Antioxidant Properties of New Phenothiazine Derivatives
Abstract
:1. Introduction
2. Materials and Methods
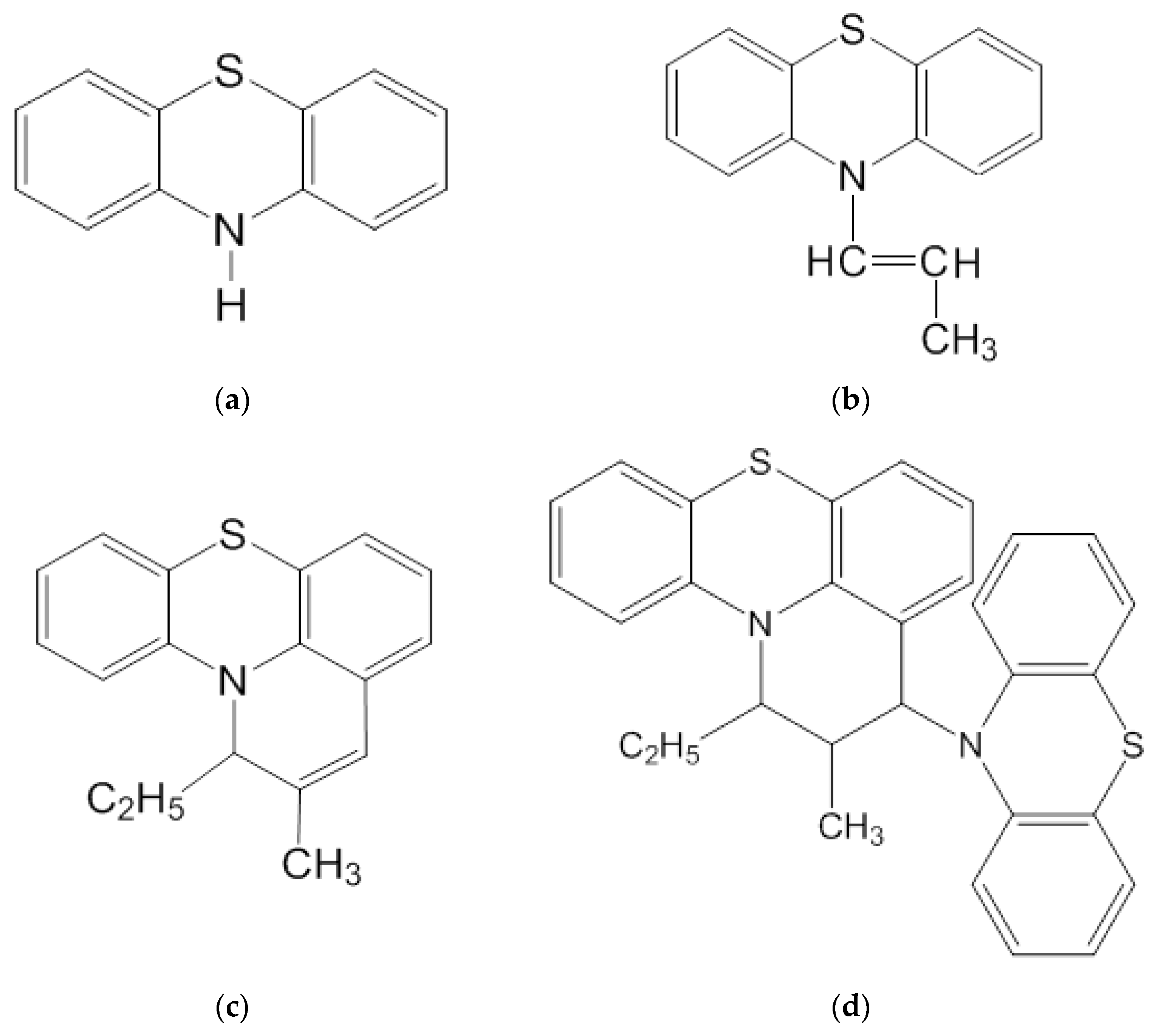
2.1. Cis-10-Propenylphenothiazine (cis-10-PPT)
2.2. 2-Methyl-1-ethyl-3-(10-phenothiazinyl)-2,3-dihydro-1H-pyrido-[3,2,1-k,l]phenothiazine
Propenylphenothiazine Dimer (DPPT)
2.3. 2-Methyl-1-ethyl-1H-pyrido-[3,2,1-k,l]-phenothiazine
Pyridophenothiazine (PyrPT)
2.4. Antioxidant Activity Measurement
3. Results
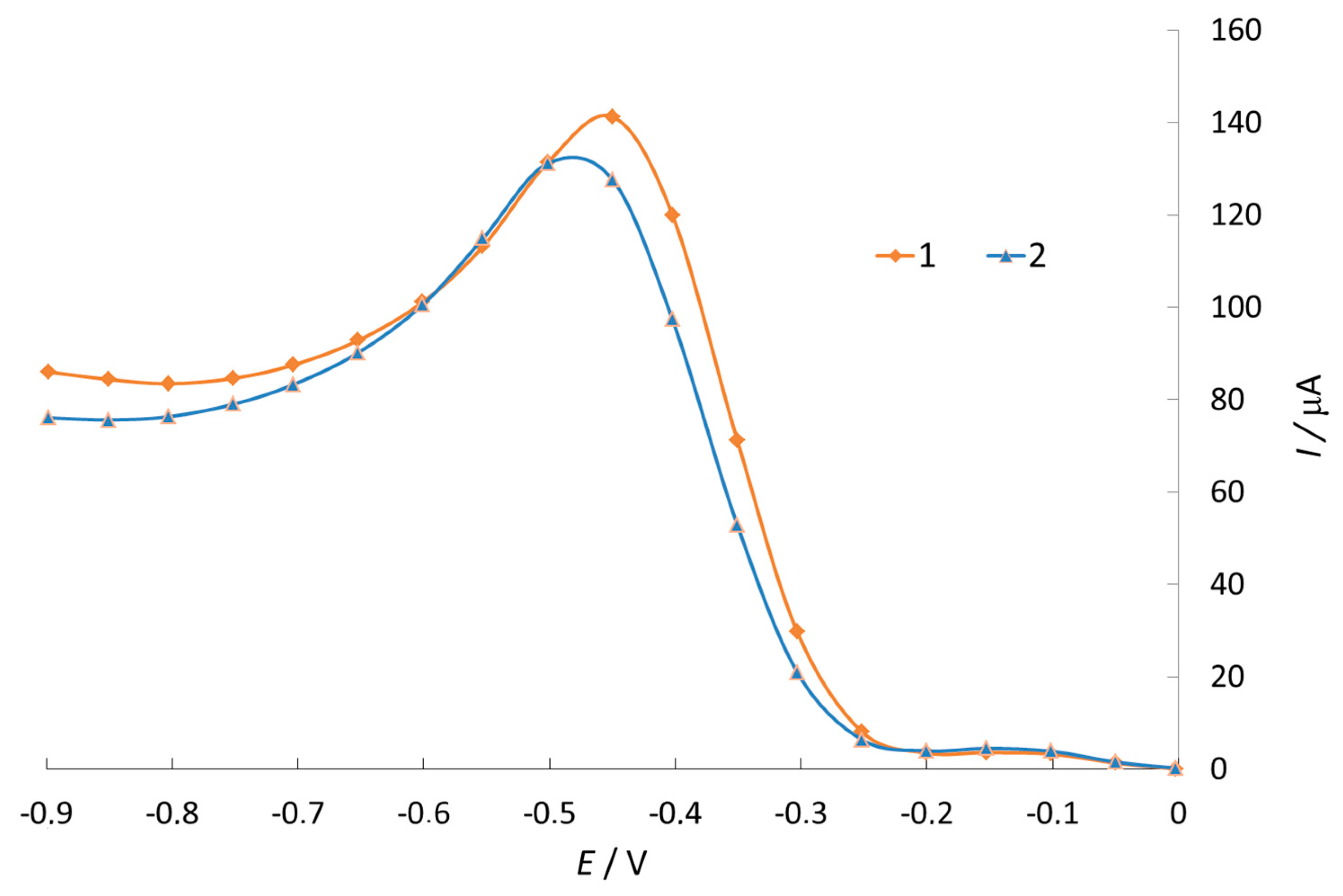
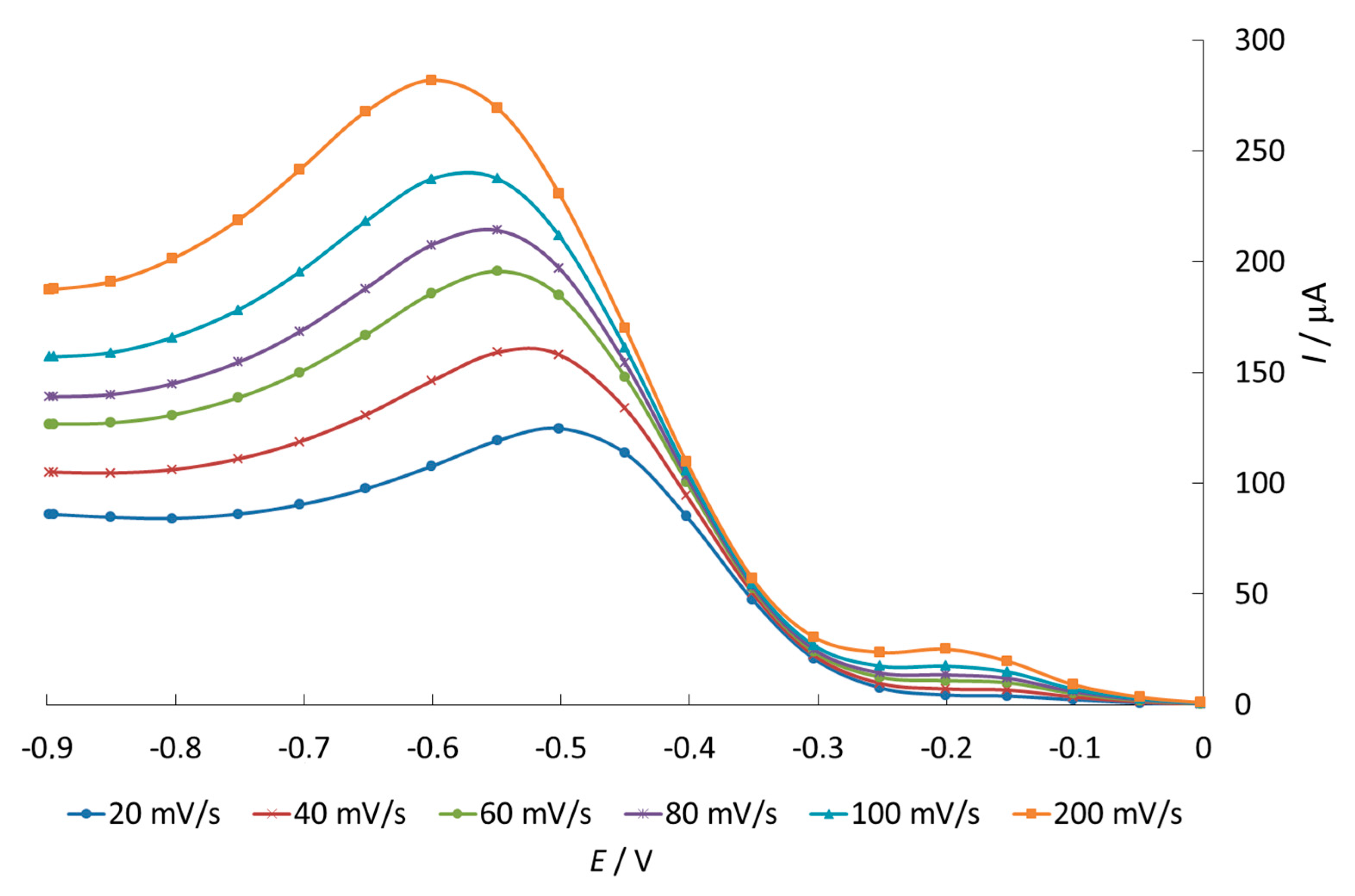
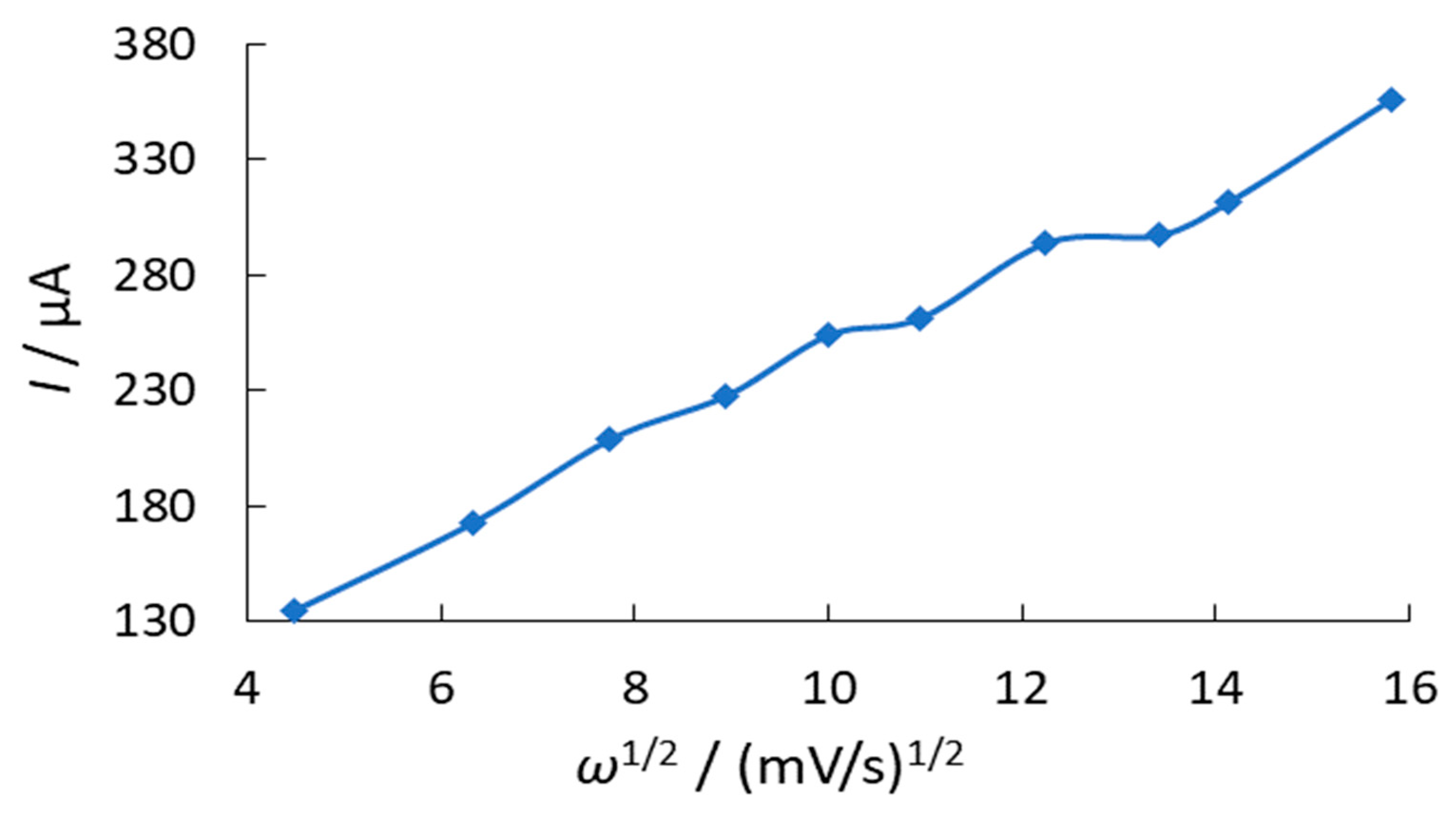
3.1. Investigation of Oxygen Electroreduction in the Presence of Phenothiazine and Its Derivatives
3.2. Determination of the Optimal Conditions for the Evaluation of Antioxidant Activity by the Experimental Design
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiau, Q. Pyridine-Embedded Phenothiazinium Dyes as Lysosome Targeted Photosensitizers for Highly Efficient Photodynamic Antitumor therapy. J. Med. Chem. 2020, 63, 4896–4907. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Phenothiazinium photosensitisers, Part VI: Photobactericidal asymmetric derivatives. Dyes Pigm. 2009, 82, 387–391. [Google Scholar] [CrossRef]
- Wilson, B. Synthesis and DNA photocleavage by mono- and bis-phenothiazinium-piperazine intercalators. Tetrahedron 2008, 64, 3429–3436. [Google Scholar] [CrossRef]
