Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis
Abstract
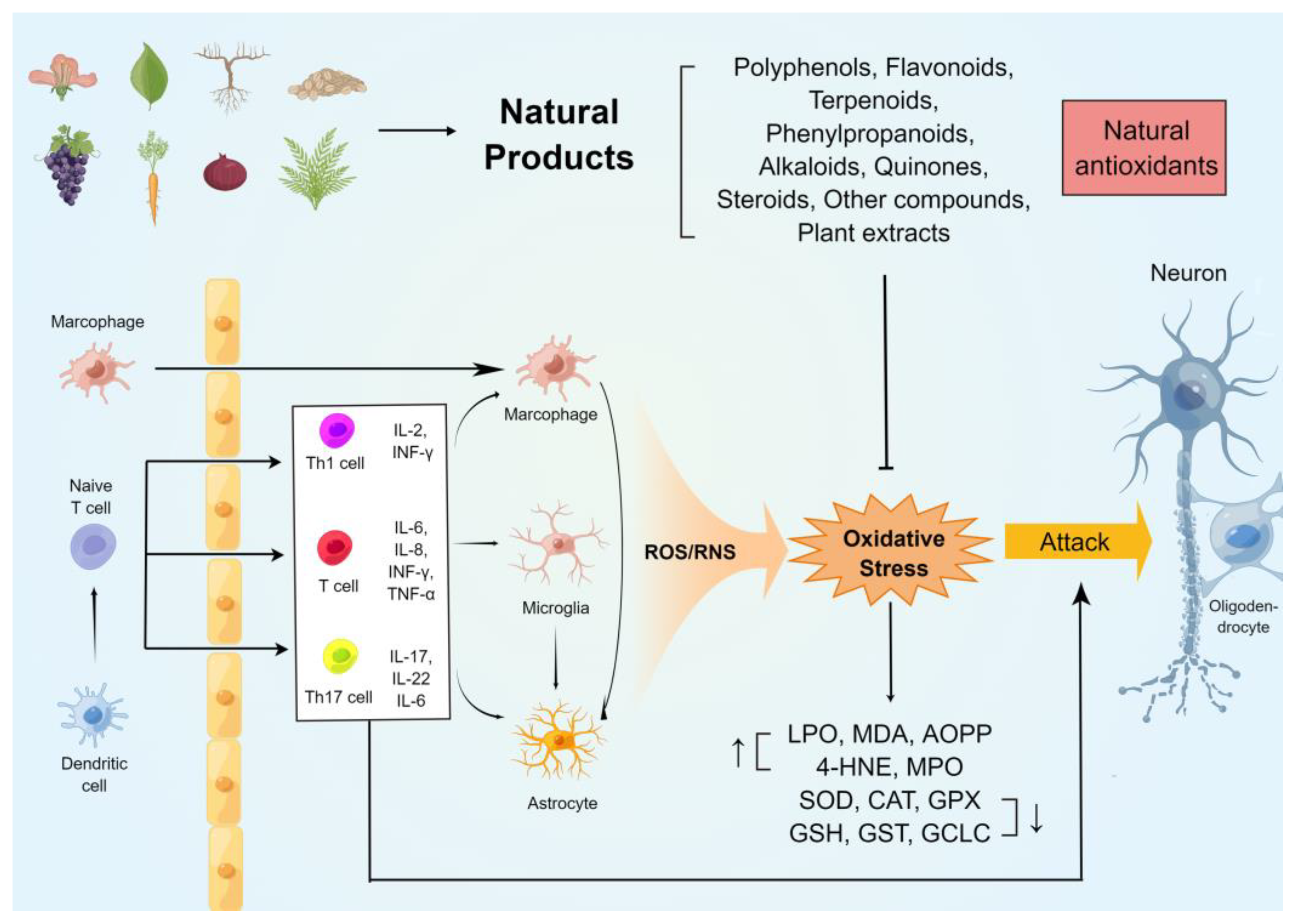
:1. Introduction
2. Oxidative Stress in the CNS of Patients with MS
3. Oxidative Stress in Animal Models of MS
4. Natural Compounds against Oxidative Stress in Animal Models of MS
4.1. Natural Phenolic Compounds
4.1.1. Flavonoids
4.1.2. Non-Flavonoids
4.2. Terpenoids
4.3. Phenylpropanoids
4.4. Alkaloids
4.5. Quinones
4.6. Steroids
4.7. Other Compounds
4.8. Plant Extracts
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Attfield, K.E.; Jensen, L.T.; Kaufmann, M.; Friese, M.A.; Fugger, L. The immunology of multiple sclerosis. Nat. Rev. Immunol. 2022, 1–17. [Google Scholar]
- Martin, D.; Pantoja, C.; Fernandez, M.A.; Valdes-Quezada, C.; Molto, E.; Matesanz, F.; Bogdanovic, O.; de la Calle-Mustienes, E.; Dominguez, O.; Taher, L.; et al. Genome-wide CTCF distribution in vertebrates defines equivalent sites that aid the identification of disease-associated genes. Nat. Struct. Mol. Biol. 2011, 18, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Ribbons, K.A.; Mcelduff, P.; Boz, C.; Trojano, M.; Izquierdo, G.; Duquette, P.; Girard, M.; Grand’Maison, F.; Hupperts, R.; Grammond, P.; et al. Male sex is independently associated with faster disability accumulation in Relapse-Onset MS but not in primary progressive MS. PLoS ONE 2015, 10, e122686. [Google Scholar]
- Correale, J.; Farez, M.F. The role of astrocytes in multiple sclerosis progression. Front. Neurol. 2015, 6, 180. [Google Scholar] [CrossRef]
- Simkins, T.J.; Duncan, G.J.; Bourdette, D. Chronic demyelination and axonal degeneration in multiple sclerosis: Pathogenesis and therapeutic implications. Curr. Neurol. Neurosci. Rep. 2021, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Ratchford, J.N.; Brock-Simmons, R.; Augsburger, A.; Steele, S.U.; Mohn, K.; Rhone, M.; Bo, J.; Costello, K. Multiple sclerosis symptom recrudescence at the end of the natalizumab dosing cycle. Int. J. MS Care 2014, 16, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissy, A.R.; Cohen, J.A. Multiple sclerosis symptom management. Expert Rev. Neurother. 2007, 7, 1213–1222. [Google Scholar] [CrossRef]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, M.; Andersen, O.; Runmarker, B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult. Scler. 2003, 9, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2017, 17, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Chakrabarty, B.; Kumar, A.; Jain, P.; Patel, H.; Saini, L. Acquired demyelinating disorders of central nervous system: A pediatric cohort. Ann. Indian Acad. Neurol. 2015, 18, S48–S55. [Google Scholar] [CrossRef]
- Lassmann, H. Multiple sclerosis pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [Green Version]
- Voet, S.; Prinz, M.; van Loo, G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte crosstalk in CNS inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Tobore, T.O. Oxidative/Nitroxidative stress and multiple sclerosis. J. Mol. Neurosci. 2021, 71, 506–514. [Google Scholar] [CrossRef]
- Pegoretti, V.; Swanson, K.A.; Bethea, J.R.; Probert, L.; Eisel, U.; Fischer, R. Inflammation and oxidative stress in multiple sclerosis: Consequences for therapy development. Oxid. Med. Cell. Longev. 2020, 2020, 7191080. [Google Scholar] [CrossRef]
- Varas, R.; Ortiz, F.C. Neuroinflammation in demyelinating diseases: Oxidative stress as a modulator of glial Cross-Talk. Curr Pharm Des. 2019, 25, 4755–4762. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Hoftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, M.E.; Mahad, D.J.; Lassmann, H.; van Horssen, J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol. Med. 2014, 20, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Acar, A.; Ugur, C.M.; Evliyaoglu, O.; Uzar, E.; Tamam, Y.; Arikanoglu, A.; Yucel, Y.; Varol, S.; Onder, H.; Tasdemir, N. Evaluation of serum oxidant/antioxidant balance in multiple sclerosis. Acta Neurol. Belg. 2012, 112, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pedro, N.; Magana-Maldonado, R.; Ramiro, A.S.; Perez-De, L.C.V.; Rangel-Lopez, E.; Sotelo, J.; Pineda, B. PAMP-DAMPs interactions mediates development and progression of multiple sclerosis. Front. Biosci. 2016, 8, 13–28. [Google Scholar]
- Kumar, V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef]
- Milatovic, D.; Zaja-Milatovic, S.; Gupta, R.C.; Yu, Y.; Aschner, M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol. Appl. Pharmacol. 2009, 240, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonsette, R.E. Neurodegeneration in multiple sclerosis: The role of oxidative stress and excitotoxicity. J. Neurol. Sci. 2008, 274, 48–53. [Google Scholar] [CrossRef]
- Signorile, A.; Ferretta, A.; Ruggieri, M.; Paolicelli, D.; Lattanzio, P.; Trojano, M.; De Rasmo, D. Mitochondria, oxidative stress, cAMP signalling and apoptosis: A crossroads in lymphocytes of multiple sclerosis, a possible role of nutraceutics. Antioxidants 2020, 10, 21. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic. Biol. Med. 2016, 100, 81–85. [Google Scholar] [CrossRef]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-mediated astrocyte-neuron signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef]
- Sun, G.Y.; He, Y.; Chuang, D.Y.; Lee, J.C.; Gu, Z.; Simonyi, A.; Sun, A.Y. Integrating cytosolic phospholipase A(2) with oxidative/nitrosative signaling pathways in neurons: A novel therapeutic strategy for AD. Mol. Neurobiol. 2012, 46, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofroniew, M.V. Reactive astrocytes in neural repair and protection. Neuroscientist 2005, 11, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Dawson, T.M.; Wesselingh, S.; Mork, S.; Choi, S.; Kong, P.A.; Hanley, D.; Trapp, B.D. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann. Neurol. 1994, 36, 778–786. [Google Scholar] [CrossRef]
- Cross, A.H.; Manning, P.T.; Stern, M.K.; Misko, T.P. Evidence for the production of peroxynitrite in inflammatory CNS demyelination. J. Neuroimmunol. 1997, 80, 121–130. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Qian, L.; O’Callaghan, J.P.; Hong, J.S. Astrogliosis in CNS pathologies: Is there a role for microglia? Mol. Neurobiol. 2010, 41, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Campbell, G.R.; Ziabreva, I.; Reeve, A.K.; Krishnan, K.J.; Reynolds, R.; Howell, O.; Lassmann, H.; Turnbull, D.M.; Mahad, D.J. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann. Neurol. 2011, 69, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Bordt, E.A.; Polster, B.M. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, M.; Hojati, Z.; Dehghanian, F. The molecular study of IFNbeta pleiotropic roles in MS treatment. Iran J. Neurol. 2013, 12, 149–156. [Google Scholar]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [Green Version]
- Herber, D.L.; Maloney, J.L.; Roth, L.M.; Freeman, M.J.; Morgan, D.; Gordon, M.N. Diverse microglial responses after intrahippocampal administration of lipopolysaccharide. Glia 2006, 53, 382–391. [Google Scholar] [CrossRef]
- Liu, B.; Gao, H.M.; Wang, J.Y.; Jeohn, G.H.; Cooper, C.L.; Hong, J.S. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann. N. Y. Acad. Sci. 2002, 962, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.R.; Mccredie, R.; Jessup, W.; Robinson, J.; Sullivan, D.; Celermajer, D.S. Oral L-arginine improves endothelium-dependent dilatation and reduces monocyte adhesion to endothelial cells in young men with coronary artery disease. Atherosclerosis 1997, 129, 261–269. [Google Scholar] [CrossRef]
