Abstract
Hyperoside (Hyp), also known as quercetin-3-O-galactoside or 3-O-β-D-galactopyranosyl, is a well-known flavonol glycoside that is abundant in various fruits, vegetables, and medicinal plants. Hyp has been suggested to exhibit a wide range of biological actions, including cardiovascular, renal, neuroprotective, antifungal, antifibrotic, and anticancer effects. Accumulating evidence supports the pharmacological activities of Hyp in improving liver pathophysiology. Hence, the present literature review aims to summarize preclinical data suggesting the beneficial effects and underlying mechanisms of Hyp. In addition, our study focuses on hepatic antioxidant defense signaling to assess the underlying mechanisms of the biological actions of Hyp that are closely associated with liver diseases. Experimental findings from an up-to-date search showed that Hyp possesses hepatoprotective, antiviral, antisteatotic, anti-inflammatory, antifibrotic, and anticancer activities in cellular and animal models related to liver dysfunction by enhancing antioxidant responses. In particular, hepatocellular antioxidant defense via activation of erythroid-related nuclear factor 2 by Hyp chiefly explains how this compound acts as a therapeutic agent in liver diseases. Thus, this review emphasizes the therapeutic potential of Hyp as a strong antioxidative substance that plays a crucial role in the regulation of various liver disorders during their pathogenesis.
1. Introduction
Liver diseases, characterized by various liver injuries from steatosis to cancer, have steadily increased the global burden as one of the leading causes of morbidity and mortality worldwide. The prevalence of chronic liver disease (CLD) is currently estimated to reach 1.5 billion across the world, and CLD ranks fourth among diseases causing death in middle-aged adults (45–64 years) [1,2]. Common risk factors that can develop and aggravate various liver diseases include viral infection, heavy alcohol use, toxin exposure, drug overuse, overnutrition, and autoimmune responses.
Among them, excessive alcohol intake, viral hepatitis, and obesity-related metabolic disorders are three crucial risk factors, accounting for over 90% of patients with CLD [3]. While viral infection has been commonly regarded as the main etiology of CLD in the past, current issues in hepatology have frequently focused on nonalcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD) [4,5].
Several signaling pathways can be intricately intertwined among pathophysiological mechanisms that cause liver diseases, and oxidative stress induced by reactive oxygen species (ROS) is considered to be one of the main pathways associated with various liver disorders [6]. In particular, the liver is vulnerable to ROS attack, and it is also known as a crucial regulator that plays key roles in the development of liver diseases with high prevalence, including NAFLD, ALD, and drug-induced liver injury (DILI) [7,8,9].
Thus, some antioxidants, such as vitamin C/E and N-acetylcysteine (NAC), have been used to control oxidative-stress-induced liver dysfunction. However, evidence is yet to confirm the efficacy and safety of antioxidants as curative therapies to prevent and treat liver diseases [9,10]. Considering the potential of antioxidative strategies for the treatment of liver diseases, natural antioxidants, to some extent, can be rational candidates with the possibility of beneficial actions. Hyperoside (Hyp, quercetin-3-O-galactoside), a flavonol glycoside, can serve as a promising antioxidant to control hepatic oxidation in liver diseases.
Hyp has been known to be derived from many herbal plants, including Crataegus pinnatifida, Abelmoschus manihot, Hypericum perforatum, Geranium carolinianum, Zanthoxylum bungeanum, the flowers of Acacia melanoxylon, Rosaceae, Rhododendraceae, Werspearaceae, and Leguminosae [11,12,13,14]. As a potential medicinal constituent, Hyp has been reported to possess a wide range of pharmacological activities against lung cancer, cardiac injury, renal injury, lung fibrosis, and diabetes [15,16,17].
Notably, recent studies have shown that Hyp can act as an effective agent that can ameliorate various liver injuries triggered by toxin exposure, drug overuse, viral infection, and a high-fat diet. However, to the best of our knowledge, previous studies have not reviewed the pharmacological activities of Hyp in diverse liver diseases. Moreover, the association between antioxidant defenses in the liver and the central mechanisms involved in the biological actions of Hyp in treating liver diseases has not yet been identified. Therefore, the present study reviews the recent experimental results on the beneficial role of Hyp and the underlying molecular mechanism by which it regulates various conditions related to liver diseases to provide preclinical evidence for further well-designed studies that can be applied in clinical settings and drug development.
2. Methods
A comprehensive search for relevant preclinical studies on Hyp and pharmacological effects related to liver diseases published from inception to May 2022 was performed using PubMed (http://pubmed.ncbi.nlm.nih.gov/, accessed on 1 June 2022), EMBASE (https://www.embase.com/, accessed on 1 June 2022), CNKI (https://oversea-cnki-net-ssl.openlink.khu.ac.kr/index/, accessed on 1 June 2022), and Google Scholar (http://scholar.google.com/, accessed on 1 June 2022). The following key search terms were entered: (“hyperoside” OR “hyperin” OR “quercetin-3-O-galactoside” OR “3-O-β-D-galactopyranosyl”) AND (“liver” OR “hepato” OR “hepatic”) NOT (“human” OR “clinical”). To retrieve as many related articles as possible, all studies obtained from reference lists as well as the above two databases were manually reviewed. After excluding duplicated or inapplicable studies, 35 articles were reviewed meticulously in this study.
3. Phytochemistry of Hyperoside
Hyp (PubChem CID: 5281643), a synonym for 3-O-galactoside of quercetin, is a well-known antioxidant flavonoid frequently found in many herbal plants. The formal name is 2-(3,4-dihydroxyphenyl)-3-(β-D-galactopyranosyloxy)-5,7-dihydroxy-4H-1-benzopyran-4-one, and its molecular formula is C21H20O12 with a molecular weight of 464.3793 g/mol (Figure 1). Hyp is a type of flavonoid possessing many biological functions and a secondary metabolite serving a role as valuable natural products [18]. It is reported that the genera Hypericum, Arbutus, Abelmoschus, Zanthoxylum, Houttuynia, etc. are rich in Hyp, and it has been extracted from over 30 herbal plants [14,19,20,21,22,23,24].
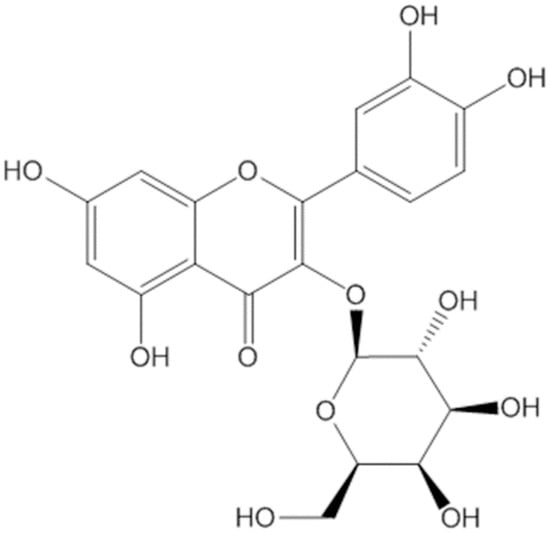
Figure 1.
The chemical structure of Hyp.
For instance, high-performance liquid chromatography (HPLC) chromatography for detecting Hyp contents showed the quantification of Hyp at 353 nm of UV and 16.13 min of retention time. The leaf parts of the plant showed larger amount of Hyp than the parts of the flower and stem. In addition, the content of Hyp in Hypericum perforatum was superior over that in Hypericum leptophyllum [24]. Similarly, 96% ethanol extract of Hypericum perforatum showed higher contents of Hyp compared with rutin and quercetin [23].
The isolation of Hyp from medicinal plant extracts mostly used ethanol solvent and the extraction methods of reflux and ultra-sonication with a range of purity from 1.91–91.41% [14]. As depicted in Figure 1, the molecular structure of Hyp is composed of multiple polar groups that include one carbonyl group, three ether linkages, and eight hydroxyl groups [25].