- Ganeev, R. Peculiarities of the nonlinear optical absorption of Methylene blue and Thionine in different solvents. Dyes Pigm. 2018, 149, 236–241. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Alamo, C.; Cuenca, E.; Shen, W.W.; Clervoy, P.; Rubio, G. History of the discovery and clinical introduction of chlorpromazine. Ann. Clin. Psychiatry 2005, 17, 113–135. [Google Scholar] [CrossRef]
- Cookson, J. Histamine in psychiatry: Promethazine as a sedative anticholinergic. BJPsych Adv. 2019, 25, 265–268. [Google Scholar] [CrossRef]
- Grimsey, E.M.; Piddock, L.J.V. Do phenothiazines possess antimicrobial and efflux inhibitory properties? FEMS Microb. Rev. 2019, 43, 577–590. [Google Scholar] [CrossRef]
- Motohashi, N.; Gollapudi, S.R.; Emrani, J.; Bhattiprolu, K.R. Antitumor properties of phenothiazines. Cancer Investig. 1991, 9, 305–319. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Jeleń, M.; Pluta, K. Phenothiazines Modified with the Pyridine Ring as Promising Anticancer Agents. Life 2021, 11, 206. [Google Scholar] [CrossRef]
- Qi, L.; Ding, Y. Potential antitumor mechanisms of phenothiazine drugs. Sci. China Life Sci. 2013, 56, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Nizi, M.G.; Desantis, J.; Nakatani, Y.; Massari, S.; Mazzarella, M.A.; Shetye, G.; Sabatinia, S.; Barrecaa, M.L.; Manfronia, G.; Felicetti, T.; et al. Antitubercular polyhalogenated phenothiazines and phenoselenazine with reduced binding to CNS receptors. Eur. J. Med. Chem. 2020, 201, 112420. [Google Scholar] [CrossRef] [PubMed]