- Wiesinger, H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog. Neurobiol. 2001, 64, 365–391. [Google Scholar] [CrossRef]
- Prinz, M.; Garbe, F.; Schmidt, H.; Mildner, A.; Gutcher, I.; Wolter, K.; Piesche, M.; Schroers, R.; Weiss, E.; Kirschning, C.J.; et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Investig. 2006, 116, 456–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soulika, A.M.; Lee, E.; Mccauley, E.; Miers, L.; Bannerman, P.; Pleasure, D. Initiation and progression of axonopathy in experimental autoimmune encephalomyelitis. J. Neurosci. 2009, 29, 14965–14979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Sackett, S.; Zhang, Y. Endocannabinoid modulation of microglial phenotypes in neuropathology. Front. Neurol. 2020, 11, 87. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Pacheco-Moises, F.P.; Bitzer-Quintero, O.K.; Ramirez-Anguiano, A.C.; Flores-Alvarado, L.J.; Ramirez-Ramirez, V.; Macias-Islas, M.A.; Torres-Sanchez, E.D. Immunology and oxidative stress in multiple sclerosis: Clinical and basic approach. Clin. Dev. Immunol. 2013, 2013, 708659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- O’Gorman, C.; Lucas, R.; Taylor, B. Environmental risk factors for multiple sclerosis: A review with a focus on molecular mechanisms. Int. J. Mol. Sci. 2012, 13, 11718–11752. [Google Scholar] [CrossRef] [Green Version]
- Giacci, M.K.; Bartlett, C.A.; Smith, N.M.; Iyer, K.S.; Toomey, L.M.; Jiang, H.; Guagliardo, P.; Kilburn, M.R.; Fitzgerald, M. Oligodendroglia are particularly vulnerable to oxidative damage after neurotrauma in vivo. J. Neurosci. 2018, 38, 6491–6504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.R.; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell. Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef]
- Miller, A.F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Juurlink, B.H.; Thorburne, S.K.; Hertz, L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia 1998, 22, 371–378. [Google Scholar] [CrossRef]
- Schulz, K.; Vulpe, C.D.; Harris, L.Z.; David, S. Iron efflux from oligodendrocytes is differentially regulated in gray and white matter. J. Neurosci. 2011, 31, 13301–13311. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Rone, M.B.; Cui, Q.L.; Fang, J.; Wang, L.C.; Zhang, J.; Khan, D.; Bedard, M.; Almazan, G.; Ludwin, S.K.; Jones, R.; et al. Oligodendrogliopathy in multiple sclerosis: Low glycolytic metabolic rate promotes oligodendrocyte survival. J. Neurosci. 2016, 36, 4698–4707. [Google Scholar] [CrossRef] [Green Version]
- Scott, G.S.; Virag, L.; Szabo, C.; Hooper, D.C. Peroxynitrite-induced oligodendrocyte toxicity is not dependent on poly(ADP-ribose) polymerase activation. Glia 2003, 41, 105–116. [Google Scholar] [CrossRef]
- Jezek, P.; Hlavata, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef]
- Pineda-Torra, I.; Siddique, S.; Waddington, K.E.; Farrell, R.; Jury, E.C. Disrupted lipid metabolism in multiple sclerosis: A role for liver x receptors? Front. Endocrinol. 2021, 12, 639757. [Google Scholar] [CrossRef]
- Kostic, M.; Zivkovic, N.; Stojanovic, I. Multiple sclerosis and glutamate excitotoxicity. Rev. Neurosci. 2013, 24, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Ramdial, K.; Franco, M.C.; Estevez, A.G. Cellular mechanisms of peroxynitrite-induced neuronal death. Brain Res. Bull. 2017, 133, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.H.; Manning, P.T.; Keeling, R.M.; Schmidt, R.E.; Misko, T.P. Peroxynitrite formation within the central nervous system in active multiple sclerosis. J. Neuroimmunol. 1998, 88, 45–56. [Google Scholar] [CrossRef]
- Jack, C.; Antel, J.; Bruck, W.; Kuhlmann, T. Contrasting potential of nitric oxide and peroxynitrite to mediate oligodendrocyte injury in multiple sclerosis. Glia 2007, 55, 926–934. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, R.T.; Machado-Vieira, R.; Zarate, C.J.; Manji, H.K. Targeting mitochondrially mediated plasticity to develop improved therapeutics for bipolar disorder. Expert Opin. Ther. Targets 2014, 18, 1131–1147. [Google Scholar] [CrossRef] [Green Version]
- Holzmeister, C.; Gaupels, F.; Geerlof, A.; Sarioglu, H.; Sattler, M.; Durner, J.; Lindermayr, C. Differential inhibition of Arabidopsis superoxide dismutases by peroxynitrite-mediated tyrosine nitration. J. Exp. Bot. 2015, 66, 989–999. [Google Scholar] [CrossRef] [Green Version]
- Jandy, M.; Noor, A.; Nelson, P.; Dennys, C.N.; Karabinas, I.M.; Pestoni, J.C.; Singh, G.D.; Luc, L.; Devyldere, R.; Perdomo, N.; et al. Peroxynitrite nitration of Tyr 56 in Hsp90 induces PC12 cell death through P2X7R-dependent PTEN activation. Redox Biol. 2022, 50, 102247. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, J.; Jimenez, D.A.; Levitan, E.S.; Aizenman, E.; Rosenberg, P.A. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J. Neurosci. 2004, 24, 10616–10627. [Google Scholar] [CrossRef] [Green Version]
- Dutta, R.; Mcdonough, J.; Yin, X.; Peterson, J.; Chang, A.; Torres, T.; Gudz, T.; Macklin, W.B.; Lewis, D.A.; Fox, R.J.; et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 2006, 59, 478–489. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef] [Green Version]
- Pandit, A.; Vadnal, J.; Houston, S.; Freeman, E.; Mcdonough, J. Impaired regulation of electron transport chain subunit genes by nuclear respiratory factor 2 in multiple sclerosis. J. Neurol. Sci. 2009, 279, 14–20. [Google Scholar] [CrossRef]
- Huang, J.B.; Hsu, S.P.; Pan, H.Y.; Chen, S.D.; Chen, S.F.; Lin, T.K.; Liu, X.P.; Li, J.H.; Chen, N.C.; Liou, C.W.; et al. Peroxisome Proliferator-Activated receptor gamma coactivator 1alpha activates vascular endothelial growth factor that protects against neuronal cell death following status epilepticus through PI3K/AKT and MEK/ERK signaling. Int. J. Mol. Sci. 2020, 21, 7247. [Google Scholar] [CrossRef]
- Witte, M.E.; Nijland, P.G.; Drexhage, J.A.; Gerritsen, W.; Geerts, D.; van Het, H.B.; Reijerkerk, A.; de Vries, H.E.; van der Valk, P.; van Horssen, J. Reduced expression of PGC-1alpha partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol. 2013, 125, 231–243. [Google Scholar] [CrossRef]
- Xiao, J.; Dong, X.; Peng, K.; Ye, F.; Cheng, J.; Dan, G.; Zou, Z.; Cao, J.; Sai, Y. PGC-1a Mediated-EXOG, a specific repair enzyme for mitochondrial DNA, plays an essential role in the Rotenone-Induced neurotoxicity of PC12 cells. J. Mol. Neurosci. 2021, 71, 2336–2352. [Google Scholar] [CrossRef]
- Hofer, A.; Noe, N.; Tischner, C.; Kladt, N.; Lellek, V.; Schauss, A.; Wenz, T. Defining the action spectrum of potential PGC-1alpha activators on a mitochondrial and cellular level in vivo. Hum. Mol. Genet. 2014, 23, 2400–2415. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.D.; Yang, D.I.; Lin, T.K.; Shaw, F.Z.; Liou, C.W.; Chuang, Y.C. Roles of oxidative stress, apoptosis, PGC-1alpha and mitochondrial biogenesis in cerebral ischemia. Int. J. Mol. Sci. 2011, 12, 7199–7215. [Google Scholar] [CrossRef] [Green Version]
