Mitochondrial Peroxiredoxin-IIF (PRXIIF) Activity and Function during Seed Aging
Abstract
:1. Introduction
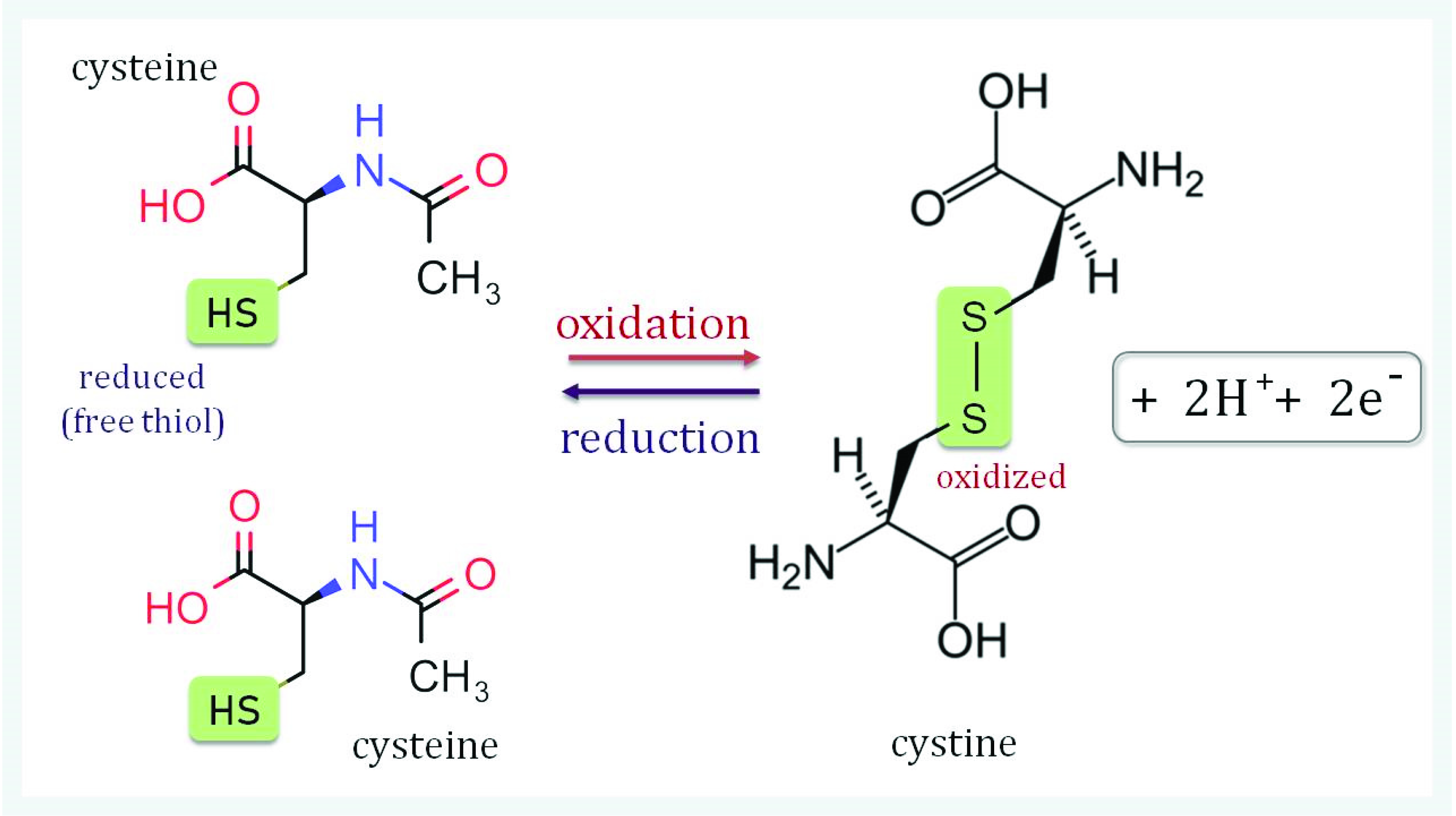
2. Seed Aging and Changes in Redox State
3. Redox Signaling in Mitochondria
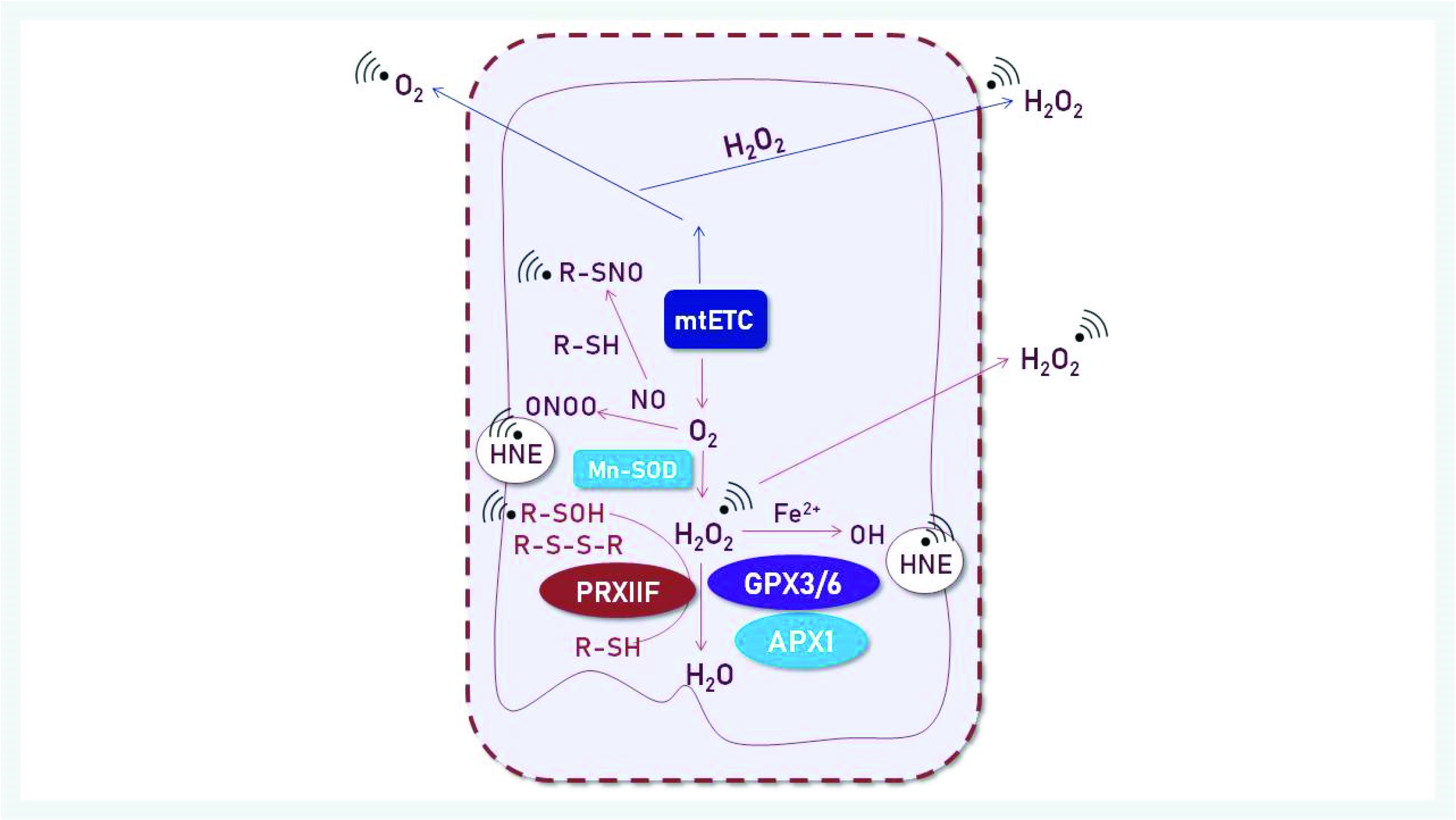
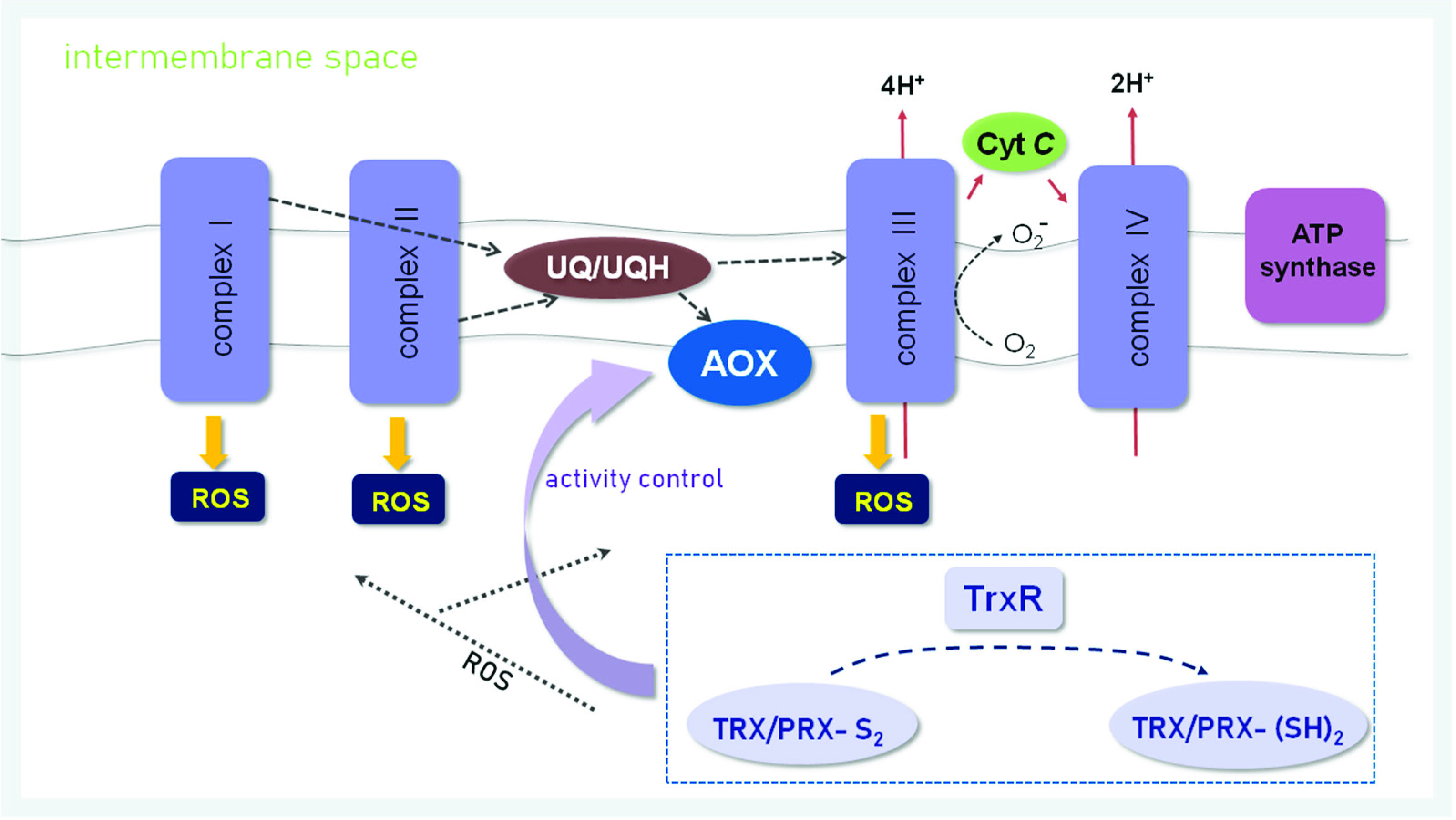
3.1. ROS Generation
3.2. The Role of Mitochondria in Cell Redox Homeostasis
3.3. Peroxide Detoxification by Peroxiredoxins
4. The Protective Function of PRXIIF in Seeds
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jacobson, F.S.; Morgan, R.W.; Christman, M.F.; Ames, B.N. An Alkyl Hydroperoxide Reductase from Salmonella Typhimurium Involved in the Defense of DNA against Oxidative Damage. Purification and Properties. J. Biol. Chem. 1989, 264, 1488–1496. [Google Scholar] [CrossRef]
- Liebthal, M.; Maynard, D.; Dietz, K.-J. Peroxiredoxins and Redox Signaling in Plants. Antioxid. Redox Signal. 2018, 28, 609–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Jang, H.H. Role of Cytosolic 2-Cys Prx1 and Prx2 in Redox Signaling. Antioxidants 2019, 8, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, S.G.; Kang, S.W.; Netto, L.E.; Seo, M.S.; Stadtman, E.R. A Family of Novel Peroxidases, Peroxiredoxins. BioFactors 1999, 10, 207–209. [Google Scholar] [CrossRef]
- Liebthal, M.; Kushwah, M.S.; Kukura, P.; Dietz, K.-J. Single Molecule Mass Photometry Reveals the Dynamic Oligomerization of Human and Plant Peroxiredoxins. iScience 2021, 24, 103258. [Google Scholar] [CrossRef]
- O’Neill, J.S.; van Ooijen, G.; Dixon, L.E.; Troein, C.; Corellou, F.; Bouget, F.-Y.; Reddy, A.B.; Millar, A.J. Circadian Rhythms Persist without Transcription in a Eukaryote. Nature 2011, 469, 554–558. [Google Scholar] [CrossRef]
- Flohé, L.; Budde, H.; Hofmann, B. Peroxiredoxins in Antioxidant Defense and Redox Regulation. BioFactors 2003, 19, 3–10. [Google Scholar] [CrossRef]
- Bréhélin, C.; Meyer, E.H.; de Souris, J.-P.; Bonnard, G.; Meyer, Y. Resemblance and Dissemblance of Arabidopsis Type II Peroxiredoxins: Similar Sequences for Divergent Gene Expression, Protein Localization, and Activity. Plant Physiol. 2003, 132, 2045–2057. [Google Scholar] [CrossRef] [Green Version]
- Okumura, M.; Saiki, M.; Yamaguchi, H.; Hidaka, Y. Acceleration of Disulfide-Coupled Protein Folding Using Glutathione Derivatives. FEBS J. 2011, 278, 1137–1144. [Google Scholar] [CrossRef]
