Sinapic Acid Inhibits Group IIA Secretory Phospholipase A2 and Its Inflammatory Response in Mice
Abstract
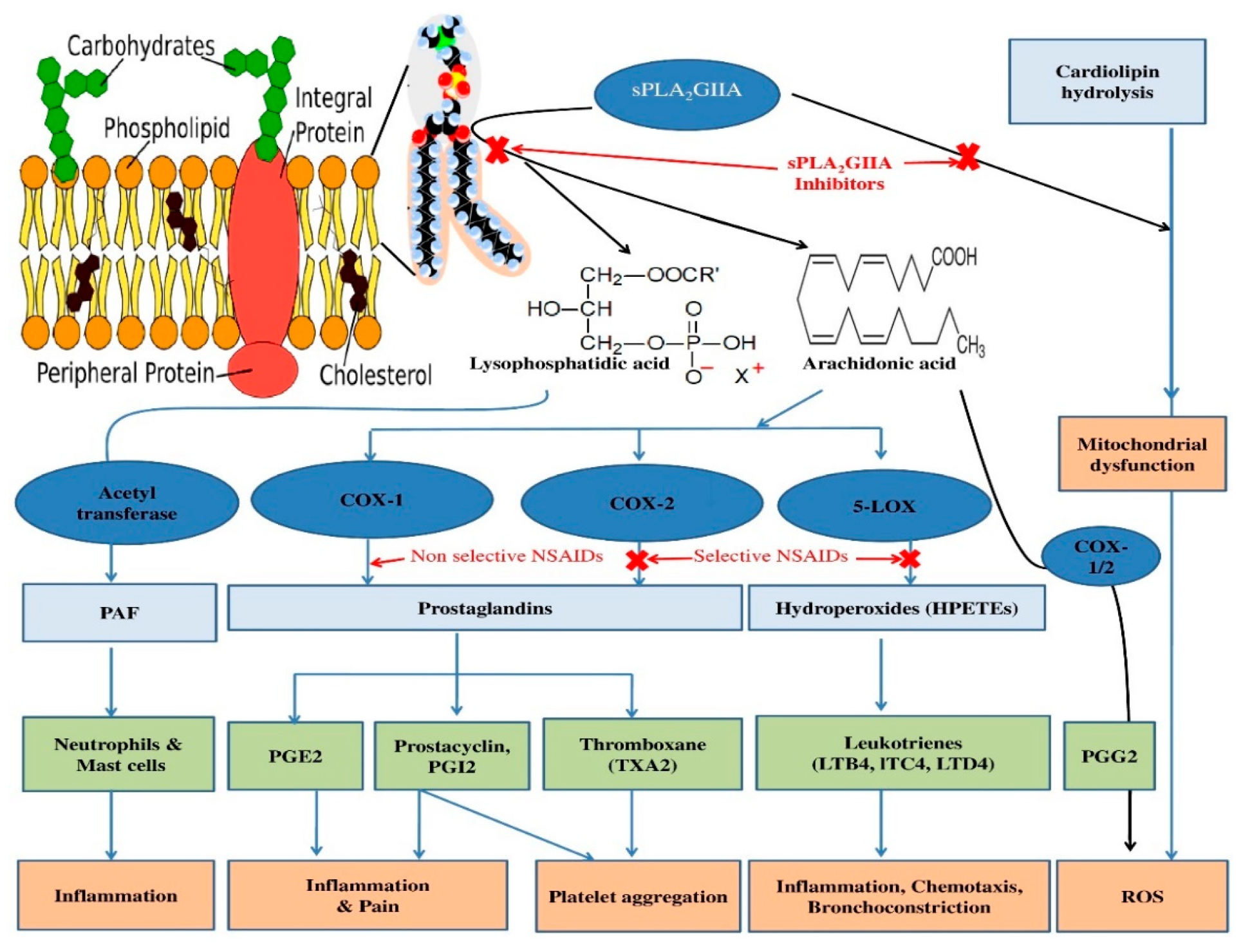
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Human Biological Fluid
2.4. Purification of sPLA2-IIA
2.5. Molecular Docking
2.6. Estimation of Antioxidant Activity
2.7. Secreted Phospholipase A2 Assay (sPLA2-IIA)
2.8. Inhibition of sPLA2-IIA Activity
2.9. The Effect of Concentrations of Substrate and Calcium on sPLA2-IIA Inhibition
2.10. Intrinsic Fluorescence Study
2.11. The Far UV-Circular Dichroism Study
2.12. Study of Reversibility of sPLA2-IIA Inhibition
2.13. Neutralization of Indirect Haemolytic Activity
2.14. Neutralization of Edema Inducing Activity of sPLA2-IIA
2.15. Hemorrhagic Activity of sPLA2-IIA
2.16. Statistical Analysis
3. Results
3.1. Molecular Docking
3.2. Estimation of Antioxidant Activities
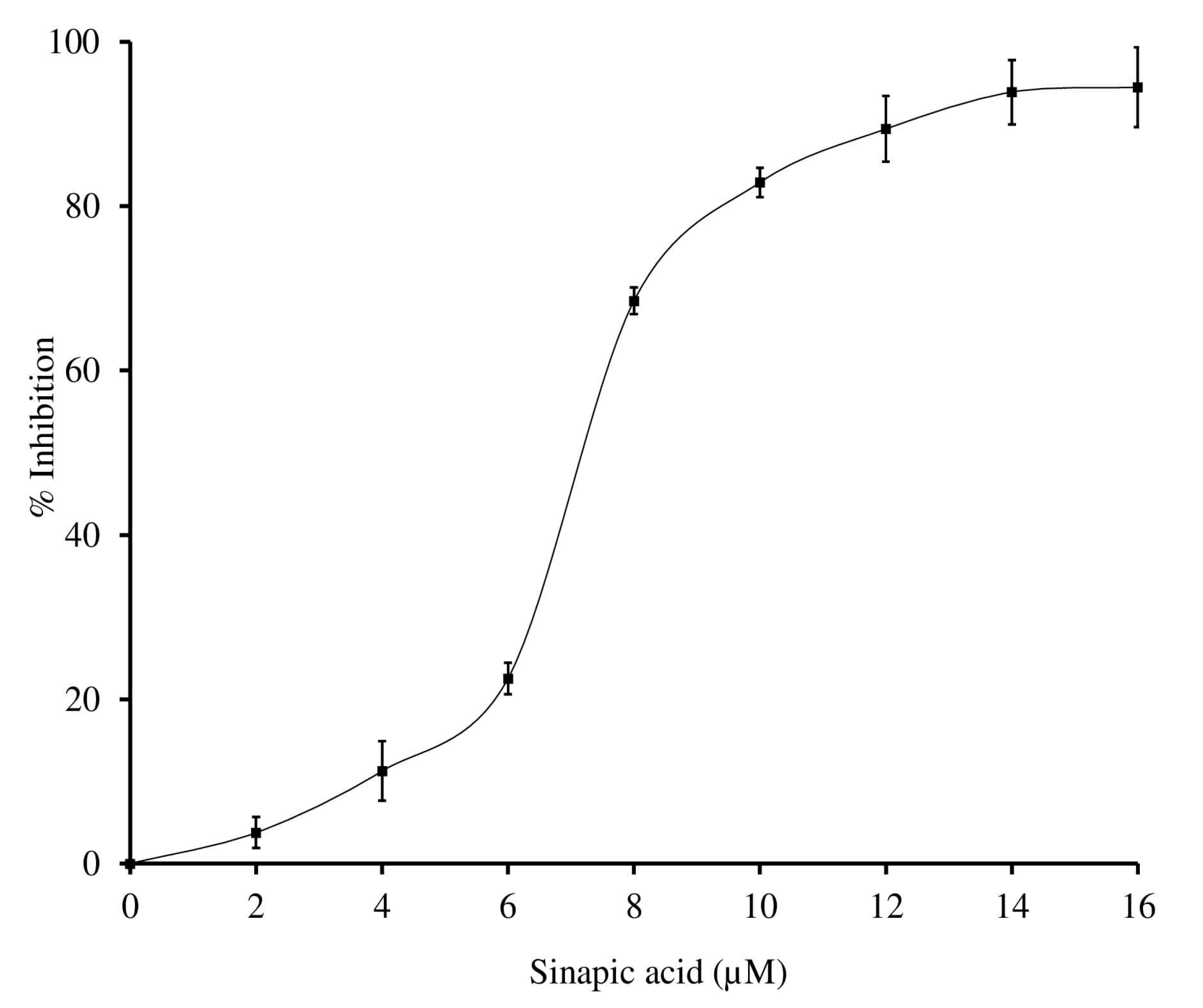
3.3. Inhibition of sPLA2-IIA
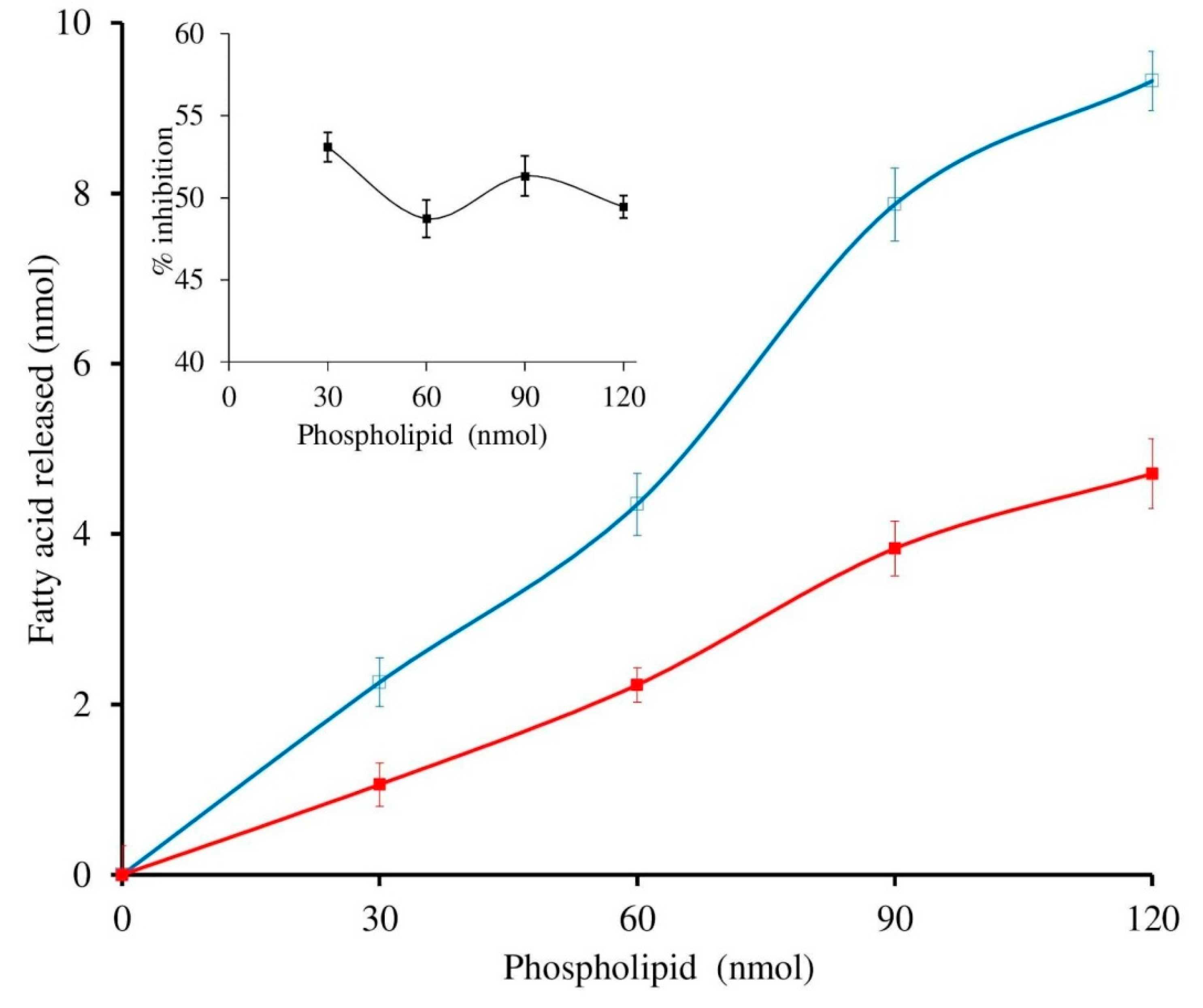
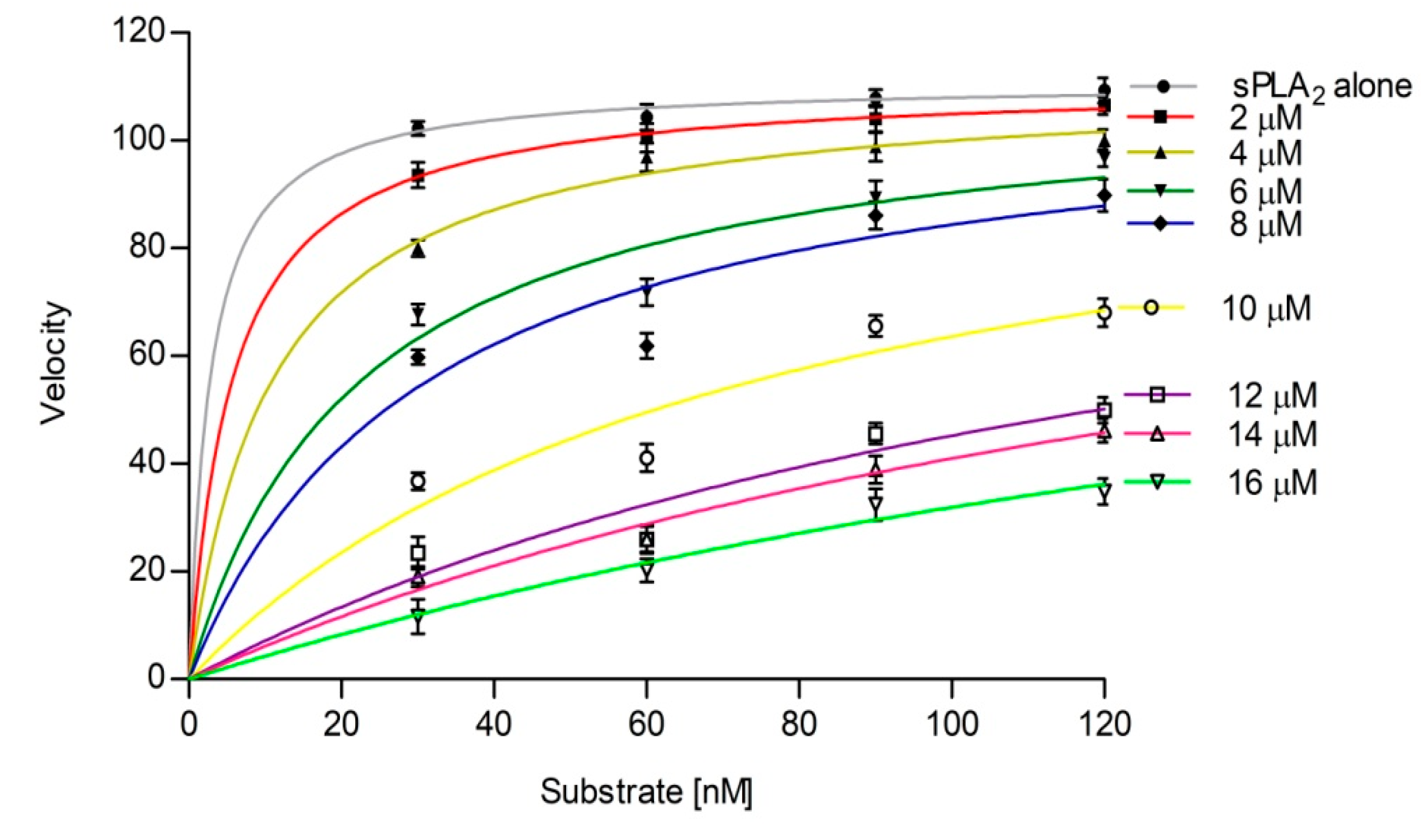
3.4. Effect of Calcium and Substrate Concentration on sPLA2 IIA Inhibition
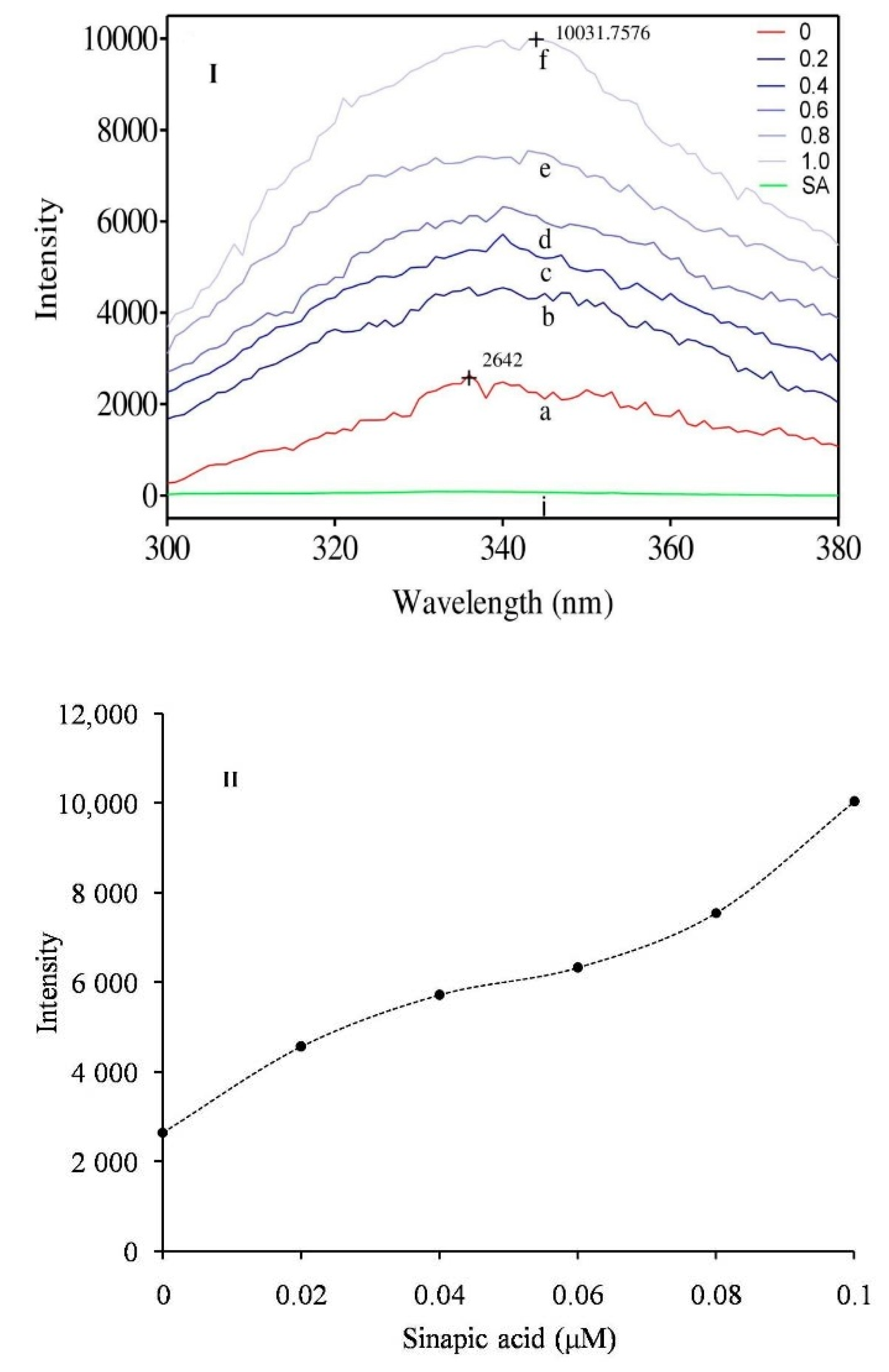
3.5. Intrinsic Fluorescence Study
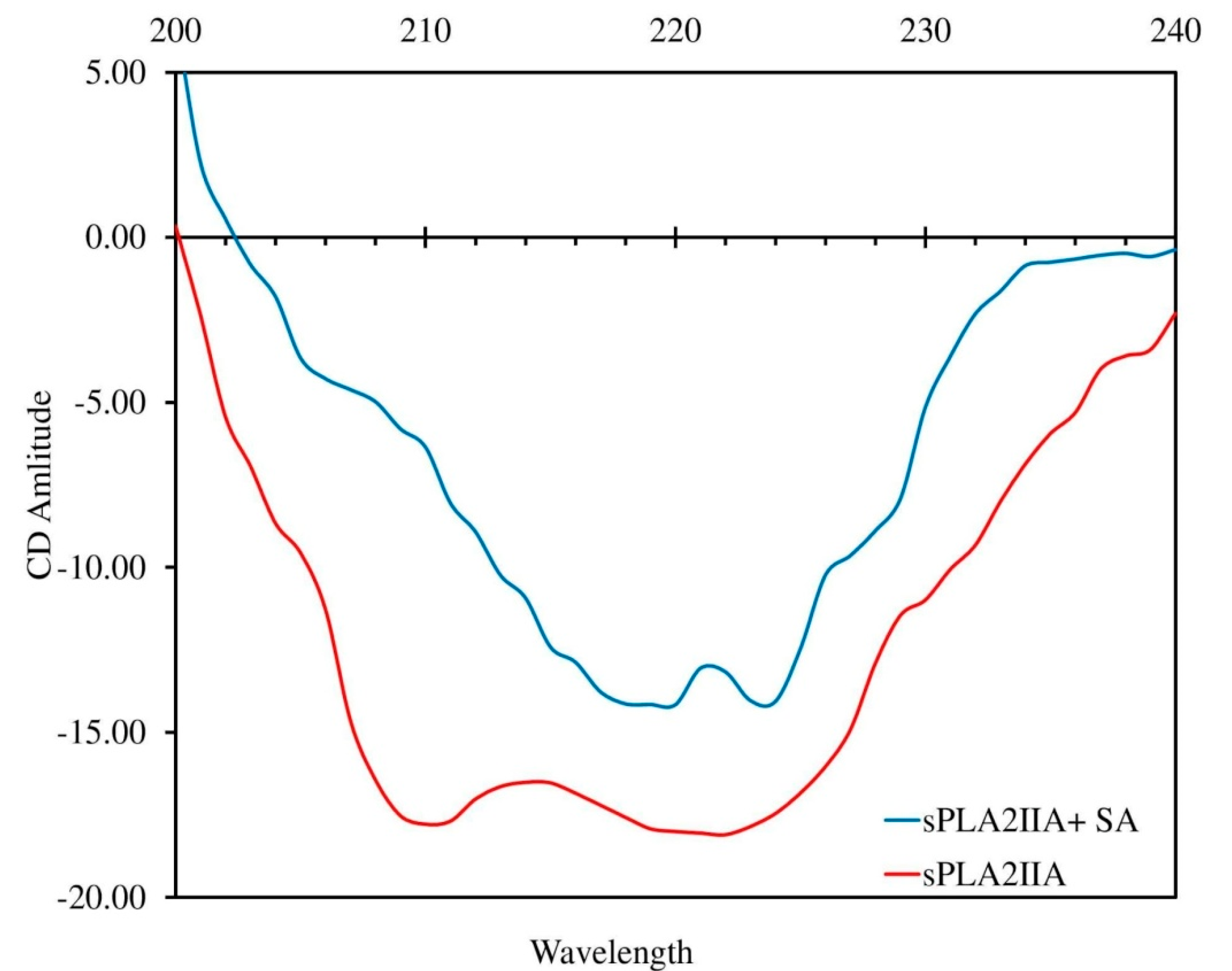
3.6. Circular Dichroism (CD) Study
3.7. Determination of Binding Characteristics
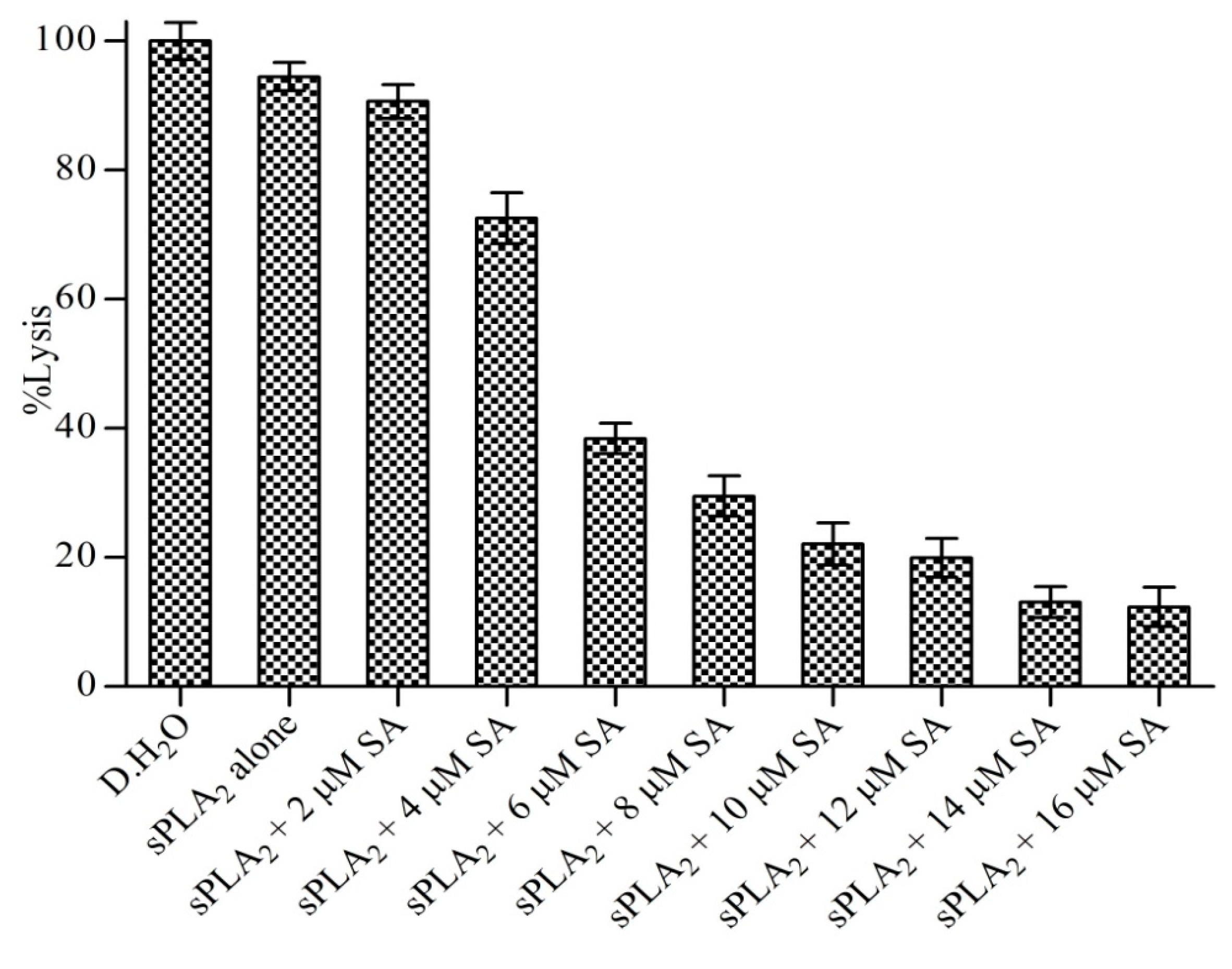
3.8. Neutralization of Indirect Haemolytic Activity
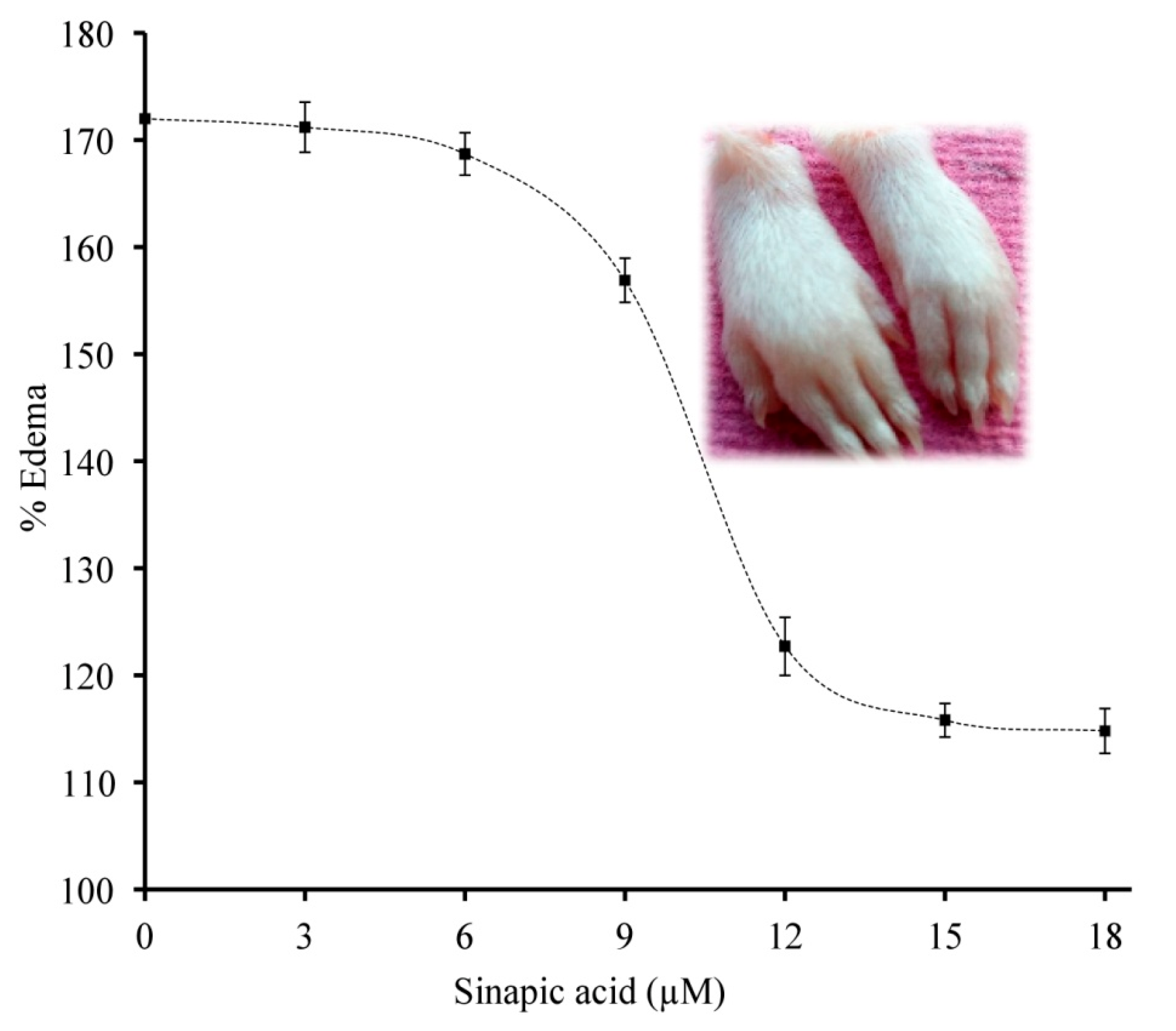
3.9. Neutralization of sPLA2 IIA Induced Mouse Paw Edema
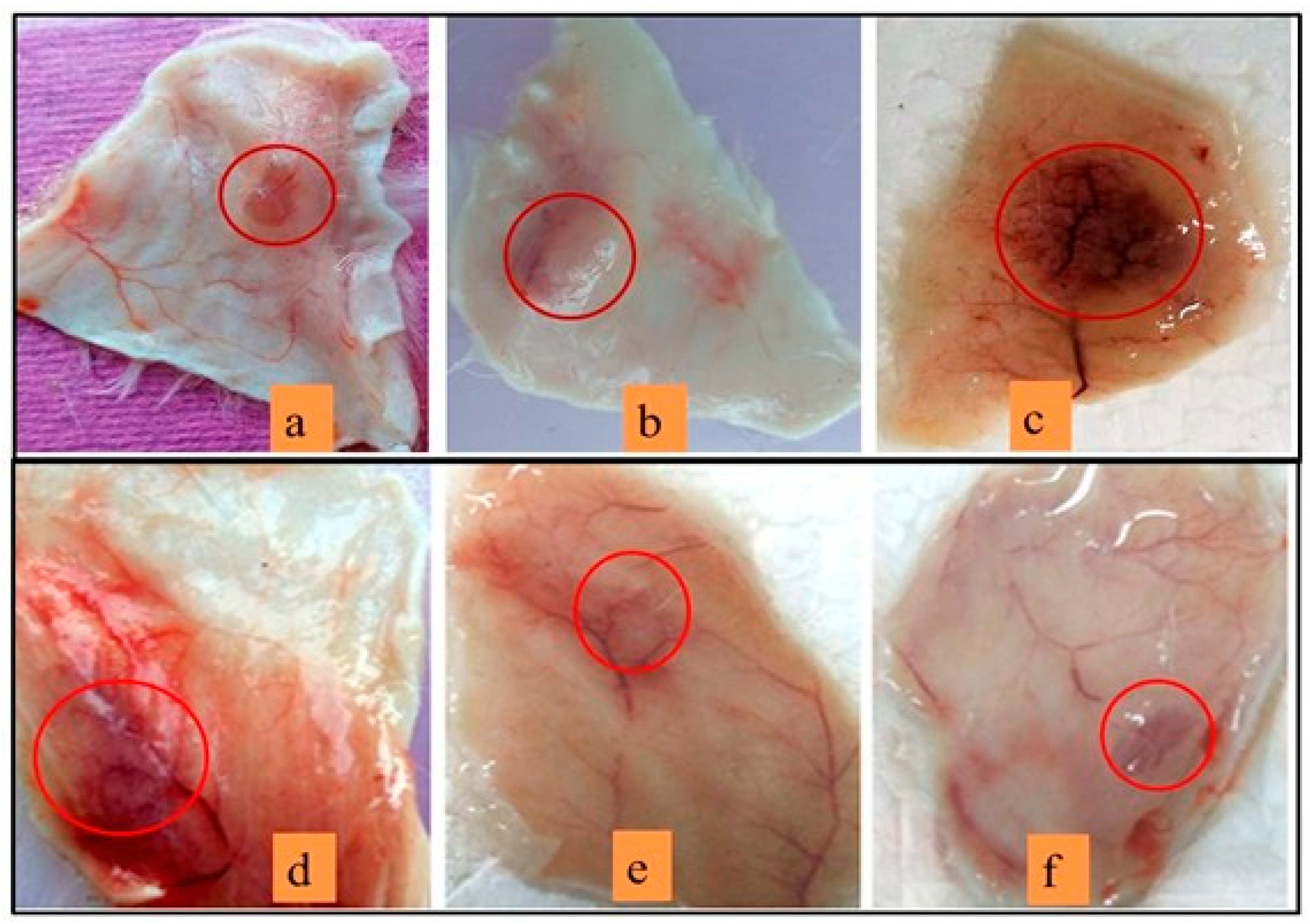
3.10. Neutralization of Haemorrhagic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammation-associated diseases in organs. Oncotarget 2018, 23, 7204. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm. Allergy-Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef]
- Tietge, U.J.; Pratico, D.; Ding, T.; Funk, C.D.; Hildebrand, R.B.; Van Berkel, T.; Van Eck, M. Macrophage-specific expression of group IIA sPLA2 results in accelerated atherogenesis by increasing oxidative stress. J. Lipid Res. 2005, 46, 1604–1614. [Google Scholar] [CrossRef]
- Bidgood, M.J.; Jamal, O.S.; Cunningham, A.M.; Brooks, P.M.; Scott, K.F. Type IIA secreted phospholipase A2 up-regulates cyclooxygenase-2 and amplifies cytokine-mediated prostaglandin production in human rheumatoid synoviocytes. J. Immunol. 2000, 165, 2790–2797. [Google Scholar] [CrossRef]
- Van Hensbergen, V.P.; Wu, Y.; van Sorge, N.M.; Touqui, L. Type IIA secreted phospholipase A2 in host defense against bacterial infections. Trends Immunol. 2020, 41, 313–326. [Google Scholar] [CrossRef]
