Low Plasma Ergothioneine Predicts Cognitive and Functional Decline in an Elderly Cohort Attending Memory Clinics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Plasma Ergothioneine Measurements
2.3. Neuropsychological Assessments
2.4. Covariate Assessments
2.5. Neuroimaging
2.6. Statistical Analyses
3. Results
3.1. Baseline Demographic and Disease Factors of the Study Cohort
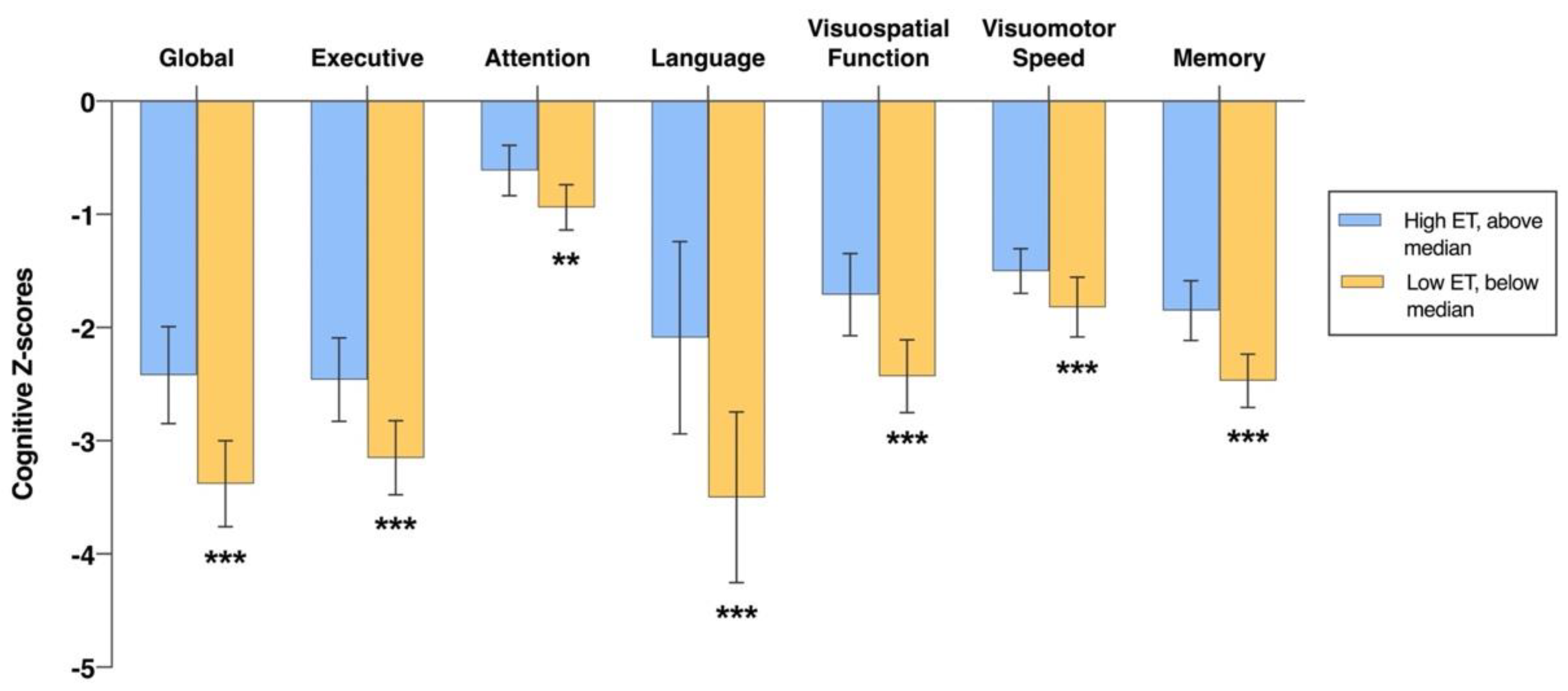
3.2. Associations of Plasma ET Levels with Cognition at Baseline
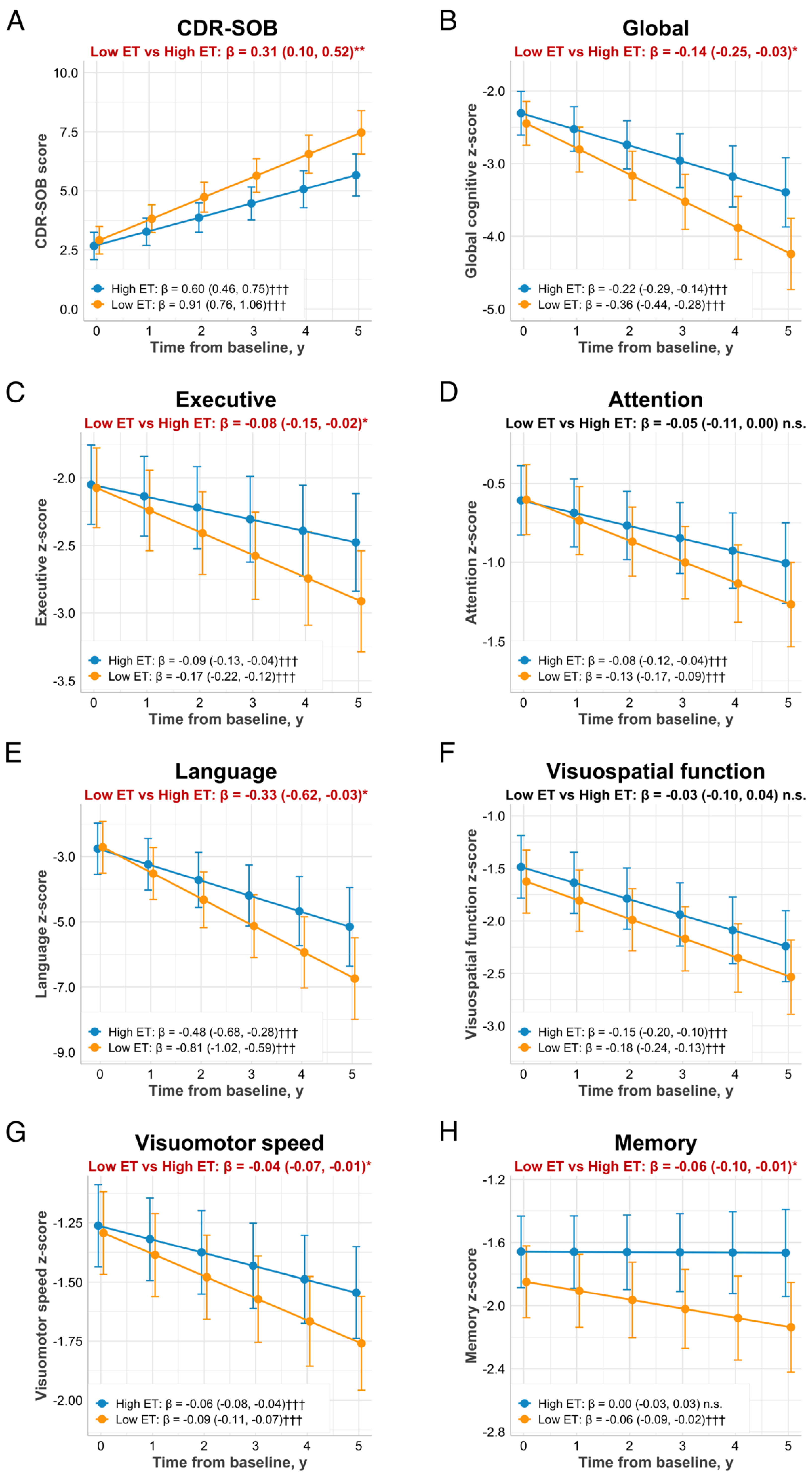
3.3. Associations of Baseline Plasma ET Levels with Cognitive Trajectories and Functional Decline
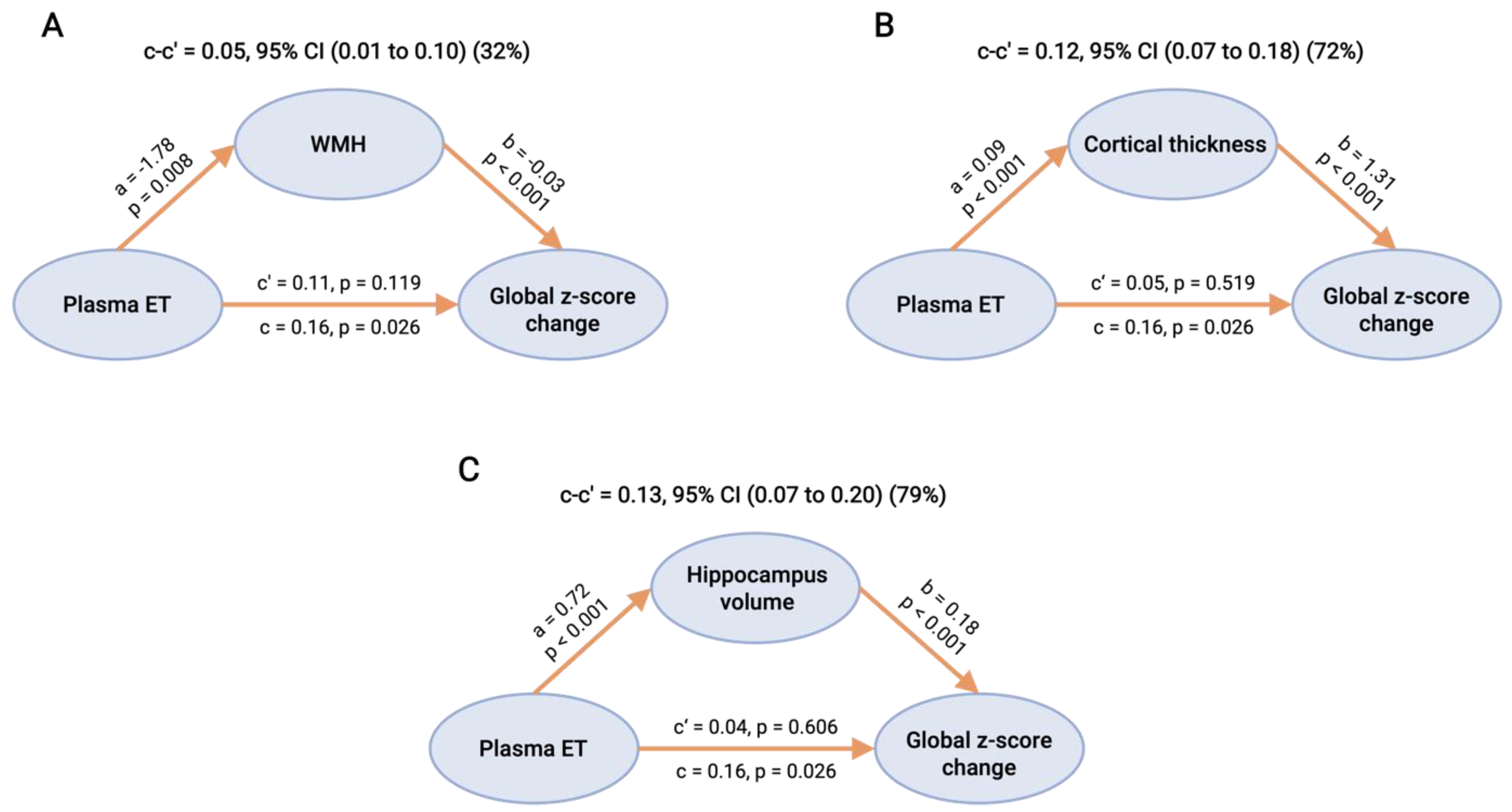
3.4. Causal Mediation Analyses of CeVD and Neurodegeneration on the Associations between ET and Cognitive Decline
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzhemer Disease International. World Azheimer Report 2021. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf (accessed on 15 July 2022).