- Lv, W.-T.; Lin, B.; Zhang, M.; Hua, X.-J. Proline Accumulation Is Inhibitory to Arabidopsis Seedlings during Heat Stress. Plant Physiol. 2011, 156, 1921–1933. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Méndez, K.J.; Sánchez Segura, L.; Chagolla, A.; Lino, B.; González de la Vara, L.E. Voltage-Dependent Anion-Selective Channels and Other Mitochondrial Membrane Proteins Form Diverse Complexes in Beetroots Subjected to Flood-Induced Programmed Cell Death. Front. Plant Sci. 2021, 12, 714847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-F.; Li, J.; Bi, F.-C.; Liu, Z.; Chang, Z.-Y.; Wang, L.-Y.; Huang, L.-Q.; Yao, N. Ceramide-Induced Cell Death Depends on Calcium and Caspase-Like Activity in Rice. Front. Plant Sci. 2020, 11, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, E.; Dietz, K.-J.; Kalemba, E.M. The Occurrence of Peroxiredoxins and Changes in Redox State in Acer platanoides and Acer pseudoplatanus During Seed Development. J. Plant Growth Regul. 2019, 38, 298–314. [Google Scholar] [CrossRef] [Green Version]
- Martí, M.C.; Jiménez, A.; Sevilla, F. Thioredoxin Network in Plant Mitochondria: Cysteine S-Posttranslational Modifications and Stress Conditions. Front. Plant Sci. 2020, 11, 6571288. [Google Scholar] [CrossRef]
- Ratajczak, E.; Małecka, A.; Ciereszko, I.; Staszak, A.M. Mitochondria Are Important Determinants of the Aging of Seeds. Int. J. Mol. Sci. 2019, 20, 1568. [Google Scholar] [CrossRef] [Green Version]
- Sweetlove, L.J.; Heazlewood, J.L.; Herald, V.; Holtzapffel, R.; Day, D.A.; Leaver, C.J.; Millar, A.H. The Impact of Oxidative Stress on Arabidopsis Mitochondria. Plant J. 2002, 32, 891–904. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring Control: The Evolution of ROS-Induced Oxidative Stress and Redox Signaling Pathways in Plant Stress Responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive Oxygen Species, Heat Stress and Oxidative-Induced Mitochondrial Damage. A Review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, e217037. [Google Scholar] [CrossRef] [Green Version]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the News: Subcellular and Organellar Reactive Oxygen Species Production and Signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J. Redox Signal Integration: From Stimulus to Networks and Genes. Physiol. Plant. 