; Herzenberg, L.A.; Vasquez, K.; Waltenbaugh, C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 3071–3076. [Google Scholar] [CrossRef]
- Corradi, M.; Folesani, G.; Andreoli, R.; Manini, P.; Bodini, A.; Piacentini, G.; Carraro, S.; Zanconato, S.; Baraldi, E. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am. J. Respir. Crit. Care Med. 2003, 167, 395–399. [Google Scholar] [CrossRef]
- Wu, W.; Samoszuk, M.K.; Comhair, S.A.; Thomassen, M.J.; Farver, C.F.; Dweik, R.A.; Kavuru, M.S.; Erzurum, S.C.; Hazen, S.L. Eosinophils generate brominating oxidants in allergen-induced asthma. J. Clin. Investig. 2000, 105, 1455–1463. [Google Scholar] [CrossRef]
- Ghosh, S.; Masri, F.; Comhair, S.; Andreadis, A.; Swaidani, S.; Aronica, M.; Aulak, K.; Erzurum, S. Nitration of proteins in murine model of asthma. Am. J. Respir. Crit. Care Med. 2003, 167, A889. [Google Scholar]
- Sackesen, C.; Ercan, H.; Dizdar, E.; Soyer, O.; Gumus, P.; Tosun, B.N.; Büyüktuncer, Z.; Karabulut, E.; Besler, T.; Kalayci, O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J. Allergy Clin. Immunol. 2008, 122, 78–85. [Google Scholar] [CrossRef]
- Wedes, S.H.; Khatri, S.B.; Zhang, R.; Wu, W.; Comhair, S.A.; Wenzel, S.; Teague, W.G.; Israel, E.; Erzurum, S.C.; Hazen, S.L. Noninvasive markers of airway inflammation in asthma. Clin. Transl. Sci. 2009, 2, 112–117. [Google Scholar] [CrossRef]
- Comhair, S.A.; Erzurum, S.C. Redox control of asthma: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 93–124. [Google Scholar] [CrossRef] [PubMed]
- Larsen, G.L.; White, C.W.; Takeda, K.; Loader, J.E.; Nguyen, D.D.; Joetham, A.; Groner, Y.; Gelfand, E.W. Mice that overexpress Cu/Zn superoxide dismutase are resistant to allergen-induced changes in airway control. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L350–L359. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Asp. Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Shalaby, K.H.; Allard-Coutu, A.; O’Sullivan, M.J.; Nakada, E.; Qureshi, S.T.; Day, B.J.; Martin, J.G. Inhaled birch pollen extract induces airway hyperresponsiveness via oxidative stress but independently of pollen-intrinsic NADPH oxidase activity, or the TLR4-TRIF pathway. J. Immunol. 2013, 191, 922–933. [Google Scholar] [CrossRef]
- Carlsten, C.; MacNutt, M.J.; Zhang, Z.; Sava, F.; Pui, M.M. Anti-oxidant N-acetylcysteine diminishes diesel exhaust-induced increased airway responsiveness in person with airway hyper-reactivity. Toxicol. Sci. 2014, 139, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Riedl, M.A.; Saxon, A.; Diaz-Sanchez, D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin. Immunol. 2009, 130, 244–251. [Google Scholar] [CrossRef]
- Sudini, K.; Diette, G.B.; Breysse, P.N.; McCormack, M.C.; Bull, D.; Biswal, S.; Zhai, S.; Brereton, N.; Peng, R.D.; Matsui, E.C. A Randomized Controlled Trial of the Effect of Broccoli Sprouts on Antioxidant Gene Expression and Airway Inflammation in Asthmatics. J. Allergy Clin. Immunol. Pract. 2016, 4, 932–940. [Google Scholar] [CrossRef]
- Teng, Y.; Sun, P.; Zhang, J.; Yu, R.; Bai, J.; Yao, X.; Huang, M.; Adcock, I.M.; Barnes, P.J. Hydrogen peroxide in exhaled breath condensate in patients with asthma: A promising biomarker? Chest 2011, 140, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Luan, G.; Li, G.; Ma, X.; Jin, Y.; Hu, N.; Li, J.; Wang, Z.; Wang, H. Dexamethasone-Induced Mitochondrial Dysfunction and Insulin Resistance-Study in 3T3-L1 Adipocytes and Mitochondria Isolated from Mouse Liver. Molecules 2019, 24, 1982. [Google Scholar] [CrossRef] [PubMed]
