Polyfunctionalized α-Phenyl-tert-butyl(benzyl)nitrones: Multifunctional Antioxidants for Stroke Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
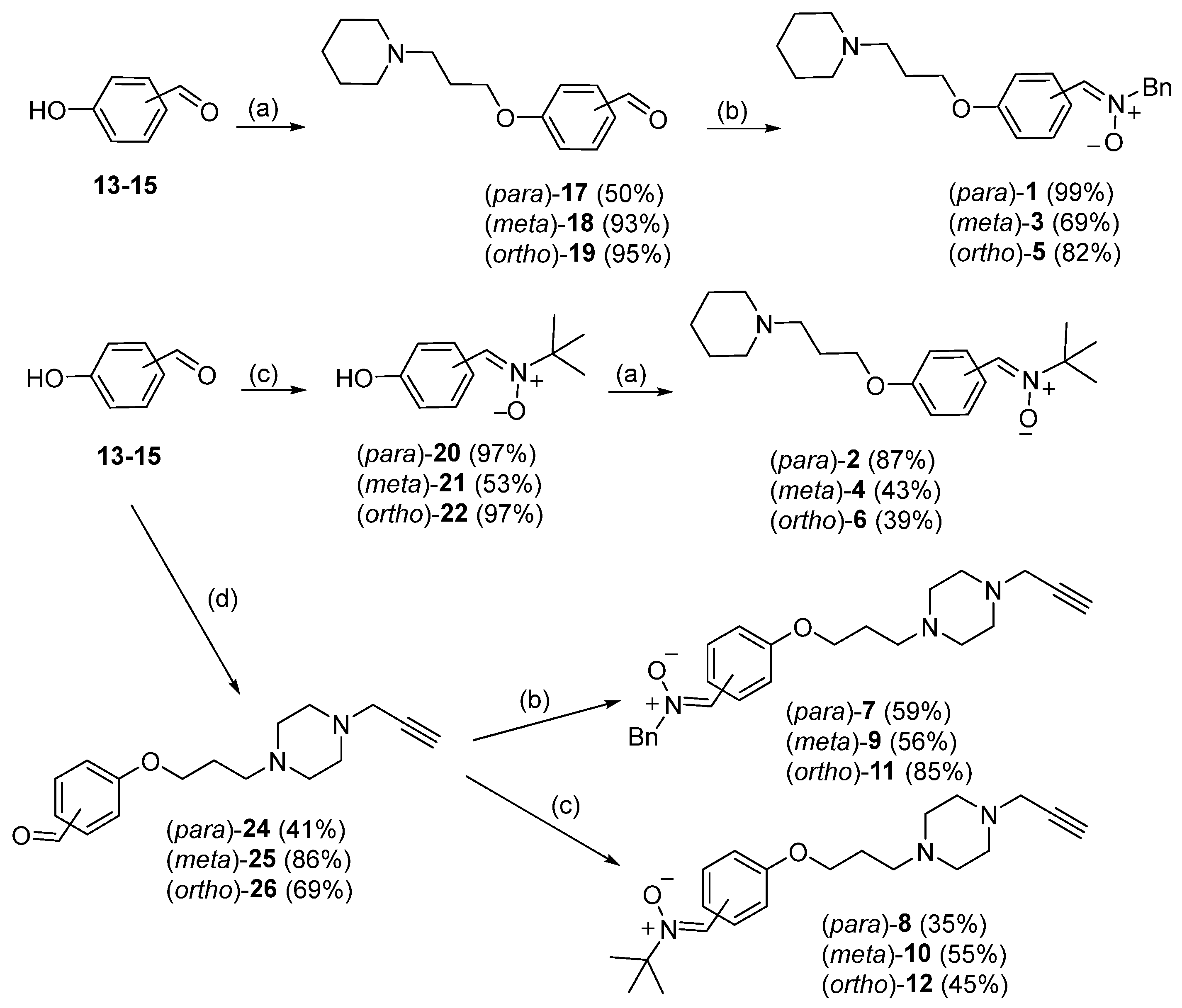
2.1.1. General Method for O-alkylation (Method A)
2.1.2. General Method for Synthesis of Nitrones (Method B)
4-(3-(Piperidin-1-yl)propoxy)benzaldehyde (17) [21]
(Z)-N-Benzyl-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)methanimine oxide (1)
3-(3-(Piperidin-1-yl)propoxy)benzaldehyde (18) [21]
(Z)-N-Benzyl-1-(3-(3-(piperidin-1-yl)propoxy)phenyl)methanimine oxide (3)
2-(3-(Piperidin-1-yl)propoxy)benzaldehyde (19) [21]
(Z)-N-Benzyl-1-(2-(3-(piperidin-1-yl)propoxy)phenyl)methanimine oxide (5)
(Z)-N-tert-Butyl-1-(4-hydroxyphenyl)methanimine oxide (20) [22]
(Z)-N-tert-Butyl-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)methanimine oxide (2)
(Z)-N-tert-Butyl-1-(3-hydroxyphenyl)methanimine oxide (21) [22]
(Z)-N-tert-Butyl-1-(3-(3-(piperidin-1-yl)propoxy)phenyl)methanimine oxide (4)
(Z)-N-tert-Butyl-1-(2-hydroxyphenyl)methanimine oxide (22) [22]
(Z)-N-tert-Butyl-1-(2-(3-(piperidin-1-yl)propoxy)phenyl)methanimine oxide (6)
1-(3-Chloropropyl)-4-(prop-2-yn-1-yl)piperazine (23) [20]
4-(3-(4-(Prop-2-yn-1-yl) piperazin-1-yl) propoxy)benzaldehyde enzaldehyde (24)
(Z)-N-Benzyl-1-(4-(3-(4-(prop-2-yn-1-yl) piperazin-1-yl) propoxy) phenyl) methanimine oxide xide (7)
3-(3-(4-(Prop-2-in-1-yl) piperazin-1-yl) propoxy)benzaldehyde (25)
(E)-N-Benzyl-1-(3-(3-(4-(prop-2-yn-1-yl) piperazin-1-yl) propoxy)phenyl)methanimine oxide xide (9)
2-(3-(4-(Prop-2-in-1-yl) piperazin-1-yl) propoxy)benzaldehyde (26)
(Z)-N-Benzyl-1-(2-(3-(4-(prop-2-yn-1-yl) piperazin-1-yl)propyl) phenyl)methanimine oxide xide (11)
(Z)-N-tert-Butyl-1-(4-(3-(4-(prop-2-yn-1-yl) piperazin-1-yl) propoxy) phenyl) methanimine oxide xide (8)
