The Synergetic Effect of Egyptian Portulaca oleracea L. (Purslane) and Cichorium intybus L. (Chicory) Extracts against Glucocorticoid-Induced Testicular Toxicity in Rats through Attenuation of Oxidative Reactions and Autophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Preparation of the P. oleracea and C. intybus Extracts
2.3.1. Preparation of P. oleracea (Purslane) Ethanolic Extract (PEE)
2.3.2. Preparation of C. Intybus (Chicory) Water Extract (CWE)
2.4. The In Vitro Study
2.4.1. Phytochemical Contents, Mineral Analysis, and Vitamins
2.4.2. HPLC Analysis for Phenolic Compounds Profile
2.4.3. In Vitro Antioxidant Activities of PEE, CWE and Their Combination
2.5. Experimental Design
2.5.1. Animals
2.5.2. Induction of Male Infertility and Treatments
2.5.3. Blood and Testicular Tissues Collection
2.6. Testicular Seminal and Biochemical Analysis
2.6.1. Seminal Analysis
2.6.2. Hormone Measurements
2.6.3. Fructose Levels and α-Mannosidase Activity
2.6.4. Glycemic Markers
2.6.5. Oxidative Stress Markers
2.6.6. ER Stress and Autophagy Regulators by ELISA Technique
2.7. Molecular Analyses
2.7.1. Quantitative Real-Time Reverse Transcription PCR Analysis (qRT-PCR)
2.7.2. Western Blot Analysis
2.8. Combination Index Analysis
2.9. Histopathological Study
2.10. Statistical Analysis
3. Results
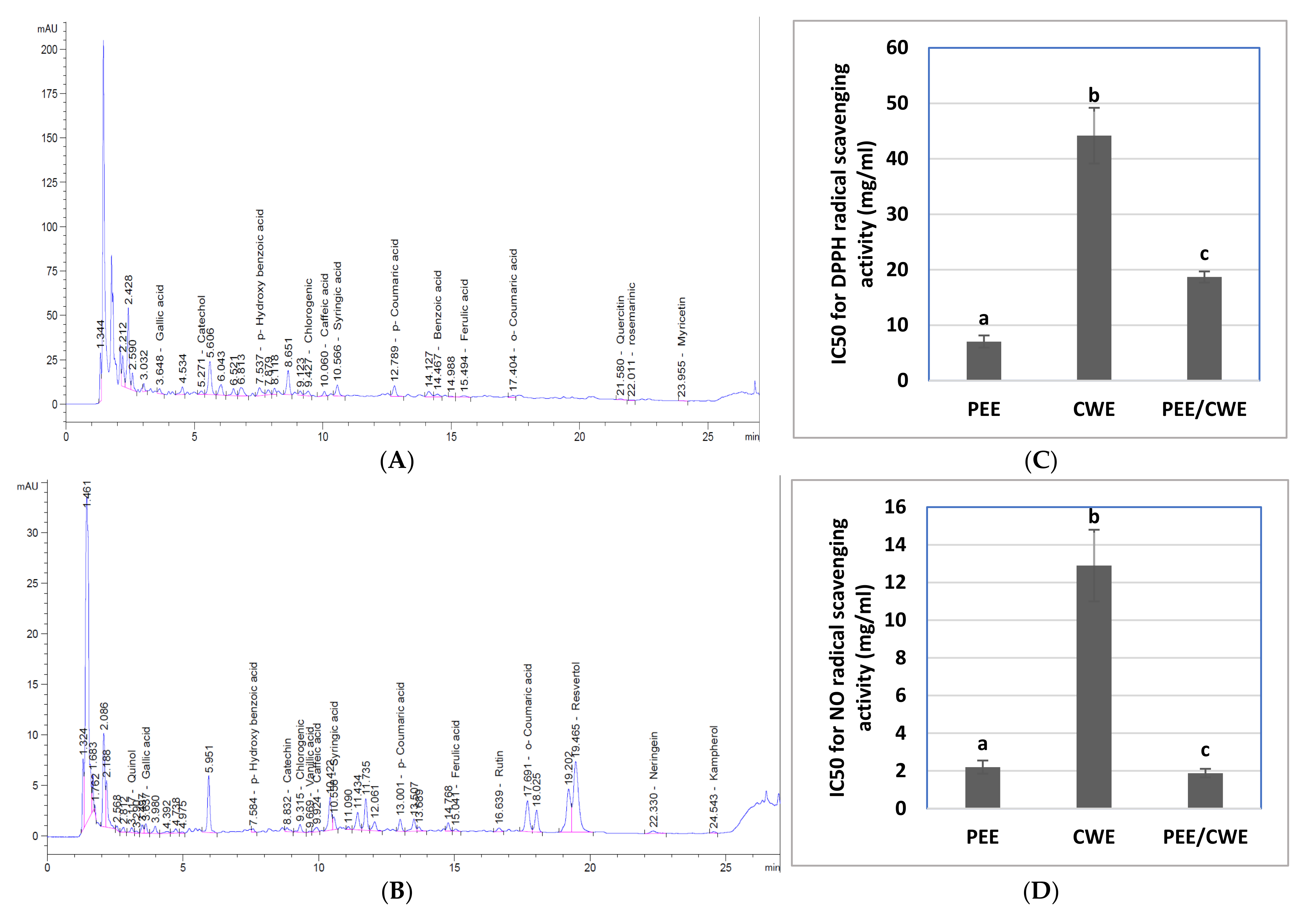
3.1. Characterization of PEE and CWE
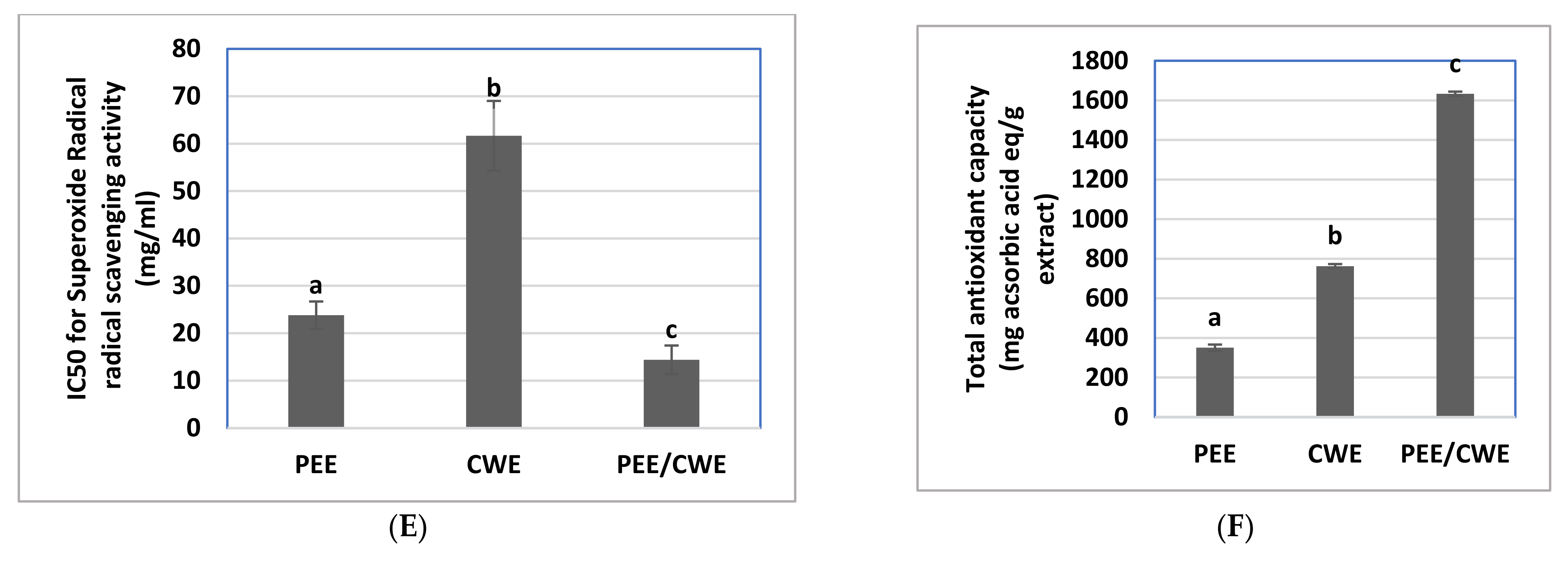
3.2. In Vitro Antioxidant Properties of PEE, CWE and Their Combination
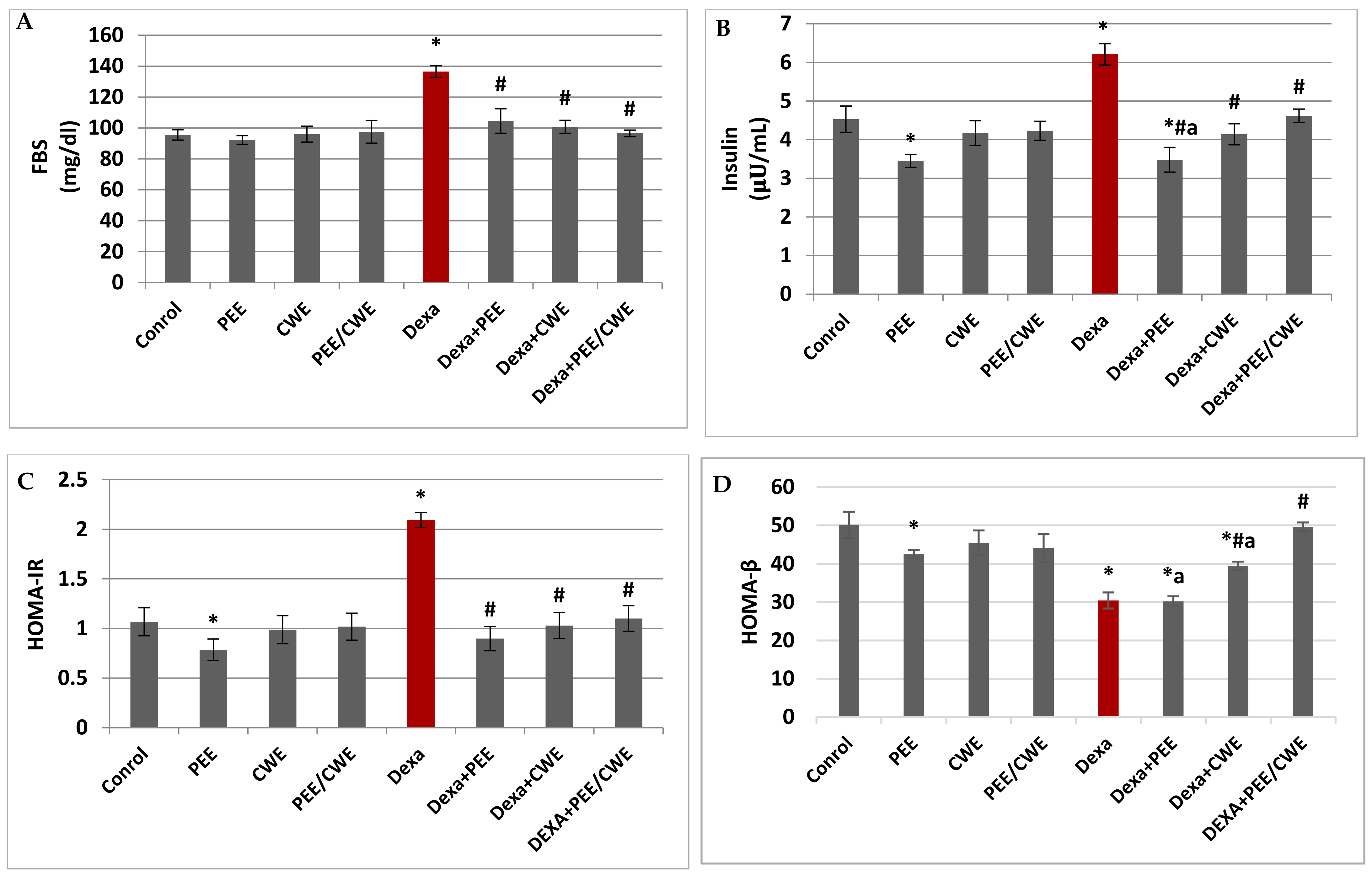
3.3. The Effects of Different Treatments on Semen Quality, Fructose Level, α-Mannosidase Activity, and Hormonal Levels
3.4. The Effects of Different Treatments on Testicular Oxidative Stress Markers
3.5. The Effects of Different Treatments on Glycemic Markers
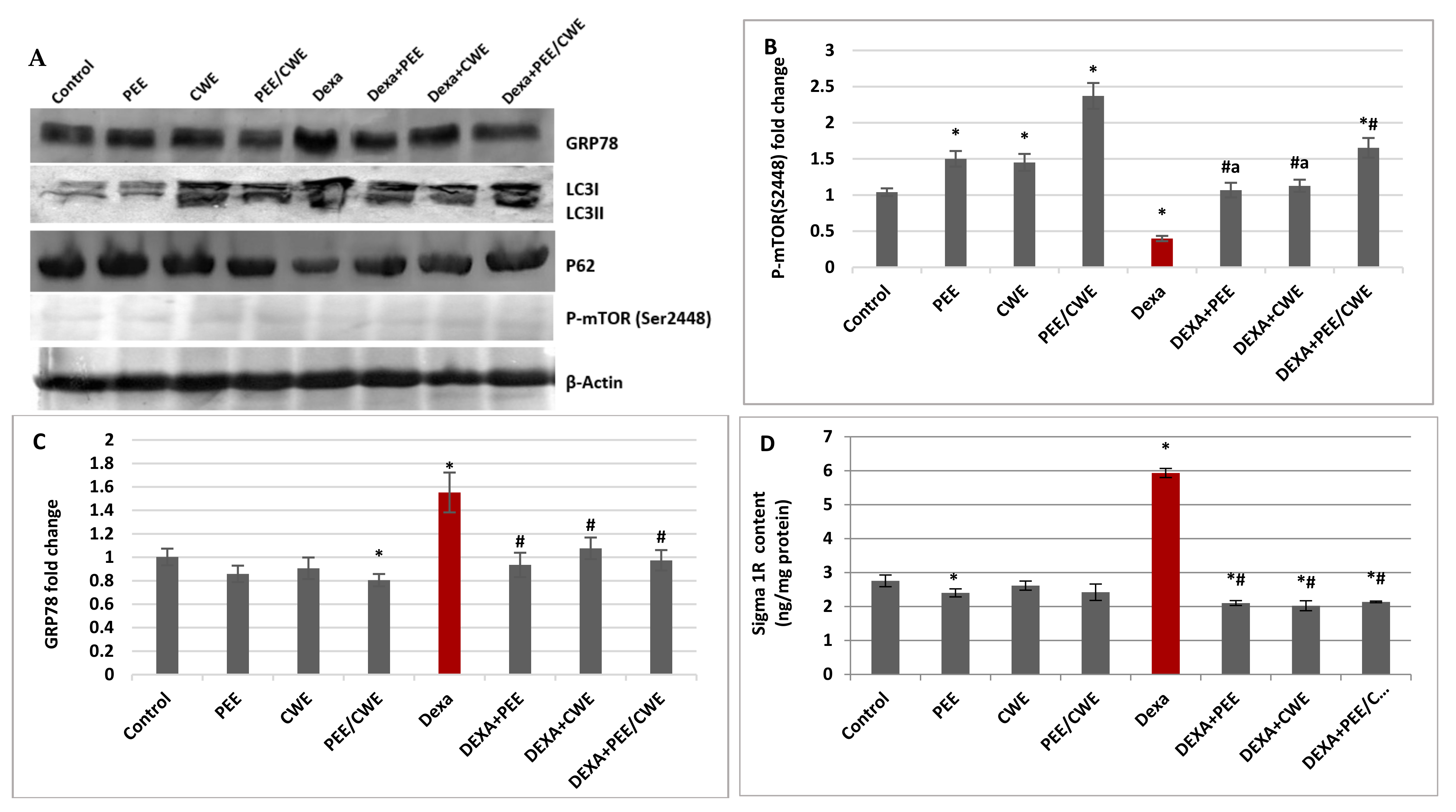
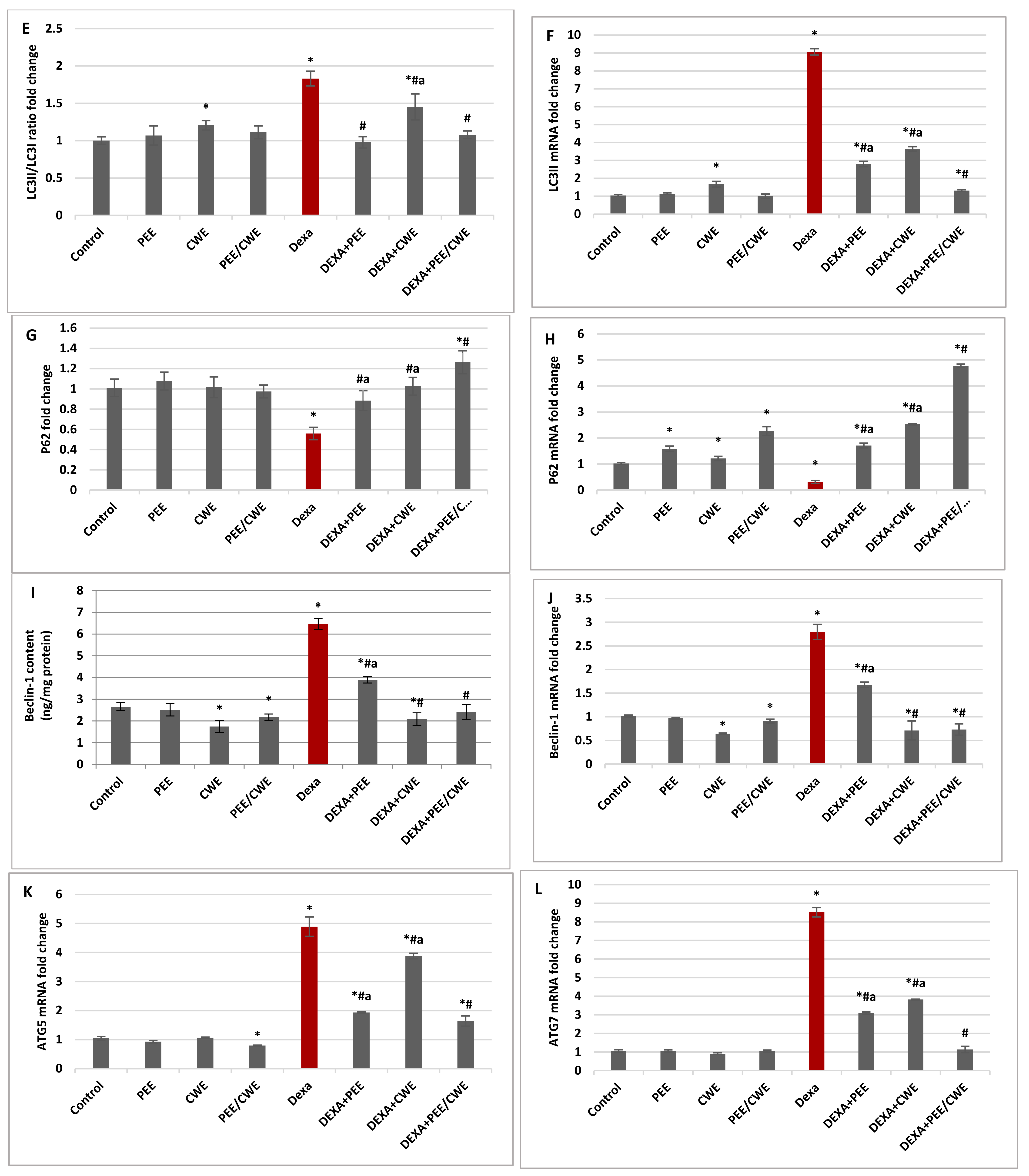
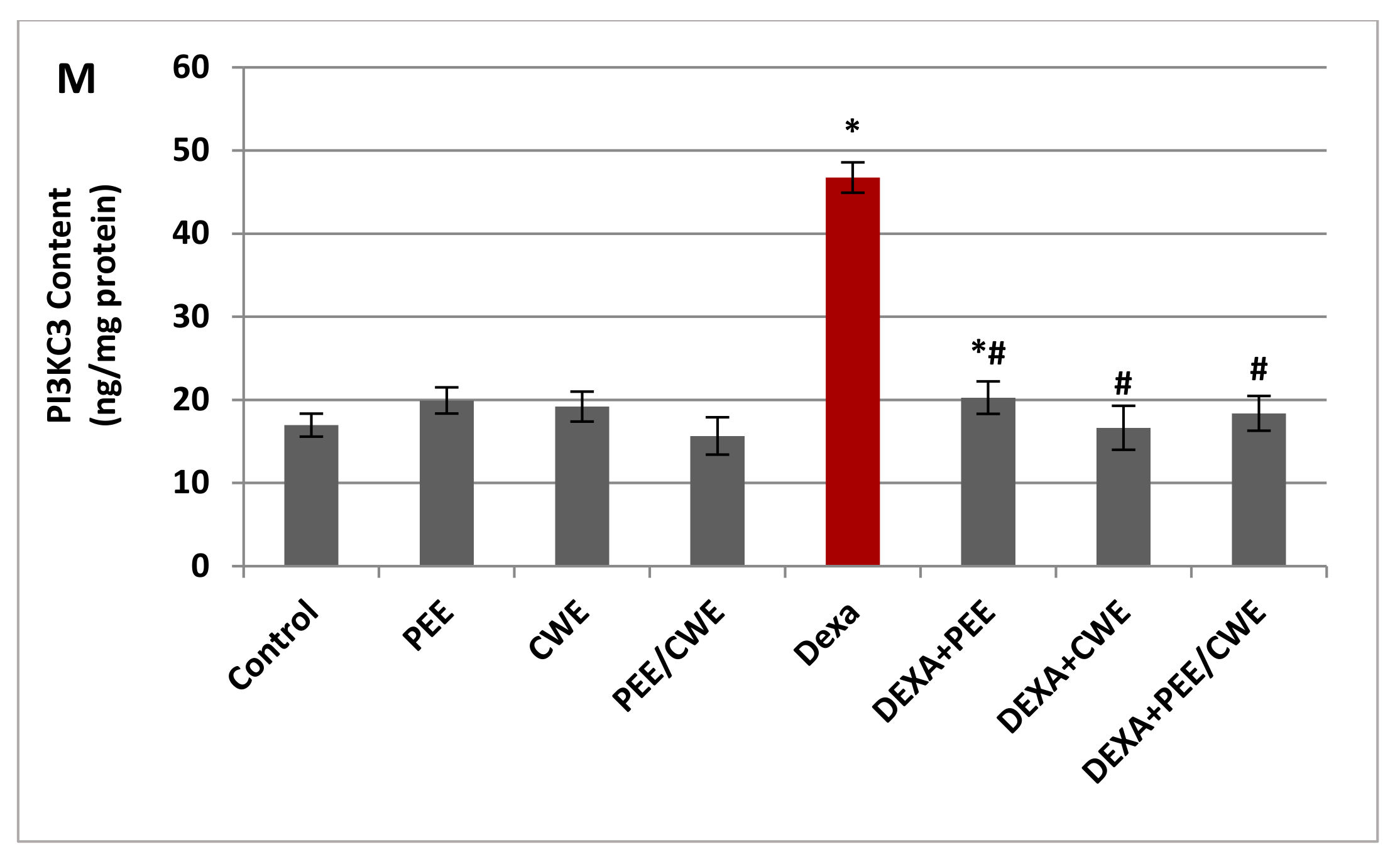
3.6. The Effects of Different Treatments on ER Stress Markers and Autophagy Regulators
3.7. Histological Changes and Morphometric Analysis in Testicular Tissues of Different Experimental Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility. Int. J. Mol. Sci. 2020, 21, 1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Madziyire, M.G.; Magwali, T.L.; Chikwasha, V.; Mhlanga, T. The causes of infertility in women presenting to gynaecology clinics in Harare, Zimbabwe; a cross sectional study. Fertil. Res. Pract. 2021, 7, 1. [Google Scholar] [CrossRef]
- Sharma, A. Male infertility; evidences, risk factors, causes, diagnosis and management in human. Ann. Clin. Lab. Res. 2017, 5, 188. [Google Scholar] [CrossRef]