- Warman, A.J.; Rito, T.S.; Fisher, N.E.; Moss, D.M.; Berry, N.G.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Antitubercular pharmacodynamics of phenothiazines. J. Antimicrob. Chemother. 2013, 68, 869–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotnikov, E.; Korotkova, E.; Voronova, O.; Sazhina, N.; Petrova, E.; Artamonov, A.; Chernyavskaya, L.; Dorozhko, E. Comparative investigation of antioxidant activity of human serum blood by amperometric, voltammetric and chemiluminescent methods. Arch. Med. Sci. 2016, 12, 1071–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Polikowska, A.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryć, R.; Michalczyk, A.; Dołęgowska, B. Importance of oxidative stress in the pathogenesis, diagnosis, and monitoring of patients with neuropsychiatric disorders, a review. Neurochem. Int. 2022, 153, 105269. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Murphy, C.M.; Ravner, H.; Smith, N.L. Mode of Action of Phenothiazine-Type Antioxidants. Ind. Eng. Chem. 1950, 42, 2479–2489. [Google Scholar] [CrossRef]
- Dalla Tiezza, M.; Hamlin, T.A.; Bickelhaupt, F.M.; Orian, L. Radical Scavenging Potential of the Phenothiazine Scaffold: A Computational Analysis. ChemMedChem 2021, 16, 3763–3771. [Google Scholar] [CrossRef]
