Antioxidant Capacities and Enzymatic Inhibitory Effects of Different Solvent Fractions and Major Flavones from Celery Seeds Produced in Different Geographic Areas in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents
2.3. Preparation of Celery Seed Extracts
2.4. Flavones Analysis by HPLC-TOF-MS
2.5. Quantitative Analysis of Flavones in the nBuOH Fraction by HPLC-UV
2.6. Measurement of Total Phenolic Content (TPC)
2.7. Measurement of Total Flavonoid Content (TFC)
2.8. DPPH Radical Scavenging Assay
2.9. ABTS Radical Scavenging Assay
2.10. Ferric Reducing Antioxidant Power (FRAP)
2.11. Cupric Reducing Antioxidant Capacity (CUPRAC)
2.12. Metal Chelating Activity on Ferrous Ions
2.13. α-Glucosidase Inhibitory Activity Assay
2.14. α-Amylase Inhibitory Activity
2.15. Molecular Modeling Docking Study
2.16. Statistical Analysis
3. Results and Discussion
3.1. TPC, TFC and Yields in Different Solvent Fractions
3.2. Analysis and Quantification of Main Flavones in the nBuOH Fractions of Celery Seeds from Different Geographic Areas
| RT (min) | Flavones | Formula | Molecular Weight | Tandem Mass Spectrometry | Flavonoids Content in nBuOH Fractions from Different Geographic Areas Celery Seeds (mg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shandong Samples | Guangxi Samples | Northeast Samples | Jiangsu Samples | Hubei Samples | ||||||
| 34.266 | Graveobioside A | C26H28O15 | 580.1428 | 579.1334, 447.0908, 285.0381 | 296.68 ± 5.35 a,A | 276.38 ± 7.39 a,B | 294.95 ± 4.22 a,A | 87.36 ± 1.97 a,D | 204.72 ± 5.50 a,C | |
| 35.276 | Luteolin-7-O-glucoside | C21H20O11 | 448.1006 | 447.0925, 285.0400 | 26.14 ± 0.41 d,B | 25.25 ± 0.66 d,C | 29.98 ± 0.46 A | 8.17 ± 0.15 d,E | 22.82 ± 0.50 d,D | |
| 37.810 | Apiin | C26H28O14 | 564.1479 | 563.1390, 269.0452 | 43.08 ± 0.89 c,A | 40.86 ± 0.95 c,B | 37.03 ± 0.24 c,C | 10.98 ± 0.06 c,E | 30.64 ± 0.57 c,D | |
| 38.394 | Graveobioside B | C27H30O15 | 594.1585 | 593.1502, 299.0554 | 176.22 ± 3.71 b,A | 165.53 ± 4.55 b,B | 171.79 ± 1.94 b,A | 45.41 ± 0.11 b,D | 107.36 ± 2.37 b,C | |
| 39.106 | Apigenin-7-O-Glucoside | C21H20O10 | 432.1056 | 431.0979, 268.0377 | 7.28 ± 0.18 e,B | 5.64 ± 0.04 e,D | 6.57 ± 0.13 d,C | 1.89 ± 0.19 e,E | 9.43 ± 0.08 e,A | |
| 39.952 | Chrysoeriol-7-O-glucosid | C22H22O11 | 462.1162 | 461.1082, 299.0560 | 3.60 ± 0.09 e,A | 3.37 ± 0.06 e,A | 3.53 ± 0.02 e,A | 1.16 ± 0.40 e,C | 2.84 ± 0.02 f,B | |
| 553.01 ± 10.58 A | 517.04 ± 13.65 B | 543.85 ± 6.77 A | 154.96 ± 2.69 D | 377.81 ± 8.97 C | ||||||
3.3. Antioxidant Capacities of Different Fractions from Five Celery Seed Samples
