Abstract
Oxidative stress is generated by the imbalance between reactive oxygen species (ROS) formation and antioxidant scavenger system’s activity. Increased ROS, such as superoxide anion, hydrogen peroxide, hydroxyl radical and peroxynitrite, likely contribute to the development and complications of atherosclerotic cardiovascular diseases (ASCVD). In genetically modified mouse models of atherosclerosis, the overexpression of ROS-generating enzymes and uncontrolled ROS formation appear to be associated with accelerated atherosclerosis. Conversely, the overexpression of ROS scavenger systems reduces or stabilizes atherosclerotic lesions, depending on the genetic background of the mouse model. In humans, higher levels of circulating biomarkers derived from the oxidation of lipids (8-epi-prostaglandin F2α, and malondialdehyde), as well as proteins (oxidized low-density lipoprotein, nitrotyrosine, protein carbonyls, advanced glycation end-products), are increased in conditions of high cardiovascular risk or overt ASCVD, and some oxidation biomarkers have been reported as independent predictors of ASCVD in large observational cohorts. In animal models, antioxidant supplementation with melatonin, resveratrol, Vitamin E, stevioside, acacetin and n-polyunsaturated fatty acids reduced ROS and attenuated atherosclerotic lesions. However, in humans, evidence from large, placebo-controlled, randomized trials or prospective studies failed to show any athero-protective effect of antioxidant supplementation with different compounds in different CV settings. However, the chronic consumption of diets known to be rich in antioxidant compounds (e.g., Mediterranean and high-fish diet), has shown to reduce ASCVD over decades. Future studies are needed to fill the gap between the data and targets derived from studies in animals and their pathogenetic and therapeutic significance in human ASCVD.
1. Introduction
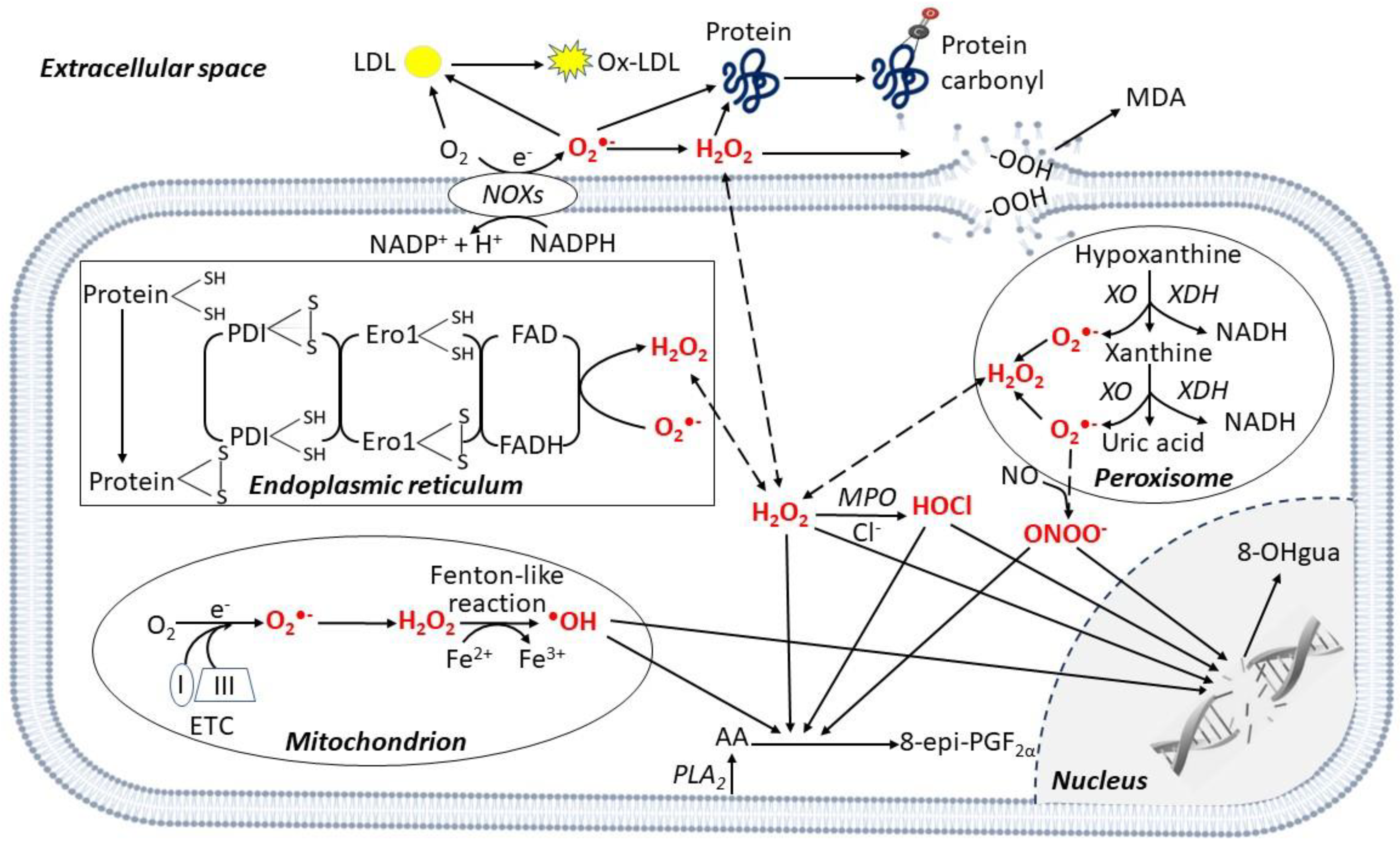
Oxidative stress is generally defined as an imbalance between formation of reactive oxygen species (ROS) [1] and their clearance by antioxidant systems [2]. ROS include molecules and free radicals (i.e., chemical species with one unpaired electron) derived from molecular oxygen (O2) formed in the cell cytoplasm, endoplasmic reticulum (ER), mitochondria, peroxisomes [3,4] and extracellular space (Figure 1).
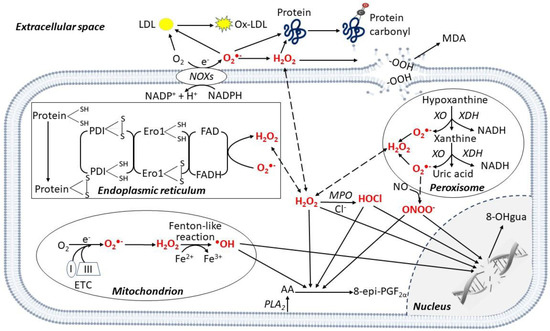
Figure 1.
Enzymatic and non-enzymatic production of reactive oxygen species in different cell compartments. Reactive oxygen species (ROS) are produced in different cellular compartments. Mitochondria generate a high quantity of ROS through the electron transport chain (ETC), mainly complexes I and III, and the •OH is produced via the Fenton-like reaction. Other ROS-producing mechanisms involve transmembrane nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs), xanthine oxidase (XO) in peroxisomes, and protein disulfide isomerase (PDI) in the endoplasmic reticulum. ROS oxidize polyunsaturated lipids from membranes releasing 8-epi-prostaglandin F2α (8-epi-PGF2α) from arachidonic acid (AA), and malondialdehyde (MDA). In the cytoplasm, myeloperoxidase (MPO) mediates HOCl formation from Cl−. In the nucleus, ROS induce DNA damage, releasing 8-hydroxy-2′-deoxyguanosine (8-OHgua). In the extracellular space, ROS mediate the oxidation of proteins, generating protein carbonylation. Specifically, in the peripheral blood the oxidation of low-density lipoprotein (LDL) generates oxidized (ox)-LDL. Abbreviations: Ero1: Endoplasmic Reticulum Oxireductin 1; FAD: Flavin Adenine Dinucleotide; PLA2: Phospholipase A2; XDH: Xanthine Dehydrogenase.
While O2 by itself is not very reactive, if one of its unpaired electrons is excited, the resulting species become powerful oxidants [5]. Superoxide anion (O2•−), is the precursor of most ROS, such as hydrogen peroxide (H2O2), which may then generate the hydroxyl radical (•OH) and the peroxynitrite (ONOO−) by reacting with nitric oxide (NO) [4] (Figure 1). O2•− can be produced during enzymatic reactions, e.g., by cytochrome P450, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs), or xanthine oxidase (XO) in the cell cytoplasm [2]. O2•− can also be non-enzymatically released along the mitochondrial electron transport chain (ETC) reactions, especially by complexes I and III [3,4] (Figure 1). Depending on their origin, type and environment, ROS-triggered signals may contribute to both cell homeostasis [3,6] or dysfunction by the non-specific damage of proteins, lipids, nucleic acids, and polysaccharides [4].
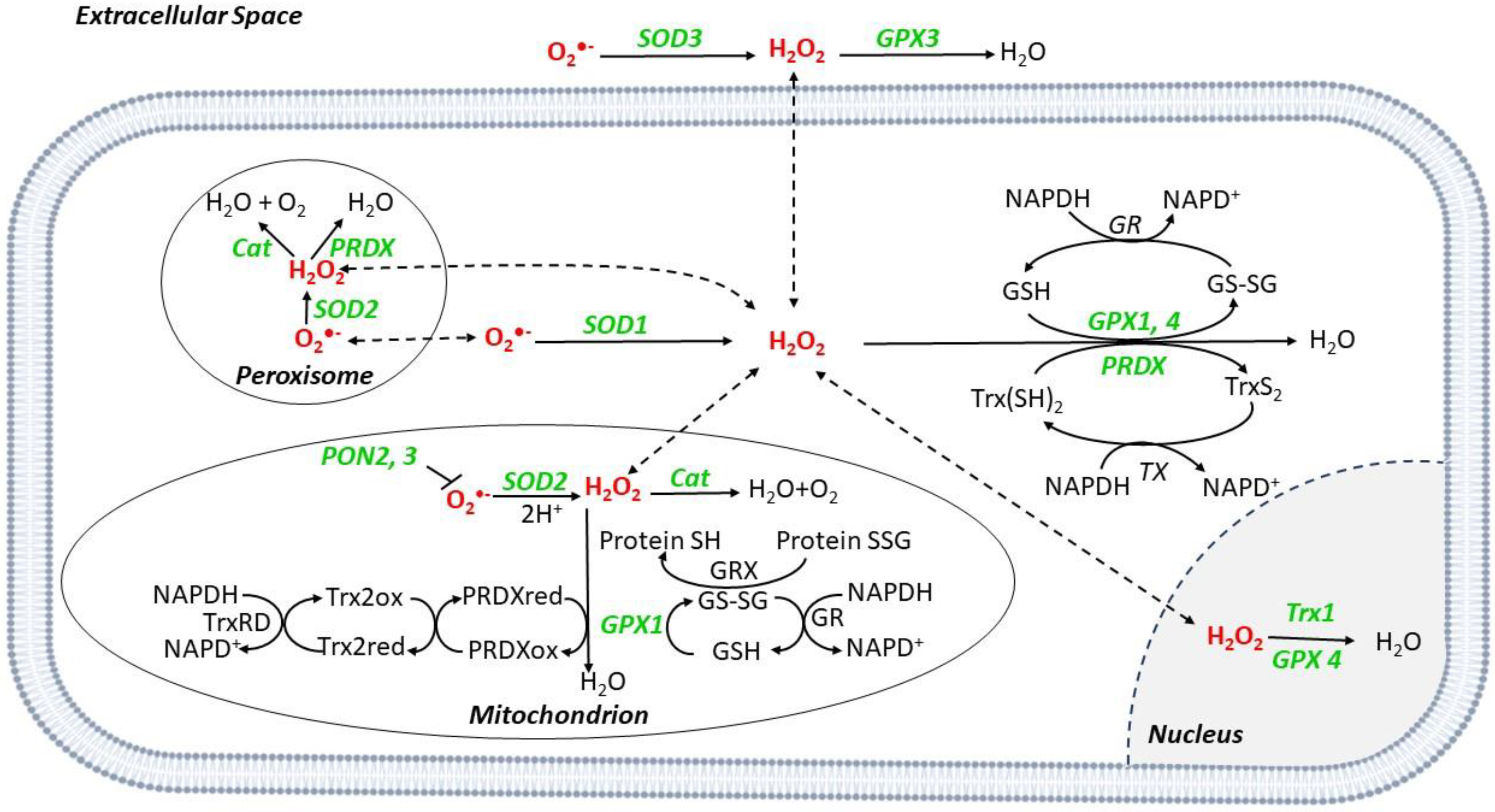
The main ROS-buffering systems in the mitochondria include glutaredoxin (GRX), glutathione (GSH) and thioredoxin (Trx) [7]. Superoxide dismutases (SOD) are metalloenzymes converting O2•− into H2O2, which can be then degraded into H2O and O2 by the GSH redox system that includes glutathione reductases (GR), glutathione peroxidase (GPX), and peroxiredoxins (PRDXs) [8] (Figure 2).
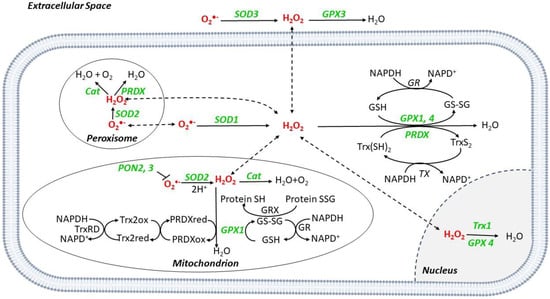
Figure 2.
ROS scavenger systems in different cell compartments. O2•− is converted to H2O2 by superoxide dismutases (SODs), SOD1 in the cytoplasm, SOD2 in the mitochondria, and peroxisome, and SOD3 in the extracellular space. Catalase (Cat) catalyzes the reduction from H2O2 to O2 and H2O in mitochondria and peroxisome. Glutathione peroxidases (GPX) catalyze the reduction in H2O2; during the reaction, glutathione (GSH) is converted to its oxidized form (GSSG), which has a decreased ability to reduce peroxide. Once oxidized, GSH can be regenerated from GSSG by the enzyme glutathione reductase (GR) using reduced nicotinamide NADPH as the electron donor. During the process, NADPH is oxidized to NADP+. Peroxiredoxins (PRDX) reduce H2O2 to H2O by utilizing electrons from NADPH via thioredoxin (Trx) and thioredoxin reductase (TR). Paraoxonase (PON) isoforms 2 and 3 can prevent mitochondrial O2•− generation. Abbreviations: GRX: Glutaredoxin; XO: Xanthine Oxidase.
SOD2 is mitochondrial, while SOD1 and 3 are cytoplasmic and extracellular, respectively [8]. Catalase (Cat) is a peroxisome scavenger enzyme, converting H2O2 into H2O and O2 [8] (Figure 2).
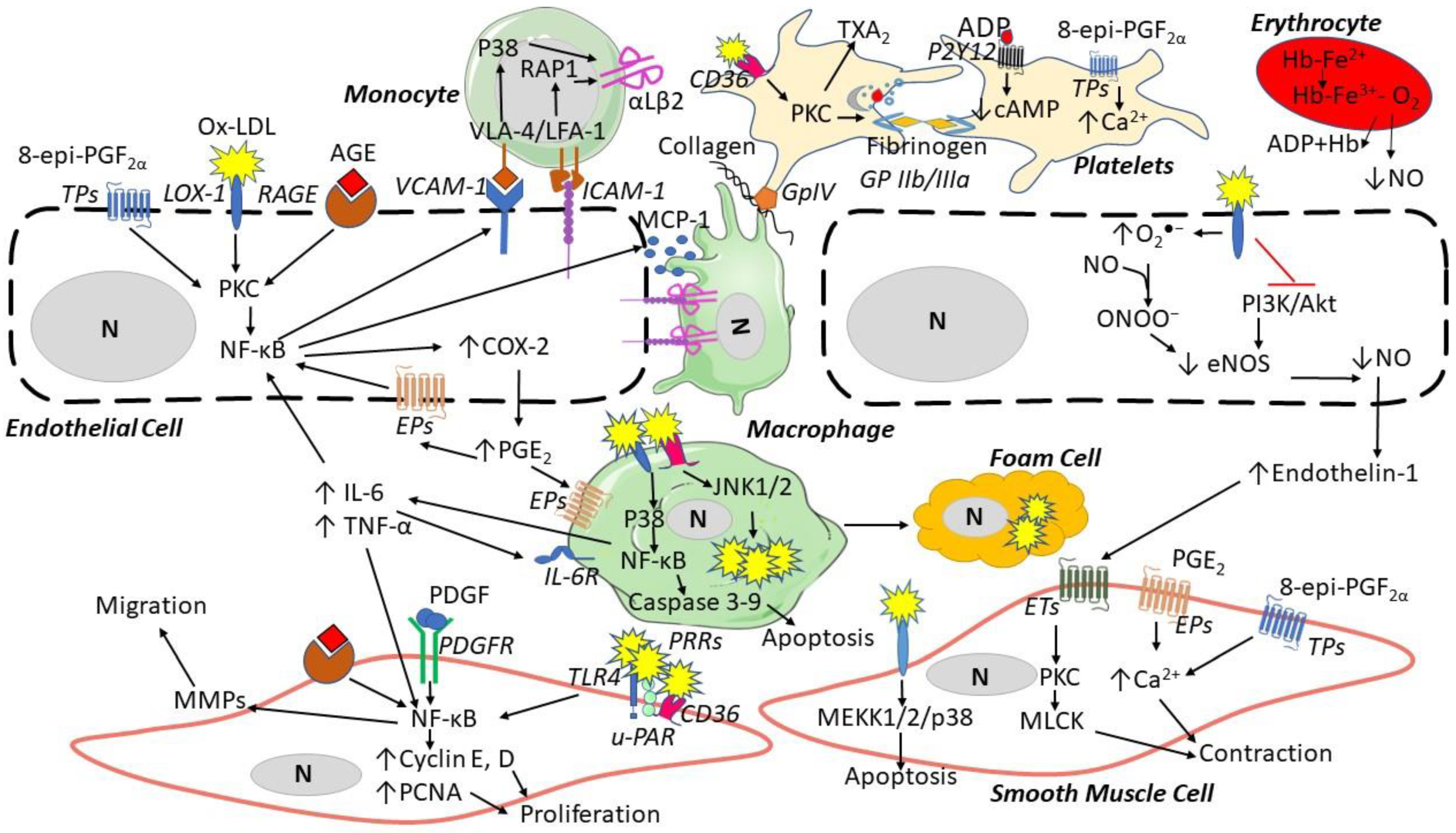
Several pre-clinical data suggest that ROS contribute to atherosclerosis through endothelial cell (EC) dysfunction, platelet activation and vascular remodeling [9] (Figure 3), while the translation of pre-clinical evidence into human atherosclerotic cardiovascular disease (ASCVD) seems more complex and less clear. The present review will revise pre-clinical, clinical and intervention evidence of ROS involvement in atherosclerosis development and its thrombotic complications.
