Promising Molecular Targets in Pharmacological Therapy for Neuronal Damage in Brain Injury
Abstract
1. Introduction
2. Brain Injury and Neurodegenerative Diseases: An Overview
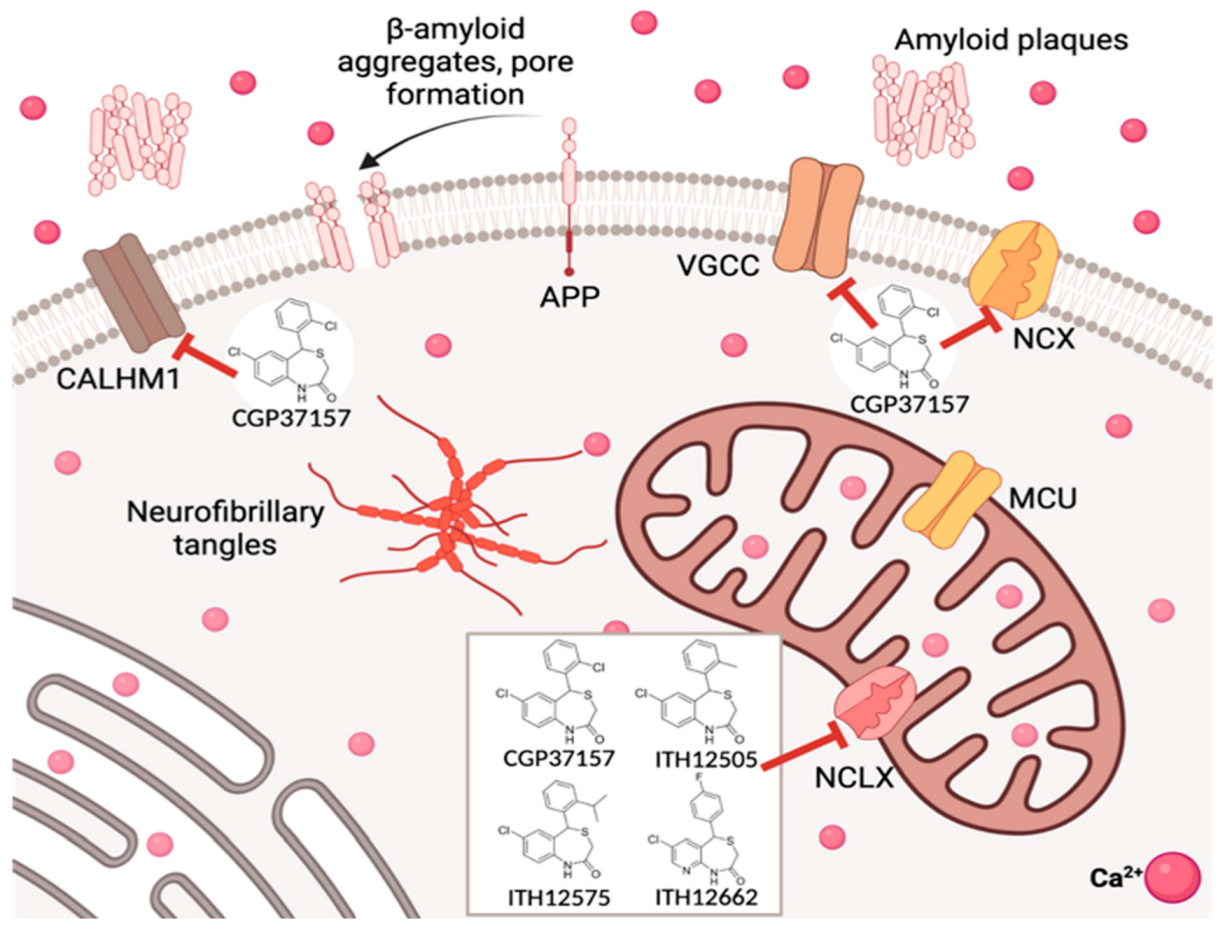
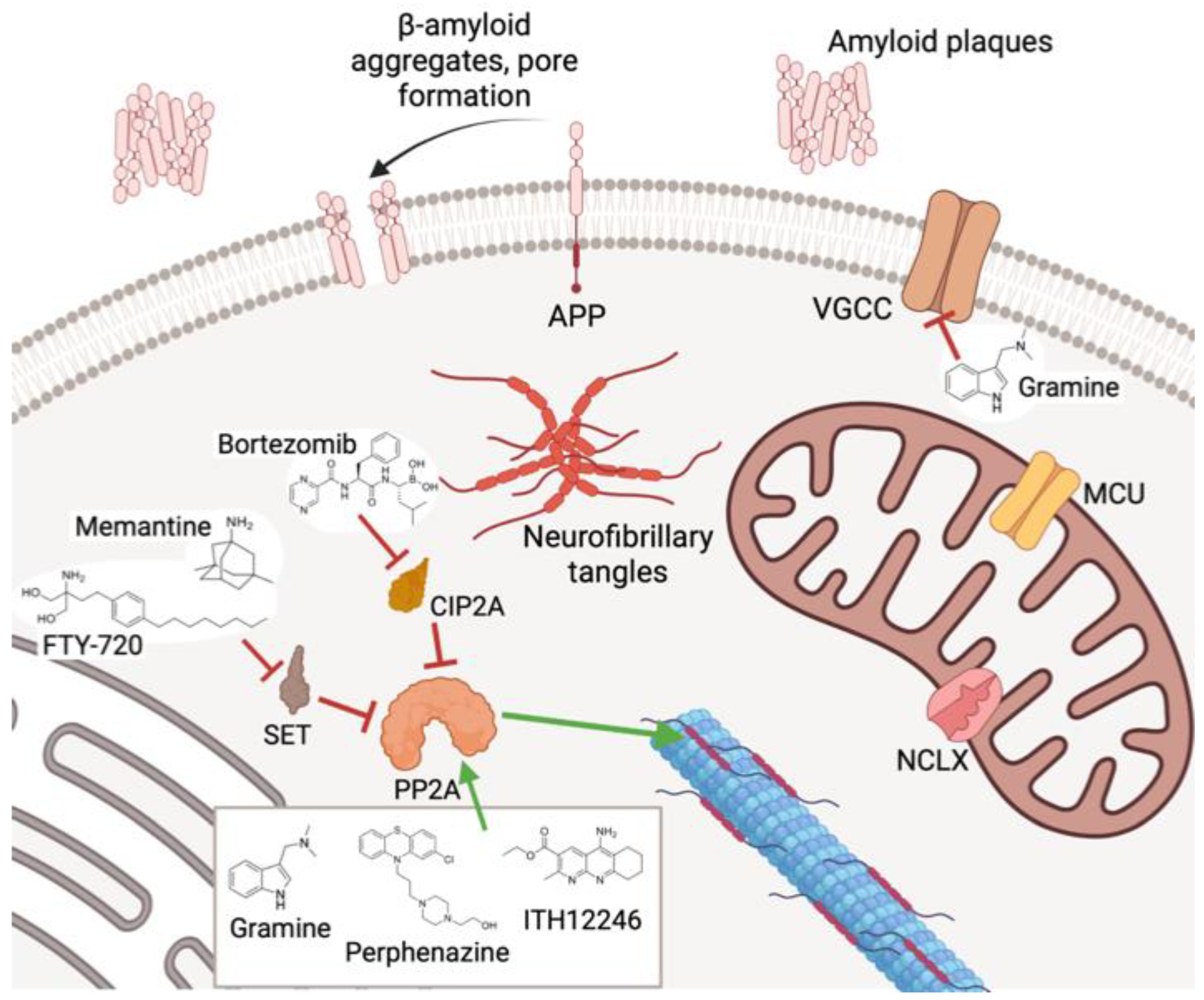
3. Cell Calcium Signal and the Role of Mitochondria
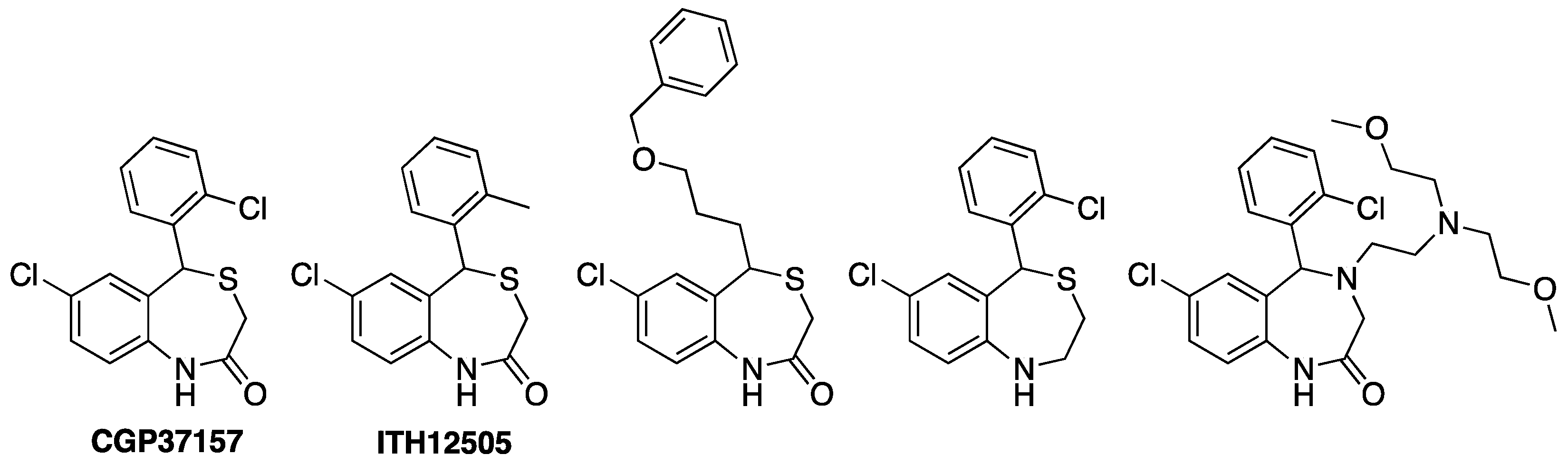
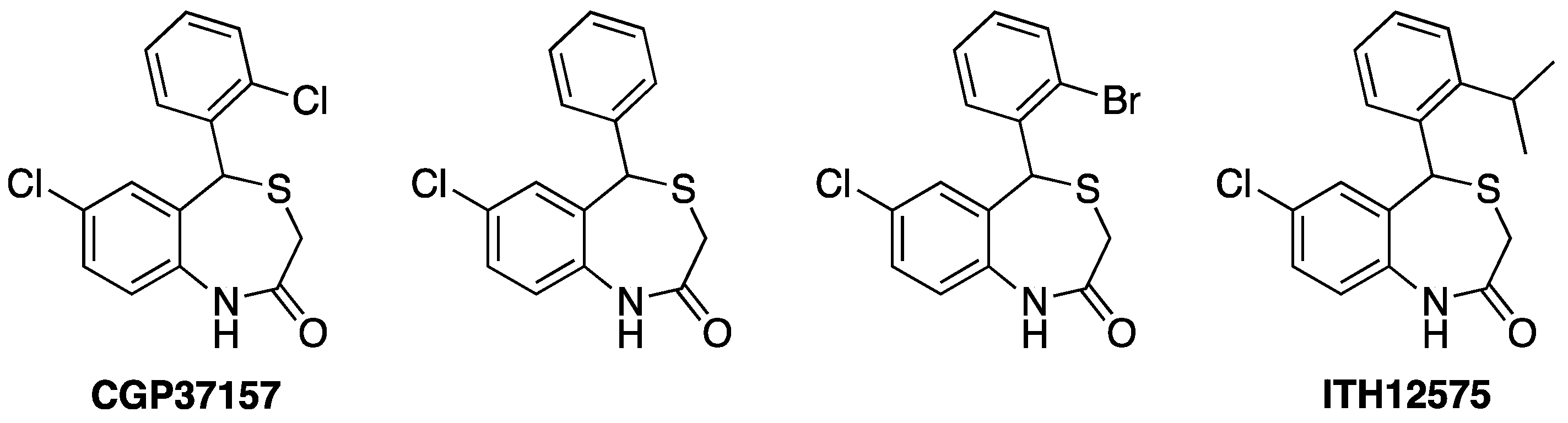
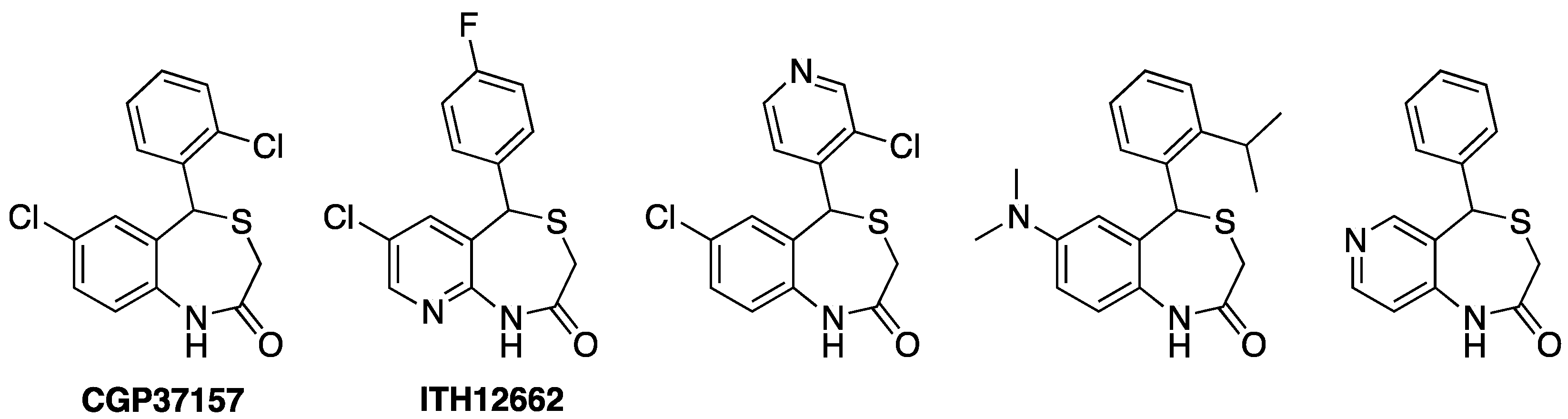
4. Drugs Acting on Mitochondrial Calcium: The 4,1-benzothiazepines
5. Dephosphorylation Processes in Neuronal Damage: The Role of PP2A
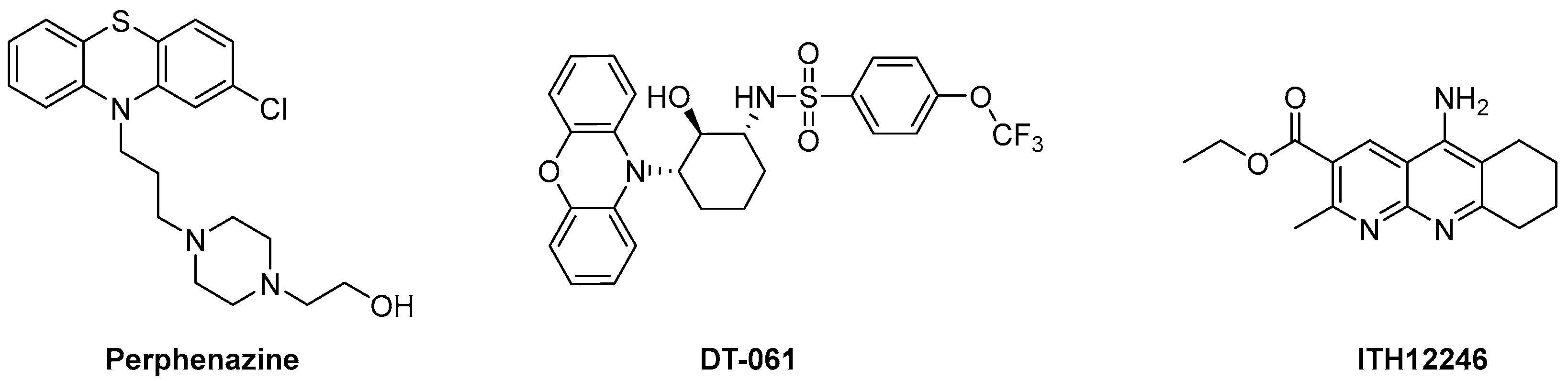
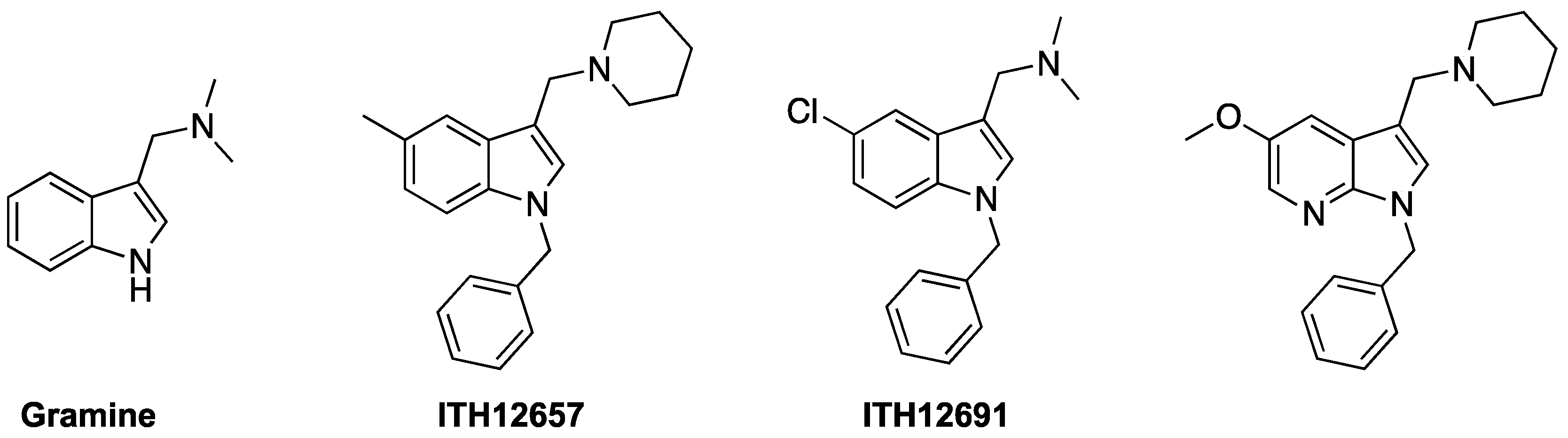
6. PP2A-Activating Drugs
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers. 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Kaye, W.; Bryan, L.; Larson, T.; Copeland, T.; Wu, J.; Muravov, O.; Horton, K. Prevalence of amyotrophic lateral sclerosis—United States, 2012–2013. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2016, 65, 1–12. [Google Scholar] [CrossRef]
- Ou, G.-y.; Lin, W.-w.; Zhao, W.-j. Neuregulins in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.; Limbucci, N.; Catalucci, A.; Caulo, M. Neurodegenerative diseases. Radiol. Clin. N. Am. 2008, 46, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.G.; Penney, J.; Tsai, L.-H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Brown, R.H., Jr.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration, and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef]
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 2017, 145, 301–307. [Google Scholar]
- Kovacs, G.G.; Budka, H. Current concepts of neuropathological diagnostics in practice: Neurodegenerative diseases. Clin. Neuropathol. 2010, 29, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Astrup, J.; Siesjo, B.K.; Symon, L. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke 1981, 12, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Somjen, G.G. Mechanisms of spreading depression-like depolarization. Physiol. Rev. 1981, 81, 1065–1096. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Excitotoxicity: Still hammering the ischemic brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Berger, C.; Schäbitz, W.R.; Georgiadis, D.; Steiner, T.; Aschoff, A.; Schwab, S. Effects of hypothermia on excitatory amino acids and metabolism in stroke patients. A microdialysis study. Stroke 2002, 33, 519–524. [Google Scholar] [CrossRef]
- Ye, H.; Jalini, S.; Zhang, L.; Charlton, M.; Carlen, P.L. Early ischemia enhances action potential-dependent, spontaneous glu-tamatergic responses in CA1 neurons. J. Cereb. Blood Flow Metab. 2010, 30, 555–565. [Google Scholar] [CrossRef]
- Yang, J.; Vitery, M.D.C.; Chen, J.; Osei-Owusu, J.; Chu, J.; Qiu, Z. Glutamate-releasing SWELL1 channel in astrocytes modulates synaptic transmission and promotes brain damage in stroke. Neuron 2019, 102, 813–827. [Google Scholar] [CrossRef]
- Rossi, D.J.; Oshima, T.; Attwell, D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 2000, 403, 316–321. [Google Scholar] [CrossRef]
- Soria, F.N.; Pérez-Samartín, A.; Martin, A.; Gona, K.B.; Llop, J.; Szczupak, B.; Chara, J.C.; Matute, C.; Domercq, M. Extrasynaptic glutamate release through cystine/glutamate antiporter contributes to ischemic damage. J. Clin. Investig. 2014, 124, 3645–3655. [Google Scholar] [CrossRef]
- Szydlowska, K.; Tymianski, M. Calcium, ischemia, and excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar]
- Andrew, R.D.; Adams, J.R.; Polischuk, T.M. Imaging NMDA- and Kainate-induced intrinsic optical signals from the hippocampal slice. J. Neurophysiol. 1996, 76, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Unterberg, A.W.; Stover, J.; Kress, B.; Kiening, K.L. Edema and brain trauma. Neuroscience 2004, 129, 1021–1029. [Google Scholar] [CrossRef]
- Castillo, J.; Loza, M.I.; Mirelman, D.; Brea, J.; Blanco, M.; Sobrino, T.; Campos, F. A novel mechanism of neuroprotection: Blood glutamate grabber. J. Cereb. Blood Flow Metab. 2016, 36, 292–301. [Google Scholar] [CrossRef]
- Butcher, S.P.; Bullock, R.; Graham, D.I.; McCulloch, J. Correlation between amino acid release and neuropathologic outcome in rat brain following middle cerebral artery occlusion. Stroke 1990, 21, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Lo, E.H.; Pierce, A.R.; Halpern, E.F.; Newcomb, R. Secondary elevation of extracellular neurotransmitter amino acids in the reperfusion phase following focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1996, 16, 114–124. [Google Scholar] [CrossRef]