- Ventimiglia, E.; Pozzi, E.; Capogrosso, P.; Boeri, L.; Alfano, M.; Cazzaniga, W.; Matloob, R.; Abbate, C.; Viganò, P.; Montorsi, F.; et al. Extensive Assessment of Underlying Etiological Factors in Primary Infertile Men Reduces the Proportion of Men With Idiopathic Infertility. Front. Endocrinol. 2021, 12, 801125. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires—A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710. [Google Scholar] [CrossRef]
- Orazizadeh, M.; Khorsandi, L.; Hashemitabar, M. Toxic effects of dexamethasone on mouse testicular germ cells. Andrologia 2010, 42, 247–253. [Google Scholar] [CrossRef]
- Hanafy, A.; Khalil, H. Influence of chronic dexamethasone administration on reproductive parameters and semen traits in male of Japanese quail. Asian J. Poult. Sci. 2015, 9, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Sadi-Guettaf, H.; Bekkouche, F.H. Dexamethasone: Impact on testicular activity. Int. J. Med. Health Sci. 2014, 8, 759–762. [Google Scholar]
- Sadeghzadeh, F.; Mehranjani, M.S.; Mahmoodi, M. Vitamin C ameliorates the adverse effects of dexamethasone on sperm motility, testosterone level, and spermatogenesis indexes in mice. Hum. Exp. Toxicol. 2019, 38, 409–418. [Google Scholar] [CrossRef]
- Chung, Y.C.; Tzeng, C.Y.; Chen, Y.I.; Chang, S.W.; Hsu, T.H.; Ho, W.J.; Kuo, Y.H.; Hung, P.H.; Chang, S.L. Improving insulin resistance with Antrodia cinnamomea mycelium powder to induce a hypoglycemic effect in dexamethasone-induced insulin-resistant rats. Mol. Med. Rep. 2018, 17, 3260–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severino, C.; Brizzi, P.; Solinas, A.; Secchi, G.; Maioli, M.; Tonolo, G. Low-dose dexamethasone in the rat: A model to study insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E367–E373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicod, N.; Giusti, V.; Besse, C.; Tappy, L. Metabolic Adaptations to Dexamethasone-Induced Insulin Resistance in Healthy Volunteers. Obes. Res. 2003, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Danek, J. Effect of dexamethasone on the changes of semen quality induced by endotoxin in stallion. Bull. Vet. Inst. Puławy 2008, 52. [Google Scholar]
- Bjelaković, G.; Beninati, S.; Pavlović, D.; Kocić, G.; Jevtović, T.; Kamenov, Β.; Šaranac, L.; Bjelaković, B.; Stojanović, I.; Bašić, J. Glucocorticoids and oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2007, 18, 115–128. [Google Scholar] [CrossRef]
- Babu, H.; Rengarajan, T.; Periyasamy, B. Dexamethasone induced alterations in lipid peroxidation, antioxidants, membrane bound ATPase in wistar albino rats. Int. J. Pharm. Pharm. Sci. 2012, 4, 73771810. [Google Scholar]
