5G Electromagnetic Radiation Attenuates Skin Melanogenesis In Vitro by Suppressing ROS Generation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
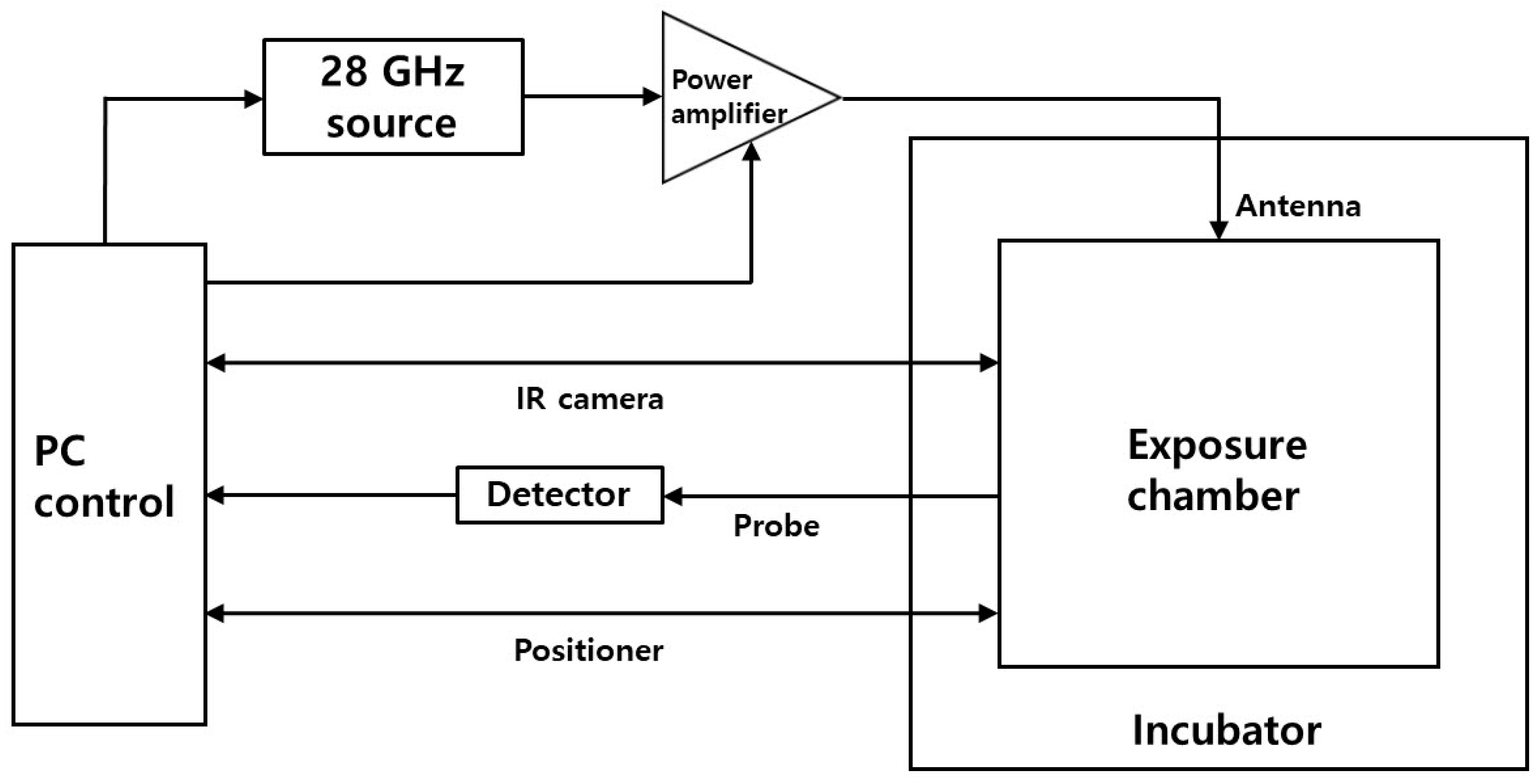
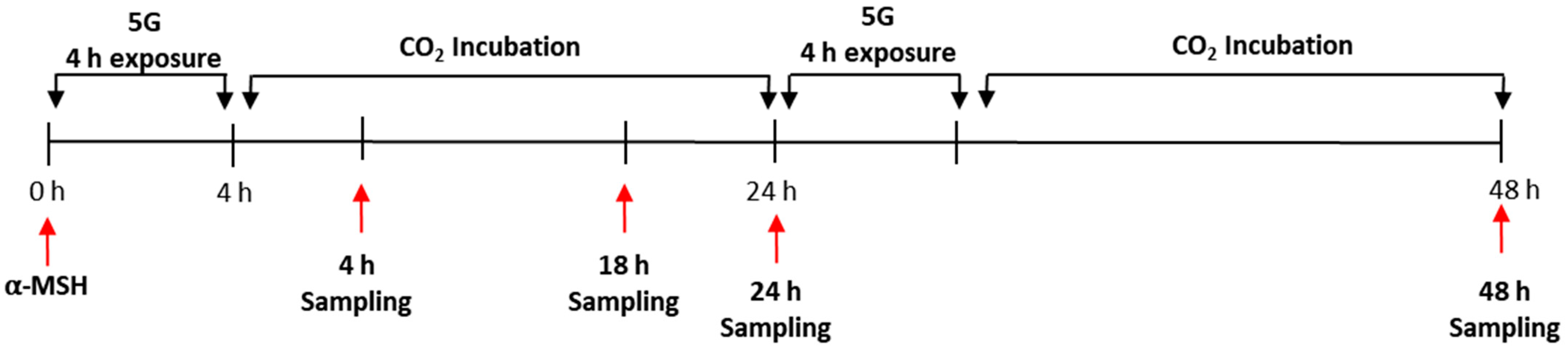
2.2. In Vitro 5G Exposure System
2.3. Melanin Content Assay and Cell Viability Assay (MTT)
2.4. Fontana–Masson Staining and Analysis
2.5. Intracellular Reactive Oxygen Species (ROS) Level
2.6. RNA Sample Preparation
2.7. Real-Time Polymerase Chain Reaction (Real-Time PCR) Assay
2.8. Evaluation of Skin Pigmentation in Melanoderm™, 3D Pigmented Human Epidermal Skin Model
2.9. Statistical Analysis
3. Results
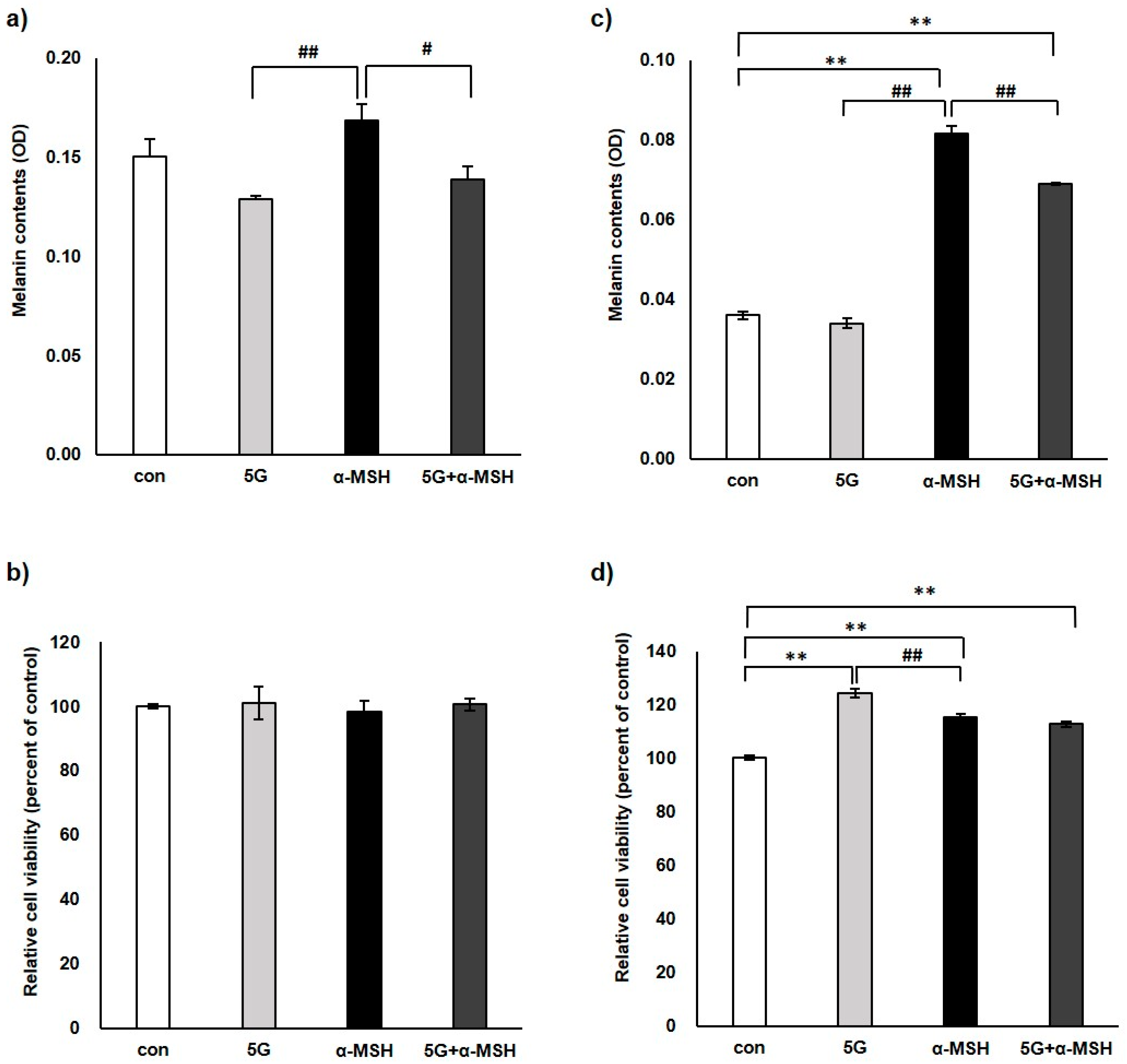
3.1. Effect of 5G EMR on Melanin Contents and Cell Viability of Murine Melanoma Cell, B16F10
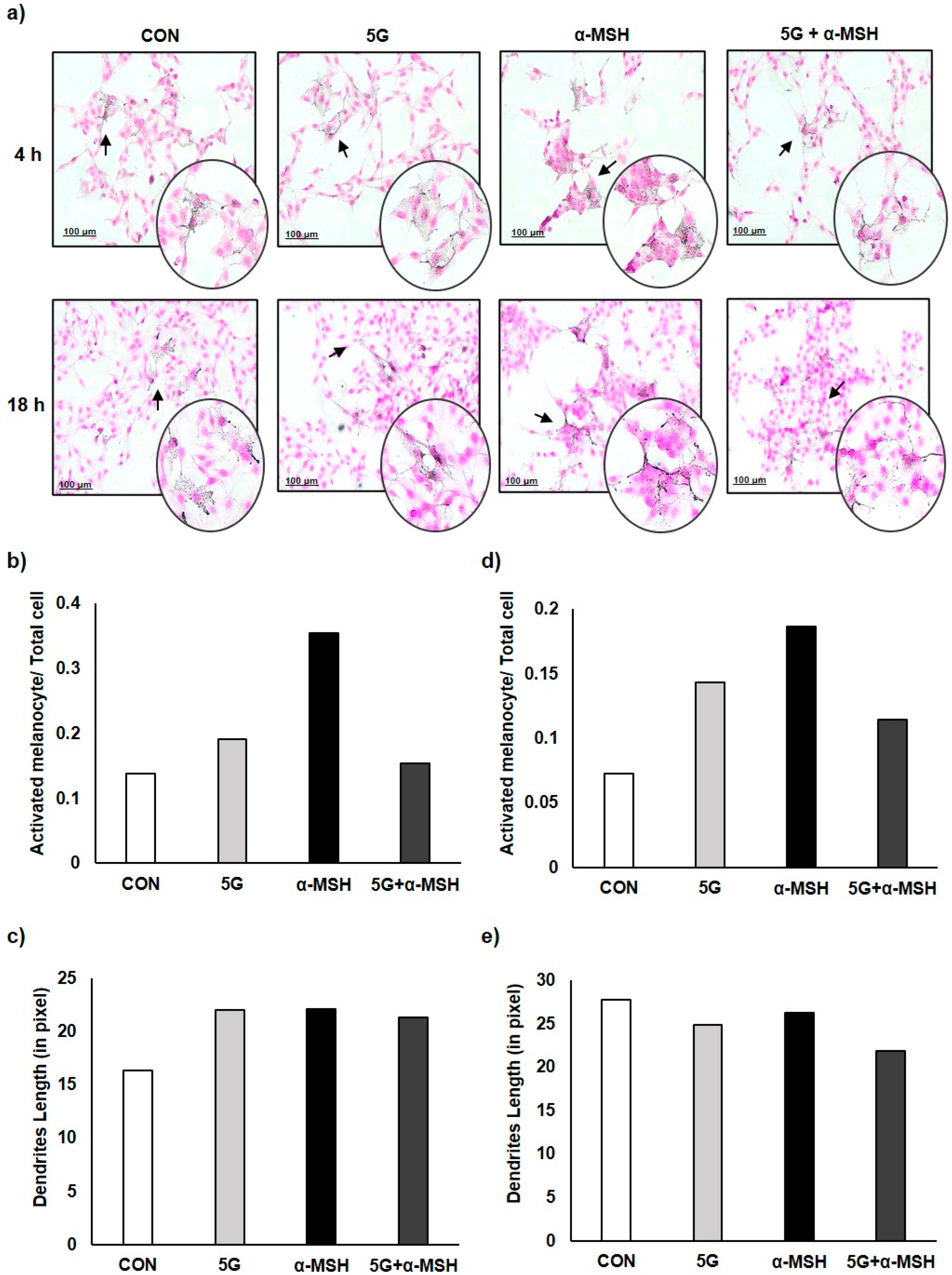
3.2. Effect of 5G EMR on Cell Morphology of Melanoma Cell, B16F10
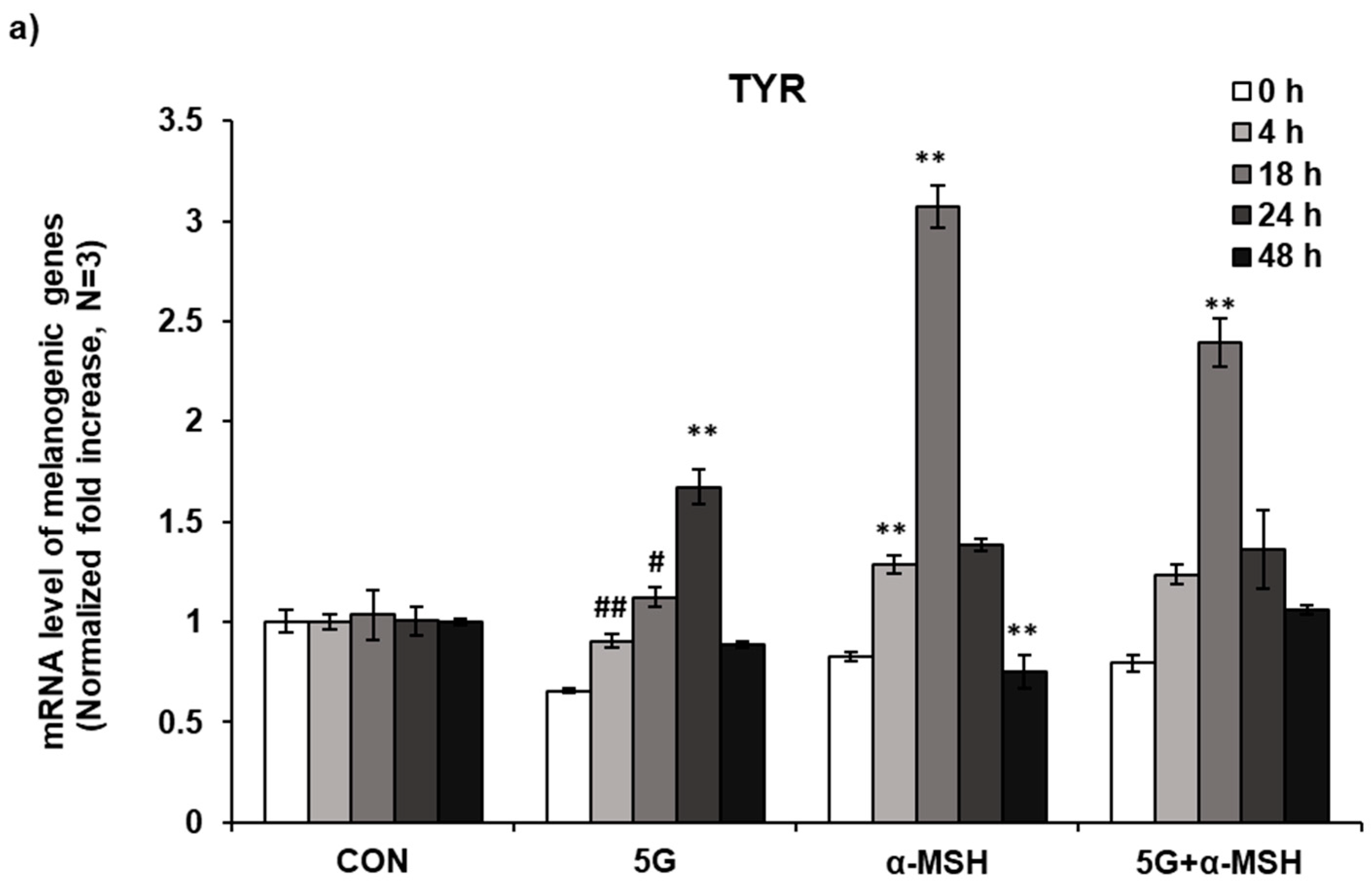
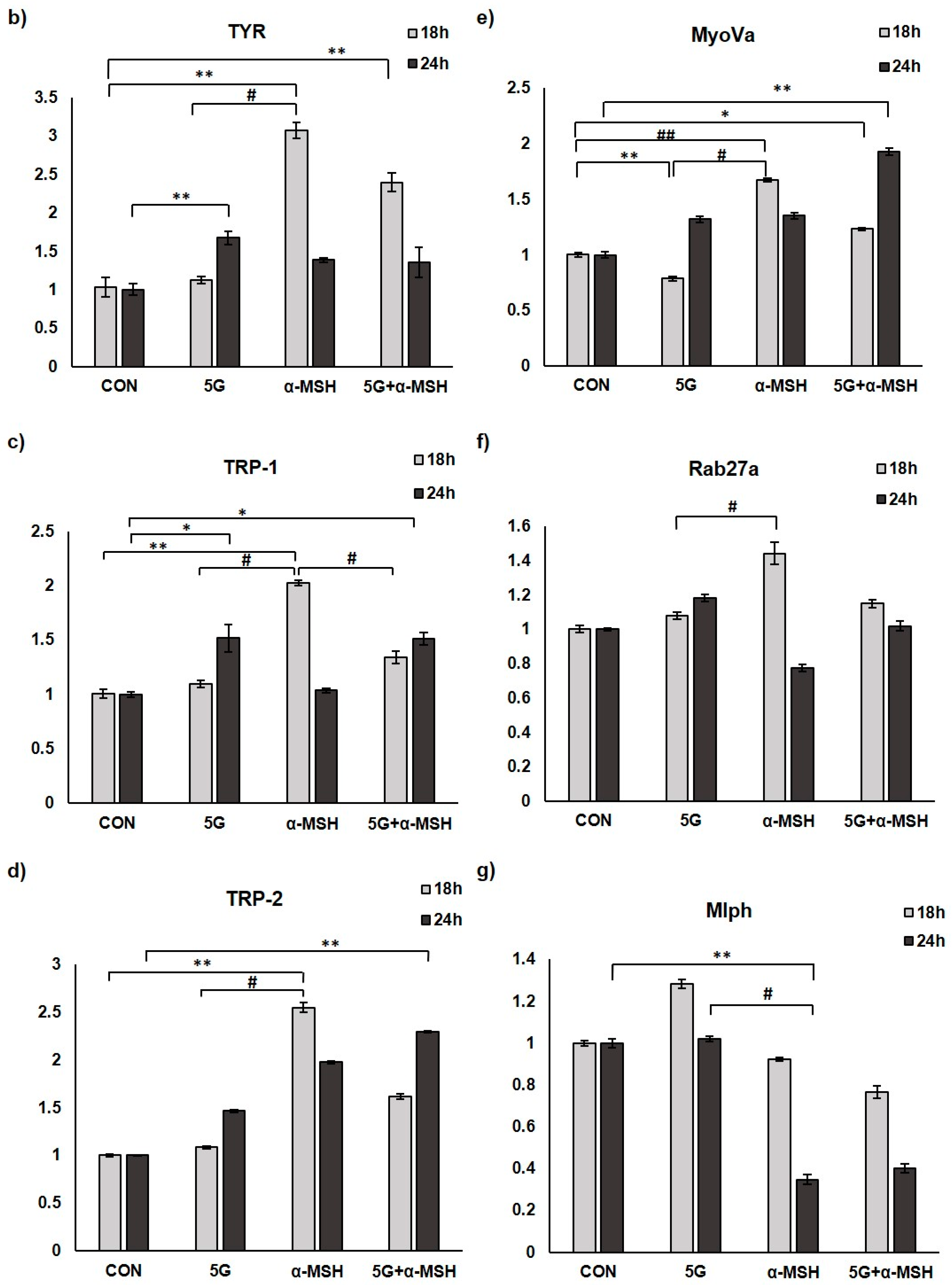
3.3. Effect of 5G EMR on mRNA Levels of Melanogenic Enzymes
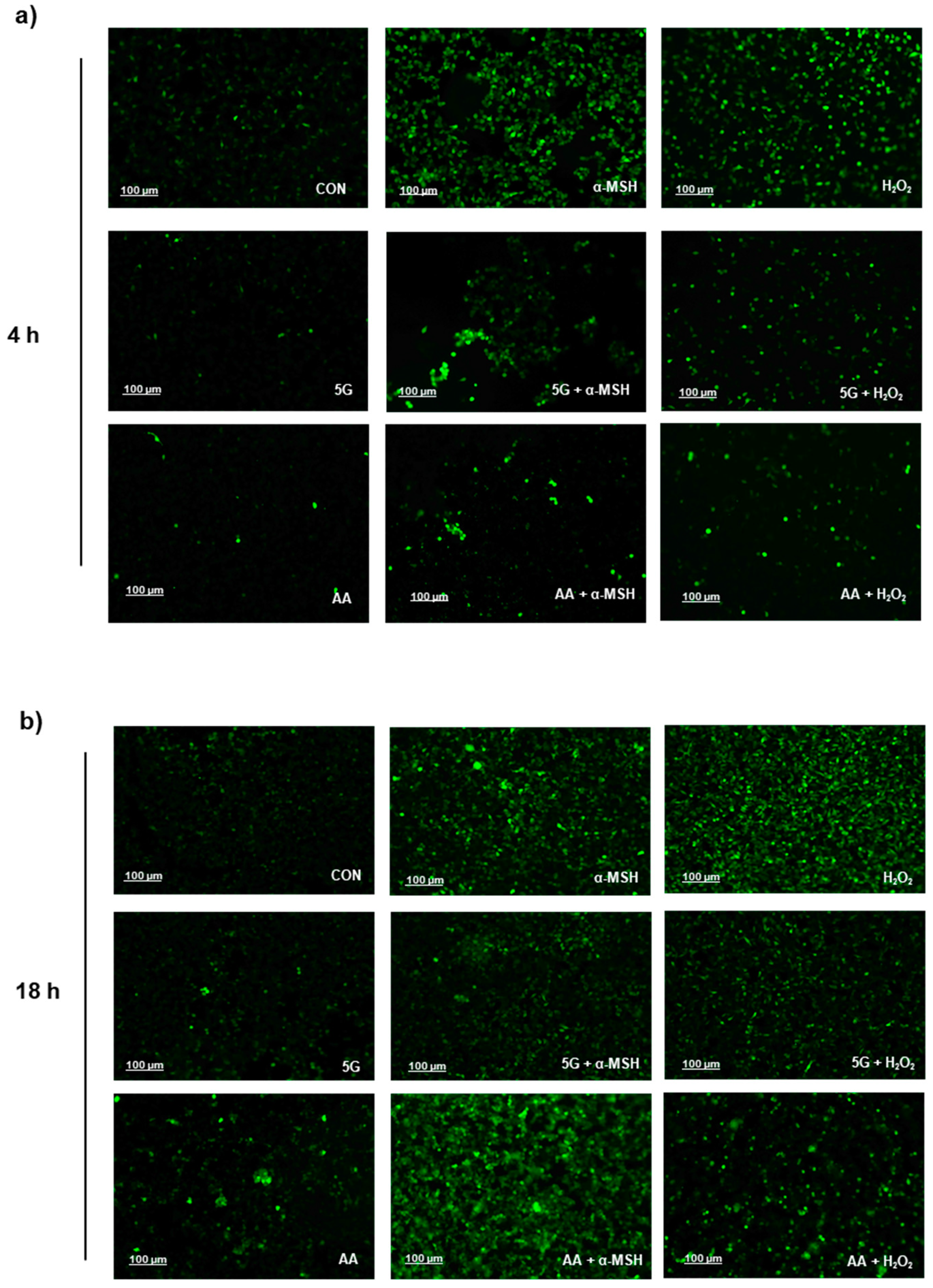
3.4. G EMR Exposure Suppresses ROS Production in α-MSH Stimulated B16F10
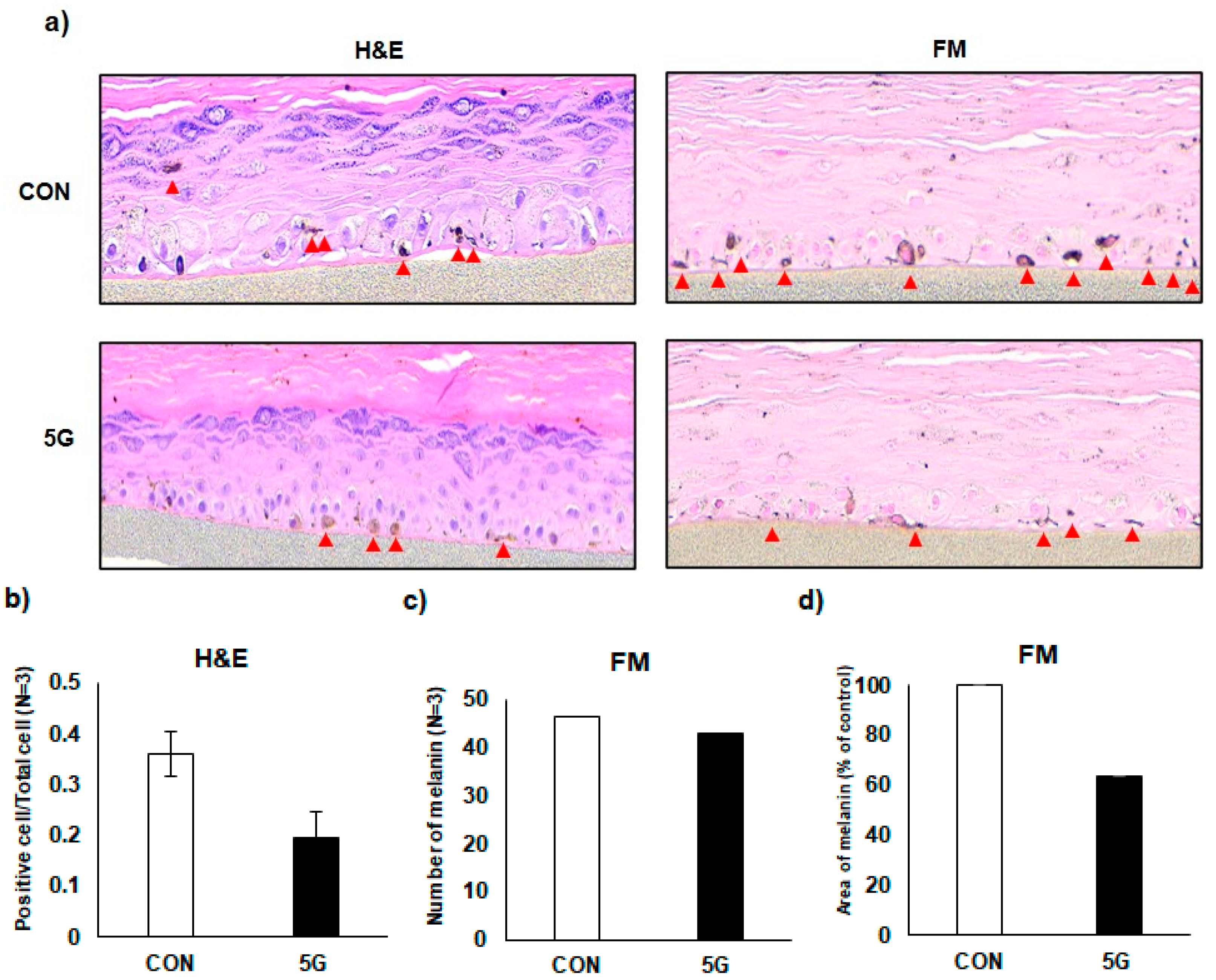
3.5. Effects of 5G on the Pigmentation in a Pigmented Human Skin Model, Melanoderm™
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- West, D.M. How 5G Technology Enables the Health Internet of Things; Brookings Center for Technology Innovation: Washington, DC, USA, 2016; Volume 3, pp. 1–20. [Google Scholar]
- Hardell, L.; Nyberg, R. [Comment] Appeals that matter or not on a moratorium on the deployment of the fifth generation, 5G, for microwave radiation. Mol. Clin. Oncol. 