Effect of Selenium on the Iron Homeostasis and Oxidative Damage in Brain and Liver of Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvents and Reagents
2.2. Experimental Animals and Exposure Protocol
2.3. Determination of Malondialdehyde Content
2.4. Determination of Se and Fe Concentrations
2.5. The Brain and Liver Homogenates Preparation
2.6. Protein Concentration Assay
2.7. Measurement of Enzyme Catalase Activity
2.8. Determination of Catalase Gene Expression
2.9. Statistical Analysis
3. Results
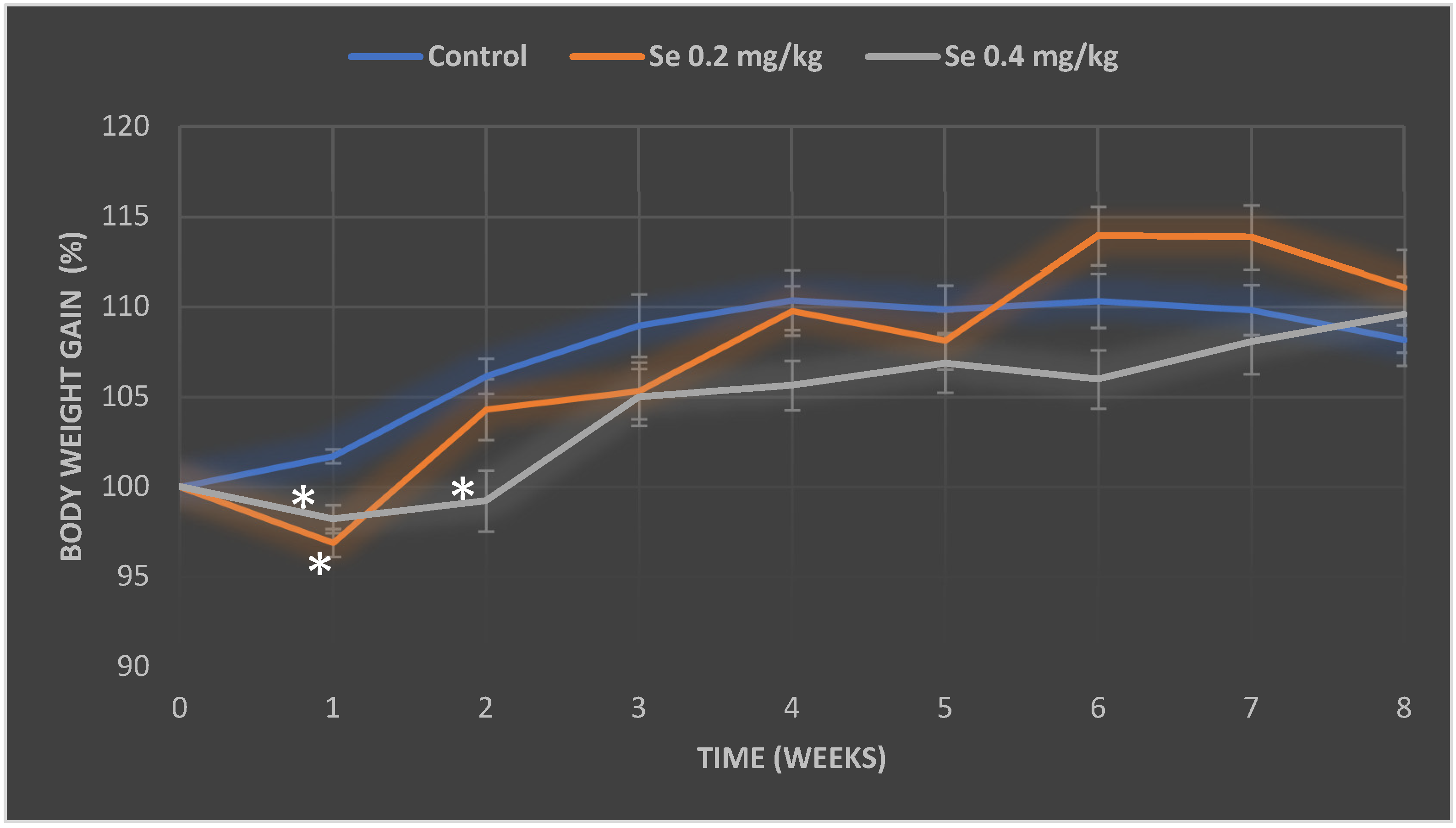
3.1. Evaluation of Selenium Effect on Body Weight and Relative Organ Mass Index of Mice
3.2. Evaluation of Selenium and Iron Concentrations in Mice Organs
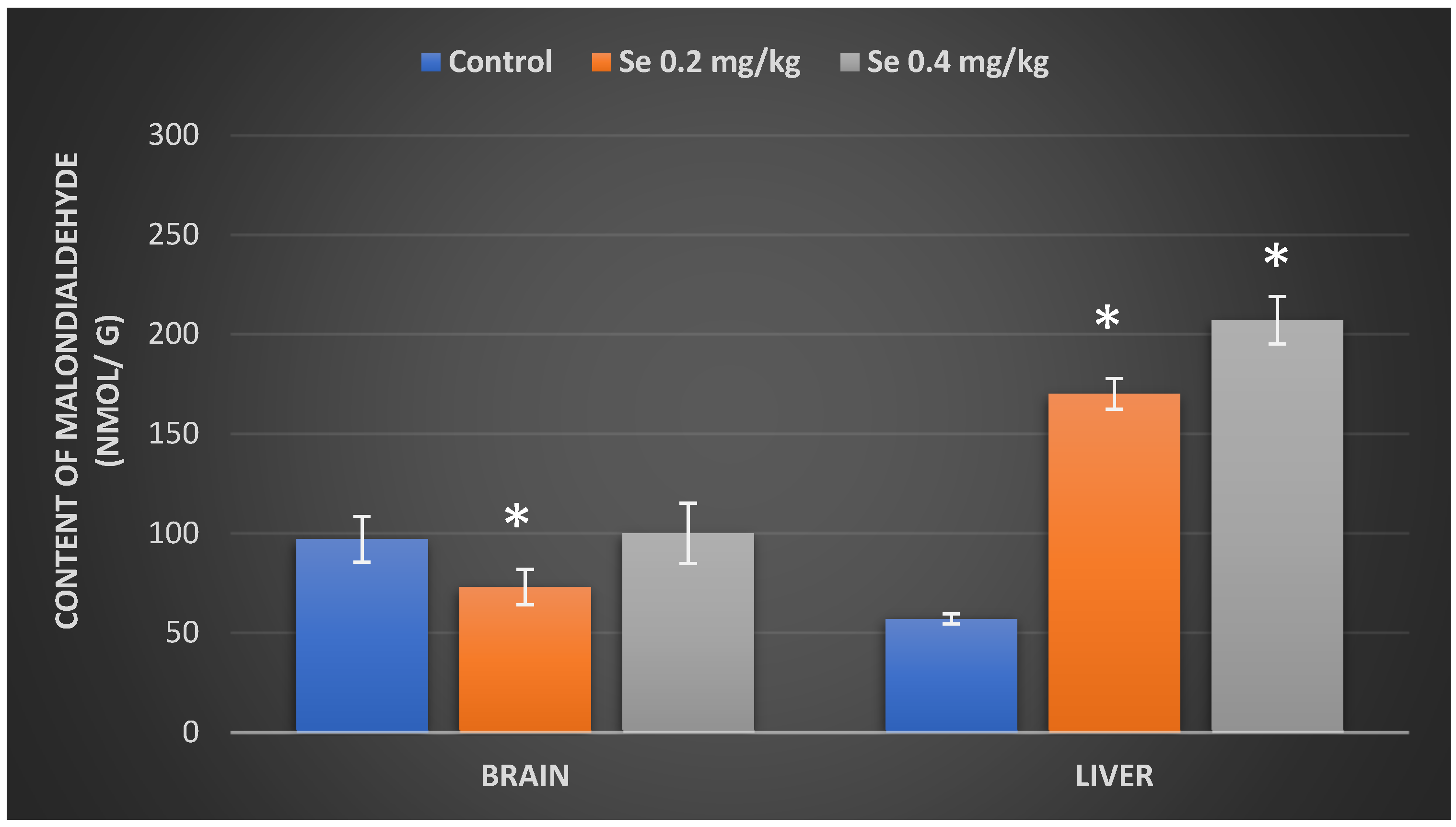
3.3. Evaluation of Malondialdehyde Content in Mice Organs after Exposure to Selenium
3.4. Determination of Catalase Activity and CAT Gene Expression in Mouse Organs after Exposure to Selenium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burk, R.F.; Hill, K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.E.; Atherton, C.; Dainty, J.R.; Lewis, D.J.; Langford, N.J.; Baxter, M.J.; Crews, H.M.; Fairweather-Tait, S.J. Absorption of selenium from wheat, garlic, and cod intrinsically labeled with Se-77 and Se-82 stable isotopes. Int. J. Vitam. Nutr. Res. 2005, 75, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, L. Forms of selenium affect its transport, uptake and glutathione peroxidase activity in the Caco-2 cell model. Biol. Trace Elem. Res. 2012, 149, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.; da Silva, F.M.; Muccillo-Baisch, A.L. Selenium content of Brazilian foods: A review of the literature values. J. Food Compos. Anal. 2017, 58, 10–15. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błazejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Barchielli, G.; Capperucci, A.; Tanini, D. The role of selenium in pathologies: An updated review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef]
- Pedrero, Z.; Madrid, Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: A review. Anal. Chim. Acta. 2009, 634, 135–152. [Google Scholar] [CrossRef]