| Code | Region | Fraction | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | FRAP (mg TE/g) | CUPRAC (mg TE/g) | Metal Chelating (mg EDTAE/g) |
|---|---|---|---|---|---|---|---|
| Sample 1 | Shandong China | DCM | 404.94 ± 14.83 e | 186.17 ± 3.69 d | 31.27 ± 2.04 k | 42.52 ± 0.91 k | 13.70 ± 0.23 d,e |
| nBuOH | 20.49 ± 0.19 l | 15.49 ± 0.32 k | 509.93 ± 20.01 b | 414.32 ± 10.02 c | 14.11 ± 0.99 d | ||
| H2O | >1000 a | 179.43 ± 4.82 d | 31.02 ± 0.13 k | 29.82 ± 2.19 l | 5.71 ± 0.21 f | ||
| Sample 2 | Guangxi China | DCM | 896.00 ± 78.14 d | 386.17 ± 25.18 a | 16.33 ± 0.90 n | 27.14 ± 0.06 l m | 16.17 ± 0.65 c |
| nBuOH | 21.97 ± 0.81 k,l | 17.15 ± 0.17 k | 486.07 ± 9.31 c | 394.32 ± 9.97 d | 18.28 ± 0.20 b | ||
| H2O | >1000 b | 260.03 ± 13.14 c | 23.24 ± 0.05 l | 24.81 ± 0.16 m,n | 4.33 ± 0.43 g | ||
| Sample 3 | Northeast China | DCM | 100.86 ± 3.40 g | 64.21 ± 5.62 g | 77.33 ± 1.38 h | 98.10 ± 3.98 h | 12.77 ± 1.06 e |
| nBuOH | 20.27 ± 0.32 l | 15.11 ± 0.22 k | 547.93 ± 9.32 a | 444.78 ± 4.22 b | 18.33 ± 0.18 b | ||
| H2O | >1000 c | 308.17 ± 21.69 b | 19.57 ± 0.11 m | 22.32 ± 0.08 n | 4.08 ± 0.47 g | ||
| Sample 4 | Jiangsu China | DCM | 87.63 ± 0.45 h | 40.49 ± 0.68 i | 117.54 ± 3.70 f | 147.86 ± 5.16 f | 20.81 ± 1.13 a |
| nBuOH | 44.13 ± 1.44 i | 33.63 ± 0.85 j | 268.33 ± 3.41 e | 234.84 ± 2.46 e | 13.16 ± 1.06 d,e | ||
| H2O | 823.25 ± 17.22 d | 141.27 ± 0.75 e | 44.86 ± 0.12 j | 56.44 ± 0.83 j | 3.41 ± 0.19 g | ||
| Sample 5 | Hubei China | DCM | 109.55 ± 0.57 f | 44.52 ± 2.92 h | 100.20 ± 1.47 g | 116.77 ± 3.39 g | 13.64 ± 0.96 d,e |
| nBuOH | 22.92 ± 0.50 k | 15.50 ± 1.26 k | 523.90 ± 3.53 b | 465.78 ± 3.09 a | 18.05 ± 0.48 b | ||
| H2O | 512.03 ± 15.45 e | 117.43 ± 1.01 f | 53.96 ± 1.19 i | 67.00 ± 0.93 i | 3.80 ± 0.84 g | ||
| VC A | 39.13 ± 0.93 j | 5.72 ± 0.10 l | 406.39 ± 4.65 d | - | - | ||
| BHT A | 5.32 ± 0.11 m | 3.79 ± 0.04 l | - | - | - |
3.4. Enzyme Inhibitory Activity of Different Fractions from Five Celery Seed Samples
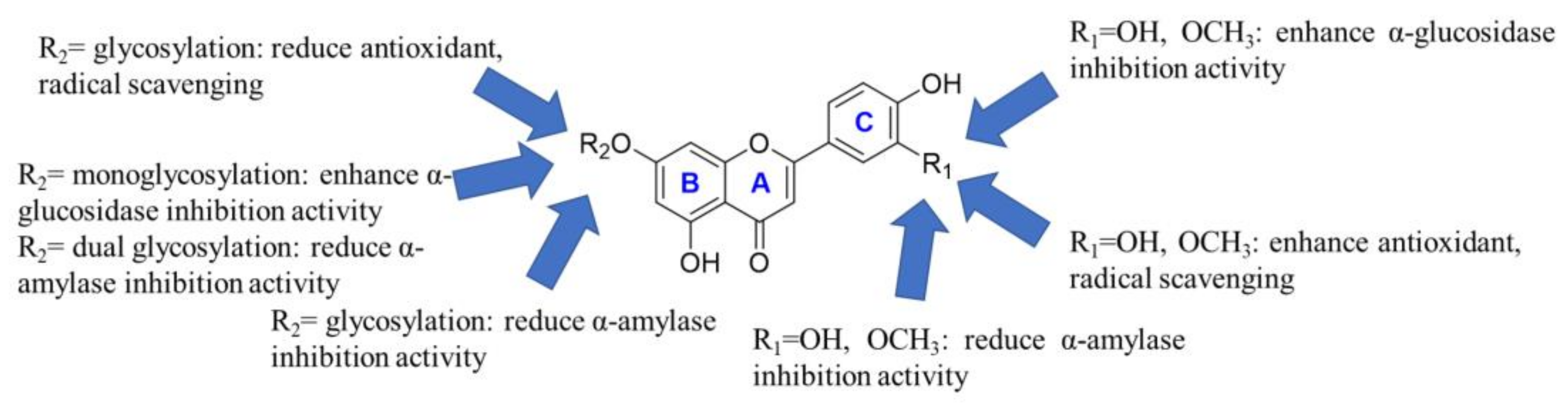
3.5. Antioxidant Activities of the Main Flavones in Celery Seeds
| No. | Compounds | DPPH IC50 (μM) | ABTS IC50 (μg/mL) | FRAP (mg TE/g) | CUPRAC (mg TE/g) | Metal Chelating (mg EDTAE/g) | α-Glucosidase IC50 (μM) | α-Amylase IC50 (mM) |
|---|---|---|---|---|---|---|---|---|
| 1 | Graveobioside A | 12.47 ± 0.08 h | 6.88 ± 0.05 h | 882.04 ± 0.43 b | 887.17 ± 8.95 c | 89.98 ± 0.42 a | 104.31 ± 5.75 b | N.A. |
| 2 | Graveobioside B | 3266.33 ± 365.08 c | 29.43 ± 1.33 e | 166.87 ± 2.97 g | 328.10 ± 14.10 e | 21.32 ± 1.51 e | 103.96 ± 1.85 b | N.A. |
| 3 | Apiin | 7686.33 ± 200.18 a | 1055.33 ± 24.68 a | 5.97 ± 1.49 j | 25.26 ± 0.18 g | 17.15 ± 1.37 f | 198.70 ± 14.91 a | N.A. |