- Eshkoor, S.A.; Hamid, T.A.; Mun, C.Y.; Ng, C.K. Mild cognitive impairment and its management in older people. Clin. Interv. Aging 2015, 10, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Jonsson, L.; Bond, J.; Prince, M.; Winblad, B. The worldwide economic impact of dementia 2010. Alzheimer’s Dement. 2013, 9, 1–11.e13. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Bennett, S.; Grant, M.M.; Aldred, S. Oxidative stress in vascular dementia and Alzheimer’s disease: A common pathology. J. Alzheimer’s Dis. 2009, 17, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Camiletti-Moirón, D.; Aparicio, V.; Aranda, P.; Radák, Z. Does exercise reduce brain oxidative stress? A systematic review. Scand. J. Med. Sci. Sports 2013, 23, e202–e212. [Google Scholar] [CrossRef]
- Meeusen, R. Exercise, nutrition and the brain. Sports Med. 2014, 44, 47–56. [Google Scholar] [CrossRef]
- Chen, J.J.; Thiyagarajah, M.; Song, J.; Chen, C.; Herrmann, N.; Gallagher, D.; Rapoport, M.J.; Black, S.E.; Ramirez, J.; Andreazza, A.C.; et al. Altered central and blood glutathione in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Alzheimer’s Res. Ther. 2022, 14, 23. [Google Scholar] [CrossRef]
- Polidori, M.C.; Mattioli, P.; Aldred, S.; Cecchetti, R.; Stahl, W.; Griffiths, H.; Senin, U.; Sies, H.; Mecocci, P. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: Relevance to Alzheimer disease and vascular dementia. Dement. Geriatr. Cogn. Disord. 2004, 18, 265–270. [Google Scholar] [CrossRef]
- Wu, L.Y.; Cheah, I.K.; Chong, J.R.; Chai, Y.L.; Tan, J.Y.; Hilal, S.; Vrooman, H.; Chen, C.P.; Halliwell, B.; Lai, M.K.P. Low plasma ergothioneine levels are associated with neurodegeneration and cerebrovascular disease in dementia. Free Radic. Biol. Med. 2021, 177, 201–211. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Denton, D.A.; Di Nisio, M.; Chong, L.Y.; Abraham, R.P.; Al-Assaf, A.S.; Anderson, J.L.; Malik, M.A.; Vernooij, R.W.; Martínez, G. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst. Rev. 2018, 12, 156. [Google Scholar] [CrossRef]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Cognitive performance among the elderly in relation to the intake of plant foods. The Hordaland Health Study. Br. J. Nutr. 2010, 104, 1190–1201. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Tang, R.M. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine, recent developments. Redox Biol. 2021, 42, 101868. [Google Scholar] [CrossRef]
- Graham, S.; Chevallier, O.; Kumar, P.; Türkoǧlu, O.; Bahado-Singh, R. Metabolomic profiling of brain from infants who died from Sudden Infant Death Syndrome reveals novel predictive biomarkers. J. Perinatol. 2017, 37, 91–97. [Google Scholar] [CrossRef]
- Tang, R.M.Y.; Cheah, I.K.-M.; Yew, T.S.K.; Halliwell, B. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci. Rep. 2018, 8, 1601. [Google Scholar] [CrossRef]
- Gründemann, D.; Hartmann, L.; Flögel, S. The ergothioneine transporter (ETT): Substrates and locations, an inventory. FEBS Lett. 2022, 596, 1252–1269. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 784–793. [Google Scholar] [CrossRef]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Could Ergothioneine Aid in the Treatment of Coronavirus Patients? Antioxidants 2020, 9, 595. [Google Scholar] [CrossRef]
- Jang, J.-H.; Aruoma, O.I.; Jen, L.-S.; Chung, H.Y.; Surh, Y.-J. Ergothioneine rescues PC12 cells from β-amyloid-induced apoptotic death. Free Radic. Biol. Med. 2004, 36, 288–299. [Google Scholar] [CrossRef]