- Kračun, D.; Klop, M.; Knirsch, A.; Petry, A.; Kanchev, I.; Chalupsky, K.; Wolf, C.M.; Görlach, A. NADPH oxidases and HIF1 promote cardiac dysfunction and pulmonary hypertension in response to glucocorticoid excess. Redox Biol. 2020, 34, 101536. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Lee, D.; Lee, S.H.; Kim, T.H. Oxidative Stress and Antioxidant Pathway in Allergic Rhinitis. Antioxidants 2021, 10, 1266. [Google Scholar] [CrossRef]
- Klain, A.; Indolfi, C.; Dinardo, G.; Licari, A.; Cardinale, F.; Caffarelli, C.; Manti, S.; Ricci, G.; Pingitore, G.; Tosca, M.; et al. United airway disease. Acta Biomed. 2021, 92, e2021526. [Google Scholar] [CrossRef]
- Lee, K.; Tai, J.; Lee, S.H.; Kim, T.H. Advances in the Knowledge of the Underlying Airway Remodeling Mechanisms in Chronic Rhinosinusitis Based on the Endotypes: A Review. Int. J. Mol. Sci. 2021, 22, 910. [Google Scholar] [CrossRef]
- Van Zele, T.; Claeys, S.; Gevaert, P.; Van Maele, G.; Holtappels, G.; Van Cauwenberge, P.; Bachert, C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006, 61, 1280–1289. [Google Scholar] [CrossRef]
- Delemarre, T.; Holtappels, G.; De Ruyck, N.; Zhang, N.; Nauwynck, H.; Bachert, C.; Gevaert, E. Type 2 inflammation in chronic rhinosinusitis without nasal polyps: Another relevant endotype. J. Allergy Clin. Immunol. 2020, 146, 337–343.e6. [Google Scholar] [CrossRef]
- Shi, L.L.; Song, J.; Xiong, P.; Cao, P.P.; Liao, B.; Ma, J.; Zhang, Y.N.; Zeng, M.; Liu, Y.; Wang, H.; et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am. J. Respir. Crit. Care Med. 2014, 190, 628–638. [Google Scholar] [CrossRef]
- Klingler, A.I.; Stevens, W.W.; Tan, B.K.; Peters, A.T.; Poposki, J.A.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; Conley, D.B.; Kern, R.C.; et al. Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J. Allergy Clin. Immunol. 2021, 147, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Kato, A. Immunopathology of chronic rhinosinusitis. Allergol. Int. 2015, 64, 121–130. [Google Scholar] [CrossRef]
- Ogasawara, N.; Klingler, A.I.; Tan, B.K.; Poposki, J.A.; Hulse, K.E.; Stevens, W.W.; Peters, A.T.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; et al. Epithelial activators of type 2 inflammation: Elevation of thymic stromal lymphopoietin, but not IL-25 or IL-33, in chronic rhinosinusitis with nasal polyps in Chicago, Illinois. Allergy 2018, 73, 2251–2254. [Google Scholar] [CrossRef]
- Zhang, N.; Van Zele, T.; Perez-Novo, C.; Van Bruaene, N.; Holtappels, G.; DeRuyck, N.; Van Cauwenberge, P.; Bachert, C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J. Allergy Clin. Immunol. 2008, 122, 961–968. [Google Scholar] [CrossRef]
- Grayson, J.W.; Hopkins, C.; Mori, E.; Senior, B.; Harvey, R.J. Contemporary Classification of Chronic Rhinosinusitis Beyond Polyps vs No Polyps: A Review. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 831–838. [Google Scholar] [CrossRef]
- Cho, S.H.; Hamilos, D.L.; Han, D.H.; Laidlaw, T.M. Phenotypes of Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, A.; Niewiadomski, P.; Olszewski, J. Influence of nasal mucosa irritants on the occurrence of chronic rhinosinusitis without /and with polyps. Otolaryngol. Pol. 2021, 75, 36–44. [Google Scholar] [CrossRef]
