Fucoidan/UVC Combined Treatment Exerts Preferential Antiproliferation in Oral Cancer Cells but Not Normal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and UVC Irradiation
2.2. Cell Culture and MTS Viability
2.3. Cell Cycle
2.4. Apoptosis
2.5. Oxidative Stress
2.6. Glutathione (GSH)
2.7. DNA Damages
2.8. Statistics
3. Results
3.1. UVC/FN versus Single Treatment on Antiproliferation
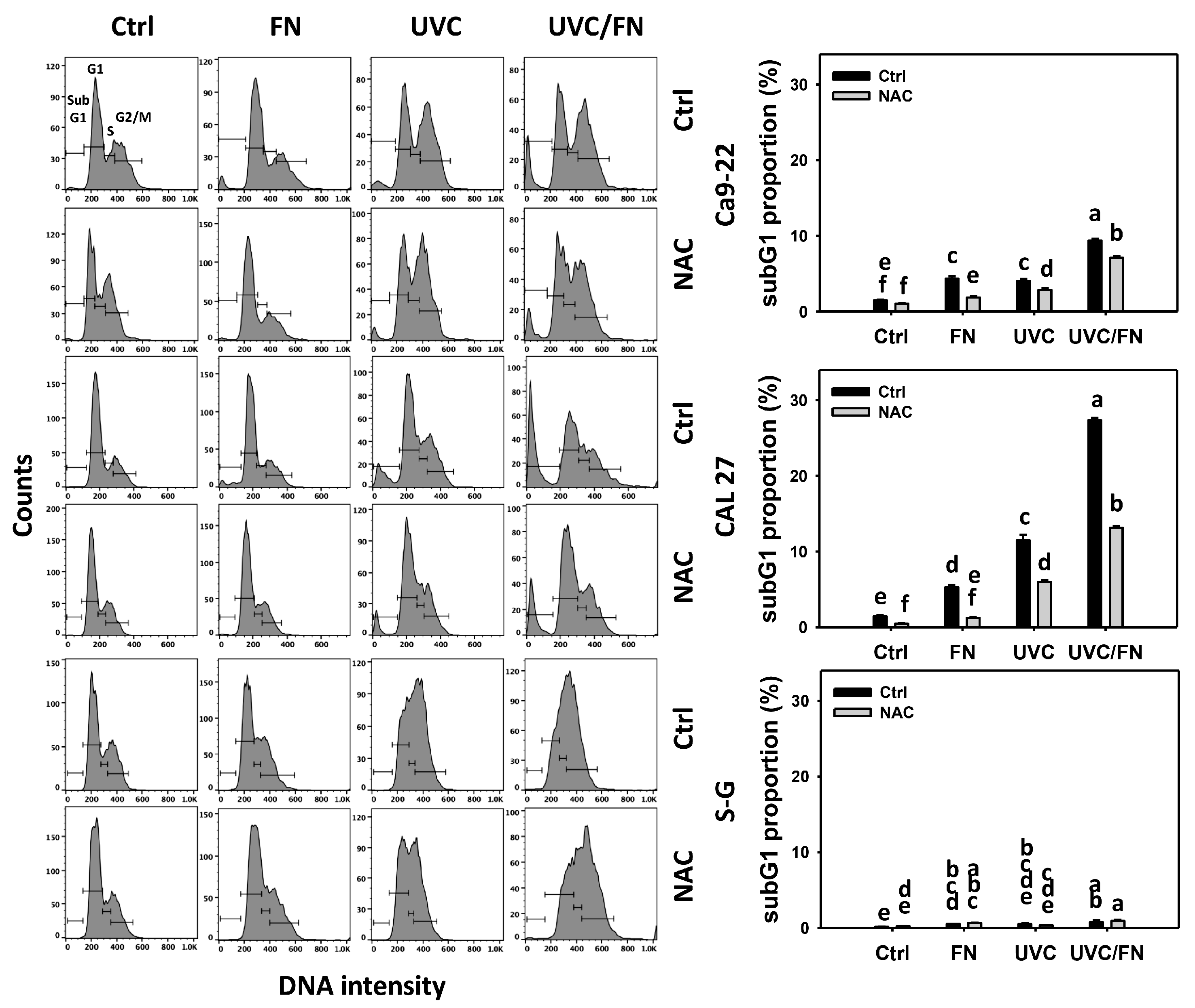
3.2. UVC/FN versus Single Treatment on SubG1 Increment
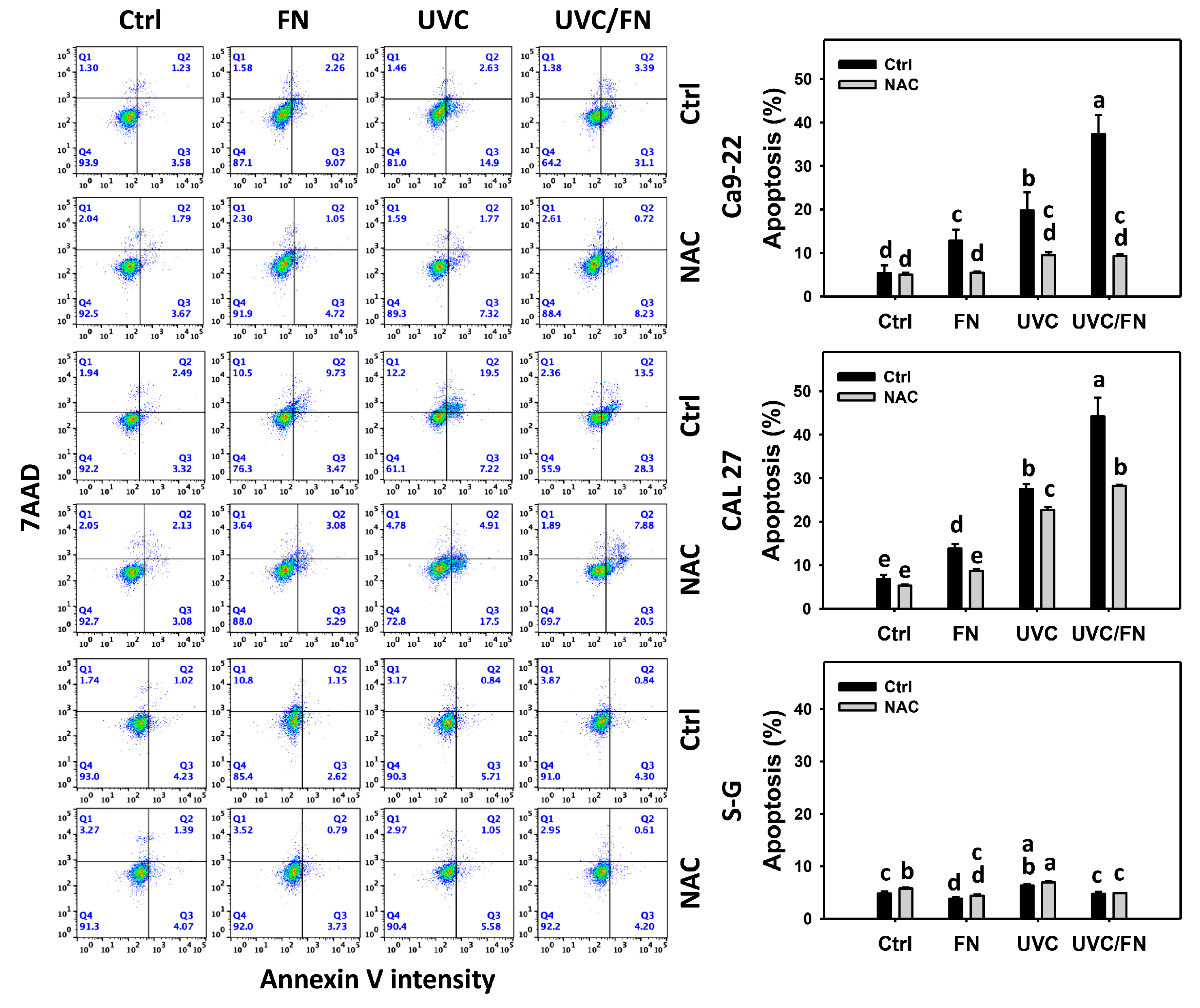
3.3. UVC/FN versus Single Treatment on Annexin V Increment
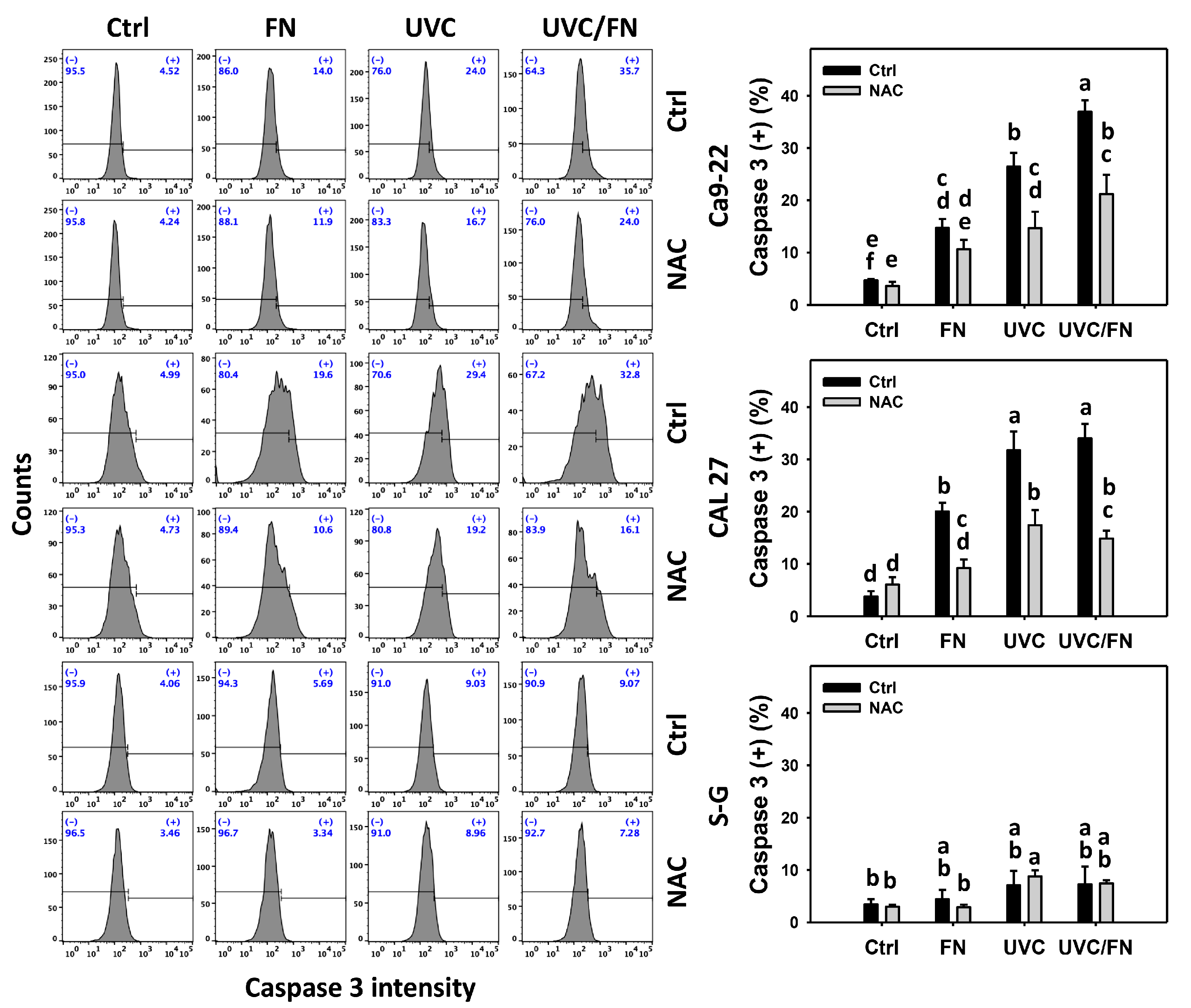
3.4. UVC/FN versus Single Treatment on Caspase 3 Activation
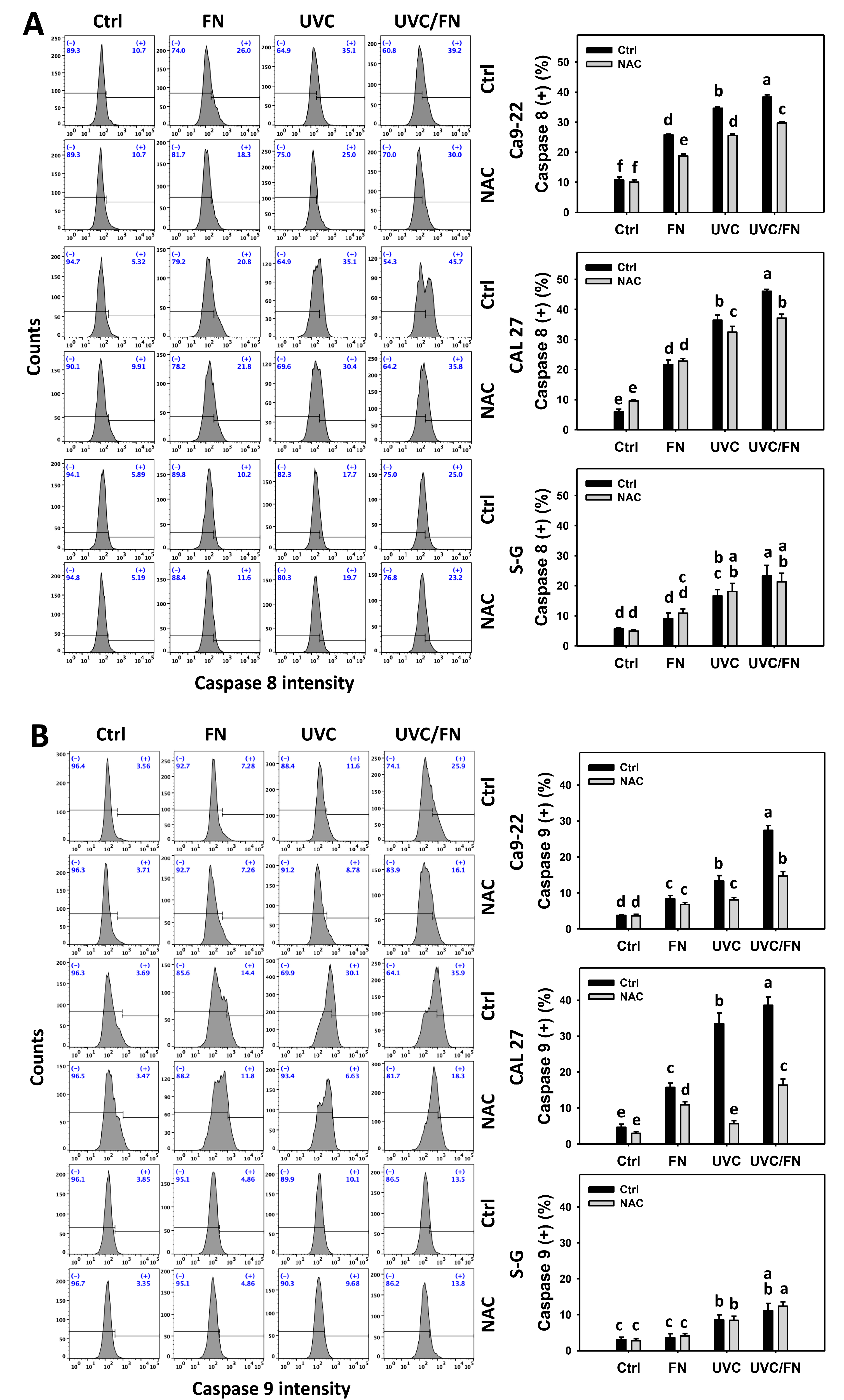
3.5. UVC/FN versus Single Treatment on Extrinsic and Intrinsic Caspase Activations
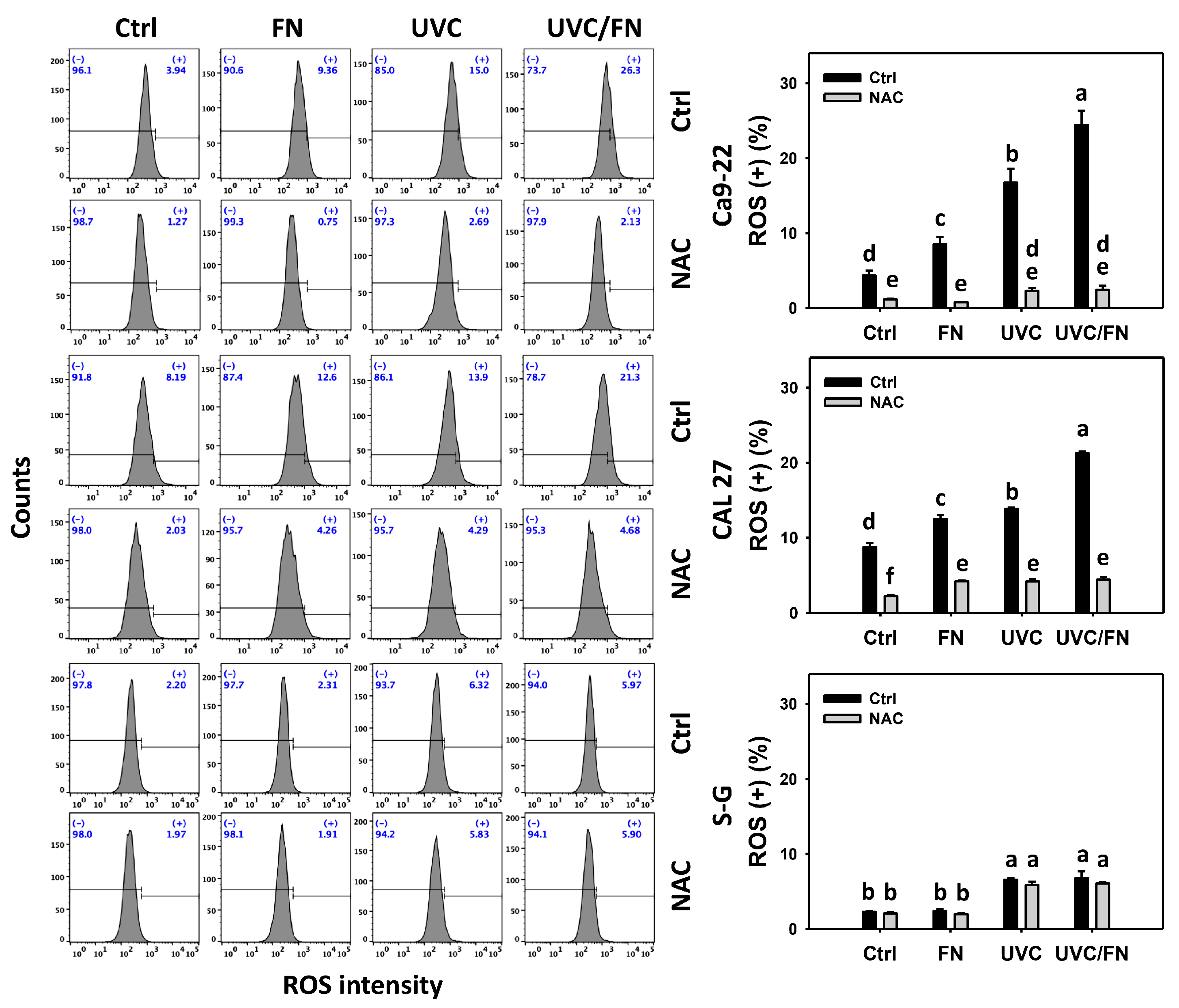
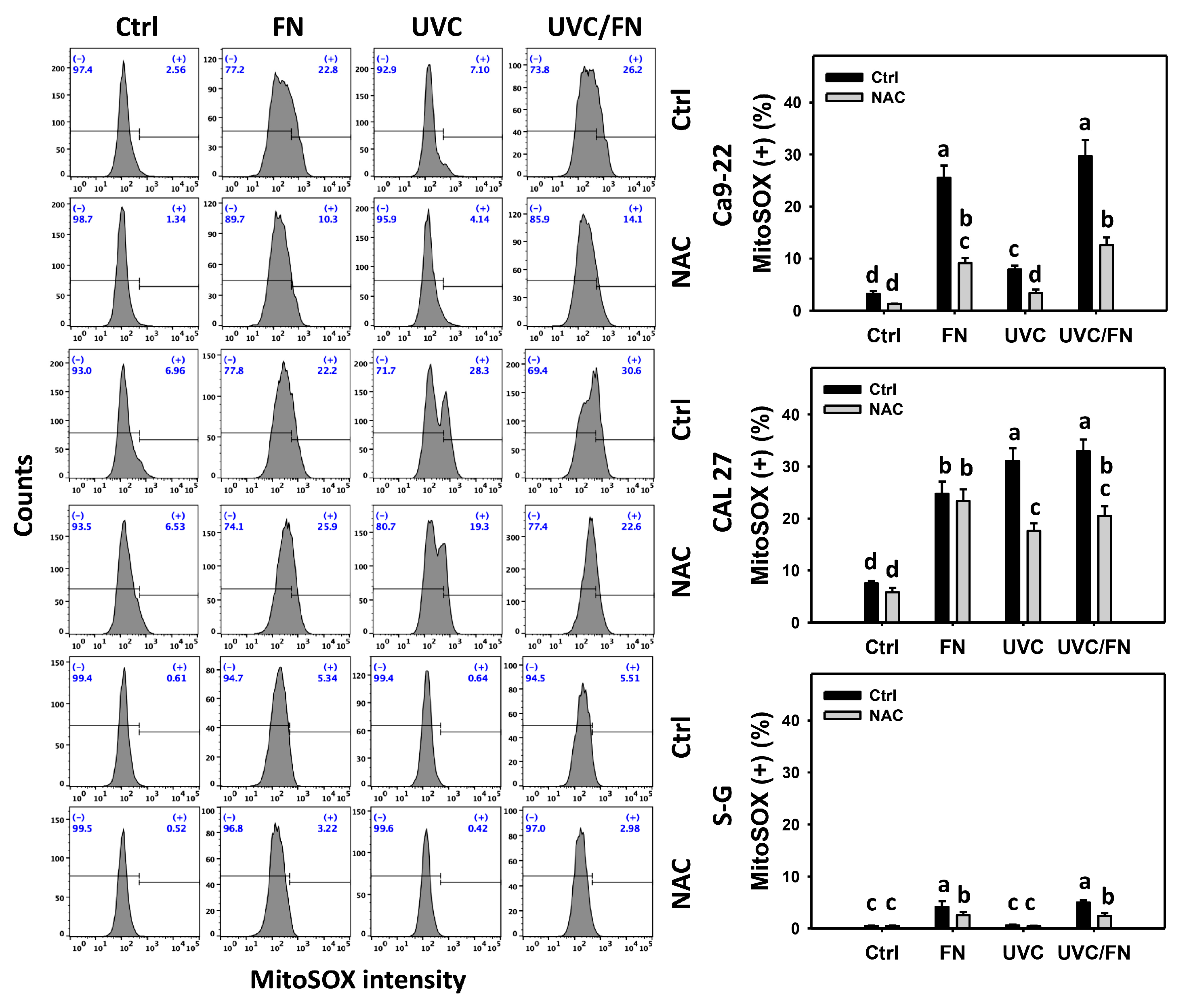
3.6. UVC/FN versus Single Treatment on ROS/MitoSOX
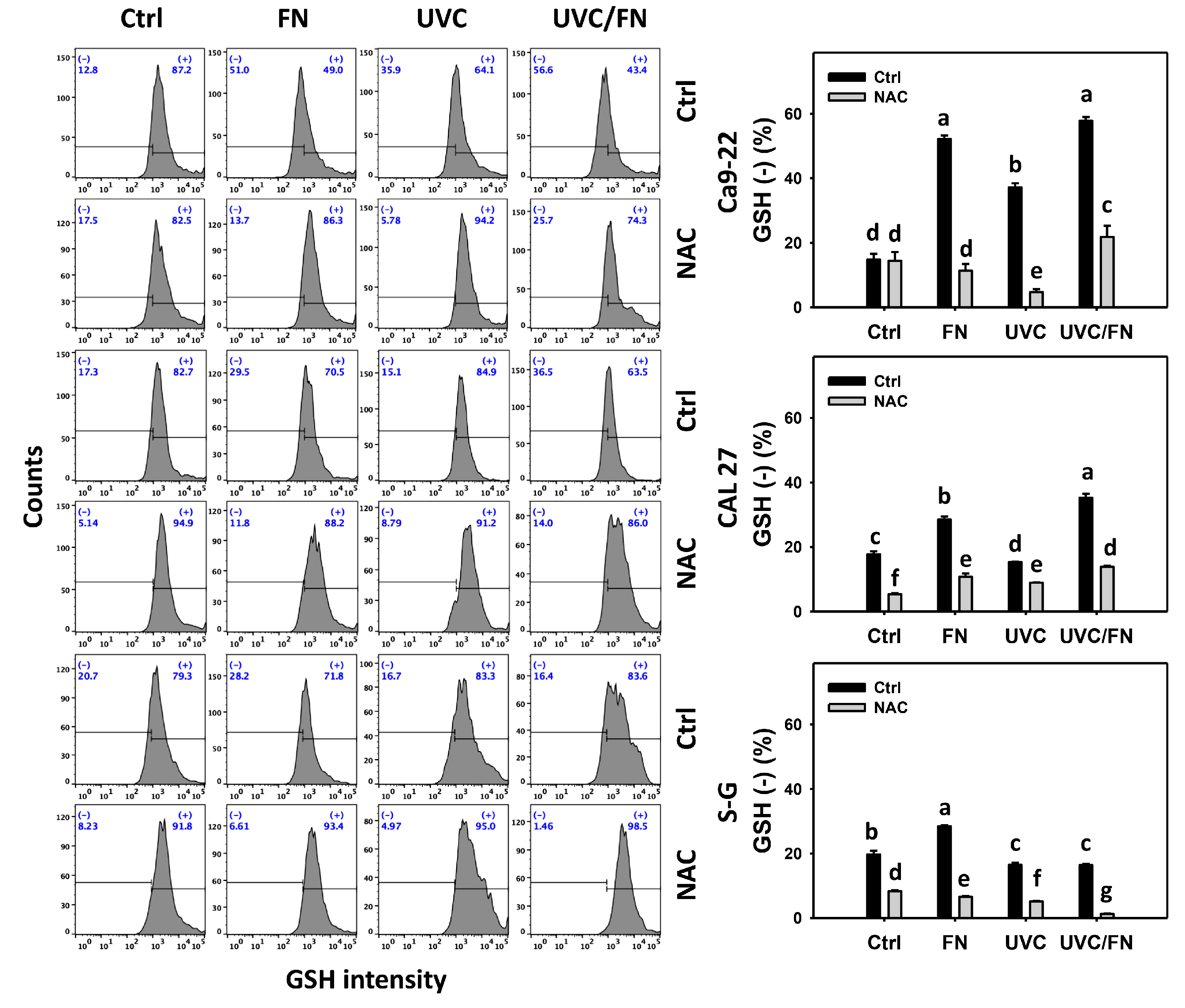
3.7. UVC/FN versus Single Treatment on GSH Depletion
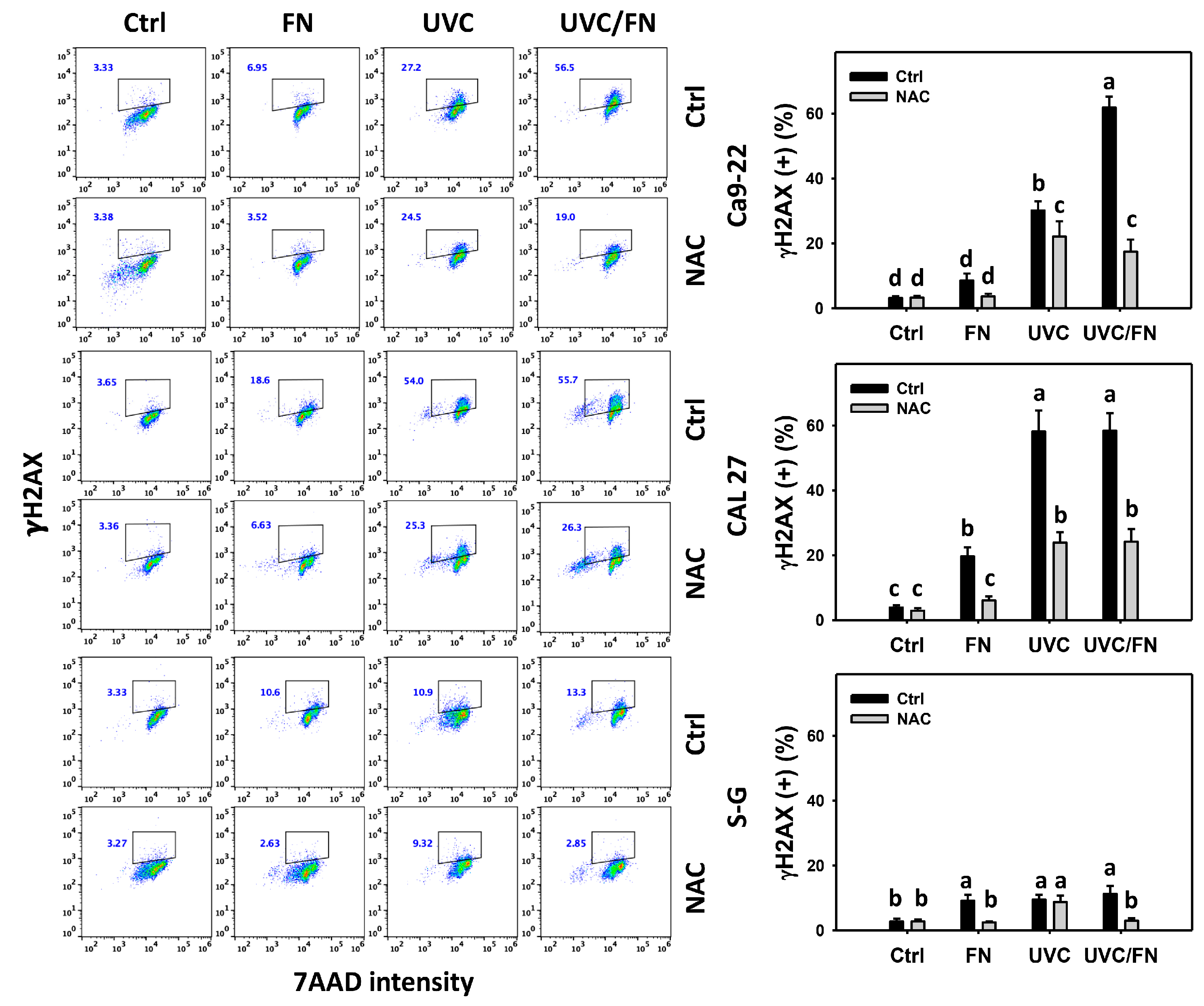
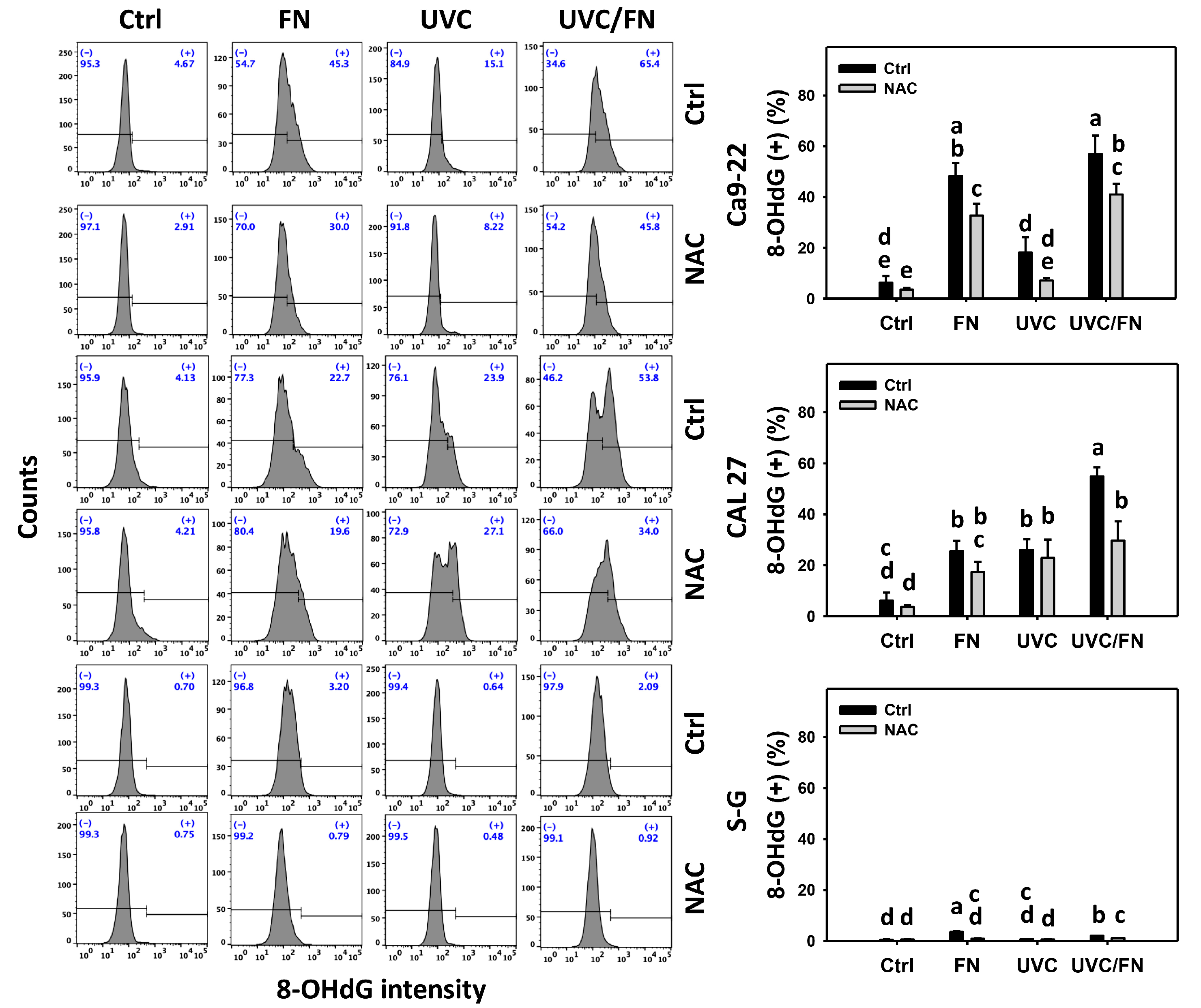
3.8. UVC/FN versus Single Treatment on DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E. Oral cancer prevention and control—The approach of the World Health Organization. Oral Oncol. 2009, 45, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Myoung, H.; Hong, S.P.; Yun, P.Y.; Lee, J.H.; Kim, M.J. Anti-cancer effect of genistein in oral squamous cell carcinoma with respect to angiogenesis and in vitro invasion. Cancer Sci. 2003, 94, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Silverman, S., Jr. Oral cancer: Complications of therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 122–126. [Google Scholar] [CrossRef]
- Mehdi, Z.; Petronek, M.S.; Stolwijk, J.M.; Mapuskar, K.A.; Kalen, A.L.; Buettner, G.R.; Cullen, J.J.; Spitz, D.R.; Buatti, J.M.; Allen, B.G. Utilization of pharmacological ascorbate to enhance hydrogen peroxide-mediated radiosensitivity in cancer therapy. Int. J. Mol. Sci. 2021, 22, 880. [Google Scholar] [CrossRef]