A recent study reported that Hyp contents obtained from Hypericum species can be analyzed quantitatively and qualitatively using high-performance thin-layer chromatography (HPTLC) methods [26]. However, the intact detection of Hyp using high-performance liquid chromatography-ultraviolet (HPLC-UV) or liquid chromatography-tandem mass spectrometry (LC-MS-MS) methods is nearly impossible because of the galactose sugar moiety of Hyp. Instead, the use of HPLC-UV analysis to detect quercetin following enzymatic hydrolysis with β-glucuronidase and sulfatase is able to show the pharmacokinetic profiles of Hyp, including tmax and Cmax [27].
However, compared to isoquercitrin (quercetin-3-O-glucoside), the absorption rate in the gastrointestinal tract and hydrolyzation of Hyp were poorer owing to the type of sugar moiety [28]. Thus, there are some limitations to the clinical application of Hyp, owing to its poor solubility, low bioavailability, and instability. Therefore, several novel tools, such as nanotechnology, microencapsulation, and eutectic mixtures, have been investigated to enhance the bioavailability and clinical potential of Hyp [29]. Moreover, further researches on the optimized extraction method, structure-based analysis, and technology improving yield of Hyp need to be conducted for clinical use.
4. Pharmacological Effects of Hyperoside in Liver Diseases
4.1. Hepatoprotective Effects
The liver is involved in various physiological functions, including the metabolism, immune responses, excretion, and detoxification [30]. Many factors, such as overnutrition, ethanol, drugs, and xenobiotics, lead to dysregulation of liver function. Hepatic injury is a major health problem worldwide [31]. Usually, liver injury leads to pathological manifestations in which abnormal serum markers and histological deterioration are observed due to hepatocyte death and the activation of hepatic stellate cells (HSCs) and Kupffer cells [31]. Considering that liver injury has been chiefly implicated in triggering various liver disorders, the beneficial roles of hepatoprotective agents have been emphasized for the treatment or prevention of liver and biliary tract diseases [32,33].
When rodents or liver cells are exposed to toxic chemicals, such as carbon tetrachloride (CCl4), hydrogen peroxide (H2O2), tert-butyl hydroperoxide (t-BHP), alcohol, and D-galactosamine (d-GalN), significant alterations in their liver function are observed, with substantial evidence that elevated serum liver enzymes correlate with the severity of liver injury. These high levels of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are mostly accompanied by histological deterioration of the liver tissue [34,35,36,37,38,39].
As depicted in Figure 2, Hyp serves as an effective hepatoprotective agent by reducing hepatocellular damage due to oxidative stress induced by chemicals [34,35,36,37,40,41,42,43]. Similar antioxidative properties of Hyp were shown in H2O2-induced intracellular oxidative stress in HepG2 cell [44]. Notably, the regulation of hepatic heme oxygenase-1 (HO-1), nuclear factor erythroid 2-related factor 2 (Nrf2), and mitogen-activated protein kinase (MAPK) in the presence of Hyp may potentiate anti-apoptosis and eventually counteract chemical-driven hepatotoxicity in rodents [34,35,36,37,38,43].
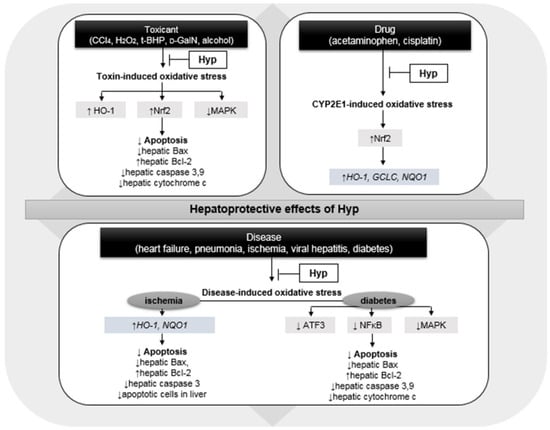
Figure 2.
Hepatoprotective effects of hyperoside (Hyp) via the regulation of oxidative stress triggered by toxic chemical, drug, and various diseases; CCl4, carbon tetrachloride; H2O2, hydrogen peroxide; t-BHP, tert-butyl hydroperoxide; d-GalN, D-galactosamine; HO-1, heme oxygenase-1; Nrf2, nuclear factor erythroid 2-related factor 2; MAPK, mitogen-activated protein kinase; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; GCLC, glutamate-cystein ligase catalytic subunit; NQO1, NAD(P)H quinone dehydrogenase 1; ATF3, cyclic AMP-dependent transcription factor; and NFκB, nuclear factor kappa B. Upward pointing arrow (↑) and downward pointing arrow (↓) represent an increase and a decrease in gene/protein expression or numerical values, respectively.
The existence of cytochrome P450 2E1 (CYP2E1)-induced hepatotoxicity by cisplatin and acetaminophen is well documented because both drugs undergo hepatic metabolism. CYP2E1, a cytochrome 450 (CYP450) involved in drug-induced liver injury, is predominantly expressed in the liver [45,46]. Although NAC has been considered a standard therapeutic agent for relieving acetaminophen poisoning, there still exist certain limitations for its use due to the possibility of inducing hepatic steatosis and systemic inflammation [47]. Several studies have investigated the efficacy of Hyp in reducing hepatotoxicity in mice and liver cells suffering from drug-induced liver injury [48,49,50,51].
These effects of Hyp were associated with the normalization of oxidative stress after high-level administration of acetaminophen or cisplatin with an increase in nuclear Nrf2 levels in the liver [48,49,50,51]. Subsequently, Hyp administration enhanced the hepatic expression of the target genes of Nrf2, such as HO-1—glutamate–cystein ligase catalytic subunit (GCLC)—and NAD(P)H quinone dehydrogenase 1 (NQO1) in LO2 liver cells and C57BL/6 mice in the presence of acetaminophen [49] (Figure 2). However, no study has reported Hyp-induced hepatic steatosis or a systemic inflammatory response similar to that induced by NAC. Hyp isolated from the leaves of Juglans sinensis exerted protective effects against hepatotoxicity in HepG2 cells induced by amiodarone and nitrofurantoin; however, the efficacy was not significant [52].
The hepatoprotective efficacy of Hyp has also been evaluated in experimental models of various diseases, including heart failure, pneumonia, hepatitis B, hepatic ischemia, and diabetes [53,54,55,56,57,58,59,60]. Hyp administration resulted in a significant reduction in serum AST and ALT levels and ameliorated the severity of hepatocellular necrosis and vacuolation. In particular, Hyp interfered with ischemia-induced oxidative stress by increasing hepatic proteins HO-1 and NQO1, thus, eventually decreasing apoptotic cells in the liver of hepatic ischemia-reperfusion injury Wistar rats [57].
A similar mechanism of Hyp, involved in reducing oxidative stress and inducing anti-apoptosis, was investigated in another study showing hepatotoxicity due to hyperglycemia [53] (Figure 2). Hyp also decreased serum AST and ALT levels by reversing the hepatic malondialdehyde (MDA) and superoxide dismutase (SOD) contents in ApoE−/− mice fed high-fat diet and concanavalin A-induced Kunming mice [61,62]. These studies suggest that Hyp treatment has considerable beneficial effects on hepatotoxicity induced by toxic substances, drugs, and diseases (Table 1). Therefore, further studies are needed to support the efficacy of Hyp and expand its clinical use as an effective hepatoprotectant for the treatment of various liver diseases.

Table 1.
Hepatoprotective activities of hyperoside (Hyp) in experimental models related to various liver diseases.
4.2. Antiviral Effects
Over the past decades, viral infection has been regarded as a major risk factor for chronic liver disease [64]. Although higher vaccination coverage and new antivirals have helped decrease the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) cases, viral hepatitis remains a crucial cause of chronic liver diseases [65]. Moreover, the incapability of viral eradication, intolerance, and adverse effects of antiviral agents have been described as limitations in the treatment of viral hepatitis.
