Structural Elucidation of Novel Stable and Reactive Metabolites of Green Tea Catechins and Alkyl Gallates by LC-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. In Vitro Incubations
2.3. LC-HRMS/MS Analysis and Data Processing
3. Results
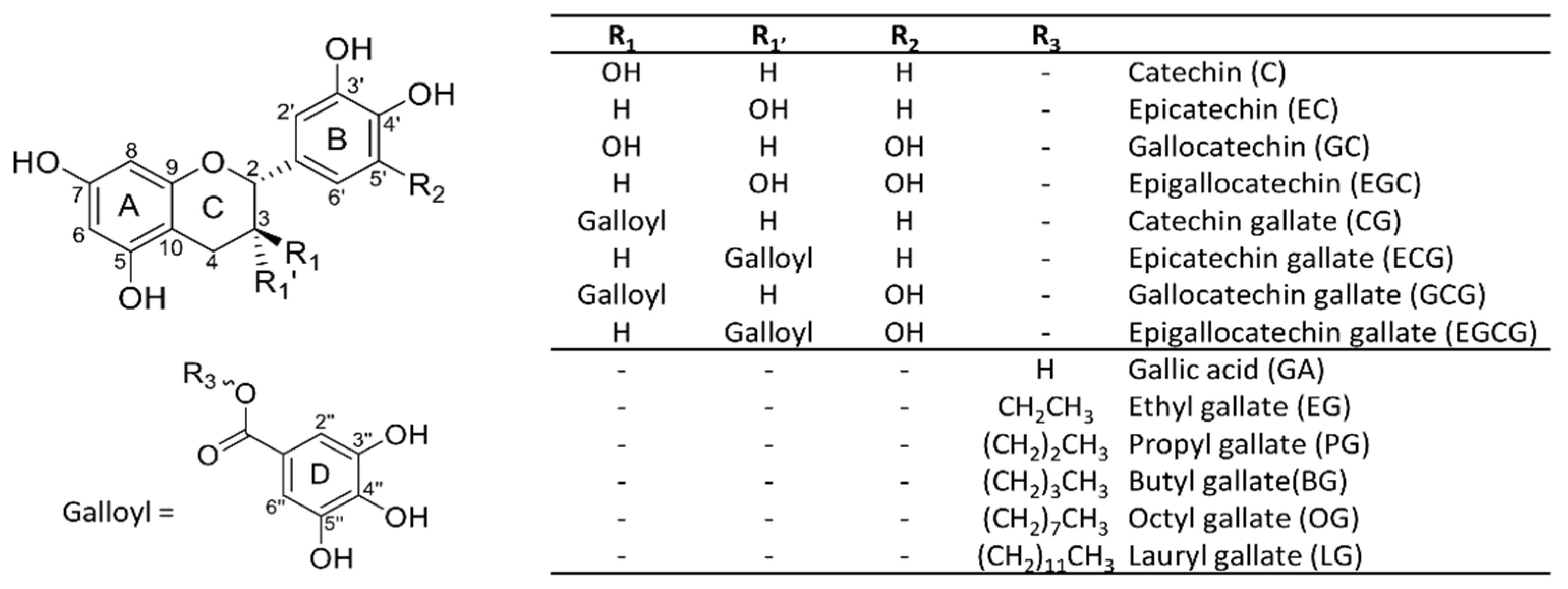
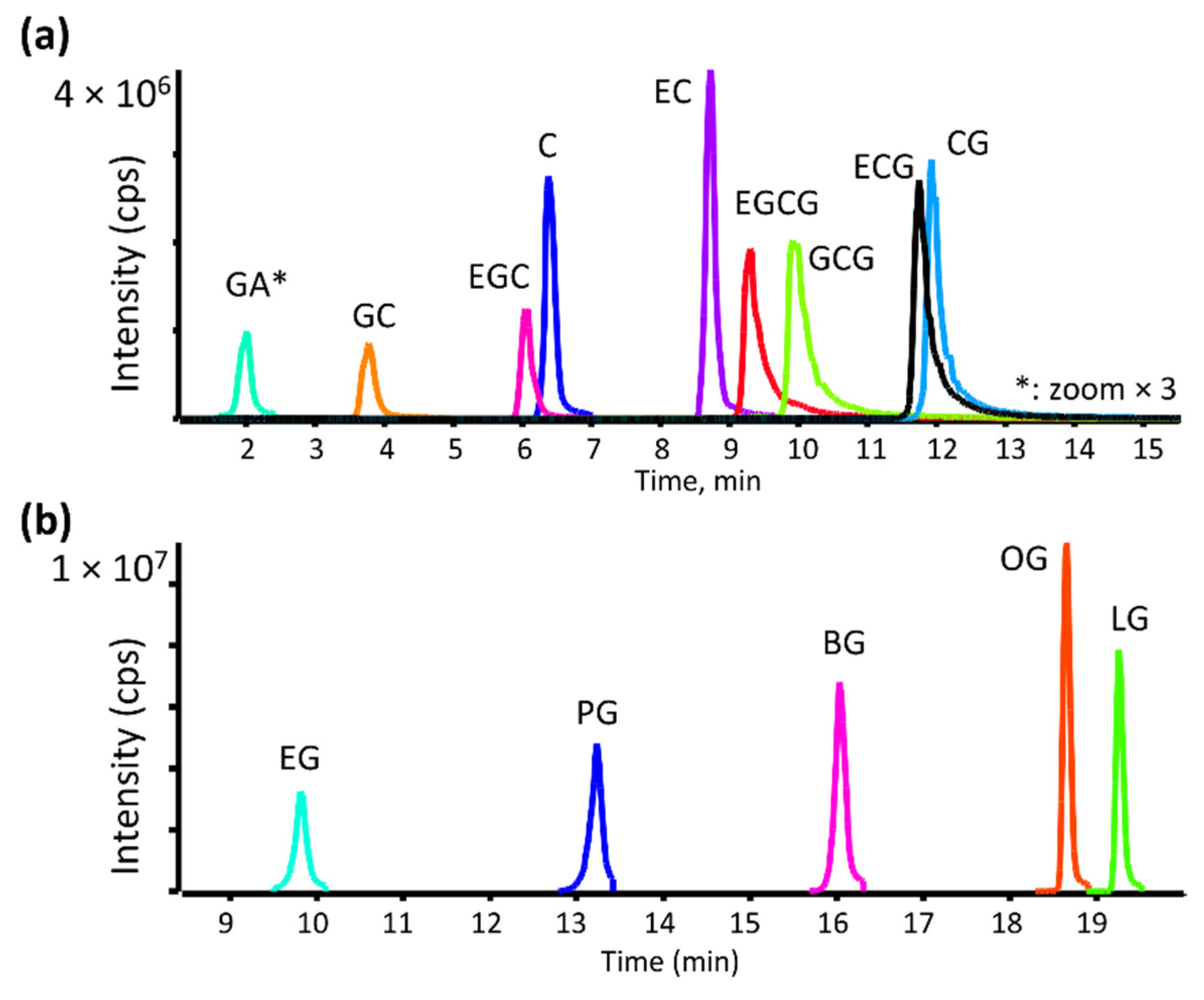
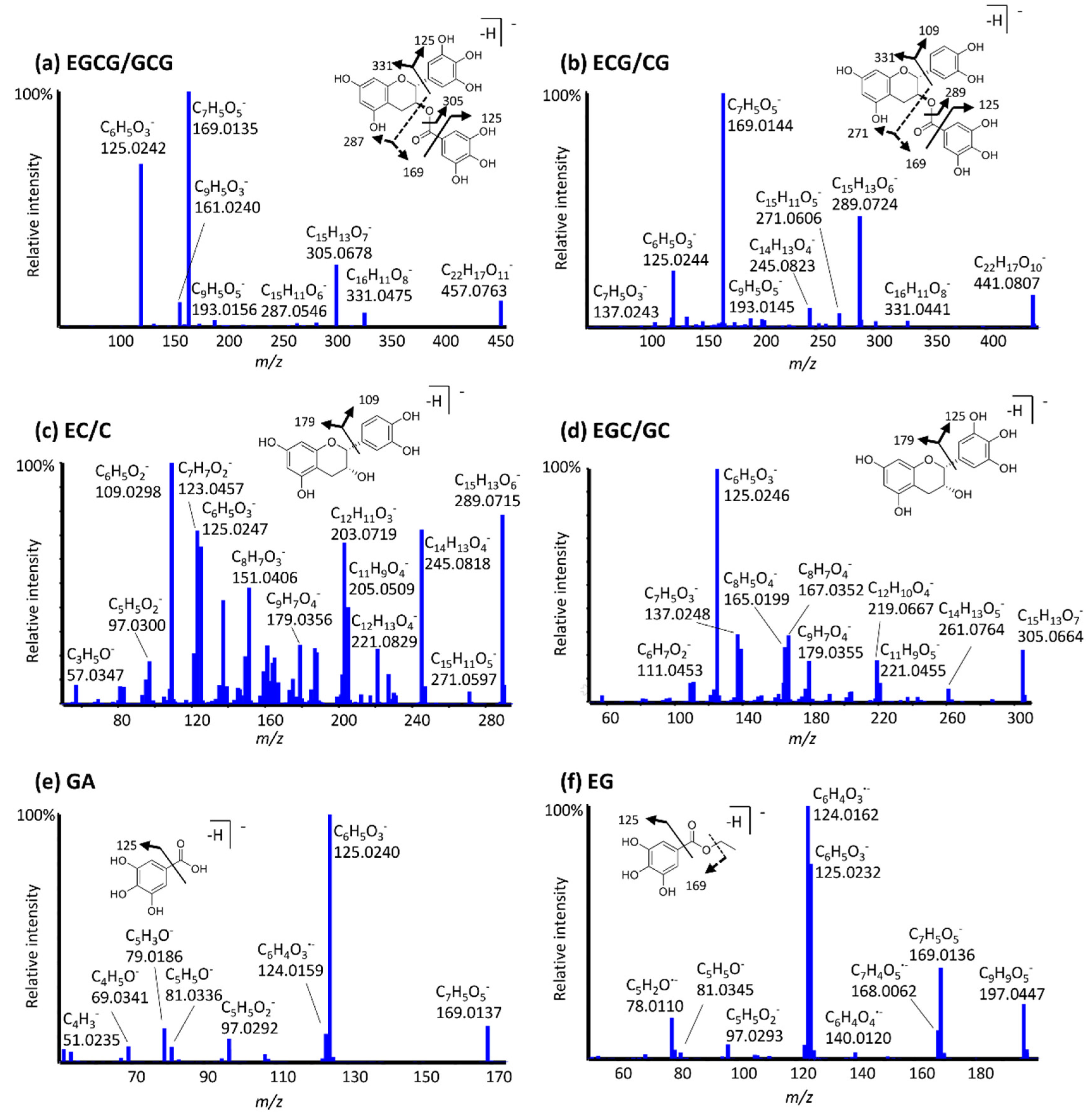
3.1. LC-MS Method Development and MS/MS Fragmentation of Catechins and Gallate Esters
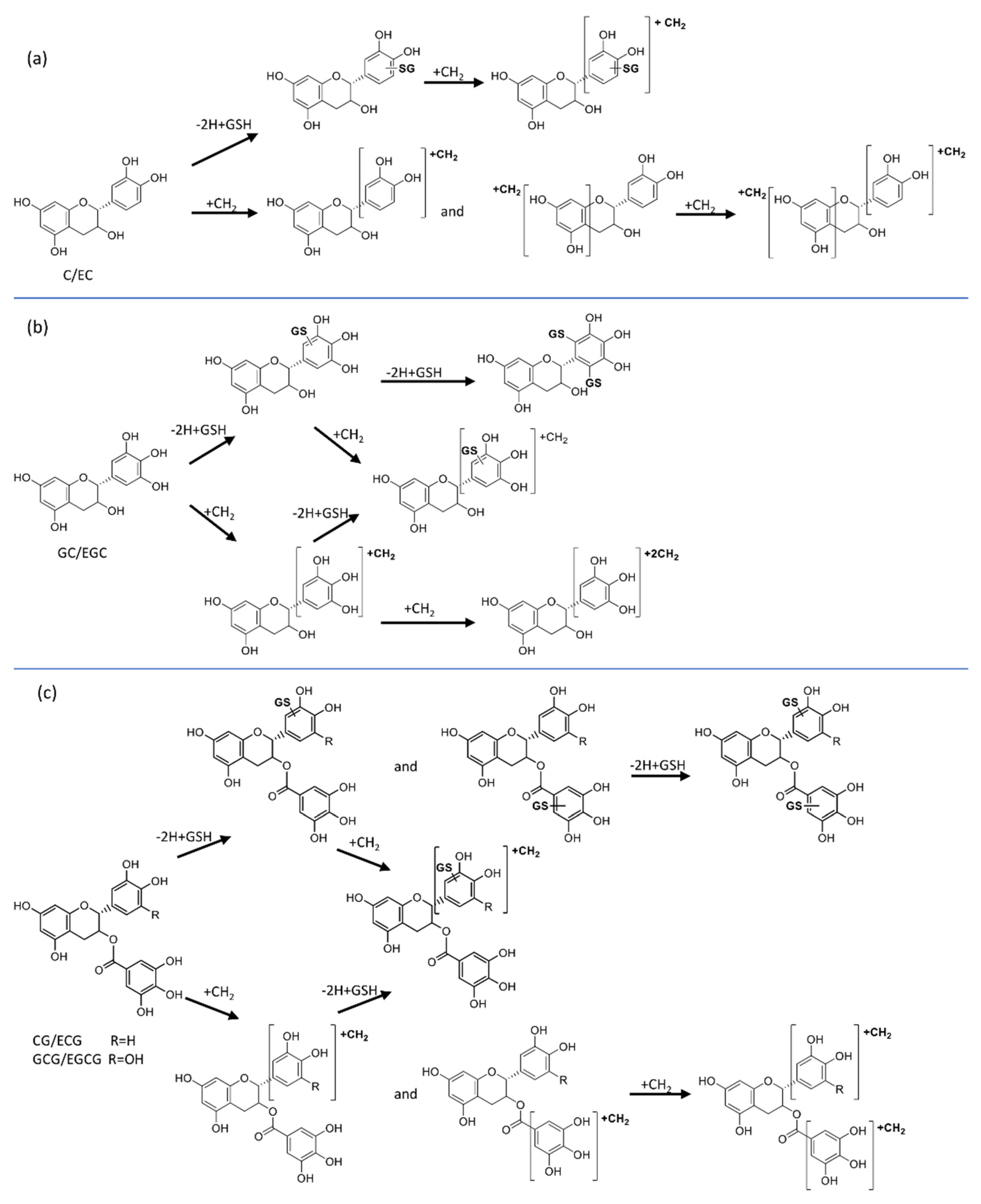
3.2. Metabolite Identification
3.2.1. Oxidative Metabolites
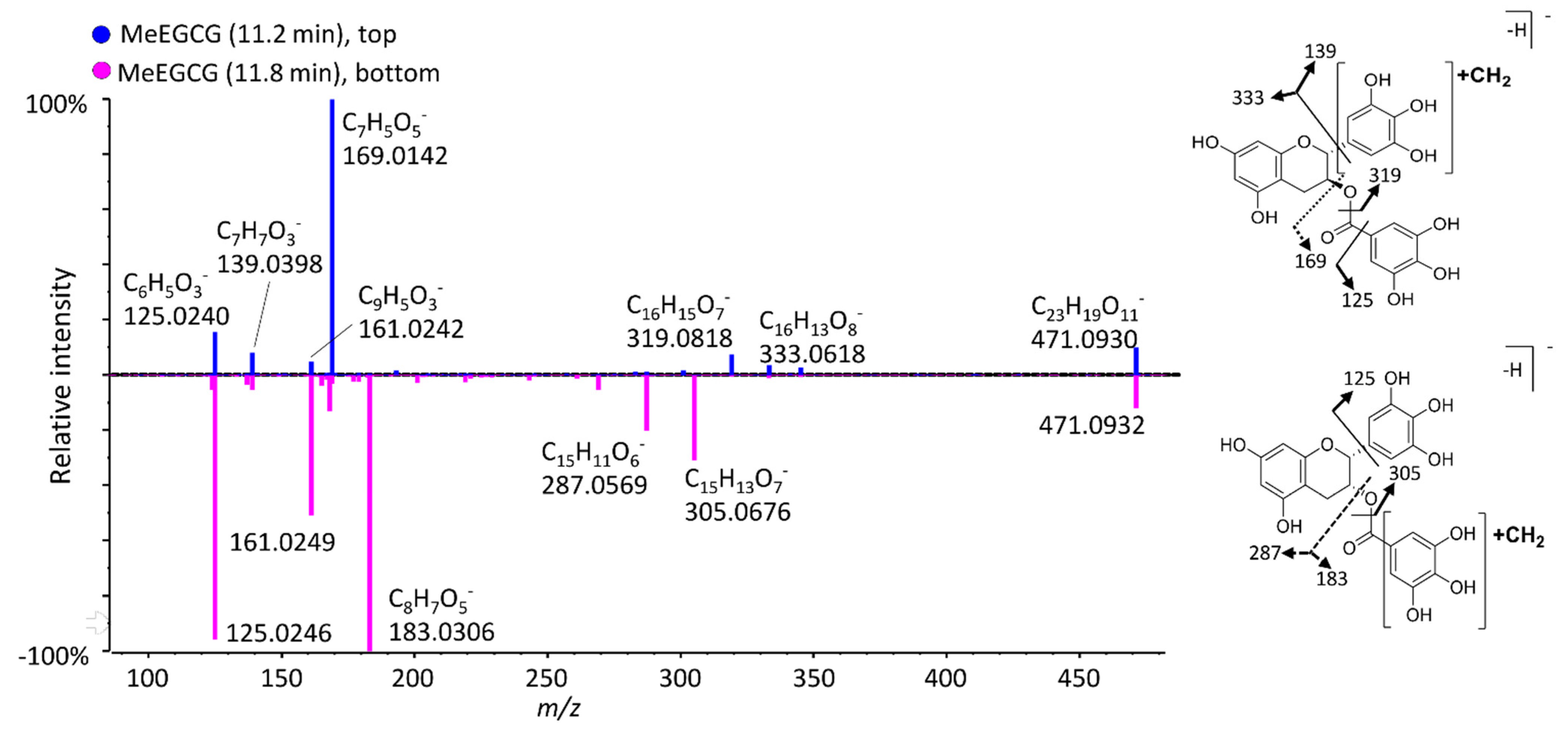
3.2.2. Methylated Metabolites
3.2.3. GSH Adducts
3.2.4. Methylated GSH Adducts
4. Discussion
4.1. Metabolism of Green Tea Catechins
4.2. Metabolism of Alkyl Gallates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brewer, M. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Viana da Silva, M.; Santos, M.R.C.; Alves Silva, I.R.; Macedo Viana, E.B.; Dos Anjos, D.A.; Santos, I.A.; Barbosa de Lima, N.G.; Wobeto, C.; Jorge, N.; Lannes, S.C.D.S. Synthetic and natural antioxidants used in the oxidative stability of edible oils: An overview. Food Rev. Int. 2021, 1, 1–24. [Google Scholar] [CrossRef]
- Mitterer-daltoé, M.B.J.; Lise, C.; Breda, L.; Casagrande, M.; Lima, V. Consumer awareness of food antioxidants. Synthetic vs. Natural. Food Sci. Technol. 2021, 41, 208–212. [Google Scholar] [CrossRef]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Pokorný, J. Are natural antioxidants better–and safer–than synthetic antioxidants? Eur. J. Lipid Sci. Technol 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Khanam, S.; Prakash, A. Promising Sources of Antioxidants from Herbs and Spices: A Review. Int. J. Adv. Res. 2021, 4, 188–195. [Google Scholar] [CrossRef]
- Basati, G.; Ghanadi, P.; Abbaszadeh, S. A review of the most important natural antioxidants and effective medicinal plants in traditional medicine on prostate cancer and its disorders. J. HerbMed Pharmacol. 2020, 9, 112–120. [Google Scholar] [CrossRef]