(Z)-N-tert-Butyl-1-(3-(3-(4-(prop-2-yn-1-yl) piperazin-1-yl) propoxy) phenyl) methanimine oxide xide (10)
(Z)-N-tert-Butyl-1-(2-(3-(4-(prop-2-yn-1-yl) piperazin-1-yl) propoxy) phenyl) methanimine oxide xide (12)
2.2. Estimation of Lipophilicity as Clog P
2.3. In Vitro Antioxidant Activity and Anti-Inflammatory Assays of Nitrones 1–12 and PBN
2.3.1. Inhibition of Linoleic Acid Peroxidation (ILPO)
2.3.2. In Vitro Inhibition of Soybean LOX
2.3.3. Competition of the Tested Compounds with DMSO for Hydroxyl Radicals
2.3.4. ABTS+–Decolorization Assay in Ethanolic Solution for Antioxidant Activity
2.3.5. DPPH Radical-Scavenging Assay
2.4. Inhibition of Cholinesterases and Monoamine Oxidases
2.5. Inhibition or Aβ1–42 Aggregation
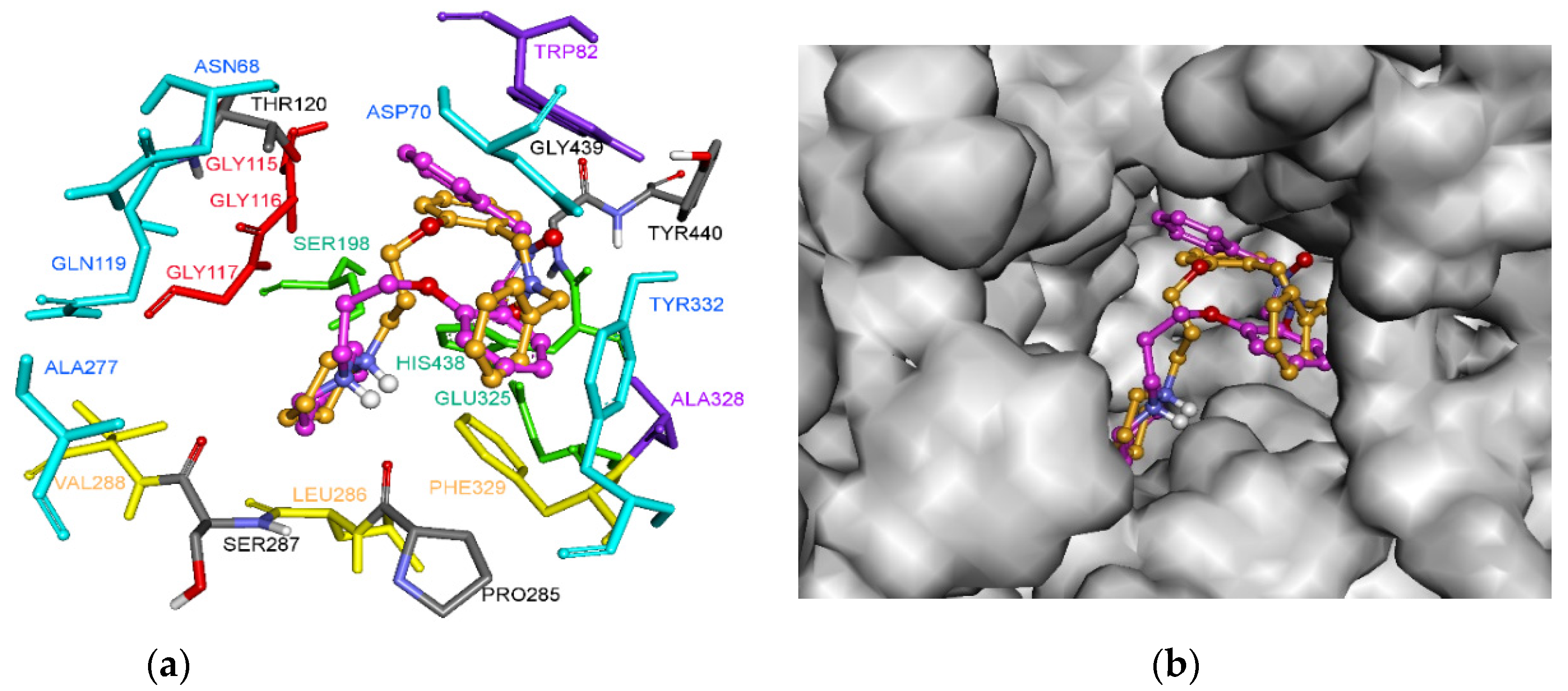
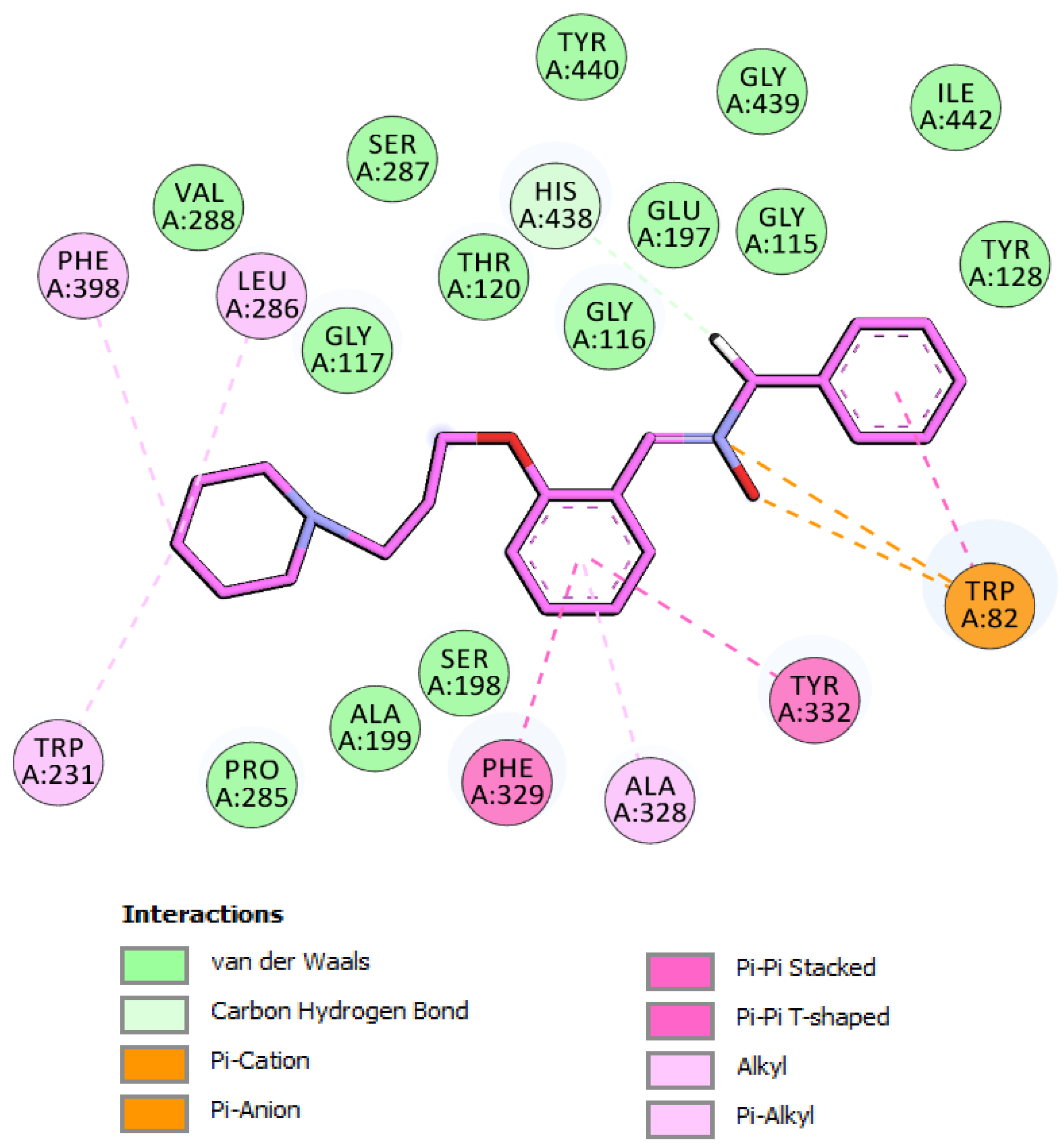
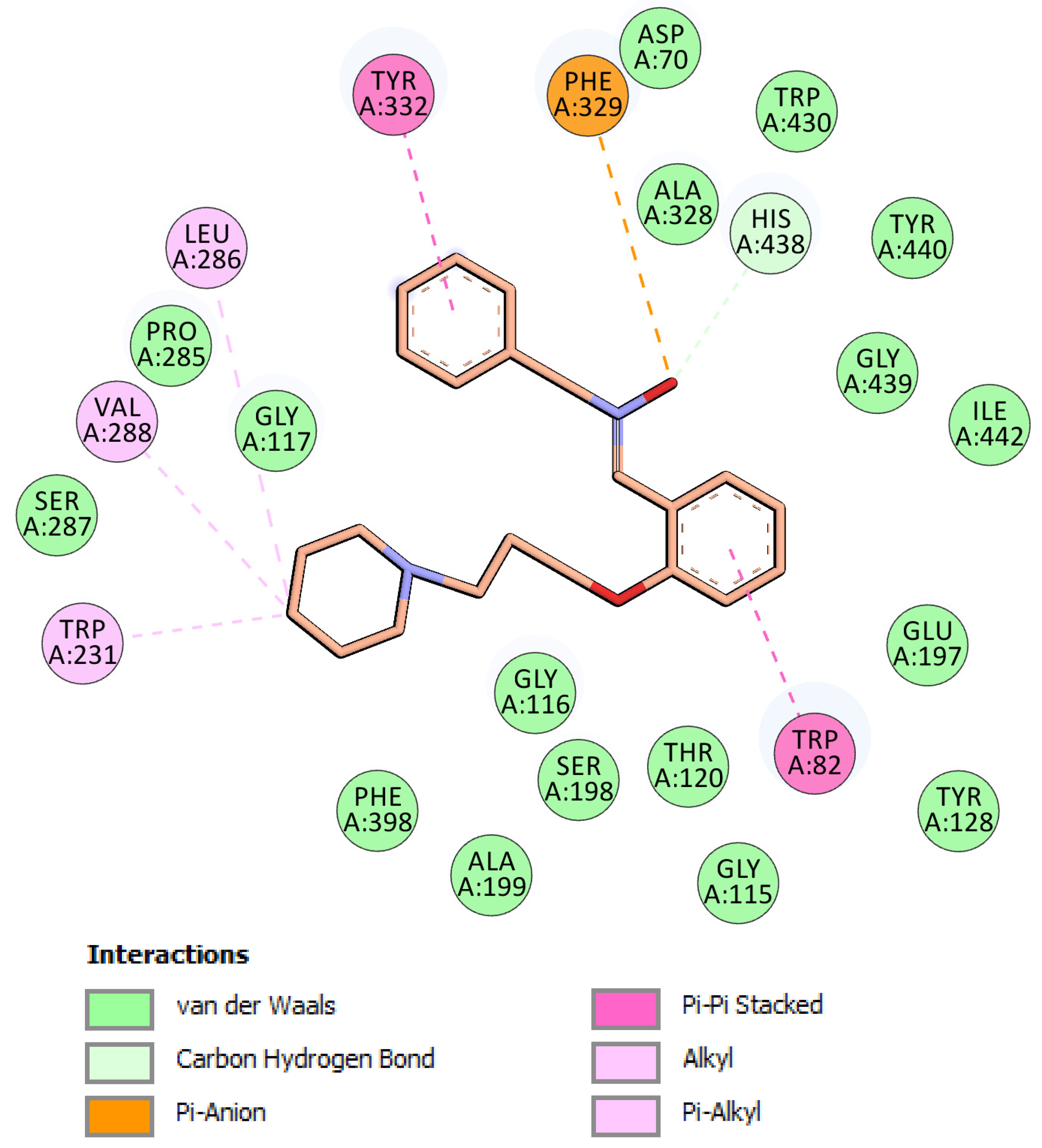
2.6. Molecular Docking of Inhibitor 5 into hBChE
2.7. Neuroprotection Experiments
3. Results and Discussion
3.1. Chemistry
3.2. Antioxidant Assays
3.3. Additional Pharmacological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Takahashi, S.; Mashima, K. Neuroprotection and disease modification by astrocytes and microglia in Parkinson disease. Antioxidants 2022, 11, 170. [Google Scholar] [CrossRef]
- Koh, J.S.; Lee, S.J.; Ryu, C.W.; Kim, H.S. Safety and efficacy of mechanical thrombectomy with solitaire stent retrieval for acute ischemic stroke: A systematic review. Neurointervention 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Nicole, O.; Docagne, F.; Ali, C.; Margaill, I.; Carmeliet, P.; MacKenzie, E.T.; Vivien, D.; Buisson, A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001, 7, 59–64. [Google Scholar] [CrossRef]