Hyp exerts antiviral effects against both HBV and HCV (Table 2). In ducklings inoculated with duck HBV DNA, Hyp at doses of 25–300 mg/kg significantly suppressed serum HBV DNA [54,66,67]. It is noteworthy that Hyp reduced intrahepatic covalently closed circular DNA (cccDNA), serving as a stable template for HBV replication in infected cells, and decreased the secretion of Th1 cytokines [68]. This involvement of Hyp in HBV cccDNA inhibition and the immune response to viral infection may be promising for the development of Hyp as an antiviral. In addition, the viral rebound of HBV in the presence of Hyp in ducklings was lower than that of ducklings treated with lamivudine, a nucleoside reverse transcriptase inhibitor against HBV [54,67].

Table 2.
Antiviral activities of hyperoside (Hyp) in experimental models related to hepatitis B virus (HBV) and hepatitis C virus (HCV) infection.
Furthermore, the antiviral effects of Hyp from Abelmoschus manihot against the secretion of HBsAg and HBeAg were stronger than lamivudine-induced suppression in HepG2.2.15 cells at the same dose of 0.05 g/kg [54]. Additionally, Hyp inhibited HCV replication by prohibiting HCV NS3 protease via docking the binding sites of NS3 in Huh-7 cells transfected with the NS3 gene of HCV [69]. Thus, further studies are needed to explore the mechanisms underlying the antiviral activities of Hyp to support its function in ameliorating viral hepatitis.
4.3. Antisteatotic Effects
The liver is one of the major organs that regulate the fat metabolism [70]. Impairment of hepatic lipid metabolism may induce abnormal lipid accumulation in liver tissue. Hepatic steatosis is an adaptive condition that responds to lipid toxicity; however, various hepatic disorders, including NAFLD, alcoholic liver disease and drug-induced liver injury, often progress from hepatic steatosis [71,72]. In particular, as the prevalence of obesity is sharply increasing, fat-rich diet-induced hepatic steatosis is emerging as a major liver disease [73].
Although previous studies on the antisteatotic effects of Hyp are scarce, this compound has been reported to reduce hepatic fat accumulation in rodents fed a high-fat diet and alcohol and in diabetes-induced rats [39,60,61,74,75,76]. The antisteatotic activity of Hyp in high-fat diet-induced C57BL/6 mice and diabetes-induced rats occurred with liver weight loss and reduced hepatic lipid contents, such as TG, TC, and NEFA [60,74].
Regarding the underlying mechanisms attributed to the antisteatotic effects of Hyp, it exerted beneficial actions in (1) maintaining hepatic lipid and glucose homeostasis by activating peroxisome proliferator-activated receptor gamma (PPARγ), which lowers glucose levels and reverses lipotoxicity; (2) reducing oxidative stress; (3) synthesizing bile acids from cholesterol by activating key catalytic enzymes CYP7A1 and CYP27A1; (4) increasing the β-oxidation of free fatty acids by the activation of nuclear farnesoid X receptor (FXR) and transcription factor liver X receptor (LXR)α implicated in lipid oxidation; and (5) inhibiting de novo lipogenesis by modulating hepatic de novo lipogenesis markers acetyl-CoA carboxylase (ACC) and sterol regulatory element binding proteins (SREBP)1,2 in vivo and in vitro [39,61,74,75,76] (Table 3).

Table 3.
Antisteatotic activities of hyperoside (Hyp) in experimental models related to hepatic steatosis.
4.4. Anti-Inflammatory Effects
The liver has recently been regarded as an important immunologic and metabolic organ [77]. Kupffer cells, innate lymphocytes, and many antigen-presenting cells are enriched in the liver tissue, and their unique immune microenvironment is closely associated with inflammatory reactions in the liver [78]. In particular, inflammatory processes are inevitably involved in the development of various liver diseases, including viral infections, autoimmune hepatitis, alcoholic hepatitis, and nonalcoholic steatohepatitis (NASH) [78]. Thus, therapeutic strategies that interfere with inflammatory markers, such as anti-tumor necrosis factor (TNF)-α treatment (e.g., infliximab and etanercept) and interleukin-24 therapy, are being investigated for the treatment of liver diseases [79,80].
The hepatic expression of several key inflammatory cytokines and chemokines, such as TNF-α, interleukin (IL)-1β, IL-6, nitric oxide (NO), inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)2, C-C motif chemokine ligand (CCL)2, and CCL5, was downregulated by the administration of Hyp during the inflammatory pathogenesis induced by CCl4 and a high-fat diet [34,38,53,60,76].
The neutralization of inflammatory markers by Hyp in hepatic tissue was accompanied by marked improvement in histological findings from abnormal conditions, including hepatic inflammatory cell infiltration and Kupffer cell hyperplasia [34,38,53,76]. Hyp obtained from Artemisia capillaris and Zanthoxylum bungeanum leaves displayed anti-inflammatory effects against CCl4 and high-carbohydrate/high-fat diet and alloxan-induced inflammation via hepatic removal of oxidative stress, respectively [34,53].
Hyp also lowered hepatic inflammatory cell infiltrations through the amelioration of hepatic oxidative stress induced by concanavalin A, high-fat diet, and alcohol [39,61,62]. In addition, the regulation of other MAPKs, NFκB, and apoptotic factors also suggests that Hyp controls inflammation [38,53,60] (Table 4). In this respect, antioxidant, anti-apoptosis, and the regulation of MAPKs and NFκB may be mechanisms that support Hyp as a beneficial anti-inflammatory agent against hepatic inflammation.

Table 4.
Anti-inflammatory activities of hyperoside (Hyp) in experimental models related to hepatic inflammation.
4.5. Antifibrotic Effects
Liver fibrosis is a vital process in wound healing that occurs during liver injury; however, it is associated with the deformation of normal hepatocytes, collagen deposition, and overaccumulation of the extracellular matrix (ECM) [81]. Fibrosis reaction and severity largely influence the overall prognosis, exacerbation, and management of CLD because liver stiffness is a significant condition that increases the risk of liver-related disorders and all-cause deaths [82,83,84]. Hence, blocking fibrogenesis pathways may play a crucial role in managing patients with CLD.
Hepatic stellate cell (HSC) activation is a common process in the pathogenesis of liver fibrosis, which subsequently triggers the excessive deposition of ECM in liver tissue. Regulation of HSC proliferation and activation is suggested to be a key therapeutic strategy that prevents the progression of hepatic fibrosis in different liver disorders. Hyp inhibits the proliferation of LX-2 human HSC line via apoptosis induction and intracellular ROS reduction [85]. The inhibition of LX-2 cells by Hyp was found to induce significant downregulation of α-smooth muscle actin (α-SMA) and collagen I mRNA and protein expression, which increased during HSC activation [85].
Similar results were observed in the presence of Hyp in transforming growth factor (TGF)-β-induced LX-2 cells [55]. TGF-β signaling is often referred to as an important pathway involved in different stages of liver disease progression, and high levels of TGF-β may be a cause and consequence of liver damage [86]. With regard to TGF-β overactivation in hepatic tissue induced by a high-fat diet and heart failure, Hyp significantly inhibited hepatic TGF-β expression. The inactivation of TGF-β signaling by Hyp may contribute to the prevention of liver cirrhosis and cancer initiation [86].
In addition to targeting TGF-β, Hyp improved histological findings exhibiting a hepatic fibrotic area increased by different etiologies, such as CCl4, a high-fat diet, and heart failure in mice and rats [35,55,76]. In particular, Hyp ameliorated CCl4-induced liver fibrosis and heart failure-induced hepatic fibrosis, accompanied by hepatocellular antioxidant defenses [35,59]. Since Hyp lowered the ROS levels in LX-2 cells, and various antioxidants are currently used to treat liver fibrosis, the antifibrotic effects of Hyp via targeting oxidative stress should be explored to counteract liver fibrosis (Table 5).

Table 5.
Antifibrotic activities of hyperoside (Hyp) in experimental models related to hepatic fibrosis.
