Methodological Optimization of Supercritical Fluid Extraction of Valuable Bioactive Compounds from the Acidophilic Microalga Coccomyxa onubensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga Strain and Biomass Production
2.2. Chemicals
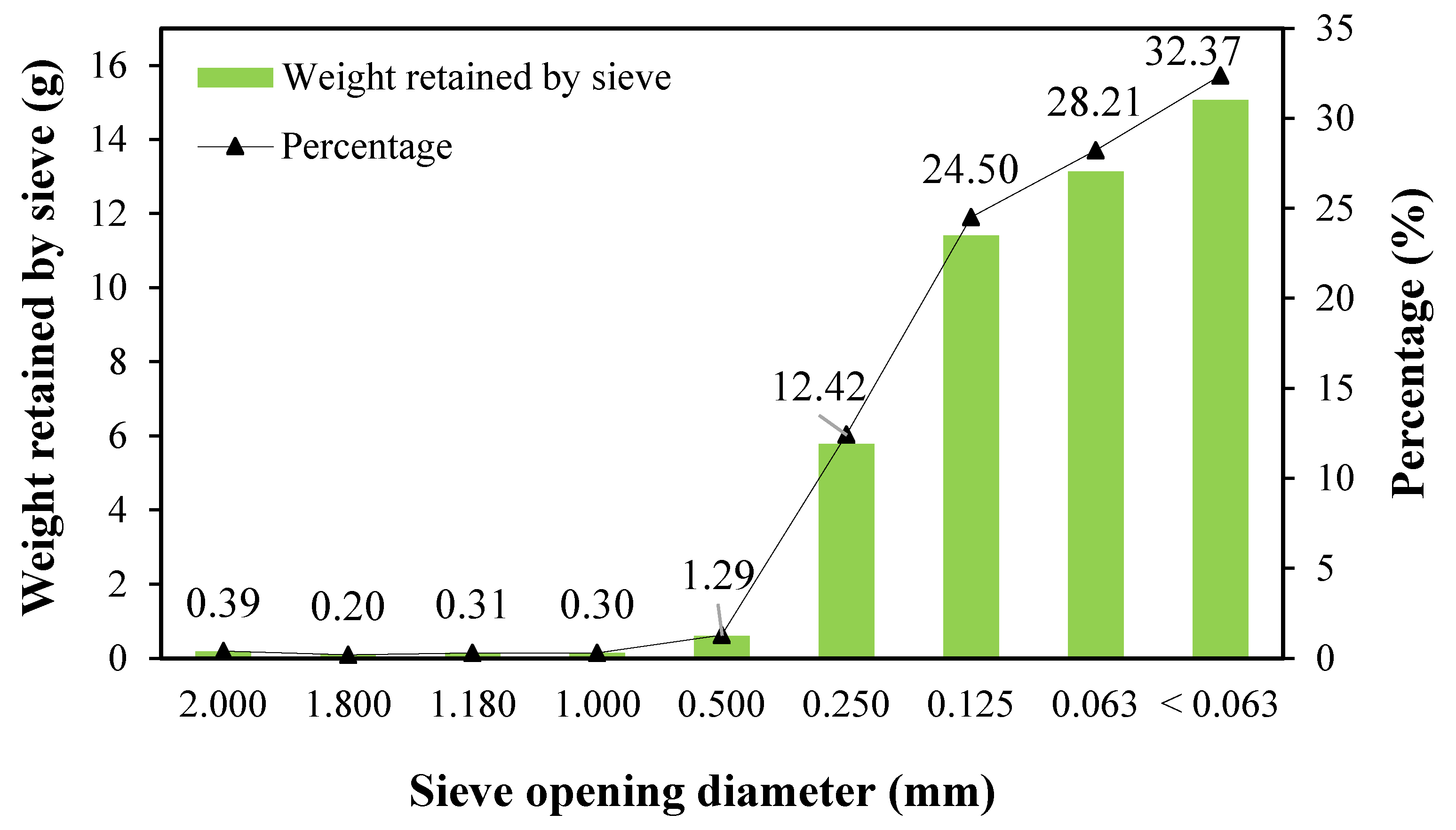
2.3. Determination of the Particle Size of Powdered Coccomyxa onubensis Biomass
2.4. SFE
2.5. Box–Behnken Experimental Design
2.6. Mathematical Modeling
2.7. Bioactive Compounds and Antioxidant Capacity
2.7.1. Lutein Quantification
2.7.2. Determination of TPC
2.7.3. Determination of Antioxidant Capacity
2.8. Statistical Analysis
3. Results
3.1. Particle Size
3.2. Mathematical Modeling and Kinetic Curve of Coccomyxa onubensis SFE Extracts
3.3. The Yield of Coccomyxa onubensis SFE Extracts
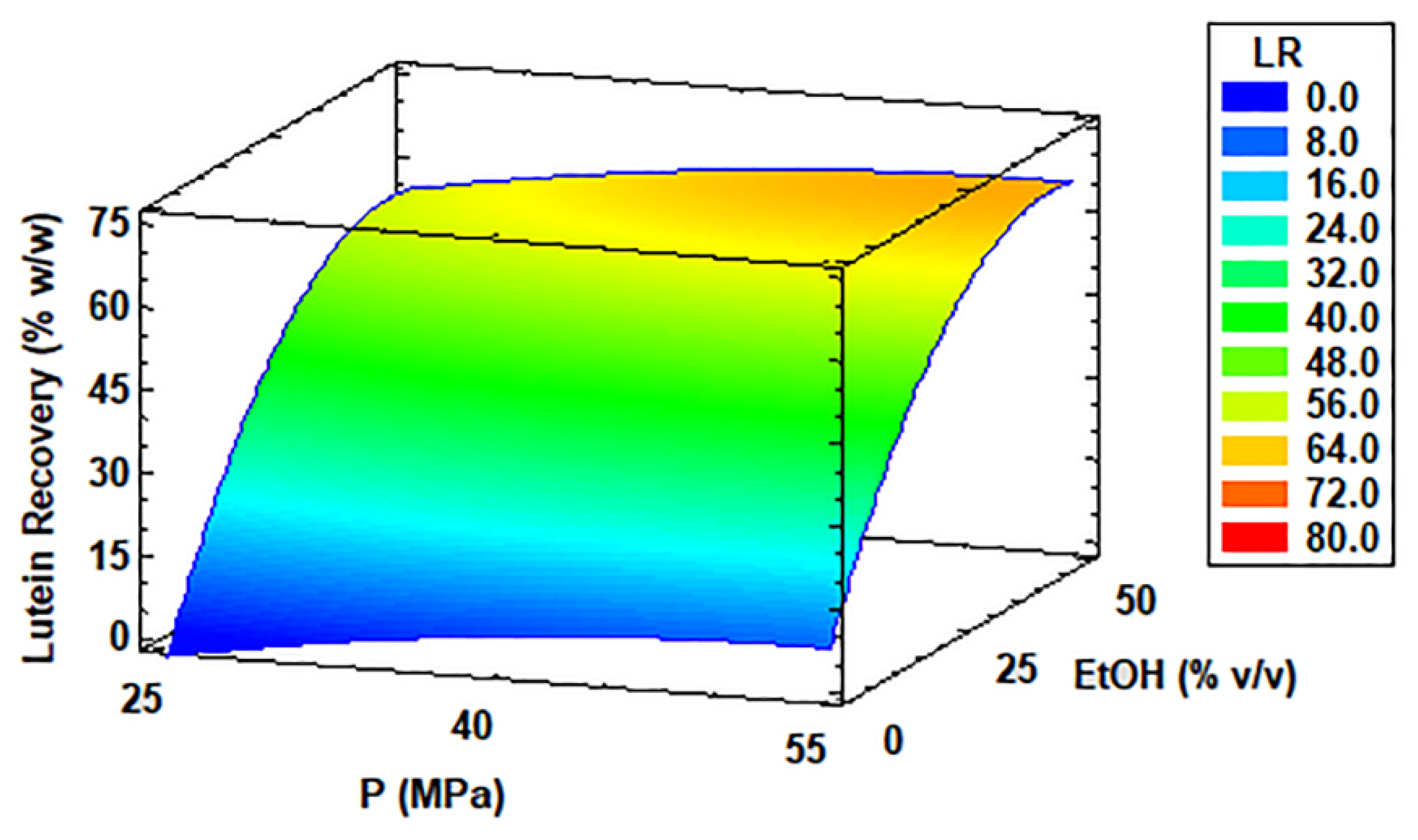
3.4. LP and LR of Coccomyxa onubensis SFE Extracts
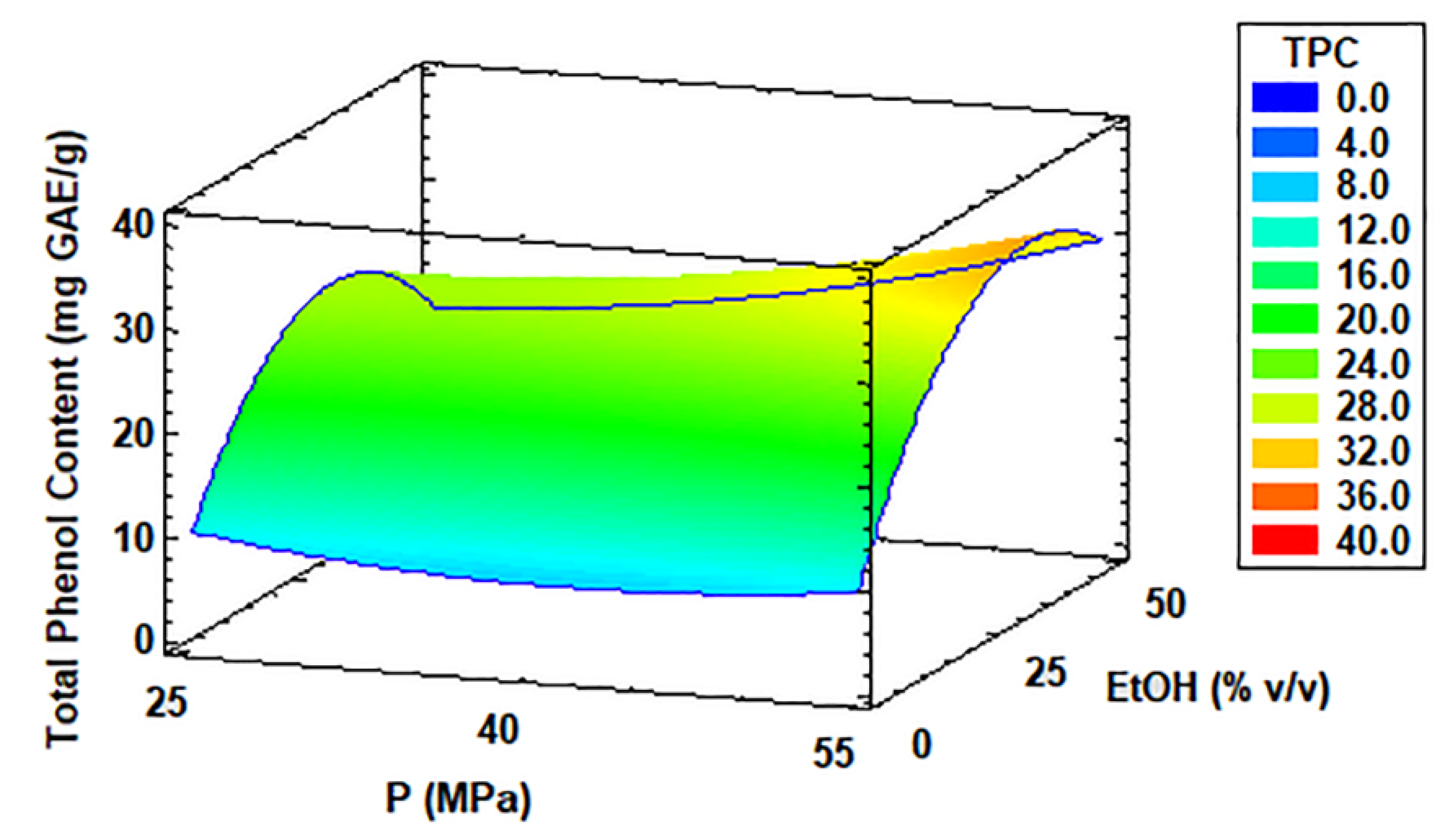
3.5. TPC of Coccomyxa onubensis SFE Extracts
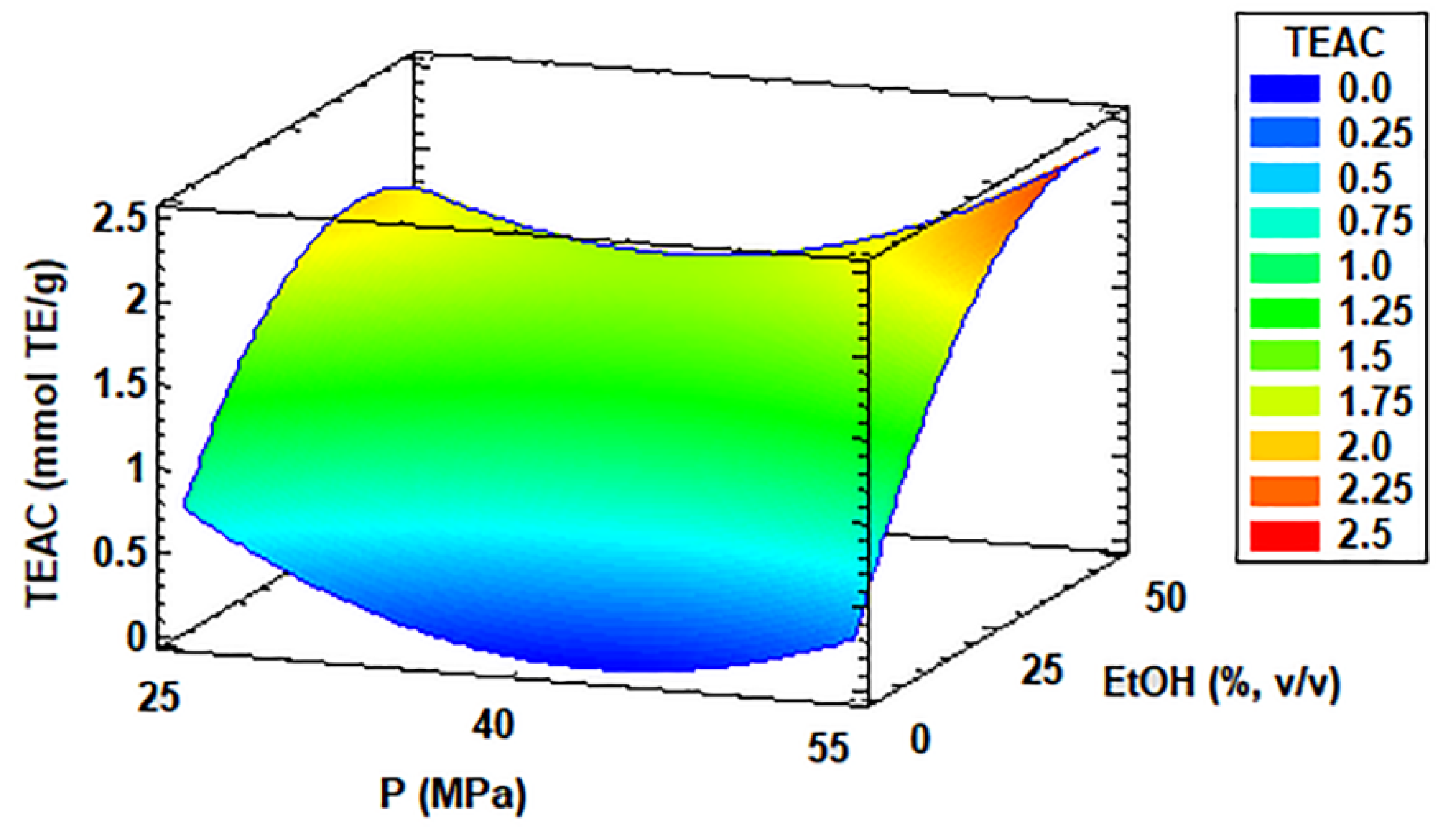
3.6. Antioxidant Activity of Coccomyxa onubensis SFE Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Barsanti, L.; Coltelli, P.; Evangelista, V.; Frassanito, A.M.; Passarelli, V.; Vesentini, N.; Gualtieri, P. Oddities and curiosities in the algal world. In Algal Toxins: Nature, Occurrence, Effect and Detection; Springer: Cham, Switzerland, 2008; pp. 353–391. [Google Scholar]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. A perspective on biotechnological applications of thermophilic microalgae and cyanobacteria. Bioresour. Technol. 2019, 278, 424–434. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Jiang, X.-R. Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 2018, 50, 94–100. [Google Scholar] [CrossRef]