- Saleh, S.R.; Attia, R.; Ghareeb, D.A. The Ameliorating Effect of Berberine-Rich Fraction against Gossypol-Induced Testicular Inflammation and Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 1056173. [Google Scholar] [CrossRef]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The Impact of Oxidative Stress in Male Infertility. Front. Mol. Biosci. 2022, 8, 799294. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Victor, P.; Sarada, D.; Ramkumar, K.M. Crosstalk between endoplasmic reticulum stress and oxidative stress: Focus on protein disulfide isomerase and endoplasmic reticulum oxidase 1. Eur. J. Pharmacol. 2021, 892, 173749. [Google Scholar] [CrossRef] [PubMed]
- Karna, K.K.; Shin, Y.S.; Choi, B.R.; Kim, H.K.; Park, J.K. The Role of Endoplasmic Reticulum Stress Response in Male Reproductive Physiology and Pathology: A Review. World J. Mens. Health 2020, 38, 484–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Song, W.; Xu, D.; Chen, X.; Li, X.; Zhao, Y. Autophagy Induced by ROS Aggravates Testis Oxidative Damage in Diabetes via Breaking the Feedforward Loop Linking p62 and Nrf2. Oxidative Med. Cell. Longev. 2020, 2020, 7156579. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yin, Q.; Wei, D.; Yang, Z.; Du, Y.; Ma, Y. Autophagy in male reproduction. Syst. Biol. Reprod. Med. 2019, 65, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ni, B.; Tian, Z.-q.; Yang, F.; Liao, W.-g.; Gao, Y.-q. Regulatory effects of autophagy on spermatogenesis. Biol. Reprod. 2017, 96, 525–530. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liao, J.; Chen, K.; Javed, M.T.; Qiao, N.; Zeng, Q.; Liu, B.; Yi, J.; Tang, Z.; et al. Long-term copper exposure promotes apoptosis and autophagy by inducing oxidative stress in pig testis. Environ. Sci. Pollut. Res. 2021, 28, 55140–55153. [Google Scholar] [CrossRef]
- Ritchie, C.; Ko, E.Y. Oxidative stress in the pathophysiology of male infertility. Andrologia 2021, 53, e13581. [Google Scholar] [CrossRef]
- Hasona, N.A. Grape seed extract attenuates dexamethasone-induced testicular and thyroid dysfunction in male albino rats. Andrologia 2018, 50, e13002. [Google Scholar] [CrossRef]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]
- Tortora, F.; Notariale, R.; Maresca, V.; Good, K.V.; Sorbo, S.; Basile, A.; Piscopo, M.; Manna, C. Phenol-Rich Feijoa sellowiana (Pineapple Guava) Extracts Protect Human Red Blood Cells from Mercury-Induced Cellular Toxicity. Antioxidants 2019, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Notariale, R.; Perrone, P.; Mele, L.; Lettieri, G.; Piscopo, M.; Manna, C. Olive Oil Phenols Prevent Mercury-Induced Phosphatidylserine Exposure and Morphological Changes in Human Erythrocytes Regardless of Their Different Scavenging Activity. Int. J. Mol. Sci. 2022, 23, 5693. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Lee, Y.J.; Lee, S.M.; Yoon, J.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Portulaca oleracea ameliorates diabetic vascular inflammation and endothelial dysfunction in db/db mice. Evid. Based Complementary Altern. Med. 2012, 2012, 741824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandagopal, S.; Kumari, B.R. Phytochemical and antibacterial studies of Chicory (Cichorium intybus L.)-A multipurpose medicinal plant. Adv. Biol. Res. 2007, 1, 17–21. [Google Scholar]
