Effects of Gold Nanoparticles Functionalized with Bioactive Compounds from Cornus mas Fruit on Aorta Ultrastructural and Biochemical Changes in Rats on a Hyperlipid Diet—A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
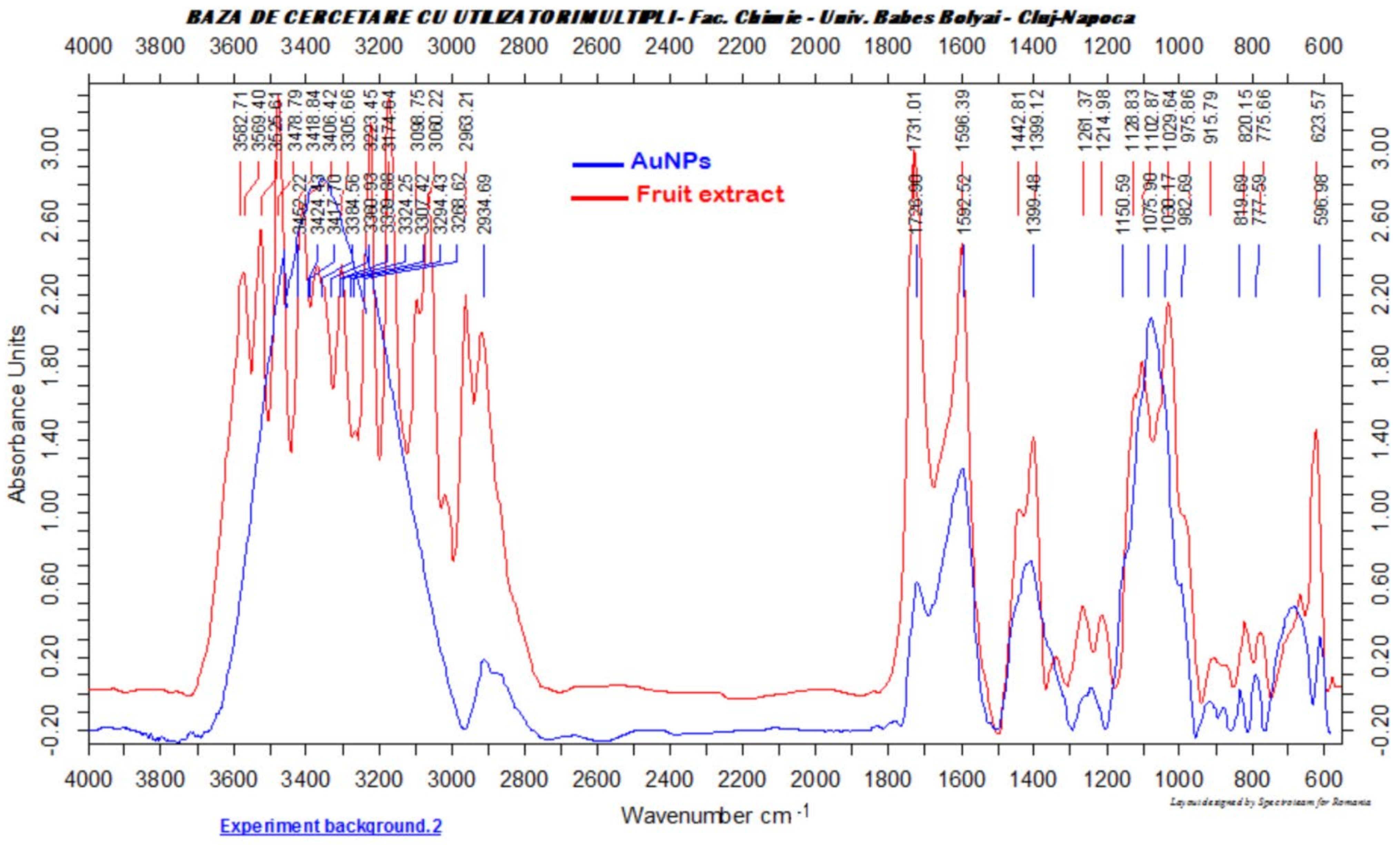
2.2. Fruit Extract Preparation and Characterization
2.3. Gold Nanoparticles Synthesis, Characterization and Tissue Determinations
2.4. Animals
2.5. High-Fat Diet (HFD)
2.6. Experimental Design
2.7. Histopathological Examination
2.8. Transmission Electron Microscopy (TEM)
2.9. Endothelin, iNOS and TNF-Alpha
2.10. Ultrasound (US) Examination
2.11. Serum Oxidative Stress Investigation
2.12. Serum Biochemical Parameters
2.13. Statistical Analysis
3. Results
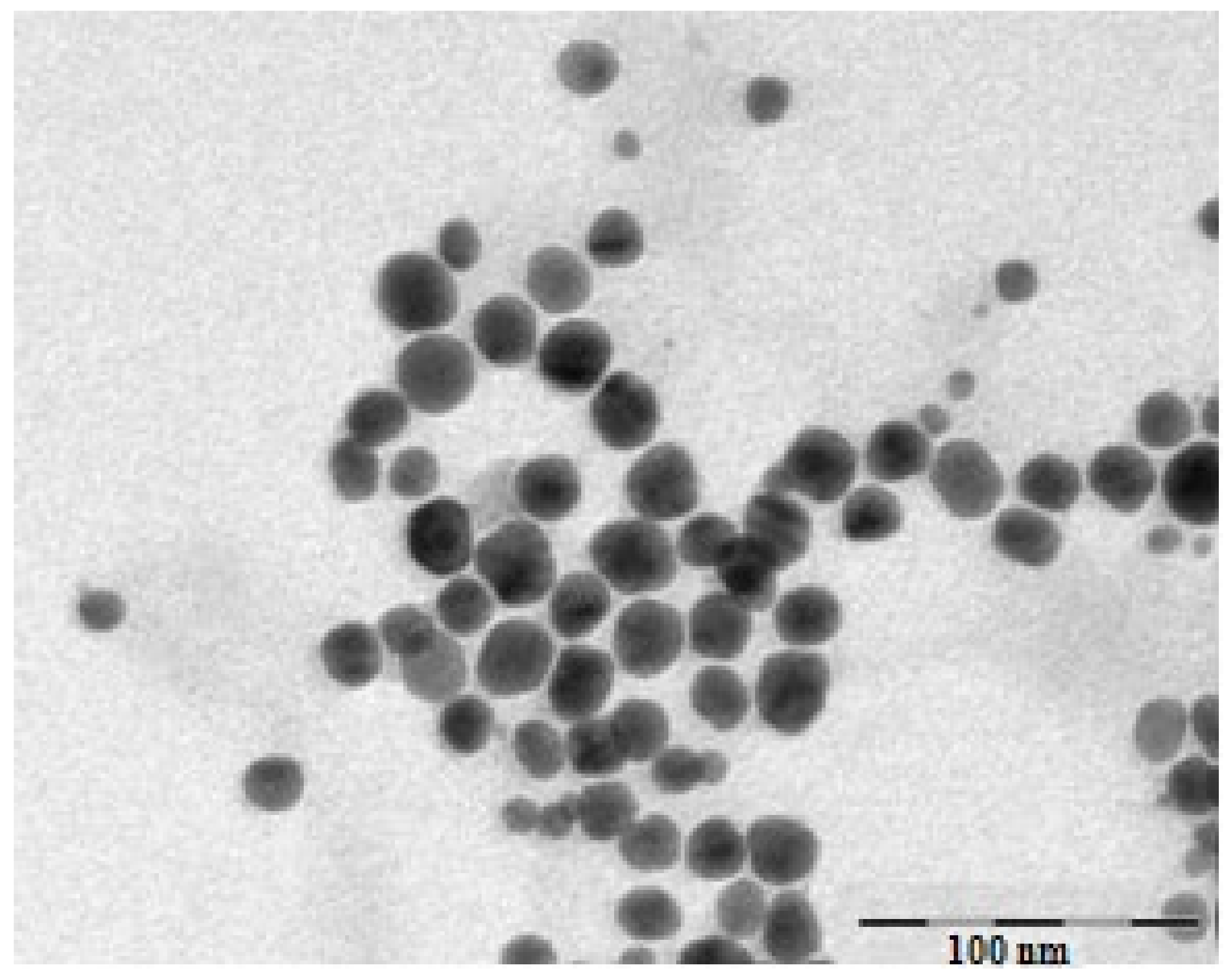
3.1. Characterization of Gold Nanoparticles Functionalized with Cornus mas L. Phytocompounds
3.2. Preliminary Experiment: AuNPsCM Tissue Distribution
3.3. Experiments in Rats with a High-Fat Diet
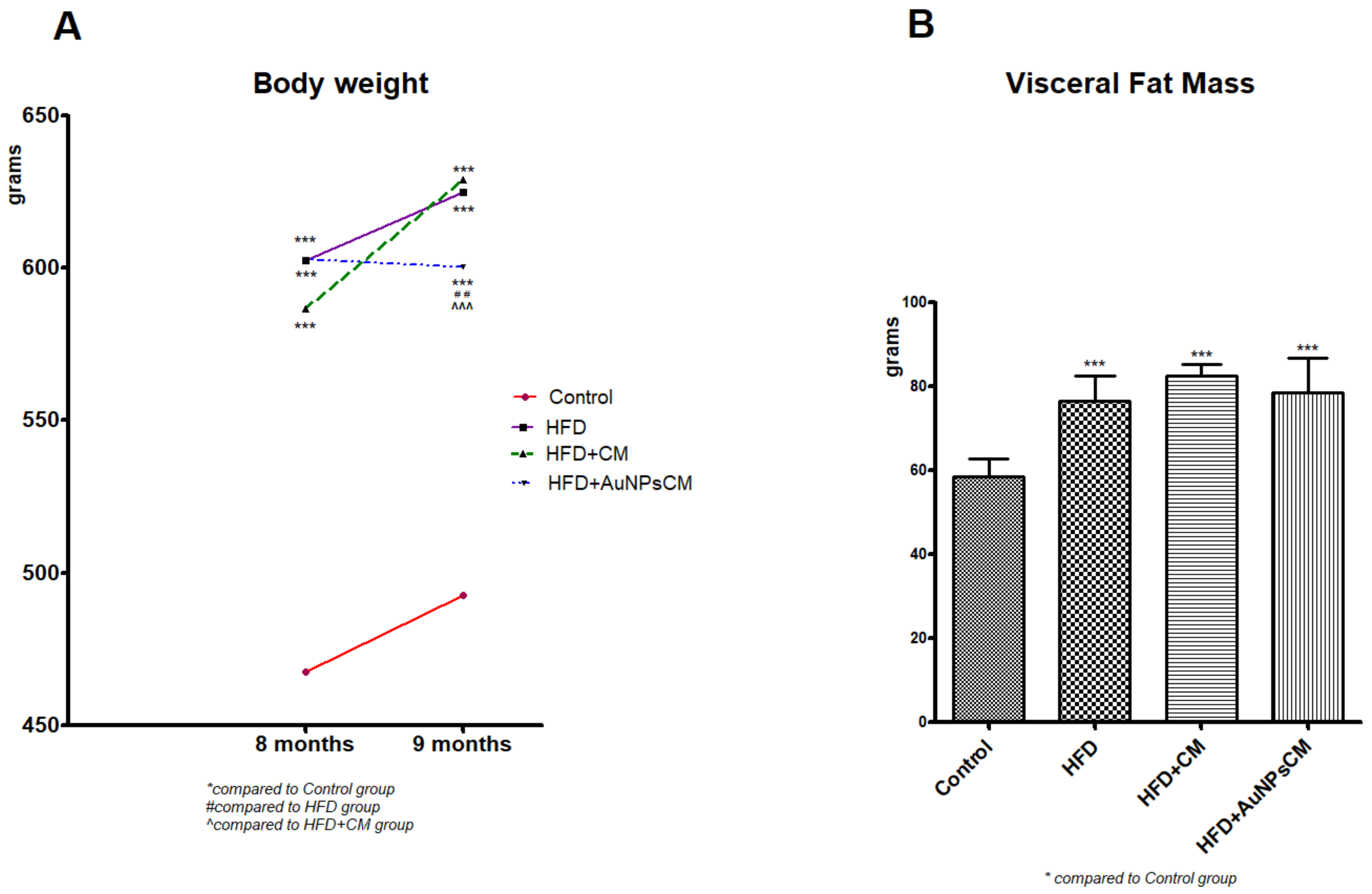
3.3.1. Weight Gain in Rats
3.3.2. Aorta Investigations
Histological Examination
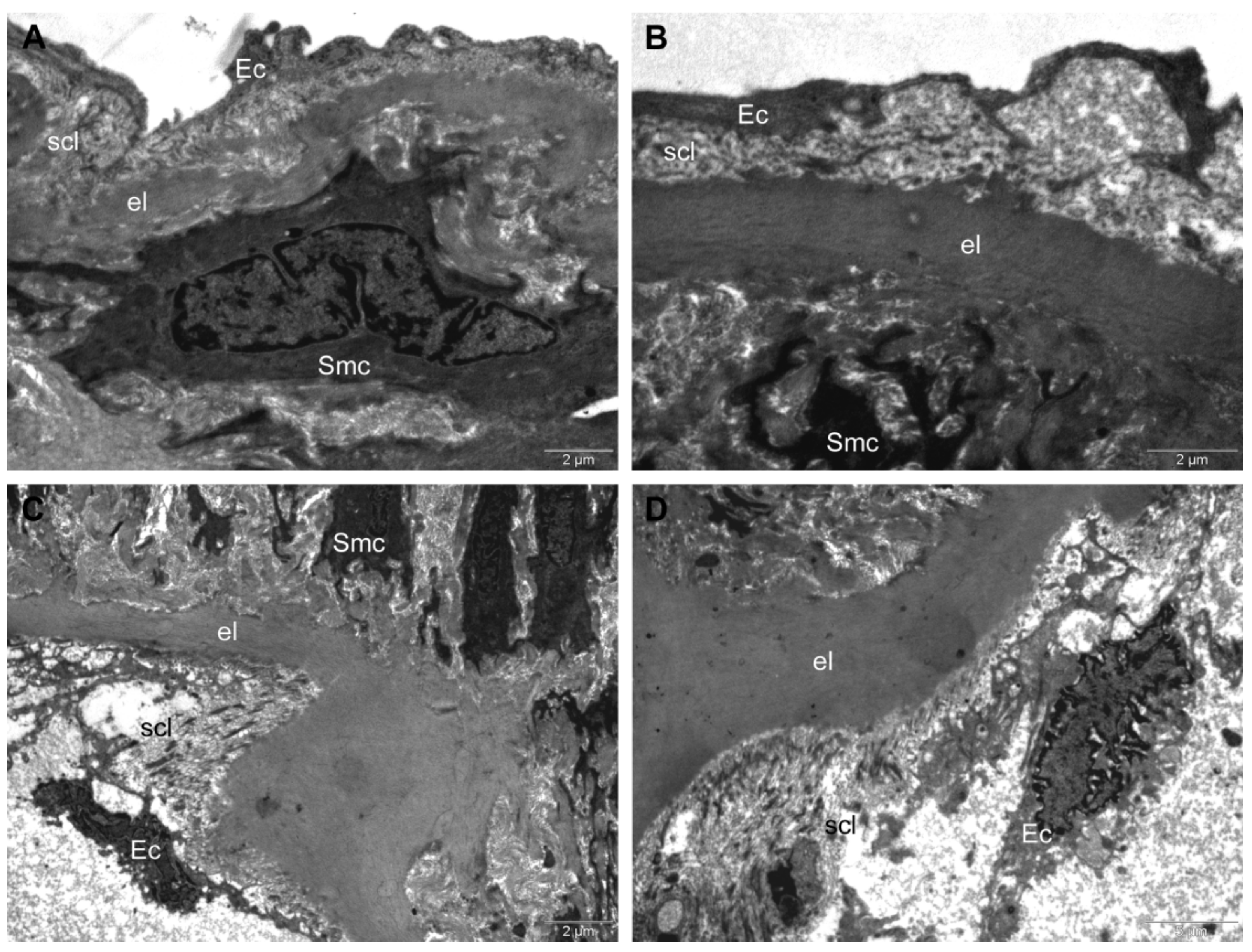
TEM Investigation
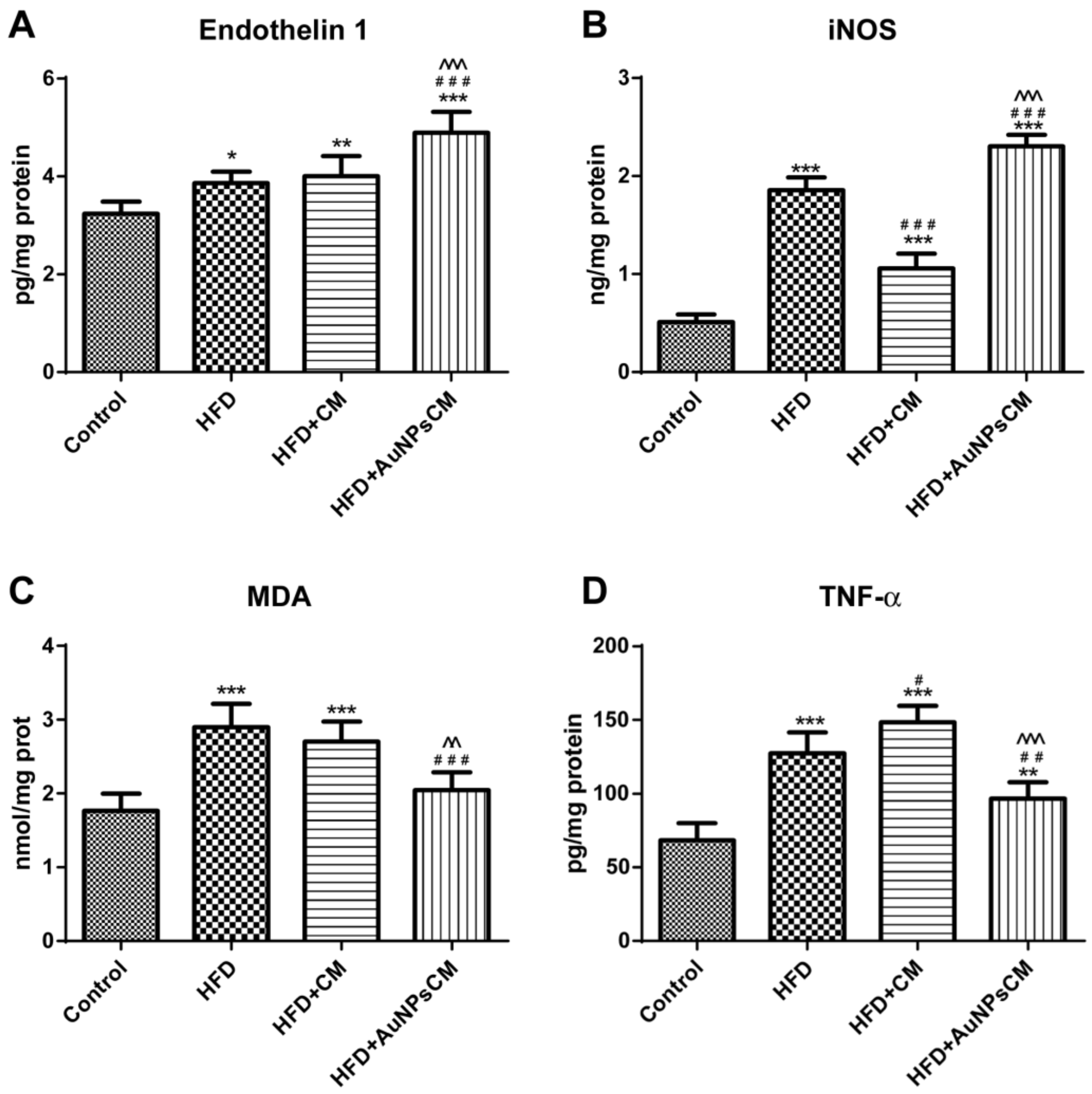
Biochemical Parameters from Aorta Homogenates
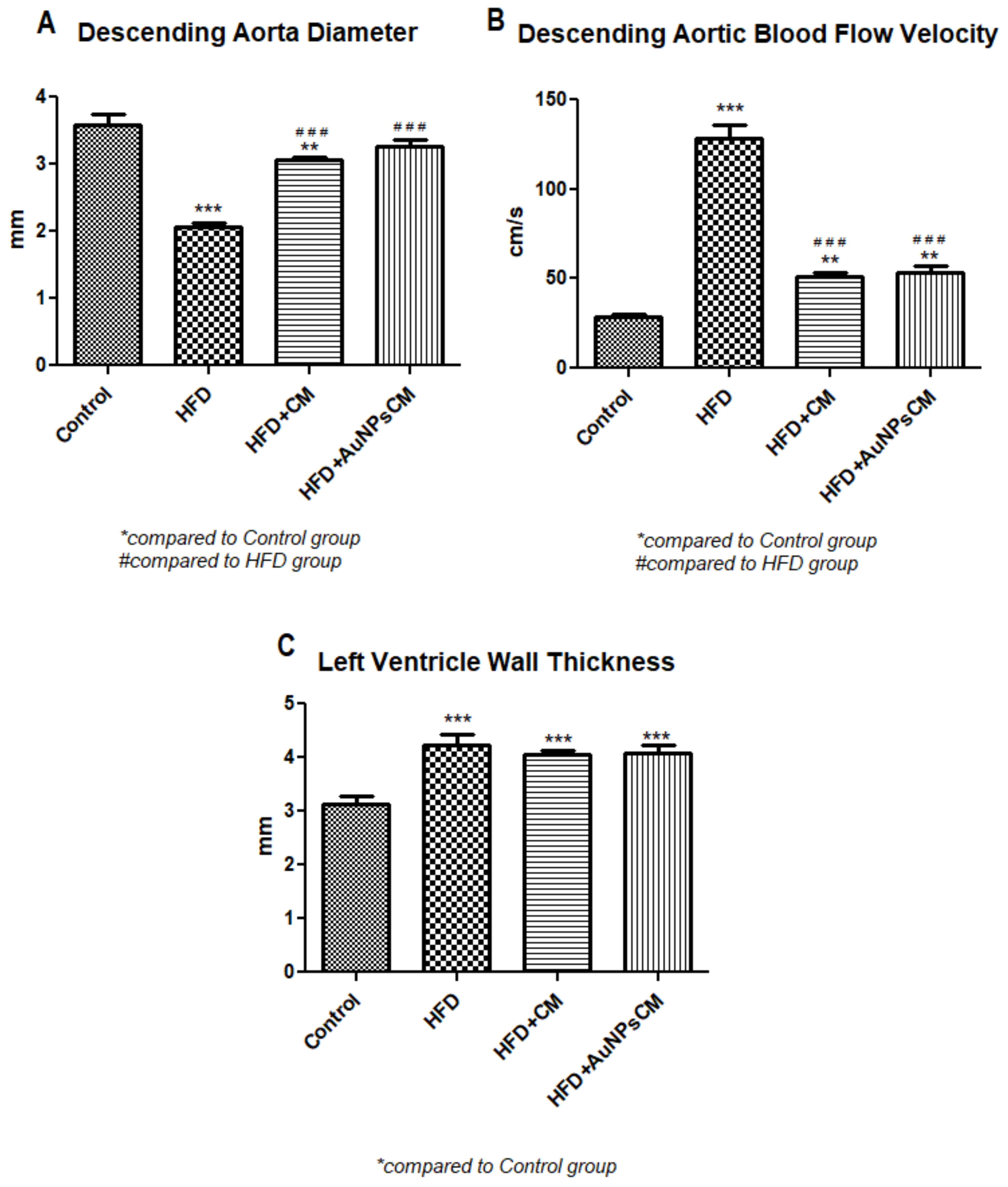
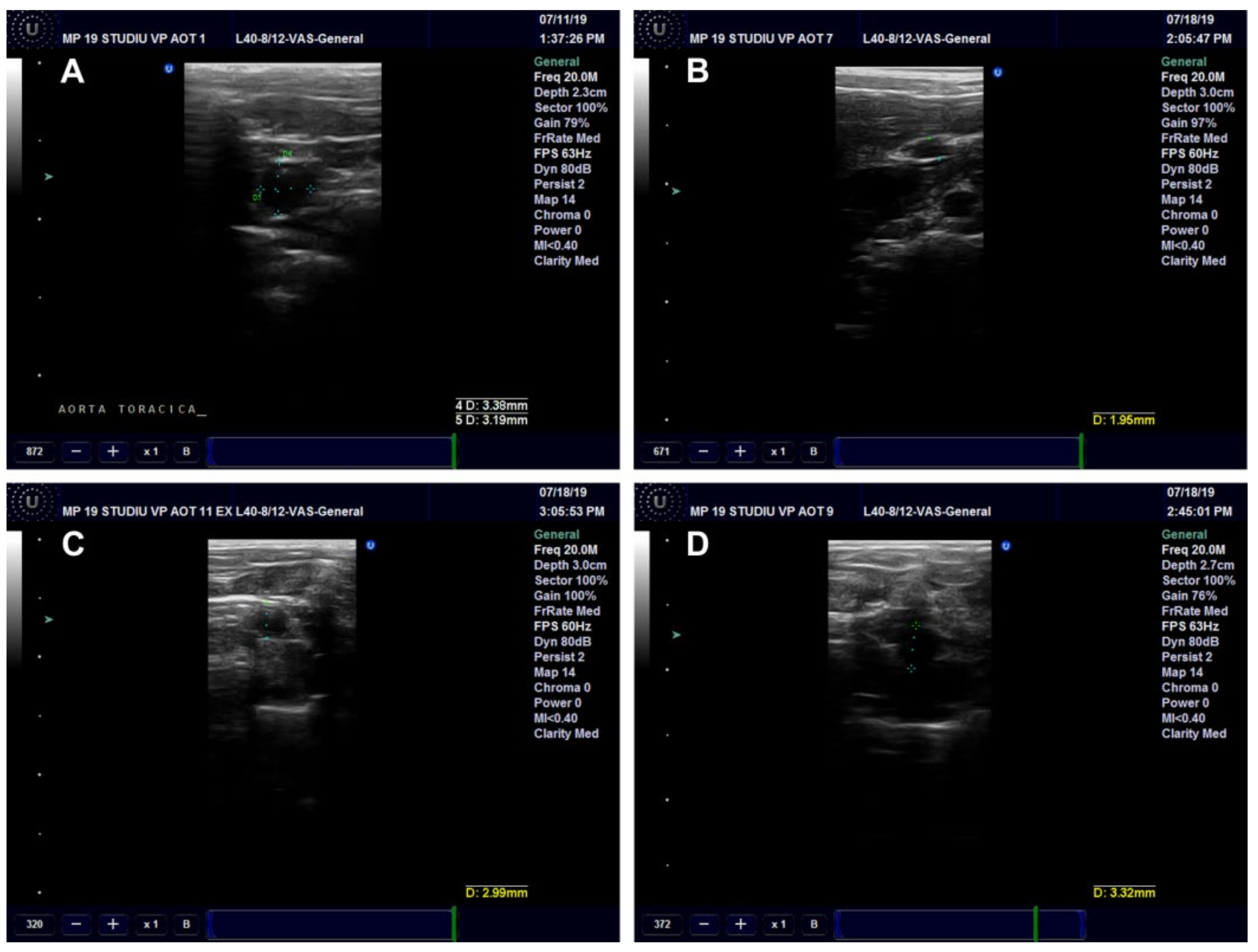
Ultrasound Examination of the Aorta and Left Ventricle
3.3.3. Serum Examination
Serum Oxidative Stress Parameters
Serum Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and cardiovascular disease: A scientific statement from the american heart association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Mengozzi, A.; Masi, S.; Virdis, A. Obesity-related endothelial dysfunction: Moving from classical to emerging mechanisms. Endocr. Metab. Sci. 2020, 1, 100063. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Xiang, G.; Li, Y.; Li, H.; Xiang, L.; Lu, J.; Xiang, L.; Dong, J.; Liu, M. GDF11 protects against endothelial injury and reduces atherosclerotic lesion formation in apolipoprotein E-null mice. Mol. Ther. 2016, 24, 1926–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomkin, G.H.; Owens, D. The chylomicron: Relationship to atherosclerosis. Int. J. Vasc. Med. 2012, 2012, 784536. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Liu, Z.; Qu, X.; Zhao, Y. Effects of yerba mate tea (Ilex paraguariensis) on vascular endothelial function and liver lipoprotein receptor gene expression in hyperlipidemic rats. Fitoterapia 2013, 84, 264–272. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Chegodaev, Y.S.; Wu, W.K.; Orekhov, A.N. Oxidative stress and antioxidants in atherosclerosis development and treatment. Biology 2020, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Cai, H.; Xu, Z.; Wang, J.; Qiu, C.; Xie, J.; Huang, W.; Sui, Z. Protective role of antioxidant huskless barley extracts on TNF-α-induced endothelial dysfunction in human vascular endothelial cells. Oxid. Med. Cell. Longev. 2018, 2018, 3846029. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Ye, P.; Li, H.; Wang, Y.; Xu, Y.; Tian, Q.; Lei, G.; Zhao, C.; Gao, Z.; Zhao, W.; et al. Lunasin attenuates oxidant-induced endothelial injury and inhibits atherosclerotic plaque progression in ApoE−/− mice by up-regulating heme oxygenase-1 via PI3K/Akt/Nrf2/ARE pathway. FASEB J. 2019, 33, 4836–4850. [Google Scholar] [CrossRef]
- Zugravu Pop, D.D.; Mitrea, D.R.; Suciu, S.; Clichici, S.V. Nanostructure-based therapies for liver fibrosis. J. Physiol. Pharmacol. 2020, 71, 771–780. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Hung, Y.C.; Liau, I.; Huang, G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yah, C.S.; Simate, G.S.; Iyuke, S.E. Nanoparticles toxicity and their routes of exposures. Pak. J. Pharm. Sci. 2012, 25, 477–491. [Google Scholar] [PubMed]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Pnyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of nanoparticles in biomedical application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, D.R.; Toader, A.M.; Hoteiuc, O.A. Oxidative stress produced by urban atmospheric nanoparticles. In Nanomaterials; Filip, A., Nascimento, G.M., Clichici, S., Eds.; IntechOpen: London, UK, 2020; pp. 55–71. [Google Scholar] [CrossRef]
- Mitrea, D.R.; Mortazavi Moschkenani, H.; Hoteiuc, O.A.; Bidian, C.; Toader, A.; Clichici, S. Antioxidant protection against cosmic radiation-induced oxidative stress at commercial flight altitude. J. Physiol. Pharmacol. 2018, 69, 30415237. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Wu, M.; Liu, L.; Xing, Y.; Yang, S.; Li, H.; Cao, Y. Roles and mechanisms of hawthorn and its extracts on atherosclerosis: A review. Front. Pharmacol. 2020, 11, 118. [Google Scholar] [CrossRef]