4.6. Anticancer Effects
Hepatocellular carcinoma (HCC) is a predominant tumor type that develops from hepatocytes and is a major cause of cancer-related mortalities [87]. Due to late diagnosis, frequent recurrence, and impaired liver function, HCC has a poor prognosis with high mortality and a low therapeutic rate [88]. In particular, the HCC disease burden is considerable in areas with a high prevalence of HBV and HCV infection because the majority of HCC cases are attributed to chronic hepatitis B and C [89,90].
Moreover, the number of patients with NASH-related HCC is steadily increasing in developed countries. However, the therapeutic efficacy of current chemotherapeutic agents remains limited, and recurrence after HCC treatment often occurs. Hence, preemptive management and modification of HCC-predisposing factors to reduce HCC development may serve as an effective option. Furthermore, complementary therapy to alleviate the adverse effects of anticancer drugs and enhance their efficacy is needed.
First, Hyp significantly suppressed the survival rates of both HepG2 and insulin-resistant HepG2 cells [75,91,92]. In particular, the cytotoxic effects of Hyp against HepG2 cells may involve cell cycle arrest at G0/G1 through the downregulation of cyclin-D1 and c-Myc expression, and apoptosis induction via the activation of p53/caspase pathway [91]. Moreover, Hyp attenuates the expression of bone morphogenetic protein (BMP)-7, p-protein kinase B (AKT), and phosphoinositide 3-kinase (PI3K), which are involved in inducing metastasis, along with the antiproliferation of HCC [91].
Second, Hyp may be a promising compound against HBV-related HCC. PLC-PRF-5 cells, the Alexander hepatoma cell line, grow continuously and secrete HBV surface antigens [93]. Hyp significantly inhibited HCC growth and increased the average survival rates of BALB/c mice injected with PLC-PRF-5 cells [94]. More importantly, Hyp treatment decreased tumor migration and invasion in PLC-PRF-5 high metastatic cells, and this antimetastatic effect of Hyp was associated with the regulation of various factors involved in the epithelial–mesenchymal transition (EMT) by increasing hepatic E-cadherin and suppressing the expression of quaking, circRNAs, and hepatic vimentin [94]. Eventually, Hyp downsized metastatic lung nodules in an HCC in vivo model [94]. Hence, Hyp may affect metastatic properties, such as migration, the invasion of other organs, the promotion of HCC EMT, and tumor growth (Table 6).

Table 6.
Anticancer activities of hyperoside (Hyp) in experimental models related to hepatocellular carcinoma (HCC).
5. Safety of Hyperoside
According to recent studies, a few studies have investigated the safety of Hyp [14,95]. In 40 healthy BALB/c mice, Hyp treatment (5,000 mg/kg, intragastrical route once) did not result in mouse poisoning, death, acute toxicity, or genotoxicity [96]. In addition to the toxicity evaluation after administration of high-dose Hyp in mice, Wistar rats were orally administered with Hyp (30, 175, and 1000 mg/kg) for 6 months to investigate the long-term toxicity of Hyp [97]. No significant changes in rat behavior, food-intake, and weight gain were observed in the presence of chronic treatment of Hyp by gavage. However, renal interstitial inflammatory cell infiltration was observed in about 20% in 20 rats of the high-dose (1000 mg/kg) group of Hyp.
At the end of the recovery period of 1 month, the renal pathological injuries were gradually diminished. Although Hyp may induce renal toxicity, this can be reversible after withdrawal of the compound. In addition, Hyp treatment (8, 65, and 500 mg/kg) for 38 weeks induced no abnormal reactions in symptoms, blood tests, and histological examination in Beagle dogs, and mild toxicity of the kidney and liver was negligible and reversible [98]. Other teams found that Hyp can contribute to the alleviation of renal cellular aging and injury in NRK-52E cells and rats exposed to D-galactose [99] as well as the protection of HK-2 (human renal proximal tubule cells) from high-glucose-induced apoptosis and inflammation [100].
Thus, it is still controversial whether Hyp induces renal and hepatic toxicity, and further studies to decide the appropriate dosages and treatment periods applicable to clinical use are needed, particularly for patients with renal or hepatic dysfunction. In terms of the impact of Hyp on fetal mice, 30 and 175 mg/kg Hyp treatment showed no significant changes in fetal appearance and growth; however, high-dose Hyp (1000 mg/kg) administration during 6–15 days after gestation significantly retarded the growth of fetus (weight and the lengths of embryo and tail) of pregnant Wistar rats [101].
On the other hand, a recent study reported that Hyp (40 mg/kg) increased the body weight of fetuses and decreased pregnancy loss in a rat model with recurrent pregnancy loss [102]. Eventually, a proper administration of Hyp based on a safe dosage setting may ensure that pregnant women can safely receive treatment depending on their indications. Consequently, although Hyp, a dietary flavonoid, showed strong antioxidant and hepatoprotective potency, further evaluation of the safety of Hyp needs to be performed due to the lack of evidence in experimental models.
6. Discussion
This review summarizes the beneficial activities of Hyp associated with liver disease and the underlying signaling mechanisms in vitro and in vivo reported in experimental studies. As illustrated in Figure 3, Hyp has been reported to exert hepatoprotective, antiviral, antisteatotic, anti-inflammatory, antifibrotic, and anticancer effects. Hyp displayed its pharmacological effects by regulating multiple mechanisms, including: (1) the depletion of hepatocellular oxidative state through the modulation of Nrf2 and ATF3; (2) the regulation of several anti-apoptotic factors; (3) the activation of hepatic metabolism on cholestasis and lipogenesis; (4) the suppression of HSC activation; and (5) the inhibition of EMT induction and the induction of cell cycle arrest (Figure 3).
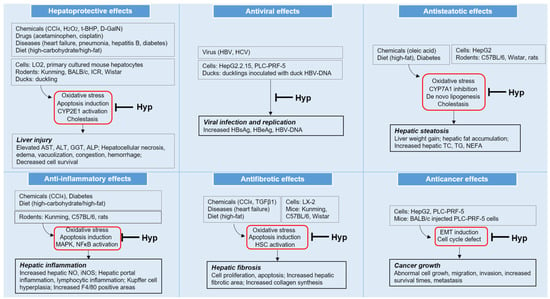
Figure 3.
Pharmacological effects and underlying mechanisms of hyperoside (Hyp) in different pathological conditions related to various liver diseases; CCl4, carbon tetrachloride; H2O2, hydrogen peroxide; t-BHP, tert-butyl hydroperoxide; d-GalN, D-galactosamine; CYP, cytochrome P450; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl peptidase; ALP, alkaline phosphatase; TC, total cholesterol; TG, triglyceride; NEFA, nonesterified fatty acids; MAPK, mitogen-activated protein kinase; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; iNOS, inducible nitric oxide synthase; HSC, hepatic stellate cell; and EMT, epithelial–mesenchymal transition.
For most liver pathologies, ROS are involved in different reactions that generate hepatic steatosis, inflammation, and fibrosis, and an impaired redox system in hepatic tissue can cause direct/indirect hepatic injury. Thus, enhancement of the antioxidant defense system by Hyp, consisting of reduced ROS generation and increased ROS elimination in hepatic tissue, may be one of the major molecular mechanisms of Hyp. In particular, major sources of oxidative stress involved in the therapeutic targets of Hyp are derived from CYP2E1 and the downregulation of the expression of antioxidant genes and Nrf2-regulated phase II enzymes occurring in liver disorders.
As depicted in Figure 4, changes in the redox and detoxifying potential of hepatocytes induced by drugs, toxic substances, diseases, and a high-fat diet can result in oxidative-stress-derived liver injury, as demonstrated by the decreased formation of hepatic antioxidant enzymes and high levels of ROS in the liver. This liver damage related to oxidative stress is represented by liver pathologies, such as hepatocyte apoptosis, hepatic steatosis, Kupffer cell activation, monocyte aggregation, HSC activation, and ECM deposition. Thus, the imbalance between antioxidant and pro-oxidant conditions can induce progressive liver diseases, including simple fatty liver, NASH, toxic hepatitis, viral hepatitis, and liver cirrhosis.