- Stefanski, E.; Pruzanski, W.; Sternby, B.; Vadas, P. Purification of a soluble phospholipase A2 from synovial fluid in rheumatoid arthritis. J. Biochem. 1986, 100, 1297–1303. [Google Scholar] [CrossRef]
- Bowton, D.L.; Seeds, M.C.; Fasano, M.B.; Goldsmith, B.; Bass, D.A. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am. J. Respir. Crit. Care Med. 1997, 155, 421–425. [Google Scholar] [CrossRef]
- Menschikowski, M.; Hagelgans, A.; Siegert, G. Secreted phospholipase A2 of group IIA: Is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases? Prostaglandins Other Lipid Mediat. 2006, 79, 1–33. [Google Scholar] [CrossRef]
- Styles, L.A.; Schalkwijk, C.G.; Aarsman, A.J.; Vichinsky, E.P.; Lubin, B.H.; Kuypers, F.A. Phospholipase A2 levels in acute chest syndrome of sickle cell disease. Blood 1996, 87, 2573–2578. [Google Scholar] [CrossRef]
- Mallat, Z.; Lambeau, G.; Tedgui, A. Lipoprotein-associated and secreted phospholipases A2 in cardiovascular disease: Roles as biological effectors and biomarkers. Circulation 2010, 122, 2183–2200. [Google Scholar] [CrossRef]
- Tietge, J.F. Extracellular phospholipases: Role in inflammation and atherosclerotic cardiovascular. Atheroscler. Risks Mech. Ther. 2015, 10, 279. [Google Scholar]
- Tan, T.L.; Goh, Y.Y. The role of group IIA secretory phospholipase A2 (sPLA2-IIA) as a biomarker for the diagnosis of sepsis and bacterial infection in adults—A systematic review. PLoS ONE 2017, 12, e0180554. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; de Boer, J.F.; Dikkers, A.; Dimova, L.G.; van der Giet, M.; Bakker, S.J.; Tietge, U.J. Group IIA Secretory Phospholipase A2 Predicts Graft Failure and Mortality in Renal Transplant Recipients by Mediating Decreased Kidney Function. J. Clin. Med. 2020, 9, 1282. [Google Scholar] [CrossRef]
- Nanda, B.L.; Nataraju, A.; Rajesh, R.; Rangappa, K.S.; Shekar, M.A.; Vishwanath, B.S. PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants-a new role as anti-inflammatory molecule. Curr. Top. Med. Chem. 2007, 7, 765–777. [Google Scholar] [CrossRef]
- Nethery, D.; Stofan, D.; Callahan, L.; DiMarco, A.; Supinski, G. Formation of reactive oxygen species by the contracting diaphragm is PLA(2) dependent. J. Appl. Physiol. 1999, 87, 792–800. [Google Scholar] [CrossRef]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.; Li, S.; Gao, Y. Occurrence, formation, environmental fate and risks of environmentally persistent free radicals in biochars. Environ. Int. 2020, 134, 105172. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Fois, A.G.; Sotgia, S.; Zinellu, E.; Bifulco, F.; Pintus, G.; Mangoni, A.A.; Carru, C.; Pirina, P. Plasma protein thiols: An early marker of oxidative stress in asthma and chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2016, 46, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of reactive oxygen species in the progression of Alzheimer’s disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef]
