Effects of Coenzyme Q10 Supplementation on Oxidative Stress Markers, Inflammatory Markers, Lymphocyte Subpopulations, and Clinical Status in Dogs with Myxomatous Mitral Valve Disease
Abstract
:1. Introduction
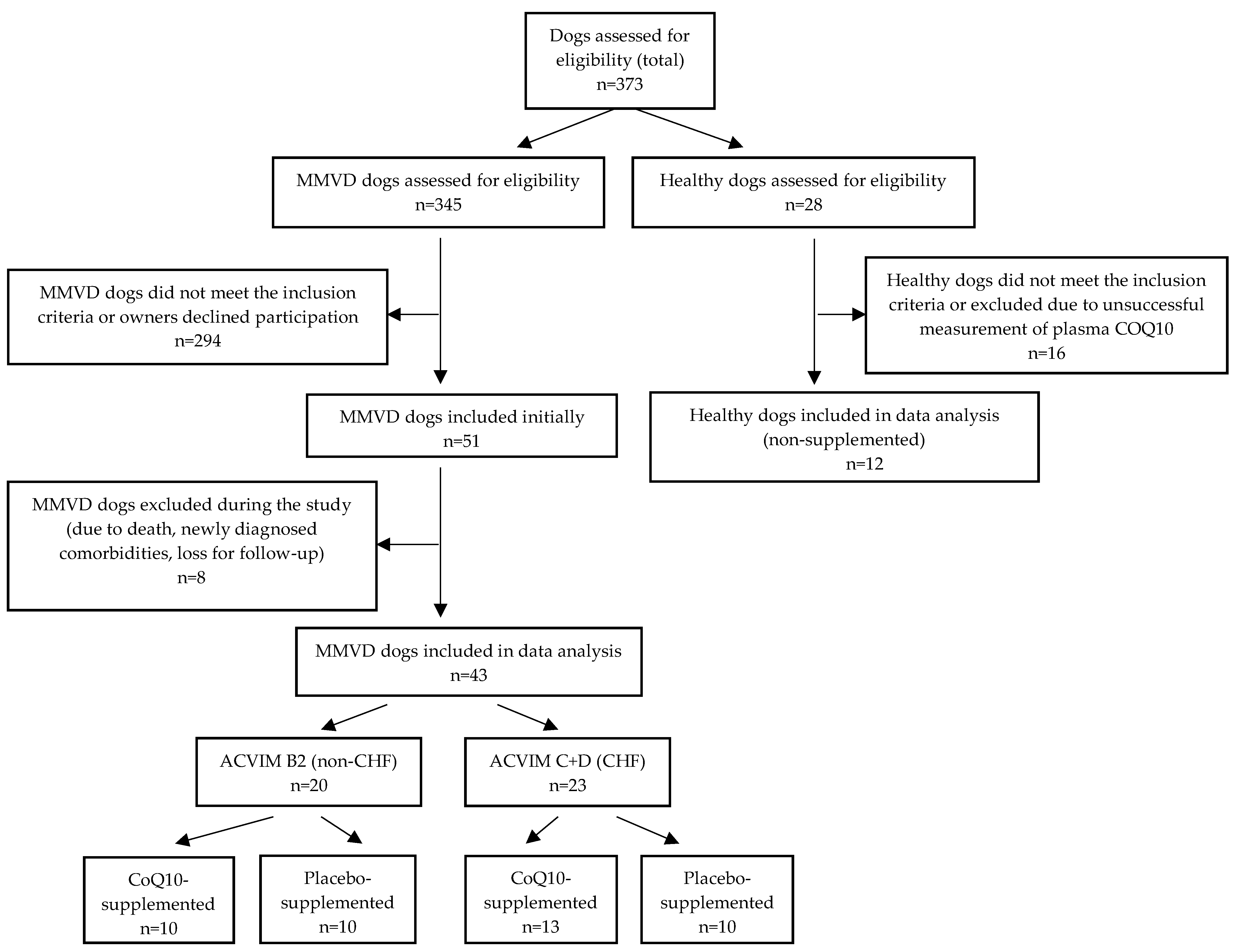
2. Materials and Methods
2.1. Animals
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Study Design
2.3. Blood Sampling
2.3.1. Routine Hematology and Biochemistry Analyses
2.3.2. Determination of Oxidative Stress Markers
2.3.3. Determination of Inflammatory Markers
2.3.4. Flow Cytometry
2.3.5. Determination of Cardiac Biomarkers
2.4. Echocardiographic and Clinical Assessment
2.5. Statistical Analysis
3. Results
3.1. Within-Group Comparisons of Measured Parameters over a Three-Month Supplementation Period
3.2. Comparisons of Measured Parameters between the CoQ10 and Placebo Groups before and after Three-Month Supplementation
3.3. Comparisons of Changes (Deltas) in Measured Parameters between CoQ10-Supplemented and Placebo-Supplemented Groups
3.4. Comparisons of Measured Parameters between ACVIM B2, CHF, and Healthy Groups
3.5. Owner-Perceived Assessment of the Condition of a Dog at the end of the Supplementation Period Compared to That at the Beginning (Subjective Assessment, Not Included in the Statistics)
3.6. Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crane, F.L. Biochemical functions of Coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta 2004, 1660, 171–199. [Google Scholar] [CrossRef] [Green Version]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and Immune Function: An Overview. Antioxidants 2021, 10, 759. [Google Scholar] [CrossRef]
- Sies, H. On the history of oxidative stress: Concept and some aspects of current development. Current Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Wang, W.; Kang, P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants 2020, 9, 1292. [Google Scholar] [CrossRef]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Folkers, K.; Littarru, G.P.; Ho, L.; Runge, T.M.; Havanonda, S.; Cooley, D. Evidence for a deficiency of coenzyme Q10 in human heart disease. Int. Z. Vitam. 1970, 40, 380–390. [Google Scholar]
- Folkers, K.; Vadhanavikit, S.; Mortensen, S.A. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc. Natl. Acad. Sci. USA 1985, 82, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, S.A.; Vadhanavikit, S.; Folkers, K. Deficiency of coenzyme Q10 in myocardial failure. Drugs Exp. Clin. Res. 1984, 10, 497–502. [Google Scholar]
- Seneş, M.; Erbay, A.R.; Yilmaz, F.M.; Topkaya, B.C.; Zengi, O.; Doğan, M.; Yücel, D. Coenzyme Q10 and high-sensitivity C-reactive protein in ischemic and idiopathic dilated cardiomyopathy. Clin. Chem. Lab. Med. 2008, 46, 382–386. [Google Scholar] [CrossRef]
- Sharma, A.; Fonarow, G.C.; Butler, J.; Ezekowitz, J.A.; Felker, G.M. Coenzyme Q10 and Heart Failure: A State-of-the-Art Review. Circ. Heart Fail. 2016, 9, 002639. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, Y. Efficacy of coenzyme Q10 in patients with cardiac failure: A meta-analysis of clinical trials. BMC Cardiovasc. Disord. 2017, 17, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Jafari, M.; Mousavi, S.M.; Asgharzadeh, A.; Yazdani, N. Coenzyme Q10 in the treatment of heart failure: A systematic review of systematic reviews. Indian Heart J. 2018, 70, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Raizner, A.E. Coenzyme Q10. Methodist DeBakey Cardiovasc. J. 2019, 15, 185–191. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F.G. Coenzyme Q10: Clinical applications in cardiovascular diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef] [Green Version]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Freeman, L.M.; Rush, J.E.; Milbury, P.E.; Blumberg, J.B. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J. Vet. Intern. Med. 2005, 19, 537–541. [Google Scholar] [CrossRef]
- Michałek, M.; Tabiś, A.; Cepiel, A.; Noszczyk-Nowak, A. Antioxidative enzyme activity and total antioxidant capacity in serum of dogs with degenerative mitral valve disease. Can. J. Vet. Res. 2020, 84, 67–73. [Google Scholar]
- Rubio, C.P.; Saril, A.; Kocaturk, M.; Tanaka, R.; Koch, J.; Ceron, J.J.; Yilmaz, Z. Changes of inflammatory and oxidative stress biomarkers in dogs with different stages of heart failure. BMC Vet. Res. 2020, 16, 433. [Google Scholar] [CrossRef]