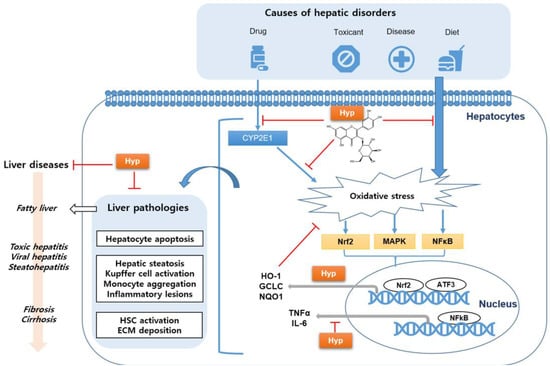
Figure 4.
Schematic diagram of hyperoside (Hyp), which improves various liver diseases by reducing oxidative stress; CYP, cytochrome P450; Nrf2, nuclear factor erythroid 2-related factor 2; MAPK, mitogen-activated protein kinase; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; HO-1, heme oxygenase-1; GCLC, glutamate–cystein ligase catalytic subunit; NQO1, NAD(P)H quinone dehydrogenase 1; TNF-α, tumor necrosis factor-alpha; IL-6, interleukin-6; ATF3, cyclic AMP-dependent transcription factor; HSC, hepatic stellate cell; and ECM, extracellular matrix.
Hyp has been demonstrated to exert marked scavenging activities against ROS attack occurring in hepatocytes induced by a variety of liver diseases. Particularly, Hyp displayed beneficial activity against CYP2E1-dependent oxidative stress and hepatotoxicity induced by drugs, such as paracetamol and cisplatin, which activated the upregulation of Nrf2 and the induction of Nrf2-targeted genes for detoxification in hepatic cells. Additionally, Hyp regulated the oxidative-stress-induced alterations of MAPK and NFκB as well as Nrf2, which impact transcription factors, thereby, resulting in the production of detoxifying enzymes and inflammatory cytokines.
Eventually, Hyp showed biological actions in experimental models against oxidative stress that disrupt the physiological liver function or normal architectural structure of liver. These antioxidant actions of Hyp may be closely associated with its chemical structure, particularly depending on the main structure and position of the galactose sugar moiety attached to this compound. For instance, Hyp has been reported to exhibit excellent antioxidant potency in in vitro and in vivo studies, and its activity was influenced by the presence of galactose at the position C3 and β-hydroxyl groups at C4 and C6 as well as the antioxidant structure–activity relationship (SAR) [44,60].
In addition, the hydroxyl groups in positions 3’ and 4’ of ring B of Hyp were found to possess high *OH-scavenging activity [103]. Hence, further investigations on SAR analysis of Hyp need to be performed to elucidate the SAR relationship of beneficial actions of Hyp against liver diseases.
In addition to the Hyp-induced alterations of oxidative stress against liver injury, it has been reported that several mechanisms are involved in the pharmacological effects of Hyp responsible for ameliorating viral infection, steatosis, and HCC. Hyp exerted antiviral effects via the inhibition of the formation of hepatic cccDNA along with the suppressed Th1 cytokine secretion in mouse spleen lymphocytes. In regard to hepatic steatosis, Hyp attenuated the lipid accumulation in liver by preserving hepatic lipid and bile acid homeostasis via the regulation of PPARγ, FXR, LXRα, ACC, SREBP, cholesterol 7α-hydroxylase (CYP7A1), and CYP27A1. It has also been demonstrated that the anticancer properties of Hyp are attributed to cell cycle arrest and the PI3K/AKT signaling pathway.
Since the early 1960s, Hyp has been reported to be isolated from over 30 herbal plants via various extraction technologies; however, Hyp involved in the effects for the treatment of liver diseases in the present study has been isolated from eight species of medicinal plants: Hyp derived from Artemisia capillaris and Zanthoxylum bungeanum showed hepatoprotective and anti-inflammatory effects; Hyp obtained from Abelmoschus manihot exerted hepatoprotective and antiviral effects; Hyp from Zanthoxylum schinifolium, Apocynum venetum, Canarium album, and Cuphobia nematocypha showed hepatoprotective activities; and Hyp from Hypericum patulum had antisteatotic effects.
Among them, further investigation is required to evaluate the role of Hyp as an active constituents of Artemisia capillaris—a well-known herbal plant frequently used for treating liver disease [104]. In addition, based on above medicinal plants containing Hyp, further studies on efficient and economic extraction methods maximizing the purity and yield are needed [14,95]. Hence, Hyp may be an active compound present in various herbal plants that can be widely applied in clinical settings owing to its hepatoprotective, antiviral, antisteatotic, anti-inflammatory, antifibrotic, and anticancer activities.
In addition, the antioxidant properties of Hyp can be extended to the suppression of glycative stress, which can take place in various liver diseases [105]. Although Hyp attenuated advanced glycation end-products (AGEs) in ECV304 bladder cancer cells and suppressed apoptosis and necrosis in AGEs-induced podocytes, there still exists no study describing the relationship of antiglycant effects of Hyp with liver disorders [106,107]. Thus, relevant further studies need to be performed to strengthen and support pharmacological effects of Hyp in liver disorders.
This review provides evidence for the use of Hyp in various liver diseases. Most liver diseases, such as NAFLD, DILI, hepatitis, and liver cirrhosis, are closely associated with oxidative-stress-induced injury, particularly in the early stages of hepatic disorders. Hence, Hyp can improve liver function and reverse pathological changes, which are mainly mediated by antioxidant signaling.