2020, 12, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Karipidis, K.; Mate, R.; Urban, D.; Tinker, R.; Wood, A. 5G mobile networks and health—A state-of-the-science review of the research into low-level RF fields above 6 GHz. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A. Towards 5G communication systems: Are there health implications? Int. J. Hyg. Environ. Health 2018, 221, 367–375. [Google Scholar] [CrossRef]
- Betzalel, N.; Ishai, P.B.; Feldman, Y. The human skin as a sub-THz receiver–Does 5G pose a danger to it or not? Environ. Res. 2018, 163, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, A.R.; Mortazavi, S. 5G Technology: Why Should We Expect a shift from RF-Induced Brain Cancers to Skin Cancers? J. Biomed. Phys. Eng. 2019, 9, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Li, W.; Seo, I.; Kim, B.; Fassih, A.; Southall, M.D.; Parsa, R. Low-level red plus near infrared lights combination induces expressions of collagen and elastin in human skin in vitro. Int. J. Cosmet. Sci. 2021, 43, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Busch, L.; Lohan, S.B. Wavelength, dose, skin type and skin model related radical formation in skin. Biophys. Rev. 2021, 13, 1091–1100. [Google Scholar] [CrossRef]
- Lim, H.W.; Kohli, I.; Granger, C.; Trullàs, C.; Piquero-Casals, J.; Narda, M.; Masson, P.; Krutmann, J.; Passeron, T. Photoprotection of the skin from visible light-induced pigmentation: Current testing methods and proposed harmonization. J. Investig. Dermatol. 2021, 141, 2569–2576. [Google Scholar] [CrossRef]
- Sutterby, E.; Thurgood, P.; Baratchi, S.; Khoshmanesh, K.; Pirogova, E. Evaluation of in vitro human skin models for studying effects of external stressors and stimuli and developing treatment modalities. View 2022, 3, 20210012. [Google Scholar] [CrossRef]