- Das, P.; Aziz, S.S.; Obbard, J.P. Two phase microalgae growth in the open system for enhanced lipid productivity. Renew. Energy 2011, 36, 2524–2528. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Eseberri, I.; Fernández-Quintela, A.; Portillo, M.P. Anti-Obesity Effects of Microalgae. Int. J. Mol. Sci. 2020, 21, 41. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Navarro, F.; Toimil, A.; Ramírez, S.; Montero, Y.; Fuentes, J.L.; Perona, J.S.; Castaño, M.Á.; Pásaro, R.; Vega, J.M.; Vílchez, C. The acidophilic microalga Coccomyxa onubensis and atorvastatin equally improve antihyperglycemic and antihyperlipidemic protective effects on rats fed on high-fat diets. J. Appl. Phycol. 2020, 32, 3923–3931. [Google Scholar] [CrossRef]
- Forján, E.; Navarro, F.; Cuaresma, M.; Vaquero, I.; Ruíz-Domínguez, M.C.; Gojkovic, Ž.; Vázquez, M.; Márquez, M.; Mogedas, B.; Bermejo, E.; et al. Microalgae: Fast-Growth Sustainable Green Factories. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1705–1755. [Google Scholar] [CrossRef]
- Garbayo, I.; Torronteras, R.; Forján, E.; Cuaresma, M.; Casal, C.; Mogedas, B.; Ruiz-Domínguez, M.C.; Márquez, C.; Vaquero, I.; Fuentes-Cordero, J.L.; et al. Identification and physiological aspects of a novel carotenoid-enriched, metal-resistant microalga isolated from an Acidic River in Huelva (Spain). J. Phycol. 2012, 48, 607–614. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Vaquero, I.; Obregón, V.; de la Morena, B.; Vílchez, C.; Vega, J.M. Lipid accumulation and antioxidant activity in the eukaryotic acidophilic microalga Coccomyxa sp. (strain onubensis) under nutrient starvation. J. Appl. Phycol. 2015, 27, 1099–1108. [Google Scholar] [CrossRef]
- Vaquero, I.; Ruiz-Domínguez, M.C.; Márquez, M.; Vílchez, C. Cu-mediated biomass productivity enhancement and lutein enrichment of the novel microalga Coccomyxa onubensis. Process. Biochem. 2012, 47, 694–700. [Google Scholar] [CrossRef]