- Kurte, M.; Luz-Crawford, P.; Vega-Letter, A.M.; Contreras, R.A.; Tejedor, G.; Elizondo-Vega, R.; Martinez-Viola, L.; Fernandez-O’Ryan, C.; Figueroa, F.E.; Jorgensen, C.; et al. IL17/IL17RA as a novel signaling axis driving mesenchymal stem cell therapeutic function in experimental autoimmune encephalomyelitis. Front. Immunol. 2018, 9, 802. [Google Scholar] [CrossRef]
- Nissen, J.C.; Tsirka, S.E. Tuftsin-driven experimental autoimmune encephalomyelitis recovery requires neuropilin-1. Glia 2016, 64, 923–936. [Google Scholar] [CrossRef] [Green Version]
- Aulova, K.S.; Urusov, A.A.; Sedykh, S.E.; Toporkova, L.B.; Lopatnikova, J.A.; Buneva, V.N.; Sennikov, S.V.; Budde, T.; Meuth, S.G.; Popova, N.A.; et al. The association between EAE development in mice and the production of autoantibodies and abzymes after immunization of mice with different antigens. J. Cell. Mol. Med. 2021, 25, 2493–2504. [Google Scholar] [CrossRef]
- Giralt, M.; Molinero, A.; Hidalgo, J. Active induction of experimental autoimmune encephalomyelitis (EAE) with MOG35-55 in the mouse. Methods Mol. Biol. 2018, 1791, 227–232. [Google Scholar]
- Marta, M.; Meier, U.C.; Lobell, A. Regulation of autoimmune encephalomyelitis by toll-like receptors. Autoimmun. Rev. 2009, 8, 506–509. [Google Scholar] [CrossRef]
- Lee, S.Y.; Goverman, J.M. The influence of T cell Ig mucin-3 signaling on central nervous system autoimmune disease is determined by the effector function of the pathogenic T cells. J. Immunol. 2013, 190, 4991–4999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libbey, J.E.; Fujinami, R.S. Experimental autoimmune encephalomyelitis as a testing paradigm for adjuvants and vaccines. Vaccine 2011, 29, 3356–3362. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.; Amor, S. Experimental autoimmune encephalomyelitis is a good model of multiple sclerosis if used wisely. Mult. Scler. Relat. Disord. 2014, 3, 555–564. [Google Scholar] [CrossRef]
- Glatigny, S.; Bettelli, E. Experimental autoimmune encephalomyelitis (EAE) as animal models of multiple sclerosis (MS). Cold Spring Harb. Perspect. Med. 2018, 8, a028977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasrado, N.; Jia, T.; Massilamany, C.; Franco, R.; Illes, Z.; Reddy, J. Mechanisms of sex hormones in autoimmunity: Focus on EAE. Biol. Sex Differ. 2020, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Mix, E.; Meyer-Rienecker, H.; Hartung, H.P.; Zettl, U.K. Animal models of multiple sclerosis--potentials and limitations. Prog. Neurobiol. 2010, 92, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, I.; Kuang, L.Q.; Theil, D.J.; Fujinami, R.S. Antibody association with a novel model for primary progressive multiple sclerosis: Induction of relapsing-remitting and progressive forms of EAE in H2s mouse strains. Brain Pathol. 2000, 10, 402–418. [Google Scholar] [CrossRef]
- Lee, M.J.; Jang, M.; Choi, J.; Chang, B.S.; Kim, D.Y.; Kim, S.H.; Kwak, Y.S.; Oh, S.; Lee, J.H.; Chang, B.J.; et al. Korean red ginseng and Ginsenoside-Rb1/-Rg1 alleviate experimental autoimmune encephalomyelitis by suppressing th1 and th17 cells and upregulating regulatory t cells. Mol. Neurobiol. 2016, 53, 1977–2002. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. Protective effects of pharmacological therapies in animal models of multiple sclerosis: A review of studies 2014–2019. Neural. Regen. Res. 2020, 15, 1220–1234. [Google Scholar]
- Lutz, N.W.; Fernandez, C.; Pellissier, J.F.; Cozzone, P.J.; Beraud, E. Cerebral biochemical pathways in experimental autoimmune encephalomyelitis and adjuvant arthritis: A comparative metabolomic study. PLoS ONE 2013, 8, e56101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brambilla, R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2019, 137, 757–783. [Google Scholar] [CrossRef] [PubMed]
- Hotta-Iwamura, C.; Benck, C.; Coley, W.D.; Liu, Y.; Zhao, Y.; Quiel, J.A.; Tarbell, K.V. Low CD25 on autoreactive Tregs impairs tolerance via low dose IL-2 and antigen delivery. J. Autoimmun. 2018, 90, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Alissafi, T.; Kalafati, L.; Lazari, M.; Filia, A.; Kloukina, I.; Manifava, M.; Lim, J.H.; Alexaki, V.I.; Ktistakis, N.T.; Doskas, T.; et al. Mitochondrial oxidative damage underlies regulatory t cell defects in autoimmunity. Cell Metab. 2020, 32, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Hasseldam, H.; Rasmussen, R.S.; Johansen, F.F. Oxidative damage and chemokine production dominate days before immune cell infiltration and EAE disease debut. J. Neuroinflamm. 2016, 13, 246. [Google Scholar] [CrossRef] [Green Version]
- Perianes-Cachero, A.; Lobo, M.; Hernandez-Pinto, A.M.; Busto, R.; Lasuncion-Ripa, M.A.; Arilla-Ferreiro, E.; Puebla-Jimenez, L. Oxidative stress and lymphocyte alterations in chronic relapsing experimental allergic encephalomyelitis in the rat hippocampus and protective effects of an ethanolamine phosphate salt. Mol. Neurobiol. 2020, 57, 860–878. [Google Scholar] [CrossRef] [Green Version]
- Radbruch, H.; Bremer, D.; Guenther, R.; Cseresnyes, Z.; Lindquist, R.; Hauser, A.E.; Niesner, R. Ongoing oxidative stress causes subclinical neuronal dysfunction in the recovery phase of EAE. Front. Immunol. 2016, 7, 92. [Google Scholar] [CrossRef] [Green Version]
- Gudi, V.; Gingele, S.; Skripuletz, T.; Stangel, M. Glial response during cuprizone-induced de- and remyelination in the CNS: Lessons learned. Front. Cell. Neurosci. 2014, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Praet, J.; Guglielmetti, C.; Berneman, Z.; Van der Linden, A.; Ponsaerts, P. Cellular and molecular neuropathology of the cuprizone mouse model: Clinical relevance for multiple sclerosis. Neurosci Biobehav Rev. 2014, 47, 485–505. [Google Scholar] [CrossRef] [Green Version]
- Hochstrasser, T.; Exner, G.L.; Nyamoya, S.; Schmitz, C.; Kipp, M. Cuprizone-Containing pellets are less potent to induce consistent demyelination in the corpus callosum of C57BL/6 mice. J. Mol. Neurosci. 2017, 61, 617–624. [Google Scholar] [CrossRef]
- Matsushima, G.K.; Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001, 11, 107–116. [Google Scholar] [CrossRef]
- Harsan, L.A.; Steibel, J.; Zaremba, A.; Agin, A.; Sapin, R.; Poulet, P.; Guignard, B.; Parizel, N.; Grucker, D.; Boehm, N.; et al. Recovery from chronic demyelination by thyroid hormone therapy: Myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J. Neurosci. 2008, 28, 14189–14201. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Ghoumari, A.M.; Bielecki, B.; Steibel, J.; Boehm, N.; Liere, P.; Macklin, W.B.; Kumar, N.; Habert, R.; Mhaouty-Kodja, S.; et al. The neural androgen receptor: A therapeutic target for myelin repair in chronic demyelination. Brain 2013, 136, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Zirngibl, M.; Assinck, P.; Sizov, A.; Caprariello, A.V.; Plemel, J.R. Oligodendrocyte death and myelin loss in the cuprizone model: An updated overview of the intrinsic and extrinsic causes of cuprizone demyelination. Mol. Neurodegener. 2022, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Biancotti, J.C.; Kumar, S.; de Vellis, J. Activation of inflammatory response by a combination of growth factors in cuprizone-induced demyelinated brain leads to myelin repair. Neurochem. Res. 2008, 33, 2615–2628. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, H.J.; Li, X.M. Differential effects of antipsychotics on the development of rat oligodendrocyte precursor cells exposed to cuprizone. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 121–129. [Google Scholar] [CrossRef]