- Zhu, D.; Lai, Y.; Shelat, P.B.; Hu, C.; Sun, G.Y.; Lee, J.C. Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J. Neurosci. 2006, 26, 11111–11119. [Google Scholar] [CrossRef]
- Gøtzsche, P.C. Non-steroidal anti-inflammatory drugs. BMJ 2000, 320, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.C.; Schnepf, G.; Barrett, M.S.; Dian, D.; Swigonski, N.L. Prevalence, attitudes, and behaviours related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in student athletes. J. Adolesc. Health 2002, 30, 150–153. [Google Scholar] [CrossRef]
- Burnett, B.P.; Levy, R.M. 5-Lipoxygenase metabolic contributions to NSAID-induced organ toxicity. Adv. Ther. 2012, 29, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Alajbegovic, A.; Gomes, A.V. NSAIDs and Cardiovascular Diseases: Role of Reactive Oxygen Species. Oxid. Med. Cell. Longev. 2015, 2015, 536962. [Google Scholar] [CrossRef]
- Fitzgerald, G.A. Coxibs and cardiovascular disease. N. Engl. J. Med. 2004, 351, 1709–1711. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 47, 78–87. [Google Scholar] [CrossRef]
- Bavry, A.A.; Khaliq, A.; Gong, Y.; Handberg, E.M.; Cooper-DeHoff, R.M.; Pepine, C.J. Harmful effects of NSAIDs among patients with hypertension and coronary artery disease. Am. J. Med. 2011, 124, 614–620. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Cavender, M.A.; Kastelein, J.J.; Schwartz, G.; Waters, D.D.; Rosenson, R.S.; Bash, D.; Hislop, C. Inhibition of secreted phospholipase A2 in patients with acute coronary syndromes: Rationale and design of the vascular inflammation suppression to treat acute coronary syndrome for 16 weeks (VISTA-16) trial. Cardiovasc. Drugs Ther. 2012, 26, 71–75. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Kastelein, J.J.; Schwartz, G.G.; Bash, D.; Rosenson, R.S.; Cavender, M.A.; Brennan, D.M.; Koenig, W.; Jukema, J.W.; Nambi, V.; et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: The VISTA-16 randomized clinical trial. JAMA 2014, 311, 252–262. [Google Scholar] [CrossRef]
- Zeiher, B.G.; Steingrub, J.; Laterre, P.F.; Dmitrienko, A.; Fukiishi, Y.; Abraham, E.; EZZI Study Group. LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholipase A2, fails to improve clinical outcome for patients with severe sepsis. Crit. Care Med. 2005, 33, 1741–1748. [Google Scholar] [CrossRef]
- Garcia-Pastor, P.; Randazzo, A.; Gomez-Paloma, L.; Alcaraz, M.J.; Paya, M. Effects of petrosaspongiolide M, a novel phospholipase A2 inhibitor, on acute and chronic inflammation. J. Pharmacol. Exp. Ther. 1999, 289, 166–172. [Google Scholar] [PubMed]