- Casal, C.; Cuaresma, M.; Vega, J.M.; Vilchez, C. Enhanced productivity of a lutein-enriched novel acidophile microalga grown on urea. Mar. Drugs 2010, 9, 29–42. [Google Scholar] [CrossRef]
- Bermejo, E.; Ruiz-Domínguez, M.C.; Cuaresma, M.; Vaquero, I.; Ramos-Merchante, A.; Vega, J.M.; Vílchez, C.; Garbayo, I. Production of lutein, and polyunsaturated fatty acids by the acidophilic eukaryotic microalga Coccomyxa onubensis under abiotic stress by salt or ultraviolet light. J. Biosci. Bioeng. 2018, 125, 669–675. [Google Scholar] [CrossRef]
- Mozaffarieh, M.; Sacu, S.; Wedrich, A. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: A review based on controversial evidence. Nutr. J. 2003, 2, 20. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Navarro, F.; Forján, E.; Vázquez, M.; Montero, Z.; Bermejo, E.; Castaño, M.; Toimil, A.; Chagüaceda, E.; García-Sevillano, M.; Sánchez, M.; et al. Microalgae as a safe food source for animals: Nutritional characteristics of the acidophilic microalga Coccomyxa onubensis. Food Nutr. Res. 2016, 60, 30472. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibañez, E.; Señoráns, F.J. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Ibáñez, E. Green processes and sustainability: An overview on the extraction of high added-value products from seaweeds and microalgae. J. Supercrit. Fluids 2015, 96, 211–216. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Huss, V.A.R.; Montero, Z.; Torronteras, R.; Cuaresma, M.; Garbayo, I.; Vílchez, C. Phylogenetic characterization and morphological and physiological aspects of a novel acidotolerant and halotolerant microalga Coccomyxa onubensis sp. nov. (Chlorophyta, Trebouxiophyceae). J. Appl. Phycol. 2016, 28, 3269–3279. [Google Scholar] [CrossRef]