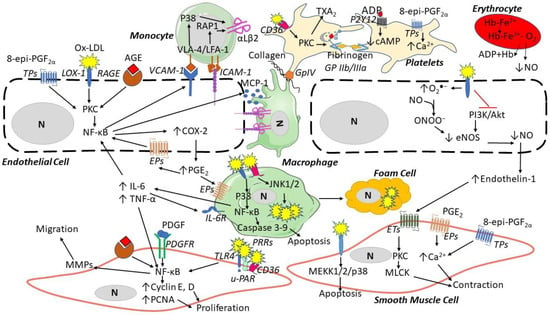
Figure 3.
ROS contribution to the formation of atherosclerotic lesions. Oxidized low-density lipoproteins (ox-LDL) and advanced glycation end-products (AGEs) can bind their receptors (LOX-1 and RAGE, respectively) and induce endothelial cell dysfunction by increasing the expression of vascular adhesion molecule-1 (VCAM-1), intracellular adhesion molecule (ICAM-1), inducing the secretion of monocyte chemotactic protein-1 (MCP-1), and reducing nitric oxide. Endothelial dysfunction then induces monocyte adhesion, the expression of αLβ2 integrin binding ICAM-1, migration to the media, and differentiation into macrophages, which then release inflammatory cytokines (e.g., interleukin(IL)-6 and tumor necrosis factor-alpha (TNF-α)). Platelets are activated by Ox-LDL through CD36 binding and 8-epi-PGF2α triggers platelet aggregation via thromboxane (TX) A2 receptors (TP), releasing adenosine diphosphate (ADP). In smooth muscle cells, ox-LDL via LOX-1, AGEs via RAGE, platelet-derived growth factor (PDGF), and endothelin-1 can induce proliferation, apoptosis, and contraction through several pathways. Abbreviations: Akt: protein kinase B; CD36: cluster of differentiation 36; COX: cyclooxygenase; eNOS: endothelial nitric oxide synthase; EPs: prostaglandin E2 receptors; ET: endothelin receptor; Hb: hemoglobin; JNK: c-Jun N-terminal kinase; LOX: lectin-like oxidized LDL receptor; LFA: lymphocyte function-associated antigen; MEKK: mitogen-activated protein kinase kinase; MMPs: matrix metalloproteinases; MLCK: myosin light-chain kinase; N: nucleus; PI3K: phosphatidylinositol 3-kinase; PAR: protease-activated receptor; PDGFR: platelet-derived growth factor receptor; PGE2: prostaglandin E2; NF-κB: nuclear factor-kappa; PCNA: proliferating cell nuclear antigen; P2Y: purinergic receptor; PKC: protein kinase C; p38: mitogen-activated protein kinases; PRRs: pattern recognition receptors; RAGE: receptors of advanced glycation end products; TLR: toll-like receptor; u-PAR: urokinase plasminogen activator receptor; VLA: vascular leukocyte adhesion molecule.
2. ROS Generation
Animal models supporting the contribution of ROS in atherosclerosis are summarized in Table 1.

Table 1.
ROS production and atherosclerosis in animal models and in human diseases.
NOX isoenzymes transport electrons across biological membranes, reducing O2 into O2•− (Figure 1), and consist of seven isoforms: NOX1 to 5, and dual oxidase 1 and 2 [34]. NOX1, 2, and 4 have been extensively investigated as ROS generators in mouse models. NOX1, which is mainly expressed in rodent’s ECs and vascular smooth muscle cells (VSMCs) [35], can be activated by different pro-thrombotic stimuli, including angiotensin II (AngII), and platelet-derived growth factor (PDGF) [36]. In apolipoprotein (Apo)E knockout (−/−) mice that develop a disease similar to human atherosclerosis, NOX activator-1 is increased in aortic atherosclerotic lesions [10], and double ApoE−/−/NOX1−/− mice show reduced O2•− in the heart and atherosclerotic lesions vs. ApoE−/− animals [3,12,13] (Table 1). In coronary arteries isolated from transplanted hearts, O2•− is higher in the coronary artery with atherosclerosis [27]. However, low NOX1 expression has also been reported in atherosclerotic coronary arteries of patients undergoing bypass grafting while NOX2 and NOX4 expression were significantly higher in vessels with coronary artery disease (CAD) vs. non-CAD [27,28] (Table 1); thus, whether NOX1 is involved in human atherogenesis is unclear.
NOX2 is highly expressed in rodent’s ECs, fibroblasts, and VSMCs [35,37], and can be activated by AngII, thrombin, endothelin, tumor necrosis factor-α (TNF-α), interleukin (IL)-1, PDGF [38,39,40]. In ApoE−/− mice, NOX2 is upregulated in aortic ECs with early vascular lesions [11] (Table 1). High-fat diet (HFD)-fed ApoE−/− mice treated with an NOX2 inhibitor show reduced O2•− in aortic lesions [14], NOX2−/− mice are protected from injury-induced neointima formation [15] and show poor platelet adhesion to injured arteries [41], double ApoE−/−/NOX2−/− mice show reduced aortic O2•− levels and atherosclerosis [11] (Table 1). EC-targeted NOX2 overexpression (+/+) in ApoE−/− mice increases O2•− levels and macrophage infiltration into early atherosclerotic lesions [17]. NOX2 requires activation through interactions between subunits, including p47 phagocyte oxidase (phox) and gp91 phox [42]. ApoE−/− p47 phox−/− or gp91 phox−/− mice have significantly decreased atherosclerosis, O2•− and increased NO in the aortas vs. ApoE−/− mice [11,18] (Table 1). Triple-NOX1−/−/NOX2−/−/NOX4−/− mice show reduced platelet O2•− formation and aggregation [19], (Table 1). In humans, congenital NOX2 deficiency is associated with a rare X-linked chronic granulomatous disease (CGD) [29]. This defect is characterized by low atherosclerosis [29], circulating oxidized low-density lipoprotein (ox-LDL), urinary 8-epi-prostaglandin F2α (8-epi-PGF2α), a non-enzymatic product of lipid oxidation [29], low O2•− and high NO generation from platelets [43] (Table 1), suggesting a role for NOX2 in human atherogenesis.
NOX4 is expressed in rodent’s VSMCs, fibroblasts, and ECs [16]. It produces H2O2 via its E-loop, accelerating O2•− dismutation [44] (Figure 1). ApoE−/−/LDL receptor (LDLr)−/− mice show increased NOX4 in aortic lesions and NOX4 knockdown, with short interfering (si)RNA in the aortic SMCs of these mice decreases H2O2, suggesting a role for NOX4-derived cellular ROS in atherosclerosis [20]. In humans, NOX4 is expressed in VSMCs, ECs and fibroblasts [45,46]. The in vitro NOX4 depletion of human umbilical vein EC (HUVECs) using small-hairpin RNA, decreases O2•− and 8-oxodeoxyguanosine, a marker of oxidative DNA damage [47] (Table 1). Human macrophages isolated from healthy individuals and treated with ox-LDL upregulate NOX4, increase O2•− and H2O2 and undergo death [48,49]. NOX4 expression in coronary artery intima increases with atherosclerosis severity in transplanted hearts [50].
Myeloperoxidase (MPO) is a leukocyte enzyme that generates hypochlorous acid (HOCl) [32,51]. HFD-fed ApoE−/− mice irradiated and reconstituted with MPO−/− bone marrow show reduced atherosclerosis [24]; MPO−/− mice and wild-type WT mice treated with an MPO inhibitor show reduced neointima formation following ischemia reperfusion (I/R) injury [24] (Table 1). However, LDLr−/− mice irradiated and reconstituted with bone marrow MPO−/− exhibit a significant increase in aortic atherosclerotic lesions vs. LDLr−/− mice [25] (Table 1). Human atherosclerotic thoracic arteries express higher MPO levels than normal ones [33] (Table 1), and MPO was significantly increased in the coronary atherosclerotic lesion of transplanted hearts [32] (Table 1).
Mitochondrial dysfunction in the cell can generate a disproportionate O2•− rate [52], which may damage mitochondrial DNA (mtDNA) [53]. Consistently in early aortic atherosclerotic lesions of ApoE−/− mice, mtDNA integrity is decreased, O2•− is increased, and treatment with a mitochondrion-targeted antioxidant significantly reduces H2O2 and atherosclerosis [54,55]. Protein kinase R-like endoplasmic reticulum resident kinase (PEAK) is a stress-sensor protein that decreases translation in response to stress [56]. In a rat cardiomyoblast cell line, PEAK silencing increases mitochondrial activity and ROS, while cardiac PEAK+/+ mice seem to be protected from I/R injury and show a significant decreased mitochondrial complex I activity [57].
MicroRNA-210 regulates cellular hypoxia response by targeting the mitochondrial energy metabolism [58]. In MicroRNA-210−/− mice, mitochondrial ROS significantly increase after I/R vs. WT [26] (Table 1). In humans, atherosclerotic carotid endarterectomies show a lower mtDNA copy number than normal vessels [59].
In conclusion, genetically modified animal models show that several enzymatic and non-enzymatic reactions that generate ROS can contribute to different phases of atherosclerosis. Human evidence on the same enzymes is more limited and often inconsistent.
3. Scavenger Systems
Studies on ROS scavenger systems are summarized in Table 2.

Table 2.
Scavenger systems and atherosclerosis in animal models and in human diseases.
Cat is localized in the peroxisomes that are adjacent to the mitochondria (Figure 2) of mammalian tissues [8,94]. In LDLr−/− mice on HFD, mitochondrial O2•− suppression in macrophages through mitochondrial Cat overexpression is associated with reduced aortic atherosclerosis [3]. Mitochondrial oxidative stress appears to be reduced by Cat+/+ targeted to macrophages or myeloid cells in LDL−/− mice, with reduced aortic lesions [63,64], and ApoE−/−/Cat+/+ mice show reduced aortic atherosclerosis and 8-epi-PGF2α expression [60,61] (Table 2). Cat+/+ in aortic VSMCs reduces apoptosis through TNF-α and metalloproteases reduction in mice [62]. Interestingly, adenovirus-mediated Cat+/+ in human aortic ECs in vitro reduces ox-LDL-induced O2•− and apoptosis via Jun N-terminal kinase inhibition and extracellular signal-regulated kinase phosphorylation, which are downstream effectors of mitogen activator protein kinase [95], with the latter being involved in atherosclerosis development in mice. In humans, Cat gene mutations cause Acatalasemia, which is characterized by low Cat levels, diabetes mellitus (DM), and increased atherosclerosis [82] which are hypothesized to be secondary to H2O2 increase [83,84].
GPXs are selenoproteins that catalyze the reduction in H2O2 and other peroxides (e.g., lipids of the cell membrane) using GSH as a substrate [96] (Figure 2). They include cytosolic and mitochondrial GPX1, extracellular GPX3, and GPX4 expressed in the cytosol, mitochondria, and nucleus [97]. In hyperhomocysteinemic cystathionine beta-synthase-deficient mice, GPX1+/+ restores normal, EC-dependent vascular function [98] (Table 2). Consistently, in ApoE−/−/GPX1+/+ mice, atherosclerotic lesions and aortic 8-epi-PGF2α content are reduced [68], while the opposite occurs in ApoE−/−/GPX1−/− mice [70,71] (Table 2). GPX1+/- mice show increased mesenteric vasoconstriction, perivascular matrix deposition, and plasma 8-epi-PGF2α [69]. The upregulation of GPX1 in human ECs in vitro decreases the expression of proatherogenic genes such as CD40, monocyte chemoattractant protein-1 (MCP-1), and vascular cell adhesion protein-1 (VCAM-1) [86] (Table 2). In 101 patients undergoing coronary stenting, erythrocytes with the 599C/T allele of the GPX1 gene exhibit low activity of GPX, ox-LDL, and a higher risk of restenosis vs. WT allele homozygotes [85] (Table 2). Other studies show that GPX1 activity in washed erythrocytes is inversely correlated with CAD and acute myocardial infarction (MI) [99,100,101].
The paraoxonase (PON) proteins seem be antioxidant by hydrolyzing lipid peroxides [102]. PON transgenic cluster (PONs 1, 2, and 3) overexpression promotes plaque stability [3]. PON1−/− mice show increased aortic O2•− and leukocyte adhesion [77]; conversely, ApoE−/−/PON1+/+ mice show reduced atherosclerosis [78] (Table 2). These data support an anti-atherosclerotic role for PON1, probably by increasing the antioxidant activity of high-density lipoprotein (HDL) and reducing ox-LDL levels in the arterial wall [78]. The overexpression of PON1 in LDL−/− adenovirus-mediated PON1 gene transfer mice reduced plaque volume [79] (Table 2). Human PON1 activity in serum is inversely related to the risk of ASCVD and stenosis requiring revascularization in patients with CAD [103,104]. The Met-Leu (M/L) 54 PON1 polymorphism is associated with reduced serum PON1 in DM subjects and correlates with increased ASCVD [87], the M/L55 and Gln-Arg (Q/R) 192 PON1 polymorphism is also associated with reduced serum PON1 activity and CAD [88,89] (Table 2).
ApoE−/−/PON2−/− mice show larger aortic atherosclerotic lesions and LDLs with higher lipid hydroperoxide content compared to ApoE−/− mice [80], and PON2−/− mice show high mitochondrial O2•− levels in peritoneal macrophages and aortas [80] (Table 2). ApoE−/− mice, injected with adenovirus-expressing human PON2, show significantly lower circulating lipid hydroperoxides. LDLs are less susceptible to oxidation, while HDL protect these from LDL oxidation [81] (Table 2). PON2 expression seems to be reduced in ECs and monocytes/macrophages from human carotid atherosclerotic lesions vs. healthy tissues [105].
Two mammalian ubiquitous Trx isoforms are known (Figure 2): Trx1 is a cytosolic and nuclear protein, whereas Trx2 is mitochondrial [106]. The Trx-related system reduces oxidized cysteine by interacting with the redox-active center of Trx (Cys-Gly-Pro-Cys), which, in turn, can be reduced by Trx reductase and NADPH [107] (Figure 2). EC-targeted Trx2+/+ mice show increased scavenging activity for H2O2 and O2•− [72], ApoE−/−/Trx2+/+ mice show improved EC function and reduced atherosclerosis [72] and mice with targeted cardiac Trx2−/− exhibit high oxidative status and vascular lesions [73,74] (Table 2). Trxs are expressed in human VSMCs of normal coronary arteries and are increased in atherosclerotic coronary arteries from autopsies, especially in macrophages [90] (Table 2), suggesting a possible role of Trx in the protection of human coronary arteries.
PRDX is a ubiquitous system of six mammalian isoforms in cytosol, mitochondria, and peroxisomes [65,108] (Figure 2). ApoE−/−/PRDX1−/− and ApoE−/−/PRDX2−/− mice display larger macrophage-rich aortic lesions [66] and accelerated plaque formation [67] (Table 2). ApoE−/−/PRDX4+/+ mice show reduced atherosclerotic lesions and ox-LDL levels [65] (Table 2).