- Hofmeijer, J.; van Putten, M. Ischemic cerebral damage. An appraisal of synaptic failure. Stroke 2012, 43, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Luengo, J.G.; Muñoz, M.D.; Álvarez-Merz, I.; Herranz, A.S.; González, J.C.; Martín del Río, R.; Hernández-Guijo, J.M.; Solís, J.M. Intra-cellular accumulation of amino acids increases synaptic potentials in rat hippocampal slices. Amino Acids 2019, 51, 1337–1351. [Google Scholar] [CrossRef]
- Álvarez-Merz, I.; Luengo, J.G.; Muñoz, M.D.; Hernández-Guijo, J.M.; Solís, J.M. Hypoxia-induced depression of synaptic transmission becomes irreversible by intracellular accumulation of non-excitatory amino acids. Neuropharmacology 2021, 190, 108557. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Merz, I.; Fomitcheva, I.V.; Sword, J.; Hernández-Guijo, J.M.; Solís, J.M.; Kirov, S.A. Novel mechanism of hypoxic neuronal injury mediated by non-excitatory amino acids and astroglial swelling. Glia 2022, 70, 2108–2130. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Green, C.; Jones, R.W.; Förstl, H.; Simsek, D.; de Reydet de Vulpillieres, F.; Luthman, S.; Adlard, N.; Bhattacharyya, S.; Wimo, A. Current issues, and future research priorities for health economic modelling across the full continuum of Alzheimer’s disease. Alzheimers Dement 2017, 13, 312–321. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2018 Alzheimer’s Disease Facts and Figures; Alzheimer’s Association: Chicago, IL, USA, 2018; pp. 367–429. [Google Scholar]
- Panche, A.; Chandra, S.; Ad, D.; Harke, S. Alzheimer’s, and current therapeutics: A review. Asian J. Pharm. Clin. Res. 2015, 8, 14–19. [Google Scholar]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A long journey into aging, brain aging, and Alzheimer’s disease following the oxidative stress tracks. J. Alzheimer’s Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P.; Myers, R.H.; Lin, C.; Srinidhi, L.; Barnes, G.; Taylor, S.A.; James, M.; Groot, N. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef] [PubMed]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 2017, 3, 17085. [Google Scholar] [CrossRef]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Logroscino, G.; Piccininni, M.; Marin, B.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Alahdab, F.; Asgedom, S.W.; Awasthi, A.; Chaiah, Y. Global, regional, and national burden of motor neuron diseases 1990–2016, A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 1083–1097. [Google Scholar] [CrossRef]
- Logroscino, G.; Traynor, B.J.; Hardiman, O.; Chiò, A.; Mitchell, D.; Swingler, R.J.; Millul, A.; Benn, E.; Beghi, E. Incidence of amyotrophic lateral sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry 2010, 81, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Manjaly, Z.R.; Scott, K.M.; Abhinav, K.; Wijesekera, L.; Ganesalingam, J.; Goldstein, L.H.; Janssen, A.; Dougherty, A.; Willey, E.; Stanton, B.R. The sex ratio in amyotrophic lateral sclerosis: A population-based study. Amyotroph. Lateral Scler. 2010, 11, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, C.; Dellve, L.; Ekbrand, H.; Zettergren, A.; Zetterberg, H.; Blennow, K.; Skoog, I.; Halleröd, B. Socioeconomic status, gender and dementia: The influence of work environment exposures and their interactions with APOE ε4. SSM Popul. Health 2018, 5, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Johansson, A.L.V.; Pedersen, N.L.; Fang, F.; Gatz, M.; Wirdefeldt, K. Socioeconomic status in relation to Parkinson’s disease risk and mortality: A population-based prospective study. Medicine 2016, 95, e4337. [Google Scholar] [CrossRef]
- Tóth, P.; Gavurová, B.; Barták, M. Alzheimer’s Disease Mortality according to Socioeconomic Factors: Country Study. Int. J. Alzheimers Dis. 2018, 2018, 8137464. [Google Scholar] [CrossRef]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef]
- Plattner, H.; Verkhratsky, A. Ca2+ signalling early in evolution—All but primitive. J. Cell Sci. 2013, 126 Pt 10, 2141–2150. [Google Scholar] [CrossRef]
- Michikawa, T.; Miyawaki, A.; Furuichi, T.; Mikoshiba, K. Inositol 1,4,5-trisphosphate receptors, and calcium signaling. Crit. Rev. Neurobiol. 1996, 10, 39–55. [Google Scholar] [CrossRef]
- Santella, L.; Lim, D.; Moccia, F. Calcium, and fertilization: The beginning of life. Trends Biochem. Sci. 2004, 29, 400–408. [Google Scholar] [CrossRef]
- Tandoğan, B.; Ulusu, N.N. Importance of calcium. Turk. J. Med. Sci. 2005, 35, 197–201. [Google Scholar]