- Perez-Torres, I.; Castrejon-Tellez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Floyd, R.A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999, 222, 236–245. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Hrelia, S.; Angeloni, C. New mechanisms of action of natural antioxidants in health and disease. Antioxidants 2020, 9, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Matsuoka, Y.; Sakai, K.; Fujiya, N.; Fujii, H.; Mano, J. Catechins in green tea powder (matcha) are heat-stable scavengers of acrolein, a lipid peroxide-derived reactive carbonyl species. Food Chem. 2021, 355, 129403. [Google Scholar] [CrossRef]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, R.; Jang, M.; Park, Y.I. Therapeutic Potential of EGCG, a Green Tea Polyphenol, for Treatment of Coronavirus Diseases. Life 2021, 11, 197. [Google Scholar] [CrossRef]
- Murtiastutik, D.; Prakoswa, S.; Rosita, C.; Tantular, I.S.; Wibisono, Y.; Hidayati, A.N.; Listiawan, M.Y. Epigallocathecingallate (EGCG) Antifungal Properties for Candida Isolates from HIV/AIDS Patients with Oral Candidiasis in Compare with Fluconazole. Indian J. Forensic Med. Toxicol. 2021, 15, 1021–1026. [Google Scholar]
- Dufresne, C.J.; Farnworth, E.R. A review of latest research findings on the health promotion properties of tea. J. Nutr. Biochem. 2001, 12, 404–421. [Google Scholar] [CrossRef]
- Velayutham, P.; Babu, A.; Liu, D. Green tea catechins and cardiovascular health: An update. Curr. Med. Chem. 2008, 15, 1840. [Google Scholar]
- Sicard, A.A.; Suarez, N.G.; Cappadocia, L.; Annabi, B. Functional targeting of the TGF-betaR1 kinase domain and downstream signaling: A role for the galloyl moiety of green tea-derived catechins in ES-2 ovarian clear cell carcinoma. J. Nutr. Biochem. 2021, 87, 108518. [Google Scholar] [CrossRef]
- Kajimoto, O.; Kajimoto, Y.; Yabune, M.; Nakamura, T.; Kotani, K.; Suzuki, Y.; Nozawa, A.; Nagata, K.; Unno, T.; Sagesaka, Y.M. Tea catechins with a galloyl moiety reduce body weight and fat. Int. J. Health Sci. 2005, 51, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Jiang, F.; Liu, P.; Chen, W.; Yi, K. Catechins containing a galloyl moiety as potential anti-HIV-1 compounds. Drug Discov. 2012, 17, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-T.; Liu, Z.-Y.; Li, A.; Zhao, G.-H.; Xie, H.-K.; Zhou, D.-Y.; Wang, T. Gallic acid and its alkyl esters emerge as effective antioxidants against lipid oxidation during hot air drying process of Ostrea talienwhanensis. LWT 2021, 139, 110551. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Yun, F.; Jiang, X.; Yu, L. Preparation and Evaluation of Gallate Ester Derivatives Used as Promising Antioxidant and Antibacterial Inhibitors. Chem. Biodivers. 2021, 18, e2000913. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Tayama, S. Cytotoxicity of propyl gallate and related compounds in rat hepatocytes. Arch. Toxicol. 1995, 69, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Meng, X.; Yang, C.S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab. Dispos. 2003, 31, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Crespy, V.; Nancoz, N.; Oliveira, M.; Hau, J.; Courtet-Compondu, M.-C.; Williamson, G. Glucuronidation of the green tea catechins, (−)-epigallocatechin-3-gallate and (-)-epicatechin-3-gallate, by rat hepatic and intestinal microsomes. Free Radic. Res. 2004, 38, 1025–1031. [Google Scholar] [CrossRef]
- OKusHio, K.; Suzuki, M.; Matsumoto, N.; Nanjo, F.; HARA, Y. Methylation of tea catechins by rat liver homogenates. Biosci. Biotechnol. Biochem. 1999, 63, 430–432. [Google Scholar] [CrossRef]
- Okushio, K.; Suzuki, M.; Matsumoto, N.; Nanjo, F.; Hara, Y. Identification of (−)-epicatechin metabolites and their metabolic fate in the rat. Drug Metab. Dispos. 1999, 27, 309–316. [Google Scholar]
- Vaidyanathan, J.B.; Walle, T. Glucuronidation and sulfation of the tea flavonoid (−)-epicatechin by the human and rat enzymes. Drug Metab. Dispos. 2002, 30, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Rha, C.-S.; Choi, Y.-M.; Kim, J.-C.; Kim, D.-O. Cost-Effective Simultaneous Separation and Quantification of Phenolics in Green and Processed Tea Using HPLC–UV–ESI Single-Quadrupole MS Detector and Python Script. Separations 2021, 8, 45. [Google Scholar] [CrossRef]
- Miketova, P.; Schram, K.H.; Whitney, J.; Li, M.; Huang, R.; Kerns, E.; Valcic, S.; Timmermann, B.N.; Rourick, R.; Klohr, S. Tandem mass spectrometry studies of green tea catechins. Identification of three minor components in the polyphenolic extract of green tea. J. Mass Spectrom. 2000, 35, 860–869. [Google Scholar] [CrossRef]
- Meng, X.; Lee, M.-J.; Li, C.; Sheng, S.; Zhu, N.; Sang, S.; Ho, C.-T.; Yang, C.S. Formation and identification of 4′-O-methyl-(−)-epigallocatechin in humans. Drug Metab. Dispos. 2001, 29, 789–793. [Google Scholar]