- Okafor, I.; Ezejindu, D. Phytochemical studies on Portulaca oleracea (purslane) plant. GJBAHS 2014, 3, 132–136. [Google Scholar]
- Varotto, S.; Lucchin, M.; Parrini, P. Immature embryos culture in Italian red chicory. Plant Cell Tissue Organ Cult. 2000, 62, 75–77. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical composition and nutritive benefits of chicory (Cichorium intybus) as an ideal complementary and/or alternative livestock feed supplement. Sci. World J. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nehir El, S.; Karakaya, S. Radical scavenging and iron-chelating activities of some greens used as traditional dishes in Mediterranean diet. Int. J. Food Sci. Nutr. 2004, 55, 67–74. [Google Scholar] [CrossRef]
- Dorostghoal, M.; Seyyednejad, S.M.; Nejad, M.N.T. Beneficial effects of Cichorium intybus L. extract on oxidative status and reproductive parameters in male Wistar rats: An experimental study. Int. J. Reprod. BioMed. 2019, 17, 425. [Google Scholar] [CrossRef]
- Ahmadi, M.; Dastyar, N.; Eftekhar Vaghefi, S.H. Effect of Aqueous Extract of Chicory (Cichorium intybus L.) Root on Sperm Parameters, Testicular Tissue and Testosterone in Adult Mice Exposed to Diazinon. Egypt. J. Vet. Sci. 2021, 52, 267–274. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Liu, Y.; Xia, Y.; Tang, T. Analysis of flavonoids in Portulaca oleracea L. by UV–vis spectrophotometry with comparative study on different extraction technologies. Food Anal. Methods 2010, 3, 90–97. [Google Scholar] [CrossRef]
- Taga, M.S.; Miller, E.; Pratt, D. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency Office of Science and Technology. Method 200.7: Trace Elements in Water, Solids, and Biosolids by Inductively Coupled Plasma-Atomic Emission Spectrometry. Revision 5. EPA-821-R-01-010. 2001; U.S. Environmental Protection Agency Office of Science and Technology: Washington, DC, USA, 2001.
- U.S. Environmental Protection Agency. Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry. Revision 4.4; EPA600/R-94-111. 1994; Environmental Monitoring Systems Laboratory Office of Research and Development U. S. Environmental Protection Agency: Cincinnati, OH, USA, 1994.
- EPA. Method 6010c Inductively Coupled Plasma-Atomic Emission Spectrometry; EPA United States Environmental Protection Agency: Washington, DC, USA, 2007.