- Engwa, G.A.; Ayuk, E.L.; Igbojekwe, B.U.; Unaegbu, M. Potential Antioxidant Activity of New Tetracyclic and Pentacyclic Nonlinear Phenothiazine Derivatives. Biochem. Res. Int. 2016, 2016, 9896575. [Google Scholar] [CrossRef] [Green Version]
- Fukuzumi, K.; Ikeda, N.; Egawa, M. Phenothiazine derivatives as new antioxidants for the autoxidation of methyl linoleate and their reaction mechanisms. J. Am. Oil Chem. Soc. 1976, 53, 623–627. [Google Scholar] [CrossRef]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Elaasser, M.M.; Asiri, A.M. Synthesis of Novel Chalcone-Based Phenothiazine Derivatives as Antioxidant and Anticancer Agents. Molecules 2020, 25, 4566. [Google Scholar] [CrossRef] [PubMed]
- Moharram, H.; Youssef, M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Fd. Sci. Technol. 2014, 11, 31–42. [Google Scholar]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT- Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Sazhina, N. Determination of Antioxidant Activity of Various Bioantioxidants and Their Mixtures by the Amperometric Method. Russ. J. Bioorg. Chem. 2017, 43, 771–775. [Google Scholar] [CrossRef]
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; Al-Farga, A.M. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Assessing Antioxidant Activity. Antioxidants 2022, 11, 584. [Google Scholar] [CrossRef]
