A Nano-Liposomal Formulation of Caffeic Acid Phenethyl Ester Modulates Nrf2 and NF-κβ Signaling and Alleviates Experimentally Induced Acute Pancreatitis in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CAPE-Loaded-NL
2.3. Characterization of CAPE-Loaded-NL
2.3.1. Vesicle Size, Polydispersity Index and Zeta Potential
2.3.2. Determination of EE%
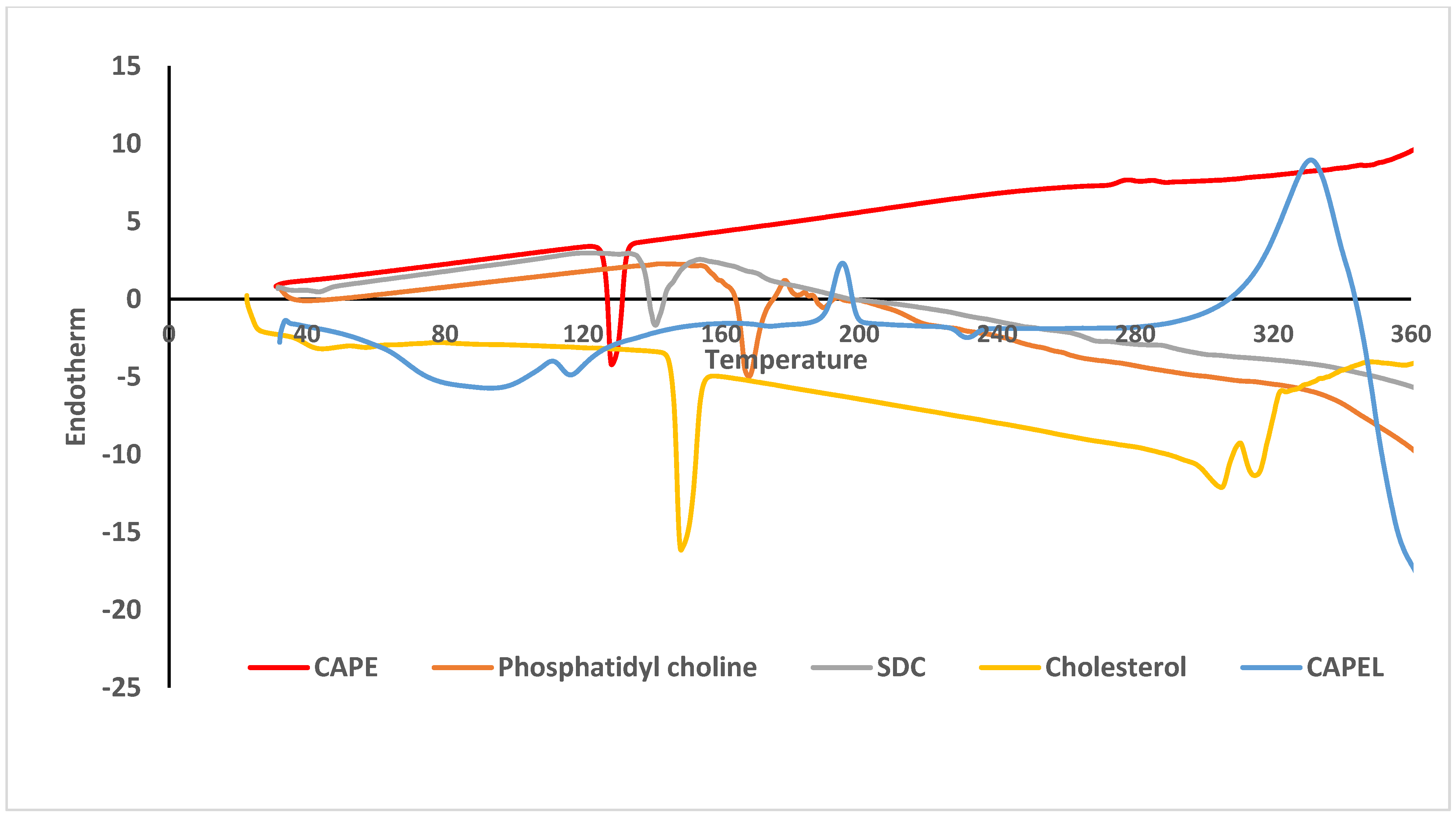
2.3.3. Differential Scanning Calorimetry (DSC)
2.3.4. Morphological Characterization
2.4. In Vivo Studies
2.4.1. Animals
2.4.2. Drug Preparation and Administration
2.4.3. Experimental Design
2.4.4. Blood Sampling and Tissue Preparation
2.4.5. Determination of Biochemical Parameters
Determination of Serum Amylase and Lipase Activities and Insulin Level
Determination of Pancreatic Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2), Heme Oxygenase-1 (HO-1), Tumor Necrosis Factor-α (TNF-α), ADP and ATP Levels
Determination of Pancreatic Myeloperoxidase (MPO) Activity
Determination of Pancreatic Oxidative Stress Biomarkers
Determination of Pancreatic Nitric Oxide (NOx) Content
Determination of Protein Content in Pancreatic Tissue
Histological and Immunohistochemical Examination
2.5. Statistical Analysis
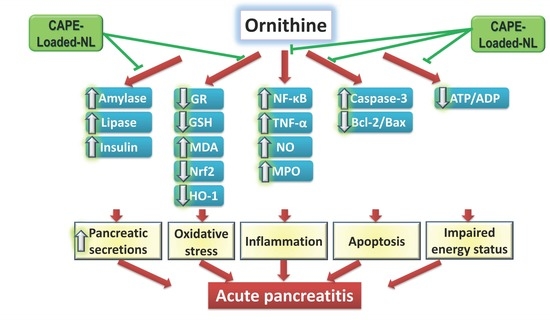
3. Results and Discussion
3.1. Preparation and Optimization of CAPE-Loaded-NL
3.2. Differential Scanning Calorimetry (DSC)
3.3. Morphological Characterization
3.4. In Vivo Studies
3.4.1. Effect of Pretreatment with CAPEL on Biochemical Parameters
Effect on Serum Amylase and Lipase Activities and Insulin Level
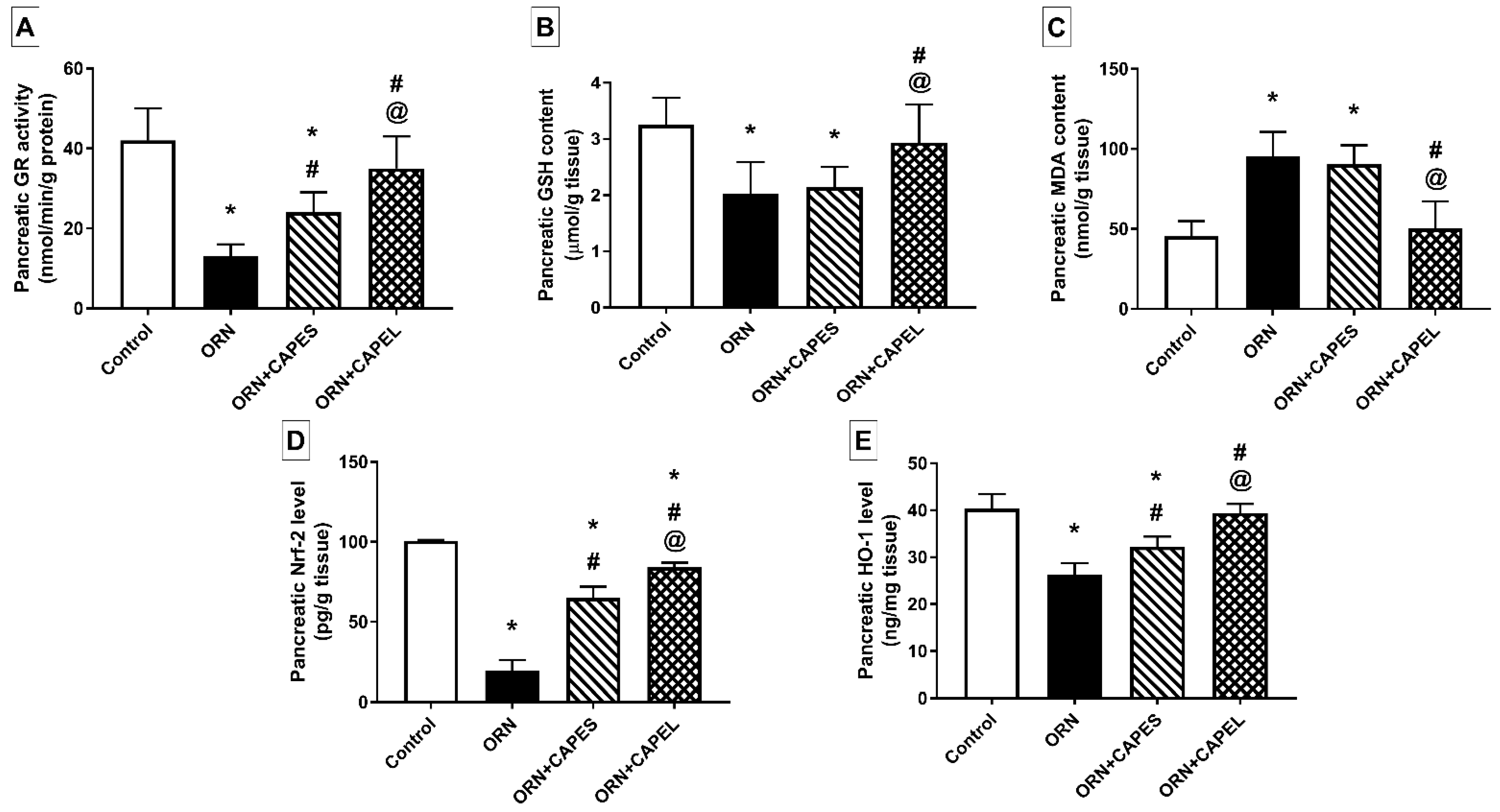
Effect on Pancreatic Oxidative Stress Biomarkers and Nrf2 Signaling
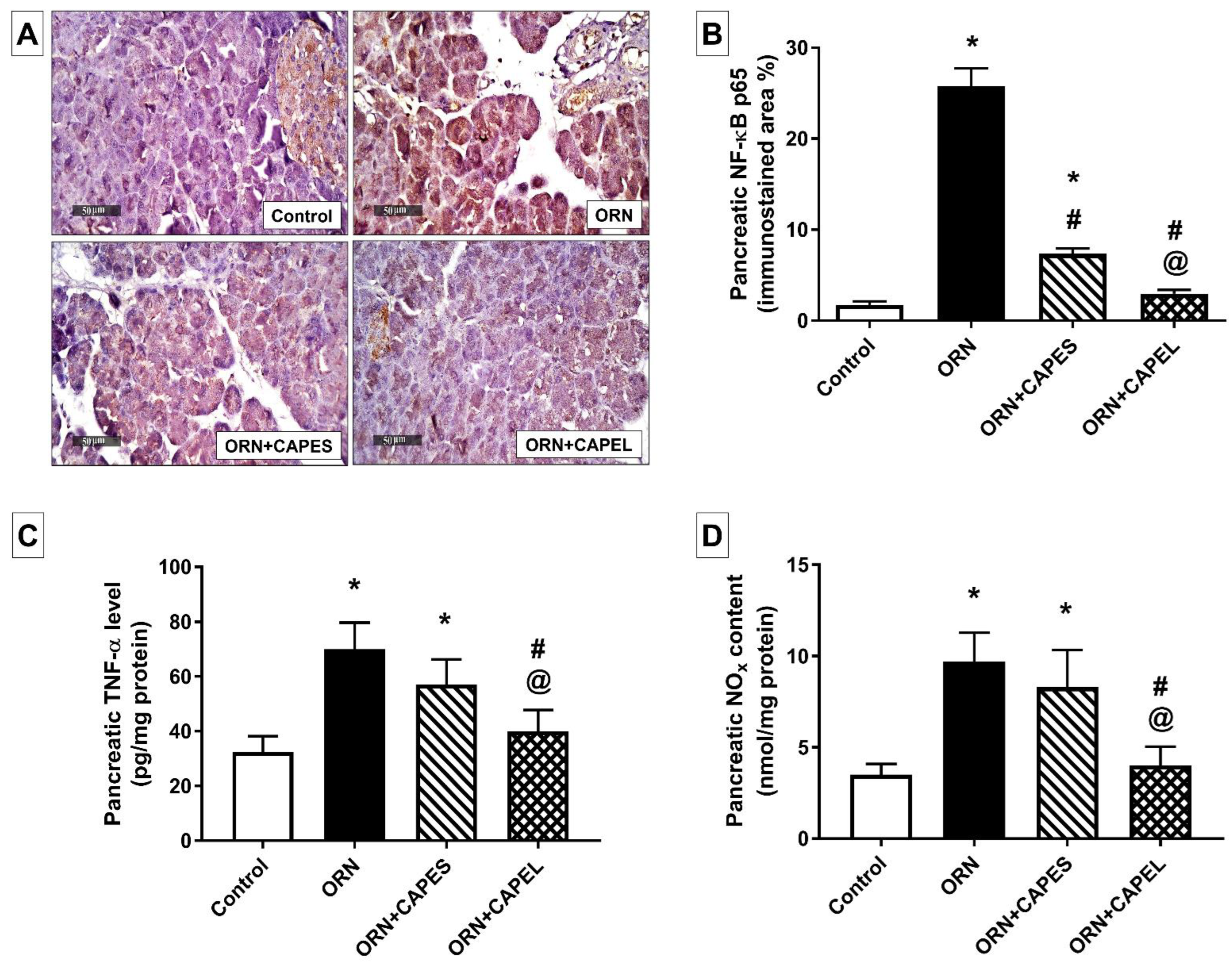
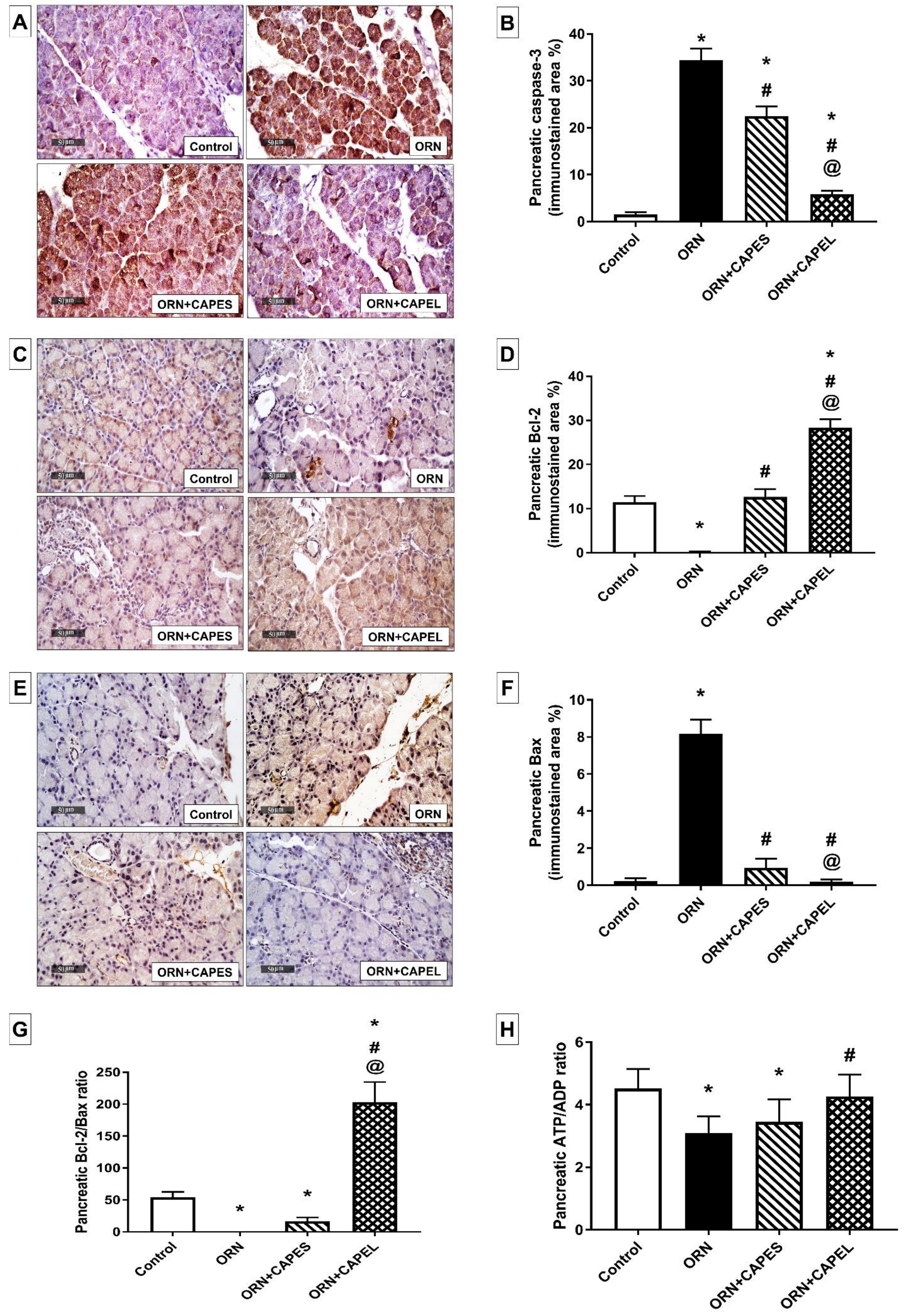
Effect on Pancreatic NF-κB Signaling and Inflammation
Effect on Pancreatic Apoptosis
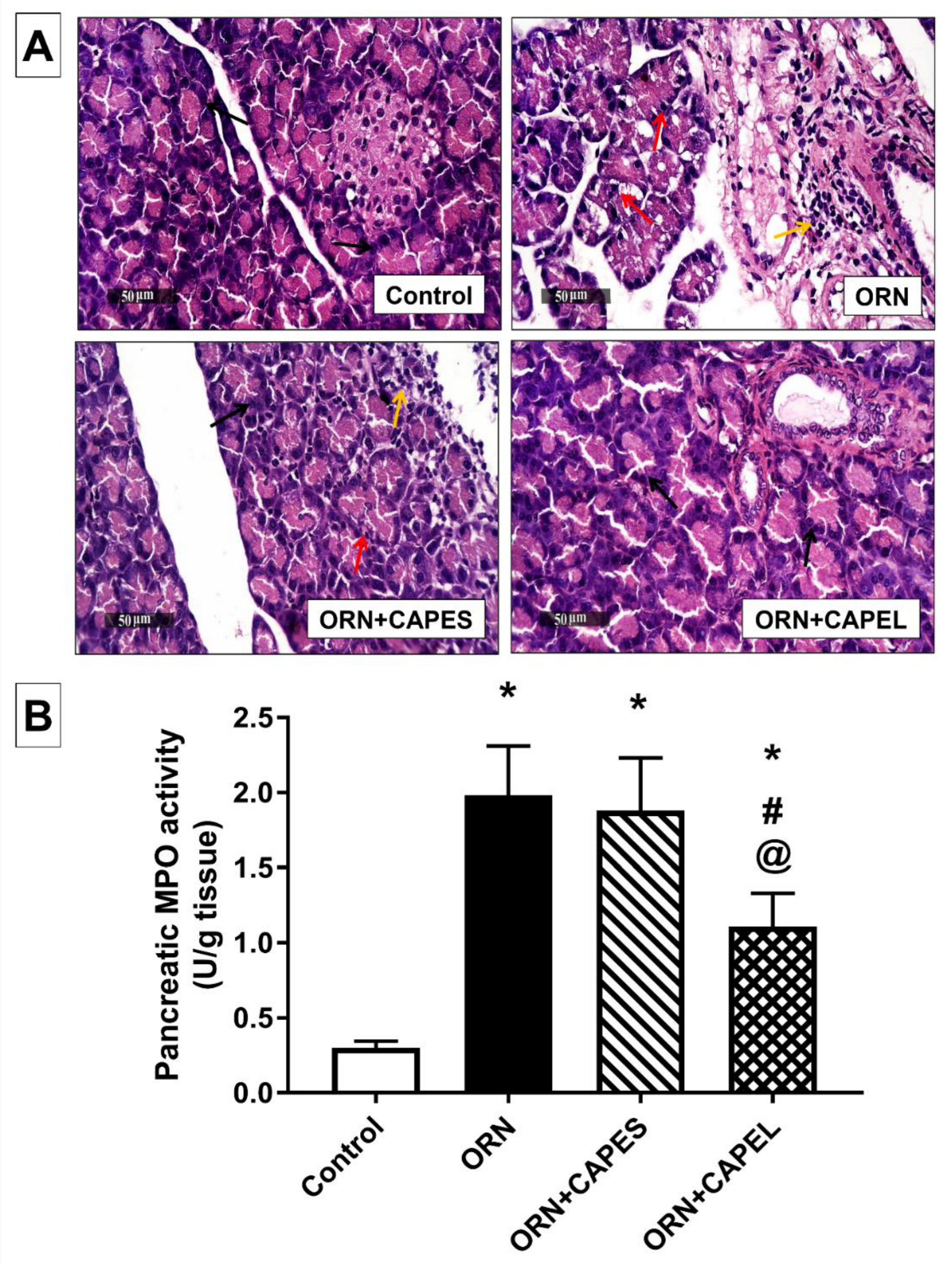
3.4.2. Effect of Pretreatment with CAPEL on Pancreatic Histological Aberrations and Neutrophil Infiltration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, B.U.; Banks, P.A. Clinical Management of Patients with Acute Pancreatitis. Gastroenterology 2013, 144, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.P.; Lam, B.Y.K.; Yip, A.W.C. APACHE System Is Better than Ranson System in the Prediction of Severity of Acute Pancreatitis. Hepatobiliary Pancreat. Dis. Int 2006, 5, 294–299. [Google Scholar]
- Whitcomb, D.C. Clinical Practice. Acute Pancreatitis. N. Engl. J. Med. 2006, 354, 2142–2150. [Google Scholar] [CrossRef]