- Calvo-Rodriguez, M.; Bacskai, B.J. Mitochondria and calcium in Alzheimer’s disease: From cell signaling to neuronal cell death. Trends Neurosci. 2021, 44, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Morales, J.-C.; Arranz-Tagarro, J.-A.; Calvo-Gallardo, E.; Maroto, M.; Padin, J.-F.; García, A.G. Stabilizers of neuronal and mitochondrial calcium cycling as a strategy for developing a medicine for Alzheimer’s disease. ACS Chem. Neurosci. 2012, 3, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.-E.; Carafoli, E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry 2005, 70, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wei, H. The role of calcium dysregulation in anesthetic-mediated neurotoxicity. Anesth. Analg. 2011, 113, 972–974. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef]
- Alexander, A.G.; Marfil, V.; Li, C. Use of Caenorhabditis elegans as a model to study Alzheimer’s disease and other neu-rodegenerative diseases. Front. Genet. 2014, 5, 279. [Google Scholar] [CrossRef]
- Cooper, J.F.; Van Raamsdonk, J.M. Modeling Parkinson’s Disease in C. elegans. J. Park. Dis. 2018, 8, 17–32. [Google Scholar] [CrossRef]
- Gaeta, A.L.; Caldwell, K.A.; Caldwell, G.A. Found in Translation: The Utility of C. elegans Alpha-Synuclein Models of Parkinson’s Disease. Brain Sci. 2019, 9, 73. [Google Scholar] [CrossRef]
- Martinez, B.A.; Caldwell, K.A.; Caldwell, G.A. C. elegans as a model system to accelerate discovery for Parkinson disease. Curr. Opin Genet. Dev. 2017, 44, 102–109. [Google Scholar] [CrossRef]
- Maulik, M.; Mitra, S.; Bult-Ito, A.; Taylor, B.E.; Vayndorf, E.M. Behavioral Phenotyping and Pathological Indicators of Parkinson’s Disease in C. elegans Models. Front. Genet. 2017, 8, 77. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, Y.; Chen, Y.; Cheng, B.; Peng, A.; Huang, K. Caenorhabditis elegans as a model system for target identification and drug screening against neurodegenerative diseases. Eur. J. Pharmacol. 2018, 819, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Le, W. Modeling neurodegenerative diseases in Caenorhabditis elegans. Exp. Neurol. 2013, 250, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Therrien, M.; Parker, J.A. Worming forward: Amyotrophic lateral sclerosis toxicity mechanisms and genetic interactions in Caenorhabditis elegans. Front. Genet. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Robberecht, W.; Van Den Bosch, L. Modelling amyotrophic lateral sclerosis: Progress and possibilities. Dis. Model Mech. 2017, 10, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Calahorro, F.; Ruiz-Rubio, M. Caenorhabditis elegans as an experimental tool for the study of complex neurological dis-eases: Parkinson’s disease, Alzheimer’s disease and autism spectrum disorder. Invert Neurosci. 2011, 11, 73–83. [Google Scholar] [CrossRef]
- Glaser, T.; Arnaud Sampaio, V.F.; Lameu, C.; Ulrich, H. Calcium signaling: A common target in neurological disorders and neurogenesis. Semin. Cell Dev. Biol. 2019, 95, 25–33. [Google Scholar] [CrossRef]
- Greotti, E.; Capitanio, P.; Wong, A.; Pozzan, T.; Pizzo, P.; Pendin, D. Familial Alzheimer’s disease-linked presenilin mutants and intracellular Ca2+ handling: A single-organelle, FRET-based analysis. Cell Calcium 2019, 79, 44–56. [Google Scholar] [CrossRef]
- Hajieva, P.; Baeken, M.W.; Moosmann, B. The role of Plasma Membrane Calcium ATPases (PMCAs) in neurodegenerative disorders. Neurosci. Lett. 2018, 663, 29–38. [Google Scholar] [CrossRef]
- Karagas, N.E.; Venkatachalam, K. Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis. Cells 2019, 8, 1232. [Google Scholar] [CrossRef]
- Alvarez, J.; Alvarez-Illera, P.; García-Casas, P.; Fonteriz, R.I.; Montero, M. The role of Ca2+ signaling in aging and neuro-degeneration: Insights from Caenorhabditis elegans models. Cells 2020, 9, 204. [Google Scholar] [CrossRef]
- Strehler, E.E.; Thayer, S.A. Evidence for a role of plasma membrane calcium pumps in neurodegenerative disease: Recent developments. Neurosci. Lett. 2018, 663, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, V.; Petrozziello, T.; Secondo, A. Calcium Dyshomeostasis and Lysosomal Ca2+ Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2019, 8, 1216. [Google Scholar] [CrossRef] [PubMed]
- Ringer, S. A further Contribution regarding the influence of the different Constituents of the Blood on the Contraction of the Heart. J. Physiol. 1883, 4, 29–42.3. [Google Scholar] [CrossRef] [PubMed]