2008, 133, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox Sensing and Signalling Associated with Reactive Oxygen in Chloroplasts, Peroxisomes and Mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Navrot, N.; Finnie, C.; Svensson, B.; Hägglund, P. Plant Redox Proteomics. J. Proteom. 2011, 74, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, P.; Masiero, S.; Eubel, H.; Braun, H.-P.; Bhushan, S.; Glaser, E.; Salamini, F.; Leister, D. Nuclear Photosynthetic Gene Expression Is Synergistically Modulated by Rates of Protein Synthesis in Chloroplasts and Mitochondria. Plant Cell 2006, 18, 970–991. [Google Scholar] [CrossRef] [Green Version]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.J.; Lehmann, M.; Schwarzländer, M.; Baxter, C.J.; Sienkiewicz-Porzucek, A.; Williams, T.C.R.; Schauer, N.; Fernie, A.R.; Fricker, M.D.; Ratcliffe, R.G.; et al. Decrease in Manganese Superoxide Dismutase Leads to Reduced Root Growth and Affects Tricarboxylic Acid Cycle Flux and Mitochondrial Redox Homeostasis. Plant Physiol. 2008, 147, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.S.; ElSayed, A.I.; Moore, M.; Dietz, K.-J. Redox and Reactive Oxygen Species Network in Acclimation for Salinity Tolerance in Sugar Beet. J. Exp. Bot. 2017, 68, 1283–1298. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Bilan, D.S.; Belousov, V.V. In Vivo Imaging of Hydrogen Peroxide with HyPer Probes. Antioxid. Redox Signal. 2018, 29, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Gerken, M.; Kakorin, S.; Chibani, K.; Dietz, K.-J. Computational Simulation of the Reactive Oxygen Species and Redox Network in the Regulation of Chloroplast Metabolism. PLoS Comput. Biol. 2020, 16, e1007102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; De Paepe, R.; Foyer, C.H. Mitochondrial Redox Biology and Homeostasis in Plants. Trends Plant Sci. 2007, 12, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Møller, I.M. Chapter 1 Oxidation of Proteins in Plants—Mechanisms and Consequences. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2009; Volume 52, pp. 1–23. [Google Scholar] [CrossRef]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial Reactive Oxygen Species. Contribution to Oxidative Stress and Interorganellar Signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juszczuk, I.M.; Rychter, A.M. Alternative Oxidase in Higher Plants. Acta Biochim. Pol. 2003, 50, 1257–1271. [Google Scholar] [CrossRef] [Green Version]