- Chan, Y.C.; Leung, P.S. Acute Pancreatitis: Animal Models and Recent Advances in Basic Research. Pancreas 2007, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C. Value of Genetic Testing in the Management of Pancreatitis. Gut 2004, 53, 1710–1717. [Google Scholar] [CrossRef]
- Abdelzaher, W.Y.; Ahmed, S.M.; Welson, N.N.; Marraiki, N.; Batiha, G.E.S.; Kamel, M.Y. Vinpocetine Ameliorates L-Arginine Induced Acute Pancreatitis via Sirt1/Nrf2/TNF Pathway and Inhibition of Oxidative Stress, Inflammation, and Apoptosis. Biomed. Pharmacother. 2021, 133, 110976. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Hong, S.-W.; Zheng, H.-M.; Lee, H.-S.; Lee, H.; Lee, D.-H.; Lee, S.Y.; Hong, S.-S. Melatonin Ameliorates Cerulein-Induced Pancreatitis by the Modulation of Nuclear Erythroid 2-Related Factor 2 and Nuclear Factor-KappaB in Rats. J. Pineal Res. 2010, 48, 239–250. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Hasanpourghadi, M.; Esa, N.M. Caffeic Acid Phenethyl Ester Attenuates Dextran Sulfate Sodium-Induced Ulcerative Colitis Through Modulation of NF-ΚB and Cell Adhesion Molecules. Appl. Biochem. Biotechnol. 2022, 194, 1091–1104. [Google Scholar] [CrossRef]
- Rakonczay, Z.; Hegyi, P.; Takács, T.; McCarroll, J.; Saluja, A.K. The Role of NF-KappaB Activation in the Pathogenesis of Acute Pancreatitis. Gut 2008, 57, 259–267. [Google Scholar] [CrossRef]
- Pérez, S.; Pereda, J.; Sabater, L.; Sastre, J. Redox Signaling in Acute Pancreatitis. Redox Biol. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Rakonczay, Z.; Hegyi, P.; Dósa, S.; Iványi, B.; Jármay, K.; Biczó, G.; Hracskó, Z.; Varga, I.S.; Karg, E.; Kaszaki, J.; et al. A New Severe Acute Necrotizing Pancreatitis Model Induced by L-Ornithine in Rats. Crit. Care Med. 2008, 36, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.U.; Niederau, C.; Klonowski-Stumpe, H.; Halangk, W.; Luthen, R.; Lippert, H. Oxidative Stress in Acute Pancreatitis. Hepato-Gastroenterol. 1999, 46, 2736–2750. [Google Scholar]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology Guideline: Management of Acute Pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Freeman, M.L. Practice Parameters Committee of the American College of Gastroenterology Practice Guidelines in Acute Pancreatitis. Am. J. Gastroenterol. 2006, 101, 2379–2400. [Google Scholar] [CrossRef] [PubMed]
- Buyukberber, M.; Savaş, M.C.; Bagci, C.; Koruk, M.; Gulsen, M.T.; Tutar, E.; Bilgic, T.; Ceylan, N.O. Therapeutic Effect of Caffeic Acid Phenethyl Ester on Cerulein-Induced Acute Pancreatitis. World J. Gastroenterol. 2009, 15, 5181–5185. [Google Scholar] [CrossRef]