- ASABE. Method of Determining and Expressing Particle Size of Chopped Forage Materials by Screening. ANSI/ASAE S424.1. Am. Soc. Agric. Biol. Eng. 2007, 663–665. [Google Scholar]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenez, D.; Munoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal-Mezquita, P. Supercritical fluid extraction of chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 161, 104824. [Google Scholar] [CrossRef]
- Meireles, M.A.A. Extraction of Bioactive Compounds from Latin American Plants. In Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 243–274. [Google Scholar]
- Ruiz-Domínguez, M.C.; Marticorena, P.; Sepúlveda, C.; Salinas, F.; Cerezal, P.; Riquelme, C. Effect of Drying Methods on Lutein Content and Recovery by Supercritical Extraction from the Microalga Muriellopsis sp. (MCH35) Cultivated in the Arid North of Chile. Mar. Drugs 2020, 18, 528. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef]
- Mouahid, A.; Crampon, C.; Toudji, S.-A.A.; Badens, E. Supercritical CO2 extraction of neutral lipids from microalgae: Experiments and modelling. J. Supercrit. Fluids 2013, 77, 7–16. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction: Topics in Physical Chemistry. Steinkopff Darmstadt; Springer: New York, NY, USA, 1994; pp. 59–145. [Google Scholar]
- Sovová, H.; Nobre, B.P.; Palavra, A. Modeling of the kinetics of supercritical fluid extraction of lipids from microalgae with emphasis on extract desorption. Materials 2016, 9, 423. [Google Scholar] [CrossRef]
- Andrich, G.; Nesti, U.; Venturi, F.; Zinnai, A.; Fiorentini, R. Supercritical fluid extraction of bioactive lipids from the microalga Nannochloropsis sp. Eur. J. Lipid Sci. Technol. 2005, 107, 381–386. [Google Scholar] [CrossRef]