Three SOD isoforms are known: cytoplasmic SOD1, mitochondrial SOD2, and extracellular SOD3 [109,110] (Figure 2). These catalyze the dismutation of O2•− into molecular O2 and H2O2 [111]. The role of SODs in atherosclerosis appears controversial [8]. In rabbit hearts perfused with high-dose SOD, the effects on atherogenesis seem to be dose-dependent [112] (Table 2). SOD1−/− mice showed increased aortic O2•− than WTs [75] (Table 2). ApoE−/−/SOD2+/− mice showed increased atherosclerosis and plaque vulnerability [76] (Table 2). SOD3 expression in atherosclerotic vessels, VSMCs, and coronary ECs in humans is reduced in DM [113,114]. SOD3R213G polymorphism is associated with reduced enzyme activity and increased ischemic heart disease [92]. A T-allele of rs2284659 variant in the promoter is associated with high SOD3 plasma levels and inversely correlates with MI incidence in type 1 (T1)DM and type 2 (T2)DM patients [93] (Table 2).
In conclusion, genetically modified mice overexpressing Cat, Cat+SOD1 [60], PRDX4 [60], or Trx2 in ECs [72] and the deletion of scavenger systems such as the nuclear factor [erythroid-derived 2]-like 2 related factors 2 (Nrf-2) [115], GPX1 [116], SOD2, and PRDX1 and 2 [3] indicate a protective role in atherosclerosis. The same patterns appear to be confirmed in fewer, descriptive human studies.
4. Human Circulating Biomarkers of Oxidative Stress
Several data arise from biomarker studies support a role for ROS in human ASCVD. Polyunsaturated lipids are susceptible to non-enzymatic oxidative damage, leading to F2-isoprostanes and malondialdehyde (MDA) [117,118] (Figure 1). The F2-isoprostane 8-epi-PGF2α is non-enzymatically derived from ROS attack to the arachidonic acid of the cell’s membranes, and is stable and measurable in human urine [118]. It exerts pro-thrombotic and vascular-damaging actions by binding to the thromboxane (TX)A2 receptor (TP), which can then activate platelets and induce EC dysfunction and VSMC contraction [119,120,121] (Figure 3). A significant and strong association has consistently been reported between urinary 8-epi-PGF2α excretion and serves as an in vivo biomarker of platelet activation, i.e., the urinary 11-dehydro-TXB2, a major enzymatic metabolite of platelet’s TXA2 [118,122], in human conditions of high CV risk, such as obesity [123,124], hypercholesterolemia [125], DM [126,127], pre-diabetes [128], essential thrombocythemia [129], hypertension [130], and cigarette-smoking (Table 3).

Table 3.
Oxidative stress biomarkers in ASCVD and related high-risk patients.
In addition, in 12,239 postmenopausal women followed over 18 years, urinary 8-epi-PGF2α independently predicted CV mortality [133] (Table 3).
MDA is a highly reactive dialdehyde generated from ROS-mediated lipid degradation (Figure 1) [157]. It can induce protein adducts and cross-linking [158], and is measurable in human blood [159]. Consistent with its lipid origin, plasma MDA and 8-epi-PGF2α have been shown to be highly correlated in some studies (Table 3) [160]. MDA levels are increased in cigarette smoking [137,139], DM [134], CAD [135,138] patients, and they independently predicted MI and revascularization in CAD patients enrolled in the Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial [136] (Table 3).
Ox-LDLs are the end-product of non-enzymatic O2•− modifications (Figure 1) to both LDL proteins and lipids and are measurable in human plasma [161,162]. Ox-LDLs contribute to foam cell development in the vessel wall and bind to macrophages via scavenger receptors [163] and to ECs through the lectin-like oxidized LDL receptor-1, increasing adhesion molecule binding [164] and platelet activation via the scavenger CD36 receptor [165] (Figure 3). Enhanced circulating ox-LDLs are reported in acute MI [140]. A meta-analysis of 8644 subjects with or without previous ASCVD showed that increased ox-LDLs are associated with ASCVD recurrence [144]; they also independently predicted carotid and femoral atherosclerosis and ASCVD in a prospective population-based survey of from 40- to 79-year-old men and women followed over 10 years [142]. They independently predicted CV death, MI, and angina in 238 CAD patients over 52 months [141], and predicted MI and CV death in acute coronary syndrome (ACS) patients [143] (Table 3).
Protein oxidation can be measured by nitrotyrosine derived from tyrosine nitration, ONOO− and NO, in serum, plasma, and urine samples [166,167]. In a case-control study with 100 CAD patients, circulating nitrotyrosine levels were higher in CAD vs. non-CAD patients, and the rates of CAD and atherosclerosis were increased in the higher nitrotyrosine quartiles [146]. Nitrotyrosine is increased in T2DM patients as compared to healthy subjects [145] (Table 3).
Protein carbonyls, the most frequent ROS-induced protein modification, are markers of the irreversible damage of lysine (Lys), arginine (Arg), proline (Pro), and threonine (Thr) residue oxidation [168], in a process named “primary protein carbonylation”. The end-product 2,4-dinitrophenylhydrazine [169,170] is stable and measurable in plasma [171]. Elevated circulating protein carbonyls were detected in T2DM [148,152], in hypercholesterolemia [151], and in CAD patients [149] (Table 3). Advanced glycation end products (AGEs) are protein carbonyls generated in the “secondary protein carbonylation” process through glycoxidation, and Nε-(carboxymethyl)lysine is the most abundant AGE [172], which is measurable in organic fluids and tissues [173]. AGEs cause cell damage by binding its receptor (RAGE), which activates nuclear factor-kappa B (NF-κB) [174], and seem to be involved in T2DM-related CV complications [147,150,153,155]. In a meta-analysis of seven prospective observational studies, including 3718 participants, increased circulating AGEs were associated with increased all-cause and CV mortality [156] (Table 3).
5. Pharmacological Interventions
5.1. Antioxidant Compounds
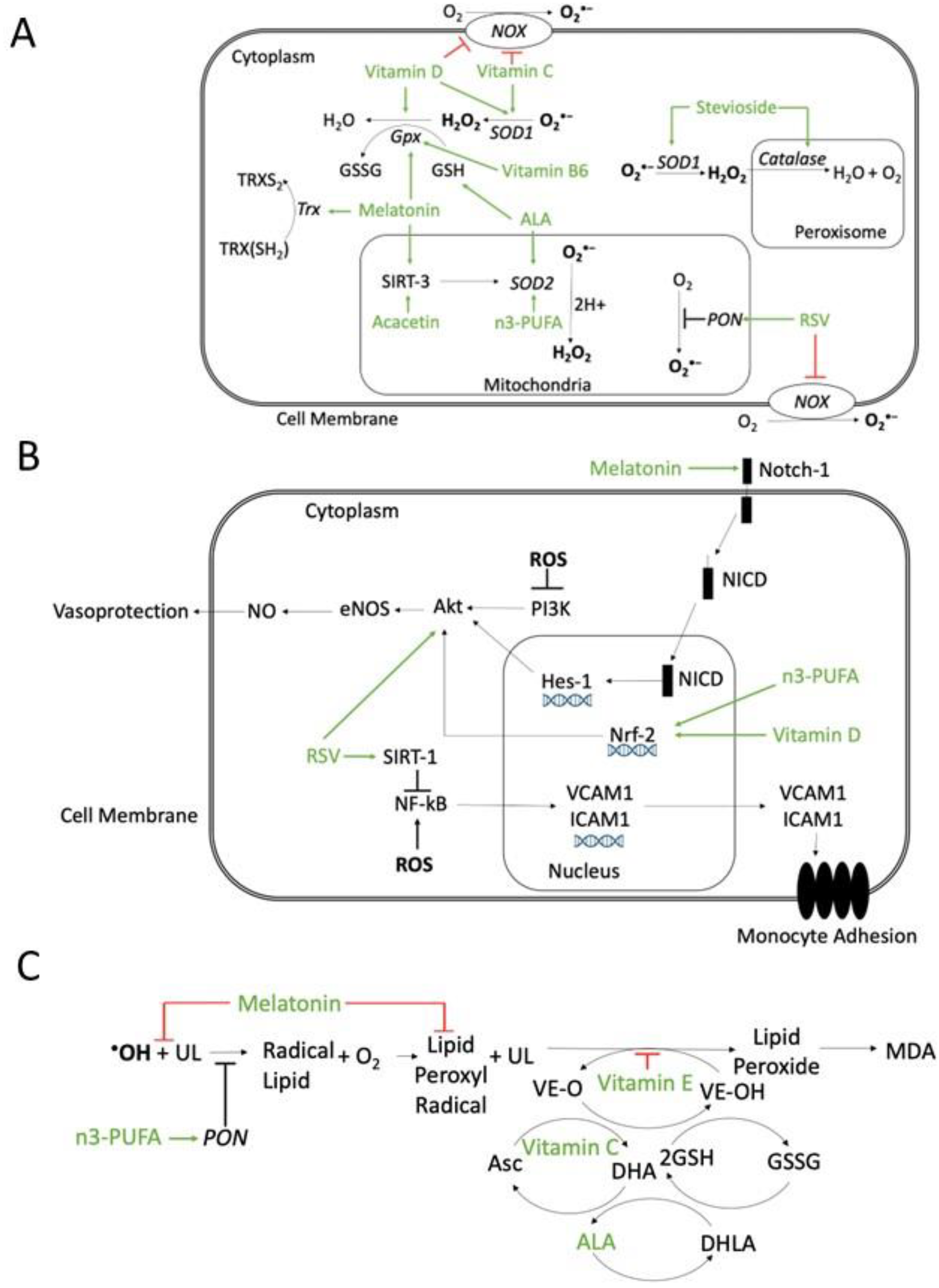
Several molecules with antioxidant properties have been studied in animal models of atherosclerosis and in humans (Figure 4).
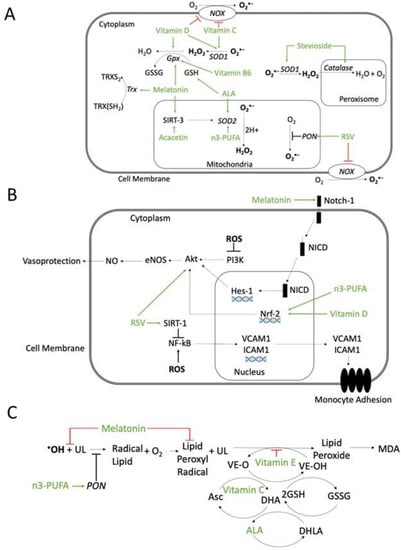
Figure 4.
Possible mechanisms of action of antioxidant compounds. (A) Effects on ROS production or scavenger systems. Resveratrol (RSV) inhibits NADPH oxidase (NOX) and increases paraoxonase (PON) activity. Vitamin D and ascorbic acid (Vitamin C) inhibit NOX and increase superoxide dismutase (SOD) activity. Vitamin B6 is involved in glutathione peroxidase (GPX) synthesis. Alpha-lipoic acid (ALA) regenerates glutathione (GSH). Melatonin enhances glutathione peroxidase (GPX) and thioredoxin (Trx), reducing ROS. Melatonin and acacetin, through the mitochondrial Sirtuin-3 (SIRT-3) pathway, increase SOD scavenger activity. Stevioside enhances both SOD and peroxisomal catalase. n-3 polyunsaturated fatty acids (n3-PUFA) enhance SOD activity. (B) Inhibition of ROS-activated pathways involved in atherosclerosis. Melatonin can activate the Notch homolog 1 (Notch-1) pathway and restore, through hairy and enhancers of split-1 (HES-1), the Phosphatidyl Inositol 3-Kinase/Protein kinase B/Endothelial nitric oxide synthase (PI3K/Akt/eNOS) pathway, which is inhibited by ROS. ROS-induced PI3K/Akt/eNOS inhibition decreases NO and vasoprotection. Vitamin D and n3-PUFA, through the activation of nuclear factor erythroid 2-related factor 2 (Nrf-2), stimulates protein kinase B/Endothelial nitric oxide synthase/NO (Akt/eNOS /NO) pathway and NO release. Resveratrol (RSV) increases Akt activity, increasing NO and vasoprotection, and through Sirtuin-1(SIRT-1) pathway, inhibits ROS-induced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) deacetylation, which upregulates the nuclear transcription of vascular cell adhesion protein-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), leading to monocyte adhesion. (C) Inhibition of lipid peroxidation. Melatonin can scavenge •OH and lipid peroxyl radicals. Vitamin E can scavenger lipid peroxyl radicals. Vitamin C can regenerate preferentially the Vitamin E radical and the ascorbic acid radical can be regenerated by GSH. ALA can regenerate both ascorbate and tocopherol radicals. n3-PUFA increases PON activity and reduces lipid peroxidation. Abbreviations: Asc: ascorbic acid (reduced); DHA: dehydroascorbic acid (oxidized); DHLA: hydrolipidic acid; GSSG: oxidized glutathione; MDA: malondialdehyde; NICD: notch 1 intracellular domain; UL: unsaturated lipid; VE-O: vitamin E oxidized; VE-OH: vitamin E reduced. Green arrows: activation; red block signs: inhibition.
Melatonin appears to increase the activity of antioxidant enzymes such as SOD and GPX, through Sirtuin (SIRT)-3 [175]. Resveratrol is a phytoalexin derived from grapes [176], likely acting via several mechanisms: the downregulation of NOX expression and activity, mitochondrial O2•− reduction [177,178], and increased PON1 activity (Figure 4A). Vitamin E refers to a group of 8 different compounds, 4 tocopherols, and 4 tocotrienols, exerting their antioxidant action by scavenging lipid peroxyl radicals through hydrogen donation from the phenolic group of the chromanol ring (Figure 4C). Vitamin E inhibits peroxyl radicals before they react with lipids such as cholesterol, cholesterol esters, fatty acids, and phospholipids [179]. Different Vitamin E forms, with the un-substituted 5-position or with the methyl-group in five positions, can also trap reactive NO species [180,181]. Vitamin D inhibits NOX, upregulates several scavenging systems, such as SOD, GPX, and Cat [182] (Figure 4A), increases NO and the activation of phosphoinositide 3-kinases/protein kinase B (PI3K/Akt) [183] (Figure 4B). Ascorbic acid, i.e., Vitamin C, appears to exert diverse anti-oxidant effects [184] through the inhibition of NOX and XO, SOD activation [185]. Ascorbic acid can preferentially regenerate the Vitamin E radical, while the ascorbic acid radical can be regenerated by GSH [186,187] (Figure 4C). Vitamin B6 is water-soluble; its active form is a cofactor [188], which catalyzes homocysteine trans-sulphuration, contributing to the homocysteine production required for GSH synthesis [189], and is involved in GPX synthesis [190] (Figure 4A). Alpha-lipoic acid (ALA) and its reduced form can regenerate anti-oxidant molecules such as GSH, Vitamin C, Vitamin E, and cofactor Q10 (CoQ10) [191] (Figure 4). Stevioside, a common sweetener [192], contains polyphenol, can increase intracellular reduced GSH, upregulates SOD and Cat and decreases lipid peroxidation [193] (Figure 4A). Acacetin is a natural flavone of plant pigments [194] and can increase SOD2 [195], and Trx activity [196] (Figure 4A). N-3 polyunsaturated fatty acids (n3-PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), seem to have different effects: in mitochondria, DHA reduces the cytochrome complex IV activity and increases SOD [197]. PUFAs upregulate the Nrf-2 transcription that leads to antioxidant gene expression [198] and enhances NO synthesis in ECs [199] (Figure 4A).
Some dietary habits appear to be associated with antioxidant properties such as fish consumption, which is likely related to high PUFAs content [200], and some fish proteins also have a scavenger effect by inhibiting lipid peroxidation [201] (Figure 4C). The Mediterranean diet is rich in green vegetables, fish, and fruit, containing polyphenolic compounds, and PUFAs [202], including nuts and virgin olive oil, which can increase PON-1 activity, reducing lipid peroxidation [203] (Figure 4C).
5.2. Studies in Animals
In streptozotocin (STZ)-treated rats that develop DM, the supplementation of melatonin (20 mg/kg once daily (od) per os) for 8 weeks could recover Notch homolog-1 translocation associated/hairy and enhancers of split/protein kinase B (Notch1/Hes/Akt) signal in an I/R injury model and enhanced SOD in aortic VSMC [204] (Figure 4B). In the same animal model, intraperitoneal melatonin (10 mg/kg od) increased SOD and decreased MDA in erythrocytes [205,206] (Figure 4A).