| 4 | Luteolin-7-O-glucoside | 9.28 ± 0.06 i | 9.41 ± 0.42 g | 830.71 ± 8.52 c | 955.05 ± 3.64 b | 8.51 ± 0.21 g | 49.87 ± 2.85 d | N.A. |
| 5 | Chrysoeriol-7-O-glucosid | 848.23 ± 143.61 e | 46.64 ± 1.24 d | 203.35 ± 6.33 f | 495.51 ± 6.64 d | 8.22 ± 0.84 g | 39.79 ± 1.05 e | 1.86 ± 0.02 b |
| 6 | Apigenin-7-O-Glucoside | 4885.33 ± 56.01 b | 461.00 ± 11.04 b | 25.90 ± 0.49 i | 33.45 ± 0.51 g | 5.69 ± 0.19 h | 77.47 ± 4.84 c | 1.74 ± 0.04 c |
| 7 | Luteolin | 4.65 ± 0.16 j | 4.58 ± 0.02 i,j | 1025.12 ± 10.78 a | 1502.63 ± 15.92 a | 88.44 ±0.64 b | 58.83 ± 2.97 d | 2.11 ± 0.01 a |
| 8 | Chrysoeriol | 580.10 ± 35.54 f | 16.69 ± 0.55 f | 337.96 ± 0.88 e | 947.43 ± 5.72 b | 36.49 ± 0.22 d | 40.49 ± 0.53 e | 1.31 ± 0.03 d |
| 9 | Apigenin | 1292.00 ± 31.00 d | 223.63 ± 10.35 c | 59.41 ± 0.24 h | 76.44 ± 1.83 f | 54.20 ± 1.28 c | 79.98 ± 0.85 c | 0.86 ± 0.01 e |
| 10 | BHT A | 39.13 ± 0.93 g | 5.72 ± 0.10 h,i | 406.39 ± 4.65 d | N. | N. | N. | N. |
| 11 | VC A | 5.32 ± 0.11 j | 3.79 ± 0.04 j | N. | N. | N. | N. | N. |
| 12 | Acarbose B | N. | N. | N. | N. | N. | 0.023 ± 0.00 f | 0.12 ± 0.00 f |
3.6. α-Glucosidase and α-Amylase Inhibition Activity of the Main Flavones in Celery Seeds
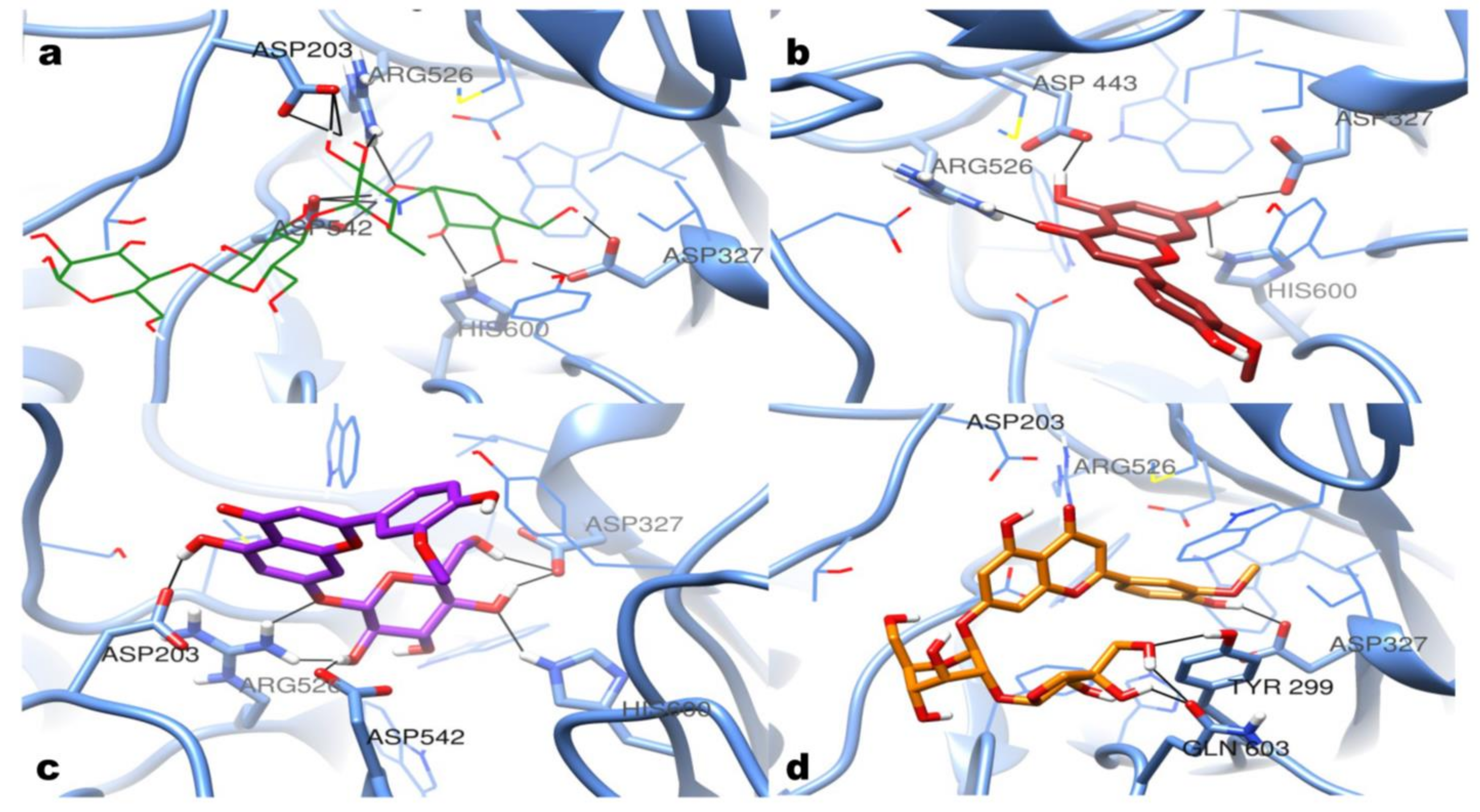
3.7. Molecular Modeling Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, G.; Shi, S.; Jia, Q.; Shi, J.; Shi, S.; Zhang, X.; Shou, X.; Zhu, X.; Hu, Y. Use of Network Pharmacology to Explore the Mechanism of Gegen (Puerariae lobatae Radix) in the Treatment of Type 2 Diabetes Mellitus Associated with Hyperlipidemia. Evid. Based Complement. Altern. Med. 2021, 2021, 6633402. [Google Scholar] [CrossRef]
- He, Y.; Al-Mureish, A.; Wu, N. Nanotechnology in the Treatment of Diabetic Complications: A Comprehensive Narrative Review. J. Diabetes Res. 2021, 2021, 6612063. [Google Scholar] [CrossRef] [PubMed]
- Kheiripour, N.; Karimi, J.; Khodadadi, I.; Tavilani, H.; Taghi Goodarzi, M.; Hashemnia, M. Hepatoprotective Effects of Silymarin on Liver Injury via Irisin Upregulation and Oxidative Stress Reduction in Rats with Type 2 Diabetes. Iran. J. Med. Sci. 2019, 44, 108–117. [Google Scholar] [PubMed]
- Cheng, D.; Liang, B.; Li, Y. Antihyperglycemic Effect of Ginkgo Biloba Extract in Streptozotocin-Induced Diabetes in Rats. BioMed Res. Int. 2012, 2013, 162724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKay-Lyons, M.; Gubitz, G.; Phillips, S.; Giacomantonio, N.; Firth, W.; Thompson, K.; Theriault, C.; Wightman, H.; Slipp, S.; Marsters, D.; et al. Program of Rehabilitative Exercise and Education to Avert Vascular Events After Non-Disabling Stroke or Transient Ischemic Attack (PREVENT Trial): A Randomized Controlled Trial. Neurorehabilit. Neural Repair 2021, 36, 119–130. [Google Scholar] [CrossRef]