- Heilbrunn, L.V.; Wiercinski, F.J. The action of various cations on muscle protoplasm. J. Cell Comp. Physiol. 1947, 29, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Csordás, G.; Hajnóczky, G. Plasticity of mitochondrial calcium signaling. J. Biol. Chem. 2003, 278, 42273–42282. [Google Scholar] [CrossRef] [PubMed]
- Csordás, G.; Thomas, A.P.; Hajnóczky, G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999, 18, 96–108. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef]
- Szabadkai, G.; Simoni, A.M.; Rizzuto, R. Mitochondrial Ca2+ uptake requires sustained Ca2+ release from the endoplasmic reticulum. J. Biol. Chem. 2003, 278, 15153–15161. [Google Scholar] [CrossRef]
- Thomas, A.P.; Bird, G.S.; Hajnóczky, G.; Robb-Gaspers, L.D.; Putney, J.W., Jr. Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996, 10, 1505–1517. [Google Scholar] [CrossRef]
- Martínez-Zaguilán, R.; Wesson, D.E. Regulation of endoplasmic reticulum-Ca-ATPase by glycolysis in eukaryotic cells. Miner Electrolyte Metab. 1996, 22, 318–335. [Google Scholar] [PubMed]
- Herrington, J.; Park, Y.B.; Babcock, D.F.; Hille, B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron 1996, 16, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Alonso, M.T.; Carnicero, E.; Cuchillo-Ibáñez, I.; Albillos, A.; García, A.G.; García-Sancho, J.; Alvarez, J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat. Cell Biol. 2000, 2, 57–61. [Google Scholar] [CrossRef]
- Villalobos, C.; Nuñez, L.; Montero, M.; García, A.G.; Alonso, M.T.; Chamero, P.; Alvarez, J.; García-Sancho, J. Redistribution of Ca2+ among cytosol and organella during stimulation of bovine chromaffin cells. FASEB J. 2002, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Naraghi, M.; Kang, H.; Neher, E. Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytosol of adrenal chromaffin cells. Biophys. J. 1997, 73, 532–545. [Google Scholar] [CrossRef]
- Neher, E. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron 1998, 20, 389–399. [Google Scholar] [CrossRef]
- Gunter, T.E.; Gunter, K.K.; Sheu, S.S.; Gavin, C.E. Mitochondrial calcium transport: Physiological and pathological relevance. Am. J. Physiol. 1994, 267 Pt 1, C313–C339. [Google Scholar] [CrossRef]
- Rizzuto, R.; Bernardi, P.; Pozzan, T. Mitochondria as all-round players of the calcium game. J. Physiol. 2000, 529 Pt 1, 37–47. [Google Scholar] [CrossRef]
- Matlib, M.A.; Schwartz, A. Selective effects of diltiazem, a benzothiazepine calcium channel blocker, and diazepam, and other benzodiazepines on the Na+/Ca2+ exchange carrier system of heart and brain mitochondria. Life Sci. 1983, 32, 2837–2842. [Google Scholar] [CrossRef]
- Chiesi, M.; Rogg, H.; Eichenberger, K.; Gazzotti, P.; Carafoli, E. Stereospecific action of diltiazem on the mitochondrial NaCa exchange system and on sarcolemmal Ca-channels. Biochem. Pharmacol. 1987, 36, 2735–2740. [Google Scholar] [CrossRef]
- Chiesi, M.; Schwaller, R.; Eichenberger, K. Structural dependency of the inhibitory action of benzodiazepines and related compounds on the mitochondrial Na+-Ca2+ exchanger. Biochem. Pharmacol. 1988, 37, 4399–4403. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.A.; Conforti, L.; Sperelakis, N.; Matlib, M.A. Selectivity of inhibition of Na (+)-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J. Cardiovasc. Pharmacol. 1993, 21, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Branca, D.; Vincenti, E.; Roberti, M.S.; Gambaretto, G.; Scutari, G. Effects of diltiazem on liver mitochondria of rats: A re-consideration. Comparative Biochemistry and physiology. C Comp. Pharmacol. Toxicol. 1993, 104, 47–49. [Google Scholar]
- Baron, K.T.; Thayer, S.A. CGP37157 modulates mitochondrial Ca2+ homeostasis in cultured rat dorsal root ganglion neurons. Eur. J. Pharmacol. 1997, 340, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, J.; Brocard, J.; Stout, A.; Reynolds, I. Pharmacological investigation of mitochondrial Ca2+ transport in central neurons: Studies with CGP-37157, an inhibitor of the mitochondrial Na+–Ca2+ exchanger. Cell Calcium. 2000, 28, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Lilly, M.J.; Owen, D.J.; D’Souza, L.J.; Tang, X.Q.; Yu, J.; Nazarbaghi, R.; Hunter, A.; Anderson, C.M.; Glasco, S.; et al. Efficient syntheses of benzothiazepines as antagonists for the mitochondrial sodium-calcium exchanger: Potential therapeutics for type II diabetes. J. Org. Chem. 2003, 68, 92–103. [Google Scholar] [CrossRef]
- Lee, B.; Miles, P.D.; Vargas, L.; Luan, P.; Glasco, S.; Kushnareva, Y.; Kornbrust, E.S.; Grako, K.A.; Wollheim, C.B.; Maechler, P.; et al. Inhibition of mitochondrial Na+-Ca2+ exchanger increases mitochondrial metabolism and poten-tiates glucose-stimulated insulin secretion in rat pancreatic islets. Diabetes 2003, 52, 965–973. [Google Scholar] [CrossRef]