- Hao, D.; Danbin, W.; Maojuan, G.; Chun, S.; Bin, L.; Lin, Y.; Yingxin, S.; Guanwei, F.; Yefei, C.; Qing, G.; et al. Ethanol extracts of Danlou tablet attenuate atherosclerosis via inhibiting inflammation and promoting lipid effluent. Pharmacol. Res. 2019, 146, 104306. [Google Scholar] [CrossRef]
- Lee, C.C.; Dudonné, S.; Dubé, P.; Desjardins, Y.; Kim, J.H.; Kim, J.S.; Kim, J.E.; Park, J.H.Y.; Lee, K.W.; Lee, K.Y. Comprehensive phenolic composition analysis and evaluation of Yak-Kong soybean (Glycine max) for the prevention of atherosclerosis. Food Chem. 2017, 234, 486–493. [Google Scholar] [CrossRef]
- Kulkarni, G.T. Herbal drug delivery systems: An emerging area in herbal drug research. J. Chronother. Drug. Deliv. 2011, 2, 113–119. [Google Scholar]
- Rafieian-Kopaei, M.; Asgary, S.; Adelnia, A.; Setorki, M.; Khazaei, M.; Kazemi, S.; Shamsi, F. The effects of cornelian cherry on atherosclerosis and atherogenic factors in hypercholesterolemic rabbits. J. Med. Plant. Res. 2011, 5, 2670–2676. [Google Scholar]
- Bayram, H.M.; Ozturkcan, A. Bioactive components and biological properties of cornelian cherry (Cornus mas L.): A comprehensive review. J. Funct. Foods 2020, 75, 104252. [Google Scholar] [CrossRef]
- Gholipour, S.; Shomali, T.; Rafieian-Kopaei, M. Anti-hypertriglyceridemic activity of cornus mas in diabetic rats. J. Clin. Diagn. Res. 2018, 12, 80802323. [Google Scholar] [CrossRef]
- Asgary, S.; Kelishadi, R.; Rafieian-Kopaei, M.; Najafi, S.; Najafi, M.; Sahebkar, A. Investigation of the lipid-modifying and antiinflammatory effects of Cornus mas L. supplementation on dyslipidemic children and adolescents. Pediatr. Cardiol. 2013, 34, 1729–1735. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Sharma, D.R.; Hazra, J.; Prajapati, P.K. Nanophytomedicines: A novel approach to improve drug delivery and pharmacokinetics of herbal medicine. Bio Bull. 2017, 3, 132–135. [Google Scholar]
- Kumar, K.; Rai, A. Miraculous therapeutic effects of herbal drugs using novel drug delivery systems. J. Pharm. 2012, 3, 27–30. [Google Scholar]
- Moldovan, B.; Filip, A.; Clichici, S.; Suharoschi, R.; Bolfa, P.; David, L. Antioxidant activity of Cornelian cherry (Cornus mas L.) fruits extract and the in vivo evaluation of its anti-inflammatory effects. J. Funct. Foods 2016, 26, 77–87. [Google Scholar] [CrossRef]