- Song, T.-Y.; Chen, C.-L.; Liao, J.-W.; Ou, H.-C.; Tsai, M.-S. Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in vivo. Food Chem. Toxicol. 2010, 48, 3492–3499. [Google Scholar] [CrossRef]
- Cheah, I.K.; Ng, L.T.; Ng, L.F.; Lam, V.Y.; Gruber, J.; Huang, C.Y.; Goh, F.Q.; Lim, K.H.; Halliwell, B. Inhibition of amyloid-induced toxicity by ergothioneine in a transgenic Caenorhabditis elegans model. FEBS Lett. 2019, 593, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-C.; Lin, H.-C.; Wu, J.-H.; Ou, H.-C.; Chai, Y.-C.; Tseng, C.-Y.; Liao, J.-W.; Song, T.-Y. Ergothioneine protects against neuronal injury induced by β-amyloid in mice. Food Chem. Toxicol. 2012, 50, 3902–3911. [Google Scholar] [CrossRef]
- Song, T.-Y.; Lin, H.-C.; Chen, C.-L.; Wu, J.-H.; Liao, J.-W.; Hu, M.-L. Ergothioneine and melatonin attenuate oxidative stress and protect against learning and memory deficits in C57BL/6J mice treated with D-galactose. Free. Radic. Res. 2014, 48, 1049–1060. [Google Scholar] [CrossRef]
- Cheah, I.K.; Feng, L.; Tang, R.M.; Lim, K.H.; Halliwell, B. Ergothioneine levels in an elderly population decrease with age and incidence of cognitive decline; a risk factor for neurodegeneration? Biochem. Biophys. Res. Commun. 2016, 478, 162–167. [Google Scholar] [CrossRef]
- Teruya, T.; Chen, Y.-J.; Kondoh, H.; Fukuji, Y.; Yanagida, M. Whole-blood metabolomics of dementia patients reveal classes of disease-linked metabolites. Proc. Natl. Acad. Sci. USA 2021, 118, e2022857118. [Google Scholar] [CrossRef]
- Paul, B.D. Ergothioneine: A Stress Vitamin with Antiaging, Vascular, and Neuroprotective Roles? Antioxid. Redox Signal. 2022, 36, 1306–1317. [Google Scholar] [CrossRef]
- Kondoh, H.; Teruya, T.; Kameda, M.; Yanagida, M. Decline of ergothioneine in frailty and cognition impairment. FEBS Lett. 2022, 596, 1270–1278. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Castellano-Escuder, P.; Carmona, F.; Lefèvre-Arbogast, S.; Low, D.Y.; Preez, A.D.; Ruigrok, S.R.; Manach, C.; Urpi-Sarda, M.; Korosi, A. Food and Microbiota Metabolites Associate with Cognitive Decline in Older Subjects: A twelve-Year Prospective Study. Mol. Nutr. Food Res. 2021, 65, 2100606. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hilal, S.; Collinson, S.L.; Chong, E.J.; Ikram, M.K.; Venketasubramanian, N.; Chen, C.L. Association of Magnetic Resonance Imaging Markers of Cerebrovascular Disease Burden and Cognition. Stroke 2015, 46, 2808–2814. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Phua, A.; Collinson, S.L.; Hilal, S.; Ikram, M.K.; Wong, T.Y.; Cheng, C.Y.; Venketasubramanian, N.; Chen, C. Additive effect of cerebral atrophy on cognition in dementia-free elderly with cerebrovascular disease. Stroke Vasc. Neurol. 2019, 4, e000202. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.; Masdeu, J.; Garcia, J.; Amaducci, L.; Orgogozo, J.-M.; Brun, A.; Hofman, A. Vascular dementia: Diagnostic criteria for research studies: Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Tang, R.M.; Yew, T.S.; Lim, K.H.; Halliwell, B. Administration of pure ergothioneine to healthy human subjects: Uptake, metabolism, and effects on biomarkers of oxidative damage and inflammation. Antioxid. Redox Signal. 2017, 26, 193–206. [Google Scholar] [CrossRef]
- Xu, X.; Chan, Q.L.; Hilal, S.; Ikram, M.K.; Venketasubramanian, N.; Tan, B.Y.; Dong, Y.; Chen, C.L.; Collinson, S.L. The Diagnostic Utility of the NINDS-CSN Neuropsychological Battery in Memory Clinics. Dement. Geriatr. Cogn. Dis. Extra 2016, 6, 276–282. [Google Scholar] [CrossRef]