- Andrich, G.; Zinnai, A.; Nesti, U.; Venturi, F.; Fiorentini, R. Supercritical fluid extraction of oil from microalga Spirulina (Arthrospira) platensis. Acta Aliment. 2006, 35, 195–203. [Google Scholar] [CrossRef]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Jesus, S.P.; Calheiros, M.N.; Hense, H.; Meireles, M.A.A. A simplified model to describe the kinetic behavior of supercritical fluid extraction from a rice bran oil byproduct. Food Public Health 2013, 3, 215–222. [Google Scholar] [CrossRef][Green Version]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess. Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Mezzomo, N.; Martínez, J.; Ferreira, S.R. Supercritical fluid extraction of peach (Prunus persica) almond oil: Kinetics, mathematical modeling and scale-up. J. Supercrit. Fluids 2009, 51, 10–16. [Google Scholar] [CrossRef]
- Sanzo, G.D.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical Carbon Dioxide Extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef]
- Yen, H.-W.; Chiang, W.-C.; Sun, C.-H. Supercritical fluid extraction of lutein from Scenedesmus cultured in an autotrophical photobioreactor. J. Taiwan Inst. Chem. Eng. 2012, 43, 53–57. [Google Scholar] [CrossRef]
- Mehariya, S.; Iovine, A.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Casella, P.; Karatza, D.; Marino, T.; Musmarra, D.; et al. Supercritical Fluid Extraction of Lutein from Scenedesmus almeriensis. Molecules 2019, 24, 1324. [Google Scholar] [CrossRef]
- del Valle, J.M. Extraction of natural compounds using supercritical CO2: Going from the laboratory to the industrial application. J. Supercrit. Fluids 2015, 96, 180–199. [Google Scholar] [CrossRef]