- Kuzin, Y.; Khadieva, A.; Padnya, P.; Khannanov, A.; Kutyreva, M.; Stoikov, I.; Evtugyn, G. Electrochemistry of new derivatives of phenothiazine: Electrode kinetics and electropolymerization conditions. Electrochim. Acta 2021, 375, 137985. [Google Scholar] [CrossRef]
- Plotnikov, E.; Voronova, O.; Linert, W.; Martemianov, D.; Korotkova, E.; Dorozhko, E.; Astashkina, A.; Martemianova, I.; Ivanova, S.; Bokhan, N. Antioxidant and Immunotropic Properties of some Lithium Salts. J. App. Pharm. Sci. 2016, 6, 086–089. [Google Scholar] [CrossRef] [Green Version]
- Plotnikov, E.; Korotkova, E.; Voronova, O.; Dorozhko, E.; Bohan, N.; Plotnikov, S. Lithium-based antioxidants: Electrochemical properties and influence on immune cells. Physiol. Pharmacol. 2015, 19, 107–113. [Google Scholar]
- Korotkova, E.I.; Karbainov, Y.A.; Shevchuk, A.V. Study of antioxidant properties by voltammetry. J. Electroanal. Chem. 2002, 518, 56–60. [Google Scholar] [CrossRef]
- Anfinogenov, V.; Napilkova, O.; Sirotkina, E.; Filimonov, V.D.; Ogorodnikov, V.D. Synthesis and cis-,trans-isomerization of 10-propenylphenothiazines. Chem. Heterocycl. Compd. 1986, 1, 121–124. [Google Scholar]
- Anfinogenov, V.; Sirotkina, E.; Zhuravkov, S. 2-Methyl-1-ethyl-3-(10-phenothiazinyl)-2,3-dihydro-1H-pyrido-[3,2,1-k,l]-phenothiazine as a Stabilizer for Polyolefins. Patent RU #2105769, 27 February 1998. [Google Scholar]