- Chai, Y.L.; Yeo, H.K.-H.; Wang, J.; Hilal, S.; Ikram, M.K.; Venketasubramanian, N.; Wong, B.-S.; Chen, C.L.-H. Apolipoprotein ε4 is associated with dementia and cognitive impairment predominantly due to Alzheimer’s disease and not with vascular cognitive impairment: A Singapore-based cohort. J. Alzheimer’s Dis. 2016, 51, 1111–1118. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Wahlund, L.-O.; Barkhof, F.; Fazekas, F.; Bronge, L.; Augustin, M.; Sjogren, M.; Wallin, A.; Adèr, H.; Leys, D.; Pantoni, L. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001, 32, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Vrooman, H.A.; Cocosco, C.A.; van der Lijn, F.; Stokking, R.; Ikram, M.A.; Vernooij, M.W.; Breteler, M.M.; Niessen, W.J. Multi-spectral brain tissue segmentation using automatically trained k-Nearest-Neighbor classification. Neuroimage 2007, 37, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Hilal, S.; Chai, Y.L.; Ikram, M.K.; Elangovan, S.; Yeow, T.B.; Xin, X.; Chong, J.Y.; Venketasubramanian, N.; Richards, A.M.; Chong, J.P.C.; et al. Markers of cardiac dysfunction in cognitive impairment and dementia. Medicine 2015, 94, e297. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Nakao, S.; Nishiyama, M.; Takeda, Y.; Ishimoto, T.; Masuo, Y.; Matsumoto, S.; Suzuki, M.; Kato, Y. Oral Administration of the Food-Derived Hydrophilic Antioxidant Ergothioneine Enhances Object Recognition Memory in Mice. Curr. Mol. Pharmacol. 2021, 14, 220–233. [Google Scholar] [CrossRef]
- Hatano, T.; Saiki, S.; Okuzumi, A.; Mohney, R.P.; Hattori, N. Identification of novel biomarkers for Parkinson’s disease by metabolomic technologies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kameda, M.; Teruya, T.; Yanagida, M.; Kondoh, H. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc. Natl. Acad. Sci. USA 2020, 117, 9483–9489. [Google Scholar] [CrossRef]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Zhang, S.; Tomata, Y.; Sugiyama, K.; Sugawara, Y.; Tsuji, I. Mushroom Consumption and Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. J. Am. Geriatr. Soc. 2017, 65, 1462–1469. [Google Scholar] [CrossRef]
- Feng, L.; Cheah, I.K.-M.; Ng, M.M.-X.; Li, J.; Chan, S.M.; Lim, S.L.; Mahendran, R.; Kua, E.-H.; Halliwell, B. The association between mushroom consumption and mild cognitive impairment: A community-based cross-sectional study in Singapore. J. Alzheimer’s Dis. 2019, 68, 197–203. [Google Scholar] [CrossRef]
- Ba, D.M.; Gao, X.; Al-Shaar, L.; Muscat, J.; Chinchilli, V.M.; Ssentongo, P.; Beelman, R.B.; Richie, J. Mushroom intake and cognitive performance among US older adults: The National Health and Nutrition Examination Survey, 2011–2014. Br. J. Nutr. 2022, 75, 1–8. [Google Scholar] [CrossRef]
- Beelman, R.B.; Phillips, A.T.; Richie, J.P., Jr.; Ba, D.M.; Duiker, S.W.; Kalaras, M.D. Health consequences of improving the content of ergothioneine in the food supply. FEBS Lett. 2022, 596, 1231–1240. [Google Scholar] [CrossRef]
- Xu, X.; Hilal, S.; Collinson, S.L.; Chan, Q.L.; Yi Chong, E.J.; Ikram, M.K.; Venketasubramanian, N.; Cheng, C.Y.; Wong, T.Y.; Chen, C.L. Validation of the Total Cerebrovascular Disease Burden Scale in a Community Sample. J. Alzheimer’s Dis. 2016, 52, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef]