- Christiansen, L.B.; Reimann, M.J.; Schou-Pedersen, A.M.V.; Larsen, S.; Lykkesfeldt, J.; Olsen, L.H. Depleted Myocardial Coenzyme Q10 in Cavalier King Charles Spaniels with Congestive Heart Failure Due to Myxomatous Mitral Valve Disease. Antioxidants 2021, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Svete, A.N.; Verk, B.; Seliškar, A.; Tomsič, K.; Križman, P.J.; Petrič, A.D. Plasma coenzyme Q10 concentration, antioxidant status, and serum N-terminal pro-brain natriuretic peptide concentration in dogs with various cardiovascular diseases and the effect of cardiac treatment on measured variables. Am. J. Vet. Res. 2017, 78, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Druzhaeva, N.; Petrič, A.D.; Tavčar-Kalcher, G.; Babič, J.; Nemec Svete, A. Randomized, double-blinded, controlled trial of the effects of coenzyme Q10 supplementation on plasma coenzyme Q10 concentration in dogs with myxomatous mitral valve disease. Am. J. Vet. Res. 2021, 82, 280–285. [Google Scholar] [CrossRef]
- Tachampa, K.; Lertwanakarn, T.; Atchariyasakchai, P.; Pumpitakkul, V.; Kireewan, S.; Buranakarl, C. Effects of coenzyme Q10 supplementation on cardiac troponin I level, heart rate variability, and echocardiographic profiles in canine with myxomatous degenerative mitral valve disease: A pilot study. Wetchasan Sattawaphaet 2018, 48, 443–452. [Google Scholar]
- Christiansen, L.B.; Morsing, M.K.; Reimann, M.J.; Martinussen, T.; Birlie, Z.; Schou-Pedersen, A.M.V.; Lykkesfeldt, J.; Olsen, L.H. Pharmacokinetics of Repeated Oral Dosing with Coenzyme Q10 in Cavalier King Charles Spaniels with Myxomatous Mitral Valve Disease. Antioxidants 2020, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.M.; Rush, J.E.; Freeman, L.M. Systemic inflammation and endothelial dysfunction in dogs with congestive heart failure. J. Vet. Intern. Med. 2012, 26, 547–557. [Google Scholar] [CrossRef]
- Zois, N.E.; Moesgaard, S.G.; Kjelgaard-Hansen, M.; Rasmussen, C.E.; Falk, T.; Fossing, C.; Häggström, J.; Pedersen, H.D.; Olsen, L.H. Circulating cytokine concentrations in dogs with different degrees of myxomatous mitral valve disease. Vet. J. 2012, 192, 106. [Google Scholar] [CrossRef]
- Domanjko Petrič, A.; Lukman, T.; Verk, B.; Nemec Svete, A. Systemic inflammation in dogs with advanced-stage heart failure. Acta Vet. Scand. 2018, 60, 20. [Google Scholar] [CrossRef] [Green Version]
- Nemec Svete, A.; Verk, B.; Čebulj-Kadunc, N.; Salobir, J.; Rezar, V.; Domanjko Petrič, A. Inflammation and its association with oxidative stress in dogs with heart failure. BMC Vet. Res. 2021, 17, 176. [Google Scholar] [CrossRef]
- Khaper, N.; Bryan, S.; Dhingra, S.; Singal, R.; Bajaj, A.; Pathak, C.M.; Singal, P.K. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid. Redox Signal. 2010, 13, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Huang, Y.C.; Chen, S.J.; Lin, P.T. Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition 2012, 28, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Tseng, Y.F.; Yen, C.H.; Lin, P.-T. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr. J. 2013, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Feng, Y.; Chen, G.C.; Qin, L.Q.; Fu, C.L.; Chen, L.H. Effects of coenzyme Q10 supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 119, 128–136. [Google Scholar] [CrossRef]
- Agnoletti, L.; Curello, S.; Malacarne, F.; Aira, A.; Cargnoni, A.; Valgimigli, M.; Ferrari, R. Immune activation in severe heart failure. Does etiology play a role? Eur. Heart J. Suppl. 2004, 6, 22–29. [Google Scholar] [CrossRef]
- Moro-García, M.A.; Echeverría, A.; Galan-Artímez, M.C.; Suárez-García, F.M.; Solano-Jaurrieta, J.J.; Avanzas-Fernandez, P.; Díaz-Molina, B.; Lambert, J.L.; López-Larrea, C.; Morris de la Tassa, C.; et al. Immunosenescence and inflammation characterize chronic heart failure patients with more advanced disease. Int. J. Cardiol. 2014, 174, 590–599. [Google Scholar] [CrossRef]
- Farabaugh, A.E.; Freeman, L.M.; Rush, J.E.; George, K.L. Lymphocyte subpopulations and hematologic variables in dogs with congestive heart failure. J. Vet. Intern. Med. 2004, 18, 505–509. [Google Scholar] [CrossRef]
- Druzhaeva, N.; Nemec Svete, A.; Ihan, A.; Pohar, K.; Domanjko Petrič, A. Peripheral blood lymphocyte subtypes in dogs with different stages of myxomatous mitral valve disease. J. Vet. Intern. Med. 2021, 35, 2112–2122. [Google Scholar] [CrossRef]
- Harker-Murray, A.K.; Tajik, A.J.; Ishikura, F.; Meyer, D.; Burnett, J.C.; Redfield, M.M. The role of coenzyme Q10 in the pathophysiology and therapy of experimental congestive heart failure in the dog. J. Card. Fail. 2000, 6, 233–242. [Google Scholar] [CrossRef]
- Prosek, M.; Smidovnik, A.; Fir, M.; Strazisar, M.; Golc Wondra, A.; Andrensek, S.; Zmitek, J. Water Soluble form of Coenzyme Q10 in the Form of an Inclusion Complex with Beta-Cyclodextrin, Process of Preparing, and Use Thereof. U.S. Patent WO 2005/111224A8, 17 August 2006. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Direct Immunofluorescence Staining of Surface Epitopes of Cells and Blood. Available online: https://www.bio-rad-antibodies.com/static/2015/flow-protocol/fc/fc4-direct-staining-intracellular-antigens-protocols.pdf (accessed on 16 June 2022).