- AOAC. International Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemist: Arlington, VA, USA, 1990. [Google Scholar]
- Abushita, A.A.; Hebshi, E.A.; Daood, H.G.; Biacs, P.A. Determination of antioxidant vitamins in tomatoes. Food Chem. 1997, 60, 207–212. [Google Scholar] [CrossRef]
- Ismail, A.; Cheah, S.F. Determination of vitamin C, β-carotene and riboflavin contents in five green vegetables organically and conventionally grown. Malays. J. Nutr. 2003, 9, 31–39. [Google Scholar]
- Kozlov, É.; Solunina, I.; Lyubareva, M.; Nadtochii, M. HPLC determination of vitamins A, D, and E in multivitamin compositions. Pharm. Chem. J. 2003, 37, 560–562. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Manzocco, L.; Anese, M.; Nicoli, M.C. Antioxidant Properties of Tea Extracts as Affected by Processing. LWT Food Sci. Technol. 1998, 31, 694–698. [Google Scholar] [CrossRef]
- Abiola, T.; Dc, D.; Oj, A.; Oa, S. Assessment of the Antidiabetic Potential of the Ethanolic Extract of Date Palm (Phoenix Dactylifera) Seed in Alloxan-Induced Diabetic Rats. J. Diabetes Metab. 2017, 9, 1–9. [Google Scholar]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droy-Lefaix, M.T.; Packer, L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef]
- Jan, S.; Khan, M.R.; Rashid, U.; Bokhari, J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of monotheca buxifolia fruit. Osong Public Health Res. Perspect. 2013, 4, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Baradaran Rahimi, V.; Rakhshandeh, H.; Raucci, F.; Buono, B.; Shirazinia, R.; Samzadeh Kermani, A.; Maione, F.; Mascolo, N.; Askari, V.R. Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury. Molecules 2019, 24, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, Y.S.; Lebda, M.A.; Hassinin, M.; Neoman, S.A. Chicory (Cichorium intybus L.) Root Extract Regulates the Oxidative Status and Antioxidant Gene Transcripts in CCl4-Induced Hepatotoxicity. PLoS ONE 2015, 10, e0121549. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, L.; Mirhoseini, M.; Mohamadpour, M.; Orazizadeh, M.; Khaghani, S. Effect of curcumin on dexamethasone-induced testicular toxicity in mice. Pharm. Biol. 2013, 51, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.C.; Mongar, J.; Gomperts, B. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature 1973, 245, 249–251. [Google Scholar] [CrossRef]
- Tulsiani, D.R.; Skudlarek, M.D.; Orgebin-Crist, M.C. Novel alpha-D-mannosidase of rat sperm plasma membranes: Characterization and potential role in sperm-egg interactions. J. Cell Biol. 1989, 109, 1257–1267. [Google Scholar] [CrossRef]
- Onishi, Y.; Hayashi, T.; Sato, K.K.; Ogihara, T.; Kuzuya, N.; Anai, M.; Tsukuda, K.; Boyko, E.J.; Fujimoto, W.Y.; Kikuchi, M. Fasting tests of insulin secretion and sensitivity predict future prediabetes in Japanese with normal glucose tolerance. J. Diabetes Investig. 2010, 1, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Tappel, A.L.; Zalkin, H. Inhibition of lipid peroxidation in mitochondria by vitamin E. Arch. Biochem. Biophys. 1959, 80, 333–336. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Transl. Res. 1967, 70, 158–169. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Maher, A.M.; Saleh, S.R.; Elguindy, N.M.; Hashem, H.M.; Yacout, G.A. Exogenous melatonin restrains neuroinflammation in high fat diet induced diabetic rats through attenuating indoleamine 2,3-dioxygenase 1 expression. Life Sci. 2020, 247, 117427. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, E.; Cordero, E.M.; Cortés, B.I.; Palominos, S.; Parra, P.; Mella, T.; Henrríquez, C.; Salazar, N.; Monasterio, G.; Cafferata, E.A.; et al. Aggregatibacter actinomycetemcomitans Induces Autophagy in Human Junctional Epithelium Keratinocytes. Cells 2020, 9, 1221. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, X.; Zhao, X.; Kong, C.; Li, Z.; Liu, Y.; Jiang, X.; Zhang, X. The autophagy-related genes Beclin1 and LC3 in the prognosis of pancreatic cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 2989–2996. [Google Scholar]
- Hu, Y.; Wen, Z.; Liu, S.; Cai, Y.; Guo, J.; Xu, Y.; Lin, D.; Zhu, J.; Li, D.; Chen, X. Ibrutinib Suppresses Intracellular Mycobacterium Tuberculosis Growth by Inducing Macrophage Autophagy. J. Infect. 2020, 80, e19–e26. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-R.; Li, L.; Pan, W. Dietary soy and tea combinations for prevention of breast and prostate cancers by targeting metabolic syndrome elements in mice. Am. J. Clin. Nutr. 2007, 86, s882–s888. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Abdelrahman, S.A.; El-Kersh, M.A.L.; Mogahed, F.A.K.; Talaat, I.M.; Habashy, N.H. The synergistic hepatoprotective potential of Beta vulgaris juice and 2,3- dimercaptosuccinic acid in lead-intoxicated rats via improving the hepatic oxidative and inflammatory stress. BMC Complement Med. Ther. 2020, 20, 268. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Sadeghi, N.; Erfani-Majd, N.; Tavalaee, M.; Tabandeh, M.R.; Drevet, J.R.; Nasr-Esfahani, M.H. Signs of ROS-Associated Autophagy in Testis and Sperm in a Rat Model of Varicocele. Oxidative Med. Cell. Longev. 2020, 2020, 5140383. [Google Scholar] [CrossRef]
- Yang, R.; Yu, Y. Glucocorticoids are double-edged sword in the treatment of COVID-19 and cancers. Int. J. Biol. Sci. 2021, 17, 1530–1537. [Google Scholar] [CrossRef]
- Jha, S.; Marko, J.; El-Maouche, D.; Mallappa, A.; Veeraraghavan, P. SUN-371 Successful Induction of Fertility with Low-Dose Dexamethasone in a Patient with Congenital Adrenal Hyperplasia and Testicular Adrenal Rest Tumor. J. Endocr. Soc. 2019, 3 (Suppl. S1), SUN-371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, A.G.; Mansour, A.A.; Ahmed, J.H. Effect of exogenous glucocorticoids on male hypogonadism. Biomed. Rep. 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of Follicle-Stimulating Hormone in Spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hu, G.; Huang, B.; Zhuo, D.; Xu, Y.; Li, H.; Zhan, X.; Ge, R.-S.; Xu, Y. Dexamethasone suppresses the differentiation of stem Leydig cells in rats in vitro. BMC Pharmacol. Toxicol. 2019, 20, 32. [Google Scholar] [CrossRef]
- Walker, W.H.; Cheng, J. FSH and testosterone signaling in Sertoli cells. Reproduction 2005, 130, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids, stress, and fertility. Minerva. Endocrinol. 2010, 35, 109–125. [Google Scholar]
- Drobnis, E.Z.; Nangia, A.K. Immunosuppressants and male reproduction. Impacts Medicat. Male Fertil. 2017, 1034, 179–210. [Google Scholar]
- Mukherjee, A.; Haldar, C.; Vishwas, D.K. Melatonin prevents dexamethasone-induced testicular oxidative stress and germ cell apoptosis in golden hamster, Mesocricetus auratus. Andrologia 2015, 47, 920–931. [Google Scholar] [CrossRef]
- Yun, H.J.; Lee, J.Y.; Kim, M.H. Prenatal exposure to dexamethasone disturbs sex-determining gene expression and fetal testosterone production in male embryos. Biochem. Biophys. Res. Commun. 2016, 471, 149–155. [Google Scholar] [CrossRef]