- Møller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH Turnover, and Metabolism of Reactive Oxygen Species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Vanlerberghe, G.C. Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Ostaszewska-Bugajska, M.; Juszczuk, I.M. Changes in the OXPHOS System in Leaf and Root Mitochondria of Arabidopsis thaliana Subjected to Long-Term Sulphur Deficiency. Acta Physiol. Plant. 2016, 38, 141. [Google Scholar] [CrossRef] [Green Version]
- Małecka, A.; Ciszewska, L.; Staszak, A.; Ratajczak, E. Relationship between Mitochondrial Changes and Seed Aging as a Limitation of Viability for the Storage of Beech Seed (Fagus Sylvatica L.). PeerJ 2021, 9, e10569. [Google Scholar] [CrossRef]
- Umbach, A.L.; Siedow, J.N. The Cyanide-Resistant Alternative Oxidases from the Fungi Pichia Stipitis and Neurospora Crassa Are Monomeric and Lack Regulatory Features of the Plant Enzyme. Arch. Biochem. Biophys. 2000, 378, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.C.; Olmos, E.; Calvete, J.J.; Díaz, I.; Barranco-Medina, S.; Whelan, J.; Lázaro, J.J.; Sevilla, F.; Jiménez, A. Mitochondrial and Nuclear Localization of a Novel Pea Thioredoxin: Identification of Its Mitochondrial Target Proteins. Plant Physiol. 2009, 150, 646–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florez-Sarasa, I.; Obata, T.; Del-Saz, N.S.F.N.; Reichheld, J.-P.; Meyer, E.H.; Rodriguez-Concepcion, M.; Ribas-Carbo, M.; Fernie, A.R. The Lack of Mitochondrial Thioredoxin TRXo1 Affects In Vivo Alternative Oxidase Activity and Carbon Metabolism under Different Light Conditions. Plant Cell Physiol. 2019, 60, 2369–2381. [Google Scholar] [CrossRef]
- Balmer, Y.; Vensel, W.H.; Tanaka, C.K.; Hurkman, W.J.; Gelhaye, E.; Rouhier, N.; Jacquot, J.-P.; Manieri, W.; Schürmann, P.; Droux, M.; et al. Thioredoxin Links Redox to the Regulation of Fundamental Processes of Plant Mitochondria. Proc. Natl. Acad. Sci. USA 2004, 101, 2642–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lázaro, J.J.; Jiménez, A.; Camejo, D.; Iglesias-Baena, I.; Martí, M.D.C.; Lázaro-Payo, A.; Barranco-Medina, S.; Sevilla, F. Dissecting the Integrative Antioxidant and Redox Systems in Plant Mitochondria. Effect of Stress and S-Nitrosylation. Front. Plant Sci. 2013, 4, 460. [Google Scholar] [CrossRef] [Green Version]