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Moise, N.S.; Moses, B.L. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, A.; Damås, J.K.; Oie, E.; Ueland, T.; Gullestad, L.; Aukrust, P. Systemic inflammation in heart failure-the whys and wherefores. Heart Fail. Rev. 2006, 11, 83–92. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation-Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Hamilton-Elliott, J.; Ambrose, E.; Christley, R.; Dukes-McEwan, J. White blood cell differentials in dogs with congestive heart failure (CHF) in comparison to those in dogs without cardiac disease. J. Small Anim. Pract. 2018, 59, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Hodge, D.O.; Rodeheffer, R.J.; McGregor, C.G.; Thomson, S.P.; Gibbons, R.J. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation 1998, 97, 19–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acanfora, D.; Gheorghiade, M.; Trojano, L.; Furgi, G.; Pasini, E.; Picone, C.; Papa, A.; Iannuzzi, G.L.; Bonow, R.O.; Rengo, F. Relative lymphocyte count: A prognostic indicator of mortality in elderly patients with congestive heart failure. Am. Heart J. 2001, 142, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Charach, G.; Grosskopf, I.; Roth, A.; Afek, A.; Wexler, D.; Sheps, D.; Weintraub, M.; Rabinovich, A.; Keren, G.; George, J. Usefulness of total lymphocyte count as predictor of outcome in patients with chronic heart failure. Am. J. Cardiol. 2011, 107, 1353–1356. [Google Scholar] [CrossRef]
- Zhai, J.; Bo, Y.; Lu, Y.; Liu, C.; Zhang, L. Effects of Coenzyme Q10 on Markers of Inflammation: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0170172. [Google Scholar]
- Zhao, Q.; Kebbati, A.H.; Zhang, Y.; Tang, Y.; Okello, E.; Huang, C. Effect of Coenzyme Q10 on the Incidence of Atrial Fibrillation in Patients with Heart Failure. J. Investig. Med. 2015, 63, 735–739. [Google Scholar] [CrossRef]
- Farsi, F.; Heshmati, J.; Keshtkar, A.; Irandoost, P.; Alamdari, N.M.; Akbari, A.; Janani, L.; Morshedzadeh, N.; Vafa, M. Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor-α and interleukin-6 levels in chronic inflammatory diseases? A systematic review and meta-analysis of randomized con- trolled trials. Pharmacol. Res. 2019, 148, 104290. [Google Scholar] [CrossRef] [PubMed]
- Van ’t Erve, T.J.; Kadiiska, M.B.; London, S.J.; Mason, R.P. Classifying oxidative stress by F2-isoprostane levels across human diseases: A meta-analysis. Redox Biol. 2017, 12, 582–599. [Google Scholar] [CrossRef] [PubMed]
- Putman, A.K.; Contreras, G.A.; Sordillo, L.M. Isoprostanes in Veterinary Medicine: Beyond a Biomarker. Antioxidants 2021, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Polidori, M.C.; Praticó, D.; Savino, K.; Rokach, J.; Stahl, W.; Mecocci, P. Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J. Card. Fail. 2004, 10, 334–338. [Google Scholar] [CrossRef]
- Nonaka-Sarukawa, M.; Yamamoto, K.; Aoki, H.; Takano, H.; Katsuki, T.; Ikeda, U.; Shimada, K. Increased urinary 15-F2t-isoprostane concentrations in patients with non-ischaemic congestive heart failure: A marker of oxidative stress. Heart 2003, 89, 871–874. [Google Scholar] [CrossRef]
- Verk, B.; Nemec Svete, A.; Salobir, J.; Rezar, V.; Domanjko Petrič, A. Markers of oxidative stress in dogs with heart failure. J. Vet. Diagn. Investig. 2017, 29, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Sangsefidi, Z.S.; Yaghoubi, F.; Hajiahmadi, S.; Hosseinzadeh, M. The effect of coenzyme Q10 supplementation on oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Food Sci. Nutr. 2020, 8, 1766–1776. [Google Scholar] [CrossRef]
- Akbari, A.; Mobini, G.R.; Agah, S.; Morvaridzadeh, M.; Omidi, A.; Potter, E.; Fazelian, S.; Ardehali, S.H.; Daneshzad, E.; Dehghani, S. Coenzyme Q10 supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Eur. J. Clin. Pharmacol. 2020, 76, 1483–1499. [Google Scholar] [CrossRef]
- Hofman-Bang, C.; Rehnqvist, N.; Swedberg, K.; Wiklund, I.; Åström, H. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure. J. Card. Fail. 1995, 1, 101–107. [Google Scholar] [CrossRef]
- Watson, P.S.; Scalia, G.M.; Galbraith, A.; Burstow, D.J.; Bett, N.; Aroney, C.N. Lack of effect of coenzyme Q on left ventricular function in patients with congestive heart failure. J. Am. Coll. Cardiol. 1999, 33, 1549–1552. [Google Scholar] [CrossRef] [Green Version]