- Domsa, E.M.; Filip, G.A.; Olteanu, D.; Baldea, I.; Clichici, S.; Muresan, A.; David, L.; Moldovan, B.; Para, I.; Suciu, M.; et al. Gold nanoparticles phytoreduced with Cornus mas extract mitigate some of gliadin effects on Caco-2 cells. J. Physiol. Pharmacol. 2020, 71, 201–212. [Google Scholar] [CrossRef]
- Baldea, I.; Florea, A.; Olteanu, D.; Clichici, S.; David, L.; Moldovan, B.; Cenariu, M.; Achim, M.; Suharoschi, R.; Danescu, S.; et al. Effects of silver and gold nanoparticles phytosynthesized with Cornus mas extract on oral dysplastic human cells. Nanomedicine 2020, 15, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Moldovan, B.; Popa, A.; David, L. Effects of storage temperature on the total phenolic content of Cornelian cherry (Cornus mas L.) fruits extracts. J. Appl. Bot. Food Qual. 2016, 89, 208–211. [Google Scholar] [CrossRef]
- Conti, M.; Morand, P.C.; Levillain, P.; Lemonnier, A. Improved fluorimetric determination of malondialdehyde. Clin. Chem. 1991, 37, 1273–1275. [Google Scholar] [CrossRef]
- Hu, M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar] [CrossRef]
- Vats, P.; Singh, V.K.; Singh, S.N.; Singh, S.B. Glutathione metabolism under high altitude stress and effect of antioxidant supplementation. Aviat. Spac. Environ. Med. 2008, 79, 1106–1111. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Bolfa, P.; Clichici, S.; Filip, G.A. Modulatory effects of Cornus sanguinea L. mediated green synthesized silver nanoparticles on oxidative stress, COX-2/NOS2 and NFkB/pNFkB expressions in experimental inflammation in Wistar rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 10, 110709. [Google Scholar] [CrossRef]
- Opris, R.; Toma, V.; Olteanu, D.; Baldea, I.; Baciu, A.M.; Lucaci, F.I.; Berghian-Sevastre, A.; Tatomir, C.; Moldovan, B.; Clichici, S.; et al. Effects of silver nanoparticles functionalized with Cornus mas L. extract on architecture and apoptosis in rat testicle. Nanomedicine 2019, 14, 275–299. [Google Scholar] [CrossRef]
- Clichici, S.; David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Filip, M.; Nagy, A.; Luca, V.; Crivii, C.; Mircea, P.; et al. Hepatoprotective effects of silymarin coated gold nanoparticles in experimental cholestasis. Mater. Sci. Eng. C 2020, 115, 111117. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, V.; Przeor, M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef] [Green Version]