- Cario, M. How hormones may modulate human skin pigmentation in melasma: An in vitro perspective. Exp. Dermatol. 2019, 28, 709–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernerd, F.; Marionnet, C.; Duval, C. Solar ultraviolet radiation induces biological alterations in human skin in vitro: Relevance of a well-balanced UVA/UVB protection. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 15. [Google Scholar] [CrossRef] [PubMed]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schütz, R. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin transfer in the epidermis: The pursuit of skin pigmentation control mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef] [PubMed]
- Benito-Martínez, S.; Zhu, Y.; Jani, R.A.; Harper, D.C.; Marks, M.S.; Delevoye, C. Research techniques made simple: Cell biology methods for the analysis of pigmentation. J. Investig. Dermatol. 2020, 140, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myung, C.H.; Kim, K.; Park, J.I.; Lee, J.E.; Lee, J.A.; Hong, S.C.; Lim, K.-M.; Hwang, J.S. 16-Kauren-2-beta-18, 19-triol inhibits melanosome transport in melanocytes by down-regulation of melanophilin expression. J. Dermatol. Sci. 2020, 97, 101–108. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.S.; Kim, N.; Choi, H.-D.; Kang, D.-J.; Kim, H.R.; Lim, K.-M. Effects of electromagnetic waves with LTE and 5g bandwidth on the skin pigmentation in vitro. Int. J. Mol. Sci. 2021, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.I.; Lee, N.K.; Kang, K.W.; Kim, K.; Lee, D.Y. The effect of smartphone usage time on posture and respiratory function. J. Phys. Ther. Sci. 2016, 28, 186–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Chung, J.Y.; Jeon, S.B.; Lee, A.K.; Choi, H.D. Proposal of 28 GHz In Vitro Exposure System Based on Field Uniformity for Three-Dimensional Cell Culture Experiments. Bioelectromagnetics 2019, 40, 445–457. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dzagbletey, P.A.; Chung, J.Y.; Jeon, S.B.; Lee, A.K.; Kim, N.; Song, S.J.; Choi, H.D. Implementation of an in vitro exposure system for 28 GHz. ETRI J. 2020, 42, 837–845. [Google Scholar] [CrossRef]
- Lee, G.H.; Jin, S.W.; Kim, S.J.; Pham, T.H.; Choi, J.H.; Jeong, H.G. Tetrabromobisphenol A Induces MMP-9 Expression via NADPH Oxidase and the activation of ROS, MAPK, and Akt Pathways in Human Breast Cancer MCF-7 Cells. Toxicol. Res. 2019, 35, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- Awad, S.S.; Moftah, N.H.; Rashed, L.A.; Touni, A.A.; Telep, R.A.A. Evaluation of the effect of narrow band-ultraviolet B on the expression of tyrosinase, TYRP-1, and TYRP-2 mRNA in vitiligo skin and their correlations with clinical improvement: A retrospective study. Dermatol. Ther. 2021, 34, e14649. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Kuroda, T.S.; Mikoshiba, K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: Implications of a tripartite protein complex for melanosome transport. J. Biol. Chem. 2002, 277, 12432–12436. [Google Scholar] [CrossRef] [Green Version]
- Hume, A.N.; Tarafder, A.K.; Ramalho, J.S.; Sviderskaya, E.V.; Seabra, M.C. A coiled-coil domain of melanophilin is essential for Myosin Va recruitment and melanosome transport in melanocytes. Mol. Biol. Cell 2006, 17, 4720–4735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hume, A.N.; Ushakov, D.S.; Tarafder, A.K.; Ferenczi, M.A.; Seabra, M.C. Rab27a and MyoVa are the primary Mlph interactors regulating melanosome transport in melanocytes. J. Cell Sci. 2007, 120, 3111–3122. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, T.S.; Itoh, T.; Fukuda, M. Functional analysis of slac2-a/melanophilin as a linker protein between Rab27A and myosin Va in melanosome transport. Methods Enzymol. 2005, 403, 419–431. [Google Scholar]