- Zhang, P.; Song, E.; Jiang, M.; Song, Y. Celecoxib and Afatinib synergistic enhance radiotherapy sensitivity on human non-small cell lung cancer A549 cells. Int. J. Radiat. Biol. 2020, 97, 1–9. [Google Scholar] [CrossRef]
- Ramesh, G.; Das, S.; Bola Sadashiva, S.R. Berberine, a natural alkaloid sensitizes human hepatocarcinoma to ionizing radiation by blocking autophagy and cell cycle arrest resulting in senescence. J. Pharm. Pharmacol. 2020, 72, 1893–1908. [Google Scholar] [CrossRef]
- Chang, H.W.; Tang, J.Y.; Yen, C.Y.; Chang, H.S.; Huang, H.W.; Chung, Y.A.; Chen, I.S.; Huang, M.Y. Synergistic anti-oral cancer effects of UVC and methanolic extracts of Cryptocarya concinna roots via apoptosis, oxidative stress and DNA damage. Int. J. Radiat. Biol. 2016, 92, 263–272. [Google Scholar] [CrossRef]
- Kawaguchi, J.; Adachi, S.; Yasuda, I.; Yamauchi, T.; Nakashima, M.; Ohno, T.; Shimizu, M.; Yoshioka, T.; Itani, M.; Kozawa, O.; et al. Cisplatin and ultra-violet-C synergistically down-regulate receptor tyrosine kinases in human colorectal cancer cells. Mol. Cancer 2012, 11, 45. [Google Scholar] [CrossRef]
- Murray, D.; Mirzayans, R. Cellular responses to platinum-based anticancer drugs and UVC: Role of p53 and implications for cancer therapy. Int. J. Mol. Sci. 2020, 21, 5766. [Google Scholar] [CrossRef] [PubMed]
- Imani, R.; Veranic, P.; Iglic, A.; Kreft, M.E.; Pazoki, M.; Hudoklin, S. Combined cytotoxic effect of UV-irradiation and TiO2 microbeads in normal urothelial cells, low-grade and high-grade urothelial cancer cells. Photochem. Photobiol. Sci. 2015, 14, 583–590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, C.Y.; Lou, D.Y.; Zhou, L.Q.; Wang, J.C.; Yang, B.; He, Q.J.; Wang, J.J.; Weng, Q.J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Func. Foods 2017, 38, 415–426. [Google Scholar]