In DM-induced KsJ-db/db mice, resveratrol added to the chow (0.3% w/w) reduced adhesive molecule expression in aortic ECs [207]. In STZ-DM LDLr−/− mice, resveratrol added to HFD (0.2% w/w) decreased monocyte MCP-1-dependent activation in the aortic root [208]. In ApoE−/− mice, resveratrol (10 mg/kg od) for 6 weeks decreased macrophage differentiation, increased monocytes GSH and decreased atherosclerosis [209]. In C57BL/6 mice on HFD, resveratrol (10 mg/kg in the chow) could restore the integrity of aortic media and recover EC function through the phosphorylation of the Akt/eNOS pathway [210].
Vitamin E (100 mg/od) halved the mortality of HFD-fed mice, and decreased macrophages in atherosclerotic lesions and circulating MDA [211].
In ApoE−/− STZ-induced-DM mice, ALA (1.65 g/kg od) reduced plasma lipid peroxidation, and increased erythrocyte GSH, and PON activity, slowing atherosclerosis [212].
In obese mice, stevioside (10 mg/kg od) for 12 weeks improved glucose transport and reduced autoantibodies against MDA-modified LDL [193], decreased ox-LDL in obese insulin-resistant LDLr−/− mouse plaques [213].
In ApoE−/− STZ-DM mice, acacetin (20 mg/kg twice daily) increased SOD and attenuated atherosclerotic lesions [214].
Compared to ApoE−/− mice fed with corn oil, ApoE−/− mice fed with fish oil containing n-3 PUFA (32.5 g/100 g total fatty acids) and n-6 PUFA (9.6 g/100 g total fatty acids) reduced atherosclerotic lesions, increased liver GSH and Cat levels [215] and lowered P-selectin and VCAM-1 expression in aorta [216]. Moreover, ApoE−/− mice fed with n3-PUFA-enriched diet had a higher expression of eNOS and reduced O2•− in the aorta versus a corn-oil-enriched diet [217]. The supplementation of a western diet with 5% EPA to LDLr−/− mice was associated with lower macrophages’ infiltration in the aorta [218]. In HFD-fed ApoE−/− mice, the antioxidant mitoquinone, a ubiquinone analogue, reduced DNA damage and atherosclerotic lesions [219].
5.3. Intervention Studies in Humans
Several studies in humans investigated a possible benefit of antioxidants by using biomarkers known as surrogates of either CV protection or CV events, which are summarized in Table 4 and Table 5, respectively.
In a small, double-blind, placebo-controlled, randomized clinical trial (RCT) in 60 DM subjects with CAD, supplementation with melatonin (10 mg od) for 12 weeks increased plasma GSH, NO, and decreased MDA and C-reactive protein (CRP) vs. placebo [220] (Table 4).

Table 4.
Randomized clinical trials and meta-analyses of antioxidant compounds and dietary intervention on cardiovascular functional surrogates or oxidative-stress biomarkers.
Table 4.
Randomized clinical trials and meta-analyses of antioxidant compounds and dietary intervention on cardiovascular functional surrogates or oxidative-stress biomarkers.
| Study (Year) | Study Population | Design and Study Duration | CV Functional Surrogates or Oxidative Stress Biomarkers | Results |
|---|---|---|---|---|
| Ashor et al. (2014) [221] | Adults with T1DM and T2DM, hypertension, heart failure and healthy subjects (n = 1129) | Meta-analysis of 44 RCT on vitamin C (<500 mg/od to >2 g/od) on endothelial function. Treatment duration: 1 day to 8 weeks | Endothelial function evaluated as FMD, plethysmography, pulse wave analysis and forearm blood flow | Standardized mean difference for endothelial function: 0.50, 95% CI = 0.34–0.66; p < 0.001 |
| Montero et al. (2014) [222] | T2DM (n = 296) | Meta-analysis of 10 trials: Vitamin E or Vitamin C (n = 148) vs. placebo (n = 148) Treatment duration: 2–52 weeks | Endothelial function, evaluated as FMD or PORH or plethysmography | Standardized mean difference for endothelial function: 0.35, 95% CI = −0.17–0.88; p = 0.18 |
| Derosa G et al. (2016) [223] | T2DM (n = 105) | Randomized study: alpha lipoic acid (ALA) 600 mg/od (n = 54) Vs. placebo (n = 51) Follow-up: 3 months | Serum SOD, erythrocyte GPX, plasma MDA | SOD comparison of within-group variations: ALA 16.7 U/mL vs. placebo 1.9 U/mL; p < 0.05 GPX comparison of within-group variations: ALA 22.4 EE/U vs. placebo 0.7 EE/U; p < 0.05 MDA comparison of within-group variations: ALA −8.9 nmol/mL vs. placebo −3.1 nmol/mL; p < 0.05 |
| Imamura et al. (2017) [224] | T2DM (n = 50) | Randomied study: Resveratrol 100 mg/od (n = 25) vs. placebo (n = 25) Treatment duration: 12 weeks | Arterial stiffness assessed by cardio-ankle vascular index | Within-group difference in cardio-ankle vascular index: resveratrol −0.4 ± 0.7 vs. placebo 0.1 ± 0.5; p < 0.01 |
| Mansournia et al. (2018) [225] | T2DM (n = 1053) | Meta-analysis of 33 studies: vitamin D vs. placebo Follow-up: 6 weeks–12 months | Serum CRP, eNOS, MDA | CRP-weighted mean difference between vitamin D vs. placebo: −0.27, 95% CI = −0.35–0.20; p < 0.001 NO-weighted mean difference between between vitamin D vs. placebo: 4.33, 95% CI = 0.96–7.70; p < 0.001 MDA-weighted mean difference between between vitamin D and placebo: –0.43, 95% CI = −0.62–0.25; p < 0.001 |
| Sattarinezhad et al. (2018) [226] | T2DM and nephropathy (n = 60) | Randomized study: Resveratrol 500 mg/od (n = 30) vs. placebo (n = 30) Follow-up: 90 days | Serum markers of NO, mSOD and MDA | NO markers’ comparison of within-group variation: resveratrol 4.4 ± 5.61 μmol/l vs. placebo −0.5 ± 5.0 μmol/L; p < 0.01 SOD comparison of within-group variation: resveratrol 4.8 ± 5.3 U/L vs. placebo −4.2 ± 9.3 U/L; p < 0.01 MDA comparison of within-group variations: resveratrol −0.4 ± 0.9 nmol/mL vs. placebo 0.9 ± 1.3 nmol/mL; p < 0.01 |
| Seyyedebrahimi et al. (2018) [227] | T2DM (n = 60) | Randomized study: Resveratrol 800 mg/od (n = 30) vs. placebo (n = 30) Follow-up: 2 months | Ferric-reducing ability in plasma (FRAP) | Percentage of FRAP change: resveratrol 44.41 ± 138.52% vs. placebo 15.30 ± 88.72%; p = 0.002 |
| Hoseini et al. (2019) [228] | T2DM (n = 46) | Randomized study: Resveratrol 500 mg/od (n = 23) vs. placebo (n = 23) Follow-up: 4 weeks | Plasma MDA and ferric-reducing ability (FRAP) | Difference between resveratrol and placebo MDA: −0.21 μmol/L, 95% CI = −0.41–0.005; p = 0.04 FRAP: 58.88 mmol/L, 95% CI = 17.33–100.44; p = 0.006 |
| Mendoza-Nùñez et al. (2019) [229] | Adults aged 60–74 years with T2DM (n = 135) | ALA 600 mg/od (n = 50) vs. placebo (n = 50) Follow-up: 6 months | Erythrocyte SOD/GPx, plasma 8-epi-PGF2α | Comparison of within-group variations SOD/GPx: ALA −0.004 vs. placebo −0.005 vs. control 0.005; p < 0.05 Comparison of within-group variations 8-epi-PGF2α: ALA −43 vs. placebo −29 vs. control 13; p < 0.05 |
| Raygan et al. (2019) [220] | T2DM with BMI ≥ 25 g/m2 and coronary heart disease, with 2- and 3- vessels (n = 60) | Randomized study: Melatonin 10 mg/od (n = 30) vs. placebo (n = 30) Follow-up:12 weeks | Plasma GSH, NO and MDA | Within-group change of GSH Melatonin +64.7 ± 105.7 mmol/L Placebo −11.1 ± 137.6 mmol/L; p = 0.02 Comparison of within-group variations NO melatonin +0.9 ± 4.7 mmol/L vs. placebo −3.3 ± 9.6 mmol/L; p = 0.03 Comparison of within-group variations MDA melatonin −0.2 ± 0.3 mmol/L vs. placebo +0.1 ± 0.5 mmol/L; p = 0.007 |
| Dalan et al. (2020) [230] | T2DM (n = 166) | Randomized study: Vitamin E 400 UI/od (n = 84) vs. placebo (n = 82) Follow-up: 24 weeks | Endothelial function assessed as peripheral arterial tonometry- reactive hyperaemia index (EndoPAT-RHI) | Difference of EndoPAT-RHI Vitamin E vs. placebo −0.02, 95% CI −0.10–0.06; p = 0.690 |
Abbreviations: ALA: alpha-lipoic acid; BMI: body mass index; CI: confidence interval; CRP: C-reactive protein; CV: cardiovascular; 8-epi-PGF2α:8-epi-prostaglandin F2α; eNOS: endothelial nitric oxide synthase; FRAP: ferric-reducing ability; FMD: flow-mediated dilation; GPX: glutathione peroxidase; GSH: glutathione; HDL: high-density lipoprotein; MDA: malondialdehyde; od: once daily; PORH: post-occlusive reactive hyperaemia; RCT: randomized clinical trial; SOD: superoxide dismutase; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus.
In another small study, patients with DM and CAD that were randomized to resveratrol (500 mg/od, n = 23) for 4 weeks showed an increased total antioxidant capacity in plasmas, as assessed by ferric-reducing ability (FRAP) and reduced MDA versus controls [228](Table 4). Two-month resveratrol (800 mg/od) increased FRAP in 48 DM subjects [227]; higher peripheral eNOS and GPX levels were reported in 60 DM subjects with nephropathy taking resveratrol (500 mg/od for 3 months) vs. placebo [226] (Table 4). Furthermore, resveratrol supplementation (100 mg/od for 12 weeks) was associated with a change in the cardio-ankle vascular index [231] in 50 subjects with T2DM vs. placebo [224] (Table 4). Moreover, in 135 T2DM patients, ALA (600 mg/od for 6 months) consistently increased erythrocyte SOD and GPX activity vs. placebo [229]; in another study on 105 T2DM subjects ALA (600 mg/od for 3 months) improved metabolic control, increased serum SOD and erythrocyte GPX activity and decreased plasma MDA [223] (Table 4).
Vitamin E (400 UI/od for 24 weeks) supplementation in 187 T2DM subjects did not modify vascular motility or ROS generation [230] (Table 4). A meta-analysis on supplementation with either Vitamin C or E in 296 subjects with T2DM did not show any difference in EC-dependent vasodilation as compared to placebo [222] (Table 4). However, the supplementation of Vitamin E 100 or 600 mg/od for 14 days in 22 hypercholesteremic patients was associated with a dose-dependent, significant decrease in urinary 8-epi-PGF2α [125]. A systematic review and meta-analysis of 1129 subjects showed a positive effect of Vitamin C on EC-dependent flow-mediated dilation, forearm blood flow, and pulse wave analysis (Table 4) [221]. Notably, the positive effect of Vitamin C was observed in healthy subjects, in whom EC dysfunction was induced by glucose, methionine and endotoxins, and a very high dose of Vitamin C (2600 mg) was used [221]. A meta-analysis of the effect of 33 placebo-controlled RCTs on 1053 DM participants showed that Vitamin D supplementation (between 200 UI/od to 50,000 UI/monthly), was associated with decreased serum CRP and MDA, and increased circulating markers of NO and GSH [225] (Table 4).
While some studies using biomarkers or indirect indexes of CV diseases showed some effect of the antioxidant compounds, RCTs with hard endpoints were largely negative. The Women’s Health Study randomized 39,000 healthy women taking Vitamin E (600 UI every other day (eod)) or placebo and failed to show any reduction in MI, stroke or CV death over a mean of 10.1 years [232] (Table 5).

Table 5.
Randomized clinical trials and meta-analyses of antioxidant compounds and dietary intervention on cardiovascular outcomes.
Table 5.
Randomized clinical trials and meta-analyses of antioxidant compounds and dietary intervention on cardiovascular outcomes.