- Molyneux, S.L.; Florkowski, C.M.; George, P.M.; Pilbrow, A.P.; Frampton, C.M.; Lever, M.; Richards, A.M. Coenzyme Q10: An independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008, 52, 1435–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, M.; Miyazaki, T.; Takagi, A.; Sugita, Y.; Ouchi, S.; Aikawa, T.; Shiozawa, T.; Hiki, M.; Takahashi, S.; Hiki, M.; et al. Low coenzyme Q10 levels in patients with acute cardiovascular disease are associated with long-term mortality. Heart Vessel. 2021, 36, 401–407. [Google Scholar] [CrossRef] [PubMed]
| ACVIM B2 | ACVIM C, D | Healthy | |
|---|---|---|---|
| Number | 20 | 23 | 12 |
| Sex (f/m) | 9/11 | 9/14 | 6/6 |
| Spayed/neutered | 9/4 | 8/4 | 5/4 |
| Age (years) Median (IQR) | 11.7 * (9.6–13.5) p = 0.001 | 10.7 * (9.3–11.8)p = 0.019 | 7.9 (6.1–9.5) |
| Weight (kg) Median (IQR) | 8.4 (6.8–11.5) | 7.8 (6.0–11.4) | 7.2 (4.8–13.4) |
| CoQ10 (mg/L) Median (IQR) | 0.176 * (0.125–0.213)p = 0.019 | 0.171 * (0.145–0.213) p = 0.008 | 0.095 (0.070–0.143) |
| Breeds | 5 CKCS, 4 MB, 1 PEK, 1 CHI, 1 MSCH, 1 LA, 1 NT, 1 MLT, 1 TS, 1 ECS, 1 SHI, 1 CC, 1 HAV | 11 CKCS, 3 SHI, 2 MB, 2 PEK, 1 MSCH, 1 MLT, 1 ACS, 1 CHI, 1 MP | 7 MB, 3 SHI, 1 YT, 1 TS |
| Treatment Pimobendan ACE inhibitor Furosemide or torasemide Spironolactone Theophylline Sildenafil Amlodipine Potassium chloride | 20 11 - - 6 1 - - | 23 23 23 4 1 - 5 3 | - - - - - - - - |
| Parameter | CoQ10 Supplemented | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 3 Months | p * | Delta | Baseline | After 3 Months | p * | Delta | p a | p b | p c | |
| CoQ10 (mg/L) | 0.190 (0.129–0.271) | 1.050 (0.560–1.364) | 0.007 | 0.790 (0.439–1.118) | 0.158 (0.123–0.199) | 0.174 (0.140–0.212) | 0.646 | −0.009 (−0.024–0.028) | 0.364 | <0.001 | <0.001 |
| GPX (U/g HGB) | 758.0 (692.8–794.8) | 765.5 (725.1–793.8) | 0.333 | 11.5 (−13.1–28.5) | 701.0 (643.7–806.1) | 668.1 (644.7–807.3) | 0.508 | −0.1 (−79.1–25.7) | 0.364 | 0.070 | 0.496 |
| F2-isoprostanes (pg/mL) | 435.7 (280.0–568.8) | 476.4 (417.8–859.6) | 0.386 | 65.2 (−92.7–246.9) | 512.8 (335.9–643.6) | 639.8 (303.7–858.8) | 0.093 | 82.4 (−10.9–198.9) | 0.364 | 0.762 | 0.650 |
| TNFSR-II (ng/mL) | 0.666 (0.214–2.693) | 0.966 6 (0.461–3.721) | 0.799 | −0.126 (−0.655–0.709) | 0.464 (0.348–0.780) | 0.662 (0.473–1.225) | 0.169 | 0.220 (−0.097–0.408) | 0.597 | 0.496 | 0.496 |
| WBC (×109/L) | 8.2 (6.1–9.5) | 8.6 (7.4–10.5) | 0.037 | 0.8 (0.0–2.2) | 10.0 (7.1–13.5) | 9.1 (7.3–12.4) | 0.445 | −0.5 (−1.8–1.0) | 0.131 | 0.762 | 0.070 |
| Neutrophils (%) | 70.1 (64.6–72.8) | 68.1 (63.7–75.0) | 0.959 | −0.3 (−4.1–2.9) | 68.9 (67.2–77.4) | 67.4 (63.8–75.7) | 0.092 | −2.9 (−4.7–0.7) | 0.970 | 0.821 | 0.241 |
| Neutrophils (×109/L) | 5.7 (4.2–6.6) | 5.9 (5.1–7.5) | 0.095 | 0.6 (−0.1–1.2) | 6.8 (4.5–9.4) | 6.4 (4.7–8.8) | 0.285 | −0.7 (−1.6–0.7) | 0.131 | 0.597 | 0.096 |
| Monocytes (%) | 4.5 (4.0–5.4) | 4.3 (4.0–4.9) | 1.000 | 0.1 (−0.8–0.2) | 4.0 (3.3–5.1) | 4.2 (3.4–5.0) | 0.330 | 0.2 (−0.3–0.7) | 0.345 | 0.705 | 0.425 |
| Monocytes (×109/L) | 0.32 (0.26–0.50) | 0.37 (0.28–0.50) | 0.241 | 0.06 (−0.03–0.13) | 0.37 (0.28–0.48) | 0.42 (0.31–0.47) | 0.799 | −0.03 (−0.09–0.11) | 0.705 | 0.880 | 0.326 |
| NLR | 3.2 (2.4–3.9) | 3.1 (2.5–4.6) | 0.878 | 0.0 (−0.6–0.5) | 3.0 (2.9–5.6) | 2.9 (2.3–4.7) | 0.139 | −0.3 (−1.0–0.1) | 1.000 | 0.821 | 0.326 |
| Lymphocytes (%) | 22.0 (18.8–28.2) | 21.4 (16.3–26.7) | 0.445 | −1.0 (−3.2–2.4) | 22.7 (14.1–23.6) | 22.9 (16.2–28.1) | 0.203 | 1.2 (−0.6–4.1) | 1.000 | 0.821 | 0.064 |
| Lymphocytes (×109/L) | 2.0 (1.2–2.2) | 2.0 (1.4–2.7) | 0.093 | 0.3 (-0.1–0.4) | 1.9 (1.5–3.2) | 2.2 (1.6–3.1) | 0.508 | 0.0 (-0.1–0.3) | 0.406 | 0.545 | 0.364 |
| T lymphocytes CD3+ (%) | 71.0 (67.1–77.9) | 69.1 (53.1–74.4) | 0.508 | –0.5 (−13.9–3.4) | 57.8 (49.2–67.9) | 59.4 (45.7–68.5) | 0.959 | −0.5 (−5.9–6.1) | 0.059 | 0.290 | 0.597 |
| T lymphocytes CD3+ (×109/L) | 1.4 (0.7–1.8) | 1.3 (0.9–1.9) | 0.241 | 0.1 (−0.1–0.3) | 1.2 (0.7–1.7) | 1.4 (0.6–1.7) | 0.445 | 0.1 (−0.2–0.3) | 0.705 | 0.940 | 0.705 |
| T helper cells CD3+CD4+ (%) | 42.0 (28.4–48.5) | 40.3 (30.0–45.6) | 0.759 | −2.0 (−2.5–2.9) | 41.5 (35.0–51.2) | 43.8 (34.5–49.8) | 0.445 | −0.2 (−2.7–1.2) | 0.880 | 0.545 | 0.940 |
| T helper cells CD3+CD4+ (×109/L) | 0.41 (0.33–0.63) | 0.45 (0.36–0.55) | 0.203 | 0.05 (−0.01–0.08) | 0.44 (0.25–0.79) | 0.61 (0.18–0.77) | 0.646 | −0.01 (−0.07–0.08) | 1.000 | 0.496 | 0.496 |
| Activated T helper cells CD3+CD4+CD25+ (%) | 35.8 (21.4–46.1) | 30.6 (22.2–44.9) | 0.515 | −0.8 (−2.5–1.7) | 25.7 (16.1–41.1) | 23.7 (19.1–35.0) | 0.878 | 2.1 (−5.6–3.7) | 0.307 | 0.290 | 0.677 |
| Activated T helper cells CD3+CD4+CD25+ (×109/L) | 0.16 (0.10–0.22) | 0.14 (0.09–0.21) | 0.575 | 0.01 (−0.02–0.03) | 0.13 (0.07–0.19) | 0.13 (0.05–0.21) | 0.959 | −0.00 (−0.03–0.03) | 0.545 | 0.650 | 0.821 |