- Hussein, H.M.; Georgiadis, A.L.; Vázquez, G.; Miley, J.T.; Memon, M.Z.; Mohammad, Y.M.; Christoforidis, G.A.; Tariq, N.; Qureshi, A.I. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: A multicenter study. AJNR Am. J. Neuroradiol. 2010, 31, 454–458. [Google Scholar] [CrossRef]
- Chan, P.H. Cellular Antioxidant Defense Mechanisms; Chow, C.K., Ed.; CRC Press: Boca Ratón, FL, USA, 1988; Volume 3, pp. 89–109. [Google Scholar]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Pakinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Esteban, G.; Brogi, S.; Shionoya, M.; Wang, L.; Campiani, G.; Unzeta, M.; Inokuchi, T.; Stefania Butini, S.; Marco-Contelles, J. Donepezi-l-like multifunctional agents: Design, synthesis, molecular modeling and biological evaluation. Eur. J. Med. Chem. 2016, 121, 864–879. [Google Scholar] [CrossRef]
- McCaig, C.; Ataliotis, P.; Shtaya, A.; Omar, A.S.; Green, A.R.; Kind, C.N.; Pereira, A.C.; Naray-Fejes-Toth, A.; Fejes-Toth, G.; Yanez-Munoz, R.J.; et al. Induction of the cell survival kinase Sgk1: A possible novel mechanism for α-phenyl-N-tert-butyl nitrone in experimental stroke. J. Cereb. Blood Flow Metab. 2019, 39, 1111–1121. [Google Scholar] [CrossRef]
- Novelli, G.P.; Angiolini, P.; Tani, R.; Consales, G.; Bordi, L. Phenyl-t-butyl-nitrone is Active Against Traumatic Shock in Rats, Free Radic. Res. Commun. 1986, 1, 321–327. [Google Scholar]
- Deletraz, A.; Zéamari, K.; Hua, K.; Combes, M.; Villamena, F.A.; Tuccio, B.; Callizot, N.; Durand, G. Substituted α-phenyl and α-naphthlyl-N-tert-butyl nitrones: Synthesis, spin-trapping, and neuroprotection evaluation. J. Org. Chem. 2020, 85, 6073–6085. [Google Scholar] [CrossRef]
- Marco-Contelles, J. Recent advances on nitrones design for stroke treatment. J. Med. Chem. 2020, 63, 13413–13427. [Google Scholar] [CrossRef]