- Cha, M.H.; Lee, S.M.; Jung, J. Lysophosphatidylcholine induces expression of genes involved in cholesterol biosynthesis in THP-1 derived macrophages. Steroids 2018, 139, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Bergholt, M.S.; Serio, A.; Mckenzie, J.S.; Boyd, A.; Soares, R.F.; Tillner, J.; Chiappini, C.; Wu, V.; Dannhorn, A.; Takats, Z.; et al. Correlated heterospectral lipidomics for biomolecular profiling of remyelination in multiple sclerosis. ACS Cent. Sci. 2018, 4, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Burrows, D.J.; Mcgown, A.; Jain, S.A.; De Felice, M.; Ramesh, T.M.; Sharrack, B.; Majid, A. Animal models of multiple sclerosis: From rodents to zebrafish. Mult. Scler. 2019, 25, 306–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Kotter, M.R. Experimental demyelination and remyelination of murine spinal cord by focal injection of lysolecithin. Methods Mol. Biol. 2018, 1791, 233–241. [Google Scholar] [PubMed]
- Zhang, M.; Wang, J.; Zhang, K.; Lu, G.; Liu, Y.; Ren, K.; Wang, W.; Xin, D.; Xu, L.; Mao, H.; et al. Ten-eleven translocation 1 mediated-DNA hydroxymethylation is required for myelination and remyelination in the mouse brain. Nat. Commun. 2021, 12, 5091. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; De Rosa, V.; Pucino, V.; Formisano, L.; Matarese, G. Animal models of multiple sclerosis. Eur. J. Pharmacol. 2015, 759, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Kalyvas, A.; David, S. Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron 2004, 41, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Tarbali, S.; Khezri, S. Vitamin D3 attenuates oxidative stress and cognitive deficits in a model of toxic demyelination. Iran. J. Basic Med. Sci. 2016, 19, 80–88. [Google Scholar]
- Tian, C.; Li, S.; He, L.; Han, X.; Tang, F.; Huang, R.; Lin, Z.; Deng, S.; Xu, J.; Huang, H.; et al. Transient receptor potential ankyrin 1 contributes to the lysophosphatidylcholine-induced oxidative stress and cytotoxicity in OLN-93 oligodendrocyte. Cell Stress Chaperones 2020, 25, 955–968. [Google Scholar] [CrossRef]
- Guazzo, E.P. A technique for producing demyelination of the rat optic nerves. J. Clin. Neurosci. 2005, 12, 54–58. [Google Scholar] [CrossRef]
- Kalakh, S.; Mouihate, A. Demyelination-Induced inflammation attracts newly born neurons to the white matter. Mol. Neurobiol. 2017, 54, 5905–5918. [Google Scholar] [CrossRef]
- Ishihara, Y.; Tsuji, M.; Kawamoto, T.; Yamazaki, T. Involvement of reactive oxygen species derived from mitochondria in neuronal injury elicited by methylmercury. J. Clin. Biochem. Nutr. 2016, 59, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Lipton, H.L.; Canto, M.C. Theiler’s virus-induced central nervous system disease in mice. UCLA Forum Med. Sci. 1976, 263–277. [Google Scholar]
- Tsunoda, I.; Fujinami, R.S. Inside-Out versus Outside-In models for virus induced demyelination: Axonal damage triggering demyelination. Springer Semin. Immunopathol. 2002, 24, 105–125. [Google Scholar] [CrossRef]
- Murray, P.D.; Pavelko, K.D.; Leibowitz, J.; Lin, X.; Rodriguez, M. CD4(+) and CD8(+) T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J. Virol. 1998, 72, 7320–7329. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.H.; Kim, C.X.; Huang, J.; Kim, B.S. Infection and activation of b cells by theiler’s murine encephalomyelitis virus (TMEV) leads to autoantibody production in an infectious model of multiple sclerosis. Cells 2020, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- Pachner, A.R.; Li, L.; Lagunoff, D. Plasma cells in the central nervous system in the Theiler’s virus model of multiple sclerosis. J. Neuroimmunol. 2011, 232, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Oleszak, E.L.; Chang, J.R.; Friedman, H.; Katsetos, C.D.; Platsoucas, C.D. Theiler’s virus infection: A model for multiple sclerosis. Clin. Microbiol. Rev. 2004, 17, 174–207. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, P.; Patel, D.C.; Wilcox, K.S.; Patel, M. Oxidative stress in murine Theiler’s virus-induced temporal lobe epilepsy. Exp. Neurol. 2015, 271, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Matthews, A.E.; Weiss, S.R.; Paterson, Y. Murine hepatitis virus—A model for virus-induced CNS demyelination. J. Neurovirol. 2002, 8, 76–85. [Google Scholar] [CrossRef]
- Matthews, A.E.; Lavi, E.; Weiss, S.R.; Paterson, Y. Neither B cells nor T cells are required for CNS demyelination in mice persistently infected with MHV-A59. J. Neurovirol. 2002, 8, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Macnamara, K.C.; Chua, M.M.; Phillips, J.J.; Weiss, S.R. Contributions of the viral genetic background and a single amino acid substitution in an immunodominant CD8+ T-cell epitope to murine coronavirus neurovirulence. J. Virol. 2005, 79, 9108–9118. [Google Scholar] [CrossRef] [Green Version]
- Lavi, E.; Gilden, D.H.; Wroblewska, Z.; Rorke, L.B.; Weiss, S.R. Experimental demyelination produced by the A59 strain of mouse hepatitis virus. Neurology 1984, 34, 597–603. [Google Scholar] [CrossRef]
- Bender, S.J.; Weiss, S.R. Pathogenesis of murine coronavirus in the central nervous system. J. Neuroimmune Pharmacol. 2010, 5, 336–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, R.S.; Dine, K.; Das, S.J.; Shindler, K.S. SIRT1 activating compounds reduce oxidative stress mediated neuronal loss in viral induced CNS demyelinating disease. Acta Neuropathol. Commun. 2014, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved release and metabolism of flavonoids by steered fermentation processes: A review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed]
- de Lima, C.D.; Buzanello, M.C.; Oliveira, F.L.; Da, S.D.L.R. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Carvalho, F.B.; Gutierres, J.M.; Bohnert, C.; Zago, A.M.; Abdalla, F.H.; Vieira, J.M.; Palma, H.E.; Oliveira, S.M.; Spanevello, R.M.; Duarte, M.M.; et al. Anthocyanins suppress the secretion of proinflammatory mediators and oxidative stress, and restore ion pump activities in demyelination. J. Nutr. Biochem. 2015, 26, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Ciftci, O.; Ozcan, C.; Kamisli, O.; Cetin, A.; Basak, N.; Aytac, B. Hesperidin, a citrus flavonoid, has the ameliorative effects against experimental autoimmune encephalomyelitis (EAE) in a C57BL/J6 mouse model. Neurochem. Res. 2015, 40, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Fontes, L.B.; Dos, S.D.D.; de Carvalho, L.S.; Mesquita, H.L.; Da, S.R.L.; Dias, A.T.; Da, S.F.A.; Do, A.C.J. Immunomodulatory effects of licochalcone a on experimental autoimmune encephalomyelitis. J. Pharm. Pharmacol. 2014, 66, 886–894. [Google Scholar] [CrossRef]
- Bitto, A.; Squadrito, F.; Irrera, N.; Pizzino, G.; Pallio, G.; Mecchio, A.; Galfo, F.; Altavilla, D. Flavocoxid, a nutraceutical approach to blunt inflammatory conditions. Med. Inflamm. 2014, 2014, 790851. [Google Scholar] [CrossRef]