- Beck, M.A.; Levander, O.A.; Handy, J. Selenium deficiency and viral infection. J. Nutr. 2003, 133, 1463–14677. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogra, Y.; Ishiwata, K.; Takayama, H.; Aimi, N.; Suzuki, K.T. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936. [Google Scholar] [CrossRef]
- Kim, H.W.; Ha, U.S.; Woo, J.C.; Kim, S.J.; Yoon, B.I.; Lee, S.J.; Cho, Y.H. Preventive effect of selenium on chronic bacterial prostatitis. J. Infect Chemother. 2012, 18, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Luo, J.; Hu, Y.; Wei, L.; Duan, M.; He, H. Selenium deficiency impairs host innate immune response and induces susceptibility to Listeria monocytogenes infection. BMC Immunol. 2009, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y. Selenoprotein P as an in vivo redox regulator: Disorders related to its deficiency and excess. J. Clin. Biochem. Nutr. 2020, 66, 1–7. [Google Scholar] [CrossRef]
- Saito, Y.; Shichiri, M.; Hamajima, T.; Ishida, N.; Mita, Y.; Nakao, S.; Hagihara, Y.; Yoshida, Y.; Takahashi, K.; Niki, E.; et al. Enhancement of lipid peroxidation and its amelioration by vitamin E in a subject with mutations in the SBP2 gene. J. Lipid Res. 2015, 56, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, L.; Guo, K.; Zheng, L.; Liu, B.; Yu, W.; Guo, C.; Liu, Z.; Chen, Y.; Tang, Z. Effects of different selenium levels on gene expression of a subset of selenoproteins and antioxidative capacity in mice. Biol. Trace Elem. Res. 2013, 154, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. German Nutrition Society (DGE). Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 19–29. [Google Scholar] [CrossRef]
- Solovyev, N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Solovyev, N.; Drobyshev, E.; Blume, B.; Michalke, B. Selenium at the neural barriers: A review. Front. Neurosci. 2021, 15, 630016. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and toxicity of sodium selenite in the treatment of patients with carcinoma in a phase I clinical trial: The SECAR study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar] [PubMed]
- Lambert, L.J.; Muzumdar, M.D.; Rideout, W.M.; Jacks, T. Basic Science Methods for Clinical Researchers; Academic Press: Cambridge, MA, USA, 2017; pp. 291–312. [Google Scholar]
- Yim, S.H.; Clish, C.B.; Gladyshev, V.N. Selenium deficiency is associated with prolongevity mechanisms. Cell Rep. 2019, 27, 2785–2797. [Google Scholar] [CrossRef]