- Anfinogenov, V.; Sirotkina, E.; Zhuravkov, S. 1-ethyl-2-methyl-1n-pyrido[3,2,1-k,l]phenothiazine as an acceptor of alkyl radicals and a method for its preparation. Patent RU #2030410, 3 October 1995. [Google Scholar]
- Miller, J.C.; Miller, J.N. Statistics for Analytical Chemistry, 6th ed.; Ellis Horwood PTR Prentice Hall: New York, NY, USA, 2010; p. 278. [Google Scholar]
- Lazic, Z.R. Design of Experiments in Chemical Engineering: A Practical Guide; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2004; p. 610. [Google Scholar]
- Iuga, C.; Campero, A.; Vivier-Bunge, A. Antioxidant vs. prooxidant action of phenothiazine in a biological environment in the presence of hydroxyl and hydroperoxyl radicals: A quantum chemistry study. RSC Adv. 2015, 5, 14678–14689. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Matralis, A.N.; Kourounakis, A.P. Antioxidant activity of newly synthesized 2,7-diazaphenothiazines. Arch. Der Pharmazie. 2010, 343, 268–273. [Google Scholar] [CrossRef]
- Hui, W.; Zuwang, W.; Chaoliang, W.; Dong, S.; Fuli, W. Antioxidant Activity of 3,7-Di-iso-octyl-phenothiazine and Its Synergistic Effect with 4,4′-Di-iso-octyl-diphenylamine. Tribol. Trans. 2007, 50, 273–276. [Google Scholar] [CrossRef]
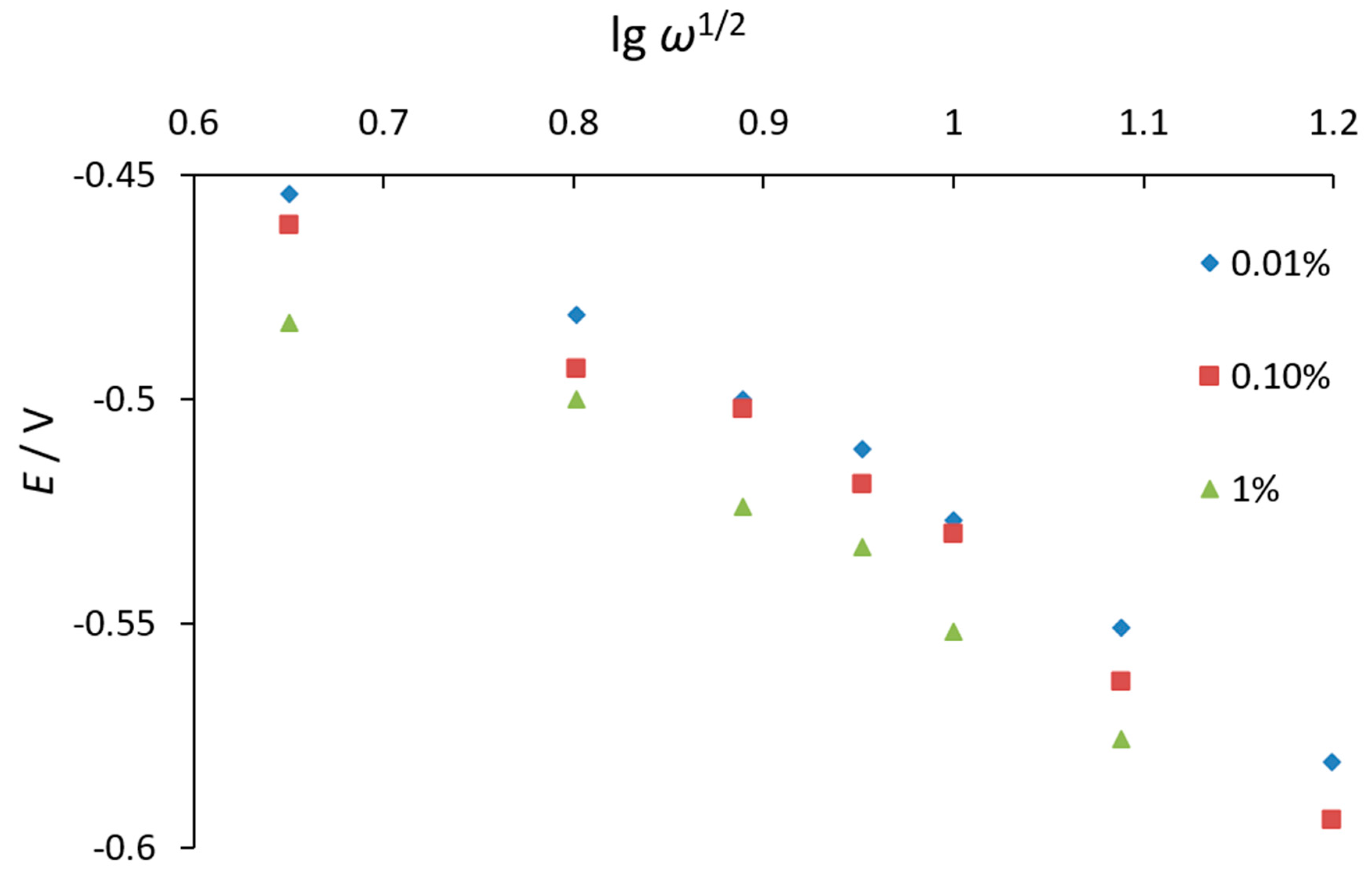
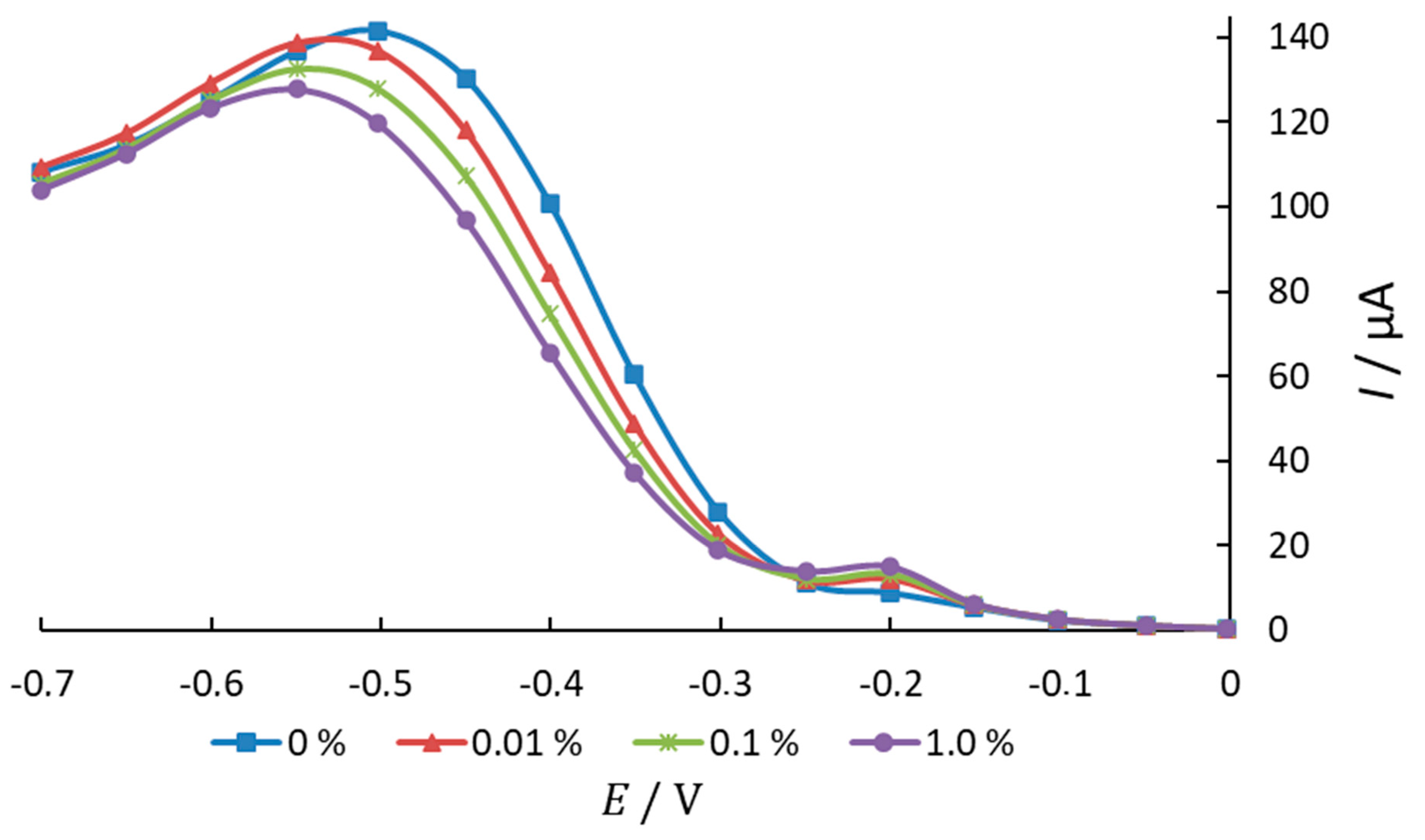
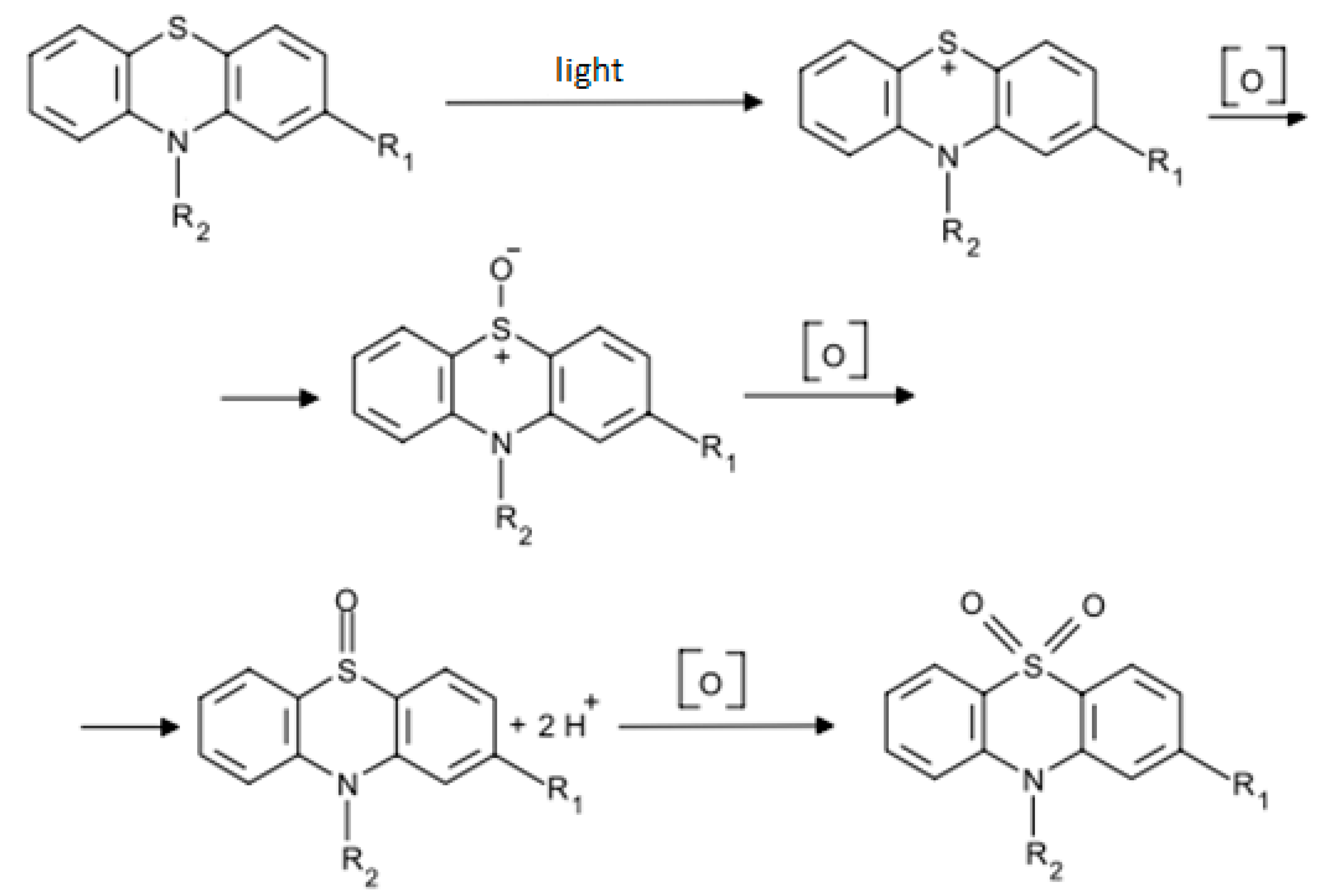