- Muzolf-Panek, M.; Gliszczynska-Swiglo, A.; de Haan, L.; Aarts, J.M.; Szymusiak, H.; Vervoort, J.M.; Tyrakowska, B.; Rietjens, I.M. Role of catechin quinones in the induction of EpRE-mediated gene expression. Chem. Res. Toxicol. 2008, 21, 2352–2360. [Google Scholar] [CrossRef]
- Mori, T.; Ishii, T.; Akagawa, M.; Nakamura, Y.; Nakayama, T. Covalent binding of tea catechins to protein thiols: The relationship between stability and electrophilic reactivity. Biosci. Biotechnol. Biochem. 2010, 74, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhong, D.; Chen, X. A fragmentation-based method for the differentiation of glutathione conjugates by high-resolution mass spectrometry with electrospray ionization. Anal. Chim. Acta 2013, 788, 89–98. [Google Scholar] [CrossRef]
- Šilarová, P.; Česlová, L.; Meloun, M. Fast gradient HPLC/MS separation of phenolics in green tea to monitor their degradation. Food Chem. 2017, 237, 471–480. [Google Scholar] [CrossRef]
- Secretan, P.-H.; Thirion, O.; Sadou Yayé, H.; Damy, T.; Astier, A.; Paul, M.; Do, B. Simple Approach to Enhance Green Tea Epigallocatechin Gallate Stability in Aqueous Solutions and Bioavailability: Experimental and Theoretical Characterizations. Pharmaceuticals 2021, 14, 1242. [Google Scholar] [CrossRef]
- Li, M.; Shen, Y.; Ling, T.; Ho, C.-T.; Li, D.; Guo, H.; Xie, Z. Analysis of Differentiated Chemical Components between Zijuan Purple Tea and Yunkang Green Tea by UHPLC-Orbitrap-MS/MS Combined with Chemometrics. Foods 2021, 10, 1070. [Google Scholar] [CrossRef]
- Moore, R.J.; Jackson, K.G.; Minihane, A.M. Green tea (Camellia sinensis) catechins and vascular function. Br. J. Nutr. 2009, 102, 1790–1802. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, H.; Wakano, K.; Kitani, S. Inhibition of NADPH oxidase subunits translocation by tea catechin EGCG in mast cell. Biochem. Biophys. Res. Commun. 2007, 362, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Aronson, W.J.; Huang, M.; Zhang, Y.; Lee, R.-P.; Heber, D.; Henning, S.M. Green tea polyphenols and metabolites in prostatectomy tissue: Implications for cancer prevention. Cancer Prev. Res. 2010, 3, 985–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qiao, L.; Zhang, X.; Wu, Z.; Weng, P. Effect of methylated tea catechins from Chinese oolong tea on the proliferation and differentiation of 3T3-L1 preadipocyte. Fitoterapia 2015, 104, 45–49. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M.; Ema, K.; Monobe, M.; Tokuda, Y.; Tachibana, H. Epicatechin-3-O-(″-O-methyl)-gallate content in various tea cultivars (Camellia sinensis L.) and its in vitro inhibitory effect on histamine release. J. Agric. Food Chem. 2012, 60, 2165–2170. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M.; Ema, K.; Tokuda, Y.; Monobe, M.; Tachibana, H.; Sameshima, Y.; Kuriyama, S. Effect of green tea powder (Camellia sinensis L. cv. Benifuuki) particle size on O-methylated EGCG absorption in rats; The Kakegawa Study. Cytotechnology 2011, 63, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Lambert, J.D.; Hong, J.; Tian, S.; Lee, M.-J.; Stark, R.E.; Ho, C.-T.; Yang, C.S. Synthesis and structure identification of thiol conjugates of (−)-epigallocatechin gallate and their urinary levels in mice. Chem. Res. Toxicol. 2005, 18, 1762–1769. [Google Scholar] [CrossRef]
- Chelcheleh, M.; Allameh, A. In vivo biotransformation of aflatoxin B1 and its interaction with cellular macromolecules in neonatal rats. Mech. Ageing Dev. 1995, 78, 189–196. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Li, W.Q.; Xia, S.; Guo, L.; Miao, Y.; Zhang, B.-K. Metabolic activation of the toxic natural products from herbal and dietary supplements leading to toxicities. Front. Pharmacol. 2021, 12, 758468. [Google Scholar] [CrossRef]
- Patra, S.; Rizzi, F.; Silva, A.; Rugina, D.; Bettuzzi, S. Molecular targets of (−)-epigallocatechin-3-gallate (EGCG): Specificity and interaction with membrane lipid rafts. J. Physiol. Pharmacol. 2008, 59, 217–235. [Google Scholar]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular targets of epigallocatechin—Gallate (EGCG): A special focus on signal transduction and cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterworth, M.; Lau, S.S.; Monks, T.J. 17β-Estradiol metabolism by hamster hepatic microsomes: Comparison of catechol estrogen O-methylation with catechol estrogen oxidation and glutathione conjugation. Chem. Res. Toxicol. 1996, 9, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Wolf, V.G.; Bonacorsi, C.; Raddi, M.S.G.; da Fonseca, L.M.; Ximenes, V.F. Octyl gallate, a food additive with potential beneficial properties to treat Helicobacter pylori infection. Food Funct. 2017, 8, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Montellano, P.R. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 2010, 110, 932–948. [Google Scholar] [CrossRef] [Green Version]