- Kong, W.; Hooper, K.M.; Ganea, D. The natural dual cyclooxygenase and 5-lipoxygenase inhibitor flavocoxid is protective in EAE through effects on Th1/Th17 differentiation and macrophage/microglia activation. Brain Behav. Immun. 2016, 53, 59–71. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors 2021, 47, 190–197. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, O.S.; Ghanem, H.B.; El-Esawy, R.O.; Sadek, M.T. The modulatory effects of luteolin on cyclic AMP/Ciliary neurotrophic factor signaling pathway in experimentally induced autoimmune encephalomyelitis. IUBMB Life 2019, 71, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.L.; Lin, L.L.; Zhang, L.; Li, L. Epimedium flavonoids ameliorate experimental autoimmune encephalomyelitis in rats by modulating neuroinflammatory and neurotrophic responses. Neuropharmacology 2012, 63, 851–862. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Salau, V.F.; Chukwuma, C.I.; Islam, M.S. Kolaviron: A biflavonoid with numerous health benefits. Curr. Pharm. Des. 2021, 27, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Omotoso, G.O.; Ukwubile, I.I.; Arietarhire, L.; Sulaimon, F.; Gbadamosi, I.T. Kolaviron protects the brain in cuprizone-induced model of experimental multiple sclerosis via enhancement of intrinsic antioxidant mechanisms: Possible therapeutic applications? Pathophysiology 2018, 25, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Garrick, J.M.; Roque, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.B.; Gutierres, J.M.; Beckmann, D.; Santos, R.P.; Thome, G.R.; Baldissarelli, J.; Stefanello, N.; Andrades, A.; Aiello, G.; Ripplinger, A.; et al. Quercetin treatment regulates the Na(+), K(+)-ATPase activity, peripheral cholinergic enzymes, and oxidative stress in a rat model of demyelination. Nutr. Res. 2018, 55, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Dierckx, T.; Haidar, M.; Grajchen, E.; Wouters, E.; Vanherle, S.; Loix, M.; Boeykens, A.; Bylemans, D.; Hardonniere, K.; Kerdine-Romer, S.; et al. Phloretin suppresses neuroinflammation by autophagy-mediated Nrf2 activation in macrophages. J. Neuroinflamm. 2021, 18, 148. [Google Scholar] [CrossRef]
- Esmaeilzadeh, E.; Soleimani, M.; Zare-Abdollahi, D.; Jameie, B.; Khorram, K.H. Curcumin ameliorates experimental autoimmune encephalomyelitis in a C57BL/6 mouse model. Drug Dev. Res. 2019, 80, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Sadeghizadeh, M.; Najafi, F.; Javan, M. Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 2015, 99, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, S.P.; Fu, J.S.; Zhang, S.; Bai, L.; Guo, L. Resveratrol defends blood-brain barrier integrity in experimental autoimmune encephalomyelitis mice. J. Neurophysiol. 2016, 116, 2173–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaiad, H.R.; Nooh, M.M.; El-Sawalhi, M.M.; Shaheen, A.A. Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: Biochemical and histological study. Mol. Neurobiol. 2017, 54, 3219–3229. [Google Scholar] [CrossRef]
- Li, W.; Deng, R.; Jing, X.; Chen, J.; Yang, D.; Shen, J. Acteoside ameliorates experimental autoimmune encephalomyelitis through inhibiting peroxynitrite-mediated mitophagy activation. Free Radic. Biol. Med. 2020, 146, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Miranda, B.; Gallardo, I.; Melliou, E.; Cabero, I.; Alvarez, Y.; Magiatis, P.; Hernandez, M.; Nieto, M.L. Oleacein attenuates the pathogenesis of experimental autoimmune encephalomyelitis through both antioxidant and Anti-Inflammatory effects. Antioxidants 2020, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Ellagic acid and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 928, 473–479. [Google Scholar] [PubMed]
- Khodaei, F.; Khoshnoud, M.J.; Heidaryfar, S.; Heidari, R.; Karimpour, B.M.; Azarpira, N.; Rashedinia, M. The effect of ellagic acid on spinal cord and sciatica function in a mice model of multiple sclerosis. J. Biochem. Mol. Toxicol. 2020, 34, e22564. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Khezri, K.; Seyed, Z.A.; Mohammadamini, H. A comprehensive review of the therapeutic potential of alpha-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim-Tabar, F.; Nazari, A.; Pouramir, M.; Ashrafpour, M.; Pourabdolhossein, F. Arbutin improves functional recovery and attenuates glial activation in Lysolecethin-Induced demyelination model in rat optic chiasm. Mol. Neurobiol. 2020, 57, 3228–3242. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.C.; Liu, L.F. Paeonol: Pharmacological effects and mechanisms of action. Int. Immunopharmacol. 2019, 72, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Pourmohammadi, S.; Roghani, M.; Kiasalari, Z.; Khalili, M. Paeonol ameliorates Cuprizone-Induced hippocampal demyelination and cognitive deficits through inhibition of oxidative and inflammatory events. J. Mol. Neurosci. 2022, 72, 748–758. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [Green Version]
- Kamisli, S.; Ciftci, O.; Taslidere, A.; Basak, T.N.; Ozcan, C. The beneficial effects of 18beta-glycyrrhetinic acid on the experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mouse model. Immunopharmacol. Immunotoxicol. 2018, 40, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Fontes, L.; Dias, D.; Aarestrup, B.; Aarestrup, F.M.; Da, S.F.A.; Correa, J. Beta-Caryophyllene ameliorates the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Biomed. Pharmacother. 2017, 91, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and antioxidant effect of ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Li, Q.Y.; Miao, Q.; Sui, R.X.; Cao, L.; Ma, C.G.; Xiao, B.G.; Xiao, W.; Yu, W.B. Ginkgolide K supports remyelination via induction of astrocytic IGF/PI3K/Nrf2 axis. Int. Immunopharmacol. 2019, 75, 105819. [Google Scholar] [CrossRef]
- Shahid-Ul-Islam; Rather, L.J.; Mohammad, F. Phytochemistry, biological activities and potential of annatto in natural colorant production for industrial applications—A review. J. Adv. Res. 2016, 7, 499–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Wu, D.M.; Li, J.; Deng, S.H.; Liu, T.; Zhang, T.; He, M.; Zhao, Y.Y.; Xu, Y. Bixin attenuates experimental autoimmune encephalomyelitis by suppressing TXNIP/NLRP3 inflammasome activity and activating NRF2 signaling. Front. Immunol. 2020, 11, 593368. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Jiang, X.; Yang, S.; Huang, Y.; Hong, J.; Ma, Y.; Fang, X.; Fang, Y.; Wu, J. The biological activity of 3-O-Acetyl-11-keto-beta-Boswellic acid in nervous system diseases. Neuromol. Med. 2022, 1–11. [Google Scholar]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Sarawi, W.; Attia, S.M.; Alanazi, W.A.; Ibrahim, K.E.; Alsanea, S.; Alqarni, S.A.; Alfardan, A.S.; et al. Acetyl-11-keto-beta-boswellic acid improves clinical symptoms through modulation of Nrf2 and NF-kappaB pathways in SJL/J mouse model of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2022, 107, 108703. [Google Scholar] [CrossRef] [PubMed]
- Nah, S.Y. Ginseng ginsenoside pharmacology in the nervous system: Involvement in the regulation of ion channels and receptors. Front. Physiol. 2014, 5, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Choi, J.H.; Oh, J.; Lee, Y.H.; In, J.G.; Chang, B.J.; Nah, S.Y.; Cho, I.H. Rg3-enriched Korean Red Ginseng extract inhibits blood-brain barrier disruption in an animal model of multiple sclerosis by modulating expression of NADPH oxidase 2 and 4. J. Ginseng Res. 2021, 45, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharmacol. 2020, 87, 89–112. [Google Scholar] [PubMed]