- Thu le, T.; Ahn, J.R.; Woo, S.H. Inhibition of L-type Ca2+ channel by mitochondrial Na+-Ca2+ exchange inhibitor CGP-37157 in rat atrial myocytes. Eur. J. Pharmacol. 2006, 552, 15–19. [Google Scholar] [CrossRef]
- Nicolau, S.M.; de Diego, A.M.; Cortés, L.; Egea, J.; González, J.C.; Mosquera, M.; López, M.G.; Hernández-Guijo, J.M.; García, A.G. Mitochondrial Na+/Ca2+-exchanger blocker CGP37157 protects against chromaffin cell death elicited by veratridine. J. Pharmacol. Exp. Ther. 2009, 330, 844–854. [Google Scholar] [CrossRef]
- Guéguinou, M.; Ibrahim, S.; Bourgeais, J.; Robert, A.; Pathak, T.; Zhang, X.; Crottès, D.; Dupuy, J.; Ternant, D.; Monbet, V.; et al. Curcumin and NCLX inhibitors share anti-tumoral mechanisms in microsatellite-instability-driven colorectal cancer. Cell Mol. Life Sci. 2022, 79, 284. [Google Scholar] [CrossRef]
- Friedli, M.J.; Inestrosa, N.C. Huperzine A and its neuroprotective molecular signaling in Alzheimer’s disease. Molecules 2021, 26, 6531. [Google Scholar] [CrossRef] [PubMed]
- González-Lafuente, L.; Egea, J.; León, R.; Martínez-Sanz, F.J.; Monjas, L.; Perez, C.; Merino, C.; García-De Diego, A.M.; Rodríguez-Franco, M.I.; García, A.G.; et al. Benzothiazepine CGP37157 and its isosteric 2’-methyl analogue provide neuroprotection and block cell calcium entry. ACS Chem. Neurosci. 2012, 3, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, F.J.; Lajarín-Cuesta, R.; Moreno-Ortega, A.J.; González-Lafuente, L.; Fernández-Morales, J.C.; López-Arribas, R.; Cano-Abad, M.F.; de los Ríos, C. Benzothiazepine CGP37157 Analogues Exert Cytoprotection in Various in Vitro Models of Neurodegeneration. ACS Chem. Neurosci. 2015, 6, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ortega, A.J.; Martínez-Sanz, F.J.; Lajarín-Cuesta, R.; de Los Rios, C.; Cano-Abad, M.F. Benzothiazepine CGP37157 and its 2’-isopropyl analogue modulate Ca2+ entry through CALHM1. Neuropharmacology 2015, 95, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Dreses-Werringloer, U.; Lambert, J.C.; Vingtdeux, V.; Zhao, H.; Vais, H.; Siebert, A.; Jain, A.; Koppel, J.; Rovelet-Lecrux, A.; Hannequin, D.; et al. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell 2008, 133, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Jadiya, P.; Kolmetzky, D.W.; Tomar, D.; Di Meco, A.; Lombardi, A.A.; Lambert, J.P.; Luongo, T.S.; Ludtmann, M.H.; Praticò, D.; Elrod, J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 2019, 10, 3885. [Google Scholar] [CrossRef] [PubMed]
- García-Casas, P.; Arias-Del-Val, J.; Alvarez-Illera, P.; Wojnicz, A.; de Los Ríos, C.; Fonteriz, R.I.; Montero, M.; Alvarez, J. The Neuroprotector Benzothiazepine CGP37157 Extends Lifespan in C. elegans Worms. Front. Aging Neurosci. 2018, 10, 440. [Google Scholar] [CrossRef]
- Ewald, C.Y.; Li, C. Understanding the molecular basis of Alzheimer’s disease using a Caenorhabditis elegans model system. Brain Struct. Funct. 2010, 214, 263–283. [Google Scholar] [CrossRef]
- Link, C.D. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1995, 92, 9368–9372. [Google Scholar] [CrossRef]
- Lublin, A.L.; Link, C.D. Alzheimer’s disease drug discovery: In vivo screening using Caenorhabditis elegans as a model for β-amyloid peptide-induced toxicity. Drug Discov. Today Technol. 2013, 10, e115–e119. [Google Scholar] [CrossRef]
- Romero, A.; Egea, J.; García, A.G.; López, M.G. Synergistic neuroprotective effect of combined low concentrations of galantamine and melatonin against oxidative stress in SH-SY5Y neuroblastoma cells. J. Pineal Res. 2010, 49, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, F.J.; Lajarín-Cuesta, R.; González-Lafuente, L.; Moreno-Ortega, A.J.; Punzón, E.; Cano-Abad, M.F.; de los Ríos, C. Neuroprotective profile of pyridothiazepines with blocking activity of the mitochondrial Na(+)/Ca2+ exchanger. Eur. J. Med. Chem. 2016, 109, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Viejo, L.; Rubio-Alarcón, M.; Arribas, R.L.; Moreno-Castro, M.; Pérez-Marín, R.; Braun-Cornejo, M.; Estrada-Valencia, M.; de Los Ríos, C. Synthesis and Biological Assessment of 4,1-Benzothiazepines with Neuroprotective Activity on the Ca2+ Overload for the Treatment of Neurodegenerative Diseases and Stroke. Molecules 2021, 26, 4773. [Google Scholar] [CrossRef] [PubMed]