- Timoszyk, A. A review of the biological synthesis of gold nanoparticles using fruit extracts: Scientific potential and application. Bull. Mater. Sci. 2018, 41, 154. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Ng, J.P.M.; Bishop, D.P.; Milthorpe, B.K.; Valenzuela, S.M. Gold nanoparticles as cell regulators: Beneficial effects of gold nanoparticles on the metabolic profile of mice with pre-existing obesity. J. Nanobiotechnol. 2018, 16, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.D.; Wu, H.Y.; Wu, D.; Wang, Y.Y.; Chang, J.H.; Zhai, Z.B.; Meng, A.M.; Liu, P.X.; Zhang, L.A.; Fan, F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. J. Nanomed. 2010, 5, 771–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palou, M.; Priego, T.; Sánchez, J.; Rodríguez, A.M.; Palou, A.; Picó, C. Gene expression patterns in visceral and subcutaneous adipose depots in rats are linked to their morphologic features. Cell. Physiol. Biochem. 2009, 24, 547–665. [Google Scholar] [CrossRef] [PubMed]
- Curry, F.E.; Adamson, R.H. Endothelial glycocalyx: Permeability barrier and mechanosensor. Ann. Biomed. Eng. 2012, 40, 828–839. [Google Scholar] [CrossRef] [Green Version]
- Rima, W.; Sancey, L.; Aloy, M.T.; Armandy, E.; Alcantara, G.B.; Epicier, T.; Malchère, A.; Joly-Pottuz, L.; Mowat, P.; Lux, F.; et al. Internalization pathways into cancer cells of gadolinium-based radiosensitizing nanoparticles. Biomaterials 2013, 34, 181–195. [Google Scholar] [CrossRef] [Green Version]
- Gromnicova, R.; Kaya, M.; Romero, I.A.; Williams, P.; Satchell, S.; Sharrack, B.; Male, D. Transport of gold nanoparticles by vascular endothelium from different human tissues. PLoS ONE 2016, 11, e0161610. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 10, 1322–1337. [Google Scholar] [CrossRef]
- Bahamonde, J.; Brenseke, B.; Chan, M.Y.; Kent, R.D.; Vikesland, P.J.; Prater, M.R. Gold nanoparticle toxicity in mice and rats: Species differences. Toxicol. Pathol. 2018, 6, 431–443. [Google Scholar] [CrossRef]
- Kruth, H.S. Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native LDL particles. Curr. Opin. Lipidol. 2011, 22, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Omelka, R.; Blahova, J.; Kovacova, V.; Babikova, M.; Mondockova, V.; Kalafova, A.; Capcarova, M.; Martiniakova, M. Cornelian cherry pulp has beneficial impact on dyslipidemia and reduced bone quality in zucker diabetic fatty rats. Animals 2020, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Baretella, O.; Chung, S.K.; Xu, A.; Vanhoutte, P.M. Endothelial overexpression of endothelin-1 modulates aortic, carotid, iliac and renal arterial responses in obese mice. Acta Pharmacol. Sin. 2017, 38, 498–512. [Google Scholar] [CrossRef] [Green Version]