7. Conclusions
Hyp can serve as a potential therapeutic agent against various hepatic pathological changes, including liver injury, steatosis, viral infection, inflammation, fibrosis, and cancer, mainly by reducing oxidative stress, inhibiting apoptosis, and regulating the lipid and cholesterol metabolism. In particular, the present review highlights the multipotent antioxidant mechanism of Hyp, and these antioxidant properties can be useful in mitigating hepatic disorders but not for cancer. Therefore, Hyp can be considered to be a beneficial compound that exhibits a wide range of biological actions with minimal hepatotoxicity in various liver diseases.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Larson, J.J.; Yawn, B.; Therneau, T.M.; Kim, W.R. Underestimation of liver-related mortality in the United States. Gastroenterology 2013, 145, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Oztumer, C.A.; Chaudhry, R.M.; Alrubaiy, L. Association between behavioural risk factors for chronic liver disease and transient elastography measurements across the UK: A cross-sectional study. BMJ Open Gastroenterol. 2020, 7, e000524. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Huang, X.; Liu, H.; Wang, Y. Interactions of hepatitis B virus infection with nonalcoholic fatty liver disease: Possible mechanisms and clinical impact. Dig. Dis. Sci. 2015, 60, 3513–3524. [Google Scholar] [CrossRef]
- Younossi, Z.; Henry, L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology 2016, 150, 1778–1785. [Google Scholar] [CrossRef]
- Singal, A.K.; Jampana, S.C.; Weinman, S.A. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011, 31, 1432–1448. [Google Scholar] [CrossRef]
- Ma, Y.; Lee, G.; Heo, S.Y.; Roh, Y.S. Oxidative stress is a key modulator in the development of nonalcoholic fatty liver disease. Antioxidants 2021, 11, 91. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef]
- Villanueva-Paz, M.; Morán, L.; López-Alcántara, N.; Freixo, C.; Andrade, R.J.; Lucena, M.I.; Cubero, F.J. Oxidative stress in drug-induced liver injury (DILI): From mechanisms to biomarkers for use in clinical practice. Antioxidants 2021, 10, 390. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, J.; An, X.; Dai, M.; Jiang, Z.; Zhang, L.; Yu, S.; Huang, X. UPLC-MS/MS Method for the Determination of Hyperoside and Application to Pharmacokinetics Study in Rat After Different Administration Routes. Chromatographia 2021, 84, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Chen, A.; Zhao, L.; Cao, F.; Ding, G.; Xiao, W. One-pot synthesis of hyperoside by a three-enzyme cascade using a UDP-galactose regeneration system. J. Agric. Food Chem. 2017, 65, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Falco, M.R.; de Vries, J.X. Isolation of hyperoside (quercetin-3-D-galactoside) from the flowers of acacia melanoxylon. Naturwissenschaften 1964, 51, 462–463. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.C.; Zhou, S.J.; Li, Y.; Zheng, T.T.; Zhou, C.Z.; Wan, X.H. Hyperoside: A review on its sources, biological activities, and molecular mechanism. Phytother. Res. 2022, 36, 2779–2802. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Wang, L.; Jin, X.; Sui, H.; Liu, Z.; Jin, Y. Hyperoside induces both autophagy and apoptosis in non-small cell lung cancer cells in vitro. Acta Pharmacol. Sin. 2016, 37, 505–518. [Google Scholar] [CrossRef]
- Xiao, R.; Xiang, A.L.; Pang, H.B.; Liu, K.Q. Hyperoside protects against hypoxia/reoxygenation induced injury in cardiomyocytes by suppressing the Bnip3 expression. Gene 2017, 629, 86–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Wang, M.; Ding, Y.; Guo, H.; Liu, J.; Cheng, Y. Hyperoside from Z. bungeanum leaves restores insulin secretion and mitochondrial function by regulating pancreatic cellular redox status in diabetic mice. Free Radic. Biol Med. 2021, 162, 412–422. [Google Scholar] [CrossRef]
- De Bruyn, F.; Van Brempt, M.; Maertens, J.; Van Bellegem, W.; Duchi, D.; De Mey, M. Metabolic engineering of Escherichia coli into a versatile glycosylation platform: Production of bio-active quercetin glycosides. Microb. Cell Fact. 2015, 14, 138. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, F.; Ding, X.; Liu, D.; Li, M. Optimization of extraction technology for hyperoside, isoquercitrin, quercetin in Abelmoschus manihot (L.) Medic.by response surface method. Sci. Technol. Food Ind. 2015, 24, 216–221. [Google Scholar]
- Qiu, Y.; Wang, M.Z. Isolation and Identification of Hyperoside in Abelmoschus manihot Flower. Adv. Mat. Res. 2013, 781, 880–883. [Google Scholar] [CrossRef]
- He, F.; Li, D.; Wang, D.; Deng, M. Extraction and purification of Quercitrin, Hyperoside, Rutin, and Afzelin from Zanthoxylum Bungeanum maxim leaves using an aqueous two-phase system. J. Food Sci. 2016, 81, C1593–C1602. [Google Scholar] [CrossRef]
- Mapoung, S.; Umsumarng, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Photoprotective Effects of a Hyperoside-Enriched Fraction Prepared from Houttuynia cordata Thunb. on Ultraviolet B-Induced Skin Aging in Human Fibroblasts through the MAPK Signaling Pathway. Plants 2021, 10, 2628. [Google Scholar] [CrossRef] [PubMed]
- Çιrak, C.; Radušienė, J.; Janulis, V.; Ivanauskas, L. Secondary metabolites in Hypericum perfoliatum: Variation among plant parts and phenological stages. Bot. Helv. 2007, 117, 29–36. [Google Scholar] [CrossRef]
- Camas, N.; Radusiene, J.; Stanius, Z.; Caliskan, O.; Cirak, C. Secondary Metabolites of Hypericum leptophyllum Hochst., an Endemic Turkish Species. Sci. World J. 2012, 2012, 501027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.; Zhang, Z.; Yang, L.; Fan, Y.; Liu, Y. Molecular structure and spectral characteristics of hyperoside and analysis of its molecular imprinting adsorption properties based on density functional theory. J. Mol. Graph. 2019, 88, 228–236. [Google Scholar] [CrossRef]
- Saçıcı, E.; Yesilada, E. Development of new and validated HPTLC methods for the qualitative and quantitative analysis of hyperforin, hypericin and hyperoside contents in Hypericum species. Phytochem. Anal. 2022, 33, 355–364. [Google Scholar] [CrossRef]
- Guo, A.; Huanng, Z.; Liu, C. Pharmacokinetic study on hyperoside in Beagle’s dogs. Chin. Herb. Med. 2012, 4, 213–217. [Google Scholar]
- Chang, Q.; Zuo, Z.; Chow, M.S.; Ho, W.K. Difference in absorption of the two structurally similar flavonoid glycosides, hyperoside and isoquercitrin, in rats. Eur. J. Pharm. Biopharm. 2005, 59, 549–555. [Google Scholar] [CrossRef]
- Zhang, X.; Su, J.; Wang, X.; Wang, X.; Liu, R.; Fu, X.; Li, Y.; Xue, J.; Li, X.; Zhang, R. Preparation and Properties of Cyclodextrin Inclusion Complexes of Hyperoside. Molecules 2022, 27, 2761. [Google Scholar] [CrossRef]
- Corless, J.K.; Middleton, H.M. Normal liver function: A basis for understanding hepatic disease. Arch. Intern. Med. 1983, 143, 2291–2294. [Google Scholar] [CrossRef]
- Jaeschke, H.; Gores, G.J.; Cederbaum, A.I.; Hinson, J.A.; Pessayre, D.; Lemasters, J.J. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002, 65, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.P.; Kala, M.; Nivsarkar, M.; Babu, R.J. Implication of formulation strategies on the bioavailability of selected plant-derived hepatoprotectants. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 489–526. [Google Scholar] [CrossRef] [PubMed]