- Sun, P.; Zhou, W.; Yue, H.; Zhang, C.; Ou, Y.; Yang, Z.; Hu, W. Compound AD110 acts as therapeutic management for Alzheimer’s disease and stroke in mouse and rat models. ACS Chem. Neurosci. 2020, 11, 929–938. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, W.; Lang, M.; Yan, R.; Li, Z.; Zhang, G.; Yu, P.; Wang, Y.; Sun, Y.; Zhang, Z. Neuroprotective effects and mechanisms of action of multifunctional agents targeting free radicals, monoamine oxidase B and cholinesterase in Parkinson’s disease model. J. Mol. Neurosci. 2017, 61, 498–510. [Google Scholar] [CrossRef]
- Bautista-Aguilera, O.M.; Hagenow, S.; Palomino-Antolìn, A.; Farré-Alìns, V.; Ismaili, L.; Joffrin, P.-L.; Jimeno, M.L.; Soukup, O.; Janockova, J.; Kalinowsky, L.; et al. Multitarget-directed ligands combining cholinesterase and monoamine oxidase inhibition with histamine H3R antagonism for neurodegenerative diseases. Angew. Chem. Int. Ed. 2017, 56, 12765–12769. [Google Scholar] [CrossRef]
- Bautista-Aguilera, O.M.; Budni, J.; Mina, F.; Medeiros, E.B.; Deuther-Conrad, W.; Entrena, J.M.; Moraleda, I.; Iriepa, I.; Lòpez-Munoz, F.; Marco-Contelles, J. Contilisant, a tetratarget small molecule for Alzheimer’s disease therapy combining cholinesterase, monoamine oxidase inhibition, and H3R antagonism with S1R agonism profile. J. Med. Chem. 2018, 61, 6937–6943. [Google Scholar] [CrossRef]
- Chioua, M.; Gonzalo-Gobernado, R.; Ayuso, M.I.; Escobar-Peso, A.; Infantes, L.; Hadjipavlou-Litina, D.; Montoya, J.J.; Montaner, J.; Alcázar, A.; Marco-Contelles, J. New quinolylnitrones for stroke therapy: Antioxidant and neuroprotective (Z)-N-tert-butyl-1-(2-chloro-6-methoxyquinolin-3-yl)methanimine oxide (as a new lead-compound for ischemic stroke treatment. J. Med. Chem. 2019, 62, 2184–2201. [Google Scholar] [CrossRef]