- Mihalj, H.; Butković, J.; Tokić, S.; Štefanić, M.; Kizivat, T.; Bujak, M.; Baus Lončar, M.; Mihalj, M. Expression of Oxidative Stress and Inflammation-Related Genes in Nasal Mucosa and Nasal Polyps from Patients with Chronic Rhinosinusitis. Int. J. Mol. Sci. 2022, 23, 5521. [Google Scholar] [CrossRef]
- Cekin, E.; Ipcioglu, O.M.; Erkul, B.E.; Kapucu, B.; Ozcan, O.; Cincik, H.; Gungor, A. The association of oxidative stress and nasal polyposis. J. Int. Med. Res. 2009, 37, 325–330. [Google Scholar] [CrossRef]
- Bozkus, F.; San, I.; Ulas, T.; Iynen, I.; Yesilova, Y.; Guler, Y.; Aksoy, N. Evaluation of total oxidative stress parameters in patients with nasal polyps. Acta Otorhinolaryngol. Ital. 2013, 33, 248–253. [Google Scholar]
- Lee, W.H.; Hong, S.N.; Kim, H.J.; Ahn, S.; Rhee, C.S.; Lee, C.H.; Kim, J.W. Effects of cigarette smoking on rhinologic diseases: Korean National Health and Nutrition Examination Survey 2008–2011. Int. Forum Allergy Rhinol. 2015, 5, 937–943. [Google Scholar] [CrossRef]
- Mady, L.J.; Schwarzbach, H.L.; Moore, J.A.; Boudreau, R.M.; Tripathy, S.; Kinnee, E.; Dodson, Z.M.; Willson, T.J.; Clougherty, J.E.; Lee, S.E. Air pollutants may be environmental risk factors in chronic rhinosinusitis disease progression. Int. Forum Allergy Rhinol. 2018, 8, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Rusznak, C.; Sapsford, R.J.; Devalia, J.L.; Justin John, R.; Hewitt, E.L.; Lamont, A.G.; Wood, A.J.; Shah, S.S.; Davies, R.J.; Lozewicz, S. Cigarette smoke potentiates house dust mite allergen-induced increase in the permeability of human bronchial epithelial cells in vitro. Am. J. Respir. Cell Mol. Biol. 1999, 20, 1238–1250. [Google Scholar] [CrossRef]
- Taub, D.D.; Oppenheim, J.J. Review of the chemokine meeting the Third International Symposium of Chemotactic Cytokines. Cytokine 1993, 5, 175–179. [Google Scholar] [CrossRef]
- Sozzani, S.; Bosisio, D.; Mantovani, A.; Ghezzi, P. Linking stress, oxidation and the chemokine system. Eur. J. Immunol. 2005, 35, 3095–3098. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Kojima, Y.; Koyanagi, A.; Yokoi, H.; Saito, T.; Kawano, K.; Furukawa, M.; Kusunoki, T.; Ikeda, K. Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope 2009, 119, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Yoshifuku, K.; Matsune, S.; Ohori, J.; Sagara, Y.; Fukuiwa, T.; Kurono, Y. IL-4 and TNF-alpha increased the secretion of eotaxin from cultured fibroblasts of nasal polyps with eosinophil infiltration. Rhinology 2007, 45, 235–241. [Google Scholar]
- Brandes, R.P.; Weissmann, N.; Schröder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef]
- Zheng, K.; Hao, J.; Xiao, L.; Wang, M.; Zhao, Y.; Fan, D.; Li, Y.; Wang, X.; Zhang, L. Expression of nicotinamide adenine dinucleotide phosphate oxidase in chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2020, 10, 646–655. [Google Scholar] [CrossRef]
- Alhawiti, N.M.; Al Mahri, S.; Aziz, M.A.; Malik, S.S.; Mohammad, S. TXNIP in Metabolic Regulation: Physiological Role and Therapeutic Outlook. Curr. Drug Targets 2017, 18, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ba, G.; Tang, R.; Li, M.; Li, Z.; Li, D.; Ye, H.; Zhang, W. Increased Expression of TXNIP Facilitates Oxidative Stress in Nasal Epithelial Cells of Patients With Chronic Rhinosinusitis With Nasal Polyps. Am. J. Rhinol. Allergy 2021, 35, 607–614. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Zhang, J.; Li, L.; Wu, X.; Ma, R.; Han, M.; Xu, G.; Wen, W.; Li, H. Expression of heme oxygenase-1 in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps: Modulation by cytokines. Int. Forum Allergy Rhinol. 2015, 5, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hao, M.; Phalen, R.F.; Hinds, W.C.; Nel, A.E. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin. Immunol. 2003, 109, 250–265. [Google Scholar] [CrossRef]