- Wu, X.; Sakamoto, T.; Zhang, F.; Sellers, J.R.; Hammer, J.A., III. In vitro reconstitution of a transport complex containing Rab27a, melanophilin and myosin Va. FEBS Lett. 2006, 580, 5863–5868. [Google Scholar] [CrossRef] [Green Version]
- Strom, M.; Hume, A.N.; Tarafder, A.K.; Barkagianni, E.; Seabra, M.C. A family of Rab27-binding proteins: Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 2002, 277, 25423–25430. [Google Scholar] [CrossRef] [Green Version]
- Koga, S.; Nakano, M.; Tero-Kubota, S. Generation of superoxide during the enzymatic action of tyrosinase. Arch. Biochem. Biophys. 1992, 292, 570–575. [Google Scholar] [CrossRef]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Nahhas, A.F.; Abdel-Malek, Z.A.; Kohli, I.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol. Photoimmunol. Photomed. 2019, 35, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, Y.; Hariu, A.; Kato, C.; Seiji, M. Radical production during tyrosinase reaction, dopa-melanin formation, and photoirradiation of dopa-melanin. J. Investig. Dermatol. 1984, 82, 573–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Huh, Y.; Lim, K.M. Anti-Pigmentary Natural Compounds and Their Mode of Action. Int. J. Mol. Sci. 2021, 22, 6206. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lim, H.-M.; Ro, H.-S.; Ki, G.-E.; Seo, Y.-K. Pulsed electromagnetic fields increase pigmentation through the p-ERK/p-p38 pathway in zebrafish (Danio rerio). Int. J. Mol. Sci. 2018, 19, 3211. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue light-induced oxidative stress in live skin. Free Radic. Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef]