| Study (Year) | Study Population | Design and Study Duration | Primary Endpoints | Results |
|---|---|---|---|---|
| De Lorgeril et al. (1994) [233] | Adults aged < 70 yrs with a MI within 6 months (n = 605) | Randomized study: Mediterranean alpha-linolenic acid-rich diet (n = 302) versus Usual diet (n = 303) Mean follow-up: 27 months | Non-fatal acute MI and CV death | Primary Endpoint Mediterranean diet n = 8 Usual diet n = 33 RR 0.27, 95% CI 0.12–0.59, p = 0.001 |
| Yusuf et al. (2000) [234] | High CV Risk for previous CV events or T2DM+1 CV risk factor (n = 9541) | Randomized study: Vitamin E 400 UI/od (n = 4761) vs. placebo (n = 4780) Mean follow-up: 4.5 years | MI, stroke, or CV death | Primary endpoint: Vitamin E n = 772 (16.2%) Placebo n = 739 (15.5%) RR: 1.05, 95% CI 0.95–1.16; p = 0.33 |
| Knoops et al. (2004) [235] | Healthy elderly from 2 European cohorts (FINE n = 726 and SENECA n = 1613) | Pooled analysis on the effect of Mediterranean diet, quitting smoking and engaging physical activity on mortality Mean follow-up: 10 years | All-cause mortality, Death from CAD, CV death | All-cause mortality Mediterranean diet HR: 0.77, 95% CI 0.68–0.88 Death from CAD Mediterranean diet HR: 0.61, 95% CI 0.43–0.88 CV Death Mediterranean Diet HR: 0.71, 95% CI 0.58–0.88 |
| Whelthon et al. (2004) [236] | Adults with and without CV disease (n = 228,864) | Metanalysis of 19 observational studies (14 cohort studies and 5 case-control studies) comparing regular fish consumption (mean intake 36 g/od or 2.2 servings/week) vs. little/no fish consumption Mean follow-up of cohort studies: 15 years | Fatal and Total CAD | Fatal CAD Regular Fish consumption RR: 0.83, 95% CI 0.76 to 0.90; p < 0.005 Total CAD Regular Fish Consumption RR: 0.86, 95% CI 0.81–0.92; p < 0.005 |
| Lee et al. (2005) [232] | Healthy women aged ≥ 45 (n = 39,876) | Randomized study: Vitamin E 600 UI/eod (n = 19,937) vs. placebo (n = 19,939) Mean follow-up: 10.1 years | Nonfatal MI, nonfatal stroke, or CV death | Primary endpoint: Vitamin E n = 482 (2.4%) Placebo n = 517 (2.5%) RR: 0.93, 95% CI 0.82–1.05; p = 0.26 |
| Cook et al. (2007) [237] | Female aged ≥ 40 with previous CV event or with ≥3 CV risk factors (hypertension, high cholesterol, DM, history of MI, BMI ≥30 kg/m2, current cigarette smoking) (n = 8171) | Randomized study, 2X2 Factorial design: Vitamin E 600 UI/eod (n = 4087), Vitamin C 500 mg/od (n = 4083) vs. placebo (n = 4084) Mean follow-up: 9.4 years | MI, stroke, CABG or PTCA, CV death | Primary endpoint: Vitamin E n = 708 (17.3%) Placebo n = 742 (18.1%) RR: 0.94, 95% CI 0.85–1.04; p = 0.23 Vitamine C n = 731 (17.9%), Placebo n = 719 (17.5%), RR: 1.02, 95% CI 0.92–1.13; p = 0.71 |
| Sesso et al. (2008) [238] | Male aged ≥ 50 years, including 5.1% with prevalent CV disease, as MI and stroke (n = 14,641) | Randomized study, 2 × 2 factorial Design: Vitamin E 400 UI/eod (n = 7329) + Vitamin C 500 mg/od (n = 7315) vs. placebo (n = 7312 vs. Vitamin E or n = 7326 vs. Vitamin C) alone Mean follow-up: 8.0 years | Non-fatal MI, non-fatal stroke, CV death | Primary endpoint: Vitamin E n = 620, 1.09 events per 1000 person–years Placebo n = 625, 1.09 events per 1000 person–year HR: 1.01, 95% CI 0.90–1.13; p = 0.86 Vitamin C n = 619, 1.08 events per 1000 person–years Placebo n = 626, 1.09 events per 1000 person–years HR: 0.99, 95% CI 0.89–1.11; p = 0.91 |
| Myung et al. (2013) [239] | Adults with and without CV disease (n = 294,478) | Metanalysis of 50 RCT evaluating the effect of several compounds (Vitamins Q10 coenzyme, calcium, n3-fatty acids) Follow-up: 6 months–12 years | CV death, MI, stroke, angina, sudden cardiac death | Primary endpoint All compounds RR 1.00, 95% CI 0.98–1.02 Vitamin B6 RR 0.92, 95% CI 0.85–0.99 |
| Bowman et al. (2018) [240] | T2DM without ASCVD (n = 15,480) | Randomized study: n-3 fatty acid 1 g/od (n = 7740) vs. placebo (n = 7740) Mean follow-up: 7.4 years | Non-fatal MI or stroke, TIA, vascular death | Primary endpoint n-3 fatty acid group n = 689 (8.9%) Placebo n = 712 (9.2%) RR: 0.97, 95% CI 0.87–1.08; p = 0.55 |
| Estruch et al. (2018) [241] | Subjects at high CV risk (T2DM or ≥3 CV risk factors, as smoking, hypertension, elevated LDL cholesterol, low HDL cholesterol, overweight or obesity, or a family history of premature CHD) (n = 7447) | Randomized study: mediterranean diet with extra-virgin olive oil integration (n = 2543) vs. mediterranean diet with mixed nuts integration (n = 2454) vs. dietary fat reduction advice as control (n = 2450) Median follow-up: 4.8 years | MI, stroke, CV death | Primary endpoint Mediterranean diet with extra-virgin olive oil n = 98 (3.8%) Incidence rate 8.1 per 1000 person–years HR vs. control: 0.69, 95% CI 0.53–0.92; p < 0.05 Mediterranean diet with nuts n = 83 (3.4%) Incidence rate 8.0 per 1000 person–years HR vs. control: 0.72, 95% CI 0.53–0.94; p < 0.05 Control group n = 109 (4.4%) Incidence 11.2 per 1000 person–years |
| Manson et al. (2019) [242] | Men aged ≥50 years and women aged ≥ 55 years without CV disease (n = 25,871) | Randomized study: Vitamin D 2000 UI/od + n-3 fatty acid 1 g/od (n = 12,927) vs. placebo (n = 12,944) Median follow-up: 5.3 years | MI, stroke, CV death | Primary endpoint Vitamin D + n-3 fatty acid group n = 96 (0.03%) Placebo group n = 409 (0.03%) HR: 0.97, 95% CI 0.85–1.12; p = 0.69 |
| Khan et al. (2021) [243] | Adults with and without CV disease (n = 149,051) | Metanalysis of 38 RCTs evaluating the effect of EPA alone (4 RCTs) or of EPA+DHA (34 RCTs) vs. placebo or low-dose fatty acid supplementation. Mean follow-up: 2.0 years | CV death, non-fatal MI, CHD | CV death Overall RR 0.93, lower limit 0.88-upper limit 0.98; p = 0.01 EPA RR 0.82, lower limit 0.68, upper limit 0.99; p = 0.04 EPA+DHA RR 0.94, lower limit 0.89, upper limit 0.99; p = 0.02 Non-fatal MI Overall RR 0.87, lower limit 0.81, upper limit 0.93; p < 0.01 EPA RR 0.72, lower limit 0.62, upper limit 0.84; p < 0.01 EPA+DHA RR 0.92, lower limit 0.85, upper limit 1.00; p = 0.05 CHD Overall RR 0.91, lower limit 0.87, upper limit 0.96; p < 0.01 EPA RR 0.73, lower limit 0.62, upper limit 0.85; p < 0.01 EPA+DHA RR 0.94, lower limit 0.89, upper limit 0.99; p = 0.01 |
| Mohan et al. (2021) [244] | Adults with and without CV event (PURE n = 147,645 ONTARGET/TRASCEND n = 31,491 ORIGIN n = 12,422) | Pooled analysis of individual participant data from a cohort study and 3 RCTs (ONTARGET, TRASCEND, ORIGIN) comparing high fish intake (≥175 g/weekly) vs. little/no fish intake (<50 g/monthly) Median follow-up: PURE: 9.1 years; ONTARGET/TRASCEND: 4.5 years; ORIGIN 6.2 years | MI, stroke, congestive heart failure, or sudden death, all-cause mortality | Primary Endpoints PURE Subjects without prior CV event >175 g/weekly fish HR: 0.94, 95% CI 0.88–1.01 Subjects with prior CV event >175 g/weekly fish HR: 0.89, 95% CI 0.74–1.06 ONTARGET/TRASCEND Subjects with prior CV event >175 g/weekly fish HR: 0.88, 95% CI 0.80–0.97; p < 0.05 ORIGIN Subjects without prior CV event >175 g/weekly fish HR: 0.94, 95% CI 0.88–1.04 Subjects with prior CV event >175 g/weekly fish HR: 0.86, 95% CI 0.80–0.92; p < 0.05 |
Abbreviations: BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CI: confidence interval; CV: cardiovascular; eod: every other day; HDL: high-density lipoprotein; HR: hazard ratio; LDL: low-density lipoprotein; MI: myocardial infarction; od: once daily; PTCA: percutaneous transluminal coronary angioplasty; RCT: randomized clinical trial; RR: relative risk; T2DM: type 2 diabetes mellitus; TIA: transient ischemic attack.
Similarly in the Heart Outcomes Prevention Evaluation (HOPE) RCT, Vitamin E (400 UI/ od) did not reduce MI, stroke, and CV death in 9541 subjects with a previous CV event or DM over 4.5 years [234] (Table 5). The Physicians’ Health Study II RCT studied a combination of Vitamin E (400 IU/eod) and C (500 mg/od) on MI, stroke, and CV death in 14,641 healthy US male physicians over 8 years, but no benefit was observed versus placebo [238] (Table 5). The Women’s Antioxidant Cardiovascular Study tested Vitamin E (600 IU/od), C (500 mg/od), and beta-carotene (50 mg/eod) on the prevention of MI, stroke, coronary revascularization, or CV death in 8171 women with a history of ASCVD or at least three CV risk factors and failed to show any benefit [237] (Table 5).
A meta-analysis of RCT on the supplementations on Vitamin A, E, C, beta-carotene, and selenium suggested that the some compounds could even increase all-cause mortality, while selenium and ascorbic acid had no effect [245]. The Vitamin D and omega-3 Trial investigated vitamin D cholecalciferol (2000 IU/od) and n-3 FA (1 g/od) on the prevention of MI, stroke, or CV death versus placebo over 5.3 years, showing no benefit [242] (Table 5).
A Study of Cardiovascular Events in Diabetes (ASCEND) RCT randomized n-3 fatty acid (1 g/od) vs. placebo, in >15,000 DM subjects with no evidence of symptomatic CV diseases, and there was no CV benefit associated with omega-3 over 7.4 years [240] (Table 5). Recently, a meta-analysis including 38 RCTs demonstrated that supplementation with EPA (from 1.8 to 4.0 g/od), or with a combination of EPA and DHA (0.4 to 5.5 g/od), was associated with a reduction in CV mortality, non-fatal MI, and CHD, with a higher reduction observed with EPA monotherapy [243]. However, results regarding the effect of EPA and DHA combination were not confirmed by the same authors when older trials with suboptimal statin therapy were excluded from the analysis: EPA plus DHA was, in fact, not associated with reduced CV death or non-fatal CV events [243].
In a large meta-analysis, including 50 studies and 294,478 participants, the supplementation of diverse antioxidants, including CoQ10, calcium, zinc, and n-3 fatty acids, did not reduce major CV events vs. no treatment or placebo in both primary and secondary CV prevention. Even in subgroup analyses of the type of intervention, outcome, quality of antioxidant, duration of treatment, and combined vs. single Vitamin administration, no CV benefit was detected, except a slight CV reduction for low-dose Vitamin B6 (RR 0.92, 95% CI from 0.85 to 0.99) [239] (Table 5).
Despite the largely negative RCT data, the Mediterranean diet and fish consumption, known for their antioxidant properties [246], have been associated with a lower risk of CV events or death in large epidemiological studies. Healthy Ageing, a longitudinal study in Europe, including 2239 healthy elderly subjects from two large surveys, followed for a mean of 10 years, showed that the Mediterranean diet was associated with significantly lower risk of all-cause mortality and CV diseases [235] (Table 5). In the Prevención con Dieta Mediterránea (PREDIMED) Study, 7447 subjects at high CV risk but without CV event were assigned to a Mediterranean diet with extra-virgin olive oil integration, a Mediterranean diet with mixed-nuts integration or a dietary fat reduction as control (Table 5). The primary endpoint of major CV events (MI, stroke, or CV death) was reduced (HR 0.69, 95% CI 0.53–0.92) for the Mediterranean diet with extra-virgin olive oil and for a Mediterranean diet with nuts (HR 0.72; 95% CI: 0.53–0.94) versus the control diet [241]. In the Lyon Diet Heart Study, a secondary prevention trial including 605 subjects with a recent MI, after a mean of 27 months, found that a Mediterranean diet was associated with significantly lower CV death and acute MI [233] (Table 5).
In a meta-analysis, including observational data, comparing regular fish consumption vs. little or no fish intake, fish consumption was associated with a relative risk of 0.83 (95% CI 0.76–0.90) for fatal CAD, and of 0.86 (95% CI 0.81–0.92) for total CHD [247] (Table 5). Recently, a meta-analysis including data from a large-scale cohort study and three RCTs showed that fish intake (at least 175 g/week) was associated with lower major CV disease, CV, non-CV and total mortality as compared with ≤50 g/month intake [248] (Table 5).
6. Conclusions
Animal studies strongly support a causal link between some enzymes and systems of generation and/or the clearance of ROS with atherosclerosis development, supporting the notion that controlling ROS is an appropriate goal for therapeutic interventions to prevent ASCVD. However, studies on antioxidant substances in humans led to inconsistent evidence regarding the effect on reducing and preventing ASCVD development or complications to date, while some studies using functional tests or soluble biomarkers have shown a positive impact on the same compounds.
Negative RCTs have helped to identify the pitfalls of the current approaches and how to design future interventions. Problems associated with RCTs can be agent concentrations, exposure time, and ASCVD status (early vs. late), while ROS are not always damaging to cell function since they can also regulate cell homeostasis, and their role is very much cell- and tissue-dependent. In addition, different ROS may have different roles (H2O2 vs. O2•−). GKT137831 (setanaxib), a promising NOX1/4 inhibitor, is currently in phase II clinical trials for DM kidney disease [249].
In conclusion, while animal models have identified several targets along the paths of ROS production and clearance, intervention RCTs are still lacking, while the dietary habits associated with a possible reduction in ROS tone have shown CV benefits. Future research will have to unravel these gaps, and find the reasons for, and the way to overcome, these inconsistent results.
Author Contributions
Conceptualization, B.R.; methodology, B.R., D.P. and G.P.; writing: original draft preparation G.P., A.R., D.H. and B.R.; review and editing A.R., B.R., D.P., G.T. and G.P.; supervision G.P. and B.R. All authors have read and agreed to the published version of the manuscript.
Funding
Supported in part by institutional funding Linea D1 2020 and Linea d1 2021 to GP and BR.
Acknowledgments
We acknowledge the editorial assistance of Carla Baffa.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, Y.; Liang, X.; Li, C.; Li, R.; Tong, X.; Zhang, R.; Shan, X.; Yang, J.; Ma, X.; Lu, W.; et al. 5-HT(2A) Receptor and 5-HT Degradation Play a Crucial Role in Atherosclerosis by Modulating Macrophage Foam Cell Formation, Vascular Endothelial Cell Inflammation, and Hepatic Steatosis. J. Atheroscler. Thromb. 2022, 29, 322–336. [Google Scholar] [CrossRef]
- Brown, D.I.; Griendling, K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015, 116, 531–549. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Andreadou, I.; Efentakis, P.; Frenis, K.; Daiber, A.; Schulz, R. Thiol-based redox-active proteins as cardioprotective therapeutic agents in cardiovascular diseases. Basic Res. Cardiol. 2021, 116, 44. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Nayor, M.; Brown, K.J.; Vasan, R.S. The Molecular Basis of Predicting Atherosclerotic Cardiovascular Disease Risk. Circ. Res. 2021, 128, 287–303. [Google Scholar] [CrossRef]
- Niu, X.-L.; Madamanchi, N.R.; Vendrov, A.E.; Tchivilev, I.; Rojas, M.; Madamanchi, C.; Brandes, R.P.; Krause, K.-H.; Humphries, J.; Smith, A.; et al. Nox activator 1: A potential target for modulation of vascular reactive oxygen species in atherosclerotic arteries. Circulation 2010, 121, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Judkins, C.P.; Diep, H.; Broughton, B.R.S.; Mast, A.E.; Hooker, E.U.; Miller, A.A.; Selemidis, S.; Dusting, G.J.; Sobey, C.G.; Drummond, G.R. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H24–H32. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, A.L.; Carrell, S.; Johnson, B.; Stanic, B.; Banfi, B.; Miller, F.J., Jr. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 2011, 216, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; de Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef] [Green Version]
- Quesada, I.M.; Lucero, A.; Amaya, C.; Meijles, D.N.; Cifuentes, M.E.; Pagano, P.J.; Castro, C. Selective inactivation of NADPH oxidase 2 causes regression of vascularization and the size and stability of atherosclerotic plaques. Atherosclerosis 2015, 242, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Keaney, J.F., Jr.; Schulz, E.; Levison, B.; Shan, L.; Sakuma, M.; Zhang, X.; Shi, C.; Hazen, S.L.; Simon, D.I. Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proc. Natl. Acad. Sci. USA 2004, 101, 13014–13019. [Google Scholar] [CrossRef] [Green Version]