- Ram, V.J.; Goel, A. Past and present scenario of hepatoprotectants. Curr. Med. Chem. 1999, 6, 217–254. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, D.W.; Yun, N.; Choi, J.S.; Islam, M.N.; Kim, Y.S.; Lee, S.M. Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J. Nat. Prod. 2011, 74, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Chen, S.; Li, L.; Wu, T. The protective effect of hyperoside on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. Exp. Toxicol. Pathol. 2017, 69, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Fu, R.; Cheng, C.; Cai, Y.; Wang, X.; Deng, D.; Gong, X.; Chen, J. Hyperoside protected against oxidative stress-induced liver injury via the PHLPP2-AKT-GSK-3β signaling pathway in vivo and in vitro. Front. Pharmacol. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Cai, Y.; Li, B.; Peng, D.; Wang, X.; Li, P.; Huang, M.; Xing, H.; Chen, J. Crm1-Dependent Nuclear Export of Bach1 is Involved in the Protective Effect of Hyperoside on Oxidative Damage in Hepatocytes and CCl4-induced Acute Liver Injury. J. Inflamm. Res. 2021, 14, 551–565. [Google Scholar] [CrossRef]
- Huang, C.; Yang, Y.; Li, W.X.; Wu, X.Q.; Li, X.F.; Ma, T.T.; Zhang, L.; Meng, X.M.; Li, J. Hyperin attenuates inflammation by activating PPAR-γ in mice with acute liver injury (ALI) and LPS-induced RAW264.7 cells. Int. Immunopharmacol. 2015, 29, 440–447. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, B.; Li, Y.; Bai, M.; Miao, M. Protective effects of hyperoside on acute alcohol-inducing liver injury in mice. Zhongguo Zhong Yao Za Zhi 2017, 33, 30–33. [Google Scholar]
- Huang, M.; Chen, J.; Hu, X.; Xia, P.; Cai, Y.; Wang, Q. Effect of hyperin on acute liver injury in rats against oxidative stress-induced by CCl4. Jujie Shushuxue Za Zhi 2013, 6, 588–590. [Google Scholar]
- Mun, S.; Ryu, H.; Choi, J. Inhibition effects of Zanthoxylum schinifolium and its active principle on lipid peroxidation and liver damage in carbon tetrachloride-treated mice. J. Korean Soc. Food Sci. Nutr. 1997, 26, 943–951. [Google Scholar]
- Ito, M.; Shimura, H.; Watanabe, N.; Tamai, M.; Hanada, K.; Takahashi, A.; Tanaka, Y.; Arai, I.; Zhang, P.L.; Rao, C. Hepatorotective Compounds from Canarium album and Euphorbia nematocypha. Chem. Pharm. Bull. 1990, 38, 2201–2203. [Google Scholar]
- Xing, H.Y.; Cai, Y.Q.; Wang, X.F.; Wang, L.L.; Li, P.; Wang, G.Y.; Chen, J.H. The cytoprotective effect of hyperoside against oxidative stress is mediated by the Nrf2-ARE signaling pathway through GSK-3β inactivation. PLoS ONE 2015, 10, e0145183. [Google Scholar] [CrossRef]
- Choi, S.J.; Tai, B.H.; Cuong, N.M.; Kim, Y.H.; Jang, H.D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587–595. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Abd Halim, S.A.S.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Ridzuan, N.R.A.; Jalil, N.A.A. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Buters, J.T.; Pineau, T.; Fernandez-Salguero, P.; Gonzalez, F.J. Role of CYP2E1 in the Hepatotoxicity of Acetaminophen. J. Biol. Chem. 1996, 271, 12063–12067. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.G.; Hsieh, C.C.; Lee, Y.J.; Li, P.H.; Tsai, M.S.; Li, C.T.; Wang, S.H. N-Acetyl cysteine overdose inducing hepatic steatosis and systemic inflammation in both propacetamol-induced hepatotoxic and normal mice. Antioxidants 2021, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Ma, M.; Han, X.; Wang, Z.; Li, H. Hyperin protects against cisplatin-induced liver injury in mice. Acta Cir. Bras. 2017, 32, 633–640. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, J.; Liu, C.; Wang, X.; Pan, J. Hyperoside alleviated N-acetyl-para-amino-phenol-induced acute hepatic injury via Nrf2 activation. Int. J. Clin. Exp. Pathol. 2019, 12, 64–76. [Google Scholar]
- Xie, W.; Jiang, Z.; Wang, J.; Zhang, X.; Melzig, M.F. Protective effect of hyperoside against acetaminophen (APAP) induced liver injury through enhancement of APAP clearance. Chem.-Biol. Interact. 2016, 246, 11–19. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Y.; Cao, Y.; Jia, Y.; Zhang, J. Metabolomics analysis reveals the protective effect of quercetin-3-O-galactoside (Hyperoside) on liver injury in mice induced by acetaminophen. J. Food Biochem. 2020, 44, e13420. [Google Scholar] [CrossRef] [PubMed]
- An, R.B.; Kim, H.C.; Tian, Y.H.; Kim, Y.C. Free radical scavenging and hepatoprotective constituents from the leaves of Juglans sinensis. Arch. Pharm. Res. 2005, 28, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.; Dong, H.; Yu, X.; Zhang, J. Anti-hypoglycemic and hepatocyte-protective effects of hyperoside from Zanthoxylum bungeanum leaves in mice with high-carbohydrate/high-fat diet and alloxan-induced diabetes. Int. J. Mol. Med. 2018, 41, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, X.; Huang, Z.; Liu, H.; Wu, G. In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol. Sin. 2007, 28, 404–409. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, C.; Liu, X.; Ge, Y.; Jiang, X.; Zhao, W. Hyperoside protects against heart failure-induced liver fibrosis in rats. Acta Histochem. 2019, 121, 804–811. [Google Scholar] [CrossRef]
- Shen, D.; Hou, J.; Yuan, F. Hyperoside suppresses injury in Mycoplasma pneumoniae pneumonia mice. Chin. J. Pathol. 2017, 12, 884–889. [Google Scholar]
- Shi, Y.; Qiu, X.; Dai, M.; Zhang, X.; Jin, G. Hyperoside attenuates hepatic ischemia-reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Transplant. Proc. 2019, 51, 2051–2059. [Google Scholar] [CrossRef]
- Lu, X.; Huang, Z.; Yang, X.; Wang, H.; Geng, M.; Li, Z. Liver protective effects of hyperin on duck hepatitis B virus infection. Zhongguo Zhong Yao Za Zhi 2007, 23, 10–12. [Google Scholar]
- Guo, X.; Qu, F.; Xin, Y.; Wang, J.; Li, H.; Ma, A.; Zhao, W. Effect and mechanism of hyperin on liver fibrosis in rats with heart failure. Shandong Yi Yao 2021, 61, 40–45. [Google Scholar]
- Abdelhameed, R.F.; Ibrahim, A.K.; Elfaky, M.A.; Habib, E.S.; Mahamed, M.I.; Mehanna, E.T.; Darwish, K.M.; Khodeer, D.M.; Ahmed, S.A.; Elhady, S.S. Antioxidant and Anti-Inflammatory Activity of Cynanchum acutum L. Isolated Flavonoids Using Experimentally Induced Type 2 Diabetes Mellitus: Biological and In Silico Investigation for NF-κB Pathway/miR-146a Expression Modulation. Antioxidants 2021, 10, 1713. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, W.; Min, D.; Liu, C.; Shang, Y.; Hu, L. Protective effect of hyperoside on non-alcoholic fatty liver disease in ApoE-/- mice induced by high-fat diet. Drug Eval. Res. 2022, 45, 281–286. [Google Scholar]
- Huang, K.; Gen, M.; Wang, J.; Huang, Z.; Yang, X.; Chen, H. Protective Effect of Hyperin on Immunological Liver Injury in Mice. Zhongguo Shiyan Fangjixue Zazhi 2015, 21, 137–141. [Google Scholar]
- Xiong, Q.; Fan, W.; Tezuka, Y.; Adnyana, I.K.; Stampoulis, P.; Hattori, M.; Namba, T.; Kadota, S. Hepatoprotective effect of Apocynum venetum and its active constituents. Planta Med. 2000, 66, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Velu, V.; Nandakumar, S.; Madhavan, V.; Shanmugasundaram, U.; Murugavel, K.G.; Balakrishnan, P.; Kumarasamy, N.; Solomon, S.; Thyagarajan, S.P. Hepatitis B virus and hepatitis C virus dual infection among patients with chronic liver disease. J. Microbiol. Immunol. Infect. 2009, 42, 122–128. [Google Scholar] [PubMed]
- Goyal, A.; Murray, J.M. The impact of vaccination and antiviral therapy on hepatitis B and hepatitis D epidemiology. PLoS ONE 2014, 9, e110143. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.; Wang, J.H.; Chen, H.Y.; Yang, X.B.; Huang, Z.M. Effects of hyperin on the cccDNA of duck hepatitis B virus and its immunological regulation. Yao Xue Xue Bao 2009, 44, 1440–1444. [Google Scholar]
- Shen, B.; Wu, N.; Shen, C.; Zhang, F.; Wu, Y.; Xu, P.; Zhang, L.; Wu, W.; Lu, Y.; Han, J. Hyperoside nanocrystals for HBV treatment: Process optimization, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2016, 42, 1772–1781. [Google Scholar] [CrossRef]