- Lucien, F.P.; Foster, N.R. Solubilities of solid mixtures in supercritical carbon dioxide: A review. J. Supercrit. Fluids 2000, 17, 111–134. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.; Welti-Chanes, J. State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef]
- Ting, S.S.; Tomasko, D.L.; Foster, N.R.; Macnaughton, S.J. Solubility of naproxen in supercritical carbon dioxide with and without cosolvents. Ind. Eng. Chem. Res. 1993, 32, 1471–1481. [Google Scholar] [CrossRef]
- Foster, N.R.; Singh, H.; Yun, S.J.; Tomasko, D.L.; Macnaughton, S.J. Polar and nonpolar cosolvent effects on the solubility of cholesterol in supercritical fluids. Ind. Eng. Chem. Res. 1993, 32, 2849–2853. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Bueno, M.; Vitali, C.; Sánchez-Martínez, J.D.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Compressed CO2 technologies for the recovery of carotenoid-enriched extracts from Dunaliella salina with potential neuroprotective activity. ACS Sustain. Chem. Eng. 2020, 8, 11413–11423. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; Broek, L.A.M.v.d.; Houweling-Tan, B.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Green compressed fluid technologies for downstream processing of Scenedesmus obliquus in a biorefinery approach. Algal Res. 2017, 24, 111–121. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Sapkale, G.; Patil, S.; Surwase, U.; Bhatbhage, P. Supercritical fluid extraction. Int. J. Chem. Sci. 2010, 8, 729–743. [Google Scholar]
- Vechpanich, J.; Shotipruk, A. Recovery of free lutein from Tagetes erecta: Determination of suitable saponification and crystallization conditions. Sep. Sci. Technol. 2010, 46, 265–271. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Vargas, M.A.; Rivas, J.N.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef]