| Characteristic | X1 (CAO, %) | X2 (Cethanol, %) |
|---|---|---|
| Zero level | 0.055 | 60 |
| Variation interval | 0.045 | 10 |
| Upper level | 0.1 | 70 |
| Lower level | 0.01 | 50 |
| Characteristic | Equations |
|---|---|
| PT | Y = 0.923 + 0.168 X1 + 0.098 X2 |
| cis-10-PPT | Y = 0.46 + 0.17 X1 + 0.065 X2 |
| PyrPT | Y = 0.23 + 0.138 X1 + 0.073 X2 |
| DPPT | Y = 0.129 + 0.027 X1 + 0.022 X2 |
| 30% Water–Ethanol Solution | C1 = 0.01% | C2 = 0.1% | C3 = 1% | |||
|---|---|---|---|---|---|---|
| K, µM/min | Sr | K, µM/min | Sr | K, µM/min | Sr | |
| PT | 1.44 | 0.01 | 1.90 | 0.01 | 5.37 | 0.02 |
| cis-10-PPT | 6.48 | 0.01 | 12.80 | 0.03 | 19.43 | 0.04 |
| PyrPT | 6.64 | 0.02 | 10.59 | 0.03 | 13.11 | 0.03 |
| DPPT | 8.53 | 0.02 | 10.74 | 0.03 | 13.90 | 0.03 |
| 50% Water–Ethanol Solution | C1 = 0.01% | C2 = 0.1% | C3 = 1% | |||
|---|---|---|---|---|---|---|
| K, µM/min | Sr | K, µM/min | Sr | K, µM/min | Sr | |
| PT | 4.73 | 0.02 | 9.65 | 0.02 | 17.11 | 0.03 |
| cis-10-PPT | 12.19 | 0.03 | 17.84 | 0.03 | 24.93 | 0.03 |
| PyrPT | 15.65 | 0.03 | 19.47 | 0.03 | 24.93 | 0.03 |
| DPPT | 15.29 | 0.03 | 23.84 | 0.04 | 44.77 | 0.04 |
| 70% Water–Ethanol Solution | C1 = 0.01% | C2 = 0.1% | C3 = 1% | |||
|---|---|---|---|---|---|---|
| K, µM/min | Sr | K, µM/min | Sr | K, µM/min | Sr | |
| PT | 6.69 | 0.02 | 15.26 | 0.03 | 20.69 | 0.06 |
| cis-10-PPT | 17.56 | 0.03 | 21.32 | 0.03 | 31.35 | 0.07 |