- Sawamura, T.; Kume, N.; Aoyama, T.; Moriwaki, H.; Hoshikawa, H.; Aiba, Y.; Tanaka, T.; Miwa, S.; Katsura, Y.; Kita, T.; et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997, 386, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Takeno, S.; Takemoto, K.; Takahara, D.; Hamamoto, T.; Ishino, T.; Kawasumi, T. Increased Tissue Expression of Lectin-Like Oxidized LDL Receptor-1 (LOX-1) Is Associated with Disease Severity in Chronic Rhinosinusitis with Nasal Polyps. Diagnostics 2020, 10, 246. [Google Scholar] [CrossRef]
- McDonald, P.P.; Bald, A.; Cassatella, M.A. Activation of the NF-kappaB pathway by inflammatory stimuli in human neutrophils. Blood 1997, 89, 3421–3433. [Google Scholar] [CrossRef] [PubMed]
- Frączek, M.; Rostkowska-Nadolska, B.; Kapral, M.; Szota, J.; Kręcicki, T.; Mazurek, U. Microarray analysis of NF-κB-dependent genes in chronic rhinosinusitis with nasal polyps. Adv. Clin. Exp. Med. 2013, 22, 209–217. [Google Scholar]
- Jung, H.J.; Zhang, Y.L.; Kim, D.K.; Rhee, C.S.; Kim, D.Y. The Role of NF-κB in Chronic Rhinosinusitis With Nasal Polyps. Allergy Asthma Immunol. Res. 2019, 11, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The Role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef]
- London, N.R., Jr.; Tharakan, A.; Rule, A.M.; Lane, A.P.; Biswal, S.; Ramanathan, M., Jr. Air pollutant-mediated disruption of sinonasal epithelial cell barrier function is reversed by activation of the Nrf2 pathway. J. Allergy Clin. Immunol. 2016, 138, 1736–1738.e4. [Google Scholar] [CrossRef]
- Tharakan, A.; Halderman, A.A.; Lane, A.P.; Biswal, S.; Ramanathan, M., Jr. Reversal of cigarette smoke extract-induced sinonasal epithelial cell barrier dysfunction through Nrf2 Activation. Int. Forum Allergy Rhinol. 2016, 6, 1145–1150. [Google Scholar] [CrossRef]
- Hudmon, A.; Schulman, H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002, 364, 593–611. [Google Scholar] [CrossRef]
- Stratton, M.; Lee, I.H.; Bhattacharyya, M.; Christensen, S.M.; Chao, L.H.; Schulman, H.; Groves, J.T.; Kuriyan, J. Activation-triggered subunit exchange between CaMKII holoenzymes facilitates the spread of kinase activity. Elife 2014, 3, e01610. [Google Scholar] [CrossRef]
- Erickson, J.R.; Joiner, M.L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Mei, Q.; Niu, R. Oxidative CaMKII as a potential target for inflammatory disease (Review). Mol. Med. Rep. 2019, 20, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Reiter, B.; Perraudin, J.P. The antibacterial activity of lactoferrin and neonatal E. coli infections. A selective and critical review. Adv. Exp. Med. Biol. 1998, 443, 175–188. [Google Scholar] [CrossRef]
- Gattas, M.V.; Forteza, R.; Fragoso, M.A.; Fregien, N.; Salas, P.; Salathe, M.; Conner, G.E. Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli. Free Radic. Biol. Med. 2009, 47, 1450–1458. [Google Scholar] [CrossRef]
- El Hassani, R.A.; Benfares, N.; Caillou, B.; Talbot, M.; Sabourin, J.C.; Belotte, V.; Morand, S.; Gnidehou, S.; Agnandji, D.; Ohayon, R.; et al. Dual oxidase2 is expressed all along the digestive tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G933–G942. [Google Scholar] [CrossRef]