- Ferreira, A.; Neves, P.; Gozzelino, R. Multilevel impacts of iron in the brain: The cross talk between neurophysiological mechanisms, cognition, and social behavior. Pharmaceuticals 2019, 12, 126. [Google Scholar] [CrossRef]
- Gozzelino, R.; Arosio, P. The importance of iron in pathophysiologic conditions. Front. Pharmacol. 2015, 6, 26. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with polyphenol reagent. J. Biol. Chem. 1951, 193, 2652–2675. [Google Scholar] [CrossRef]
- Sadauskiene, I.; Liekis, A.; Bernotiene, R.; Sulinskiene, J.; Kasauskas, A.; Zekonis, G. The efects of Buckwheat leaf and flower extracts on antioxidant status in mice organs. Oxid. Med. Cell. Longev. 2018, 2018, 6712407. [Google Scholar] [CrossRef] [PubMed]
- Crissman, J.W.; Goodman, D.G.; Hildebrandt, P.K.; Maronpot, R.R.; Prater, D.A.; Riley, J.H.; Seaman, W.J.; Thake, D.C. Best practices guideline: Toxicologic histopathology. Toxicol. Pathol. 2004, 32, 126–131. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Selenium. In Fact Sheet for Health Professionals; National Institutes of Health; Bethesda: Maryland, MD, USA, 2018. [Google Scholar]
- Preedy, V.R.; Watson, R.R. (Eds.) Reviews in Food and Nutrition Toxicity; CRC Press: Boca Raton, FL, USA, 2005; Volume 4. [Google Scholar]
- National Research Council (US) Subcommittee on Laboratory Animal Nutrition. Nutrient Requirements of Laboratory Animals, 4th Revised ed.; The National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Sharadamma, K.C.; Purushotham, B.; Radhakrishna, P.M.; Abhilekha, P.M.; Vagdevi, H.M. Role of selenium in pets health and nutrition: A review. Asian J. Anim. Sci. 2011, 5, 64–70. [Google Scholar] [CrossRef][Green Version]
- Edens, F.W.; Sefton, A.E. Organic selenium in animal nutrition–utilisation, metabolism, storage and comparison with other selenium sources. J. Appl. Anim. Nutr. 2016, 4, 9. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Egrise, D.; Nève, J.; Pasteels, J.-L.; Schoutens, A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Bone Miner. Res. 2001, 6, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R.; Nicol, F.; Beckeit, G.J. Selenium deficiency, thyroid hormone metabolism, and thyroid hormone deiodinases. Am. J. Clin. Nutr. 1993, 57, 236–239. [Google Scholar] [CrossRef]