- Reid, R.C. Inhibitors of secreted phospholipase A2 group IIA. Curr. Med. Chem. 2005, 12, 3011–3026. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Martins, M.; Silva, P.; Garrido, E.M.; Garrido, J.; Firuzi, O.; Miri, R.; Saso, L.; Borges, F. Dietary phenolic acids and derivatives. Evaluation of the antioxidant activity of sinapic acid and its alkyl esters. J. Agric. Food Chem. 2010, 58, 11273–11280. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. J. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.J.; Koh, D.J.; Kim, S.H.; Park, S.J.; Ryu, J.H.; Kim, D.G.; Lee, J.Y.; Lee, K.T. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-κB inactivation. J. Agric. Food Chem. 2008, 56, 10265–19272. [Google Scholar] [CrossRef]
- Huang, X.; Pan, Q.; Mao, Z.; Zhang, R.; Ma, X.; Xi, Y.; You, H. Sinapic acid inhibits the IL-1β-induced inflammation via MAPK downregulation in rat chondrocytes. Inflammation 2018, 41, 562–568. [Google Scholar] [CrossRef]
- Lee, J.Y. Anti-inflammatory effects of sinapic acid on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. Arch. Pharm. Res. 2018, 41, 243–250. [Google Scholar] [CrossRef]
- Kasturi, S.; Gowda, T.V. Purification and characterization of a major phospholipase A2 from Russell’s viper (Viperarusselli) venom. Toxicon 1989, 27, 229–237. [Google Scholar] [CrossRef]
- Laemmli, U.K. SDS-page Laemmli method. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Davidson, F.F.; Dennis, E.A. Evolutionary relationships and implications for the regulation of phospholipase A2 from snake venom to human secreted forms. J. Mol. Evol. 1990, 31, 228–238. [Google Scholar] [CrossRef]
- Teixeira, C.D.; Landucci, E.C.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon 2003, 42, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Sales, T.A.; Marcussi, S.; Da Cunha, E.F.; Kuca, K.; Ramalho, T.C. Can inhibitors of snake venom phospholipases A2 lead to new insights into anti-inflammatory therapy in humans? A theoretical study. Toxins 2017, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Schüttelkopf, W.; van Aalten, D.M.F. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chemistr. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Jinadatta, P.; Rajshekarappa, S.; Rao, K.S.R.; Subbaiah, S.G.P.; Shastri, S. In silico, in vitro: Antioxidant and antihepatotoxic activity of gnetol from Gnetum ula Brongn. Bioimpacts 2019, 9, 239–249. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Gutteridge, J.M.; Halliwell, B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 1990, 15, 129–135. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Patriarca, P.; Beckerdite, S.; Pettis, P.; Elsbach, P. Phospholipid metabolism by phagocytic cells: VII. The degradation and utilization of phospholipids of various microbial species by rabbit granulocytes. Biochim. Biophys. Acta BBA Lipids Lipid Metab. 1972, 280, 45–56. [Google Scholar] [CrossRef]