- Ricardo, L. Male Accessory Glands and Sperm Function; IntechOpen Limited: London, UK, 2018. [Google Scholar]
- Dzugan, M.; Ksiazkiewicz, J. Activity of alpha- and beta-mannosidases in semen and reproductive organs of the drake. Reprod. Biol. 2009, 9, 25–37. [Google Scholar] [CrossRef]
- Srikanth, V.; Malini, T.; Govindarajulu, P.; Balasubramanian, K. Effects of ethanol ingestion on epididymal glycosidases and fertility in the rat. Int. J. Androl. 1998, 21, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia 2021, 53, e13577. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [Green Version]
- Shaban, N.Z.; Yehia, S.A.; Awad, D.; Shaban, S.Y.; Saleh, S.R. A Titanium (IV)-Dithiophenolate Complex and Its Chitosan Nanocomposite: Their Roles towards Rat Liver Injuries In Vivo and against Human Liver Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 11219. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Tappy, L.; Randin, D.; Vollenweider, P.; Vollenweider, L.; Paquot, N.; Scherrer, U.; Schneiter, P.; Nicod, P.; Jéquier, E. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J. Clin. Endocrinol. Metab. 1994, 79, 1063–1069. [Google Scholar]
- Ferris, H.A.; Kahn, C.R. New mechanisms of glucocorticoid-induced insulin resistance: Make no bones about it. J. Clin. Investig. 2012, 122, 3854–3857. [Google Scholar] [CrossRef]
- Burén, J.; Lai, Y.C.; Lundgren, M.; Eriksson, J.W.; Jensen, J. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch Biochem. Biophys. 2008, 474, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wego, M.T.; Poualeu Kamani, S.L.; Miaffo, D.; Nchouwet, M.L.; Kamanyi, A.; Wansi Ngnokam, S.L. Protective Effects of Aqueous Extract of Baillonella toxisperma Stem Bark on Dexamethasone-Induced Insulin Resistance in Rats. Adv. Pharmacol. Sci. 2019, 2019, 8075163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasieka, A.M.; Rafacho, A. Impact of Glucocorticoid Excess on Glucose Tolerance: Clinical and Preclinical Evidence. Metabolites 2016, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geer, E.B.; Islam, J.; Buettner, C. Mechanisms of glucocorticoid-induced insulin resistance: Focus on adipose tissue function and lipid metabolism. Endocrinol. Metab. Clin. North Am. 2014, 43, 75–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.S.; Chou, M.C.; Yeh, Y.C.; Yang, Y.H.; Lai, C.L.; Yen, C.F.; Liu, C.K.; Liao, Y.C. Plasma homocysteine levels and major depressive disorders in Alzheimer disease. Am. J. Geriatr. Psychiatry 2010, 18, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Weide, T.; Huber, T.B. Implications of autophagy for glomerular aging and disease. Cell Tissue Res. 2011, 343, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, L.; Geng, Y.; He, J.; Chen, X.; Xu, H.; Li, R.; Wang, Y.; Ding, Y.; Liu, X. Rapamycin inhibits spermatogenesis by changing the autophagy status through suppressing mechanistic target of rapamycin-p70S6 kinase in male rats. Mol. Med. Rep. 2017, 16, 4029–4037. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, I.; Espino, J.; Bejarano, I.; Gallardo-Soler, A.; Campo, M.; Salido, G.; Pariente, J.; Peña, F.; Tapia, J. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci. Rep. 2016, 6, 1–19. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Lin, S.; Wang, K.; Wang, H.; Liu, Z. Protective effect of naringenin against cadmium-induced testicular toxicity in male SD rats. J. Inorg. Biochem. 2021, 214, 111310. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, F.; Hu, L.; Zhang, J.; Chen, D.; Zhao, S. Inhibition of endoplasmic reticulum stress-related autophagy attenuates MCLR-induced apoptosis in zebrafish testis and mouse TM4 cells. Ecotoxicol. Environ. Saf. 2021, 221, 112438. [Google Scholar] [CrossRef]
- Duzgun, A.; Bedir, A.; Ozdemir, T.; Nar, R.; Kilinc, V.; Salis, O.; Alacam, H.; Gulten, S. Effect of dexamethasone on unfolded protein response genes (MTJ1, Grp78, Grp94, CHOP, HMOX-1) in HEp2 cell line. Indian J. Biochem. Biophys. 2013, 50, 505–510. [Google Scholar]
- Tesei, A.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Zanoni, M.; Paolillo, M.; Curti, D.; Rui, M.; Rossi, D.; et al. Sigma Receptors as Endoplasmic Reticulum Stress “Gatekeepers” and their Modulators as Emerging New Weapons in the Fight Against Cancer. Front. Pharmacol. 2018, 9, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zhu, L.; Liu, D.; Chi, T.; Ji, X.; Liu, P.; Yang, X.; Tian, X.; Zou, L. Sigma-1 receptor protects against endoplasmic reticulum stress-mediated apoptosis in mice with cerebral ischemia/reperfusion injury. Apoptosis 2019, 24, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zode, G.S.; Sharma, A.B.; Lin, X.; Searby, C.C.; Bugge, K.; Kim, G.H.; Clark, A.F.; Sheffield, V.C. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J. Clin. Investig. 2014, 124, 1956–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Xie, W.; Yin, D.; Luo, R.; Liu, M.; Guo, F. ATG5 and ATG7 induced autophagy interplays with UPR via PERK signaling. Cell Commun. Signal 2019, 17, 42. [Google Scholar] [CrossRef] [Green Version]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2503–2518. [Google Scholar] [CrossRef]
- Sun, Q.; Fan, W.; Zhong, Q. Regulation of Beclin 1 in autophagy. Autophagy 2009, 5, 713–716. [Google Scholar] [CrossRef] [Green Version]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Menon, M.B.; Dhamija, S. Beclin 1 Phosphorylation—At the Center of Autophagy Regulation. Front. Cell Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-Y.; Gertner, M.; Pontarelli, F.; Court-Vazquez, B.; Bennett, M.V.L.; Ofengeim, D.; Zukin, R.S. Global ischemia induces lysosomal-mediated degradation of mTOR and activation of autophagy in hippocampal neurons destined to die. Cell Death Differ. 2017, 24, 317–329. [Google Scholar] [CrossRef]
- Liang, X.-f.; Guan, X.-r. p62/SQSTM1: A potential molecular target for treatment of atherosclerosis. Front. Lab. Med. 2017, 1, 104–106. [Google Scholar] [CrossRef]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.R.; Masry, A.M.; Ghareeb, D.A.; Newairy, A.A.; Sheta, E.; Maher, A.M. Trichoderma reesei fungal degradation boosted the potentiality of date pit extract in fighting scopolamine-induced neurotoxicity in male rats. Sci. Rep. 2021, 11, 14872. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Z.; Zhang, D.; Li, H. Antioxidant activity of purslane extract and its inhibitory effect on the lipid and protein oxidation of rabbit meat patties during chilled storage. J. Sci. Food Agric. 2021, 101, 1953–1962. [Google Scholar] [CrossRef]
- Seif, M.M.; Madboli, A.-N.; Marrez, D.A.; Aboulthana, W.M.K. Hepato-Renal protective Effects of Egyptian Purslane Extract against Experimental Cadmium Toxicity in Rats with Special Emphasis on the Functional and Histopathological Changes. Toxicol. Rep. 2019, 6, 625–631. [Google Scholar] [CrossRef]