- Hilenski, L.L.; Clempus, R.E.; Quinn, M.T.; Lambeth, J.D.; Griendling, K.K. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 677–683. [Google Scholar] [CrossRef]
- Douglas, G.; Bendall, J.K.; Crabtree, M.J.; Tatham, A.L.; Carter, E.E.; Hale, A.B.; Channon, K.M. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE−/− mice. Cardiovasc. Res. 2012, 94, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Barry-Lane, P.A.; Patterson, C.; van der Merwe, M.; Hu, Z.; Holland, S.M.; Yeh, E.T.; Runge, M.S. p47phox is required for atherosclerotic lesion progression in ApoE−/− mice. J. Clin. Investig. 2001, 108, 1513–1522. [Google Scholar] [CrossRef]
- Vara, D.; Mailer, R.K.; Tarafdar, A.; Wolska, N.; Heestermans, M.; Konrath, S.; Spaeth, M.; Renné, T.; Schröder, K.; Pula, G. NADPH Oxidases Are Required for Full Platelet Activation In Vitro and Thrombosis In Vivo but Dispensable for Plasma Coagulation and Hemostasis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 683–697. [Google Scholar] [CrossRef]
- Xu, S.; Chamseddine, A.H.; Carrell, S.; Miller, F.J. Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol. 2014, 2, 642–650. [Google Scholar] [CrossRef] [Green Version]
- Ohara, Y.; Peterson, T.E.; Harrison, D.G. Hypercholesterolemia increases endothelial superoxide anion production. J. Clin. Investig. 1993, 91, 2546–2551. [Google Scholar] [CrossRef] [Green Version]
- Schröder, K.; Vecchione, C.; Jung, O.; Schreiber, J.G.; Shiri-Sverdlov, R.; van Gorp, P.J.; Busse, R.; Brandes, R.P. Xanthine oxidase inhibitor tungsten prevents the development of atherosclerosis in ApoE knockout mice fed a Western-type diet. Free Radic. Biol. Med. 2006, 41, 1353–1360. [Google Scholar] [CrossRef]
- Nomura, J.; Busso, N.; Ives, A.; Matsui, C.; Tsujimoto, S.; Shirakura, T.; Tamura, M.; Kobayashi, T.; So, A.; Yamanaka, Y. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci. Rep. 2014, 4, 4554. [Google Scholar] [CrossRef] [Green Version]
- Tiyerili, V.; Camara, B.; Becher, M.U.; Schrickel, J.W.; Lütjohann, D.; Mollenhauer, M.; Baldus, S.; Nickenig, G.; Andrié, R.P. Neutrophil-derived myeloperoxidase promotes atherogenesis and neointima formation in mice. Int. J. Cardiol. 2016, 204, 29–36. [Google Scholar] [CrossRef]
- Brennan, M.-L.; Anderson, M.M.; Shih, D.M.; Qu, X.-D.; Wang, X.; Mehta, A.C.; Lim, L.L.; Shi, W.; Hazen, S.L.; Jacob, J.S. Increased atherosclerosis in myeloperoxidase-deficient mice. J. Clin. Investig. 2001, 107, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Dasgupta, C.; Mulder, C.; Zhang, L. MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction. Circulation 2022, 145, 1140–1153. [Google Scholar] [CrossRef]
- Guzik, T.J.; Sadowski, J.; Kapelak, B.; Jopek, A.; Rudzinski, P.; Pillai, R.; Korbut, R.; Channon, K.M. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1614–1620. [Google Scholar] [CrossRef] [Green Version]
- Guzik, T.J.; Sadowski, J.; Guzik, B.; Jopek, A.; Kapelak, B.; Przybylowski, P.; Wierzbicki, K.; Korbut, R.; Harrison, D.G.; Channon, K.M. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Sibley, C.T.; Estwick, T.; Zavodni, A.; Huang, C.Y.; Kwan, A.C.; Soule, B.P.; Long Priel, D.A.; Remaley, A.T.; Rudman Spergel, A.K.; Turkbey, E.B.; et al. Assessment of atherosclerosis in chronic granulomatous disease. Circulation 2014, 130, 2031–2039. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, P.; Sanguigni, V.; Lenti, L.; Ferro, D.; Finocchi, A.; Rossi, P.; Violi, F. gp91phox-Dependent Expression of Platelet CD40 Ligand. Circulation 2004, 110, 1326–1329. [Google Scholar] [CrossRef] [Green Version]
- Manea, A.; Manea, S.-A.; Gan, A.M.; Constantin, A.; Fenyo, I.M.; Raicu, M.; Muresian, H.; Simionescu, M. Human monocytes and macrophages express NADPH oxidase 5; a potential source of reactive oxygen species in atherosclerosis. Biochem. Biophys. Res. Commun. 2015, 461, 172–179. [Google Scholar] [CrossRef]
- Sugiyama, S.; Okada, Y.; Sukhova, G.K.; Virmani, R.; Heinecke, J.W.; Libby, P. Macrophage Myeloperoxidase Regulation by Granulocyte Macrophage Colony-Stimulating Factor in Human Atherosclerosis and Implications in Acute Coronary Syndromes. Am. J. Pathol. 2001, 158, 879–891. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Investig. 1994, 94, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Lassègue, B.; Clempus, R.E. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R277–R297. [Google Scholar] [CrossRef] [Green Version]
- Gimenez, M.; Schickling, B.M.; Lopes, L.R.; Miller, F.J., Jr. Nox1 in cardiovascular diseases: Regulation and pathophysiology. Clin. Sci. 2016, 130, 151–165. [Google Scholar] [CrossRef]
- Zafari, A.M.; Ushio-Fukai, M.; Akers, M.; Yin, Q.; Shah, A.; Harrison, D.G.; Taylor, W.R.; Griendling, K.K. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 1998, 32, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Lassègue, B.; Griendling, K.K. NADPH oxidases: Functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 653–661. [Google Scholar] [CrossRef]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef] [Green Version]
- Johar, S.; Cave, A.C.; Narayanapanicker, A.; Grieve, D.J.; Shah, A.M. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006, 20, 1546–1548. [Google Scholar] [CrossRef] [Green Version]
- Delaney, M.K.; Kim, K.; Estevez, B.; Xu, Z.; Stojanovic-Terpo, A.; Shen, B.; Ushio-Fukai, M.; Cho, J.; Du, X. Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.; Geng, X.; Li, F.; Ding, Y. NOX Activation by Subunit Interaction and Underlying Mechanisms in Disease. Front. Cell. Neurosci. 2017, 10, 301. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, P.; Carnevale, R.; Di Santo, S.; Bartimoccia, S.; Sanguigni, V.; Lenti, L.; Finocchi, A.; Mendolicchio, L.; Soresina, A.R.; Plebani, A.; et al. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Takac, I.; Schröder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef] [Green Version]
- Touyz, R.M.; Chen, X.; Tabet, F.; Yao, G.; He, G.; Quinn, M.T.; Pagano, P.J.; Schiffrin, E.L. Expression of a Functionally Active gp91phox-Containing Neutrophil-Type NAD(P)H Oxidase in Smooth Muscle Cells From Human Resistance Arteries. Circ. Res. 2002, 90, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Ago, T.; Kitazono, T.; Ooboshi, H.; Iyama, T.; Han, Y.H.; Takada, J.; Wakisaka, M.; Ibayashi, S.; Utsumi, H.; Iida, M. Nox4 as the Major Catalytic Component of an Endothelial NAD(P)H Oxidase. Circulation 2004, 109, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Lener, B.; Kozieł, R.; Pircher, H.; Hütter, E.; Greussing, R.; Herndler-Brandstetter, D.; Hermann, M.; Unterluggauer, H.; Jansen-Dürr, P. The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem. J. 2009, 423, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.F.; Qiao, M.; Schröder, K.; Zhao, Q.; Asmis, R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ. Res. 2010, 106, 1489–1497. [Google Scholar] [CrossRef] [Green Version]
- Asmis, R.; Begley, J.G. Oxidized LDL promotes peroxide-mediated mitochondrial dysfunction and cell death in human macrophages: A caspase-3-independent pathway. Circ. Res. 2003, 92, e20–e29. [Google Scholar] [CrossRef] [Green Version]
- Sorescu, D.; Weiss, D.; Lassègue, B.; Clempus, R.E.; Szöcs, K.; Sorescu, G.P.; Valppu, L.; Quinn, M.T.; Lambeth, J.D.; Vega, J.D.; et al. Superoxide Production and Expression of Nox Family Proteins in Human Atherosclerosis. Circulation 2002, 105, 1429–1435. [Google Scholar] [CrossRef] [Green Version]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012, 48, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial Dysfunction in Atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tabas, I. Emerging roles of mitochondria ROS in atherosclerotic lesions: Causation or association? J. Atheroscler. Thromb. 2014, 21, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Vendrov, K.C.; Simmons, B.P.; Schuck, R.N.; Stouffer, G.A.; Lee, C.R. Urinary 11-dehydro-thromboxane B2 levels are associated with vascular inflammation and prognosis in atherosclerotic cardiovascular disease. Prostaglandins Other Lipid Mediat. 2018, 134, 24–31. [Google Scholar] [CrossRef]
- Han, A.-P.; Yu, C.; Lu, L.; Fujiwara, Y.; Browne, C.; Chin, G.; Fleming, M.; Leboulch, P.; Orkin, S.H.; Chen, J.-J. Heme-regulated eIF2α kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 2001, 20, 6909–6918. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, X.; Li, C.; Li, Q.; An, Y.A.; Luo, X.; Deng, Y.; Gillette, T.G.; Scherer, P.E.; Wang, Z.V. Integrated Stress Response Couples Mitochondrial Protein Translation With Oxidative Stress Control. Circulation 2021, 144, 1500–1515. [Google Scholar] [CrossRef]
- He, M.; Lu, Y.; Xu, S.; Mao, L.; Zhang, L.; Duan, W.; Liu, C.; Pi, H.; Zhang, Y.; Zhong, M.; et al. MiRNA-210 modulates a nickel-induced cellular energy metabolism shift by repressing the iron–sulfur cluster assembly proteins ISCU1/2 in Neuro-2a cells. Cell Death Dis. 2014, 5, e1090. [Google Scholar] [CrossRef]
- Yu, E.P.K.; Reinhold, J.; Yu, H.; Starks, L.; Uryga, A.K.; Foote, K.; Finigan, A.; Figg, N.; Pung, Y.-F.; Logan, A.; et al. Mitochondrial Respiration Is Reduced in Atherosclerosis, Promoting Necrotic Core Formation and Reducing Relative Fibrous Cap Thickness. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2322–2332. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Roberts, L.J.; Shi, M.J.; Zhou, L.C.; Ballard, B.R.; Richardson, A.; Guo, Z.M. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ. Res. 2004, 95, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhou, L.; Wang, Z.; Roberts, L.J., 2nd; Lin, X.; Zhao, Y.; Guo, Z. Overexpression of antioxidant enzymes in ApoE-deficient mice suppresses benzo(a)pyrene-accelerated atherosclerosis. Atherosclerosis 2009, 207, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Parastatidis, I.; Weiss, D.; Joseph, G.; Taylor, W.R. Overexpression of catalase in vascular smooth muscle cells prevents the formation of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2389–2396. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, G.Z.; Rabinovitch, P.S.; Tabas, I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB-mediated inflammation in macrophages. Circ. Res. 2014, 114, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, W.; Wang, N.; Tall, A.R.; Tabas, I. Mitochondrial Oxidative Stress Promotes Atherosclerosis and Neutrophil Extracellular Traps in Aged Mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e99–e107. [Google Scholar] [CrossRef] [Green Version]
- Guo, X. Overexpression of Peroxiredoxin 4 Attenuates Atherosclerosis in Apolipoprotein E Knockout Mice. Antioxid. Redox Signal. 2012, 17, 1362–1375. [Google Scholar] [CrossRef] [Green Version]
- Kisucka, J.; Chauhan, A.K.; Patten, I.S.; Yesilaltay, A.; Neumann, C.; Van Etten, R.A.; Krieger, M.; Wagner, D.D. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circ. Res. 2008, 103, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Park, J.G.; Yoo, J.Y.; Jeong, S.J.; Choi, J.H.; Lee, M.R.; Lee, M.N.; Hwa Lee, J.; Kim, H.C.; Jo, H.; Yu, D.Y.; et al. Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Circ. Res. 2011, 109, 739–749. [Google Scholar] [CrossRef]
- Kader, T.; Porteous, C.M.; Jones, G.T.; Dickerhof, N.; Narayana, V.K.; Tull, D.; Taraknath, S.; McCormick, S.P.A. Ribose-cysteine protects against the development of atherosclerosis in apoE-deficient mice. PLoS ONE 2020, 15, e0228415. [Google Scholar] [CrossRef]
- Forgione, M.A.; Cap, A.; Liao, R.; Moldovan, N.I.; Eberhardt, R.T.; Lim, C.C.; Jones, J.; Goldschmidt-Clermont, P.J.; Loscalzo, J. Heterozygous Cellular Glutathione Peroxidase Deficiency in the Mouse. Circulation 2002, 106, 1154–1158. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Torzewski, M.; Degreif, A.; Rossmann, H.; Canisius, A.; Lackner, K.J. Impact of Glutathione Peroxidase-1 Deficiency on Macrophage Foam Cell Formation and Proliferation: Implications for Atherogenesis. PLoS ONE 2013, 8, e72063. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.; Stefanovic, N.; Pete, J.; Calkin, A.C.; Giunti, S.; Thallas-Bonke, V.; Jandeleit-Dahm, K.A.; Allen, T.J.; Kola, I.; Cooper, M.E.; et al. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation 2007, 115, 2178–2187. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Luo, Y.; Zhang, W.; He, Y.; Dai, S.; Zhang, R.; Huang, Y.; Bernatchez, P.; Giordano, F.J.; Shadel, G.; et al. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am. J. Pathol. 2007, 170, 1108–1120. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Zhou, H.J.; Zhang, H.; Huang, Y.; Hinojosa-Kirschenbaum, F.; Fan, P.; Yao, L.; Belardinelli, L.; Tellides, G.; Giordano, F.J.; et al. Thioredoxin-2 Inhibits Mitochondrial Reactive Oxygen Species Generation and Apoptosis Stress Kinase-1 Activity to Maintain Cardiac Function. Circulation 2015, 131, 1082–1097. [Google Scholar] [CrossRef] [Green Version]
- Kameritsch, P.; Singer, M.; Nuernbergk, C.; Rios, N.; Reyes, A.M.; Schmidt, K.; Kirsch, J.; Schneider, H.; Müller, S.; Pogoda, K.; et al. The mitochondrial thioredoxin reductase system (TrxR2) in vascular endothelium controls peroxynitrite levels and tissue integrity. Proc. Natl. Acad. Sci. USA 2021, 118, e1921828118. [Google Scholar] [CrossRef]
- Dayal, S.; Gu, S.X.; Hutchins, R.D.; Wilson, K.M.; Wang, Y.; Fu, X.; Lentz, S.R. Deficiency of Superoxide Dismutase Impairs Protein C Activation and Enhances Susceptibility to Experimental Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1798–1804. [Google Scholar] [CrossRef] [Green Version]
- Vendrov, A.E.; Stevenson, M.D.; Alahari, S.; Pan, H.; Wickline, S.A.; Madamanchi, N.R.; Runge, M.S. Attenuated Superoxide Dismutase 2 Activity Induces Atherosclerotic Plaque Instability During Aging in Hyperlipidemic Mice. J. Am. Heart Assoc. 2017, 6, e006775. [Google Scholar] [CrossRef] [Green Version]
- Ng, D.S.; Chu, T.; Esposito, B.; Hui, P.; Connelly, P.W.; Gross, P.L. Paraoxonase-1 deficiency in mice predisposes to vascular inflammation, oxidative stress, and thrombogenicity in the absence of hyperlipidemia. Cardiovasc. Pathol. 2008, 17, 226–232. [Google Scholar] [CrossRef]
- Tward, A.; Xia, Y.-R.; Wang, X.-P.; Shi, Y.-S.; Park, C.; Castellani, L.W.; Lusis, A.J.; Shih, D.M. Decreased Atherosclerotic Lesion Formation in Human Serum Paraoxonase Transgenic Mice. Circulation 2002, 106, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Mackness, B.; Quarck, R.; Verreth, W.; Mackness, M.; Holvoet, P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1545–1550. [Google Scholar] [CrossRef] [Green Version]
- Devarajan, A.; Bourquard, N.; Hama, S.; Navab, M.; Grijalva, V.R.; Morvardi, S.; Clarke, C.F.; Vergnes, L.; Reue, K.; Teiber, J.F.; et al. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid. Redox Signal. 2011, 14, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.J.; Hama, S.Y.; Bourquard, N.; Navab, M.; Reddy, S.T. Adenovirus mediated expression of human paraoxonase 2 protects against the development of atherosclerosis in apolipoprotein E-deficient mice. Mol. Genet. Metab. 2006, 89, 368–373. [Google Scholar] [CrossRef]
- Góth, L. A new type of inherited catalase deficiencies: Its characterization and comparison to the Japanese and Swiss type of acatalasemia. Blood Cells Mol. Dis. 2001, 27, 512–517. [Google Scholar] [CrossRef]
- Góth, L.; Eaton, J.W. Hereditary catalase deficiencies and increased risk of diabetes. Lancet 2000, 356, 1820–1821. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Ollosson, R.; Stewart, V.C.; Clark, J.B. Oxidation of nitric oxide by oxomanganese-salen complexes: A new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem. J. 2002, 366, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Shuvalova, Y.; Kaminnyi, A.I.; Meshkov, A.; Shirokov, R.; Samko, A. Association between polymorphisms of eNOS and GPx-1 genes, activity of free-radical processes and in-stent restenosis. Mol. Cell. Biochem. 2012, 370, 241–249. [Google Scholar] [CrossRef]
- Wagner, A.H.; Kautz, O.; Fricke, K.; Zerr-Fouineau, M.; Demicheva, E.; Güldenzoph, B.; Bermejo, J.L.; Korff, T.; Hecker, M. Upregulation of Glutathione Peroxidase Offsets Stretch-Induced Proatherogenic Gene Expression in Human Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1894–1901. [Google Scholar] [CrossRef]