| Cytotoxic T lymphocytes CD3+CD8+ (%) | 44.2 (33.9–56.7) | 44.5 (36.9–58.7) | 0.285 | 2.2 (−2.0–4.6) | 36.7 (27.8–44.0) | 34.2 (30.3–41.7) | 0.721 | 0.9 (−2.1–2.6) | 0.290 | 0.131 | 0.408 |
| Cytotoxic T lymphocytes CD3+CD8+ (×109/L) | 0.57 (0.26–0.89) | 0.57 (0.35–1.02) | 0.093 | 0.07 (−0.02–0.13) | 0.39 (0.24–0.64) | 0.46 (0.21–0.70) | 0.386 | 0.04 (−0.08–0.12) | 0.290 | 0.406 | 0.650 |
| Activated cytotoxic T lymphocytes CD3+CD8+CD25+ (%) | 8.8 (5.7–15.4) | 7.4 (4.3–12.4) | 0.241 | −1.3 (−2.8–0.9) | 9.5 (3.8–14.1) | 7.8 (3.1–16.7) | 0.646 | 0.0 (−5.1–1.5) | 0.821 | 0.940 | 0.733 |
| Activated cytotoxic T lymphocytes CD3+CD8+CD25+ (×109/L) | 0.05 (0.03–0.08) | 0.04 (0.03–0.06) | 0.878 | −0.00 (−0.01–0.01) | 0.04 (0.01–0.07) | 0.04 (0.02–0.06) | 0.646 | −0.00 (−0.03–0.01) | 0.496 | 0.762 | 0.940 |
| T helper cells/cytotoxic T lymphocytes ratio (CD4/CD8) | 1.0 (0.5–1.4) | 0.9 (0.5–1.2) | 0.508 | −0.0 (−0.1–0.1) | 1.1 (0.9–1.6) | 1.3 (0.9–1.5) | 0.508 | −0.0 (−0.2–0.1) | 0.450 | 0.257 | 1.000 |
| DPT CD3+CD4+CD8+ (%) | 0.6 (0.5–1.7) | 0.7 (0.5–1.3) | 0.766 | 0.1 (−0.3–0.2) | 1.0 (0.6–2.1) | 1.0 (0.7–2.7) | 0.528 | 0.1 (−0.3–0.5) | 0.206 | 0.266 | 0.733 |
| DPT CD3+CD4+CD8+ (×109/L) | 0.008 (0.007–0.016) | 0.010 (0.007–0.019) | 0.386 | 0.000 (−0.001–0.005) | 0.013 (0.009–0.018) | 0.015 (0.007–0.027) | 0.386 | 0.001 (−0.002–0.012) | 0.326 | 0.257 | 0.762 |
| DNT CD3+CD4-CD8- (%) | 15.2 (11.9–17.0) | 14.6 (10.6–16.2) | 0.285 | −1.5 (−4.0–0.8) | 16.5 (10.8–20.4) | 18.2 (10.4–20.8) | 0.953 | −0.1 (−1.0–1.3) | 0.820 | 0.450 | 0.199 |
| DNT CD3+CD4-CD8- (×109/L) | 0.17 (0.11–0.27) | 0.16 (0.13–0.23) | 0.799 | 0.02 (−0.07–0.02) | 0.15 (0.10–0.24) | 0.16 (0.09–0.27) | 0.878 | −0.01 (−0.03–0.05) | 1.000 | 0.940 | 0.880 |
| B lymphocytes CD45+CD21+ (%) | 12.3 (8.3–17.2) | 13.9 (7.4–16.7) | 0.919 | −0.1 (−1.4–2.0) | 16.9 (10.2–24.6) | 13.5 (10.1–22.0) | 0.110 | −1.5 (−2.3–0.2) | 0.130 | 0.427 | 0.089 |
| B lymphocytes CD45+CD21+ (×109/L) | 0.19 (0.13–0.32) | 0.22 (0.14–0.32) | 0.285 | 0.03 (−0.03–0.06) | 0.32 (0.13–0.78) | 0.29 (0.13–0.68) | 0.575 | -0.00 (−0.10–0.03) | 0.290 | 0.364 | 0.364 |
| NT-proBNP (pmol/L) | 646.5 (440.3–1031.3) | 712.0 (455.0–1044.0) | 0.541 | 0.0 (−143.0–236.3) | 974.0 (867.0–1299.0) | 953.0 (602.5–1247.5) | 0.515 | 3.0 (−238.0–49.5) | 0.102 | 0.288 | 0.450 |
| cTnI (µg/L) | 0.048 (0.037–0.099) | 0.044 (0.031–0.067) | 0.959 | 0.003 (−0.016–0.011) | 0.043 (0.030–0.075) | 0.039 (0.025–0.066) | 0.678 | −0.004 (−0.010–0.008) | 0.450 | 0.762 | 0.970 |
| LA/Ao | 2.0 (1.8–2.0) | 1.8 (1.5–2.0) | 0.333 | −0.1 (−0.4–0.1) | 1.9 (1.8–2.2) | 2.0 (1.7–2.3) | 0.721 | −0.0 (−0.2–0.2) | 0.970 | 0.272 | 0.650 |
| nLVIDd | 1.7 (1.6–1.8) | 1.7 (1.6–1.8) | 0.878 | −0.0 (−0.1–0.2) | 1.9 (1.8–2.0) | 1.9 (1.9–2.0) | 0.878 | 0.0 (−0.1–0.1) | 0.034 | 0.082 | 1.000 |
| nLVIDs | 0.9 (0.8–1.0) | 1.0 (0.8–1.0) | 0.333 | 0.0 (−0.1–0.2) | 1.0 (0.8–1.2) | 1.0 (0.8–1.1) | 0.878 | 0.0 (−0.1–0.1) | 0.290 | 0.597 | 0.290 |
| MV E velocity (m/s) | 0.96 (0.88–1.09) | 0.90 (0.82–1.00) | 0.161 | −0.10 (-0.21–0.05) | 0.87 (0.84–1.14) | 1.04 (0.80–1.20) | 0.475 | 0.01 (−0.09–0.17) | 0.657 | 0.328 | 0.197 |
| MV A velocity (m/s) | 0.84 (0.59–0.90) | 0.75 (0.65–0.91) | 0.889 | 0.01 (−0.13–0.12) | 0.75 (0.60–0.98) | 0.74 (0.59–0.82) | 0.507 | −0.03 (−0.12–0.10) | 0.965 | 0.756 | 0.503 |
| MV E/A | 1.18 (1.05–1.61) | 1.08 (1.00–1.30) | 0.575 | −0.04 (−0.40–0.27) | 1.15 (0.96–1.56) | 1.25 (1.05–1.69) | 0.114 | 0.17 (−0.01–0.27) | 0.477 | 0.286 | 0.286 |
| TR PG (mmHg) | 28.0 (20.0–33.0) | 29.0 (26.0–51.0) | 0.259 | 6.0 (−6.0–16.5) | 28.0 (25.0–50.0) | 33.0 (27.0–34.0) | 1.000 | 3.0 (−13.0–7.9) | 0.424 | 0.915 | 0.367 |
| FS (%) | 47.5 (41.8–49.3) | 45.0 (38.0–51.0) | 0.261 | −2.0 (−8.5–2.3) | 44.0 (40.5–55.3) | 44.0 (41.0–53.5) | 0.799 | 0.0 (−2.3–1.5) | 0.791 | 0.363 | 0.448 |
| HR (bpm) | 130.0 (119.0–150.0) | 124.0 (100.0–142.5) | 0.321 | −2.0 (−12.5–8.5) | 135.0 (117.5–140.0) | 125.0 (110.0–142.5) | 0.713 | −10.0 (−10.0–12.5) | 0.818 | 0.676 | 0.907 |
| Murmur (grade) | 4.0 (3.8–4.0) | 4.0 (3.0–4.0) | 0.157 | 0.0 (−0.3–0.0) | 4.0 (4.0–4.0) | 4.0 (4.0–4.0) | 0.317 | 0.0 (0.0–0.0) | 0.542 | 0.194 | 0.542 |
| Weight (kg) | 7.7 (6.0–10.7) | 8.2 (5.7–11.2) | 0.799 | 0.0 (−0.2–0.3) | 9.2 (7.2–13.8) | 9.1 (7.1–14.3) | 0.721 | −0.0 (−0.3–0.2) | 0.257 | 0.384 | 0.791 |
| BCS | 5.0 (5.0–6.3) | 5.0 (5.0–6.3) | 1.000 | 0.0 (0.0–0.0) | 5.0 (4.8–5.3) | 5.0 (4.0–6.0) | 0.655 | 0.00 (0.00–0.00) | 0.170 | 0.319 | 0.957 |
| Parameter | CoQ10 Supplemented | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 3 Months | p * | Delta | Baseline | After 3 Months | p * | Delta | p a | p b | p c | |