- Laloi, C.; Rayapuram, N.; Chartier, Y.; Grienenberger, J.-M.; Bonnard, G.; Meyer, Y. Identification and Characterization of a Mitochondrial Thioredoxin System in Plants. Proc. Natl. Acad. Sci. USA 2001, 98, 14144–14149. [Google Scholar] [CrossRef] [Green Version]
- Gelhaye, E.; Rouhier, N.; Gérard, J.; Jolivet, Y.; Gualberto, J.; Navrot, N.; Ohlsson, P.-I.; Wingsle, G.; Hirasawa, M.; Knaff, D.B.; et al. A Specific Form of Thioredoxin h Occurs in Plant Mitochondria and Regulates the Alternative Oxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 14545–14550. [Google Scholar] [CrossRef] [Green Version]
- Barranco-Medina, S.; Krell, T.; Finkemeier, I.; Sevilla, F.; Lázaro, J.-J.; Dietz, K.-J. Biochemical and Molecular Characterization of the Mitochondrial Peroxiredoxin PsPrxII F from Pisum Sativum. Plant Physiol. Biochem. 2007, 45, 729–739. [Google Scholar] [CrossRef]
- Attacha, S.; Solbach, D.; Bela, K.; Moseler, A.; Wagner, S.; Schwarzländer, M.; Aller, I.; Müller, S.J.; Meyer, A.J. Glutathione Peroxidase-like Enzymes Cover Five Distinct Cell Compartments and Membrane Surfaces in Arabidopsis Thaliana. Plant Cell Environ. 2017, 40, 1281–1295. [Google Scholar] [CrossRef]
- Beer, S.M.; Taylor, E.R.; Brown, S.E.; Dahm, C.C.; Costa, N.J.; Runswick, M.J.; Murphy, M.P. Glutaredoxin 2 Catalyzes the Reversible Oxidation and Glutathionylation of Mitochondrial Membrane Thiol Proteins: Implications for Mitochondrial Redox regulation and antioxidant defense. J. Biol. Chem. 2004, 279, 47939–47951. [Google Scholar] [CrossRef] [Green Version]
- Moseler, A.; Aller, I.; Wagner, S.; Nietzel, T.; Przybyla-Toscano, J.; Mühlenhoff, U.; Lill, R.; Berndt, C.; Rouhier, N.; Schwarzländer, M.; et al. The Mitochondrial Monothiol Glutaredoxin S15 Is Essential for Iron-Sulfur Protein Maturation in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2015, 112, 13735–13740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseler, A.; Kruse, I.; Maclean, A.E.; Pedroletti, L.; Franceschetti, M.; Wagner, S.; Wehler, R.; Fischer-Schrader, K.; Poschet, G.; Wirtz, M.; et al. The Function of Glutaredoxin GRXS15 Is Required for Lipoyl-Dependent Dehydrogenases in Mitochondria. Plant Physiol. 2021, 186, 1507–1525. [Google Scholar] [CrossRef] [PubMed]
- Delledonne, M. NO News Is Good News for Plants. Curr. Opin. Plant Biol. 2005, 8, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Kumari, A.; Florez-Sarasa, I.; Fernie, A.R.; Igamberdiev, A.U. Interaction of Nitric Oxide with the Components of the Plant Mitochondrial Electron Transport Chain. J. Exp. Bot. 2018, 69, 3413–3424. [Google Scholar] [CrossRef] [Green Version]