- Vishwanath, B.S.; Frey, F.J.; Bradbury, M.J.; Dallman, M.F.; Frey, B.M. Glucocorticoid deficiency increases phospholipase A2 activity in rats. J. Clin. Investig. 1993, 92, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Dharmappa, K.K.; Mohamed, R.; Shivaprasad, H.V.; Vishwanath, B.S. Genistein, a potent inhibitor of secreted phospholipase A 2: A new insight in down regulation of inflammation. Inflammopharmacology 2010, 18, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Prigent-Dachary, J.; Boffa, M.C.; Boisseau, M.R.; Dufourcq, J. Snake venom phospholipases A2. A fluorescence study of their binding to phospholipid vesicles correlation with their anticoagulant activities. J. Biol. Chem. 1980, 255, 7734–7739. [Google Scholar] [CrossRef]
- Boman, H.G.; Kaletta, U. Chromatography of rattlesnake venom: A separation of three phosphodiesterases. Biochim. Biophys. Acta 1957, 24, 619–631. [Google Scholar] [CrossRef]
- Yamakawa, M.; Nozaki, M.; Hokama, Z. Fractionation of sakishima habu (Trimeresurus elegans) venom and lethal, hemorrhagic and edema-forming activities of the fractions. Anim. Plant Microb. Toxins 1976, 1, 97–109. [Google Scholar]
- Vishwanath, B.S.; Gowda, T.V. Interaction of aristolochic acid with Viperarusselli phospholipase A2: Its effect on enzymatic and pathological activities. Toxicon 1987, 25, 929–937. [Google Scholar] [CrossRef]
- Kondo, H.; Kondo, S.; Ikezawa, H.; Murata, R.; Ohsaka, A. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol. 1960, 13, 43–51. [Google Scholar] [CrossRef]
- Venkatesh, M.; Gowda, V. Synergistically acting PLA2: Peptide hemorrhagic complex from Daboia russelii venom. Toxicon 2013, 73, 111–120. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; de Sanctis, D.; Pereira, P.J.B. The tick-derived anticoagulant madanin is processed by thrombin and factor Xa. PLoS ONE 2013, 8, e71866. [Google Scholar] [CrossRef]
- Burlingham, B.T.; Widlanski, T.S. An intuitive look at the relationship of Ki and IC50: A more general use for the Dixon plot. J. Chem. Educ. 2003, 80, 214. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Chuang, Y.C.; Ku, Y.H. Quantitation of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chem. 2007, 102, 1163–1171. [Google Scholar] [CrossRef]