- Kamuhabwa, A.R.; Charles, E. Predictors of Poor Glycemic Control in Type 2 Diabetic Patients Attending Public Hospitals in Dar es Salaam. Drug Healthc. Patient Safety. 2014, 6, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, J.; Li, N.; Liu, J.; Zhou, J.; Zhuang, P.; Chen, H. A Systematic Review of Orthosiphon Stamineus Benth. in the Treatment of Diabetes and Its Complications. Molecules 2022, 27, 444. [Google Scholar] [CrossRef]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlcek, J.; Balla, S.; Szekeres, L.; Adam, V.; Kizek, R. Polyphenolic Profile and Biological Activity of Chinese Hawthorn (Crataegus pinnatifida BUNGE) Fruits. Molecules 2012, 17, 14490–14509. [Google Scholar] [CrossRef] [Green Version]
- Balli, D.; Cecchi, L.; Khatib, M.; Bellumori, M.; Cairone, F.; Carradori, S.; Zengin, G.; Cesa, S.; Innocenti, M.; Mulinacci, N. Characterization of Arils Juice and Peel Decoction of Fifteen Varieties of Punica granatum L.: A Focus on Anthocyanins, Ellagitannins and Polysaccharides. Antioxidants 2020, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Thao, N.P.; Binh, P.T.; Luyen, N.T.; Hung, T.M.; Dang, N.H.; Dat, N.T. α-Amylase and α-Glucosidase Inhibitory Activities of Chemical Constituents from Wedelia chinensis (Osbeck.) Merr. Leaves. J. Anal. Methods Chem. 2018, 2018, 2794904. [Google Scholar] [CrossRef] [Green Version]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, Z.; Liu, G.; Wu, Y.; Ouyang, J. Inhibitory Effect of Chestnut (Castanea Mollissima Blume) Inner Skin Extract on the Activity Of A-Amylase, A-Glucosidase, Dipeptidyl Peptidase IV And In Vitro Digestibility of Starches. Food Chem. 2020, 324, 126847. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 541–555. [Google Scholar] [CrossRef]
- Lung, M.-Y.; Chang, Y.-C. Antioxidant Properties of the Edible Basidiomycete Armillaria mellea in Submerged Cultures. Int. J. Mol. Sci. 2011, 12, 6367–6384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooti, W.; Daraei, N. A Review of the Antioxidant Activity of Celery (Apium graveolens L.). J. Evid.-Based Complementary Altern. Med. 2017, 22, 1029–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghadam, M.H.; Imenshahidi, M.; Mohajeri, S.A. Antihypertensive Effect of Celery Seed on Rat Blood Pressure in Chronic Administration. J. Med. Food 2013, 16, 558–563. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Lu, S.; Harnly, J.M. Detection and Quantification of Glycosylated Flavonoid Malonates in Celery, Chinese Celery, and Celery Seed by LC-DAD-ESI/MS. J. Agric. Food Chem. 2007, 55, 1321–1326. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Meenu, M.; Chen, P.; Xu, B. Comparative Study on Phytochemical Profiles and Antioxidant Capacities of Chestnuts Produced in Different Geographic Area in China. Antioxidants 2020, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Osés, S.M.; Marcos, P.; Azofra, P.; De Pablo, A.; Fernández-Muíño, M.Á.; Sancho, M.T. Phenolic Profile, Antioxidant Capacities and Enzymatic Inhibitory Activities of Propolis from Different Geographical Areas: Needs for Analytical Harmonization. Antioxidants 2020, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.E.; El Gedaily, R.A.; Mocan, A.; Farag, M.A.; El-Seedi, H.R. Profiling Metabolites and Biological Activities of Sugarcane (Saccharum officinarum Linn.) Juice and its Product Molasses via a Multiplex Metabolomics Approach. Molecules 2019, 24, 934. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-T.; Lin, H.-R.; Yang, C.-S.; Liaw, C.-C.; Sung, P.-J.; Kuo, Y.-H.; Cheng, M.-J.; Chen, J.-J. Antioxidant and Anti-α-Glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Fruits of Crataegus pinnatifida. Antioxidants 2022, 11, 320. [Google Scholar] [CrossRef]
- Gao, S.H.; Zhao, T.R.; Liu, Y.P.; Wang, Y.F.; Cheng, G.G.; Cao, J.X. Phenolic constituents, antioxidant activity and neuroprotective effects of ethanol extracts of fruits, leaves and flower buds from Vaccinium dunalianum Wight. Food Chem. 2022, 374, 131752. [Google Scholar] [CrossRef] [PubMed]
- Drouet, S.; Leclerc, E.A.; Garros, L.; Tungmunnithum, D.; Kabra, A.; Abbasi, B.H.; Lainé, É.; Hano, C. A Green Ultrasound-Assisted Extraction Optimization of the Natural Antioxidant and Anti-Aging Flavonolignans from Milk Thistle Silybum marianum (L.) Gaertn. Fruits for Cosmetic Applications. Antioxidants 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapatinha, M.; Oliveira, A.; Costa, S.; Pedro, S.; Gonçalves, A.; Mendes, R.; Bandarra, N.; Pires, C. Red and brown seaweeds extracts: A source of biologically active compounds. Food Chem. 2022, 393, 133453. [Google Scholar] [CrossRef] [PubMed]
- Etsassala, N.; Badmus, J.A.; Marnewick, J.L.; Egieyeh, S.; Iwuoha, E.I.; Nchu, F.; Hussein, A.A. Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities, Molecular Docking, and Antioxidant Capacities of Plectranthus ecklonii Constituents. Antioxidants 2022, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, M.A.H.; Ismail, A.; Kassim, N.K.; Hamid, M.; Ali, M.S.M. Phenolic profiling and evaluation of in vitro antioxidant, α-glucosidase and α-amylase inhibitory activities of Lepisanthes fruticosa (Roxb) Leenh fruit extracts. Food chemistry 2020, 331, 127240. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Sim, L.; Quezada-Calvillo, R.; Sterchi, E.E.; Nichols, B.L.; Rose, D.R. Human Intestinal Maltase–Glucoamylase: Crystal Structure of the N-Terminal Catalytic Subunit and Basis of Inhibition and Substrate Specificity. J. Mol. Biol. 2008, 375, 782–792. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Mu, B.; Song, Z.; Ma, Z.; Mu, T. The In Vitro Antioxidant Activity and Inhibition of Intracellular Reactive Oxygen Species of Sweet Potato Leaf Polyphenols. Oxidative Med. Cell. Longev. 2018, 2018, 9017828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Xin, X.; Yuan, Q.; Su, D.; Liu, W. Phytochemical Properties and Antioxidant Capacities of Various Colored Berries. J. Sci. Food Agric. 2013, 94, 180–188. [Google Scholar] [CrossRef]
- Klepacka, J.; Gujska, E.; Michalak, J. Phenolic Compounds as Cultivar- and Variety-distinguishing Factors in Some Plant Products. Mater. Veg. 2011, 66, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Bala, A.; Chakraborty, M.; Karmakar, I.; Haldar, S.; Das, A.; Haldar, P.K. Amelioration of oxidative DNA damage in mouse peritoneal macrophages by Hippophae salicifolia due to its proton (H+) donation capability: Ex vivo and in vivo studies. J. Pharm. Bioallied Sci. 2016, 8, 210–216. [Google Scholar] [CrossRef]
- Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Heo, H.J. Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX-Induced Melanogenesis in B16/F10 Melanoma Cells. Nutrients 2020, 12, 832. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, H.; Zhang, D.; Liu, J.; Wang, J.; Wang, S.; Sun, B. Baijiu Vinasse Extract Scavenges Glyoxal and Inhibits the Formation of N(ε)-Carboxymethyllysine in Dairy Food. Molecules 2019, 24, 1526. [Google Scholar] [CrossRef] [Green Version]
- Vitale, G.A.; Coppola, D.; Esposito, F.P.; Buonocore, C.; Ausuri, J.; Tortorella, E.; De Pascale, D. Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives. Antioxidants 2020, 9, 1183. [Google Scholar] [CrossRef]
- Zhenbao, J.; Fei, T.; Ling, G.; Guanjun, T.; Xiaolin, D. Antioxidant properties of extracts from juemingzi (Cassia tora L.) evaluated in vitro. LWT 2007, 40, 1072–1077. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Screening for in vitro antioxidant properties and fatty acid profiles of five Centaurea L. species from Turkey flora. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2914–2920. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant Capacity and Major Phenolic Compounds of Spices Commonly Consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Soleymaninejad, M.; Joursaraei, S.G.; Feizi, F.; Anarkooli, I.J. The Effects of Lycopene and Insulin on Histological Changes and the Expression Level of Bcl-2 Family Genes in the Hippocampus of Streptozotocin-Induced Diabetic Rats. J. Diabetes Res. 2017, 2017, 4650939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farid, N.; Inbal, D.; Nakhoul, N.; Evgeny, F.; Miller-Lotan, R.; Levy, A.P.; Rabea, A. Vitamin E and Diabetic Nephropathy in Mice Model and Humans. World J. Nephrol. 2013, 2, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, O.O.; Igbinosa, I.H.; Chigor, V.N.; Uzunuigbe, O.E.; Oyedemi, S.O.; Odjadjare, E.E.; Okoh, A.I.; Igbinosa, E.O. Polyphenolic Contents and Antioxidant Potential of Stem Bark Extracts from Jatropha curcas (Linn). Int. J. Mol. Sci. 2011, 12, 2958–2971. [Google Scholar] [CrossRef]
- Dolati, K.; Rakhshandeh, H.; Golestani, M.; Forouzanfar, F.; Sadeghnia, R.; Sadeghnia, H.R. Inhibitory Effects of Apium graveolens on Xanthine Oxidase Activity and Serum Uric Acid Levels in Hyperuricemic Mice. Prev. Nutr. Food Sci. 2018, 23, 127–133. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Yusoff, N.A.; Ahmad, M.; al Hindi, B.; Widyawati, T.; Yam, M.F.; Mahmud, R.; Razak, K.N.A.; Asmawi, M.Z. Aqueous Extract of Nypa fruticans Wurmb. Vinegar Alleviates Postprandial Hyperglycemia in Normoglycemic Rats †. Nutrients 2015, 7, 7012–7026. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 2019, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhu, L.; Chen, Y.; Tian, J.; Wang, Y. In Vivo And In Vitro Antioxidant Activity and A-Glucosidase, A-Amylase Inhibitory Effects of Flavonoids from Cichorium Glandulosum Seeds. Food Chem. 2013, 139, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as Alpha-Amylase and Alpha-Glucosidase Inhibitors and Their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Mencherini, T.; Mancini, E.; Aquino, R.P.; De Almeida, L.F.R.; De Feo, V. In Vitro Phytotoxicity and Antioxidant Activity of Selected Flavonoids. Int. J. Mol. Sci. 2012, 13, 5406–5419. [Google Scholar] [CrossRef] [PubMed]

| Code | Region | Fraction | TPC (mg GAE/g) | TFC (mg RE/g) | Yields (%) |
|---|---|---|---|---|---|
| Sample 1 | Shandong China | DCM | 8.82 ± 0.39 k | 26.40 ± 1.23 g,h | 4.53 |
| nBuOH | 71.90 ± 0.90 c | 652.57 ± 12.14 a | 1.75 | ||
| H2O | 7.79 ± 0.82 k | 22.49 ± 0.76 h | 4.20 | ||
| 10.48 | |||||
| Sample 2 | Guangxi China | DCM | 6.08 ± 0.11 l,m | 19.30 ± 0.54 h,i | 3.55 |
| nBuOH | 65.17 ± 0.12 d | 589.31 ± 3.94 c | 1.85 | ||
| H2O | 7.24 ± 0.42 k,l | 13.08 ± 0.79 i,j | 2.83 | ||
| 8.23 | |||||
| Sample 3 | Northeast China | DCM | 24.42 ± 0.40 h | 34.10 ± 1.16 f,g | 3.68 |
| nBuOH | 80.17 ± 0.57 a | 632.21 ± 6.56 b | 3.10 | ||