- Onori, P.; DeMorrow, S.; Gaudio, E.; Franchitto, A.; Mancinelli, R.; Venter, J.; Kopriva, S.; Ueno, Y.; Alvaro, D.; Savage, J.; et al. Caffeic Acid Phenethyl Ester Decreases Cholangiocarcinoma Growth by Inhibition of NF-ΚB and Induction of Apoptosis. Int. J. Cancer 2009, 125, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Yu, C.H.; Chang, Y.P.; Lin, Y.T.; Huang, C.J.; Kuo, Y.H.; Tsai, P.J. Protective Effect of Caffeic Acid Derivatives on Tert-Butyl Hydroperoxide-Induced Oxidative Hepato-Toxicity and Mitochondrial Dysfunction in HepG2 Cells. Molecules 2017, 22, 702. [Google Scholar] [CrossRef]
- Teke, Z.; Bostanci, E.B.; Yenisey, C.; Sacar, M.; Simsek, N.G.; Akoglu, M. Caffeic Acid Phenethyl Ester Alleviates Mesenteric Ischemia/Reperfusion Injury. J. Investig. Surg. 2012, 25, 354–365. [Google Scholar] [CrossRef]
- Yang, N.; Shi, J.-J.; Wu, F.-P.; Li, M.; Zhang, X.; Li, Y.-P.; Zhai, S.; Jia, X.-L.; Dang, S.-S. Caffeic Acid Phenethyl Ester Up-Regulates Antioxidant Levels in Hepatic Stellate Cell Line T6 via an Nrf2-Mediated Mitogen Activated Protein Kinases Pathway Basic Study. World J. Gastroenterol. 2017, 21, 1203–1214. [Google Scholar] [CrossRef]
- Turkyilmaz, S.; Alhan, E.; Ercin, C.; Kural Vanizor, B.; Kaklikkaya, N.; Ates, B.; Erdogan, S.; Topaloglu, S. Effects of Caffeic Acid Phenethyl Ester on Pancreatitis in Rats. J. Surg. Res. 2008, 145, 19–24. [Google Scholar] [CrossRef]
- Celli, N.; Dragani, L.K.; Murzilli, S.; Pagliani, T.; Poggi, A. In Vitro and in Vivo Stability of Caffeic Acid Phenethyl Ester, a Bioactive Compound of Propolis. J. Agric. Food Chem. 2007, 55, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Derman, S. Caffeic Acid Phenethyl Ester Loaded PLGA Nanoparticles: Effect of Various Process Parameters on Reaction Yield, Encapsulation Efficiency, and Particle Size. J. Nanomater. 2015, 16, 318. [Google Scholar] [CrossRef]
- Lee, H.Y.; Il Jeong, Y.; Kim, E.J.; Lee, K.D.; Choi, S.H.; Kim, Y.J.; Kim, D.H.; Choi, K.C. Preparation of Caffeic Acid Phenethyl Ester-Incorporated Nanoparticles and Their Biological Activity. J. Pharm. Sci. 2015, 104, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; Rashed, H.M.; Fayez, H.; Farouk, F.; Shamma, R.N. Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver. Pharmaceutics 2020, 12, 107. [Google Scholar] [CrossRef]