- Russell, W.R.; Labat, A.; Scobbie, L.; Duncan, G.J.; Duthie, G.G. Phenolic acid content of fruits commonly consumed and locally produced in Scotland. Food Chem. 2009, 115, 100–104. [Google Scholar] [CrossRef]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Drzewiecki, J.; Cvikrová, M.; Martincová, O.; Katrich, E.; Trakhtenberg, S. Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols. J. Agric. Food Chem. 2008, 56, 4418–4426. [Google Scholar] [CrossRef]
- Ferreres, F.; Sousa, C.; Vrchovská, V.; Valentão, P.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Chemical composition and antioxidant activity of tronchuda cabbage internal leaves. Eur. Food Res. Technol. 2006, 222, 88–98. [Google Scholar] [CrossRef]
- Kawsar, S.M.; Huq, E.; Nahar, N.; Ozeki, Y. Identification and quantification of phenolic acids in Macrotyloma uniflorum by reversed phase-HPLC. Am. J. Plant Physiol. 2010, 5, 204–211. [Google Scholar] [CrossRef]
- Dabas, D.; Ziegler, G.R.; Lambert, J.D. Anti-Inflammatory Properties of a Colored Avocado Seed Extract. Adv. Food Technol. Nutr. Sci. Open J. 2019, 5, 8–12. [Google Scholar] [CrossRef]
- Giresha, A.S.; Narayanappa, M.; Joshi, V.; Vishwanath, B.S.; Dharmappa, K.K. Human secreted phospholipase A2 (spla2) inhibition by aqueous extract of Macrotyloma uniflorum (seed) as anti-inflammatory activity. Int. J. Pharm. Pharm. Sci. 2015, 7, 217–222. [Google Scholar]
- Hameed, H.; Aydin, S.; Başaran, A.A.; Başaran, N. Assessment of cytotoxic properties of sinapic acid in vitro. Turk. J. Pharm. Sci. 2016, 13, 225–232. [Google Scholar] [CrossRef]
- Qiao, H.Y.; Dahiya, J.P.; Classen, H.L. Nutritional and physiological effects of dietary sinapic acid (4-hydroxy-3, 5-dimethoxy-cinnamic acid) in broiler chickens and its metabolism in the digestive tract. Poult. Sci. 2008, 87, 719–726. [Google Scholar] [CrossRef]
- Ntie-Kang, F.; Lifongo, L.L.; Mbah, J.; Owono, L.C.O.; Megnassan, E.; Mbaze, L.M.; Judson, P.N.; Sippl, W.; Efange, S.M.N. In silico drug metabolism and pharmacokinetic profiles of natural products from medicinal plants in the Congo basin. Silico Pharmacol. 2013, 1, 12. [Google Scholar] [CrossRef]
- Kim, R.R.; Chen, Z.; Mann, T.; Bastard, K.F.; Scott, K.; Church, W.B. Structural and functional aspects of targeting the secreted human group IIA phospholipase A2. Molecules 2020, 25, 4459. [Google Scholar] [CrossRef] [PubMed]
- Adibhatla, R.M.; Hatcher, J.F. Phospholipase A2, reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008, 41, 560. [Google Scholar] [CrossRef] [PubMed]