- Hu, X.; Chandler, J.D.; Orr, M.L.; Hao, L.; Liu, K.; Uppal, K.; Go, Y.M.; Jones, D.P. Selenium supplementation alters hepatic energy and fatty acid metabolism in mice. J. Nutr. 2018, 148, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Raines, A.M.; Sunde, R.A. Selenium toxicity but not deficient or super-nutritional selenium status vastly alters the transcriptome in rodents. BMC Genom. 2011, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Tos-Luty, S.; Obuchowska-Przebirowska, D.; Latuszynska, J.; Musik, I.; Tokarska-Rodak, M. Comparison of histological and ultrastructural changes in mice organs after supplementation with inorganic and organic selenium. Ann. Agric. Environ. Med. 2003, 10, 87–91. [Google Scholar] [PubMed]
- Li, K.X.; Wang, J.S.; Yuan, D.; Zhao, R.X.; Wang, Y.X.; Zhan, X.A. Effects of different selenium sources and levels on antioxidant status in broiler breeders. Asian-Australas. J. Anim. Sci. 2018, 31, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Grotto, D.; Carneiro, M.F.H.; de Castro, M.M.; Garcia, S.C.; Junior, F.B. Long-term excessive selenium supplementation induces hypertension in rats. Biol. Trace Elem. Res. 2018, 182, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Stolwijk, J.M.; Garje, R.; Sieren, J.C.; Buettner, G.R.; Zakharia, Y. Understanding the redox biology of selenium in the search of targeted cancer therapies. Antioxidants 2020, 9, 420. [Google Scholar] [CrossRef]
- Shen, H.-M.; Yang, C.-F.; Ding, W.-X.; Liu, J.; Ong, C.-N. Superoxide radical-initiated apoptotic signalling pathway in selenite-treated HepG2 cells: Mitochondria serve as the main target. Free Radic. Biol. Med. 2001, 30, 9–21. [Google Scholar] [CrossRef]
- Spallholz, J.E. On the narute of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 1994, 17, 45–64. [Google Scholar] [CrossRef]
- Hwang, I.; Lee, J.; Huh, J.Y.; Park, J.; Lee, H.B.; Ho, Y.S.; Ha, H. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes 2012, 61, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.M. Detoxification Pathways in the liver. J. Inherit. Metab. Dis. 1991, 14, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.C.; Manuel Pérez de la Lastra, J.; Plou, F.J.; Pérez-Lebeña, E.; Reinbothe, S. Molecular sciences the chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar]