| PyrPT | 19.86 | 0.03 | 26.13 | 0.03 | 29.47 | 0.07 |
| DPPT | 25.50 | 0.05 | 38.46 | 0.07 | 71.27 | 0.08 |
| 85% Water–Ethanol Solution | C1 = 0.01% | C2 = 0.1% | C3 = 1% | |||
|---|---|---|---|---|---|---|
| K, µM/min | Sr | K, µM/min | Sr | K, µM/min | Sr | |
| PT | 17.78 | 0.05 | 23.03 | 0.06 | 24.85 | 0.06 |
| cis-10-PPT | 27.36 | 0.06 | 35.11 | 0.07 | 39.90 | 0.07 |
| PyrPT | 22.57 | 0.06 | 29.87 | 0.07 | 33.06 | 0.07 |
| DPPT | 39.90 | 0.07 | 65.44 | 0.08 | 82.76 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronova, O.; Zhuravkov, S.; Korotkova, E.; Artamonov, A.; Plotnikov, E. Antioxidant Properties of New Phenothiazine Derivatives. Antioxidants 2022, 11, 1371. https://doi.org/10.3390/antiox11071371
Voronova O, Zhuravkov S, Korotkova E, Artamonov A, Plotnikov E. Antioxidant Properties of New Phenothiazine Derivatives. Antioxidants. 2022; 11(7):1371. https://doi.org/10.3390/antiox11071371
Chicago/Turabian StyleVoronova, Olesya, Sergey Zhuravkov, Elena Korotkova, Anton Artamonov, and Evgenii Plotnikov. 2022. "Antioxidant Properties of New Phenothiazine Derivatives" Antioxidants 11, no. 7: 1371. https://doi.org/10.3390/antiox11071371
APA StyleVoronova, O., Zhuravkov, S., Korotkova, E., Artamonov, A., & Plotnikov, E. (2022). Antioxidant Properties of New Phenothiazine Derivatives. Antioxidants, 11(7), 1371. https://doi.org/10.3390/antiox11071371