- Fabrowska, J.; Ibañez, E.; Łęska, B.; Herrero, M. Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res. 2016, 19, 237–245. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Cerezal, P.; Salinas, F.; Medina, E.; Renato-Castro, G. Application of Box-Behnken Design and Desirability Function for Green Prospection of Bioactive Compounds from Isochrysis galbana. Appl. Sci. 2020, 10, 2789. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
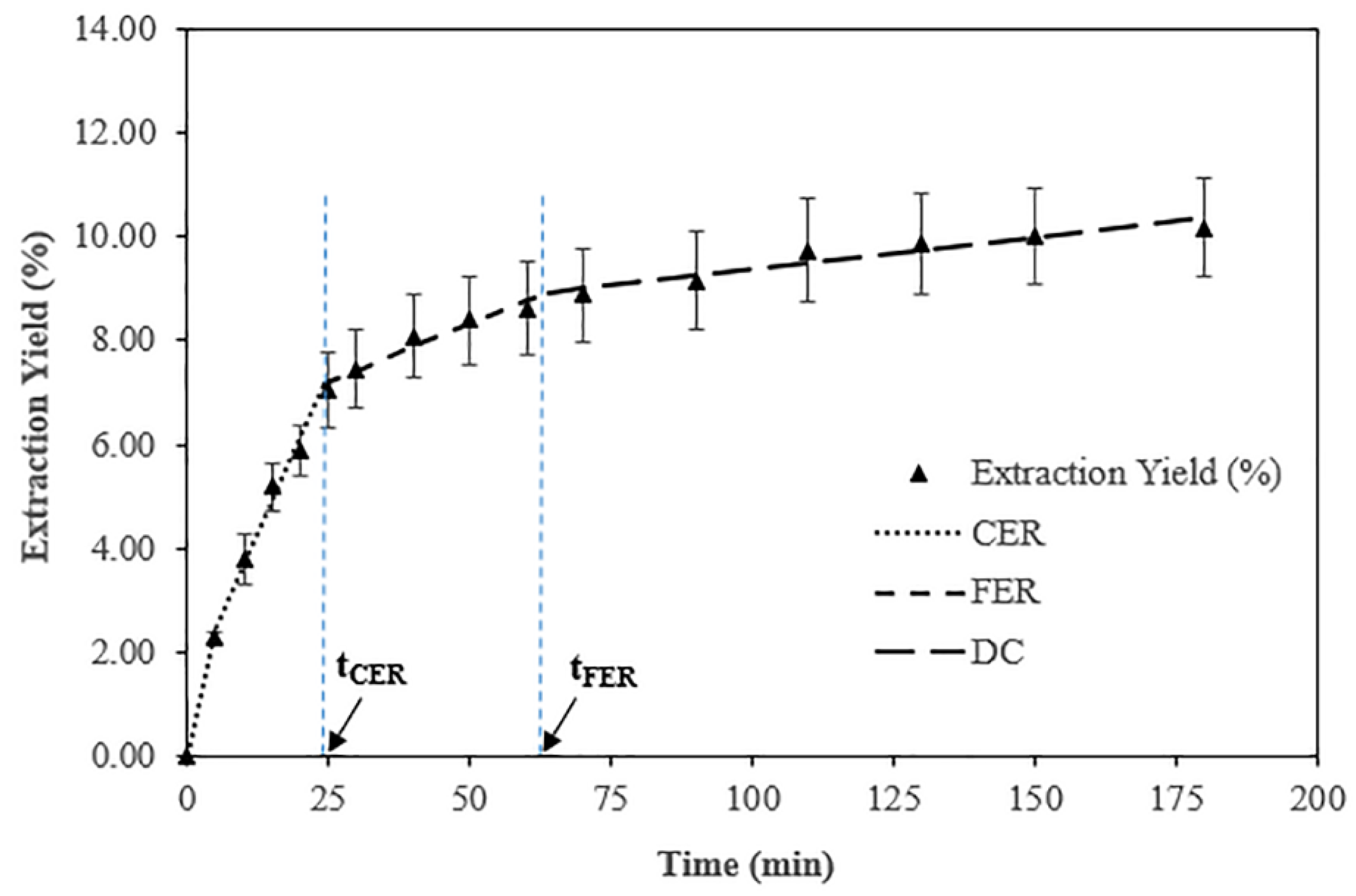


| Parameters | Stages of the General Extraction Curve | ||||
| CER | FER | DC | |||
| Time (min.) | 24.33 | 63.86 | 180.0 | ||
| Accumulated (%) | 7.18 | 1.76 | 1.42 | ||
| Accumulated Extract (%) | 7.18 | 8.94 | 10.36 | ||
| Recovery (%) | 68.53 | 17.32 | 14.15 | ||
| Total Recovery (%) | 68.53 | 85.85 | 100.0 | ||
| MEXT (g/min) | 6.0 × 10–3 | 9.3 × 10−4 | 2.6 × 10−4 | ||
| Y (mg extract/g biomass) | 70.27 | 17.56 | 14.73 | ||
| Y (g extract/g (CO2 75% + ethanol 25%)) | 1.91 × 10–3 | 3.0 × 10−4 | 8.5 × 10−5 | ||
| R2 | 0.9793 | 1.0000 | 1.0000 | ||
| Linear Coefficients of Spline model | b0 | a1 | a2 | a3 | |
| 1.2223 | 0.2449 | –0.2003 | –0.0324 | ||
| Run | T (°C) | P (MPa) | EtOH (%, v/v) | Y (%, w/w) | LP (mg/g Extract) | LR (%, w/w) | TPC (mg GAE/g Extract) | TEAC (mmol TE/g Extract) |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 40 | 25 | 7.95 | 16.07 | 40.71 | 22.64 | 0.693 |
| 2 | 30 | 25 | 25 | 5.92 | 12.37 | 23.33 | 10.45 | 0.285 |
| 3 | 70 | 25 | 25 | 7.31 | 16.23 | 37.81 | 25.96 | 1.708 |
| 4 | 30 | 55 | 25 | 4.68 | 18.55 | 27.66 | 19.13 | 2.237 |
| 5 | 70 | 55 | 25 | 6.58 | 25.39 | 53.22 | 36.08 | 2.149 |
| 6 | 30 | 40 | 0 | 0.79 | 4.82 | 1.22 | 7.34 | 0.104 |
| 7 | 70 | 40 | 0 | 2.99 | 8.22 | 7.84 | 6.23 | 0.011 |
| 8 | 50 | 40 | 25 | 8.28 | 15.15 | 39.99 | 27.51 | 1.148 |
| 9 | 30 | 40 | 50 | 13.28 | 1.49 | 6.33 | 3.94 | 0.387 |