- Alonso, J.M.; Escobar-Peso, A.; Palomino-Antolín, A.; Diez-Iriepa, D.; Chioua, M.; Martínez-Alonso, E.; Iriepa, I.; Egea, J.; Alcázar, A.; Marco-Contelles, J. Privileged quinolylnitrones for the combined therapy of ischemic stroke and Alzheimer’s disease. Pharmaceuticals 2021, 14, 861. [Google Scholar] [CrossRef]
- Naruto, S.; Mizuta, H.; Sawayama, T.; Yoshida, T.; Uno, H.; Kawashima, K.; Sohji, Y.; Kadokawa, T.; Nishimura, H. Synthesis and spasmolytic activities of 2-(1,2-benzisoxazol-3-yl)-3{[ω-(dialkylamino)alkoxy]phenyl}acrylonitriles. J. Med. Chem. 1982, 25, 1240–1245. [Google Scholar] [CrossRef]
- Hinton, R.D.; Janzen, E.G. Synthesis and characterization of phenyl-substituted C-phenyl-N-tert-butylnitrones and some of their radical adducts. J. Org. Chem. 1992, 57, 2646–2651. [Google Scholar] [CrossRef]
- Kuo, G.-H.; Prouty, C.; Wang, A.; Emanuel, S.; DeAngelis, A.; Zhang, Y.; Song, F.; Beall, L.; Connolly, P.J.; Karnachi, P.; et al. Synthesis and structure-activity relationships of pyrazine-pyridine biheteroaryls as novel, potent, and selective vascular endothelial growth factor receptor-2 inhibitors. J. Med. Chem. 2005, 48, 4892–4909. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Romagnoli, R.; Nuñez, M.C.; Perretti, M.; Paul-Clark, M.J.; Ferrario, M.; Govoni, M.; Benedini, F.; Ongini, E. Synthesis of nitro esters of prednisolone, new compounds combining pharmacological properties of both glucocorticoids and nitric oxide. J. Med. Chem. 2004, 47, 711–719. [Google Scholar] [CrossRef]