- Goossen, L.J.; Rodriguez, N.; Gooßen, K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 2008, 47, 3100–3120. [Google Scholar] [CrossRef]
- Sadler, J.C.; Humphreys, L.D.; Snajdrova, R.; Burley, G.A. A Tandem Enzymatic sp2-C-Methylation Process: Coupling in Situ S-Adenosyl-l-Methionine Formation with Methyl Transfer. ChemBioChem 2017, 18, 992–995. [Google Scholar] [CrossRef] [Green Version]
- Nes, W.D.; Song, Z.; Dennis, A.L.; Zhou, W.; Nam, J.; Miller, M.B. Biosynthesis of phytosterols: Kinetic mechanism for the enzymatic C-methylation of sterols. J. Biol. Chem. 2003, 278, 34505–34516. [Google Scholar] [CrossRef] [Green Version]



| Biotransformation | Formula | RT (min) | m/z (ppm) | RT (min) | m/z (ppm) |
|---|---|---|---|---|---|
| C/EC | C | EC | |||
| Parent | C15H14O6 | 6.5 | 289.0722 (1.5) | 8.8 | 289.0720 (0.8) |
| +CH2 | C16H16O6 | 10.1 | 303.0879 (1.6) | 11.6 | 303.0877 (1) |
| 11.3 | 303.0878 (1.3) | 13.0 | 303.0876 (0.6) | ||
| +2CH2 | C17H18O6 | 13.0 | 317.1030 (−0.2) | 14.1 | 317.1032 (0.4) |
| −2H+GSH | C25H29N3O12S | 5.8 | 594.1403 (0.6) | 5.1 | 594.1400 (0.1) |
| 6.7 | 594.1400 (0.1) | 8.7 | 594.1404 (0.8) | ||
| +CH2-2H+GSH | C26H31N3O12S | 7.4 | 608.1531 (−4.1) | 9.0 | 608.1548 (−1.3) |
| 8.0 | 608.1554 (−0.3) | 10.1 | 608.1549 (−1.1) | ||
| GC/EGC | GC | EGC | |||
| Parent | C15H14O7 | 3.8 | 305.0673 (2) | 6.0 | 305.0669 (0.7) |
| +CH2 | C16H16O7 | 6.9 | 319.0829 (1.8) | 9.1 | 319.0829 (1.8) |
| 7.8 | 319.0835 (3.7) | 9.8 | 319.0827 (1.2) | ||
| +2CH2 | C17H18O7 | 10.9 | 333.0989 (2.8) | 13.2 | 333.0985 (1.6) |
| 11.9 | 333.0979 (−0.2) | 11.5 | 333.0983 (−1.1) | ||
| −2H+GSH | C25H29N3O13S | 2.5 | 610.1346 (−0.4) | 4.6 | 610.1342 (-1) |
| 3.4 | 610.1350 (0.3) | 5.8 | 610.1345 (−0.5) | ||
| −4H+2GSH | C35H44N6O19S2 | 3.6 | 457.0982 * (0.7) | 5.5 | 457.0983 * (1) |
| +CH2-2H+GSH | C26H31N3O13S | 5.9 | 624.1509 (0.7) | 7.1 | 624.1501 (−0.6) |
| 6.9 | 624.1498 (−1.1) | 7.9 | 624.1503 (−0.3) | ||
| CG/ECG | CG | ECG | |||
| Parent | C22H18O10 | 11.9 | 441.0824 (−0.7) | 11.7 | 441.0824 (−0.7) |
| +CH2 | C23H20O10 | 14.1 | 455.0988 (0.9) | 12.7 | 455.0978 (1.1) |
| 14.6 | 455.0983 (−0.2) | 13.3 | 455.0985 (−0.3) | ||