- Shelat, P.B.; Chalimoniuk, M.; Wang, J.H.; Strosznajder, J.B.; Lee, J.C.; Sun, A.Y.; Simonyi, A.; Sun, G.Y. Amyloid β peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J. Neurochem. 2008, 106, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Arao, Y.; Sun, S.J.; Kikuchi, A.; Kayama, F. Oral administration of soy-derived genistin suppresses lipopolysaccharide-induced acute liver inflammation but does not induce thymic atrophy in the rat. Life Sci. 2006, 78, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Ko, J.W.; Jeon, S.; Kwon, Y.H. Protective effect of genistein against neuronal degeneration in ApoE−/− mice fed a high-fat diet. Nutrients 2016, 8, 692. [Google Scholar] [CrossRef]
- Rumman, M.; Pandey, S.; Singh, B.; Gupta, M.; Ubaid, S.; Mahdi, A.A. Genistein Prevents Hypoxia-Induced Cognitive Dysfunctions by Ameliorating Oxidative Stress and Inflammation in the Hippocampus. Neurotox. Res. 2021, 39, 1123–1133. [Google Scholar] [CrossRef]
- Bastian, B.C.; Sellert, C.; Seekamp, A.; Römisch, J.; Pâques, E.P.; Bröcker, E.B. Inhibition of human skin phospholipase A2 by “lipocortins” is an indirect effect of substrate/lipocortin interaction. J. Investig. Dermatol. 1993, 101, 359–363. [Google Scholar] [CrossRef]
- Nemec, K.N.; Pande, A.H.; Qin, S.; Urbauer, R.J.B.; Tan, S.; Moe, D.; Tatulian, S.A. Structural and functional effects of tryptophans inserted into the membrane-binding and substrate-binding sites of human group IIA phospholipase A2. Biochemistry 2006, 45, 12448–12460. [Google Scholar] [CrossRef]
- Matveeva, E.G.; Morisseau, C.; Goodrow, M.H.; Mullin, C.; Hammock, B.D. Tryptophan fluorescence quenching by enzyme inhibitors as a tool for enzyme active site structure investigation: Epoxide hydrolase. Curr. Pharm. Biotechnol. 2009, 10, 589–599. [Google Scholar] [CrossRef]
- Joshi, B.N.; Sainani, M.N.; Bastawade, K.B.; Deshpande, V.V.; Gupta, V.S.; Ranjekar, P.K. Pearl millet cysteine protease inhibitor: Evidence for the presence of two distinct sites responsible for anti-fungal and anti-feedentactivities. Eur. J. Biochem. 1999, 265, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta BBA Proteins Proteom. 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Yousefpour, A.; Amjad Iranagh, S.; Nademi, Y.; Modarress, H. Molecular dynamics simulation of nonsteroidal antiinflammatory drugs, naproxen and relafen, in a lipid bilayer membrane. Int. J. Quantum Chem. 2013, 113, 1919–1930. [Google Scholar] [CrossRef]
- Badyal, D.K.; Desai, C. Animal use in pharmacology education and research: The changing scenario. Indian J. Pharmacol. 2014, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hakes, L.; Pinney, J.W.; Robertson, D.L.; Lovell, S.C. Protein-protein interaction networks and biology—What’s the connection? Nat. Biotechnol. 2008, 26, 69–72. [Google Scholar] [CrossRef]
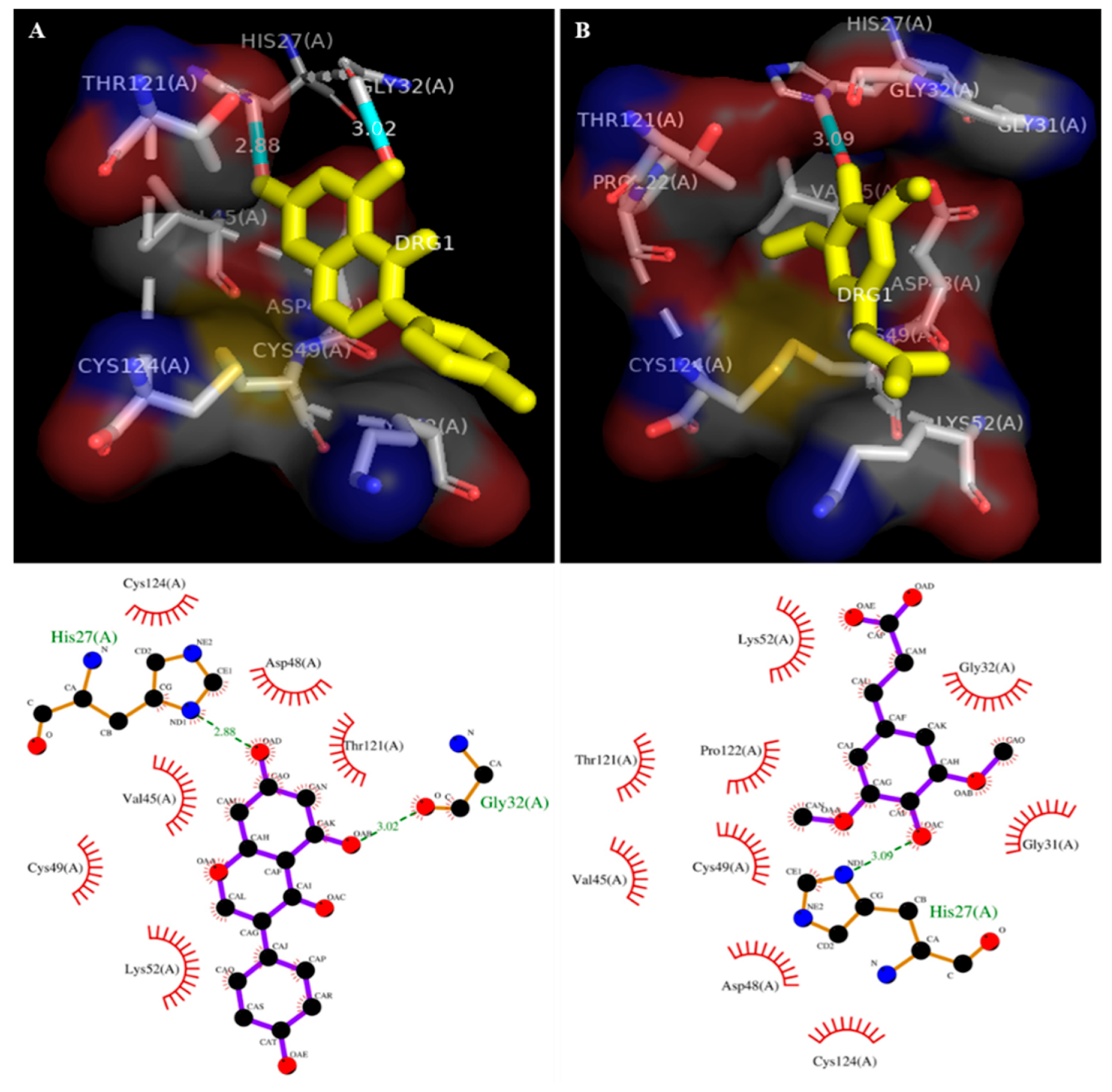