- Chamorro, B.; Diez-Iriepa, D.; Merás-Sáiz, B.; Chioua, M.; García-Vieira, D.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Martínez-Murillo, R.; González-Nieto, D.; et al. Synthesis, antioxidant properties and neuroprotection of phenyl-tert-butylnitrone derived homobisnitrones in in vitro and in vivo ischemia models. Sci. Rep. 2020, 10, 14150. [Google Scholar] [CrossRef]
- Knez, D.; Sosič, I.; Pislar, A.; Mitrović, A.; Jukic, M.; Kos, J.; Gobec, S. 8-Hydroxyquinoline-based anti-Alzheimer multimodal agents. Monatsh. Chem. 2020, 151, 1111–1120. [Google Scholar] [CrossRef]
- Hebda, M.; Bajda, M.; Wieckowska, A.; Szałaj, N.; Pasieka, A.; Panek, D.; Godyn, J.; Wichur, T.; Knez, D.; Gobec, S.; et al. Synthesis, molecular modelling and biological evaluation of novel heterodimeric, multiple ligands targeting cholinesterases and amyloid beta. Molecules 2016, 21, 410. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A program for macromolecular energy minimization and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Viejo, L.; Rubio-Alarcón, M.; Arribas, R.L.; Moreno-Castro, M.; Pérez-Marín, R.; Braun-Cornejo, M.; Estrada-Valencia, M.; de los Ríos, C. Synthesis and Biological Assessment of 4,1-Benzothiazepines with Neuroprotective Activity on the Ca2+ Overload for the Treatment of Neurodegenera-tive Diseases and Stroke. Molecules 2021, 26, 4473. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Duffy, E.M.; Jorgensen, W.L. Prediction of properties from simulations: Free energies of solvation in hexadecane, octanol, and water. J. Am. Chem. Soc. 2000, 122, 2878–2888. [Google Scholar] [CrossRef]
- Kamat, P.K.; Rai, S.; Nath, C. Okadaic acid induced neurotoxicity: An emerging tool to study Alzheimer’s disease pathology. Neurotoxicol. 2013, 37, 163–172. [Google Scholar] [CrossRef]