- Cho, D.Y.; Nayak, J.V.; Bravo, D.T.; Le, W.; Nguyen, A.; Edward, J.A.; Hwang, P.H.; Illek, B.; Fischer, H. Expression of dual oxidases and secreted cytokines in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2013, 3, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, T.; Schleimer, R.P. Formation of nasal polyps: The roles of innate type 2 inflammation and deposition of fibrin. J. Allergy Clin. Immunol. 2020, 145, 740–750. [Google Scholar] [CrossRef]
- Topal, O.; Kulaksızoglu, S.; Erbek, S.S. Oxidative stress and nasal polyposis: Does it affect the severity of the disease? Am. J. Rhinol. Allergy 2014, 28, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. Oxidative stress, nitric oxide synthase, and superoxide dismutase: A matter of imbalance underlies endothelial dysfunction in the human coronary circulation. Hypertension 2008, 51, 31–32. [Google Scholar] [CrossRef]
- Wu, M.M.; Sun, H.; He, G.X.; Wang, T.S.; Xiao, Z.Q.; Feng, X.P. Determination of differentially expressed proteins and it’s significance among chronic sinusitis, nasal polyps and normal nasal mucosa. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2006, 41, 171–175. [Google Scholar] [PubMed]
- Kwon, H.S.; Bae, Y.J.; Moon, K.A.; Lee, Y.S.; Lee, T.; Lee, K.Y.; Kim, T.B.; Park, C.S.; Moon, H.B.; Cho, Y.S. Hyperoxidized peroxiredoxins in peripheral blood mononuclear cells of asthma patients is associated with asthma severity. Life Sci. 2012, 90, 502–508. [Google Scholar] [CrossRef]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Hosoyama, T.; Mikamo, A.; Kurazumi, H.; Nishimoto, A.; Ueno, K.; Shirasawa, B.; Hamano, K. Hypoxic preconditioning of human cardiosphere-derived cell sheets enhances cellular functions via activation of the PI3K/Akt/mTOR/HIF-1α pathway. Am. J. Transl. Res. 2017, 9, 664–673. [Google Scholar]
- Yadav, U.C.; Naura, A.S.; Aguilera-Aguirre, L.; Boldogh, I.; Boulares, H.A.; Calhoun, W.J.; Ramana, K.V.; Srivastava, S.K. Aldose reductase inhibition prevents allergic airway remodeling through PI3K/AKT/GSK3β pathway in mice. PLoS ONE 2013, 8, e57442. [Google Scholar] [CrossRef]
- Jia, M.; Chen, X.; Liu, J.; Chen, J. PTEN promotes apoptosis of H2O2-injured rat nasal epithelial cells through PI3K/Akt and other pathways. Mol. Med. Rep. 2018, 17, 571–579. [Google Scholar] [CrossRef]
- Wu, M.M.; Chen, T.; Xiong, W.; Huang, C.J.; Shi, Z.Q.; Pu, H.J. The role of PACAP protein in chronic sinusitis with or without nasal polyps. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2016, 30, 1608–1611. [Google Scholar] [CrossRef]
- Dagli, M.; Eryilmaz, A.; Besler, T.; Akmansu, H.; Acar, A.; Korkmaz, H. Role of free radicals and antioxidants in nasal polyps. Laryngoscope 2004, 114, 1200–1203. [Google Scholar] [CrossRef]
- Cheng, Y.K.; Tsai, M.H.; Lin, C.D.; Hwang, G.Y.; Hang, L.W.; Tseng, G.C.; Shen, P.S.; Chang, W.C. Oxidative stress in nonallergic nasal polyps associated with bronchial hyperresponsiveness. Allergy 2006, 61, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Hariri, B.M.; McMahon, D.B.; Chen, B.; Freund, J.R.; Mansfield, C.J.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Reed, D.R.; et al. Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor. J. Biol. Chem. 2017, 292, 8484–8497. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, D.W.; Khalmuratova, R.; Kim, J.H.; Jung, M.H.; Chang, D.Y.; Shin, E.C.; Lee, H.K.; Shin, H.W.; Rhee, C.S.; et al. Resveratrol prevents development of eosinophilic rhinosinusitis with nasal polyps in a mouse model. Allergy 2013, 68, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Koennecke, M.; Benecke, F.; Masche, A.; Linke, R.; Bruchhage, K.L.; Pries, R.; Klimek, L.; Wollenberg, B. Increased phosphorylation of eNOS in nasal polyps of chronic rhinosinusitis patients can be diminished by 1,8-cineol. Nitric Oxide 2018, 78, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Smith, N.; Schuster, D.; Azbell, C.; Sorscher, E.J.; Rowe, S.M.; Woodworth, B.A. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: Therapeutic implications for chronic rhinosinusitis. Am. J. Rhinol. Allergy 2011, 25, 307–312. [Google Scholar] [CrossRef]
- Hoza, J.; Salzman, R.; Starek, I.; Schalek, P.; Kellnerova, R. Efficacy and safety of erdosteine in the treatment of chronic rhinosinusitis with nasal polyposis-a pilot study. Rhinology 2013, 51, 323–327. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Sato, Y.; Shibata, H.; Arai, T.; Yamamoto, A.; Okimura, Y.; Arakaki, N.; Higuti, T. Variation in synergistic activity by flavone and its related compounds on the increased susceptibility of various strains of methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics. Int. J. Antimicrob. Agents 2004, 24, 226–233. [Google Scholar] [CrossRef]
- Pezzuto, J.M. The phenomenon of resveratrol: Redefining the virtues of promiscuity. Ann. N. Y. Acad. Sci. 2011, 1215, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A. Anti-inflammatory effects of resveratrol: Possible role in prevention of age-related cardiovascular disease. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.; Valero, A.; Picado, C. Nasal nitric oxide. Arch. Bronconeumol. 2004, 40, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.; Rogliani, P.; Calzetta, L.; Matera, M.G. Multifaceted Beneficial Effects of Erdosteine: More than a Mucolytic Agent. Drugs 2020, 80, 1799–1809. [Google Scholar] [CrossRef]
| Exogenous Oxidants | Endogenous Oxidants | Reduced Antioxidants |
|---|---|---|
| Air pollution | Hydrogen peroxide | Thioredoxin |
| Tobacco smoke | Peroxynitrite | Nrf2 |
| Biomass smoke | Xanthine oxidase | Glutathione |
| Allergens | Superoxide anions | Forkhead box protein |
| Particulate matter | Mitochondrial oxidants | Vitamins |
| PM2.5 (<10 μ, <2.5 μ, <0.1 μ) | Myeloperoxidase | SOD |
| Targets | Biomarkers |
|---|---|
| Exhaled breath condensate | Hydrogen ions |
| Hydrogen peroxide | |
| Nitric oxide | |
| Oxides of nitrogen | |
| 8-Isoprostanes | |
| Glutathione | |
| Bronchoalveolar fluid | 3-Bromotyrosine |
| Catalase | |
| Systemic circulation | Reduced glutathione |
| Ascorbic acid | |
| α-Tocopherol | |
| Lycopene | |
| β-Carotene | |
| Urine | Bromotyrosine |
| F2-isoprostane | |
| Experimental detection | Copper-zinc SOD |
| Manganese SOD |
| Research Type | Key Findings of Basic Research Study and General Information of Clinical Trial | Reference |
|---|---|---|
| Basic research studies | Eotaxin immunoreactivity in endothelial cells of NPs | Yao et al. [77] |
| Eotaxin-1 plays a key role in the selective recruitment of eosinophils in NPs | Yoshifuku et al. [78] | |
| Expression of p67phox and 4-HNE were higher in NP tissues than healthy mucosae | Zheng et al. [80] | |
| TXNIP expression is upregulated in CRSwNP | Lin et al. [82] | |