- Cho, S.-E.; Kim, Y.-M.; Kang, K.-H.; Kim, S.-C.; Park, J.-K.; Seo, Y.-K. Pigmentation effect of electromagnetic fields at various intensities to melanocytes. Tissue Eng. Regen. Med. 2016, 13, 560–567. [Google Scholar] [CrossRef]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Sahu, R.K.; Matlam, M.; Deshmukh, V.K.; Dwivedi, J.; Jha, A.K. In vitro techniques to assess the proficiency of skin care cosmetic formulations. Pharmacogn. Rev. 2013, 7, 97. [Google Scholar]
- Nappi, A.J.; Vass, E. Hydrogen peroxide generation associated with the oxidations of the eumelanin precursors 5, 6-dihydroxyindole and 5, 6-dihydroxyindole-2-carboxylic acid. Melanoma Res. 1996, 6, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tonissen, K.F.; Di Trapani, G. Modulating skin colour: Role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci. Rep. 2021, 41, BSR20210427. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic acid in skin health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [Green Version]
- Chou, H.-Y.; Wang, H.-M.D.; Kuo, C.-H.; Lu, P.-H.; Wang, L.; Kang, W.; Sun, C.-L. Antioxidant graphene oxide nanoribbon as a novel whitening agent inhibits microphthalmia-associated transcription factor-related melanogenesis mechanism. ACS Omega 2020, 5, 6588–6597. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.M.; Ko, Y.; Park, M.; Kim, B.; Park, J.E.; Kim, D.; Moon, Y.L.; Lim, W. Comparison of the alendronate and irradiation with a light-emitting diode (LED) on murine osteoclastogenesis. Lasers Med. Sci. 2017, 32, 189–200. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Machado, C.D.S.M.; De Marchi, T.; Casalechi, H.L.; Bjordal, J.M.; Carvalho, P.D.T.C.D.; Leal-Junior, E.C.P. Infrared low-level laser therapy (photobiomodulation therapy) before intense progressive running test of high-level soccer players: Effects on functional, muscle damage, inflammatory, and oxidative stress markers—A randomized controlled trial. Oxidative Med. Cell. Longev. 2019, 2019, 6239058. [Google Scholar] [CrossRef]
- Hopkins, J.T.; McLoda, T.A.; Seegmiller, J.G.; Baxter, G.D. Low-level laser therapy facilitates superficial wound healing in humans: A triple-blind, sham-controlled study. J. Athl. Train. 2004, 39, 223–229. [Google Scholar]
- Hussein, A.J.; Alfars, A.A.; Falih, M.A.J.; Hassan, A.-N.A. Effects of a low level laser on the acceleration of wound healing in rabbits. N. Am. J. Med. Sci. 2011, 3, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.; Simões, A.; Sá, P.H.R.N.; Eduardo, C.D.P. Improvement in quality of life of an oncological patient by laser phototherapy. Photomed. Laser Surg. 2009, 27, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Tatmatsu-Rocha, J.C.; Ferraresi, C.; Hamblin, M.R.; Maia, F.D.; Nascimento, N.R.F.D.; Driusso, P.; Parizotto, N.A. Low-level laser therapy (904 nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J. Photochem. Photobiol. B Biol. 2016, 164, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Castro, J.R.; da Silva Pereira, F.; Chen, L.; Arana-Chavez, V.E.; Ballester, R.Y.; DiPietro, L.A.; Simões, A. Improvement of full-thickness rat skin wounds by photobiomodulation therapy (PBMT): A dosimetric study. J. Photochem. Photobiol. B Biol. 2020, 206, 111850. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.B.; Kim, J.S.; Ko, Y.J.; Kwon, H.; Kim, S.W.; Min, H.K.; Kim, O.; Choi, H.R.; Kim, O.J. Effects of 635nm light-emitting diode irradiation on angiogenesis in CoCl2-exposed HUVECs. Lasers Surg. Med. 2011, 43, 344–352. [Google Scholar] [CrossRef]