- Hayne, M.; Di Antonio, A. Protein phosphatase 2A restrains DLK signaling to promote proper Drosophila synaptic development and mammalian cortical neuron survival. Neurobiol. Dis. 2022, 163, 105586. [Google Scholar] [CrossRef]
- Zimmer, E.R.; Kalinine, E.; Haas, C.B.; Torrez, V.R.; Souza, D.O.; Muller, A.P.; Portela, L.V. Pretreatment with memantine prevents Alzheimer-like alterations induced by intrahippocampal okadaic acid administration in rats. Curr. Alzheimer Res. 2012, 9, 1182–1190. [Google Scholar] [CrossRef]


| Nitrones/Standard | ClogP a | ILPO b (%) | LOX Inhibition (% b or IC50 [μM]) | OH Scav. Capacity (%) b | ABTS+ (%) b | DPPH b |
|---|---|---|---|---|---|---|
| PBN | 3.02 | 11 | 23% | no | 5 | no |
| 1 | 3.75 | n.a. | 60 μM | 7 | 27 | 0 |
| 2 | 3.22 | n.a. | n.a. | 54 | n.a. | 0 |
| 3 | 3.75 | 29 | n.a. | n.a. | 31 | 2 |
| 4 | 3.22 | 45 | 37% | 54 | 23 | 0 |
| 5 | 3.68 | n.a. | 10 μM | 9 | 19 | 0 |
| 6 | 3.15 | n.a. | 39% | 13 | n.a. | 0 |
| 7 | 2.90 | n.a. | 27.2% | n.a. | 28 | 0 |
| 8 | 2.37 | n.a. | 23.3% | 78 | 10 | 0 |
| 9 | 2.90 | n.a. | 100 μM | 81 | n.a. | 1 |
| 10 | 2.37 | 31 | no | 20 | 13 | 0 |
| 11 | 2.83 | 6 | 38% | 71 | 15 | 0 |
| 12 | 2.30 | 31 | 25% | 9.8 | 13 | o |
| NDGA | 3.92 | n.d. | 0.45 μM | n.d. | n.d. | n.d. |
| Trolox | 3.09 | 93 | n.d. | 88 | 91 | n.d. |
| Nitrones | hMAO-A | hMAO-B | hAChE | hBChE |
|---|---|---|---|---|
| IC50 [µM] ± SEM a | ||||
| 1 | n.a. b | n.a. b | n.a. b | 45.82 ± 0.96 |
| 2 | n.a. b | n.a. b | n.a. b | n.a. b |
| 3 | n.a. b | n.a. b | n.a. b | 16.35 ± 1.03 |
| 4 | n.a. b | n.a. b | n.a. b | 28.87 ± 3.41 |
| 5 | n.a. b | n.a. b | n.a. b | 3.46 ± 0.27 |
| 6 | n.a. b | n.a. b | n.a. b | 6.52 ± 1.12 |
| 7 | n.a. b | n.a. b | n.a. b | n.a. b |
| 8 | n.a. b | n.a. b | n.a. b | n.a. b |
| 9 | n.a. b | n.a. b | n.a. b | n.a. b |
| 10 | n.a. b | n.a. b | n.a. b | 51.04 ± 2.21 |