- Aksoy, Y.; Balk, M.; Ögüs, I.H.; Özer, N. The mechanism of inhibition of human erythrocyte catalase by azide. Turk. J. Biol. 2004, 28, 65–70. [Google Scholar]
- Agarwal, R.; Behari, J.R. Role of selenium in mercury intoxication in mice. Ind. Health 2007, 45, 388–395. [Google Scholar] [CrossRef] [PubMed]


| Mice Group | Concentration of Selenium | |
|---|---|---|
| Brain (µg/g) | Liver (µg/g) | |
| Control | 0.061 ± 0.012 | 0.615 ± 0.095 |
| Se 0.2 mg/kg BW | 0.145 ± 0.013 * | 1.946 ± 0.069 * |
| Se 0.4 mg/kg BW | 0.154 ± 0.015 * | 2.108 ± 0.045 * |
| Concentration of Iron | ||
| Brain (µg/g) | Liver (µg/g) | |
| Control | 27.265 ± 1.895 | 119.068 ± 11.259 |
| Se 0.2 mg/kg BW | 32.404 ± 1.301 * | 252.673 ± 10.613 * |
| Se 0.4 mg/kg BW | 32.731 ± 1.512 * | 275.093 ± 10.613 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staneviciene, I.; Sulinskiene, J.; Sadauskiene, I.; Liekis, A.; Ruzgaite, A.; Naginiene, R.; Baranauskiene, D.; Simakauskiene, V.; Krusnauskas, R.; Viezeliene, D. Effect of Selenium on the Iron Homeostasis and Oxidative Damage in Brain and Liver of Mice. Antioxidants 2022, 11, 1216. https://doi.org/10.3390/antiox11071216
Staneviciene I, Sulinskiene J, Sadauskiene I, Liekis A, Ruzgaite A, Naginiene R, Baranauskiene D, Simakauskiene V, Krusnauskas R, Viezeliene D. Effect of Selenium on the Iron Homeostasis and Oxidative Damage in Brain and Liver of Mice. Antioxidants. 2022; 11(7):1216. https://doi.org/10.3390/antiox11071216
Chicago/Turabian StyleStaneviciene, Inga, Jurgita Sulinskiene, Ilona Sadauskiene, Arunas Liekis, Ausrine Ruzgaite, Rima Naginiene, Dale Baranauskiene, Vaida Simakauskiene, Raulas Krusnauskas, and Dale Viezeliene. 2022. "Effect of Selenium on the Iron Homeostasis and Oxidative Damage in Brain and Liver of Mice" Antioxidants 11, no. 7: 1216. https://doi.org/10.3390/antiox11071216
APA StyleStaneviciene, I., Sulinskiene, J., Sadauskiene, I., Liekis, A., Ruzgaite, A., Naginiene, R., Baranauskiene, D., Simakauskiene, V., Krusnauskas, R., & Viezeliene, D. (2022). Effect of Selenium on the Iron Homeostasis and Oxidative Damage in Brain and Liver of Mice. Antioxidants, 11(7), 1216. https://doi.org/10.3390/antiox11071216