- Liu, M.; Liu, Y.; Cao, M.J.; Liu, G.M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Hwang, P.A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 15. [Google Scholar] [CrossRef]
- Jin, J.O.; Song, M.G.; Kim, Y.N.; Park, J.I.; Kwak, J.Y. The mechanism of fucoidan-induced apoptosis in leukemic cells: Involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol. Carcinog. 2010, 49, 771–782. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Sheu, J.H.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Brown algae-derived fucoidan exerts oxidative stress-dependent antiproliferation on oral cancer cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef]
- Banafa, A.M.; Roshan, S.; Liu, Y.Y.; Chen, H.J.; Chen, M.J.; Yang, G.X.; He, G.Y. Fucoidan induces G1 phase arrest and apoptosis through caspases-dependent pathway and ROS induction in human breast cancer MCF-7 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Lee, D.S.; Jeong, J.W.; Hong, S.H.; Choi, I.W.; Cha, H.J.; Kim, S.; Kim, H.S.; Park, C.; Kim, G.Y.; et al. Fucoidan induces ROS-dependent apoptosis in 5637 human bladder cancer cells by downregulating telomerase activity via inactivation of the PI3K/Akt signaling pathway. Drug Dev. Res. 2017, 78, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xue, M.; Wang, Q.; Liang, H.; Yang, J.; Pei, Z.; Qin, K. Inhibition of fucoidan on breast cancer cells and potential enhancement of their sensitivity to chemotherapy by regulating autophagy. Phytother. Res. 2021, 35, 6904–6917. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.L.; Tseng, A.J.; Hsu, H.Y.; Hsu, W.H.; Lin, Z.H.; Hua, W.J.; Lin, T.Y. Fucoidan increased the sensitivity to gefitinib in lung cancer cells correlates with reduction of TGFbeta-mediated Slug expression. Int. J. Biol. Macromol. 2020, 153, 796–805. [Google Scholar] [CrossRef]