- Shahpiri, A.; Svensson, B.; Finnie, C. The NADPH-Dependent Thioredoxin Reductase/Thioredoxin System in Germinating Barley Seeds: Gene Expression, Protein Profiles, and Interactions between Isoforms of Thioredoxin h and Thioredoxin Reductase. Plant Physiol. 2008, 146, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, A.; Treffon, P.; Basiry, D.; Jozefowicz, A.M.; Matros, A.; Mock, H.-P.; Dietz, K.-J. Function and Regulation of Chloroplast Peroxiredoxin IIE. Antioxidants 2021, 10, 152. [Google Scholar] [CrossRef]
- Horling, F.; König, J.; Dietz, K.-J. Type II Peroxiredoxin C, a Member of the Peroxiredoxin Family of Arabidopsis Thaliana: Its Expression and Activity in Comparison with Other Peroxiredoxins. Plant Physiol. Biochem. 2002, 40, 491–499. [Google Scholar] [CrossRef]
- Finkemeier, I.; Goodman, M.; Lamkemeyer, P.; Kandlbinder, A.; Sweetlove, L.J.; Dietz, K.-J. The Mitochondrial Type II Peroxiredoxin F Is Essential for Redox Homeostasis and Root Growth of Arabidopsis thaliana under Stress. J. Biol. Chem. 2005, 280, 12168–12180. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.; Karplus, P.A.; Poole, L.B. Typical 2-Cys Peroxiredoxins—Structures, Mechanisms and Functions. FEBS J. 2009, 276, 2469–2477. [Google Scholar] [CrossRef] [Green Version]
- Vaseghi, M.-J.; Chibani, K.; Telman, W.; Liebthal, M.F.; Gerken, M.; Schnitzer, H.; Mueller, S.M.; Dietz, K.-J. The Chloroplast 2-Cysteine Peroxiredoxin Functions as Thioredoxin Oxidase in Redox Regulation of Chloroplast Metabolism. eLife 2018, 7, e38194. [Google Scholar] [CrossRef]
- O’Neill, J.S.; Reddy, A.B. Circadian Clocks in Human Red Blood Cells. Nature 2011, 469, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Han, X.; Yang, J.; Jiang, Y.; Hu, Y. The Arabidopsis Circadian Clock Protein PRR5 Interacts with and Stimulates ABI5 to Modulate Abscisic Acid Signaling during Seed Germination. Plant Cell 2021, 33, 3022–3041. [Google Scholar] [CrossRef] [PubMed]
- Puerto-Galán, L.; Pérez-Ruiz, J.M.; Guinea, M.; Cejudo, F.J. The Contribution of NADPH Thioredoxin Reductase C (NTRC) and Sulfiredoxin to 2-Cys Peroxiredoxin Overoxidation in Arabidopsis Thaliana Chloroplasts. J. Exp. Bot. 2015, 66, 2957–2966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veal, E.A.; Underwood, Z.E.; Tomalin, L.E.; Morgan, B.A.; Pillay, C.S. Hyperoxidation of Peroxiredoxins: Gain or Loss of Function? Antioxid. Redox Signal. 2018, 28, 574–590. [Google Scholar] [CrossRef]