- Ismail, M.F.; Elmeshad, A.N.; Salem, N.A.H. Potential Therapeutic Effect of Nanobased Formulation of Rivastigmine on Rat Model of Alzheimer’s Disease. Int. J. Nanomed. 2013, 8, 393–406. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Lu, H.; Li, S.; Dai, D.; Zhang, Q.; Min, Z.; Yang, C.; Sun, S.; Ye, L.; Teng, C.; Cao, X.; et al. Enhanced Treatment of Cerebral Ischemia–Reperfusion Injury by Intelligent Nanocarriers through the Regulation of Neurovascular Units. Acta Biomater. 2022, 147, 314–326. [Google Scholar] [CrossRef]
- Wei, X.; Dai, J.; Zhong, Y.; Zhang, D.; Liu, L.; Wang, L.; Huang, Y.; Chen, P.; Zhou, Z.; Chen, X.; et al. Caffeic Acid Phenethyl Ester Loaded in Nano-Targeted Delivery System with Casein: Physicochemical Characterization, in Vitro Release, and Binding Mechanisms. LWT 2021, 150, 111938. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–400. [Google Scholar]
- Priyanka, K.; Abdul Hasan Sathali, A. Preparation and Evaluation of Montelukast Sodium Loaded Solid Lipid Nanoparticles. J. Young Pharm. 2012, 4, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Koyu, A.; Gokalp, O.; Gumral, N.; Oktem, F.; Karahan, N.; Yilmaz, N.; Saygin, M. Impact of Caffeic Acid Phenethyl Ester Treatment on Vancomycin-Induced Pancreatic Damage in Rats. Toxicol. Ind. Health 2016, 32, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of Cutaneous Inflammation: Estimation of Neutrophil Content with an Enzyme Marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Mihara, M. Determination of Malonaldehyde Precursor in Tissues by Thiobarbituric Acid Test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Long, W.K.; Carson, P.E. Increased Erythrocyte Glutathione Reductase Activity in Diabetes Mellitus. Biochem. Biophys. Res. Commun. 1961, 5, 394–399. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide Biol. Chem. 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Culling, C.F.A. Handbook of Histopathological and Histochemical Techniques; Elsevier: Amsterdam, The Netherlands, 1974. [Google Scholar]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Cieszkowski, J.; Dembinski, M.; Sendur, R.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Pretreatment with Obestatin Inhibits the Development of Cerulein-Induced Pancreatitis. J. Physiol. Pharmacol. 2009, 60, 95–101. [Google Scholar]
- Elsayed, H.E.; Ebrahim, H.Y.; Mady, M.S.; Khattab, M.A.; El-Sayed, E.K.; Moharram, F.A. Ethnopharmacological Impact of Melaleuca Rugulosa (Link) Craven Leaves Extract on Liver Inflammation. J. Ethnopharmacol. 2022, 292, 115215. [Google Scholar] [CrossRef]