- López-Gil, A.; Nanclares, C.; Méndez-López, I.; Martínez-Ramírez, C.; de Los Rios, C.; Padín-Nogueira, J.F.; Montero, M.; Gandía, L.; García, A.G. The quantal catecholamine release from mouse chromaffin cells challenged with repeated ACh pulses is regulated by the mitochondrial Na(+) /Ca2+ exchanger. J. Physiol. 2017, 595, 2129–2146. [Google Scholar] [CrossRef]
- Kurosawa, M. Phosphorylation and dephosphorylation of protein in regulating cellular function. J. Pharmacol. Toxicol. Methods 1994, 31, 135–139. [Google Scholar] [CrossRef]
- Biundo, F.; Del Prete, D.; Zhang, H.; Arancio, O.; D’Adamio, L. A role for tau in learning, memory and synaptic plasticity. Sci. Rep. 2018, 8, 3184. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Avila, J.; Lucas, J.J.; Perez, M.; Hernandez, F. Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef]
- Goedert, M.; Wischik, C.M.; Crowther, R.A.; Walker, J.E.; Klug, A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: Identification as the microtubule-associated protein tau. Proc. Natl. Acad. Sci. USA 1988, 85, 4051–4055. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Ferrer, I.; Gomez-Isla, T.; Puig, B.; Freixes, M.; Ribé, E.; Dalfó, E.; Avila, J. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr. Alzheimer Res. 2005, 2, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Sangodkar, J.; Farrington, C.C.; McClinch, K.; Galsky, M.D.; Kastrinsky, D.B.; Narla, G. All roads lead to PP2A: Exploiting the therapeutic potential of this phosphatase. FEBS J. 2016, 283, 1004–1024. [Google Scholar] [CrossRef]
- Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005, 22, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Janssens, V.; Goris, J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001, 353 Pt 3, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yogesha, S.D.; Mayfield, J.E.; Gill, G.N.; Zhang, Y. Viewing serine/threonine protein phosphatases through the eyes of drug designers. FEBS J. 2013, 280, 4739–4760. [Google Scholar] [CrossRef]
- Lambrecht, C.; Haesen, D.; Sents, W.; Ivanova, E.; Janssens, V. Structure, regulation, and pharmacological modulation of PP2A phosphatases. Methods Mol. Biol. 2013, 1053, 283–305. [Google Scholar]
- Kurimchak, A.; Haines, D.S.; Garriga, J.; Wu, S.; De Luca, F.; Sweredoski, M.J.; Deshaies, R.J.; Hess, S.; Graña, X. Activation of p107 by fibroblast growth factor, which is essential for chondrocyte cell cycle exit, is mediated by the protein phosphatase 2A/B55α holoenzyme. Mol. Cell Biol. 2013, 33, 3330–3342. [Google Scholar] [CrossRef][Green Version]
- Sontag, J.M.; Sontag, E. Protein phosphatase 2A dysfunction in Alzheimer’s disease. Front. Mol. Neurosci. 2014, 7, 16. [Google Scholar] [CrossRef]
- Kong, M.; Ditsworth, D.; Lindsten, T.; Thompson, C.B. Alpha4 is an essential regulator of PP2A phosphatase activity. Mol. Cell 2009, 36, 51–60. [Google Scholar] [CrossRef]
- Sents, W.; Ivanova, E.; Lambrecht, C.; Haesen, D.; Janssens, V. The biogenesis of active protein phosphatase 2A holoenzymes: A tightly regulated process creating phosphatase specificity. FEBS J. 2013, 280, 644–661. [Google Scholar] [CrossRef]
- Li, M.; Guo, H.; Damuni, Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phos-phatase 2A from bovine kidney. Biochemistry 1995, 34, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Puustinen, P.; Niemelä, M.; Ahola, R.; Arnold, H.; Böttzauw, T.; Ala-aho, R.; Nielsen, C.; Ivaska, J.; Taya, Y.; et al. CIP2A inhibits PP2A in human malignancies. Cell 2007, 130, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Pimanda, J.E.; Westermarck, J. Cancerous inhibitor of protein phosphatase 2A, an emerging human oncoprotein and a potential cancer therapy target. Cancer Res. 2013, 73, 6548–6553. [Google Scholar] [CrossRef] [PubMed]
- Tanimukai, H.; Grundke-Iqbal, I.; Iqbal, K. Up-regulation of inhibitors of protein phosphatase-2A in Alzheimer’s disease. Am. J. Pathol. 2005, 166, 1761–1771. [Google Scholar] [CrossRef]
- Haesen, D.; Sents, W.; Lemaire, K.; Hoorne, Y.; Janssens, V. The Basic Biology of PP2A in Hematologic Cells and Malig-nancies. Front. Oncol. 2014, 4, 347. [Google Scholar] [CrossRef]
- Neviani, P.; Harb, J.G.; Oaks, J.J.; Santhanam, R.; Walker, C.J.; Ellis, J.J.; Ferenchak, G.; Dorrance, A.M.; Paisie, C.A.; Eiring, A.M.; et al. PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J. Clin. Investig. 2013, 123, 4144–4157. [Google Scholar] [CrossRef]
- Voronkov, M.; Braithwaite, S.P.; Stock, J.B. Phosphoprotein phosphatase 2A: A novel druggable target for Alzheimer’s disease. Future Med. Chem. 2011, 3, 821–833. [Google Scholar] [CrossRef]