- Sicari, V.; Loizzo, M.R.; Tundis, R.; Mincione, A.; Pellicano, T.M. Portulaca oleracea L.(Purslane) extracts display antioxidant and hypoglycaemic effects. J. Appl. Bot. Food Qual. 2018, 91, 39–46. [Google Scholar]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Saleh, S.R.; Ghareeb, D.A.; Masoud, A.A.; Sheta, E.; Nabil, M.; Masoud, I.M.; Maher, A.M. Phoenix dactilyfera L. Pits Extract Restored Bone Homeostasis in Glucocorticoid-Induced Osteoporotic Animal Model through the Antioxidant Effect and Wnt5a Non-Canonical Signaling. Antioxidants 2022, 11, 508. [Google Scholar] [CrossRef]
- Wołonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postępy Hig. Med. Doświadczalnej 2016, 70, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Snaith, S.M.; Levvy, G.A. α-Mannosidase as a Zinc-dependent Enzyme. Nature 1968, 218, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.U.; Ali, M.E.; Rahman, M.M. Purslane Weed (Portulaca oleracea): A Prospective Plant Source of Nutrition, Omega-3 Fatty Acid, and Antioxidant Attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Khalaf, H.; El-Saadani, R.; El-Desouky, A.; Abdeldaiem, M.; Elmehy, M. Antioxidant and antimicrobial activity of gamma-irradiated chicory (Cichorium intybus L.) leaves and roots. J. Food Meas. Charact. 2018, 12, 1843–1851. [Google Scholar] [CrossRef]
- Li, G.Y.; Zheng, Y.X.; Sun, F.Z.; Huang, J.; Lou, M.M.; Gu, J.K.; Wang, J.H. In Silico Analysis and Experimental Validation of Active Compounds from Cichorium intybus L. Ameliorating Liver Injury. Int. J. Mol. Sci. 2015, 16, 22190–22204. [Google Scholar] [CrossRef] [Green Version]
- Esmaillzadeh, A.; Zakizadeh, E.; Faghihimani, E.; Gohari, M.; Jazayeri, S. The effect of purslane seeds on glycemic status and lipid profiles of persons with type 2 diabetes: A randomized controlled cross-over clinical trial. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2015, 20, 47. [Google Scholar]
- Nasimi Doost Azgomi, R.; Karimi, A.; Tutunchi, H.; Moini Jazani, A. A comprehensive mechanistic and therapeutic insight into the effect of chicory (Cichorium intybus) supplementation in diabetes mellitus: A systematic review of literature. Int. J. Clin. Pract. 2021, 75, e14945. [Google Scholar] [CrossRef]
- Nowrouzi, P.S.; Mazani, M.; Rezagholizadeh, L.; Banaei, S. Mechanism and clinical aspects of the effects of chicory on diabetes. Asian J. Res. Med. Pharm. Sci. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Dehghan, P.; Pourghassem Gargari, B.; Asgharijafarabadi, M. Effects of high performance inulin supplementation on glycemic status and lipid profile in women with type 2 diabetes: A randomized, placebo-controlled clinical trial. Health Promot. Perspect 2013, 3, 55–63. [Google Scholar]
- Miao, M.; Dai, Y.; Rui, C.; Fan, Y.; Wang, X.; Fan, C.; Mu, J.; Hou, W.; Dong, Z.; Li, P.; et al. Dietary supplementation of inulin alleviates metabolism disorders in gestational diabetes mellitus mice via RENT/AKT/IRS/GLUT4 pathway. Diabetol. Metab. Syndr. 2021, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Rahimiyan-Heravan, M.; Roshangar, L.; Karimi, P.; Sefidgari-Abrasi, S.; Morshedi, M.; Saghafi-Asl, M.; Bavafa-Valenlia, K. The potential therapeutic effects of Lactobacillus plantarum and inulin on serum and testicular reproductive markers in diabetic male rats. Diabetol. Metab. Syndr. 2020, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Lamoochi, Z.; Fathi Moghaddam, H.; Mansouri, S.M.T. Effects of Portulaca oleracea ethanolic extract on reproductive system of aging female mice. Int. J. Reprod. Biomed. 2016, 14, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayaka, H.B.; Londonkar, R.; Andumesh, M.K. Evaluation of Portulaca oleracea l for anti-fertility effect in female albino rats. Int. J. Pharm. Pharm. Sci. 2014, 6, 86–89. [Google Scholar]
- Okafor, I.A.; Nnamah, U.S.; Ahiatrogah, S.; Serwaa, D.; Nnaka, J. Reproductive toxicity potentials of methanolic extract of Portulaca oleracea in male rats: An experimental study. Int. J. Reprod. Biomed. 2021, 19, 245–254. [Google Scholar]
- Zhang, Z.; Qiao, D.; Zhang, Y.; Chen, Q.; Chen, Y.; Tang, Y.; Que, R.; Chen, Y.; Zheng, L.; Dai, Y.; et al. Portulaca oleracea L. Extract Ameliorates Intestinal Inflammation by Regulating Endoplasmic Reticulum Stress and Autophagy. Mol. Nutr. Food Res. 2022, 66, 2100791. [Google Scholar] [CrossRef]


| Gene Name/ Accession Number | Primer Sequence | Annealing Temperature (°C) | Ref. | |
|---|---|---|---|---|
| GAPDH/ NM_017008.4 | F | AGATCCACAACGGATACATT | 52 | [67] |
| R | TCCCTCAAGATTGTCAGCAA | |||
| LC3II/NM_199500.2 | F | GAGAAGCAGCTTCCTGTTCTGG | 60 | [68] |
| R | GTGTCCGTTCACCAACAGGAAG | |||
| Beclin-1/ NM_053739.2 | F | GAGAAGCAGCTTCCTGTTCTGG | 60 | [69] |
| R | GTGTCCGTTCACCAACAGGAAG | |||
| P62/ NM_175843.5 | F | TGCCCAGACTACGACTTGTG | 56 | [68] |
| R | AGTGTCCGTGTTTCACCTTCC | |||
| ATG5/ NM_001014250.2 | F | GCAGATGGACAGTTGCACACAC | 56 | [70] |
| R | GAGGTGTTTCCAACATTGGCTCA | |||
| ATG7/ NM_001012097.1 | F | CGTTGCCCACAGCATCATCTTC | 56 | [70] |
| R | TCCCATGCCTCCTTTCTGGTTC | |||
| GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LC3II, microtubule-associated protein light chain 3II; P62, ubiquitin-binding protein p62; ATG5, autophagy-related protein 5; ATG7, autophagy-related protein 7 | ||||
| Phytochemicals | Concentration | |
|---|---|---|
| PEE | CWE | |
| Yield (g%) | 15.72 ± 0.72 | 9.36 ± 0.50 |
| Total phenolics (mg catechin Eq/g extract) | 49.642 ± 0.910 | 16.428 ± 0.793 |
| Total flavonoids (mg gallic acid Eq/g extract) | 113.285 ± 3.011 | 105.071 ± 1.105 |
| HPLC analysis of the phenolic compounds (μg/g extract) | ||
| Pyrogallol | ND | ND |
| Quinol | ND | 12.50323 |
| Gallic acid | 27.68568 | 9.87210 |
| Catechol | 67.76362 | ND |
| p-Hydroxybenzoic acid | 180.47324 | 4.27578 |
| Catechin | ND | 0.00000 |
| Chlorogenic acid | 39.94523 | 8.35102 |
| Vanillic acid | ND | 0.00000 |
| Caffeic acid | 14.27399 | 4.48349 |
| Syringic acid | 56.37858 | 9.67627 |
| p-Coumaric acid | 31.63395 | 0.00000 |
| Benzoic acid | 394.93195 | ND |
| Ferulic acid | 13.97240 | 2.27579 |
| Rutin | ND | 0.00000 |
| Ellagic | ND | ND |
| o-Coumaric acid | 18.32819 | 25.75831 |
| Resveratrol | ND | 941.10714 |
| Cinnamic acid | ND | ND |
| Quercetin | 101.22068 | ND |
| Rosmarinic acid | 72.48203 | ND |
| Naringin | ND | 333.97923 |
| Myricetin | 112.91257 | ND |
| Kaempferol | ND | 26.01053 |
| Total | 1132.00209 | 1378.29289 |
| Minerals (µg/g extract) | ||
| Ca | 895089.527 | 104208.430 |
| Cu | 1975.3378 | 967.96511 |
| Fe | 108850.8108 | 31512.7095 |
| K | 205133.2432 | 1015929.7674 |
| Mg | 901662.2297 | 32384.18605 |
| Mn | 887.7027 | 577.2674 |
| Na | 2009375.5405 | 220631.2209 |
| P | 649056.4189 | 146762.7325 |
| Se | 45.2027 | 22.1512 |
| Zn | 4350.6081 | 933.9535 |
| Vitamins (value/g extract) | ||
| Folic acid (µg) | 56.99 | 68.98 |