- Shahin, N.N.; El-Nabarawy, N.A.; Gouda, A.S.; Mégarbane, B. The Protective Role of Spermine against Male Reproductive Aberrations Induced by Exposure to Electromagnetic Field—An Experimental Investigation in the Rat. Toxicol. Appl. Pharmacol. 2019, 370, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered Nanoparticles Interacting with Cells: Size Matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Ahmed, I.S.; Campbell, P.; Kondo, T. Enhanced Gene Transfection Using Calcium Phosphate Co-Precipitates and Low-Intensity Pulsed Ultrasound. Eur. J. Pharm. Sci. 2012, 47, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Guinedi, A.S. Liposomes as an Ocular Delivery System for Acetazolamide: In Vitro and in Vivo Studies. AAPS PharmSciTech 2007, 8, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.; Abd El-Alim, S.H.; Shamma, R.N.; Awad, G.E.A. Design and Optimization of Surfactant-Based Nanovesicles for Ocular Delivery of Clotrimazole. J. Liposome Res. 2013, 23, 203–210. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as Particulate Drug Formulations in Therapy. Rationale for Development and What We Can Expect for the Future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Abdel Hady, M.; Awad, G.A.S. Brain Delivery of Olanzapine by Intranasal Administration of Transfersomal Vesicles. J. Liposome Res. 2012, 22, 336–345. [Google Scholar] [CrossRef]
- Nasr, M.; Mansour, S.; Mortada, N.D.; Elshamy, A.A. Vesicular Aceclofenac Systems: A Comparative Study between Liposomes and Niosomes. J. Microencapsul. 2008, 25, 499–512. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan Nanoparticles: Preparation, Size Evolution and Stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Stošić, B.; Janković, R.; Stošić, M.; Marković, D.; Veselinović, I.; Ilić, I.; Sokolović, D. Caffeic Acid Phenethyl Ester Attenuates Changes in Pancreatic Tissue Damage Biomarkers Induced by Cisplatin. Can. J. Physiol. Pharmacol. 2020, 98, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-P.; Guo, W.-Y.; Wang, W.-X.; Zhao, L.; Xiang, M.-W.; Mei, F.-C.; Abliz, A.; Hu, P.; Deng, W.-H.; Yu, J. 4-Phenylbutyric Acid Attenuates Pancreatic Beta-Cell Injury in Rats with Experimental Severe Acute Pancreatitis. Int. J. Endocrinol. 2016, 2016, 4592346. [Google Scholar] [CrossRef] [PubMed]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin Activates the Haem Oxygenase-1 Gene via Regulation of Nrf2 and the Antioxidant-Responsive Element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Stavchansky, S.; Kerwin, S.M.; Bowman, P.D. Structure-Activity Relationships in the Cytoprotective Effect of Caffeic Acid Phenethyl Ester (CAPE) and Fluorinated Derivatives: Effects on Heme Oxygenase-1 Induction and Antioxidant Activities. Eur. J. Pharmacol. 2010, 635, 16–22. [Google Scholar] [CrossRef]