| H2O | 5.17 ± 0.97 m | 8.83 ± 0.12 j | 3.07 | ||
| 11.90 | |||||
| Sample 4 | Jiangsu China | DCM | 42.15 ± 2.56 f | 39.82 ± 1.20 f | 2.33 |
| nBuOH | 44.55 ± 0.29 e | 201.92 ± 0.08 e | 2.76 | ||
| H2O | 10.79 ± 0.38 j | 5.94 ± 0.10 j | 5.00 | ||
| 10.09 | |||||
| Sample 5 | Hubei China | DCM | 31.88 ± 0.14 g | 42.36 ± 2.56 f | 3.33 |
| nBuOH | 74.71 ± 0.93 b | 467.20 ± 10.74 d | 1.82 | ||
| H2O | 14.47 ± 1.46 i | 11.00 ± 0.18 j | 6.63 | ||
| 10.48 |
| Code | Region | Fraction | α-Glucosidase IC50 (μg/mL) | α-Amylase IC50 (μg/mL) |
|---|---|---|---|---|
| Sample 1 | Shandong province | DCM | N.A. | 349.03 ± 2.43 f |
| nBuOH | 80.57 ± 3.96 d | 749.73 ± 15.51 b | ||
| H2O | N.A. | N.A. | ||
| Sample 2 | Guangxi province | DCM | - | 95.96 ± 4.43 g,h |
| nBuOH | 89.20 ± 3.45 c | 626.93 ± 26.04 d | ||
| H2O | N.A. | N.A. | ||
| Sample 3 | Northeast China | DCM | 70.24 ± 4.69 e | 34.69 ± 1.62 i |
| nBuOH | 57.00 ± 3.93 f | 391.07 ± 12.92 e | ||
| H2O | N.A. | N.A. | ||
| Sample 4 | Jiangsu province | DCM | 235.17 ± 0.51 b | 119.43 ± 2.32 g |
| nBuOH | 90.72 ± 2.06 c | 984.27 ± 26.67 a | ||
| H2O | N.A. | N.A. | ||
| Sample 5 | Hubei province | DCM | 302.10 ± 1.82 a | 103.23 ±3.04 g |
| nBuOH | 48.79 ± 2.65 g | 675.13 ± 11.27 c | ||
| H2O | N.A. | N.A. | ||
| Acarbose B | 0.015 ± 0.001 h | 75.48 ± 2.50 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yu, J.; Tu, Q.; Yan, F.; Hu, Z.; Zhang, Y.; Song, C. Antioxidant Capacities and Enzymatic Inhibitory Effects of Different Solvent Fractions and Major Flavones from Celery Seeds Produced in Different Geographic Areas in China. Antioxidants 2022, 11, 1542. https://doi.org/10.3390/antiox11081542
Zhang C, Yu J, Tu Q, Yan F, Hu Z, Zhang Y, Song C. Antioxidant Capacities and Enzymatic Inhibitory Effects of Different Solvent Fractions and Major Flavones from Celery Seeds Produced in Different Geographic Areas in China. Antioxidants. 2022; 11(8):1542. https://doi.org/10.3390/antiox11081542
Chicago/Turabian StyleZhang, Chao, Jing Yu, Qiang Tu, Fu Yan, Zhao Hu, Youming Zhang, and Chun Song. 2022. "Antioxidant Capacities and Enzymatic Inhibitory Effects of Different Solvent Fractions and Major Flavones from Celery Seeds Produced in Different Geographic Areas in China" Antioxidants 11, no. 8: 1542. https://doi.org/10.3390/antiox11081542
APA StyleZhang, C., Yu, J., Tu, Q., Yan, F., Hu, Z., Zhang, Y., & Song, C. (2022). Antioxidant Capacities and Enzymatic Inhibitory Effects of Different Solvent Fractions and Major Flavones from Celery Seeds Produced in Different Geographic Areas in China. Antioxidants, 11(8), 1542. https://doi.org/10.3390/antiox11081542