- Babaei, S.; Picard, P.; Ravandi, A.; Monge, J.C.; Lee, T.C.; Cernacek, P.; Stewart, D.J. Blockade of endothelin receptors markedly reduces atherosclerosis in LDL receptor deficient mice: Role of endothelin in macrophage foam cell formation. Cardiovasc. Res. 2000, 48, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Hosseinpour-Jaghdani, F.; Shomali, T.; Gholipour-Shahraki, S.; Rahimi-Madiseh, M.; Rafieiman-Kopaei, M. Cornus mas: A review on traditional uses and pharmacological properties. JCIM 2017, 14, 20160137. [Google Scholar] [CrossRef] [PubMed]
- Bujor, A.; Miron, A.; Luca, S.V.; Skalicka-Wozniak, K.; Silion, M.; Ancuceanu, R.; Dinu, M.; Girard, C.; Demougeot, C.; Totoson, P. Metabolite profiling, arginase inhibition and vasorelaxant activity of Cornus mas, Sorbus aucuparia and Viburnum opulus fruit extracts. Food Chem. Toxicol. 2019, 133, 110764. [Google Scholar] [CrossRef]
- Wilcox, J.N.; Subramanian, R.R.; Sundell, C.L.; Tracey, W.R.; Pollock, J.S.; Harrison, D.G.; Marsden, P.A. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2479–2488. [Google Scholar] [CrossRef]
- Nussler, A.K.; Di Silvio, M.; Billiar, T.R.; Hoffman, R.A.; Geller, D.A.; Selby, R.; Madariaga, J.; Simmons, R.L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J. Exp. Med. 1992, 176, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [Green Version]
- Ridnour, L.A.; Thomas, D.D.; Mancardi, D.; Espey, M.G.; Miranda, K.M.; Paolocci, N.; Feelisch, M.; Fukuto, J.; Wink, D.A. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol. Chem. 2004, 385, 1–10. [Google Scholar] [CrossRef]
- Detmers, P.A.; Hernandez, M.; Mudgett, J.; Hassing, H.; Burton, C.; Mundt, S.; Chun, S.; Fletcher, D.; Card, D.J.; Lisnock, J.; et al. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J. Immunol. 2000, 165, 3430–3435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lietava, J.; Beerova, N.; Klymenko, S.V.; Panghyova, E.; Varga, I.; Pechanova, O. Effects of Cornelian Cherry on Atherosclerosis and Its Risk Factors. Oxid. Med. Cell. Longev. 