- Danckwardt, S.; Hentze, M.W.; Kulozik, A.E. Pathologies at the Nexus of Blood Coagulation and Inflammation: Thrombin in Hemostasis, Cancer, and Beyond. J. Mol. Med. 2013, 91, 1257–1271. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Dembinski, A.; Warzecha, Z.; Dembinski, M.; Cieszkowski, J.; Rembiasz, K.; Konturek, S.J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Pawlik, W.W. Protective and Therapeutic Effect of Heparin in Acute Pancreatitis. J. Physiol. Pharmacol. 2008, 59 (Suppl. 4), 103–125. [Google Scholar]
- Maduzia, D.; Ceranowicz, P.; Cieszkowski, J.; Chmura, A.; Galazka, K.; Kusnierz-Cabala, B.; Warzecha, Z. Administration of Warfarin Accelerates the Recovery in Ischemia/Reperfusion-Induced Acute Pancreatitis. J. Physiol. Pharmacol. 2020, 71, 417–427. [Google Scholar] [CrossRef]
- Warzecha, Z.; Sendur, P.; Ceranowicz, P.; Cieszkowski, J.; Dembiński, M.; Sendur, R.; Bonior, J.; Jaworek, J.; Ambroży, T.; Olszanecki, R.; et al. Therapeutic Effect of Low Doses of Acenocoumarol in the Course of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats. Int. J. Mol. Sci. 2017, 18, 882. [Google Scholar] [CrossRef]
- Gebhard, C.; Stähli, B.E.; Largiadèr, S.; Holy, E.W.; Akhmedov, A.; Camici, G.G.; Lüscher, T.F.; Tanner, F.C. Caffeic Acid Phenethyl Ester Inhibits Endothelial Tissue Factor Expression. Biol. Pharm. Bull. 2013, 36, 1032–1035. [Google Scholar] [CrossRef][Green Version]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Stachura, J.; Tomaszewska, R.; Konturek, S.J.; Sendur, R.; Dembiński, M.; Pawlik, W.W. Pancreatic Damage and Regeneration in the Course of Ischemia-Reperfusion Induced Pancreatitis in Rats. J. Physiol. Pharmacol. 2001, 52, 221–235. [Google Scholar]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Konturek, S.J.; Tomaszewska, R.; Stachura, J.; Konturek, P.C. IGF-1 Stimulates Production of Interleukin-10 and Inhibits Development of Caerulein-Induced Pancreatitis. J. Physiol. Pharmacol. 2003, 54, 575–590. [Google Scholar] [PubMed]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Konturek, P.C.; Stachura, J.; Konturek, S.J.; Niemiec, J. Protective Effect of Calcitonin Gene-Related Peptide against Caerulein-Induced Pancreatitis in Rats. J. Physiol. Pharmacol. 1997, 48, 775–787. [Google Scholar] [PubMed]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Kuśnierz-Cabala, B.; Naskalski, J.W.; Jaworek, J.; Konturek, S.J.; Pawlik, W.W.; et al. Influence of Ischemic Preconditioning on Blood Coagulation, Fibrinolytic Activity and Pancreatic Repair in the Course of Caerulein-Induced Acute Pancreatitis in Rats. J. Physiol. Pharmacol. 2007, 58, 303–319. [Google Scholar]