- He, Y.X.; Du, M.; Shi, H.L.; Huang, F.; Liu, H.S.; Wu, H.; Zhang, B.B.; Dou, W.; Wu, X.J.; Wang, Z.T. Astragalosides from Radix Astragali benefits experimental autoimmune encephalomyelitis in C57BL/6 mice at multiple levels. BMC Complement. Altern. Med. 2014, 14, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, L.; Quan, M.Y.; Wang, D.; Jia, Z.; Li, Z.F.; Li, B.; Guo, L.; Tan, G.J. Nordihydroguaiaretic acid can suppress progression of experimental autoimmune encephalomyelitis. IUBMB Life 2018, 70, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Cui, H.; Ma, Z.; Liu, X.; Yang, L. Recent progresses in the pharmacological activities of caffeic acid phenethyl ester. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, A.; Akyol, O.; Gurel, A.; Armutcu, F.; Iraz, M.; Oztas, E. Protective effects of caffeic acid phenethyl ester against experimental allergic encephalomyelitis-induced oxidative stress in rats. Free Radic. Biol. Med. 2004, 37, 386–394. [Google Scholar] [CrossRef]
- Kilic, I.; Yesiloglu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Behrangi, N.; Namvar, N.; Ataei, M.; Dizaji, S.; Javdani, G.; Sanati, M.H. MMP9 gene expression variation by ingesting tart cherry and P-Coumaric acid during remyelination in the cuprizone mouse model. Acta Med. Iran. 2017, 55, 539–549. [Google Scholar] [PubMed]
- Bibi, T.; Khan, A.; Khan, A.U.; Shal, B.; Ali, H.; Seo, E.K.; Khan, S. Magnolol prevented brain injury through the modulation of Nrf2-dependent oxidative stress and apoptosis in PLP-induced mouse model of multiple sclerosis. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Singh, A.P.; Bhatti, R. Mechanistic interplay of various mediators involved in mediating the neuroprotective effect of daphnetin. Pharmacol. Rep. 2021, 73, 1220–1229. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, B.; Liu, X.; Han, Q.; Ge, W.; Zhang, W.; Lu, Y.; Wu, Q.; Shi, L. Daphnetin ameliorates experimental autoimmune encephalomyelitis through regulating heme oxygenase-1. Neurochem. Res. 2020, 45, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Nasrnezhad, R.; Halalkhor, S.; Sadeghi, F.; Pourabdolhossein, F. Piperine improves experimental autoimmune encephalomyelitis (EAE) in lewis rats through its neuroprotective, anti-inflammatory, and antioxidant effects. Mol. Neurobiol. 2021, 58, 5473–5493. [Google Scholar] [CrossRef]
- Roshanbakhsh, H.; Elahdadi, S.M.; Dehghan, S.; Nazari, A.; Javan, M.; Pourabdolhossein, F. Piperine ameliorated memory impairment and myelin damage in lysolecethin induced hippocampal demyelination. Life Sci. 2020, 253, 117671. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, F.; Wu, L.; Xu, Y.; Du, Q. Matrine exerts pharmacological effects through multiple signaling pathways: A comprehensive review. Drug Des. Dev. Ther. 2022, 16, 533–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.R.; Zhang, X.J.; Liu, H.C.; Ma, W.D.; Zhang, M.L.; Zhang, Y.; Li, X.; Dou, M.M.; Jing, Y.L.; Chu, Y.J.; et al. Matrine protects oligodendrocytes by inhibiting their apoptosis and enhancing mitochondrial autophagy. Brain Res. Bull. 2019, 153, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, S.M.; Biglari, S.; Khorram, K.H.; Esmaeilzadeh, E. Shikonin ameliorates experimental autoimmune encephalomyelitis (EAE) via immunomodulatory, anti-apoptotic and antioxidative activity. J. Pharm. Pharmacol. 2020, 72, 1970–1976. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Fakhoury, I.H.; Zaki, A.M.; El-Baba, C.O.; Gali-Muhtasib, H.U. Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discov. Today 2014, 19, 18–30. [Google Scholar] [CrossRef]
- Spate, S.; Ramadan, H.H.; Mohamed, A. Interleukins (IL-7 and IL-7r) gene expression and thymoquinones role in the amelioration of eae symptoms—Biomed 2010. Biomed. Sci. Instrum. 2010, 46, 185–190. [Google Scholar]
- Mohamed, A.; Shoker, A.; Bendjelloul, F.; Mare, A.; Alzrigh, M.; Benghuzzi, H.; Desin, T. Improvement of experimental allergic encephalomyelitis (EAE) by thymoquinone; An oxidative stress inhibitor. Biomed. Sci. Instrum. 2003, 39, 440–445. [Google Scholar]
- Baig, M.W.; Nasir, B.; Waseem, D.; Majid, M.; Khan, M.; Haq, I.U. Withametelin: A biologically active withanolide in cancer, inflammation, pain and depression. Saudi Pharm. J. 2020, 28, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Shal, B.; Khan, A.U.; Bibi, T.; Islam, S.U.; Baig, M.W.; Haq, I.U.; Ali, H.; Ahmad, S.; Khan, S. Withametelin, a novel phytosterol, alleviates neurological symptoms in EAE mouse model of multiple sclerosis via modulation of Nrf2/HO-1 and TLR4/NF-kappaB signaling. Neurochem. Int. 2021, 151, 105211. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sugimoto, K. Guggulsterone and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 329–361. [Google Scholar] [PubMed]
- Kumar, N.; Sharma, N.; Khera, R.; Gupta, R.; Mehan, S. Guggulsterone ameliorates ethidium bromide-induced experimental model of multiple sclerosis via restoration of behavioral, molecular, neurochemical and morphological alterations in rat brain. Metab. Brain Dis. 2021, 36, 911–925. [Google Scholar] [CrossRef]
- Yoo, I.H.; Kim, M.J.; Kim, J.; Sung, J.J.; Park, S.T.; Ahn, S.W. The Anti-Inflammatory effect of sulforaphane in mice with experimental autoimmune encephalomyelitis. J. Korean Med. Sci. 2019, 34, e197. [Google Scholar] [CrossRef]
- Li, B.; Cui, W.; Liu, J.; Li, R.; Liu, Q.; Xie, X.H.; Ge, X.L.; Zhang, J.; Song, X.J.; Wang, Y.; et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp. Neurol. 2013, 250, 239–249. [Google Scholar] [CrossRef]
- Kuo, P.C.; Brown, D.A.; Scofield, B.A.; Yu, I.C.; Chang, F.L.; Wang, P.Y.; Yen, J.H. 3H-1,2-dithiole-3-thione as a novel therapeutic agent for the treatment of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2016, 57, 173–186. [Google Scholar] [CrossRef]
- Penton-Rol, G.; Lagumersindez-Denis, N.; Muzio, L.; Bergami, A.; Furlan, R.; Fernandez-Masso, J.R.; Nazabal-Galvez, M.; Llopiz-Arzuaga, A.; Herrera-Rolo, T.; Veliz-Rodriguez, T.; et al. Comparative neuroregenerative effects of C-Phycocyanin and IFN-Beta in a model of multiple sclerosis in mice. J. Neuroimmune Pharmacol. 2016, 11, 153–167. [Google Scholar] [CrossRef]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Safari, H.; Anani, S.G.; Naseri, M. Artemisia dracunculus L. modulates the immune system in a multiple sclerosis mouse model. Nutr. Neurosci. 2021, 24, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Grubic-Kezele, T. Olive leaf polyphenols attenuate the clinical course of experimental autoimmune encephalomyelitis and provide neuroprotection by reducing oxidative stress, regulating microglia and SIRT1, and preserving myelin integrity. Oxid. Med. Cell. Longev. 2020, 2020, 6125638. [Google Scholar] [CrossRef]
- Merighi, S.; Travagli, A.; Tedeschi, P.; Marchetti, N.; Gessi, S. Antioxidant and antiinflammatory effects of Epilobium parviflorum, Melilotus officinalis and Cardiospermum halicacabum plant extracts in macrophage and microglial cells. Cells 2021, 10, 2691. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Soleimani, M.; Esmaeilzadeh, E.; Zare-Abdollahi, D.; Khorram, K.H. Healing influence of Melilotus officinalis herbal extract on experimental autoimmune encephalomyelitis in C57BL/6 mice. Iran. J. Pharm. Res. 2020, 19, 321–329. [Google Scholar] [PubMed]