2019, 2019, 2515270. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, R.; Mitrea, D.R.; Florea, A.; David, L.; Moldovan, B.E.; Mureşan, L.E.; Suciu, Ş.; Lenghel, M.; Hărănguş, I.C.; Ungur, R.A.; et al. Aorta modifications in oral gold nanoparticles administration in rats. JHSRM 2021, 4, 210–218. [Google Scholar] [CrossRef]
- Tsai, D.-H.; Elzey, S.; DelRio, F.W.; Keene, A.M.; Tyner, K.M.; Clogston, J.D.; MacCuspie, R.I.; Guha, S.; Zachariah, M.R.; Hackley, V.A. Tumor necrosis factor interaction with gold nanoparticles. Nanoscale 2012, 4, 3208–3217. [Google Scholar] [CrossRef]
- McAdams, R.M.; McPherson, R.J.; Dabestani, N.M.; Gleason, C.A.; Juul, S.E. Left ventricular hypertrophy is prevalent in Sprague-Dawley rats. Comp. Med. 2010, 60, 357–363. [Google Scholar] [PubMed]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef] [PubMed]
- Chaicherd, S.; Killingsworth, M.C.; Pissuwan, D. Toxicity of gold nanoparticles in a commercial dietary supplement drink on connective tissue fibroblast cells. SN Appl. Sci. 2019, 1, 336. [Google Scholar] [CrossRef] [Green Version]
- Milanezi, F.G.; Meireles, L.M.; de Christo Scherer, M.M.; de Oliveira, J.P.; da Silva, A.R.; de Araujo, M.L.; Endringer, D.C.; Fronza, M.; Guimarães, M.; Scherer, R. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with quercetin. Saudi Pharm. J. 2019, 27, 968–974. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Danielewski, M.; Kucharska, A.Z.; Matuszewska, A.; Rapak, A.; Gomułkiewicz, A.; Dzimira, S.; Dzięgiel, P.; Nowak, B.; Trocha, M.; Magdalan, J.; et al. Cornelian cherry (Cornus mas L.) iridoid and anthocyanin extract enhances PPAR-α, PPAR-γ expression and reduces I/M ratio in aorta, increases LXR-α expression and alters adipokines and triglycerides levels in cholesterol-rich diet rabbit model. Nutrients 2021, 13, 3621. [Google Scholar] [CrossRef]
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological behaviour of gold nanoparticles on various models: Influence of physicochemical properties and other factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, A.; Bundschuh, M.; Klingelhofer, D.; Groneberg, A. Gold nanoparticles: Recent aspects for human toxicology. J. Occup. Med. Toxicol. 2013, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
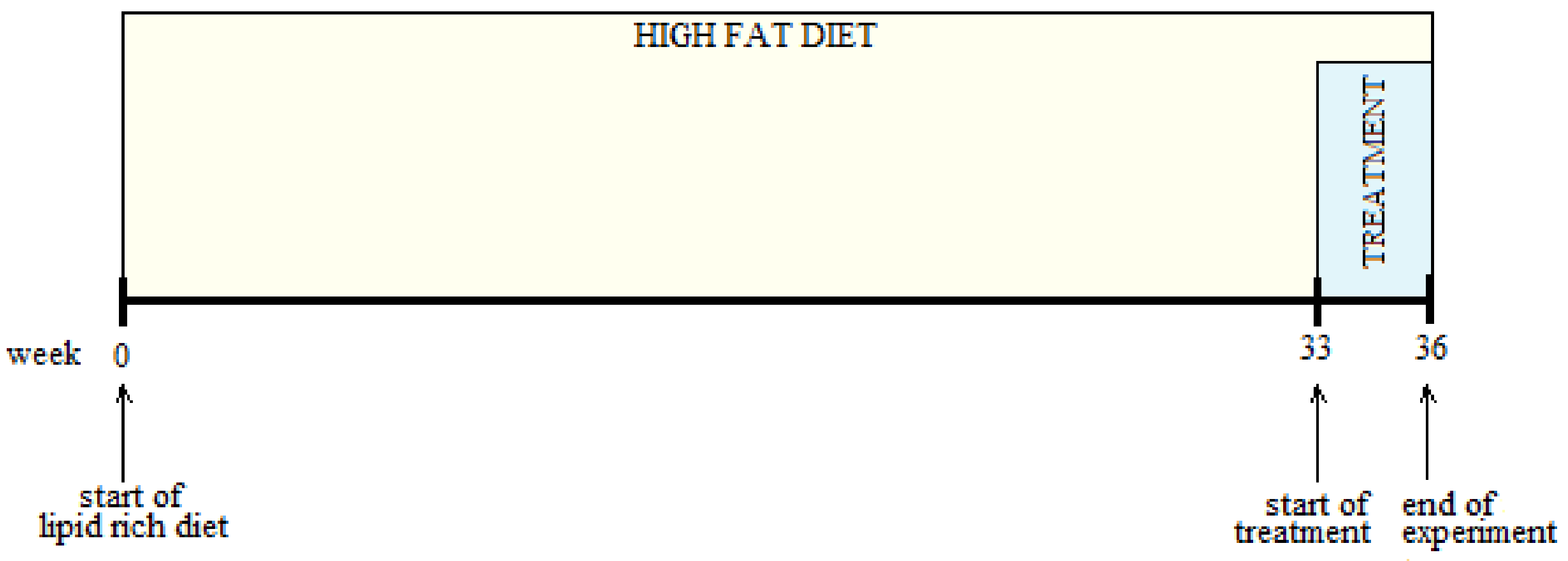



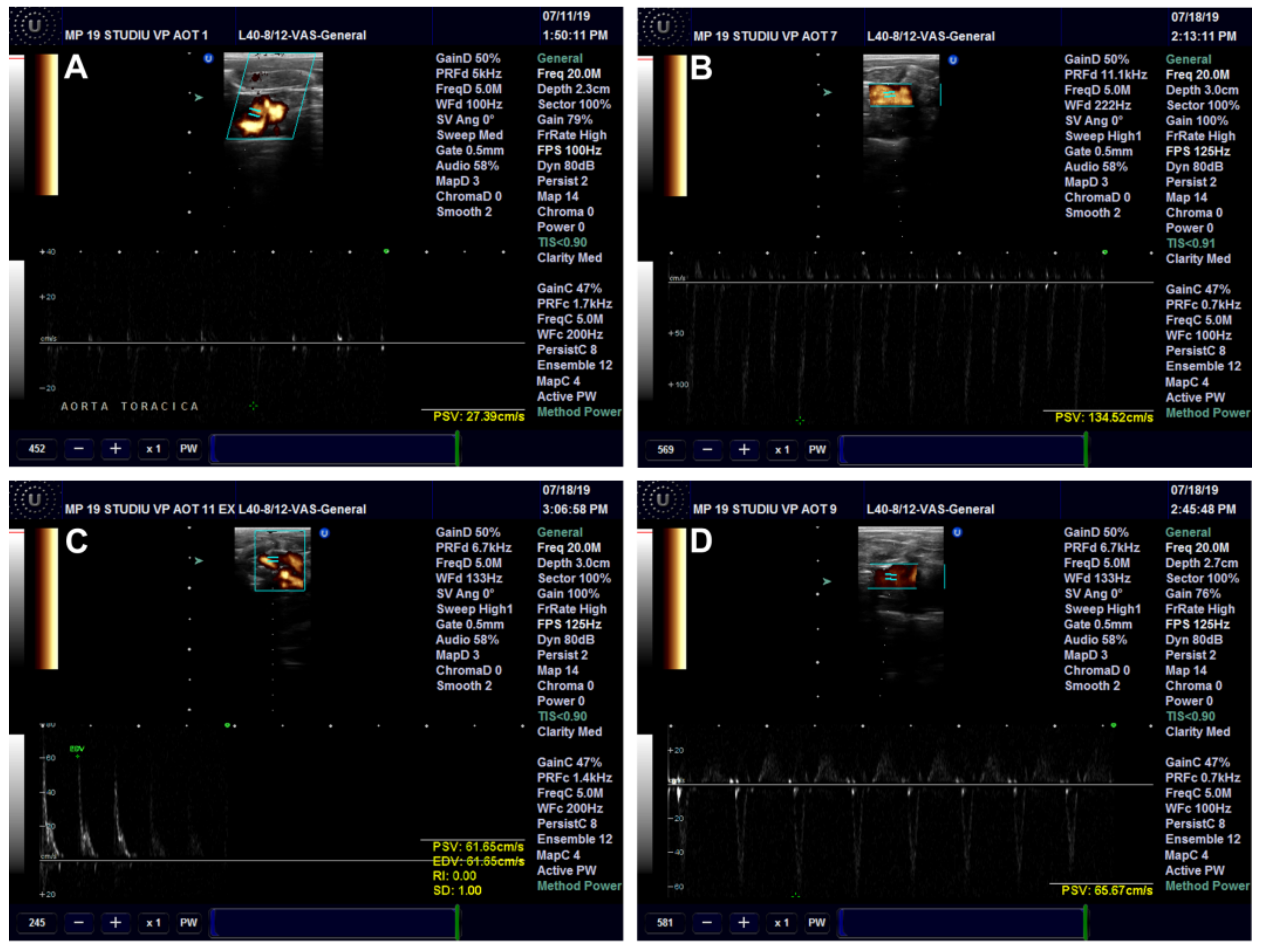