- Al Monla, R.; Dassouki, Z.; Sari-Chmayssem, N.; Mawlawi, H.; Gali-Muhtasib, H. Fucoidan and alginate from the brown algae Colpomenia sinuosa and their combination with vitamin C trigger apoptosis in colon cancer. Molecules 2022, 27, 358. [Google Scholar] [CrossRef]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Peng, B.R.; Hsu, K.C.; El-Shazly, M.; Shih, S.P.; Lin, T.E.; Kuo, F.W.; Chou, Y.C.; Lin, H.Y.; Lu, M.C. 13-Acetoxysarcocrassolide exhibits cytotoxic activity against oral cancer cells through the interruption of the Keap1/Nrf2/p62/SQSTM1 pathway: The need to move beyond classical concepts. Mar. Drugs 2020, 18, 382. [Google Scholar]
- Shih, H.C.; El-Shazly, M.; Juan, Y.S.; Chang, C.Y.; Su, J.H.; Chen, Y.C.; Shih, S.P.; Chen, H.M.; Wu, Y.C.; Lu, M.C. Cracking the cytotoxicity code: Apoptotic induction of 10-acetylirciformonin B is mediated through ROS generation and mitochondrial dysfunction. Mar. Drugs 2014, 12, 3072–3090. [Google Scholar] [CrossRef]
- Kasten, F.H.; Pineda, L.F.; Schneider, P.E.; Rawls, H.R.; Foster, T.A. Biocompatibility testing of an experimental fluoride releasing resin using human gingival epithelial cells in vitro. In Vitro Cell Dev. Biol. 1989, 25, 57–62. [Google Scholar] [CrossRef]
- Kasten, F.H.; Soileau, K.; Meffert, R.M. Quantitative evaluation of human gingival epithelial cell attachment to implant surfaces in vitro. Int. J. Periodontics Restor. Dent. 1990, 10, 68–79. [Google Scholar]
- Hsieh, P.L.; Liao, Y.W.; Hsieh, C.W.; Chen, P.N.; Yu, C.C. Soy isoflavone genistein impedes cancer stemness and mesenchymal transition in head and neck cancer through activating miR-34a/RTCB axis. Nutrients 2020, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Wang, Y.Y.; Lan, T.H.; Lin, L.C.; Yuan, S.F.; Tang, J.Y.; Chang, H.W. Low dose combined treatment with ultraviolet-C and withaferin a enhances selective killing of oral cancer cells. Antioxidants 2020, 9, 1120. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.C.; Hsieh, Y.C.; Li, L.H.; Chang, C.C.; Janouskova, K.; Ramani, M.V.; Subbaraju, G.V.; Cheng, K.T.; Chang, C.C. Dehydroxyhispolon methyl ether, a hispolon derivative, inhibits WNT/beta-catenin signaling to elicit human colorectal carcinoma cell apoptosis. Int. J. Mol. Sci. 2020, 21, 8839. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chan, H.S.; Tsay, H.S.; Funayama, S.; Kuo, C.L.; Chung, J.G. Ethyl acetate fraction from methanol extraction of Vitis thunbergii var. taiwaniana induced G0/G1 phase arrest via inhibition of cyclins D and E and induction of apoptosis through caspase-dependent and -independent pathways in human prostate carcinoma DU145 cells. Environ. Toxicol. 2018, 33, 41–51. [Google Scholar]
- Liu, S.L.; Yang, K.H.; Yang, C.W.; Lee, M.Y.; Chuang, Y.T.; Chen, Y.N.; Chang, F.R.; Chen, C.Y.; Chang, H.W. Burmannic acid inhibits proliferation and induces oxidative stress response of oral cancer cells. Antioxidants 2021, 10, 1588. [Google Scholar] [CrossRef]
- Wu, C.F.; Lee, M.G.; El-Shazly, M.; Lai, K.H.; Ke, S.C.; Su, C.W.; Shih, S.P.; Sung, P.J.; Hong, M.C.; Wen, Z.H.; et al. Isoaaptamine induces T-47D cells apoptosis and autophagy via oxidative stress. Mar. Drugs 2018, 16, 18. [Google Scholar] [CrossRef]
- Shih, S.P.; Lu, M.C.; El-Shazly, M.; Lin, Y.H.; Chen, C.L.; Yu, S.S.F.; Liu, Y.C. The antileukemic and anti-prostatic effect of aeroplysinin-1 is mediated through ROS-induced apoptosis via NOX activation and inhibition of HIF-1a activity. Life 2022, 12, 687. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef]
- Oh, B.; Kim, J.; Lu, W.; Rosenthal, D. Anticancer effect of fucoidan in combination with tyrosine kinase inhibitor lapatinib. Evid. Based Complementary Altern. Med. 2014, 2014, 865375. [Google Scholar] [CrossRef]
- Perse, M. Cisplatin mouse models: Treatment, toxicity and translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef]
- Lorizio, W.; Wu, A.H.; Beattie, M.S.; Rugo, H.; Tchu, S.; Kerlikowske, K.; Ziv, E. Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res. Treat. 2012, 132, 1107–1118. [Google Scholar] [PubMed]
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef]