| Expression of HO-1 mRNA and proteins was higher in the NPs than that in control | Yu et al. [83] | |
| LOX-1 mRNA expression was higher in CRSwNP than that in healthy controls | Nishida et al. [86] | |
| CRSwNP group possessed a higher number of NF-κB p65-positive cells and higher mRNA expression levels of IL-6, IL-8, and eotaxin than control group | Jung et al. [89] | |
| The barrier dysfunction of nasal sinus epithelial cells can be reversed through Nrf2 activation | Tharakan et al. [92] | |
| DUOX1 mRNA levels were increased in CRSwNP compared to those in control | Cho et al. [100] | |
| Oxidative stress level was related to the nasal congestion and disease severity | Topal et al. [102] | |
| Compared to the control tis-sues, NP samples contained a higher level of malondialde-hyde and lower levels of SOD and nitric oxide | Cekin et al. [70] | |
| Clinical trial | 25 Caucasian patients (10 females and 15 males, aged 51–62 years). Moderate to high preoperative Malm endoscopy and Lund–Mackay CT scores. The treatment lasted for 6 months | Mihalj et al. [69] |
| Biomarkers of Oxidative Stress | Biomarkers of Antioxidants |
|---|---|
| Eotaxin-1 [77,78] | HO-1 [23,83] |
| NADPH [79,80] | LOX-1 [85,86] |
| TXNIP [81,82] | Nrf2 [90,91,92] |
| NF-κB [87,88,89] | SOD [70,102,104] |
| Lactoperoxidase [97,98] | PRDX2 [69,111] |
| DUOX1, DUOX2 [99,100] | BPIFA1 [69,111] |
| Malondialdehyde [70,102,103] | ADCYAP1 [69,111] PTEN, PI3K, Akt [107,108,109,110] |
| Antioxidants | Roles in the Nasal Mucosa and NPs | Reference |
|---|---|---|
| Several representative flavones (apigenin, wogonin, chrysin, tangeritin) | Inhibits the upregulation of Muc5AC and inducible nitric oxide synthase, as well as the release of cytokines (e.g., IL-8) | Hariri et al. [114] |
| Resveratrol | Decreases the degree of eosinophilic infiltration and subepithelial fibrosis, as well as levels of IL-4, IL-5, prostaglandin D synthase, and leukotriene C4 synthase | Kim et al. [115] |
| Terpenoids | Monoterpene oxide 1,8-cineol decreases the excessive eNOS phosphorylation typically found in NPs | Koennecke et al. [116] |
| Quercetin | Increases transepithelial Cl− transport and ciliary beat frequency in culture models of sinonasal epithelium | Zhang et al. [117] |
| Erdosteine | This study compared patients with CRSwNP treated with Erdosteine alone or Erdosteine in combination with nasal corticosteroid spray and found that the response was significantly better in the Erdosteine-only group | Hoza et al. [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, J.; Shin, J.-M.; Park, J.; Han, M.; Kim, T.H. Oxidative Stress and Antioxidants in Chronic Rhinosinusitis with Nasal Polyps. Antioxidants 2023, 12, 195. https://doi.org/10.3390/antiox12010195
Tai J, Shin J-M, Park J, Han M, Kim TH. Oxidative Stress and Antioxidants in Chronic Rhinosinusitis with Nasal Polyps. Antioxidants. 2023; 12(1):195. https://doi.org/10.3390/antiox12010195
Chicago/Turabian StyleTai, Junhu, Jae-Min Shin, Jaehyung Park, Munsoo Han, and Tae Hoon Kim. 2023. "Oxidative Stress and Antioxidants in Chronic Rhinosinusitis with Nasal Polyps" Antioxidants 12, no. 1: 195. https://doi.org/10.3390/antiox12010195
APA StyleTai, J., Shin, J.-M., Park, J., Han, M., & Kim, T. H. (2023). Oxidative Stress and Antioxidants in Chronic Rhinosinusitis with Nasal Polyps. Antioxidants, 12(1), 195. https://doi.org/10.3390/antiox12010195