- Rhee, S.G.; Kil, I.S. Mitochondrial H2O2 Signaling Is Controlled by the Concerted Action of Peroxiredoxin III and Sulfiredoxin: Linking Mitochondrial Function to Circadian Rhythm. Free. Radic. Biol. Med. 2016, 99, 120–127. [Google Scholar] [CrossRef]
- Barranco-Medina, S.; Krell, T.; Bernier-Villamor, L.; Sevilla, F.; Lázaro, J.-J.; Dietz, K.-J. Hexameric Oligomerization of Mitochondrial Peroxiredoxin PrxIIF and Formation of an Ultrahigh Affinity Complex with Its Electron Donor Thioredoxin Trx-o. J. Exp. Bot. 2008, 59, 3259–3269. [Google Scholar] [CrossRef] [Green Version]
- Barranco-Medina, S.; López-Jaramillo, F.J.; Bernier-Villamor, L.; Sevilla, F.; Lázaro, J.-J. Cloning, Overexpression, Purification and Preliminary Crystallographic Studies of a Mitochondrial Type II Peroxiredoxin from Pisum Sativum. Acta Crystallogr. Sect. F 2006, 62, 695–698. [Google Scholar] [CrossRef] [Green Version]
- Horling, F.; Lamkemeyer, P.; König, J.; Finkemeier, I.; Kandlbinder, A.; Baier, M.; Dietz, K.-J. Divergent Light-, Ascorbate-, and Oxidative Stress-Dependent Regulation of Expression of the Peroxiredoxin Gene Family in Arabidopsis. Plant Physiol. 2003, 131, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Gama, F.; Keech, O.; Eymery, F.; Finkemeier, I.; Gelhaye, E.; Gardeström, P.; Dietz, K.J.; Rey, P.; Jacquot, J.-P.; Rouhier, N. The Mitochondrial Type II Peroxiredoxin from Poplar. Physiol. Plant. 2007, 129, 196–206. [Google Scholar] [CrossRef]
- Chen, H.; Osuna, D.; Colville, L.; Lorenzo, O.; Graeber, K.; Küster, H.; Leubner-Metzger, G.; Kranner, I. Transcriptome-Wide Mapping of Pea Seed Ageing Reveals a Pivotal Role for Genes Related to Oxidative Stress and Programmed Cell Death. PLoS ONE 2013, 8, e78471. [Google Scholar] [CrossRef] [Green Version]
- Pukacka, S.; Ratajczak, E. Age-Related Biochemical Changes during Storage of Beech (Fagus sylvatica L. ) Seeds. Seed Sci. Res. 2007, 17, 45–53. [Google Scholar] [CrossRef]