| CoQ10 (mg/L) | 0.171 (0.144–0.222) | 0.863 (0.598–1.247) | 0.001 | 0.705 (0.371–1.071) | 0.164 (0.142–0.230) | 0.163 (0.138–0.208) | 0.575 | 0.007 (−0.033–0.034) | 0.804 | <0.001 | <0.001 |
| GPX (U/g HGB) | 807.3 (690.8–823.9) | 765.9 (711.1–812.3) | 0.701 | −14.8 (−41.6–32.1) | 786.4 (723.3–854.3) | 827.8 (729.2–894.1) | 0.139 | 30.8 (7.7–57.7) | 0.664 | 0.137 | 0.137 |
| F2-isoprostanes (pg/mL) | 510.6 (405.7–680.8) | 534.1 (467.7–649.8) | 0.917 | 7.4 (−104.2–90.9) | 468.2 (338.1–737.8) | 658.5 (399.1–813.3) | 0.169 | 85.7 (−35.7–169.5) | 0.804 | 0.756 | 0.239 |
| TNFSR-II (ng/mL) | 2.144 (1.292–3.278) | 1.113 (0.802–1.683) | 0.071 | −0.650 (−2.059–0.270) | 0.983 (0.607–4.312) | 1.149 (0.713–2.947) | 0.515 | −0.007 (−1.559–0.322) | 0.598 | 0.843 | 0.391 |
| WBC (×109/L) | 8.8 (6.5–10.0) | 9.8 (7.2–11.3) | 0.249 | 0.8 (−0.7–1.3) | 9.9 (7.1–12.7) | 9.3 (8.0–13.1) | 0.799 | −0.6 (−1.2–1.4) | 0.385 | 0.577 | 0.420 |
| Neutrophils (%) | 69.3 (63.9–73.9) | 67.4 (64.7–72.5) | 0.363 | −2.2 (−4.6–3.0) | 66.4 (58.1–71.4) | 68.6 (62.7–76.2) | 0.241 | 4.2 (−1.1–10.3) | 0.336 | 0.852 | 0.041 |
| Neutrophils (×109/L) | 5.9 (4.4–7.6) | 6.0 (4.9–8.1) | 0.345 | 0.5 (−0.6–0.9) | 5.9 (4.8–8.6) | 6.9 (5.5–7.8) | 0.721 | 0.0 (−1.0–1.4) | 0.756 | 0.577 | 0.901 |
| Monocytes (%) | 5.1 (4.3–6.2) | 5.2 (4.1–6.4) | 0.527 | −0.2 (−0.5–0.7) | 5.1 (4.0–6.3) | 4.8 (3.1–5.4) | 0.386 | −0.6 (−1.5–0.6) | 0.664 | 0.351 | 0.192 |
| Monocytes (×109/L) | 0.46 (0.36–0.61) | 0.47 (0.40–0.59) | 0.311 | 0.01 (−0.04–0.09) | 0.49 (0.35–0.66) | 0.45 (0.37–0.56) | 0.445 | −0.01 (−0.21–0.13) | 0.535 | 0.664 | 0.121 |
| NLR | 3.3 (2.5–4.6) | 3.2 (2.6–3.8) | 0.345 | −0.3 (−0.8–0.5) | 3.0 (2.3–3.8) | 3.7 (2.5–4.9) | 0.093 | 0.8 (0.3–1.2) | 0.620 | 0.577 | 0.055 |
| Lymphocytes (%) | 21.5 (16.2–24.4) | 22.3 (18.6–25.3) | 0.311 | 1.3 (−2.5–3.9) | 22.1 (17.7–28.3) | 18.7 (15.7–25.8) | 0.139 | −3.5 (−9.0– −0.3) | 0.598 | 0.438 | 0.044 |
| Lymphocytes (×109/L) | 1.6 (1.4–2.1) | 2.0 (1.4–2.6) | 0.152 | 0.0 (−0.2–0.5) | 2.0 (1.6–3.2) | 1.9 (1.1–3.1) | 0.203 | −0.3 (−0.7–0.1) | 0.107 | 0.804 | 0.041 |
| T lymphocytes CD3+ (%) | 61.6 (50.3–70.7) | 63.2 (42.4–74.5) | 0.600 | 1.5 (−5.3–6.6) | 58.6 (22.9–64.9) | 58.7 (33.5–66.1) | 0.169 | 5.4 (−4.5–15.0) | 0.264 | 0.420 | 0.264 |
| T lymphocytes CD3+ (×109/L) | 1.0 (0.8–1.3) | 1.0 (0.8–1.5) | 0.152 | 0.0 (−0.1–0.3) | 0.9 (0.4–1.9) | 1.2 (0.5–1.8) | 0.878 | −0.0 (−0.1–0.1) | 0.951 | 0.951 | 0.352 |
| T helper cells CD3+CD4+ (%) | 50.5 (46.5–60.0) | 53.1 (45.5–54.7) | 0.093 | −2.8 (−6.4–1.1) | 52.3 (41.7–54.5) | 49.3 (44.6–55.7) | 0.721 | 0.5 (−3.6–3.5) | 0.385 | 0.951 | 0.154 |
| T helper cells CD3+CD4+ (×109/L) | 0.47 (0.38–0.72) | 0.46 (0.37–0.76) | 0.861 | −0.00 (−0.09–0.08) | 0.45 (0.19–1.00) | 0.58 (0.28–0.82) | 0.646 | −0.01 (−0.09–0.09) | 0.804 | 0.852 | 1.000 |
| Activated T helper cells CD3+CD4+CD25+ (%) | 22.6 (19.2–42.3) | 22.7 (17.9–30.6) | 0.505 | −1.5 (−4.6–2.0) | 30.5 (26.8–33.3) | 27.2 (25.2–32.6) | 0.037 | −3.5 (−5.6– −0.9) | 0.131 | 0.313 | 0.402 |
| Activated T helper cells CD3+CD4+CD25+ (×109/L) | 0.12 (0.08–0.24) | 0.11 (0.09–0.17) | 0.650 | −0.01 (−0.04–0.03) | 0.15 (0.05–0.25) | 0.15 (0.09–0.20) | 0.445 | −0.01 (−0.09–0.03) | 1.000 | 0.535 | 0.951 |
| Cytotoxic T lymphocytes CD3+CD8+ (%) | 29.1 (23.3–33.3) | 32.3 (23.0–39.9) | 0.075 | 2.1 (−0.6–8.4) | 28.1 (23.8–34.2) | 30.3 (25.7–35.9) | 0.343 | 1.3 (−1.7–4.9) | 0.877 | 0.901 | 0.515 |
| Cytotoxic T lymphocytes CD3+CD8+ (×109/L) | 0.28 (0.21–0.39) | 0.37 (0.18–0.54) | 0.075 | 0.01 (−0.01–0.19) | 0.27 (0.12–0.65) | 0.36 (0.14–0.59) | 0.386 | 0.02 (−0.04–0.06) | 0.852 | 0.756 | 0.420 |
| Activated cytotoxic T lymphocytes CD3+CD8+CD25+ (%) | 9.2 (6.0–14.7) | 8.3 (5.7–10.6) | 0.239 | −0.5 (−3.9–1.4) | 12.2 (8.7–16.7) | 12.4 (5.7–15.7) | 0.168 | −1.1 (−1.7–0.8) | 0.251 | 0.264 | 0.901 |
| Activated cytotoxic T lymphocytes CD3+CD8+CD25+ (×109/L) | 0.03 (0.02–0.04) | 0.03 (0.02–0.04) | 0.917 | −0.00 (−0.01–0.01) | 0.03 (0.01–0.07) | 0.03 (0.02–0.06) | 0.799 | −0.00 (−0.01–0.01) | 1.000 | 0.951 | 0.710 |
| T helper cells/cytotoxic T lymphocytes ratio (CD4/CD8) | 1.6 (1.4–2.6) | 1.6 (1.2–2.5) | 0.133 | –0.3 (–0.5–0.1) | 1.9 (1.3–2.1) | 1.6 (1.4–2.0) | 0.445 | −0.0 (−0.5–0.2) | 0.710 | 0.804 | 0.535 |
| DPT CD3+CD4+CD8+ (%) | 1.2 (0.8–2.1) | 1.0 (0.8–1.9) | 0.592 | −0.1 (−0.3–0.2) | 1.2 (0.8–2.0) | 1.2 (1.0–2.2) | 0.252 | 0.1 (−0.1–0.3) | 0.975 | 0.454 | 0.235 |
| DPT CD3+CD4+CD8+ (×109/L) | 0.011 (0.005–0.020) | 0.012 (0.005–0.024) | 0.972 | −0.001 (−0.002–0.004) | 0.012 (0.007–0.018) | 0.014 (0.007–0.022) | 0.575 | 0.000 (−0.002–0.005) | 0.901 | 0.951 | 0.852 |