- Wu, X.; George, R.L.; Huang, W.; Wang, H.; Conway, S.J.; Leibach, F.H.; Ganapathy, V. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim. Et Biophys. Biomembr. 2000, 1466, 315–327. [Google Scholar] [CrossRef]
- Nakamichi, N.; Nakayama, K.; Ishimoto, T.; Masuo, Y.; Wakayama, T.; Sekiguchi, H.; Sutoh, K.; Usumi, K.; Iseki, S.; Kato, Y. Food-derived hydrophilic antioxidant ergothioneine is distributed to the brain and exerts antidepressant effect in mice. Brain Behav. 2016, 6, e00477. [Google Scholar] [CrossRef]
- Smith, E.; Ottosson, F.; Hellstrand, S.; Ericson, U.; Orho-Melander, M.; Fernandez, C.; Melander, O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 2020, 106, 691–697. [Google Scholar] [CrossRef]
- Grochowski, C.; Litak, J.; Kamieniak, P.; Maciejewski, R. Oxidative stress in cerebral small vessel disease. Role of reactive species. Free Radic. Res. 2018, 52, 1–13. [Google Scholar] [CrossRef]
- Martin, K.R. The bioactive agent ergothioneine, a key component of dietary mushrooms, inhibits monocyte binding to endothelial cells characteristic of early cardiovascular disease. J. Med. Food 2010, 13, 1340–1346. [Google Scholar] [CrossRef]
- Li, R.W.; Yang, C.; Sit, A.S.; Kwan, Y.-W.; Lee, S.M.; Hoi, M.P.; Chan, S.-W.; Hausman, M.; Vanhoutte, P.M.; Leung, G.P. Uptake and protective effects of ergothioneine in human endothelial cells. J. Pharmacol. Exp. Ther. 2014, 350, 691–700. [Google Scholar] [CrossRef]
- Koh, S.S.; Ooi, S.C.-Y.; Lui, N.M.-Y.; Qiong, C.; Ho, L.T.-Y.; Cheah, I.K.-M.; Halliwell, B.; Herr, D.R.; Ong, W.-Y. Effect of ergothioneine on 7-ketocholesterol-induced endothelial injury. Neuromolecular Med. 2021, 23, 184–198. [Google Scholar] [CrossRef]
- Karas, G.; Sluimer, J.; Goekoop, R.; Van Der Flier, W.; Rombouts, S.; Vrenken, H.; Scheltens, P.; Fox, N.; Barkhof, F. Amnestic mild cognitive impairment: Structural MR imaging findings predictive of conversion to Alzheimer disease. Am. J. Neuroradiol. 2008, 29, 944–949. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chan, Y.H.; Chan, Q.L.; Gyanwali, B.; Hilal, S.; Tan, B.Y.; Ikram, M.K.; Venketasubramanian, N.; Chen, C.L. Global cerebrovascular burden and long-term clinical outcomes in Asian elderly across the spectrum of cognitive impairment. Int. Psychogeriatr. 2018, 30, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Hilal, S.; Liu, S.; Wong, T.Y.; Vrooman, H.; Cheng, C.-Y.; Venketasubramanian, N.; Chen, C.L.; Zhou, J.H. White matter network damage mediates association between cerebrovascular disease and cognition. J. Cereb. Blood Flow Metab. 2021, 41, 1858–1872. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I. Ergothioneine, where are we now? FEBS Lett. 2022, 596, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.; Sumien, N.; Johnson, L.; D’Agostino, D.; Edwards, M.; Paxton, R.J.; Hall, J.R.; O’Bryant, S. Vitamin C Supplementation, APOE4 Genotype and Cognitive Functioning in a Rural-Dwelling Cohort. J. Nutr. Health Aging 2016, 20, 841–844. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Total Sample (n = 470) | Clinical Status | ||
|---|---|---|---|---|
| No Dementia (n = 281) | Dementia (n = 189) | p Value | ||
| Age, y, mean (SD) | 73 (8) | 72 (8) | 75 (7) | <0.001 *** |
| Education, y, median (IQR) | 6 (7) | 9 (6) | 5 (8) | <0.001 *** |
| Female, n (%) | 248 (52.8) | 141 (50.2) | 107 (56.6) | 0.171 |
| APOE ε4 status (ε4+), n (%) | 137 (29.1) | 71 (25.3) | 66 (34.9) | 0.024 * |
| Body mass index, kg/m2, mean (SD) | 23.8 (3.9) | 24.0 (3.7) | 23.4 (4.0) | 0.112 |
| Hypertension, n (%) | 327 (70) | 183 (65.4) | 144 (77) | 0.007 ** |