| Vitamin C (µg) | 40.05 | 63.25 |
| Vitamin A (IU) | 55.41 | 5.91 |
| Results are presented as the mean ± SD (n = 3). Eq—Equivalent; ND—Not detected. | ||
| Parameters | CI | Effect | |
|---|---|---|---|
| In vitro antioxidant assays | |||
| DPPH (mg/mL) | 0.730± 0.001 | Synergistic | |
| NO scavenging (mg/mL) | 0.249± 0.020 | Synergistic | |
| Superoxide radical scavenging (mg/mL) | 0.337± 0.017 | Synergistic | |
| T. antioxidant (mg/mL) | 0.341± 0.022 | Synergistic | |
| Seminal quality and oxidative stress markers | |||
| Sperm count (106/mL) | 1.076 ± 0.006 | Additive | |
| Morphology index (%) | 0.985 ± 0.002 | Synergistic | |
| Motility type (%) | Progressive | 0.981 ± 0.011 | Synergistic |
| Non-progressive | 0.959 ± 0.015 | Synergistic | |
| Motility (%) | 1st h | 0.919 ± 0.013 | Synergistic |
| 2nd h | 0.911 ± 0.003 | Synergistic | |
| 3rd h | 0.000 ± 0.000 | Synergistic | |
| Serum fructose level (mg/dL) | 1.064 ± 0.011 | Additive | |
| Seminal fructose level (mg/g tissue) | 1.019 ± 0.009 | Additive | |
| α-Mannosidase activity (U/mg protein) | 0.123 ± 0.002 | Synergistic | |
| FSH (μIU/mL) | 0.890 ± 0.001 | Synergistic | |
| LH (μIU/mL) | 0.837 ± 0.004 | Synergistic | |
| Testosterone (ng/dL) | 0.618 ± 0.011 | Synergistic | |
| MDA (μmol/mg protein) | 0.748 ± 0.041 | Synergistic | |
| GSH (mM/mg protein) | 0.763 ± 0.012 | Synergistic | |
| GPx activity (U/mg protein) | 0.520 ± 0.011 | Synergistic | |
| GST activity (U/mg protein) | 0.543 ± 0.039 | Synergistic | |
| Insulin resistance markers | |||
| FBS (mg/dL) | 0.951 ± 0.021 | Synergistic | |
| Insulin (𝛍𝐔/mL) | 0.983 ± 0.011 | Synergistic | |
| HOMA-IR | 0.755 ± 0.019 | Synergistic | |
| HOMA-β | 1.227 ± 0.028 | Antagonistic | |
| ER stress and autophagy parameters | |||
| GRP78 (Protein expression fold) | 0.580 ± 0.018 | Synergistic | |
| Sigma 1R (ng/mg protein) | 0.898 ± 0.011 | Synergistic | |
| PI3KC3 (ng/mg protein) | 1.076 ± 0.018 | Additive | |
| Beclin-1 | ng/mg protein | 0.898 ± 0.005 | Synergistic |
| mRNA expression fold | 0.557 ± 0.011 | Synergistic | |
| LC3II | Protein expression fold | 0.543 ± 0.016 | Synergistic |
| mRNA expression fold | 0.332 ± 0.022 | Synergistic | |
| ATG5 (mRNA expression fold) | 0.384 ± 0.008 | Synergistic | |
| ATG7 (mRNA expression fold) | 0.271 ± 0.027 | Synergistic | |
| P62 | Protein expression fold | 0.485 ± 0.148 | Synergistic |
| mRNA expression fold | 0.595 ± 0.016 | Synergistic | |
| P-mTOR Ser2448 (Protein expression fold) | 0.434 ± 0.018 | Synergistic | |
| CI < 1, =1, and >1 indicate synergistic, additive, and antagonistic effects, respectively. | |||
| Control | PEE | CWE | PEE/CWE | Dexa | Dexa + PEE | Dexa + CWE | Dexa + PEE/CWE | ||
| Sperm Count (106/mL) | 178 ± 8.83 | 189 ± 5.66 * | 163 ± 8.66 | 169 ± 3.41 | 206 ± 4.24 * | 168 ± 7.07 #,a | 185 ± 5.55 #,a | 200 ± 1.14 *,# | |
| Morphology Index (%) | 90.0 ± 5.0 | 92.5 ± 3.5 | 90.0 ± 0.0 | 82.5 ± 4.04 | 75.0 ± 0.0 * | 90.9 ± 2.35 # | 85.6 ± 7.07 # | 85.5 ± 3.5 # | |
| Motility Type (%) | Progressive | 86.65 ± 4.71 | 90.00 ± 0.00 | 85.00 ± 4.08 | 90.00 ± 0.00 | 47.50 ± 6.45 * | 85.00 ± 1.07 #,a | 90.75 ± 2.50 # | 93.32 ± 2.35 *,# |
| Non-progressive | 13.32 ± 4.01 | 10.00± 0.00 | 15.00 ± 4.08 | 10.00 ± 0.00 | 52.54 ± 6.45 * | 15.00 ± 2.07 #,a | 10.00 ± 1.08 #,a | 6.65 ± 2.05 *,# | |
| Motility (%) | 1st h | 92.0 ± 4.5 | 90.0 ± 0.00 | 85.0 ± 7.07 | 90.0 ± 0.00 | 45.0 ± 5.77 * | 87.0 ± 10.61 # | 93.3 ± 2.35 #,a | 87.5 ± 2.53 # |
| 2nd h | 65.0 ± 8.14 | 67.5 ± 7.68 | 50.5 ± 9.85 | 53.1 ± 7.07 | 11.7 ± 2.07 * | 42.5 ± 10.6 *,# | 75.5 ± 13.43 # | 72.1 ± 10.60 # | |
| 3rd h | 5.0 ± 0.5 | 5.0 ± 0.0 | 5.0 ± 0.0 | 4.5 ± 0.4 | 0.0 + 0.0 * | 5.0 ± 0.0 # | 5.1 ± 0.0 # | 5.5 ± 0.0 # | |
| Serum Fructose level (mg/dL) | 124.34 ± 10.15 | 145.57 ± 13.96 | 148.67 ± 4.34* | 150.88 ± 8.75 * | 85.13 ± 8.799 * | 136.73 ± 6.68 # | 133.19 ± 10.35 # | 143.36 ± 5.01 *,# | |
| Seminal Fructose level (mg/g tissue) | 148.23± 8.59 | 150.50 ± 8.28 | 156.08 ± 6.78 | 163.71 ± 8.23 | 115.79 ± 7.26 * | 145.13 ± 4.09 # | 139.38 ± 3.64 # | 143.36 ± 4.90 # | |
| α-Mannosidase activity (U/mg protein) | 0.18 ± 0.011 | 0.17 ± 0.017 | 0.18 ± 0.014 | 0.18 ± 0.018 | 0.13 ± 0.012 * | 0.16 ± 0.016 # | 0.15 ± 0.030 # | 0.17 ± 0.016 # | |
| FSH (μIU/mL) | 49.5 ± 1.80 | 48.4 ± 1.71 | 61.6 ± 4.04 * | 67.65 ± 2.93 * | 11 ± 1.41 * | 43.9 ± 3.88 #,a | 53.1 ± 2.34 # | 50.8 ± 0.93 # | |
| LH (μIU/mL) | 37.17 ± 2.02 | 38.5 ± 0.99 | 45.03 ± 2.88 * | 44.425 ± 4.49 * | 13.67± 2.05 * | 27.47 ± 1.6 *,#,a | 38.83 ± 1.02 *,# | 34.2 ± 1.04 # | |
| Testosterone (ng/dL) | 2.51 ± 0.30 | 2.19 ± 0.19 | 2.23 ± 0.30 | 2.39 ± 0.41 | 0.91 ± 0.10 * | 1.05 ± 0.11* | 1.29 ± 0.25 * | 1.51 ± 0.17 *,# | |
| MDA (μmol/mg protein) | 0.883 ± 0.19 | 0.819 ± 0.06 | 0.654 ± 0.24 | 0.325 ± 0.05 * | 3.064 ± 0.42 * | 0.821 ± 0.12 # | 0.703 ± 0.15 # | 0.726 ± 0.11 # | |
| GSH (mM/mg protein) | 3.83 ± 0.20 | 3.79 ± 0.18 | 3.71 ± 0.32 | 3.99± 0.44 | 2.11± 0.18 * | 3.31 ± 0.25 *,# | 3.28 ± 0.13 *,#,a | 3.70 ± 0.18 # | |
| GPx activity (U/mg protein) | 4.61 ± 0.31 | 4.09 ± 0.30 | 4.38 ± 0.29 | 4.91 ± 0.24 | 1.27 ± 0.21 * | 3.6 ± 0.24 *,# | 3.7 ± 0.2 *,# | 3.4 ± 0.17 *,# | |
| GST activity (U/mg protein) | 1.87 ± 0.05 | 1.80 ± 0.07 | 1.78 ± 0.06 | 1.85 ± 0.09 | 1.0 ± 0.03 * | 1.66 ± 0.09 *,# | 1.58 ± 0.07 *,#a | 1.76 ± 0.09 # | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, S.R.; Manaa, A.; Sheta, E.; Ghareeb, D.A.; Abd-Elmonem, N.M. The Synergetic Effect of Egyptian Portulaca oleracea L. (Purslane) and Cichorium intybus L. (Chicory) Extracts against Glucocorticoid-Induced Testicular Toxicity in Rats through Attenuation of Oxidative Reactions and Autophagy. Antioxidants 2022, 11, 1272. https://doi.org/10.3390/antiox11071272
Saleh SR, Manaa A, Sheta E, Ghareeb DA, Abd-Elmonem NM. The Synergetic Effect of Egyptian Portulaca oleracea L. (Purslane) and Cichorium intybus L. (Chicory) Extracts against Glucocorticoid-Induced Testicular Toxicity in Rats through Attenuation of Oxidative Reactions and Autophagy. Antioxidants. 2022; 11(7):1272. https://doi.org/10.3390/antiox11071272
Chicago/Turabian StyleSaleh, Samar R., Ashraf Manaa, Eman Sheta, Doaa A. Ghareeb, and Nihad M. Abd-Elmonem. 2022. "The Synergetic Effect of Egyptian Portulaca oleracea L. (Purslane) and Cichorium intybus L. (Chicory) Extracts against Glucocorticoid-Induced Testicular Toxicity in Rats through Attenuation of Oxidative Reactions and Autophagy" Antioxidants 11, no. 7: 1272. https://doi.org/10.3390/antiox11071272
APA StyleSaleh, S. R., Manaa, A., Sheta, E., Ghareeb, D. A., & Abd-Elmonem, N. M. (2022). The Synergetic Effect of Egyptian Portulaca oleracea L. (Purslane) and Cichorium intybus L. (Chicory) Extracts against Glucocorticoid-Induced Testicular Toxicity in Rats through Attenuation of Oxidative Reactions and Autophagy. Antioxidants, 11(7), 1272. https://doi.org/10.3390/antiox11071272