- Garin, M.C.; James, R.W.; Dussoix, P.; Blanché, H.; Passa, P.; Froguel, P.; Ruiz, J. Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J. Clin. Investig. 1997, 99, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Morita, H.; Kurihara, H.; Sugiyama, T.; Kato, N.; Ebihara, A.; Hamada, C.; Kurihara, Y.; Shindo, T.; Oh-hashi, Y.; et al. Evidence for association between paraoxonase gene polymorphisms and atherosclerotic diseases. Atherosclerosis 2000, 149, 435–442. [Google Scholar] [CrossRef]
- Fortunato, G.; Rubba, P.; Panico, S.; Trono, D.; Tinto, N.; Mazzaccara, C.; De Michele, M.; Iannuzzi, A.; Vitale, D.F.; Salvatore, F.; et al. A paraoxonase gene polymorphism, PON 1 (55), as an independent risk factor for increased carotid intima-media thickness in middle-aged women. Atherosclerosis 2003, 167, 141–148. [Google Scholar] [CrossRef]
- Okuda, M.; Inoue, N.; Azumi, H.; Seno, T.; Sumi, Y.; Hirata, K.; Kawashima, S.; Hayashi, Y.; Itoh, H.; Yodoi, J.; et al. Expression of glutaredoxin in human coronary arteries: Its potential role in antioxidant protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1483–1487. [Google Scholar] [CrossRef] [Green Version]
- Urbonavicius, S.; Lindholt, J.S.; Vorum, H.; Urbonaviciene, G.; Henneberg, E.W.; Honoré, B. Proteomic identification of differentially expressed proteins in aortic wall of patients with ruptured and nonruptured abdominal aortic aneurysms. J. Vasc. Surg. 2009, 49, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Juul, K.; Tybjærg-Hansen, A.; Marklund, S.; Heegaard, N.H.H.; Steffensen, R.; Sillesen, H.; Jensen, G.; Nordestgaard, B.G. Genetically Reduced Antioxidative Protection and Increased Ischemic Heart Disease Risk. Circulation 2004, 109, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Mohammedi, K.; Bellili-Muñoz, N.; Marklund, S.L.; Driss, F.; Le Nagard, H.; Patente, T.A.; Fumeron, F.; Roussel, R.; Hadjadj, S.; Marre, M.; et al. Plasma extracellular superoxide dismutase concentration, allelic variations in the SOD3 gene and risk of myocardial infarction and all-cause mortality in people with type 1 and type 2 diabetes. Cardiovasc. Diabetol. 2015, 14, 845. [Google Scholar] [CrossRef] [Green Version]
- del Río, L.A.; Sandalio, L.M.; Palma, J.M.; Bueno, P.; Corpas, F.J. Metabolism of oxygen radicals in peroxisomes and cellular implications. Free Radic. Biol. Med. 1992, 13, 557–580. [Google Scholar] [CrossRef]
- Lin, S.J.; Shyue, S.K.; Liu, P.L.; Chen, Y.H.; Ku, H.H.; Chen, J.W.; Tam, K.B.; Chen, Y.L. Adenovirus-mediated overexpression of catalase attenuates oxLDL-induced apoptosis in human aortic endothelial cells via AP-1 and C-Jun N-terminal kinase/extracellular signal-regulated kinase mitogen-activated protein kinase pathways. J. Mol. Cell. Cardiol. 2004, 36, 129–139. [Google Scholar] [CrossRef]
- Bellinger, F.P.; Raman, A.V.; Reeves, M.A.; Berry, M.J. Regulation and function of selenoproteins in human disease. Biochem. J. 2009, 422, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Arthur, J.R. The glutathione peroxidases. Cell. Mol. Life Sci. 2000, 57, 1825–1835. [Google Scholar] [CrossRef]
- Weiss, N.; Zhang, Y.Y.; Heydrick, S.; Bierl, C.; Loscalzo, J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 12503–12508. [Google Scholar] [CrossRef] [Green Version]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J. Glutathione Peroxidase 1 Activity and Cardiovascular Events in Patients with Coronary Artery Disease. N. Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef] [Green Version]
- Loeper, J.; Goy, J.; Rozensztajn, L.; Bedu, O.; Moisson, P. Lipid peroxidation and protective enzymes during myocardial infarction. Clin. Chim. Acta 1991, 196, 119–125. [Google Scholar] [CrossRef]
- Espinola-Klein, C.; Rupprecht, H.J.; Bickel, C.; Schnabel, R.; Genth-Zotz, S.; Torzewski, M.; Lackner, K.; Munzel, T.; Blankenberg, S.; Investigators, A. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am. J. Cardiol. 2007, 99, 808–812. [Google Scholar] [CrossRef]
- Rubattu, S.; Forte, M.; Raffa, S. Circulating Leukocytes and Oxidative Stress in Cardiovascular Diseases: A State of the Art. Oxid. Med. Cell. Longev. 2019, 2019, 2650429. [Google Scholar] [CrossRef]
- Mackness, B.; Davies, G.K.; Turkie, W.; Lee, E.; Roberts, D.H.; Hill, E.; Roberts, C.; Durrington, P.N.; Mackness, M.I. Paraoxonase Status in Coronary Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Mackness, B.; Durrington, P.; McElduff, P.; Yarnell, J.; Azam, N.; Watt, M.; Mackness, M. Low Paraoxonase Activity Predicts Coronary Events in the Caerphilly Prospective Study. Circulation 2003, 107, 2775–2779. [Google Scholar] [CrossRef] [Green Version]
- Fortunato, G.; Taranto, M.D.D.; Bracale, U.M.; Guercio, L.D.; Carbone, F.; Mazzaccara, C.; Morgante, A.; D’Armiento, F.P.; D’Armiento, M.; Porcellini, M.; et al. Decreased Paraoxonase-2 Expression in Human Carotids During the Progression of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Turanov, A.A.; Kehr, S.; Marino, S.M.; Yoo, M.-H.; Carlson, B.A.; Hatfield, D.L.; Gladyshev, V.N. Mammalian thioredoxin reductase 1: Roles in redox homoeostasis and characterization of cellular targets. Biochem. J. 2010, 430, 285–293. [Google Scholar] [CrossRef]
- Sun, Q.A.; Kirnarsky, L.; Sherman, S.; Gladyshev, V.N. Selenoprotein oxidoreductase with specificity for thioredoxin and glutathione systems. Proc. Natl. Acad. Sci. USA 2001, 98, 3673–3678. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [Green Version]
- Faraci, F.M.; Didion, S.P. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Tainer, J.A.; Getzoff, E.D.; Richardson, J.S.; Richardson, D.C. Structure and mechanism of copper, zinc superoxide dismutase. Nature 1983, 306, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.K.; Bose, S.K.; McCord, J.M. The toxicity of high-dose superoxide dismutase suggests that superoxide can both initiate and terminate lipid peroxidation in the reperfused heart. Free Radic. Biol. Med. 1994, 16, 195–200. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, M.; Li, Y.; Traverse, J.H.; Zhang, P.; Salvemini, D.; Fukai, T.; Bache, R.J. Increased superoxide production causes coronary endothelial dysfunction and depressed oxygen consumption in the failing heart. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H133–H141. [Google Scholar] [CrossRef] [PubMed]
- Sudhahar, V.; Okur, M.N.; Bagi, Z.; O’Bryan, J.P.; Hay, N.; Makino, A.; Patel, V.S.; Phillips, S.A.; Stepp, D.; Ushio-Fukai, M.; et al. Akt2 (Protein Kinase B Beta) Stabilizes ATP7A, a Copper Transporter for Extracellular Superoxide Dismutase, in Vascular Smooth Muscle. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Barajas, B.; Che, N.; Yin, F.; Rowshanrad, A.; Orozco, L.D.; Gong, K.W.; Wang, X.; Castellani, L.W.; Reue, K.; Lusis, A.J.; et al. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Chew, P.; Yuen, D.Y.; Stefanovic, N.; Pete, J.; Coughlan, M.T.; Jandeleit-Dahm, K.A.; Thomas, M.C.; Rosenfeldt, F.; Cooper, M.E.; de Haan, J.B. Antiatherosclerotic and renoprotective effects of ebselen in the diabetic apolipoprotein E/GPx1-double knockout mouse. Diabetes 2010, 59, 3198–3207. [Google Scholar] [CrossRef] [Green Version]
- Habib, A.; Petrucci, G.; Rocca, B. Pathophysiology of Thrombosis in Peripheral Artery Disease. Curr. Vasc. Pharmacol. 2020, 18, 204–214. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis. Cardiovasc. Res. 2021, 117, 2001–2015. [Google Scholar] [CrossRef]
- Patrono, C.; FitzGerald, G.A. Isoprostanes: Potential Markers of Oxidant Stress in Atherothrombotic Disease. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2309–2315. [Google Scholar] [CrossRef]
- Audoly, L.P.; Rocca, B.; Fabre, J.E.; Koller, B.H.; Thomas, D.; Loeb, A.L.; Coffman, T.M.; FitzGerald, G.A. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation 2000, 101, 2833–2840. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-S.; Ramamurthy, S.K.; Lin, X.; Le Breton, G.C. Cell signalling through thromboxane A2 receptors. Cell. Signal. 2004, 16, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; Rocca, B. Measurement of Thromboxane Biosynthesis in Health and Disease. Front Pharmacol 2019, 10, 1244. [Google Scholar] [CrossRef]
- Petrucci, G.; Zaccardi, F.; Giaretta, A.; Cavalca, V.; Capristo, E.; Cardillo, C.; Pitocco, D.; Porro, B.; Schinzari, F.; Toffolo, G.; et al. Obesity is associated with impaired responsiveness to once-daily low-dose aspirin and in vivo platelet activation. J. Thromb. Haemost. 2019, 17, 885–895. [Google Scholar] [CrossRef]
- Davi, G.; Guagnano, M.T.; Ciabattoni, G.; Basili, S.; Falco, A.; Marinopiccoli, M.; Nutini, M.; Sensi, S.; Patrono, C. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA 2002, 288, 2008–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davi, G.; Alessandrini, P.; Mezzetti, A.; Minotti, G.; Bucciarelli, T.; Costantini, F.; Cipollone, F.; Bon, G.B.; Ciabattoni, G.; Patrono, C. In vivo formation of 8-Epi-prostaglandin F2 alpha is increased in hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3230–3235. [Google Scholar] [CrossRef]
- Zaccardi, F.; Rizzi, A.; Petrucci, G.; Ciaffardini, F.; Tanese, L.; Pagliaccia, F.; Cavalca, V.; Ciminello, A.; Habib, A.; Squellerio, I.; et al. In Vivo Platelet Activation and Aspirin Responsiveness in Type 1 Diabetes. Diabetes 2016, 65, 503–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davì, G.; Chiarelli, F.; Santilli, F.; Pomilio, M.; Vigneri, S.; Falco, A.; Basili, S.; Ciabattoni, G.; Patrono, C. Enhanced Lipid Peroxidation and Platelet Activation in the Early Phase of Type 1 Diabetes Mellitus. Circulation 2003, 107, 3199–3203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santilli, F.; Zaccardi, F.; Liani, R.; Petrucci, G.; Simeone, P.; Pitocco, D.; Tripaldi, R.; Rizzi, A.; Formoso, G.; Pontecorvi, A.; et al. In vivo thromboxane-dependent platelet activation is persistently enhanced in subjects with impaired glucose tolerance. Diabetes/Metab. Res. Rev. 2020, 36, e3232. [Google Scholar] [CrossRef]
- Pascale, S.; Petrucci, G.; Dragani, A.; Habib, A.; Zaccardi, F.; Pagliaccia, F.; Pocaterra, D.; Ragazzoni, E.; Rolandi, G.; Rocca, B.; et al. Aspirin-insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. Blood 2012, 119, 3595–3603. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Jung, S.; Kim, S.Y.; Lee, S.-H.; Lee, J.H. Prehypertension-Associated Elevation in Circulating Lysophosphatidlycholines, Lp-PLA2 Activity, and Oxidative Stress. PLoS ONE 2014, 9, e96735. [Google Scholar] [CrossRef] [Green Version]
- Keaney, J.F.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.F.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and Systemic Oxidative Stress. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwedhelm, E.; Bartling, A.; Lenzen, H.; Tsikas, D.; Maas, R.; Brümmer, J.; Gutzki, F.M.; Berger, J.; Frölich, J.C.; Böger, R.H. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: A matched case-control study. Circulation 2004, 109, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roest, M.; Voorbij, H.A.M.; Van der Schouw, Y.T.; Peeters, P.H.M.; Teerlink, T.; Scheffer, P.G. High levels of urinary F2-isoprostanes predict cardiovascular mortality in postmenopausal women. J. Clin. Lipidol. 2008, 2, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Noberasco, G.; Odetti, P.; Boeri, D.; Maiello, M.; Adezati, L. Malondialdehyde (MDA) level in diabetic subjects. Relationship with blood glucose and glycosylated hemoglobin. Biomed. Pharmacother. 1991, 45, 193–196. [Google Scholar] [CrossRef]
- Cavalca, V.; Cighetti, G.; Bamonti, F.; Loaldi, A.; Bortone, L.; Novembrino, C.; De Franceschi, M.; Belardinelli, R.; Guazzi, M.D. Oxidative Stress and Homocysteine in Coronary Artery Disease. Clin. Chem. 2001, 47, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.F.; Jacob, R.F.; Jeffers, B.; Ghadanfar, M.M.; Preston, G.M.; Buch, J.; Mason, R.P. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: A longitudinal analysis of the PREVENT study. J. Am. Coll. Cardiol. 2004, 44, 1996–2002. [Google Scholar] [CrossRef] [Green Version]
- Tanriverdi, H.; Evrengul, H.; Kuru, O.; Tanriverdi, S.; Seleci, D.; Enli, Y.; Kaftan, H.A.; Kilic, M. Cigarette Smoking Induced Oxidative Stress may Impair Endothelial Function and Coronary Blood Flow in Angiographically Normal Coronary Arteries. Circ. J. 2006, 70, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Kotur-Stevuljevic, J.; Memon, L.; Stefanovic, A.; Spasic, S.; Spasojevic-Kalimanovska, V.; Bogavac-Stanojevic, N.; Kalimanovska-Ostric, D.; Jelić-Ivanovic, Z.; Zunic, G. Correlation of oxidative stress parameters and inflammatory markers in coronary artery disease patients. Clin. Biochem. 2007, 40, 181–187. [Google Scholar] [CrossRef]
- Kubihal, C.; Naik, H. Effect of smoking on vitamin C and MDA: A cross sectional comparative study. Int. J. Res. Med. Sci. 2019, 7, 746–749. [Google Scholar] [CrossRef]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef]
- Shimada, K.; Mokuno, H.; Matsunaga, E.; Miyazaki, T.; Sumiyoshi, K.; Miyauchi, K.; Daida, H. Circulating oxidized low-density lipoprotein is an independent predictor for cardiac event in patients with coronary artery disease. Atherosclerosis 2004, 174, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Kiechl, S.; Willeit, J.; Mayr, M.; Miller, E.R.; Kronenberg, F.; Xu, Q.; Bergmark, C.; Weger, S.; Oberhollenzer, F.; et al. Oxidized Phospholipids Predict the Presence and Progression of Carotid and Femoral Atherosclerosis and Symptomatic Cardiovascular Disease. J. Am. Coll. Cardiol. 2006, 47, 2219–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-C.; Tang, Y.; Chen, Y.; Huang, X.-H.; Zhang, M.; Chen, J.; Sun, Y.-G.; Li, Y.-G. Oxidized Low-Density Lipoprotein and C-Reactive Protein Have Combined Utility for Better Predicting Prognosis After Acute Coronary Syndrome. Cell Biochem. Biophys. 2014, 68, 379–385. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association Between Circulating Oxidized LDL and Atherosclerotic Cardiovascular Disease: A Meta-analysis of Observational Studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Mercuri, F.; Quagliaro, L.; Assaloni, R.; Motz, E.; Tonutti, L.; Taboga, C. Detection of nitrotyrosine in the diabetic plasma: Evidence of oxidative stress. Diabetologia 2001, 44, 834–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishehbor, M.H.; Aviles, R.J.; Brennan, M.-L.; Fu, X.; Goormastic, M.; Pearce, G.L.; Gokce, N.; Keaney, J.; John, F.; Penn, M.S.; et al. Association of Nitrotyrosine Levels With Cardiovascular Disease and Modulation by Statin Therapy. JAMA 2003, 289, 1675–1680. [Google Scholar] [CrossRef] [Green Version]
- Kilhovd, B.K.; Berg, T.J.; Birkeland, K.I.; Thorsby, P.; Hanssen, K.F. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care 1999, 22, 1543–1548. [Google Scholar] [CrossRef]
- De Cristofaro, R.; Rocca, B.; Vitacolonna, E.; Falco, A.; Marchesani, P.; Ciabattoni, G.; Landolfi, R.; Patrono, C.; Davì, G. Lipid and protein oxidation contribute to a prothrombotic state in patients with type 2 diabetes mellitus. J. Thromb. Haemost. 2003, 1, 250–256. [Google Scholar] [CrossRef]
- Mutlu-Türkoglu, Ü.; Akalýn, Z.; Ýlhan, E.; Yýlmaz, E.; Bilge, A.; Niþancý, Y.; Uysal, M. Increased plasma malondialdehyde and protein carbonyl levels and lymphocyte DNA damage in patients with angiographically defined coronary artery disease. Clin. Biochem. 2005, 38, 1059–1065. [Google Scholar] [CrossRef]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Beck, J.; Dalal, M.; Varadhan, R.; Walston, J.; Guralnik, J.M.; Fried, L.P. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin. Exp. Res. 2009, 21, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Pirinccioglu, A.G.; Gökalp, D.; Pirinccioglu, M.; Kizil, G.; Kizil, M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin. Biochem. 2010, 43, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Vegi, P.; Kutty, A. Protein carbonyl content as a stable Oxidative stress marker in Type II Diabetes. Int. J. Biol. Med. Res. 2012, 3, 2362–2365. [Google Scholar]
- van Eupen, M.G.A.; Schram, M.T.; Colhoun, H.M.; Scheijen, J.L.J.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. Plasma levels of advanced glycation endproducts are associated with type 1 diabetes and coronary artery calcification. Cardiovasc. Diabetol. 2013, 12, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNair, E.; Qureshi, M.; Prasad, K.; Pearce, C. Atherosclerosis and the Hypercholesterolemic AGE-RAGE Axis. Int. J. Angiol. 2016, 25, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Kopytek, M.; Ząbczyk, M.; Mazur, P.; Undas, A.; Natorska, J. Accumulation of advanced glycation end products (AGEs) is associated with the severity of aortic stenosis in patients with concomitant type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 92. [Google Scholar] [CrossRef]