| Hyperlipidemia, n (%) | 348 (74.2) | 210 (75.0) | 138 (73) | 0.630 |
| Diabetes, n (%) | 169 (36) | 92 (32.7) | 77 (40.7) | 0.076 |
| Cardiovascular disease, n (%) | 57 (12.2) | 32 (11.4) | 25 (13.4) | 0.530 |
| MMSE score, median (IQR) | 23 (10) | 26 (5) | 16 (7) | <0.001 *** |
| CDR-SOB score, median (IQR) | 1.0 (5.0) | 0 (1.0) | 6.0 (5.0) | <0.001 *** |
| Cerebrovascular disease markers ¶ | ||||
| Cortical infarcts, n (%) | 53 (12.3) | 25 (9.3) | 28 (17.4) | 0.013 * |
| Lacunes ≥ 2, n (%) | 58 (13.5) | 28 (10.4) | 30 (18.6) | 0.016 * |
| WMH, ARWMC ≥ 8, n (%) | 175 (40.7) | 82 (30.5) | 93 (57.8) | <0.001 *** |
| Neurodegenerative markers | ||||
| Global cortical thickness, mm, mean (SD) | 2.3 (0.1) | 2.3 (0.1) | 2.2 (0.1) | <0.001 *** |
| Hippocampus volume, ml, mean (SD) | 6.4 (1.2) | 6.8 (1.0) | 5.6 (1.1) | <0.001 *** |
| Total intracranial volume, ml, mean (SD) | 1095 (112) | 1099 (108) | 1088 (121) | 0.335 |
| Ergothioneine, nM, median (IQR) | 784 (855) | 896 (882) | 588 (549) | <0.001 *** |
| Mean Difference in Baseline Cognitive Z-Scores per Unit Increase in Log-Transformed Plasma ET Levels | ||
|---|---|---|
| β (95% CI) * | p Value | |
| Global | 2.02 (1.36, 2.68) | <0.001 |
| Executive function | 1.44 (0.87, 2.01) | <0.001 |
| Attention | 0.56 (0.21, 0.91) | 0.002 |
| Language | 2.90 (1.58, 4.22) | <0.001 |
| Visuospatial function | 1.54 (0.99, 2.10) | <0.001 |
| Visuomotor speed | 0.79 (0.48, 1.09) | <0.001 |
| Memory | 1.32 (0.91, 1.73) | <0.001 |
| Mean Difference in the Rate of Change of Outcome Scores per Unit Increase in Log-Transformed Plasma ET Levels | ||
|---|---|---|
| β (95% CI) * | p Value | |
| CDR-SOB a | −0.55 (−0.87, −0.22) | 0.001 |
| Neuropsychological z-scores b | ||
| Global | 0.23 (0.06, 0.40) | 0.008 |
| Executive function | 0.12 (0.02, 0.23) | 0.019 |
| Attention | 0.12 (0.04, 0.21) | 0.004 |
| Language | 0.49 (0.03, 0.94) | 0.037 |
| Visuospatial function | 0.07 (−0.04, 0.18) | 0.210 |
| Visuomotor speed | 0.05 (0.01, 0.09) | 0.028 |
| Memory | 0.10 (0.03, 0.17) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.-Y.; Kan, C.N.; Cheah, I.K.; Chong, J.R.; Xu, X.; Vrooman, H.; Hilal, S.; Venketasubramanian, N.; Chen, C.P.; Halliwell, B.; et al. Low Plasma Ergothioneine Predicts Cognitive and Functional Decline in an Elderly Cohort Attending Memory Clinics. Antioxidants 2022, 11, 1717. https://doi.org/10.3390/antiox11091717
Wu L-Y, Kan CN, Cheah IK, Chong JR, Xu X, Vrooman H, Hilal S, Venketasubramanian N, Chen CP, Halliwell B, et al. Low Plasma Ergothioneine Predicts Cognitive and Functional Decline in an Elderly Cohort Attending Memory Clinics. Antioxidants. 2022; 11(9):1717. https://doi.org/10.3390/antiox11091717
Chicago/Turabian StyleWu, Liu-Yun, Cheuk Ni Kan, Irwin K. Cheah, Joyce Ruifen Chong, Xin Xu, Henri Vrooman, Saima Hilal, Narayanaswamy Venketasubramanian, Christopher P. Chen, Barry Halliwell, and et al. 2022. "Low Plasma Ergothioneine Predicts Cognitive and Functional Decline in an Elderly Cohort Attending Memory Clinics" Antioxidants 11, no. 9: 1717. https://doi.org/10.3390/antiox11091717
APA StyleWu, L.-Y., Kan, C. N., Cheah, I. K., Chong, J. R., Xu, X., Vrooman, H., Hilal, S., Venketasubramanian, N., Chen, C. P., Halliwell, B., & Lai, M. K. P. (2022). Low Plasma Ergothioneine Predicts Cognitive and Functional Decline in an Elderly Cohort Attending Memory Clinics. Antioxidants, 11(9), 1717. https://doi.org/10.3390/antiox11091717