| +2CH2 | C24H22O10 | 16.4 | 469.1142 (0.4) | 15.7 | 469.1143 (−0.6) |
| −2H+GSH | C32H33N3O16S | 10.4 | 746.1476 (−4.4) | 8.4 | 746.1514 (0.7) |
| 11.1 | 746.1504 (−0.6) | 10.1 | 746.1512 (0.4) | ||
| 11.4 | 746.1491 (-2.4) | 11.2 | 746.1514 (0.7) | ||
| −4H+2GSH | C42H48N6O22S2 | 10.7 | 525.1049 * (−1.9) | 10.0 | 525.1061 * (0.4) |
| +CH2-2H+GSH | C33H35N3O16S | 11.5 | 760.1648 (−2.3) | 11.3 | 760.1672 (0.9) |
| 11.9 | 760.1633 (−4.2) | 11.7 | 760.1670 (0.6) | ||
| GCG/EGCG | GCG | EGCG | |||
| Parent | C22H18O11 | 9.9 | 457.0775 (−0.3) | 9.3 | 457.0772 (−1) |
| +CH2 | C23H20O11 | 12.3 | 471.0937 (0.9) | 11.2 | 471.0929 (−0.8) |
| 12.7 | 471.0937 (0.9) | 11.8 | 471.0938 (1.1) | ||
| +2CH2 | C24H22O11 | 14.2 | 485.1091 (0.3) | 13.5 | 485.1085 (−0.9) |
| 14.8 | 485.1094 (0.9) | 14.6 | 485.1093 (0.7) | ||
| −2H+GSH | C32H33N3O17S | 7.9 | 762.1434 (−3.1) | 7.6 | 762.1451 (−0.9) |
| 8.6 | 762.1432 (−3.4) | 8.8 | 762.1469 (1.5) | ||
| −4H+2GSH | C42H48N6O23S2 | 6.6 | 533.1026 * (−1.4) | 7.3 | 533.1033 * (−0.1) |
| +CH2-2H+GSH | C33H35N3O17S | 9.6 | 776.1592 (−2.9) | 9.5 | 776.1610 (−0.6) |
| 10.2 | 776.1606 (−1.1) | 10.2 | 776.1607 (−1) | ||
| Biotransformation | Formula | RT (min) | m/z (ppm) |
|---|---|---|---|
| GA a (Parent) | C7H6O5 | 3.5 | 169.0142 (−0.3) |
| +CH2 | C8H8O5 | 7.2 | 183.0302 (1.6) |
| 6.5 | 183.0298 (−0.5) | ||
| +2CH2 | C9H10O5 | 11.3 | 197.0459 (1.8) |
| −2H+GSH | C17H21N3O11S | 3.1 | 474.0828 (0.8) |
| +CH2-2H+GSH | C18H23N3O11S | 4.2 | 488.0980 (−0.1) |
| EG (Parent) | C9H10O5 | 9.8 | 197.0460 (−2.4) |
| +CH2 | C10H12O5 | 13.2 | 211.0617 (−2.7) |
| 14.4 | 211.0620 (−3.9) | ||
| −2H+GSH | C19H25N3O11S | 8.1 | 502.1133 (0.7) |
| +CH2-2H+GSH | C20H27N3O11S | 10.4 | 516.1296 (−0.6) |
| 11.5 | 516.1298 (−1) | ||
| PG (Parent) | C10H12O5 | 13.2 | 211.0612 (−0.2) |
| +CH2 | C11H14O5 | 15.9 | 225.0770 (−0.9) |
| 16.9 | 225.0768 (−0.2) | ||
| −2H+GSH | C20H27N3O11S | 10.9 | 516.1283 (1.9) |
| +CH2-2H+GSH | C21H29N3O11S | 12.9 | 530.1452 (−0.5) |
| 13.9 | 530.1441 (1.5) | ||
| 14.4 | 530.1446 (0.7) | ||
| −4H+2GSH | C30H42N6O17S2 | 10.1 | 410.0951 * (−0.1) |
| BG (Parent) | C11H14O5 | 16.0 | 225.0775 (−3.2) |
| +CH2 | C12H16O5 | 17.5 | 239.0928 (−1.6) |
| 17.8 | 239.0929 (−1.7) | ||
| +O | C11H14O6 | 8.4 | 241.0725 (−3.2) |