| 10 | 70 | 40 | 50 | 13.27 | 15.83 | 66.98 | 18.67 | 1.490 |
| 11 | 50 | 25 | 0 | 1.58 | 10.56 | 5.33 | 13.44 | 0.297 |
| 12 | 50 | 55 | 0 | 2.47 | 7.48 | 5.88 | 7.19 | 0.198 |
| 13 | 50 | 25 | 50 | 16.32 | 7.09 | 36.89 | 15.02 | 0.809 |
| 14 | 50 | 55 | 50 | 10.14 | 11.54 | 37.29 | 20.97 | 1.744 |
| 15 | 50 | 40 | 25 | 8.73 | 16.32 | 45.44 | 21.35 | 0.723 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Domínguez, M.C.; Medina, E.; Salinas, F.; Bugueño, W.; Fuentes, J.-L.; Vílchez, C.; Garbayo, I.; Cerezal-Mezquita, P. Methodological Optimization of Supercritical Fluid Extraction of Valuable Bioactive Compounds from the Acidophilic Microalga Coccomyxa onubensis. Antioxidants 2022, 11, 1248. https://doi.org/10.3390/antiox11071248
Ruiz-Domínguez MC, Medina E, Salinas F, Bugueño W, Fuentes J-L, Vílchez C, Garbayo I, Cerezal-Mezquita P. Methodological Optimization of Supercritical Fluid Extraction of Valuable Bioactive Compounds from the Acidophilic Microalga Coccomyxa onubensis. Antioxidants. 2022; 11(7):1248. https://doi.org/10.3390/antiox11071248
Chicago/Turabian StyleRuiz-Domínguez, Mari Carmen, Elena Medina, Francisca Salinas, Waldo Bugueño, Juan-Luis Fuentes, Carlos Vílchez, Inés Garbayo, and Pedro Cerezal-Mezquita. 2022. "Methodological Optimization of Supercritical Fluid Extraction of Valuable Bioactive Compounds from the Acidophilic Microalga Coccomyxa onubensis" Antioxidants 11, no. 7: 1248. https://doi.org/10.3390/antiox11071248
APA StyleRuiz-Domínguez, M. C., Medina, E., Salinas, F., Bugueño, W., Fuentes, J.-L., Vílchez, C., Garbayo, I., & Cerezal-Mezquita, P. (2022). Methodological Optimization of Supercritical Fluid Extraction of Valuable Bioactive Compounds from the Acidophilic Microalga Coccomyxa onubensis. Antioxidants, 11(7), 1248. https://doi.org/10.3390/antiox11071248