- Chen, X.; Lei, Z.; Cao, J.; Zhang, W.; Wu, R.; Cao, F.; Guo, Q.; Wang, J. Traditional uses, phytochemistry, pharmacology and current uses of underutilized Xanthoceras sorbifolium bunge: A review. J. Ethnopharmacol. 2022, 283, 114747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Ye, Z.Q.; Zhang, F.; Han, J.J.; Yang, T.; Wang, Z.Z.; Zhang, Y. Nutshell extracts of Xanthoceras sorbifolia: A new potential source of bioactive phenolic compounds as a natural antioxidant and immunomodulator. J. Agric. Food Chem. 2018, 66, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, A.; Mosayebi, G.; Salehi, H.; Abtahi, H. Effect of ethanol extract of saffron (Crocus sativus L.) on the inhibition of experimental autoimmune encephalomyelitis in C57bl/6 mice. Pak. J. Biol. Sci. 2009, 12, 690–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omotoso, G.O.; Gbadamosi, I.T.; Afolabi, T.T.; Abdulwahab, A.B.; Akinlolu, A.A. Ameliorative effects of Moringa on cuprizone-induced memory decline in rat model of multiple sclerosis. Anat. Cell Biol. 2018, 51, 119–127. [Google Scholar] [CrossRef]
- Conde, C.; Escribano, B.M.; Luque, E.; Aguilar-Luque, M.; Feijoo, M.; Ochoa, J.J.; Latorre, M.; Giraldo, A.I.; Lillo, R.; Aguera, E.; et al. The protective effect of extra-virgin olive oil in the experimental model of multiple sclerosis in the rat. Nutr. Neurosci. 2020, 23, 37–48. [Google Scholar] [CrossRef]
- Dias, D.S.; Fontes, L.B.; Crotti, A.E.; Aarestrup, B.J.; Aarestrup, F.M.; Da, S.F.A.; Correa, J.O. Copaiba oil suppresses inflammatory cytokines in splenocytes of C57Bl/6 mice induced with experimental autoimmune encephalomyelitis (EAE). Molecules 2014, 19, 12814–12826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosayebi, G.; Ghazavi, A.; Salehi, H.; Payani, M.A.; Khazae, M.R. Effect of sesame oil on the inhibition of experimental autoimmune encephalomyelitis in C57BL/6 mice. Pak. J. Biol. Sci. 2007, 10, 1790–1796. [Google Scholar] [CrossRef] [Green Version]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening primrose (Oenothera biennis) biological activity dependent on chemical composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selek, S.; Esrefoglu, M.; Meral, I.; Bulut, H.; Caglar, H.G.; Sonuc, G.; Yildiz, C.; Teloglu, E.S.; Dogan, N.; Yuce, B.; et al. Effects of Oenothera biennis L. and Hypericum perforatum L. extracts on some central nervous system myelin proteins, brain histopathology and oxidative stress in mice with experimental autoimmune encephalomyelitis. Biotech. Histochem. 2019, 94, 75–83. [Google Scholar] [CrossRef]
| Compound | Experimental Model | Treatment Time Point and Path | Dosage | Antioxidative Stress Effect | Major Results |
|---|---|---|---|---|---|
| Anthocyanins | EB (male Wistar rats) | Starting from EB injection day (oral) | 30, 100 mg/kg for 7 days | ↑ HNE-His, MDA, NOX ↑ NPSH, GSH, SOD | ↓ IL-6, IL-1β, TNF-α, IFN-γ. ↑ IL-10. ↑ Na+, K+-ATPase, Ca2+-ATPase. |
| Hesperidin | EAE (female C57BL/6J mice) | After 14th day induction of EAE (i.p. injection) | 100 mg/kg/d for 7 days | ↓ MDA. ↑ GPx, SOD, CAT, GSH | ↓ Caspase-3, IL-17, TNF-α and IL-1β. ↓ inflammatory infiltration. |
| Licochalcone A | EAE (female C57BL/6 mice) | Starting at 10th days post-immunization (oral) | 15, 30 mg/kg/d for 10 days | ↓ H2O2, NO | ↓ TNF-α, IFN-γ and IL-17. ↓ Th1 and Th17 cells. |
| Flavocoxid | EAE (female C57BL/6 mice) | Prevention protocol: Starting from immunization day therapeutic treatment protocol: from day 14 post immunization (i.p. injection) | 100 mg/kg/d every other day Prevention protocol: for 28 days. therapeutic treatment protocol: for 19 days. | ↓ iNOS, COX2, 5-LOX. | ↓ IL-6, IL-12, IL-23, IL-17, IFN-γ. ↓ Th1/Th17 cells. ↑ Arg1, Ym1, CD206, TGF-β, IL-10. |
| Luteolin | EAE (female Wistar rats) | Starting at 10th day post-immunization (i.p. injection) | 10 mg/kg/d for 33 day | ↑ TAC | ↑ CNTF, cAMP. ↓ c-caspase-3, NF-κB, MIP-1α. |
| Epimedium flavonoids | EAE (Female Lewis rats) | Starting from immunization day (oral) | 20, 60 mg/kg/d for 15 days | ↓ NO, iNOS | ↓ IL-1β, TNF-α, IκB-α, Iba1, CD3, GFAP. ↑ CNPase, NGF. |
| Kolaviron | CPZ (male Wistar rats) | Starting from cuprizone diet fed (oral) | 200 mg/kg/d both cuprizone and kolaviron for 42 days | ↓ MDA. ↑ GPx, SOD | ↑ the neuronal integrity |
| Quercetin | EB (male Wistar rats) | Starting from EB injection day (i.p. injection) | 50 mg/kg/d for 22 days | ↓ MDA. ↑ CAT, SOD. | ↓ AChE. ↓ Na+, K+-ATPase |
| Phloretin | EAE (C57BL/6J mice) | Starting after 6 days of immunization or after disease onset (i.p. injection) | 50 mg/kg/d for 10 days or 20 days | ↓ ROS, NO, NOS2, ↑ Nrf2, NQO1 | ↓ MHC-II, CD86, TNF-α, IL-6, CCL4, CCL5, CXCL2. ↑ IL-4, CNTF, IGF-1, p-AMK, P62, LC3-II. |
| Curcumin | EAE (female C57BL/6 mice) | Starting from immunization day (i.p. injection) | 20 mg/kg/d for 21 days | ↑ GPx, SOD. | ↓ IL-6, IL-17, TNF-α, IFN-γ. ↑ TGF-β. |
| EAE (female Lewis rats) | Starting at 12th day post-immunization | 12.5 mg/kg/d for 18 days | ↓ iNOS, ↑ HO-1, Nrf2 | ↓ IL-1, IL-17. ↑ IL-4, IL-10, TGF-β, BDNF, NGF, MBP, Nestin. | |
| Resveratrol | EAE (female C57BL/6 mice) | Starting from immunization day (i.p. injection) | 10, 25, 50 mg/kg/d for 20 days | ↓ iNOS, NOX2, NOX4. ↓ NADPH activity | ↑ Arg1 and IL-10 ↑ ZO-1, Occludin, claudin-5, ICAM-1, VCAM-1. ↑ the BBB integrity |
| CPZ (male C57BL/6 mice) | cuprizone diet for 7 days, followed by 3 weeks on 0.2 % cuprizone diet plus resveratrol (oral) | 250 mg/kg/d for 21 days | ↓ LPO. ↑ GSH, SOD, cytochrome oxidase | ↓ Rel-A, pIκB-α, TNF-α. ↑ MBP, CNP, Olig1. | |
| Acteoside | EAE (female C57BL/6N mice) | Prevention protocol: Starting from day 2 post immunization therapeutic treatment protocol: from day 11 post immunization (oral) | Prevention protocol: 30 mg/kg/d for 29 days therapeutic treatment protocol: 5, 10 and 30 mg/kg/d for 19 days | ↓ ONOO-, iNOS, NADPH oxidases. | ↓ LC3-II to LC3-I in mitochondrial fraction. ↓ the translocation of Drp1 to the mitochondria. ↓ neuronal apoptotic. |
| Oleacein | EAE (female C57BL/6J mice) | Starting from immunization day (i.p. injection) | 10 mg/kg/d for 24 days | ↓ O2−, MDA, AOPP, ROS, iNOS, COX2. ↑ FRAP, Sestrin-3 | ↓ TNFα, IL-13, IL-33, IL-1β, MCP-1. ↑ IL-10. ↑ the BBB integrity. ↓ inflammatory infiltration. |
| Ellagic acid | CPZ (male C57BL/6 mice) | Starting from cuprizone diet fed (oral) | 5, 50, 100 mg/kg/d coadministration of CPZ and Ellagic acid for 6 weeks | ↓ MDA, ROS. ↑ CAT, SOD. | ↑ the integrity of myelin in spinal cord and sciatica. |
| Arbutin | LPC (male Wistar rats) | Starting from LPC injection day (i.p. injection) | 50 mg/kg/d for 14 days | ↑ Nrf2, HO-1. | ↓ IL-1β, IL-17, TNF-α, GFAP. ↑ IL-10, MBP, Olig2. |
| Paeonol | CPZ (male C57BL/6 mice) | Started 1 week after beginning cuprizone challenge till the end of week 6 post-cuprizone (oral) | 25 mg, 100 mg/kg/d for 42 days | ↓ MDA, ROS, MPO. ↑ CAT, SOD, GSH. | ↓ NF-κB, TNF-α. ↑ MBP. |
| 18β-glycyrrhetinic acid | EAE (female C57BL/6 mice) | 14 days after induction of EAE (i.p. injection) | 100 mg/kg/d for 7 days | ↓ MDA. ↑ GPx, SOD, CAT, GSH | ↓ Caspase-3, IL-17, TNF-α, IL-1β. |
| β-Caryophyllene | EAE (female C57BL/6 mice) | Starting at 10th day post-immunization (oral) | 25, 50mg/kg/d for 9 days | ↓ H2O2, NO | ↓ TNF-α, IFN-γ, IL-17 |
| Ginkgolide K | CPZ (male C57BL/6 mice) | Starting from 5th weeks until the end of 6th weeks (oral) | 20 mg/kg/d for 14 days | ↑ Nrf2, HO-1. ↓ NO, iNOS. | ↓ IL-1β, IL-6, TNF-α. ↓ p-NF-kB/p65, caspase-3, TUNEL. ↑ IGF/PI3K. |