| 8.6 | 241.0724 (−3) | ||
| +O+CH2 | C12H16O6 | 11.6 | 255.0880 (−2.4) |
| −2H+GSH | C21H29N3O11S | 13.5 | 530.1436 (2.6) |
| +CH2-2H+GSH | C22H31N3O11S | 15.2 | 544.1587 (3.5) |
| 16.2 | 544.1590 (3) | ||
| −4H+2GSH | C31H44N6O17S2 | 12.2 | 417.1032 * (−0.6) |
| OG (Parent) | C15H22O5 | 18.6 | 281.1394 (−0.1) |
| +CH2 | C16H24O5 | 18.8 | 295.1544 (2.3) |
| 19.0 | 295.1544 (2.3) | ||
| +O | C15H22O6 | 17.1 | 297.1345 (−0.7) |
| 17.3 | 297.1340 (1) | ||
| +O+CH2 | C16H24O6 | 17.8 | 311.1499 (0.4) |
| +2O | C15H22O7 | 14.2 | 313.1291 (−0.4) |
| OG aldehyde (+O-2H) | C15H20O6 | 17.4 | 295.1190 (−1) |
| OG acid (+2O-2H) | C15H20O7 | 17.2 | 311.1139 (−1) |
| −2H+GSH | C25H37N3O11S | 18.1 | 586.2066 (1.7) |
| +CH2-2H+GSH | C26H39N3O11S | 18.4 | 600.2228 (0.7) |
| +O-2H+GSH | C25H37N3O12S | 15.5 | 602.2018 (1.1) |
| 15.1 | 602.2025 (0.4) | ||
| +O+CH2-2H+GSH | C26H39N3O12S | 16.9 | 616.2180 (0.1) |
| LG (Parent) | C19H30O5 | 19.2 | 337.2017 (1) |
| +CH2 | C20H32O5 | 19.3 | 351.2177 (−0.1) |
| 19.4 | 351.2172 (1.4) | ||
| +O | C19H30O6 | 18.5 | 353.1981 (3.5) |
| 18.7 | 353.1966 (0.9) | ||
| LG aldehyde (+O-2H) | C19H28O6 | 18.8 | 351.1811 (0.6) |
| LG acid (+2O-2H) | C19H28O7 | 18.6 | 367.1759 (0.7) |
| −2H+GSH | C29H45N3O11S | 18.9 | 642.2707 (−0.8) |
| +CH2-2H+GSH | C30H47N3O11S | 19.0 | 656.2857 (−1.4) |
| +O-2H+GSH | C29H45N3O12S | 18.2 | 658.2657 (−0.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ousji, O.; Sleno, L. Structural Elucidation of Novel Stable and Reactive Metabolites of Green Tea Catechins and Alkyl Gallates by LC-MS/MS. Antioxidants 2022, 11, 1635. https://doi.org/10.3390/antiox11091635
Ousji O, Sleno L. Structural Elucidation of Novel Stable and Reactive Metabolites of Green Tea Catechins and Alkyl Gallates by LC-MS/MS. Antioxidants. 2022; 11(9):1635. https://doi.org/10.3390/antiox11091635
Chicago/Turabian StyleOusji, Ons, and Lekha Sleno. 2022. "Structural Elucidation of Novel Stable and Reactive Metabolites of Green Tea Catechins and Alkyl Gallates by LC-MS/MS" Antioxidants 11, no. 9: 1635. https://doi.org/10.3390/antiox11091635
APA StyleOusji, O., & Sleno, L. (2022). Structural Elucidation of Novel Stable and Reactive Metabolites of Green Tea Catechins and Alkyl Gallates by LC-MS/MS. Antioxidants, 11(9), 1635. https://doi.org/10.3390/antiox11091635