- Schreiner, S.; Nassal, M. A role for the host DNA damage response in hepatitis B virus cccDNA formation—and beyond? Viruses 2017, 9, 125. [Google Scholar] [CrossRef]
- Rehman, S.; Ashfaq, U.A.; Ijaz, B.; Riazuddin, S. Anti-hepatitis C virus activity and synergistic effect of Nymphaea alba extracts and bioactive constituents in liver infected cells. Microb. Pathog. 2018, 121, 198–209. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef]
- Ding, W.X.; Yang, L. Alcohol and drug-induced liver injury: Metabolism, mechanisms, pathogenesis and potential therapies. Liver Res. 2019, 3, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; Larter, C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.Y.; Zhai, Z.Z.; Li, Z.F.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem.-Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, R.; Liang, Z.; Fan, A.; Kang, D. Hyperoside attenuates non-alcoholic fatty liver disease through targeting Nr4A1 in macrophages. Int. Immunopharmacol. 2021, 94, 107438. [Google Scholar] [CrossRef]
- Duan, J.Y.; Chen, W.; Zhao, Y.Q.; He, L.L.; Li, E.C.; Bai, Z.H.; Wang, Y.J.; Zhang, C.P. Flavonoids from Hypericum patulum enhance glucose consumption and attenuate lipid accumulation in HepG2 cells. J. Food Biochem. 2021, 45, e13898. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sheng, F.; Zou, L.; Xiao, J.; Li, P. Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism. J. Adv. Res. 2021, 34, 109–122. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Szabo, G.; Csak, T. Inflammasomes in liver diseases. J. Hepatol. 2012, 57, 642–654. [Google Scholar] [CrossRef]
- Tilg, H.; Kaser, A.; Moschen, A.R. How to modulate inflammatory cytokines in liver diseases. Liver Int. 2006, 26, 1029–1039. [Google Scholar] [CrossRef]
- Petrescu, A.D.; DeMorrow, S. Interleukin-24 therapy-a potential new strategy against liver fibrosis. EBioMedicine 2021, 65, 103245. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 2011, 6, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, M.; Rombouts, K.; Colagrande, S. Fibrosis in chronic liver diseases: Diagnosis and management. J. Hepatol. 2005, 42, S22–S36. [Google Scholar] [CrossRef]
- Singh, S.; Fujii, L.L.; Murad, M.H.; Wang, Z.; Asrani, S.K.; Ehman, R.L.; Kamath, P.S.; Talwalkar, J.A. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Zhou, Q.; Zhang, D.; Bi, Q.; Wu, Y.; Huang, W. Liver stiffness measurement predicted liver-related events and all-cause mortality: A systematic review and nonlinear dose-response meta-analysis. Hepatol. Commun. 2018, 2, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yue, Z.; Guo, M.; Fang, L.; Bai, L.; Li, X.; Tao, Y.; Wang, S.; Liu, Q.; Zhi, D. Dietary flavonoid hyperoside induces apoptosis of activated human LX-2 hepatic stellate cell by suppressing canonical NF-κB signaling. Biomed. Res. Int. 2016, 2016, 1068528. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Ten Dijke, P.; Consortium, I.L. TGF-β signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef]
- Severi, T.; Van Malenstein, H.; Verslype, C.; Van Pelt, J.F. Tumor initiation and progression in hepatocellular carcinoma: Risk factors, classification, and therapeutic targets. Acta Pharmacol. Sin. 2010, 31, 1409–1420. [Google Scholar] [CrossRef]
- EASL. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B. Epidemiology of HCC: Consider the Population. J. Clin. Gastroenterol. 2013, 47, S2–S6. [Google Scholar] [CrossRef]
- Amarapurkar, D. Asia-Pacific working party on prevention of hepatocellular carcinoma. Application of surveillance programs for hepatocellular carcinoma in the Asia-Pacific Region. J. Gastroenterol. Hepatol. 2009, 24, 955–961. [Google Scholar] [CrossRef]
- Wei, S.; Sun, Y.; Wang, L.; Zhang, T.; Hu, W.; Bao, W.; Mao, L.; Chen, J.; Li, H.; Wen, Y. Hyperoside suppresses BMP-7-dependent PI3K/AKT pathway in human hepatocellular carcinoma cells. Ann. Transl. Med. 2021, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xiong, H.; Wu, H.; Wen, J.; Liang, L. Regulatory effect of hyperoside on proliferation and apoptosis of hepatic carcinoma cell HepG2 via mitochondrial P53/Caspase signaling pathway. Chin. J. Immunol. 2018, 34, 1832–1836. [Google Scholar]
- Sattler, F.R.; Paolucci, S.; Kreider, J.W.; Ladda, R.L. A human hepatoma cell line (PLC/PRF/5) produces lung metastases and secretes HBsAg in nude mice. Eur. J. Cancer Clin. Oncol. 1982, 18, 381–389. [Google Scholar] [CrossRef]
- Han, J.; Meng, J.; Chen, S.; Wang, X.; Yin, S.; Zhang, Q.; Liu, H.; Qin, R.; Li, Z.; Zhong, W. YY1 complex promotes quaking expression via super-enhancer binding during EMT of hepatocellular carcinoma. Cancer Res. 2019, 79, 1451–1464. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef] [PubMed]
- Ai, G.; Huang, Z.; Wang, D.; Zhang, H. Acute toxicity and genotoxicity evaluation of hyperoside extracted from Abelmoschus manihot (L.) Medic. J. Chin. Pharm. Sci. 2012, 21, 477. [Google Scholar] [CrossRef]
- Ai, G.; Huang, Z.; Wang, D.; Zhang, H. Toxicity of hyperoside after long-term oral administration in Sistar rats. Chin. J. New Drugs 2012, 21, 2811–2816. [Google Scholar]
- Ai, G.; Wang, D.; Huang, Z.; Zhang, H. Long-term toxicity of hyperoside in Beagle dogs. Chin. J. New Drugs 2015, 24, 1641–1647. [Google Scholar]
- Liu, B.; Tu, Y.; He, W.; Liu, Y.; Wu, W.; Fang, Q.; Tang, H.; Tang, R.; Wan, Z.; Sun, W.; et al. Hyperoside attenuates renal aging and injury induced by D-galactose via inhibiting AMPK-ULK1 signaling-mediated autophagy. Aging 2018, 10, 4197–4212. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Sun, X.; Lou, Y.; Yu, J. Hyperoside protects HK-2 cells against high glucose-induced apoptosis and inflammation via the miR-499a-5p/NRIP1 pathway. Pathol. Oncol. Res. 2021, 27, 629829. [Google Scholar] [CrossRef]
- Ai, G.; Huang, Z.; Wang, D.; Liu, Z. Study on toxicity of hyperoside in rat embryo-fetal development. Zhongguo Zhong Yao Za Zhi 2012, 37, 2452–2455. [Google Scholar] [PubMed]
- Wei, A.; Song, Y.; Ni, T.; Xiao, H.; Wan, Y.; Ren, X.; Li, H.; Xu, G. Hyperoside attenuates pregnancy loss through activating autophagy and suppressing inflammation in a rat model. Life Sci. 2020, 254, 117735. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Zhu, Z.Q.; Hu, T.X.; Zhu, D.Y. Structure-activity relationship of natural flavonoids in hydroxyl radical-scavenging effects. Acta Pharmacol. Sin. 2002, 23, 667–672. [Google Scholar] [PubMed]
- Jang, E.; Kim, B.J.; Lee, K.T.; Inn, K.S.; Lee, J.H. A survey of therapeutic effects of Artemisia capillaris in liver diseases. Evid. Based Complement. Alternat. Med. 2015, 2015, 728137. [Google Scholar] [CrossRef] [PubMed]
- Hyogo, H.; Yamagishi, S. Advanced glycation end products (AGEs) and their involvement in liver disease. Curr. Pharm. Des. 2008, 14, 969–972. [Google Scholar] [CrossRef]
- Zhang, Z.; Sethiel, M.S.; Shen, W.; Liao, S.; Zou, Y. Hyperoside downregulates the receptor for advanced glycation end products (RAGE) and promotes proliferation in ECV304 cells via the c-Jun N-terminal kinases (JNK) pathway following stimulation by advanced glycation end-products in vitro. Int. J. Mol. Sci. 2013, 14, 22697–22707. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; An, X.F.; Teng, S.C.; Liu, J.S.; Shang, W.B.; Zhang, A.H.; Yuan, Y.G.; Yu, J.Y. Pretreatment with the total flavone glycosides of Flos Abelmoschus manihot and hyperoside prevents glomerular podocyte apoptosis in streptozotocin-induced diabetic nephropathy. J. Med. Food 2012, 15, 461–468. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).