| No. | Ligands | Enzyme Name | Binding Energy (kcal/Mol) | No. of H Bonds | RMSD | Inhibition Constant (Ki) μM | Amino Acids Involved in Interactions Nteractions |
|---|---|---|---|---|---|---|---|
| 1 | Genistein | 1POE (Human PLA2) | −7.2 | 02 | 80.96 | 1.95 | Val45, Asp48, Cys49, Lys52, Thr121, Cys124 |
| 2 | Sinapic acid | −7.6 | 01 | 83.44 | 2.71 | Gly31, Gly32, Asp48, Cys49, Lys52, Thr121, Pro122, Cys124, |
| Antioxidant Assays | Sinapic Acid (25 µM) | Ascorbic Acid (25 µM) | Quercetin (25 µM) | α-Lipoic Acid (25 µM) |
|---|---|---|---|---|
| DPPH free radical | 89% ± 2.13 | 96% ± 1.78 | NT | NT |
| Reducing power | 63.5% ± 2.05 | NT | 66.5% ± 1.82 | NT |
| Anti-lipid peroxidation | 80.6% ± 1.07 | NT | NT | 89% ± 1.69 |
| Enzyme | Specific Activity * (nmol/mg/min at 37 °C) | IC50 (μM) # | Type of Inhibition | |
|---|---|---|---|---|
| Sinapic Acid | Genistein | |||
| sPLA2-IIA | 160.0 | 4.16 ± 0.13 | 11.75 [58] | Both are competitive inhibitors bound to the active site of the sPLA2-IIA. |
| * Secondary Structure of sPLA2-IIA | sPLA2-IIA Alone | sPLA2-IIA + Sinapic Acid (IC50) |
|---|---|---|
| α-helix | 43.66% | 21.47% |
| β-strand | 10.62% | 31.65% |
| Random coil | 45.72% | 46.88% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giresha, A.S.; Urs, D.; Pundalik, S.; Meti, R.S.; Pramod, S.N.; Supreetha, B.H.; Somegowda, M.; Dharmappa, K.K.; El-Shehawi, A.M.; Albogami, S.; et al. Sinapic Acid Inhibits Group IIA Secretory Phospholipase A2 and Its Inflammatory Response in Mice. Antioxidants 2022, 11, 1251. https://doi.org/10.3390/antiox11071251
Giresha AS, Urs D, Pundalik S, Meti RS, Pramod SN, Supreetha BH, Somegowda M, Dharmappa KK, El-Shehawi AM, Albogami S, et al. Sinapic Acid Inhibits Group IIA Secretory Phospholipase A2 and Its Inflammatory Response in Mice. Antioxidants. 2022; 11(7):1251. https://doi.org/10.3390/antiox11071251
Chicago/Turabian StyleGiresha, Aladahalli S., Deepadarshan Urs, Sophiya Pundalik, Rajkumar S. Meti, Siddanakoppalu N. Pramod, Ballenahalli H. Supreetha, Madhusudana Somegowda, Kattepura K. Dharmappa, Ahmed M. El-Shehawi, Sarah Albogami, and et al. 2022. "Sinapic Acid Inhibits Group IIA Secretory Phospholipase A2 and Its Inflammatory Response in Mice" Antioxidants 11, no. 7: 1251. https://doi.org/10.3390/antiox11071251
APA StyleGiresha, A. S., Urs, D., Pundalik, S., Meti, R. S., Pramod, S. N., Supreetha, B. H., Somegowda, M., Dharmappa, K. K., El-Shehawi, A. M., Albogami, S., Elseehy, M. M., Alaklabi, A., Elansary, H. O., Mehder, A. O. A., & Mahmoud, E. A. (2022). Sinapic Acid Inhibits Group IIA Secretory Phospholipase A2 and Its Inflammatory Response in Mice. Antioxidants, 11(7), 1251. https://doi.org/10.3390/antiox11071251