- Ajaykumar, C. Overview on the Side Effects of Doxorubicin; IntechOpen: London, UK, 2020. [Google Scholar]
- Frankel, C.; Palmieri, F.M. Lapatinib side-effect management. Clin. J. Oncol. Nurs. 2010, 14, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, R.J. Gefitinib: An adverse effects profile. Expert Opin. Drug Saf. 2006, 5, 469–479. [Google Scholar] [CrossRef]
- Wang, S.C.; Wang, Y.Y.; Lin, L.C.; Chang, M.Y.; Yuan, S.F.; Tang, J.Y.; Chang, H.W. Combined treatment of sulfonyl chromen-4-ones (CHW09) and ultraviolet-C (UVC) enhances proliferation inhibition, apoptosis, oxidative stress, and DNA damage against oral cancer cells. Int. J. Mol. Sci. 2020, 21, 6443. [Google Scholar] [CrossRef]
- Takac, P.; Kello, M.; Vilkova, M.; Vaskova, J.; Michalkova, R.; Mojzisova, G.; Mojzis, J. Antiproliferative effect of acridine chalcone is mediated by induction of oxidative stress. Biomolecules 2020, 10, 345. [Google Scholar] [CrossRef]
- Kim, U.; Shin, C.; Kim, C.Y.; Ryu, B.; Kim, J.; Bang, J.; Park, J.H. Albendazole exerts antiproliferative effects on prostate cancer cells by inducing reactive oxygen species generation. Oncol. Lett. 2021, 21, 395. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.B.; Wang, D.; Zhu, Q.L.; Zheng, J.L. Combined effects of polystyrene microplastics and cadmium on oxidative stress, apoptosis, and GH/IGF axis in zebrafish early life stages. Sci. Total Environ. 2022, 813, 152514. [Google Scholar]
- Peng, S.Y.; Lin, L.C.; Yang, Z.W.; Chang, F.R.; Cheng, Y.B.; Tang, J.Y.; Chang, H.W. Combined treatment with low cytotoxic ethyl acetate Nepenthes extract and ultraviolet-C improves antiproliferation to oral cancer cells via oxidative stress. Antioxidants 2020, 9, 873. [Google Scholar] [CrossRef]
- Nasihun, T.; Widayati, E. Administration of purwoceng (Pimpinella alpina Molk) improves oxidative stress biomarker following UVC irradiation in spargue-dawley male rats. J. Nat. Remedies 2016, 16, 115–124. [Google Scholar] [CrossRef]