- Szymiczek, A.; Pastorino, S.; Larson, D.; Tanji, M.; Pellegrini, L.; Xue, J.; Li, S.; Giorgi, C.; Pinton, P.; Takinishi, Y.; et al. FTY720 inhibits mesothelioma growth in vitro and in a syngeneic mouse model. J. Transl. Med. 2017, 15, 58. [Google Scholar] [CrossRef]
- Enjeti, A.K.; D’Crus, A.; Melville, K.; Verrills, N.M.; Rowlings, P. A systematic evaluation of the safety and toxicity of fingolimod for its potential use in the treatment of acute myeloid leukaemia. Anticancer Drugs 2016, 27, 560–568. [Google Scholar] [CrossRef]
- Agarwal, A.; MacKenzie, R.J.; Pippa, R.; Eide, C.A.; Oddo, J.; Tyner, J.W.; Sears, R.; Vitek, M.P.; Odero, M.D.; Christensen, D.J.; et al. Antagonism of SET using OP449 enhances the efficacy of tyrosine kinase inhibitors and overcomes drug resistance in myeloid leukemia. Clin. Cancer Res. 2014, 20, 2092–2103. [Google Scholar] [CrossRef]
- Wang, S.; Xie, W.; Wang, D.; Peng, Z.; Zheng, Y.; Liu, N.; Dai, W.; Wang, Y.; Wang, Z.; Yang, Y.; et al. Discovery of a small molecule targeting SET-PP2A interaction to overcome BCR-ABLT315I mutation of chronic myeloid leukemia. Oncotarget 2015, 6, 12128–12140. [Google Scholar] [CrossRef] [PubMed]
- Chohan, M.O.; Khatoon, S.; Iqbal, I.G.; Iqbal, K. Involvement of I2PP2A in the abnormal hyperphosphorylation of tau and its reversal by Memantine. FEBS Lett. 2006, 580, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, L.; Wen, Z.S.; Hu, Z.; Wu, F.Q.; Li, W.; Liu, J.; Zhou, G.B. Cancerous inhibitor of PP2A is targeted by natural compound celastrol for degradation in non-small-cell lung cancer. Carcinogenesis 2014, 35, 905–914. [Google Scholar] [CrossRef]
- Tseng, L.M.; Liu, C.Y.; Chang, K.C.; Chu, P.Y.; Shiau, C.W.; Chen, K.F. CIP2A is a target of bortezomib in human triple negative breast cancer cells. Breast Cancer Res. 2012, 14, R68. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, L.; Wen, Z.S.; Cheng, Y.X.; Zhou, G.B. Ethoxysanguinarine Induces Inhibitory Effects and Downregulates CIP2A in Lung Cancer Cells. ACS Med. Chem. Lett. 2014, 5, 113–118. [Google Scholar] [CrossRef][Green Version]
- Kim, M.O.; Choe, M.H.; Yoon, Y.N.; Ahn, J.; Yoo, M.; Jung, K.Y.; An, S.; Hwang, S.G.; Oh, J.S.; Kim, J.S. Antihelminthic drug niclosamide inhibits CIP2A and reactivates tumor suppressor protein phosphatase 2A in non-small cell lung cancer cells. Biochem. Pharmacol. 2017, 144, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, I.; Rincón, R.; Manso, R.; Madoz-Gúrpide, J.; Caramés, C.; del Puerto-Nevado, L.; Rojo, F.; García-Foncillas, J. Hyperphosphorylation of PP2A in colorectal cancer and the potential therapeutic value showed by its forskolin-induced dephosphorylation and activation. Biochim. Biophys. Acta 2014, 1842, 1823–1829. [Google Scholar] [CrossRef]
- Basurto-Islas, G.; Blanchard, J.; Tung, Y.C.; Fernandez, J.R.; Voronkov, M.; Stock, M.; Zhang, S.; Stock, J.B.; Iqbal, K. Thera-peutic benefits of a component of coffee in a rat model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2701–2712. [Google Scholar] [CrossRef]
- Kickstein, E.; Krauss, S.; Thornhill, P.; Rutschow, D.; Zeller, R.; Sharkey, J.; Williamson, R.; Fuchs, M.; Köhler, A.; Glossmann, H.; et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 21830–21835. [Google Scholar] [CrossRef]
- Koh, P.O. Melatonin attenuates decrease of protein phosphatase 2A subunit B in ischemic brain injury. J. Pineal Res. 2012, 52, 57–61. [Google Scholar] [CrossRef]
- Arribas, R.L.; Romero, A.; Egea, J.; de Los Ríos, C. Modulation of serine/threonine phosphatases by melatonin: Therapeutic approaches in neurodegenerative diseases. Br. J. Pharmacol. 2018, 175, 3220–3229. [Google Scholar] [CrossRef]
- Pelech, S.; Cohen, P. The protein phosphatases involved in cellular regulation. 1. Modulation of protein phosphatases-1 and 2A by histone H1, protamine, polylysine and heparin. Eur. J. Biochem. 1985, 148, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Pan, L.; Groen, R.W.; Baleydier, F.; Kentsis, A.; Marineau, J.; Grebliunaite, R.; Kozakewich, E.; Reed, C.; Pflumio, F.; et al. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J. Clin. Investig. 2014, 124, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Kauko, O.; O’Connor, C.M.; Kulesskiy, E.; Sangodkar, J.; Aakula, A.; Izadmehr, S.; Yetukuri, L.; Yadav, B.; Padzik, A.; Laajala, T.D.; et al. PP2A inhibition is a druggable MEK inhibitor resistance mechanism in KRAS-mutant lung cancer cells. Sci. Transl. Med. 2018, 10, eaaq1093. [Google Scholar] [CrossRef] [PubMed]