| DNT CD3+CD4-CD8- (%) | 17.1 (12.9–24.3) | 17.2 (11.2–22.7) | 0.046 | −1.7 (−2.6–-0.2) | 19.5 (15.0–20.9) | 16.9 (13.0–19.4) | 0.028 | −2.1 (−3.0– −0.2) | 0.828 | 0.975 | 0.576 |
| DNT CD3+CD4-CD8- (×109/L) | 0.17 (0.12–0.22) | 0.17 (0.11–0.22) | 0.972 | -0.00 (−0.03–0.03) | 0.18 (0.09–0.34) | 0.19 (0.10–0.27) | 0.241 | −0.01 (−0.07–0.01) | 0.901 | 0.804 | 0.385 |
| B lymphocytes CD45+CD21+ (%) | 14.8 (11.8–26.0) | 18.5 (13.0–23.2) | 0.221 | 1.0 (−2.0–5.4) | 17.3 (15.8–20.1) | 14.2 (12.8–21.1) | 0.285 | −3.3 (−4.6–2.5) | 0.535 | 0.336 | 0.107 |
| B lymphocytes CD45+CD21+ (×109/L) | 0.31 (0.18–0.40) | 0.34 (0.25–0.53) | 0.152 | 0.02 (−0.03–0.25) | 0.39 (0.27–0.58) | 0.26 (0.15–0.67) | 0.333 | −0.04 (−0.14–0.06) | 0.215 | 0.664 | 0.121 |
| NT-proBNP (pmol/L) | 1600.0 (647.5–2356.5) | 1344.0 (767.5–4472.0) | 0.382 | 117.0 (−177.5–1798.0) | 2688.0 (1730.0–4271.5) | 2647.0 (1215.5–4917.3) | 0.799 | −35.0 (−867.0–1848.0) | 0.137 | 0.385 | 0.577 |
| cTnI (µg/L) | 0.060 (0.030–0.090) | 0.064 (0.039–0.166) | 0.249 | 0.013 (−0.010–0.035) | 0.090 (0.062–0.173) | 0.135 (0.051–0.263) | 0.326 | 0.004 (−0.023–0.076) | 0.117 | 0.216 | 0.841 |
| LA/Ao | 2.2 (2.1–2.4) | 2.2 (2.0–2.4) | 0.646 | 0.0 (−0.2–0.2) | 2.2 (2.0–2.3) | 2.0 (1.8–2.5) | 0.760 | −0.1 (−0.2–0.4) | 0.344 | 0.256 | 0.940 |
| nLVIDd | 2.0 (2.0–2.2) | 2.1 (1.9–2.3) | 0.695 | 0.0 (−0.1–0.2) | 2.1 (1.7–2.4) | 1.8 (1.6–2.4) | 0.333 | −0.1 (−0.4–0.1) | 0.895 | 0.391 | 0.391 |
| nLVIDs | 1.0 (0.7–1.2) | 1.1 (0.9–1.4) | 0.060 | 0.2 (0.0–0.3) | 1.0 (0.8–1.5) | 0.8 (0.7–1.3) | 0.022 | −0.1 (−0.2– −0.0) | 0.553 | 0.391 | 0.006 |
| MV E velocity (m/s) | 1.29 (1.12–1.51) | 1.18 (1.01–1.68) | 0.638 | 0.05 (−0.14–0.18) | 1.19 (1.03–1.53) | 1.15 (0.81–1.40) | 0.074 | −0.13 (−0.22–0.06) | 0.368 | 0.420 | 0.162 |
| MV A velocity (m/s) | 0.79 (0.72–1.02) | 0.84 (0.65–0.97) | 0.328 | -0.04 (−0.15–0.10) | 0.85 (0.79–0.96) | 0.90 (0.60–1.13) | 0.646 | −0.05 (−0.15–0.13) | 0.620 | 0.828 | 0.780 |
| MV E/A | 1.49 (1.14–1.85) | 1.57 (1.21–1.73) | 0.972 | 0.06 (−0.43–0.33) | 1.49 (1.18–1.61) | 1.29 (1.06–1.71) | 0.285 | −0.04 (−0.29–0.12) | 0.710 | 0.239 | 0.756 |
| TR PG (mmHg) | 40.0 (35.8–51.8) | 41.0 (35.5–50.0) | 0.859 | −2.0 (−12.3–10.0) | 38.0 (36.5–53.5) | 34.0 (26.5–48.5) | 0.373 | −5.9 (−14.0–7.5) | 0.902 | 0.306 | 0.567 |
| FS (%) | 47.5 (44.0–61.8) | 47.0 (36.0–48.8) | 0.049 | −5.5 (−10.3–-4) | 44.7 (33.8–52.0) | 45.5 (38.3–58.5) | 0.105 | 2.5 (−0.3–8.8) | 0.321 | 0.843 | 0.007 |
| HR (bpm) | 140.0 (110.0–145.0) | 130.0 (120.0–150.0) | 0.632 | 10.0 (−15.0–10.0) | 133.0 (120.0–142.5) | 130.0 (110.0–142.5) | 0.671 | −5.0 (−16.5–20.0) | 0.850 | 0.684 | 0.490 |
| Murmur (grade) | 4.00 (4.0–5.0) | 4.00 (4.0–4.5) | 0.083 | 0.0 (−0.5–0.0) | 4.0 (4.0–5.0) | 4.5 (4.0–5.0) | 0.564 | 0.0 (0.0–0.3) | 0.442 | 0.365 | 0.136 |
| Weight (kg) | 8.0 (5.2–11.0) | 8.0 (4.7–10.5) | 0.328 | −0.2 (−0.6–0.1) | 7.8 (6.9–11.6) | 8.1 (7.1–11.6) | 0.285 | 0.2 (−0.3–0.6) | 0.620 | 0.535 | 0.163 |
| BCS | 5.0 (4.5–6.5) | 5.0 (3.5–6.5) | 0.206 | 0.0 (−1.0–0.0) | 4.0 (3.75–6.0) | 4.5 (3.75–6.25) | 0.083 | 0.0 (0.0–1.0) | 0.127 | 0.775 | 0.062 |
| Parameter | ACVIM B2 | CHF | Healthy | p * | p a | p b | p c |
|---|---|---|---|---|---|---|---|
| CoQ10 (mg/L) | 0.176 (0.125–0.213) | 0.171 (0.145–0.213) | 0.095 (0.070–0.143) | 0.006 | 0.019 | 0.008 | 1.000 |
| GPX (U/g HGB) | 746.5 (675.8–792.1) | 792.6 (692.0–827.7) | 775.4 (684.1–827.5) | 0.317 | / | / | / |
| F2-isoprostanes (pg/mL) | 468.9 (323.2–586.1) | 510.6 (356.2–727.0) | 536.0 (420.2–884.2) | 0.373 | / | / | / |
| TNFSR-II (ng/mL) | 0.539 (0.247–0.949) | 1.716 (0.683–3.426) | 0.752 (0.495–0.876) | 0.040 | 1.000 | 0.048 | 0.006 |
| WBC (×109/L) | 8.4 (6.9–10.7) | 9.0 (7.1–10.9) | 8.3 (5.9–9.5) | 0.463 | / | / | / |
| Neutrophils (%) | 69.4 (66.8–74.7) | 68.9 (63.3–72.5) | 66.9 (62.6–72.9) | 0.642 | / | / | / |
| Neutrophils (×109/L) | 5.7 (4.9–8.1) | 5.9 (4.8–7.8) | 5.6 (4.1–6.6) | 0.556 | / | / | / |
| Monocytes (%) | 4.3 (3.6–5.1) | 5.1 (4.1–6.2) | 4.1 (3.3–5.7) | 0.120 | / | / | / |
| Monocytes (×109/L) | 0.35 (0.28–0.46) | 0.47 (0.36–0.63) | 0.34 (0.27–0.49) | 0.044 | 1.000 | 0.122 | 0.099 |
| NLR | 3.0 (2.9–4.5) | 3.2 (2.6–4.3) | 2.7 (2.3–4.0) | 0.654 | / | / | / |
| Lymphocytes (%) | 22.5 (16.9–23.7) | 21.5 (16.9–25.4) | 24.3 (18.6–27.7) | 0.624 | / | / | / |
| Lymphocytes (×109/L) | 1.9 (1.3–2.6) | 1.8 (1.5–2.5) | 1.8 (1.4–2.5) | 0.877 | / | / | / |
| T lymphocytes CD3+ (%) | 67.1 (53.6–75.9) | 61.0 (30.5–67.2) | 60.5 (53.9–71.7) | 0.180 | / | / | / |
| T lymphocytes CD3+ (×109/L) | 1.22 (0.76–1.73) | 0.96 (0.62–1.55) | 1.04 (0.72–1.66) | 0.640 | / | / | / |