- Sharifi-Zahabi, E.; Sharafabad, F.H.; Abdollahzad, H.; Malekahmadi, M.; Rad, N.B. Circulating Advanced Glycation End Products and Their Soluble Receptors in Relation to All-Cause and Cardiovascular Mortality: A Systematic Review and Meta-analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 2157–2171. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free. Radic. Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- Nair, V.; Cooper, C.S.; Vietti, D.E.; Turner, G.A. The chemistry of lipid peroxidation metabolites: Crosslinking reactions of malondialdehyde. Lipids 1986, 21, 6–10. [Google Scholar] [CrossRef]
- Tsikas, D.; Suchy, M.-T.; Niemann, J.; Tossios, P.; Schneider, Y.; Rothmann, S.; Gutzki, F.-M.; Frölich, J.C.; Stichtenoth, D.O. Glutathione promotes prostaglandin H synthase (cyclooxygenase)-dependent formation of malondialdehyde and 15(S)-8-iso-prostaglandin F2α. FEBS Lett. 2012, 586, 3723–3730. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, G.; Song, X.; Wang, L.; Li, Z.; Wu, H.; Dong, H. Golgi apparatus fragmentation participates in oxidized low-density lipoprotein-induced endothelial cell injury. J. Cell. Biochem. 2019, 120, 18862–18870. [Google Scholar] [CrossRef]
- Wraith, K.S.; Magwenzi, S.; Aburima, A.; Wen, Y.; Leake, D.; Naseem, K.M. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase–signaling pathways. Blood 2013, 122, 580–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsikas, D.; Mitschke, A.; Gutzki, F.M. Measurement of 3-nitro-tyrosine in human plasma and urine by gas chromatography-tandem mass spectrometry. Methods Mol. Biol. 2012, 828, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, F.J.; Baker, P.R.S.; Freeman, B.A. NO-dependent protein nitration: A cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003, 28, 646–654. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein carbonylation. Antioxid. Redox Signal. 2010, 12, 323–325. [Google Scholar] [CrossRef]
- Levine, R.L. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic. Biol. Med. 2002, 32, 790–796. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.-J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, Q.; Gao, W.; Li, B.; Xia, Z.; Zhao, B. Melatonin protects against focal cerebral ischemia-reperfusion injury in diabetic mice by ameliorating mitochondrial impairments: Involvement of the Akt-SIRT3-SOD2 signaling pathway. Aging 2021, 13, 16105–16123. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Liu, X.; Ahmad, N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann. N. Y. Acad. Sci. 2015, 1348, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [Green Version]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef] [Green Version]
- Niki, E. Lipid oxidation that is, and is not, inhibited by vitamin E: Consideration about physiological functions of vitamin E. Free Radic. Biol. Med. 2021, 176, 1–15. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, C.; Rizzi, M.; Squarzanti, D.F.; Pittarella, P.; Vacca, G.; Renò, F. 1α,25-Dihydroxycholecalciferol (Vitamin D3) induces NO-dependent endothelial cell proliferation and migration in a three-dimensional matrix. Cell. Physiol. Biochem. 2013, 31, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra-de Man, A.M.E.; Elbers, P.W.G.; Oudemans-Van Straaten, H.M. Vitamin C: Should we supplement? Curr. Opin. Crit. Care 2018, 24, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buettner, G.R.; Jurkiewicz, B.A. Ascorbate free radical as a marker of oxidative stress: An EPR study. Free Radic. Biol. Med. 1993, 14, 49–55. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit. Care 2014, 18, 460. [Google Scholar] [CrossRef] [Green Version]
- Endo, N.; Nishiyama, K.; Otsuka, A.; Kanouchi, H.; Taga, M.; Oka, T. Antioxidant activity of vitamin B6 delays homocysteine-induced atherosclerosis in rats. Br. J. Nutr. 2006, 95, 1088–1093. [Google Scholar] [CrossRef] [Green Version]
- Dalto, D.B.; Matte, J.J. Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, C.S.; Reed, D.J. Glutathione redox cycle-driven recovery of reduced glutathione after oxidation by tertiary-butyl hydroperoxide in preimplantation mouse embryos. Arch. Biochem. Biophys. 1995, 321, 6–12. [Google Scholar] [CrossRef]
- Packer, L.; Kraemer, K.; Rimbach, G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001, 17, 888–895. [Google Scholar] [CrossRef]
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural sweetener Stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI J 2021, 20, 1412–1430. [Google Scholar] [CrossRef] [PubMed]
- Prata, C.; Zambonin, L.; Rizzo, B.; Maraldi, T.; Angeloni, C.; Vieceli Dalla Sega, F.; Fiorentini, D.; Hrelia, S. Glycosides from Stevia rebaudiana Bertoni Possess Insulin-Mimetic and Antioxidant Activities in Rat Cardiac Fibroblasts. Oxid. Med. Cell. Longev. 2017, 2017, 3724545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punia, R.; Raina, K.; Agarwal, R.; Singh, R.P. Acacetin enhances the therapeutic efficacy of doxorubicin in non-small-cell lung carcinoma cells. PLoS ONE 2017, 12, e0182870. [Google Scholar] [CrossRef]
- Wu, Y.; Song, F.; Li, Y.; Li, J.; Cui, Y.; Hong, Y.; Han, W.; Wu, W.; Lakhani, I.; Li, G.; et al. Acacetin exerts antioxidant potential against atherosclerosis through Nrf2 pathway in apoE−/− Mice. J. Cell. Mol. Med. 2021, 25, 521–534. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Wu, H.J.; Chen, K.H.; Lin, F.; Li, G.; Sun, H.Y.; Xiao, G.S.; Wang, Y.; Li, G.R. Water-soluble acacetin prodrug confers significant cardioprotection against ischemia/reperfusion injury. Sci. Rep. 2016, 6, 36435. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 Signaling. React. Oxyg. Species 2019, 8, 312–322. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Dong, Y.; Wang, S.; Song, P.; Viollet, B.; Zou, M.H. Activation of the AMP-activated protein kinase by eicosapentaenoic acid (EPA, 20:5 n-3) improves endothelial function in vivo. PLoS ONE 2012, 7, e35508. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, Z.R.; Luo, H.Y. Influence of Amino Acid Compositions and Peptide Profiles on Antioxidant Capacities of Two Protein Hydrolysates from Skipjack Tuna (Katsuwonus pelamis) Dark Muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef]
- Nadtochiy, S.M.; Redman, E.K. Mediterranean diet and cardioprotection: The role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition 2011, 27, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, M.A.; Osada, J. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068–4092. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.C.; Zhang, W.; Zhang, W.; Ma, J.X.; Pei, F.; Li, B.Y. Melatonin attenuates aortic oxidative stress injury and apoptosis in STZ-diabetes rats by Notch1/Hes1 pathway. J. Steroid. Biochem. Mol. Biol. 2021, 212, 105948. [Google Scholar] [CrossRef]
- Perneby, C.; Granström, E.; Beck, O.; Fitzgerald, D.; Harhen, B.; Hjemdahl, P. Optimization of an Enzyme Immunoassay for 11-Dehydro-Thromboxane B2 in Urine: Comparison with GC-MS. Thromb. Res. 1999, 96, 427–436. [Google Scholar] [CrossRef]
- Vural, H.; Sabuncu, T.; Arslan, S.O.; Aksoy, N. Melatonin inhibits lipid peroxidation and stimulates the antioxidant status of diabetic rats. J. Pineal Res. 2001, 31, 193–198. [Google Scholar] [CrossRef]
- Guo, R.; Liu, B.; Wang, K.; Zhou, S.; Li, W.; Xu, Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-κB pathway. Diabetes Vasc. Dis. Res. 2014, 11, 92–102. [Google Scholar] [CrossRef]
- Ullevig, S.L.; Zhao, Q.; Zamora, D.; Asmis, R. Ursolic acid protects diabetic mice against monocyte dysfunction and accelerated atherosclerosis. Atherosclerosis 2011, 219, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Vasamsetti, S.B.; Karnewar, S.; Gopoju, R.; Gollavilli, P.N.; Narra, S.R.; Kumar, J.M.; Kotamraju, S. Resveratrol attenuates monocyte-to-macrophage differentiation and associated inflammation via modulation of intracellular GSH homeostasis: Relevance in atherosclerosis. Free Radic. Biol. Med. 2016, 96, 392–405. [Google Scholar] [CrossRef]
- Huang, J.P.; Hsu, S.C.; Li, D.E.; Chen, K.H.; Kuo, C.Y.; Hung, L.M. Resveratrol Mitigates High-Fat Diet-Induced Vascular Dysfunction by Activating the Akt/eNOS/NO and Sirt1/ER Pathway. J. Cardiovasc. Pharmacol. 2018, 72, 231–241. [Google Scholar] [CrossRef]
- Otero, P.; Bonet, B.; Herrera, E.; Rabano, A. Development of atherosclerosis in the diabetic BALB/c mice: Prevention with Vitamin E administration. Atherosclerosis 2005, 182, 259–265. [Google Scholar] [CrossRef]
- Yi, X.; Maeda, N. alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes 2006, 55, 2238–2244. [Google Scholar] [CrossRef]
- Geeraert, B.; Crombé, F.; Hulsmans, M.; Benhabilès, N.; Geuns, J.M.; Holvoet, P. Stevioside inhibits atherosclerosis by improving insulin signaling and antioxidant defense in obese insulin-resistant mice. Int. J. Obes. 2010, 34, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Han, W.-M.; Chen, X.-C.; Li, G.-R.; Wang, Y. Acacetin protects against high glucose-induced endothelial cells injury by preserving mitochondrial function via activating Sirt1/Sirt3/AMPK signals. Front. Pharmacol. 2020, 11, 2179. [Google Scholar] [CrossRef]
- Wang, H.-H.; Hung, T.-M.; Wei, J.; Chiang, A.-N. Fish oil increases antioxidant enzyme activities in macrophages and reduces atherosclerotic lesions in apoE-knockout mice. Cardiovasc. Res. 2004, 61, 169–176. [Google Scholar] [CrossRef]
- Casós, K.; Sáiz, M.P.; Ruiz-Sanz, J.I.; Mitjavila, M.T. Atherosclerosis prevention by a fish oil-rich diet in apoE−/− mice is associated with a reduction of endothelial adhesion molecules. Atherosclerosis 2008, 201, 306–317. [Google Scholar] [CrossRef]
- Casós, K.; Zaragozá, M.C.; Zarkovic, N.; Zarkovic, K.; Andrisic, L.; Portero-Otín, M.; Cacabelos, D.; Mitjavila, M.T. A fish oil-rich diet reduces vascular oxidative stress in apoE–/– mice. Free Radic. Res. 2010, 44, 821–829. [Google Scholar] [CrossRef]
- Matsumoto, M.; Sata, M.; Fukuda, D.; Tanaka, K.; Soma, M.; Hirata, Y.; Nagai, R. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis 2008, 197, 524–533. [Google Scholar] [CrossRef]
- Mercer, J.R.; Yu, E.; Figg, N.; Cheng, K.K.; Prime, T.A.; Griffin, J.L.; Masoodi, M.; Vidal-Puig, A.; Murphy, M.P.; Bennett, M.R. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/− mice. Free Radic. Biol. Med. 2012, 52, 841–849. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Reiter, R.J.; Asemi, Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 191–196. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Mathers, J.C.; Siervo, M. Effect of vitamin C on endothelial function in health and disease: A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2014, 235, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Walther, G.; Stehouwer, C.D.; Houben, A.J.; Beckman, J.A.; Vinet, A. Effect of antioxidant vitamin supplementation on endothelial function in type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2014, 15, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. A Clinical Trial about a Food Supplement Containing α-Lipoic Acid on Oxidative Stress Markers in Type 2 Diabetic Patients. Int. J. Mol. Sci. 2016, 17, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imamura, H.; Yamaguchi, T.; Nagayama, D.; Saiki, A.; Shirai, K.; Tatsuno, I. Resveratrol Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Patients With Type 2 Diabetes Mellitus. Int. Heart J. 2017, 58, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Mansournia, M.A.; Ostadmohammadi, V.; Doosti-Irani, A.; Ghayour-Mobarhan, M.; Ferns, G.; Akbari, H.; Ghaderi, A.; Talari, H.R.; Asemi, Z. The Effects of Vitamin D Supplementation on Biomarkers of Inflammation and Oxidative Stress in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2018, 50, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Sattarinezhad, A.; Roozbeh, J.; Shirazi Yeganeh, B.; Omrani, G.R.; Shams, M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef]
- Seyyedebrahimi, S.; Khodabandehloo, H.; Nasli Esfahani, E.; Meshkani, R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018, 55, 341–353. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Mendoza-Núñez, V.M.; García-Martínez, B.I.; Rosado-Pérez, J.; Santiago-Osorio, E.; Pedraza-Chaverri, J.; Hernández-Abad, V.J. The Effect of 600 mg Alpha-lipoic Acid Supplementation on Oxidative Stress, Inflammation, and RAGE in Older Adults with Type 2 Diabetes Mellitus. Oxid. Med. Cell. Longev. 2019, 2019, 3276958. [Google Scholar] [CrossRef] [Green Version]
- Dalan, R.; Goh, L.L.; Lim, C.J.; Seneviratna, A.; Liew, H.; Seow, C.J.; Xia, L.; Chew, D.E.K.; Leow, M.K.S.; Boehm, B.O. Impact of Vitamin E supplementation on vascular function in haptoglobin genotype stratified diabetes patients (EVAS Trial): A randomised controlled trial. Nutr. Diabetes 2020, 10, 13. [Google Scholar] [CrossRef]
- Ishida, K.; Morimoto, S.; Horiuchi, S.; Kimura, M.; Ishikawa, T.; Kimura, S.; Yamashita, K.; Takano, N.; Seki, Y.; Bokuda, K.; et al. Comparison of the usefulness of the cardio-ankle vascular index and augmentation index as an index of arteriosclerosis in patients with essential hypertension. Hypertens Res. 2022, 45, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef] [PubMed]
- de Lorgeril, M.; Renaud, S.; Mamelle, N.; Salen, P.; Martin, J.L.; Monjaud, I.; Guidollet, J.; Touboul, P.; Delaye, J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994, 343, 1454–1459. [Google Scholar] [CrossRef]
- Yusuf. Vitamin E Supplementation and Cardiovascular Events in High-Risk Patients. N. Engl. J. Med. 2000, 342, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.T.; de Groot, L.C.; Kromhout, D.; Perrin, A.E.; Moreiras-Varela, O.; Menotti, A.; van Staveren, W.A. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: The HALE project. JAMA 2004, 292, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Whelton, S.P.; He, J.; Whelton, P.K.; Muntner, P. Meta-Analysis of observational studies on fish intake and coronary heart disease. Am. J. Cardiol. 2004, 93, 1119–1123. [Google Scholar] [CrossRef]
- Cook, N.R.; Albert, C.M.; Gaziano, J.M.; Zaharris, E.; MacFadyen, J.; Danielson, E.; Buring, J.E.; Manson, J.E. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the Women’s Antioxidant Cardiovascular Study. Arch. Intern. Med. 2007, 167, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef] [Green Version]
- Myung, S.K.; Ju, W.; Cho, B.; Oh, S.W.; Park, S.M.; Koo, B.K.; Park, B.J. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 346, f10. [Google Scholar] [CrossRef] [Green Version]
- Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; Cox, J.; et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.; Wei, L.; et al. Associations of Fish Consumption With Risk of Cardiovascular Disease and Mortality Among Individuals With or Without Vascular Disease From 58 Countries. JAMA Intern. Med. 2021, 181, 631–649. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Aranda, N.; Valls, R.M.; Romeu, M.; Sánchez-Martos, V.; Albaladejo, R.; Fernández-Castillejo, S.; Nogués, R.; Catalán, Ú.; Pedret, A.; Espinel, A.; et al. Consumption of seafood and its estimated heavy metals are associated with lipid profile and oxidative lipid damage on healthy adults from a Spanish Mediterranean area: A cross-sectional study. Environ. Res. 2017, 156, 644–651. [Google Scholar] [CrossRef]
- Hungate, B.A.; Van GROENIGEN, K.J.; Six, J.; Jastrow, J.D.; Luo, Y.; De GRAAFF, M.A.; van Kessel, C.; Osenberg, C.W. Assessing the effect of elevated carbon dioxide on soil carbon: A comparison of four meta-analyses. Glob. Chang. Biol. 2009, 15, 2020–2034. [Google Scholar] [CrossRef]
- Jayedi, A.; Shab-Bidar, S. Fish Consumption and the Risk of Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Adv. Nutr. 2020, 11, 1123–1133. [Google Scholar] [CrossRef]
- Lee, S.R.; An, E.J.; Kim, J.; Bae, Y.S. Function of NADPH Oxidases in Diabetic Nephropathy and Development of Nox Inhibitors. Biomol. Ther. 2020, 28, 25–33. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).