| Bixin | EAE (female C57BL/6 mice) | Starting at 12th days post-immunization (i.p. injection) | 50, 100, 200 mg/kg/d for 18 days | ↓ ROS, Nrf2, TXNIP, NLRP3 | ↓ TNF-α, IL-6, IL-8, IL-17, and IFN-γ. ↑ IL-10. ↓ Th1 and Th17 cells. |
| Acetyl-11-keto-β-boswellic acid | EAE (female SJL/J mice) | starting at day 11 after immunization (oral) | 20 mg/kg/d for 30 days | ↑ Nrf2, HO-1, TAC ↓ iNOS, lipid peroxides | ↓ p-NF-κB, NF-κB, IL-6 |
| Ginsenoside-Rg3 | EAE (female C57BL/6N mice) | Starting from 7 days before immunization (oral) | 37.5, 75, 150mg/kg/d for 28 day | ↓ MitoSOX, 4-HNE, NOX2, NOX4, NADPH activity, COX2, iNOS | ↑ occludens-1, claudin-3, and claudin-5. ↓ TNF-α, IL-6, IL-1β. |
| Astragaloside | EAE (female C57BL/6 mice) | Starting from immunization day (i.p. injection) | 10, 25, 50 mg/kg/d for 15 days | ↓ ROS, MDA, iNOS ↑ GPx, SOD | ↓ p53, p-tau ↑ Bcl-2/Bax ratio. ↑ the BBB integrity. |
| Nordihydroguaiaretic acid | EAE (female C57BL/6 mice) | Starting from immunization day (i.p. injection) | 10 mg/kg/d for 30 days | ↓ MDA. ↑ HO-1 | ↓ IL-17 ↓ p38MAPK, SGK1. ↓ inflammatory infiltration. |
| Caffeic acid phenethyl ester | EAE (female Wistar rats) | Starting from the first day of immunization (i.p. injection) | 25 μmol/kg/d for 14 days | ↑ GPx, ADA, SOD. ↓ MDA, NO, XO. | ↓ inflammatory infiltration. |
| P-Coumaric Acid | CPZ (female and male C57BL/6 mice) | Starting from 7th weeks until the end of 12th weeks (oral) | 21.6 ppm/mouse/d for 42 days | ↓ free radical (DPPH) | ↓ MMP-9 |
| Magnolol | EAE (female Swiss mice) | Starting from immunization day (oral) | 0.1, 1, 10 mg/kg/d for 21 days | ↓ MDA, MPO, NO, iNOS. ↑ GSH, GST, SOD, Nrf2. | ↓ CD8+ T cell. ↓ c-caspase-3. |
| Daphnetin | EAE (female C57BL/6 mice) | Starting from immunization day (i.p. injection) | 8 mg/kg/d for 21 days | ↓MDA, HO-1 | ↓ IFN-γ, IL-17, IL-1β, IL-6, TNF-α. ↑ IL-10. inflammatory infiltration. |
| Piperine | EAE (female Lewis rats) | Starting at 8th day post-immunization (i.p. injection) | 5 mg/kg/d for 22 days | ↓ iNOS, MDA. ↑ Nrf2, HO-1 FRAP. | ↓ TNF-, IL-1β, caspase-3. ↑ IL-10, BDNF, MBP. |
| LPC (Male Wistar rats) | Starting at 3 days post LPC injection (i.p. injection) | 5, 10, 20 mg/kg/d for 10 days | ↑ Nrf2, HO1, FRAP. ↓ iNOS. | ↓ TNF-α, IL-1β, NF-κB, Foxp3. ↑ IL-10, BDNF, MBP. | |
| Matrine | EAE (Female Wistar rats) | starting from day 11 post immunization (i.p. injection) | 250 mg/kg/d for 7 days | ↓ MDA. ↑ GPx | ↓ caspase-3, α-B-crystallin, Cyt. ↑ Beclin1, LC3. |
| Shikonin | EAE (female C57BL/6 mice) | After EAE was induced (i.p. injection) | 20 mg/kg/d for 21 days | ↑ GPx1 | ↓ TNF-α, IFN-γ and Bax. ↑ TGF-β and Bcl2. |
| Thymoquinone | EAE (female Lewis rats) | at days 1–5 post immunization or at day 12–17 post immunization (i.v. injection) | 1 mg/kg for 5 days or 6 days | ↑ GSH | ↓ IL-7, IL-7R ↓ inflammatory infiltration. |
| Withametelin | EAE (Female Swiss mice) | starting at day 9 through day 25 (i.p. injection) | 10, 100, and 1000 μg/kg/d for 17 days | ↓ NO, MPO, iNOS ↑ GSH, SOD, Nrf2, HO-1 | ↓ TLR4, NF-κB, AP-1. ↑ IκB-α, Bcl-2. |
| Guggulsterone | EB (Wistar rats) | Starting at 8th day post EB injection (oral) | 30, 60 mg/kg/d for 28 days | ↓ AchE, MDA, NO. ↑ SOD, CAT, GSH. | ↓ IL-1β, TNF-α. ↓ caspase-3, Bax, STAT3. ↑ Bcl2, PPAR-ϒ, MBP. |
| Sulforaphane | EAE (female C57BL/6N mice) | 14 days before EAE induction (oral) | 50 mg/kg/d for 28 days | ↓ iNOS | ↓ CD4, CD68, GFAP ↓ inflammatory infiltration. |
| EAE (female C57BL/6 mice) | Starting from immunization day (i.p. injection) | 50 mg/kg every other day up to 22 days | ↓ HO-1, NQO1 ↑ Nrf2 | ↑ Occludin, claudin-5. ↑IL-10. ↓ MMP9, Th17 cells. | |
| 3H-1,2-dithiole-3-thione | EAE (female C57BL/6N mice) | starting at day 1 post immunization (i.p. injection) | 10 mg/kg/d for 30 days | ↑ Nrf2, NQO1, GCLC, HO-1. ↓ iNOS | ↓ IL-23. ↓ Th1 and Th17 differentiation. |
| C-Phycocyanin | EAE (female C57BL/6 mice) | Treatments started at disease onset (i.p. injection) | 2, 4, 8 mg/kg/d for 15 days | ↓ MDA, peroxidation potential, CAT/SOD ratio. ↑ GSH. | ↓ CD3, Mac-3 ↓ IL-17, IL-6, Foxp3 ↓ inflammatory infiltration |
| Artemisia dracunculus L. | EAE (female C57BL/6 mice) | Starting at 11th day post immunization (oral) | 500 mg/kg/d for 23 days | ↑ TAC (FRAP) | ↓ IL-17, IL-23. ↑ TGF-β. ↓ inflammatory infiltration |
| Olive leaf | EAE (female C57BL/6 mice) | starting from the first day after EAE induction. (olive leaf tea, oral) starting from the 8th day after EAE induction (olive leaf extract, i.p. injection) | Olive leaf tea: ad libitum for 20 or 30 days Olive leaf extract: 1024 mg/kg/d for 10 days | ↓ MDA ↑ SOD1, SOD2, GPx1 | ↑ SIRT. ↓ M1 microglia. ↑ M2 microglia. |
| Melilotus officinalis | EAE (female C57BL/6 mice) | starting from first day post-immunization (i.p. injection) | 10 mg/kg/d for 21 days | ↑ CAT, GPx1 | ↓ IL-6, TNF-α, IFN-γ, IL-17. ↑ TGF-β, IL-5. |
| Nutshell of Xanthoceras sorbifolia | EAE (female C57BL/6 mice) | Starting from 5 days before immunization (oral) | 50, 100, 150 mg/kg/d for 35 days | ↓ free radical (DPPH) | ↓ Th1, Th17 cells. ↓ p-STAT1, p-STAT3, p-STAT4. ↓ IL-17, IL-1α, IL-1β, TNF-α, CCL1, CCL2, CXCL1, CXCL10, CXCL11. |
| Crocus sativus L. | EAE (male C57BL/6 mice) | Starting from immunization day (oral) | 500 mg/kg/d for 21 days | ↑ TAC (FRAP). ↓ NO | ↓ inflammatory infiltration |
| Moringa oleifera. | CPZ (Wistar rats) | Starting from cuprizone diet fed (oral) | 1.875 mg/mL/mouse/d for 35 days | ↓ NO ↑ CAT, SOD | ↑ neuronal integrity. |
| Olive oil | EAE (Dark Agouti rats) | starting at 11th day post immunization (oral) | representing 10% of calorie intake in the total standard diet for 54 days | ↑ GPx. ↓ LPO, NO. | ↓ NF-κBp65, TNF-α. ↓ LPS, LBP. |
| Copaiba oil | EAE (female C57BL/6 mice) | Splenocytes were obtained from EAE mice at day 20 post immunization (in vitro) | 100, 50 and 25 µg/mL for 24 h | ↓ H2O2, NO. | ↓ TNF-α, INF-γ, IL-17 |
| Sesame oil | EAE (male C57BL/6 mice) | Starting from day 3 before the immunization (oral) | 4 mL/kg/d for 28 days | ↓ NO ↑ TAC (FRAP). | ↓ inflammatory infiltration. |
| Hypericum perforatum L. extract | EAE (C57BL/6 mice) | Starting from immunization day (oral) | 18−21 g/kg/d for 42 days | ↑ TSA. ↓ TOS, OSI. | ↑ MOG, MBP. ↓ inflammatory infiltration. |
| Oenothera biennis L. extract | EAE (C57BL/6 mice) | Starting from immunization day (oral) | 18−21 g/kg/d for 42 days | ↑ TSA. ↓ TOS, OSI. | ↑ MOG, MBP. ↓ inflammatory infiltration. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, Z.; Liu, S.; Liu, Y.; Li, C.; Wang, L. Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis. Antioxidants 2022, 11, 1495. https://doi.org/10.3390/antiox11081495
Zha Z, Liu S, Liu Y, Li C, Wang L. Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis. Antioxidants. 2022; 11(8):1495. https://doi.org/10.3390/antiox11081495
Chicago/Turabian StyleZha, Zheng, Sisi Liu, Yijiang Liu, Chen Li, and Lei Wang. 2022. "Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis" Antioxidants 11, no. 8: 1495. https://doi.org/10.3390/antiox11081495
APA StyleZha, Z., Liu, S., Liu, Y., Li, C., & Wang, L. (2022). Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis. Antioxidants, 11(8), 1495. https://doi.org/10.3390/antiox11081495