- Ratajczak, E.; Ströher, E.; Oelze, M.-L.; Kalemba, E.M.; Pukacka, S.; Dietz, K.-J.; Ratajczak, E.; Ströher, E.; Oelze, M.-L.; Kalemba, E.M.; et al. The Involvement of the Mitochondrial Peroxiredoxin PRXIIF in Defining Physiological Differences between Orthodox and Recalcitrant Seeds of Two Acer Species. Funct. Plant Biol. 2013, 40, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, E.; Staszak, A.; Wojciechowska, N.; Bagniewska-Zadworna, A.; Dietz, K. Regulation of Thiol Metabolism as a Factor That Influences the Development and Storage Capacity of Beech Seeds. J. Plant Physiol. 2019, 239, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Birtić, S.; Anderson, K.M.; Pritchard, H.W. Glutathione Half-Cell Reduction Potential: A Universal Stress Marker and Modulator of Programmed Cell Death? Free Radic. Biol. Med. 2006, 40, 2155–2165. [Google Scholar] [CrossRef]
- Gray, M.W. Mitochondria. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 430–432. [Google Scholar] [CrossRef]
- Mao, C.; Zhu, Y.; Cheng, H.; Yan, H.; Zhao, L.; Tang, J.; Ma, X.; Mao, P. Nitric Oxide Regulates Seedling Growth and Mitochondrial Responses in Aged Oat Seeds. Int. J. Mol. Sci. 2018, 19, 1052. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From Intracellular Signaling Networks to Cell Death: The Dual Role of Reactive Oxygen Species in Seed Physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Nietzel, T.; Mostertz, J.; Ruberti, C.; Née, G.; Fuchs, P.; Wagner, S.; Moseler, A.; Müller-Schüssele, S.J.; Benamar, A.; Poschet, G.; et al. Redox-Mediated Kick-Start of Mitochondrial Energy Metabolism Drives Resource-Efficient Seed Germination. Proc. Natl. Acad. Sci. USA 2020, 117, 741–751. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klupczyńska, E.A.; Dietz, K.-J.; Małecka, A.; Ratajczak, E. Mitochondrial Peroxiredoxin-IIF (PRXIIF) Activity and Function during Seed Aging. Antioxidants 2022, 11, 1226. https://doi.org/10.3390/antiox11071226
Klupczyńska EA, Dietz K-J, Małecka A, Ratajczak E. Mitochondrial Peroxiredoxin-IIF (PRXIIF) Activity and Function during Seed Aging. Antioxidants. 2022; 11(7):1226. https://doi.org/10.3390/antiox11071226
Chicago/Turabian StyleKlupczyńska, Ewelina A., Karl-Josef Dietz, Arleta Małecka, and Ewelina Ratajczak. 2022. "Mitochondrial Peroxiredoxin-IIF (PRXIIF) Activity and Function during Seed Aging" Antioxidants 11, no. 7: 1226. https://doi.org/10.3390/antiox11071226
APA StyleKlupczyńska, E. A., Dietz, K.-J., Małecka, A., & Ratajczak, E. (2022). Mitochondrial Peroxiredoxin-IIF (PRXIIF) Activity and Function during Seed Aging. Antioxidants, 11(7), 1226. https://doi.org/10.3390/antiox11071226