- Kim, H. Cerulein Pancreatitis: Oxidative Stress, Inflammation, and Apoptosis. Rev. Gut Liver 2008, 2, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Amodio, R.; De Ruvo, C.; Di Matteo, V.; Poggi, A.; Di Santo, A.; Martelli, N.; Lorenzet, R.; Rotilio, D.; Cacchio, M.; Esposito, E. Caffeic Acid Phenethyl Ester Blocks Apoptosis Induced by Low Potassium in Cerebellar Granule Cells. Int. J. Dev. Neurosci. 2003, 21, 379–389. [Google Scholar] [CrossRef]
- Turan, D.; Abdik, H.; Sahin, F.; Avşar Abdik, E. Evaluation of the Neuroprotective Potential of Caffeic Acid Phenethyl Ester in a Cellular Model of Parkinson’s Disease. Eur. J. Pharmacol. 2020, 883, 173342. [Google Scholar] [CrossRef]
- Schipper, R.G.; Penning, L.C.; Verhofstad, A.A.J. Involvement of Polyamines in Apoptosis. Facts and Controversies: Effectors or Protectors? Semin. Cancer Biol. 2000, 10, 55–68. [Google Scholar] [CrossRef]
- Kaiser, A.M.; Saluja, A.K.; Sengupta, A.; Saluja, M.; Steer, M.L. Relationship between Severity, Necrosis, and Apoptosis in Five Models of Experimental Acute Pancreatitis. Am. J. Physiol. Cell Physiol. 1995, 269, C1295–C1304. [Google Scholar] [CrossRef]
- Meier, R.F.; Beglinger, C. Nutrition in Pancreatic Diseases. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 507–529. [Google Scholar] [CrossRef]
- Siech, M.; Davis, M.A.; Beger, H.G. Different Changes in High-Energy Phosphates in Alcoholic Acute Pancreatitis and Taurocholate Acute Pancreatitis in Rats Using NMR Spectroscopy at 2.0 T. Pancreas 1997, 15, 350–357. [Google Scholar] [CrossRef]
- Ahmed, I.S.; El-Hosary, R.; Shalaby, S.; Abd-Rabo, M.M.; Elkhateeb, D.G.; Nour, S. PD-PK Evaluation of Freeze-Dried Atorvastatin Calcium-Loaded Poly-ε-Caprolactone Nanoparticles. Int. J. Pharm. 2016, 504, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; El Hosary, R.; Hassan, M.A.; Haider, M.; Abd-Rabo, M.M. Efficacy and Safety Profiles of Oral Atorvastatin-Loaded Nanoparticles: Effect of Size Modulation on Biodistribution. Mol. Pharm. 2018, 15, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Nyman, G.; Oldberg Wagner, S.; Prystupa-Chalkidis, K.; Ryberg, K.; Hagvall, L. Contact Allergy in Western Sweden to Propolis of Four Different Origins. Acta Derm. Venereol. 2020, 100, 1–5. [Google Scholar] [CrossRef]