| T helper cells CD3+CD4+ (%) | 41.7 (31.2–47.9) | 52.0 (44.7–56.6) | 53.6 (45.2–62.8) | 0.003 | 0.012 | 1.000 | 0.013 |
| T helper cells CD3+CD4+ (×109/L) | 0.41 (0.31–0.73) | 0.47 (0.35–1.55) | 0.55 (0.42–0.73) | 0.314 | / | / | / |
| Activated T helper cells CD3+CD4+CD25+ (%) | 30.2 (19.5–44.7) | 26.0 (21.4–32.8) | 35.7 (23.0–42.0) | 0.497 | / | / | / |
| Activated T helper cells CD3+CD4+CD25+ (×109/L) | 0.14 (0.08–0.20) | 0.13 (0.07–0.25) | 0.19 (0.16–0.24) | 0.910 | / | / | / |
| Cytotoxic T lymphocytes CD3+CD8+ (%) | 39.1 (31.1–50.1) | 29.1 (24.0–33.2) | 26.0 (20.3–34.8) | 0.003 | 0.020 | 1.000 | 0.007 |
| Cytotoxic T lymphocytes CD3+CD8+ (×109/L) | 0.47 (0.26–0.76) | 0.28 (0.14–0.40) | 0.30 (0.19–0.50) | 0.080 | / | / | / |
| Activated cytotoxic T lymphocytes CD3+CD8+CD25+ (%) | 9.1 (5.2–14.0) | 10.1 (6.2–14.7) | 12.5 (10.6–22.6) | 0.178 | / | / | / |
| Activated cytotoxic T lymphocytes CD3+CD8+CD25+ (×109/L) | 0.05 (0.03–0.07) | 0.03 (0.01–0.05) | 0.07 (0.03–0.09) | 0.456 | / | / | / |
| T helper cells/cytotoxic T lymphocytes ratio (CD4/CD8) | 1.05 (0.72–1.40) | 1.75 (1.42–2.28) | 2.17 (1.31–2.76) | 0.001 | 0.007 | 1.000 | 0.003 |
| DPT CD3+CD4+CD8+ (%) | 0.7 (0.5–1.8) | 1.2 (0.8–2.0) | 1.2 (0.9–1.5) | 0.295 | / | / | / |
| DPT CD3+CD4+CD8+ (×109/L) | 0.011 (0.007–0.015) | 0.011 (0.007–0.019) | 0.014 (0.008–0.027) | 0.719 | / | / | / |
| DNT CD3+CD4-CD8- (%) | 15.9 (11.7–18.1) | 17.8 (13.5–23.9) | 15.8 (12.1–20.5) | 0.255 | / | / | / |
| DNT CD3+CD4-CD8- (×109/L) | 0.15 (0.11–0.25) | 0.17 (0.11–0.26) | 0.15 (0.10–0.31) | 0.985 | / | / | / |
| B lymphocytes CD45+CD21+ (%) | 14.1 (9.9–18.4) | 16.9 (12.6–23.1) | 13.7 (10.7–16.8) | 0.174 | / | / | / |
| B lymphocytes CD45+CD21+ (×109/L) | 0.21 (0.14–0.46) | 0.32 (0.20–0.49) | 0.25 (0.18–0.35) | 0.349 | / | / | / |
| NT-proBNP (pmol/L) | 950.0 (492.0–1116.0) | 1956.0 (1093.0–2924.0) | 1452.0 (1110.0–1853.0) | 0.004 | 0.341 | 0.690 | 0.001 |
| Cardiac TnI (µg/L) | 0.044 (0.034–0.086) | 0.078 (0.037–0.112) | 0.022 (0.012–0.189) | 0.087 | / | / | / |
| LA/Ao | 1.95 (1.79–2.05) | 2.21 (1.98–2.32) | 1.36 (1.17–1.60) | <0.001 | 0.002 | <0.001 | 0.047 |
| nLVIDd | 1.81 (1.64–1.91) | 2.04 (1.85–2.18) | 1.36 (1.19–1.66) | <0.001 | 0.031 | <0.001 | 0.050 |
| nLVIDs | 0.90 (0.83–1.03) | 1.01 (0.83–1.29) | 0.80 (0.66–0.92) | 0.066 | / | / | / |
| MV E velocity (m/s) | 0.92 (0.85–1.12) | 1.28 (1.10–1.53) | 0.60 (0.54–0.70) | <0.001 | 0.006 | <0.001 | 0.015 |
| MV A velocity (m/s) | 0.79 (0.60–0.91) | 0.83 (0.74–0.97) | 0.46 (0.39–0.62) | <0.001 | 0.003 | <0.001 | 0.718 |
| MV E/A | 1.17 (1.01–1.56) | 1.49 (1.22–1.68) | 1.31 (1.04–1.50) | 0.175 | / | / | / |
| TR PG (mmHg)d | 28.0 (21.5–43.3) | 39.0 (37.0–49.0) | – | / | / | / | 0.008 |
| FS (%) | 45.5 (41.0–50.8) | 44.7 (39.3–58.8) | 39.5 (37.0–51.5) | 0.556 | / | / | / |
| HR (bpm) | 130.0 (120.0–140.0) | 136.0 (120.0–140.0) | 110.0 (100.0–130.0) | 0.011 | 0.036 | 0.011 | 1.000 |
| Murmur (grade)d | 4.0 (4.0–4.0) | 4.0 (4.0–5.0) | – | / | / | / | <0.001 |
| BCS | 5.0 (5.0–6.0) | 5.0 (4.0–6.0) | 5.0 (5.0–6.00) | 0.205 | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druzhaeva, N.; Nemec Svete, A.; Tavčar-Kalcher, G.; Babič, J.; Ihan, A.; Pohar, K.; Krapež, U.; Domanjko Petrič, A. Effects of Coenzyme Q10 Supplementation on Oxidative Stress Markers, Inflammatory Markers, Lymphocyte Subpopulations, and Clinical Status in Dogs with Myxomatous Mitral Valve Disease. Antioxidants 2022, 11, 1427. https://doi.org/10.3390/antiox11081427
Druzhaeva N, Nemec Svete A, Tavčar-Kalcher G, Babič J, Ihan A, Pohar K, Krapež U, Domanjko Petrič A. Effects of Coenzyme Q10 Supplementation on Oxidative Stress Markers, Inflammatory Markers, Lymphocyte Subpopulations, and Clinical Status in Dogs with Myxomatous Mitral Valve Disease. Antioxidants. 2022; 11(8):1427. https://doi.org/10.3390/antiox11081427
Chicago/Turabian StyleDruzhaeva, Natalia, Alenka Nemec Svete, Gabrijela Tavčar-Kalcher, Janja Babič, Alojz Ihan, Katka Pohar, Uroš Krapež, and Aleksandra Domanjko Petrič. 2022. "Effects of Coenzyme Q10 Supplementation on Oxidative Stress Markers, Inflammatory Markers, Lymphocyte Subpopulations, and Clinical Status in Dogs with Myxomatous Mitral Valve Disease" Antioxidants 11, no. 8: 1427. https://doi.org/10.3390/antiox11081427
APA StyleDruzhaeva, N., Nemec Svete, A., Tavčar-Kalcher, G., Babič, J., Ihan, A., Pohar, K., Krapež, U., & Domanjko Petrič, A. (2022). Effects of Coenzyme Q10 Supplementation on Oxidative Stress Markers, Inflammatory Markers, Lymphocyte Subpopulations, and Clinical Status in Dogs with Myxomatous Mitral Valve Disease. Antioxidants, 11(8), 1427. https://doi.org/10.3390/antiox11081427