- Chan, W.H.; Yu, J.S. Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. J. Cell. Biochem. 2000, 78, 73–84. [Google Scholar] [CrossRef]
- Chan, W.H.; Wu, C.C.; Yu, J.S. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J. Cell. Biochem. 2003, 90, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, Y.P.; Huang, X.Z.; He, M.; Chen, Y.Y.; Shi, G.Y.; Li, H.; Yi, J.; Wang, J. Emodin enhances sensitivity of gallbladder cancer cells to platinum drugs via glutathion depletion and MRP1 downregulation. Biochem. Pharmacol. 2010, 79, 1134–1140. [Google Scholar] [CrossRef]
- Khan, M.; Yi, F.; Rasul, A.; Li, T.; Wang, N.; Gao, H.; Gao, R.; Ma, T. Alantolactone induces apoptosis in glioblastoma cells via GSH depletion, ROS generation, and mitochondrial dysfunction. IUBMB Life 2012, 64, 783–794. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Zampagni, M.; Evangelisti, E.; Conti, S.; D’Adamio, G.; Goti, A.; Becatti, M.; Fiorillo, C.; Taddei, N.; Cecchi, C.; et al. Protective properties of novel S-acyl-glutathione thioesters against ultraviolet-induced oxidative stress. Photochem. Photobiol. 2013, 89, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Khattab, S.A.; Hussien, W.F.; Raafat, N.; Ahmed Alaa El-Din, E. Effects of catechin hydrate in benzo[a]pyrene-induced lung toxicity: Roles of oxidative stress, apoptosis, and DNA damage. Toxicol. Mech. Methods 2021, 31, 467–475. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Hacioglu, C.; Kar, F.; Kacar, S.; Sahinturk, V.; Kanbak, G. Bexarotene inhibits cell proliferation by inducing oxidative stress, DNA damage and apoptosis via PPARgamma/NF-kappaB signaling pathway in C6 glioma cells. Med. Oncol. 2021, 38, 31. [Google Scholar] [CrossRef]
- Dunkern, T.R.; Fritz, G.; Kaina, B. Ultraviolet light-induced DNA damage triggers apoptosis in nucleotide excision repair-deficient cells via Bcl-2 decline and caspase-3/-8 activation. Oncogene 2001, 20, 6026–6038. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.K.; Chang, W.C.; Chan, W.H.; Yang, S.D.; Ni, M.H.; Yu, J.S. Proteolytic cleavage and activation of PAK2 during UV irradiation-induced apoptosis in A431 cells. J. Cell. Biochem. 1998, 70, 442–454. [Google Scholar] [CrossRef]