- Lubart, R.; Lavi, R.; Friedmann, H.; Rochkind, S. Photochemistry and photobiology of light absorption by living cells. Photomed. Laser Ther. 2006, 24, 179–185. [Google Scholar] [CrossRef]
- Kanzaki, H.; Shinohara, F.; Kajiya, M.; Kodama, T. The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J. Biol. Chem. 2013, 288, 23009–23020. [Google Scholar] [CrossRef] [Green Version]
- Mosca, R.C.; Santos, S.N.; Nogueira, G.E.C.; Pereira, D.L.; Costa, F.C.; Pereira, J.X.; Zeituni, C.A.; Arany, P.R. The Efficacy of Photobiomodulation Therapy in Improving Tissue Resilience and Healing of Radiation Skin Damage. Photonics 2022, 9, 10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Lee, Y.S.; Kim, N.; Choi, H.-D.; Lim, K.-M. 5G Electromagnetic Radiation Attenuates Skin Melanogenesis In Vitro by Suppressing ROS Generation. Antioxidants 2022, 11, 1449. https://doi.org/10.3390/antiox11081449
Kim K, Lee YS, Kim N, Choi H-D, Lim K-M. 5G Electromagnetic Radiation Attenuates Skin Melanogenesis In Vitro by Suppressing ROS Generation. Antioxidants. 2022; 11(8):1449. https://doi.org/10.3390/antiox11081449
Chicago/Turabian StyleKim, Kyuri, Young Seung Lee, Nam Kim, Hyung-Do Choi, and Kyung-Min Lim. 2022. "5G Electromagnetic Radiation Attenuates Skin Melanogenesis In Vitro by Suppressing ROS Generation" Antioxidants 11, no. 8: 1449. https://doi.org/10.3390/antiox11081449
APA StyleKim, K., Lee, Y. S., Kim, N., Choi, H.-D., & Lim, K.-M. (2022). 5G Electromagnetic Radiation Attenuates Skin Melanogenesis In Vitro by Suppressing ROS Generation. Antioxidants, 11(8), 1449. https://doi.org/10.3390/antiox11081449