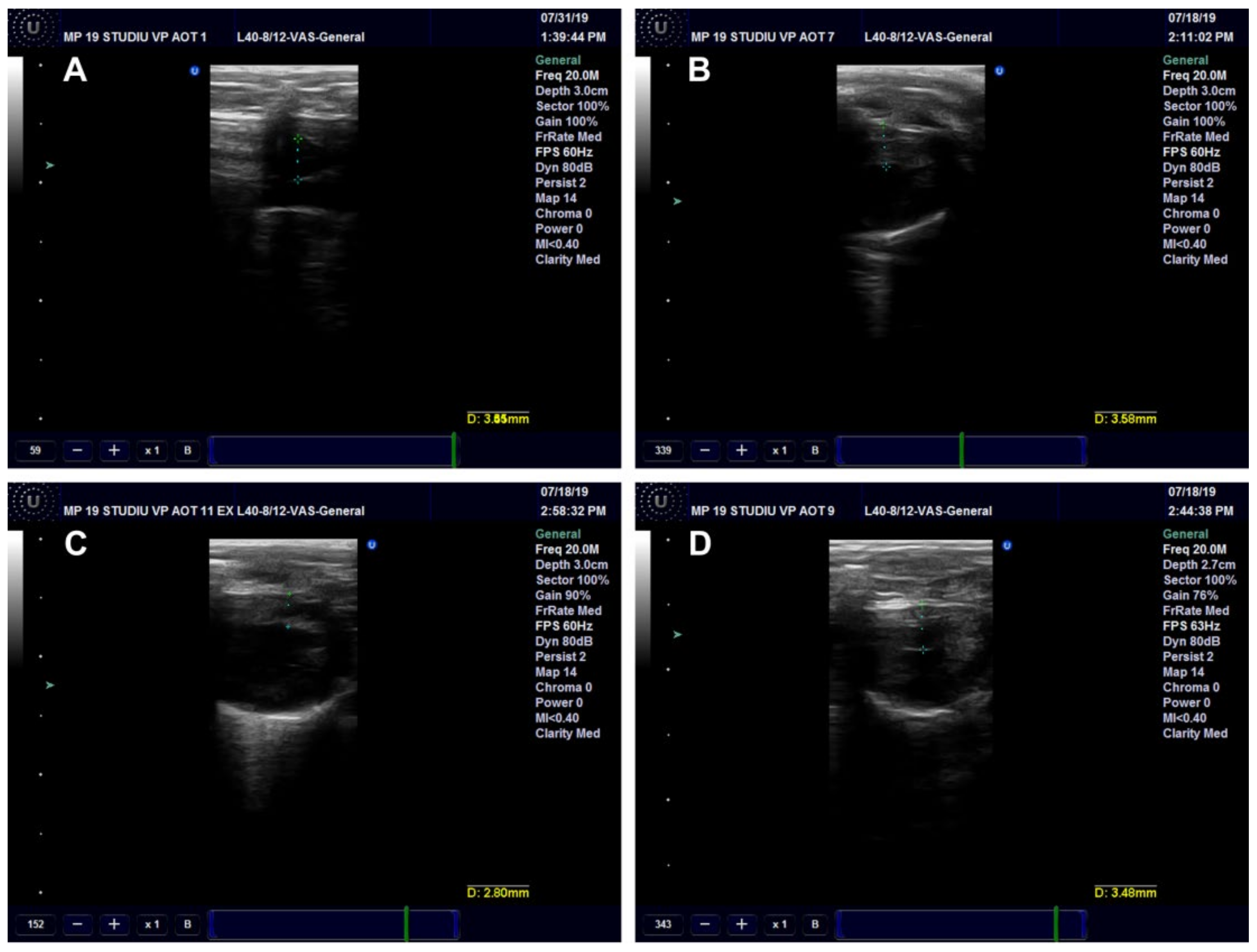

| Serum Parameter | Control | HFD | HFD + CM | HFD + AuNPsCM |
|---|---|---|---|---|
| CRP | 1.67 ± 0.21 | 1.46 ± 0.18 | 1.01 ± 0.25 c,d | 0.42 ± 0.17 c,e,g |
| Triglycerides | 136.30 ± 9.25 | 266.01 ± 31.25 c | 151.00 ± 38.56 e | 81.57 ± 32.82 a,e,f |
| Cholesterol | 61.86 ± 4.05 | 71.83 ± 9.78 | 80.43 ± 17.61 | 84.57 ± 15.03 a |
| HDL | 45.39 ± 3.60 | 57.52 ± 6.55 | 56.00 ± 10.29 | 62.24 ± 13.37 a |
| LDL | 7.48 ± 0.60 | 10.63 ± 1.85 a | 13.00 ± 3.16 c | 12.43 ± 1.61 c |
| Gamma-GT | 1.28 ± 0.48 | 1.71 ± 0.48 | 1.42 ± 0.53 | 1.71 ± 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, R.; Mitrea, D.-R.; Florea, A.; Chiş, I.-C.; Suciu, Ş.; David, L.; Moldovan, B.E.; Mureşan, L.E.; Lenghel, M.; Ungur, R.A.; et al. Effects of Gold Nanoparticles Functionalized with Bioactive Compounds from Cornus mas Fruit on Aorta Ultrastructural and Biochemical Changes in Rats on a Hyperlipid Diet—A Preliminary Study. Antioxidants 2022, 11, 1343. https://doi.org/10.3390/antiox11071343
Moldovan R, Mitrea D-R, Florea A, Chiş I-C, Suciu Ş, David L, Moldovan BE, Mureşan LE, Lenghel M, Ungur RA, et al. Effects of Gold Nanoparticles Functionalized with Bioactive Compounds from Cornus mas Fruit on Aorta Ultrastructural and Biochemical Changes in Rats on a Hyperlipid Diet—A Preliminary Study. Antioxidants. 2022; 11(7):1343. https://doi.org/10.3390/antiox11071343
Chicago/Turabian StyleMoldovan, Remus, Daniela-Rodica Mitrea, Adrian Florea, Irina-Camelia Chiş, Şoimiţa Suciu, Luminiţa David, Bianca Elena Moldovan, Laura Elena Mureşan, Manuela Lenghel, Rodica Ana Ungur, and et al. 2022. "Effects of Gold Nanoparticles Functionalized with Bioactive Compounds from Cornus mas Fruit on Aorta Ultrastructural and Biochemical Changes in Rats on a Hyperlipid Diet—A Preliminary Study" Antioxidants 11, no. 7: 1343. https://doi.org/10.3390/antiox11071343
APA StyleMoldovan, R., Mitrea, D.-R., Florea, A., Chiş, I.-C., Suciu, Ş., David, L., Moldovan, B. E., Mureşan, L. E., Lenghel, M., Ungur, R. A., Opriş, R. V., Decea, N., & Clichici, S. V. (2022). Effects of Gold Nanoparticles Functionalized with Bioactive Compounds from Cornus mas Fruit on Aorta Ultrastructural and Biochemical Changes in Rats on a Hyperlipid Diet—A Preliminary Study. Antioxidants, 11(7), 1343. https://doi.org/10.3390/antiox11071343