- Ruffmann, R.; Wendel, A. GSH rescue by N-acetylcysteine. Klin Wochenschr. 1991, 69, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Davies, M.J. Actions of ultraviolet light on cellular structures. EXS 2006, 96, 131–157. [Google Scholar]
- Bagan, J.; Sarrion, G.; Jimenez, Y. Oral cancer: Clinical features. Oral. Oncol. 2010, 46, 414–417. [Google Scholar] [CrossRef]
- Mittal, A.; Kumar, M.; Gopishankar, N.; Kumar, P.; Verma, A.K. Quantification of narrow band UVB radiation doses in phototherapy using diacetylene based film dosimeters. Sci. Rep. 2021, 11, 684. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, Y.-T.; Shiau, J.-P.; Yen, C.-Y.; Hou, M.-F.; Jeng, J.-H.; Tang, J.-Y.; Chang, H.-W. Fucoidan/UVC Combined Treatment Exerts Preferential Antiproliferation in Oral Cancer Cells but Not Normal Cells. Antioxidants 2022, 11, 1797. https://doi.org/10.3390/antiox11091797
Chuang Y-T, Shiau J-P, Yen C-Y, Hou M-F, Jeng J-H, Tang J-Y, Chang H-W. Fucoidan/UVC Combined Treatment Exerts Preferential Antiproliferation in Oral Cancer Cells but Not Normal Cells. Antioxidants. 2022; 11(9):1797. https://doi.org/10.3390/antiox11091797
Chicago/Turabian StyleChuang, Ya-Ting, Jun-Ping Shiau, Ching-Yu Yen, Ming-Feng Hou, Jiiang-Huei Jeng, Jen-Yang Tang, and Hsueh-Wei Chang. 2022. "Fucoidan/UVC Combined Treatment Exerts Preferential Antiproliferation in Oral Cancer Cells but Not Normal Cells" Antioxidants 11, no. 9: 1797. https://doi.org/10.3390/antiox11091797
APA StyleChuang, Y.-T., Shiau, J.-P., Yen, C.-Y., Hou, M.-F., Jeng, J.-H., Tang, J.-Y., & Chang, H.-W. (2022). Fucoidan/UVC Combined Treatment Exerts Preferential Antiproliferation in Oral Cancer Cells but Not Normal Cells. Antioxidants, 11(9), 1797. https://doi.org/10.3390/antiox11091797