- De Groot, A.C. Propolis: A Review of Properties, Applications, Chemical Composition, Contact Allergy, and Other Adverse Effects. Dermat. Contact Atopic Occup. Drug 2013, 24, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Basista-Sołtys, K. Allergy to Propolis in Beekeepers-A Literature Review. Occup. Med. Health Aff. 2013, 1, 105. [Google Scholar] [CrossRef]
- Kalkan Yıldırım, H.; Canbay, E.; Öztürk, Ş.; Aldemir, O.; Sözmen, E.Y. Biotransformation of Propolis Phenols by L. Plantarum as a Strategy for Reduction of Allergens. Food Sci. Biotechnol. 2018, 27, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Konturek, S.J.; Dembinski, M.; Pawlik, W.W.; Tomaszewska, R.; Stachura, J.; Kusnierz-Cabala, B.; Naskalski, J.W.; et al. Ischemic Preconditioning Inhibits Development of Edematous Cerulein-Induced Pancreatitis: Involvement of Cyclooxygenases and Heat Shock Protein 70. World J. Gastroenterol. 2005, 11, 5958–5965. [Google Scholar] [CrossRef] [PubMed]


| EA | VS (nm) | PDI | EE (%) | ZP (mV) |
|---|---|---|---|---|
| Tween 80 | 476 ± 9 b*,c* | 0.42 ± 0.04 b* | 77.1 ± 1.44 b*,c* | −49.3 ± 3.68 b* |
| SC | 1211 ± 433 a*,c* | 0.90 ± 0.06 a*,c* | 93.5 ± 0.29 a*,c* | −57.05 ± 0.78 a*,c* |
| SDC | 309 ± 54 a*,b* | 0.46 ± 0.12 b* | 86.9 ± 0.98 a*,b* | −47.15 ± 0.78 b* |
| Control | ORN | ORN+CAPES | ORN+CAPEL | |
| Acinar degenerative changes | 0 | 3 | 1 | 0 |
| Congested vasculature | 0 | 1 | 0 | 0 |
| Inflammatory cell infiltration | 0 | 2 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahin, N.N.; Shamma, R.N.; Ahmed, I.S. A Nano-Liposomal Formulation of Caffeic Acid Phenethyl Ester Modulates Nrf2 and NF-κβ Signaling and Alleviates Experimentally Induced Acute Pancreatitis in a Rat Model. Antioxidants 2022, 11, 1536. https://doi.org/10.3390/antiox11081536
Shahin NN, Shamma RN, Ahmed IS. A Nano-Liposomal Formulation of Caffeic Acid Phenethyl Ester Modulates Nrf2 and NF-κβ Signaling and Alleviates Experimentally Induced Acute Pancreatitis in a Rat Model. Antioxidants. 2022; 11(8):1536. https://doi.org/10.3390/antiox11081536
Chicago/Turabian StyleShahin, Nancy Nabil, Rehab Nabil Shamma, and Iman Saad Ahmed. 2022. "A Nano-Liposomal Formulation of Caffeic Acid Phenethyl Ester Modulates Nrf2 and NF-κβ Signaling and Alleviates Experimentally Induced Acute Pancreatitis in a Rat Model" Antioxidants 11, no. 8: 1536. https://doi.org/10.3390/antiox11081536
APA StyleShahin, N. N., Shamma, R. N., & Ahmed, I. S. (2022). A Nano-Liposomal Formulation of Caffeic Acid Phenethyl Ester Modulates Nrf2 and NF-κβ Signaling and Alleviates Experimentally Induced Acute Pancreatitis in a Rat Model. Antioxidants, 11(8), 1536. https://doi.org/10.3390/antiox11081536