- de Los Ríos, C.; Egea, J.; Marco-Contelles, J.; León, R.; Samadi, A.; Iriepa, I.; Moraleda, I.; Gálvez, E.; García, A.G.; López, M.G.; et al. Synthesis, inhibitory activity of cholinesterases, and neuroprotective profile of novel 1,8-naphthyridine derivatives. J. Med. Chem. 2010, 53, 5129–5143. [Google Scholar] [CrossRef]
- Kamat, P.K.; Nath, C. Okadaic acid: A tool to study regulatory mechanisms for neurodegeneration and regeneration in Alzheimer’s disease. Neural Regen. Res. 2015, 10, 365–367. [Google Scholar] [CrossRef]
- Suganuma, M.; Fujiki, H.; Suguri, H.; Yoshizawa, S.; Hirota, M.; Nakayasu, M.; Ojika, M.; Wakamatsu, K.; Yamada, K.; Sugimura, T. Okadaic acid: An additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc. Natl. Acad. Sci. USA 1988, 85, 1768–1771. [Google Scholar] [CrossRef]
- Kumagai, M.; Yanagi, T.; Murata, M.; Yasumoto, T.; Kat, M.; Lassus, P.; Rodriguez-Vazquez, J.A. Okadaic Acid as the Causative Toxin of Diarrhetic Shellfish Poisoning in Europe. Agric. Biol. Chem. 1986, 50, 2853–2857. [Google Scholar]
- Medina, M.; Avila, J.; Villanueva, N. Use of okadaic acid to identify relevant phosphoepitopes in pathology: A focus on neurodegeneration. Mar Drugs 2013, 11, 1656–1668. [Google Scholar] [CrossRef]
- Lorrio, S.; Romero, A.; González-Lafuente, L.; Lajarín-Cuesta, R.; Martínez-Sanz, F.J.; Estrada, M.; Samadi, A.; Mar-co-Contelles, J.; Rodríguez-Franco, M.I.; Villarroya, M.; et al. PP2A ligand ITH12246 protects against memory impairment and focal cerebral ischemia in mice. ACS Chem. Neurosci. 2013, 4, 1267–1277. [Google Scholar] [CrossRef]
- Taleski, G.; Sontag, E. Protein phosphatase 2A and tau: An orchestrated ‘Pas de Deux’. FEBS Lett. 2018, 592, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Romero, A.; Egea, J.; Marco-Contelles, J.; Del Pino, J.; de Los Ríos, C. Analysis of gene expression profiles of CR80, a neuroprotective 1,8-Naphthyridine. Future Med. Chem. 2018, 10, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Madinaveitia, J. The alkaloids of Arundo donax L. J. Chem. Soc. 1937, 59, 1927–1929. [Google Scholar] [CrossRef]
- Lajarín-Cuesta, R.; Nanclares, C.; Arranz-Tagarro, J.A.; González-Lafuente, L.; Arribas, R.L.; Araujo de Brito, M.; Gandía, L.; de Los Ríos, C. Gramine Derivatives Targeting Ca2+ Channels and Ser/Thr Phosphatases: A New Dual Strategy for the Treatment of Neurodegenerative Diseases. J. Med. Chem. 2016, 59, 6265–6280. [Google Scholar] [CrossRef] [PubMed]
- Lajarín-Cuesta, R.; Arribas, R.L.; Nanclares, C.; García-Frutos, E.M.; Gandía, L.; de Los Ríos, C. Design and synthesis of multipotent 3-aminomethylindoles and 7-azaindoles with enhanced protein phosphatase 2A-activating profile and neuroprotec-tion. Eur. J. Med. Chem. 2018, 157, 294–309. [Google Scholar] [CrossRef] [PubMed]
- García-Vázquez, R.; Rebitski, E.P.; Viejo, L.; de los Ríos, C.; Darder, M.; García-Frutos, E.M. Clay-based hybrids for controlled release of 7-azaindole derivatives as neuroprotective drugs in the treatment of Alzheimer’s disease. Appl. Clay Sci. 2020, 189, 105541. [Google Scholar] [CrossRef]
- Arribas, R.L.; Bordas, A.; Domènech Omella, J.; Cedillo, J.L.; Janssens, V.; Montiel, C.; de Los Ríos, C. An okadaic acid fragment analogue prevents nicotine-induced resistance to cisplatin by recovering PP2A activity in non-small cell lung cancer cells. Bioorg. Chem. 2020, 100, 103874. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de los Ríos, C.; Viejo, L.; Carretero, V.J.; Juárez, N.H.; Cruz-Martins, N.; Hernández-Guijo, J.M. Promising Molecular Targets in Pharmacological Therapy for Neuronal Damage in Brain Injury. Antioxidants 2023, 12, 118. https://doi.org/10.3390/antiox12010118
de los Ríos C, Viejo L, Carretero VJ, Juárez NH, Cruz-Martins N, Hernández-Guijo JM. Promising Molecular Targets in Pharmacological Therapy for Neuronal Damage in Brain Injury. Antioxidants. 2023; 12(1):118. https://doi.org/10.3390/antiox12010118
Chicago/Turabian Stylede los Ríos, Cristóbal, Lucía Viejo, Victoria Jiménez Carretero, Natalia Hernández Juárez, Natália Cruz-Martins, and Jesús M. Hernández-Guijo. 2023. "Promising Molecular Targets in Pharmacological Therapy for Neuronal Damage in Brain Injury" Antioxidants 12, no. 1: 118. https://doi.org/10.3390/antiox12010118
APA Stylede los Ríos, C., Viejo, L., Carretero, V. J., Juárez, N. H., Cruz-Martins, N., & Hernández-Guijo, J. M. (2023). Promising Molecular Targets in Pharmacological Therapy for Neuronal Damage in Brain Injury. Antioxidants, 12(1), 118. https://doi.org/10.3390/antiox12010118