| 11 | n.a. b | n.a. b | n.a. b | 67.30 ± 6.48 |
| 12 | n.a. b | n.a. b | n.a. b | n.a. b |
| QPPCaco | PSA | QPlogBB | metab | QPlogKhsa | % HOA | ROF | ROT |
| 1388.113 | 32.545 | 0.131 | 3 | 0.919 | 100.000 | 1 | 0 |
| MW | SASA | volume | donorHB | accptHB | QPlogPo/w | QPlogS | MW |
| 352.475 | 725.561 | 1269.781 | 0.000 | 3.750 | 5.441 | –5.318 | 352.475 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diez-Iriepa, D.; Knez, D.; Gobec, S.; Iriepa, I.; de los Ríos, C.; Bravo, I.; López-Muñoz, F.; Marco-Contelles, J.; Hadjipavlou-Litina, D. Polyfunctionalized α-Phenyl-tert-butyl(benzyl)nitrones: Multifunctional Antioxidants for Stroke Treatment. Antioxidants 2022, 11, 1735. https://doi.org/10.3390/antiox11091735
Diez-Iriepa D, Knez D, Gobec S, Iriepa I, de los Ríos C, Bravo I, López-Muñoz F, Marco-Contelles J, Hadjipavlou-Litina D. Polyfunctionalized α-Phenyl-tert-butyl(benzyl)nitrones: Multifunctional Antioxidants for Stroke Treatment. Antioxidants. 2022; 11(9):1735. https://doi.org/10.3390/antiox11091735
Chicago/Turabian StyleDiez-Iriepa, Daniel, Damijan Knez, Stanislav Gobec, Isabel Iriepa, Cristóbal de los Ríos, Isaac Bravo, Francisco López-Muñoz, José Marco-Contelles, and Dimitra Hadjipavlou-Litina. 2022. "Polyfunctionalized α-Phenyl-tert-butyl(benzyl)nitrones: Multifunctional Antioxidants for Stroke Treatment" Antioxidants 11, no. 9: 1735. https://doi.org/10.3390/antiox11091735
APA StyleDiez-Iriepa, D., Knez, D., Gobec, S., Iriepa, I., de los Ríos, C., Bravo, I., López-Muñoz, F., Marco-Contelles, J., & Hadjipavlou-Litina, D. (2022). Polyfunctionalized α-Phenyl-tert-butyl(benzyl)nitrones: Multifunctional Antioxidants for Stroke Treatment. Antioxidants, 11(9), 1735. https://doi.org/10.3390/antiox11091735












