Antioxidants in Arrhythmia Treatment—Still a Controversy? A Review of Selected Clinical and Laboratory Research
Abstract
1. Introduction
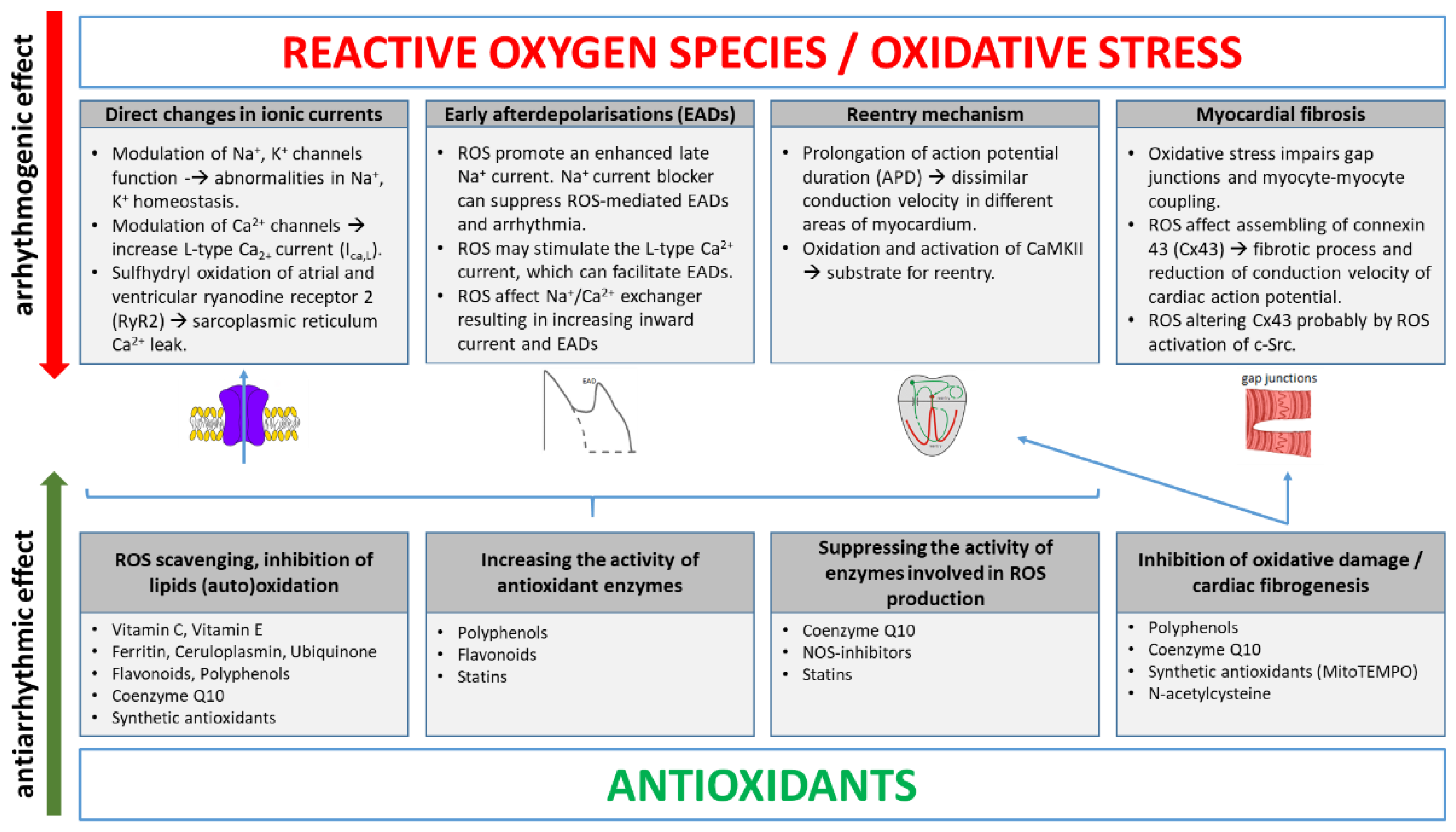
2. Main ROS Molecular Mechanisms Inducing Arrhythmias
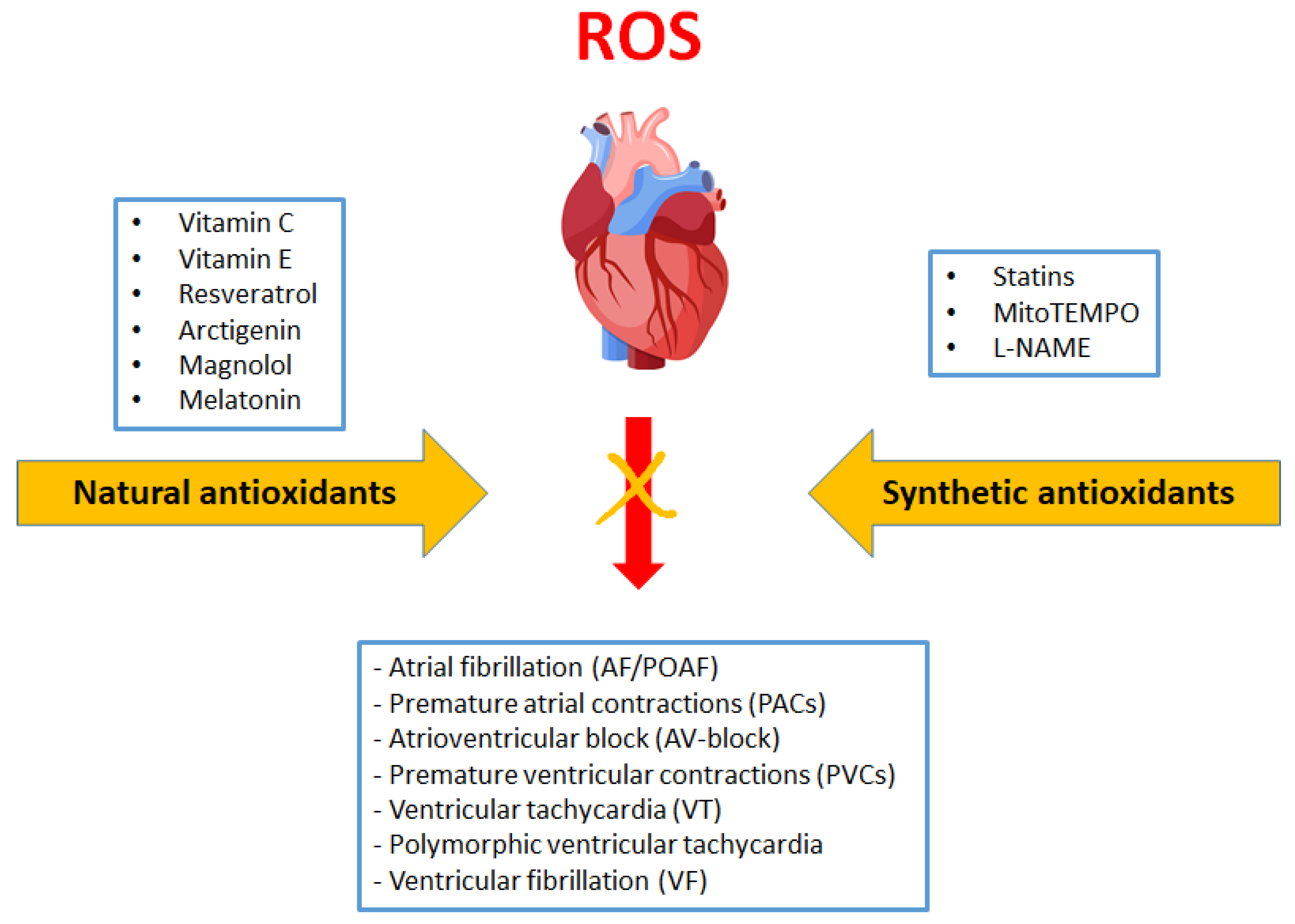
3. Antioxidants in Arrhythmia Treatment
3.1. Vitamins C and E in Atrial Fibrillation and Other Supraventricular Arrhythmias
3.1.1. Animal Studies
3.1.2. Human Studies
3.2. Vitamins C and E in Ventricular Arrhythmias
3.2.1. Animal Studies
3.2.2. Human Studies
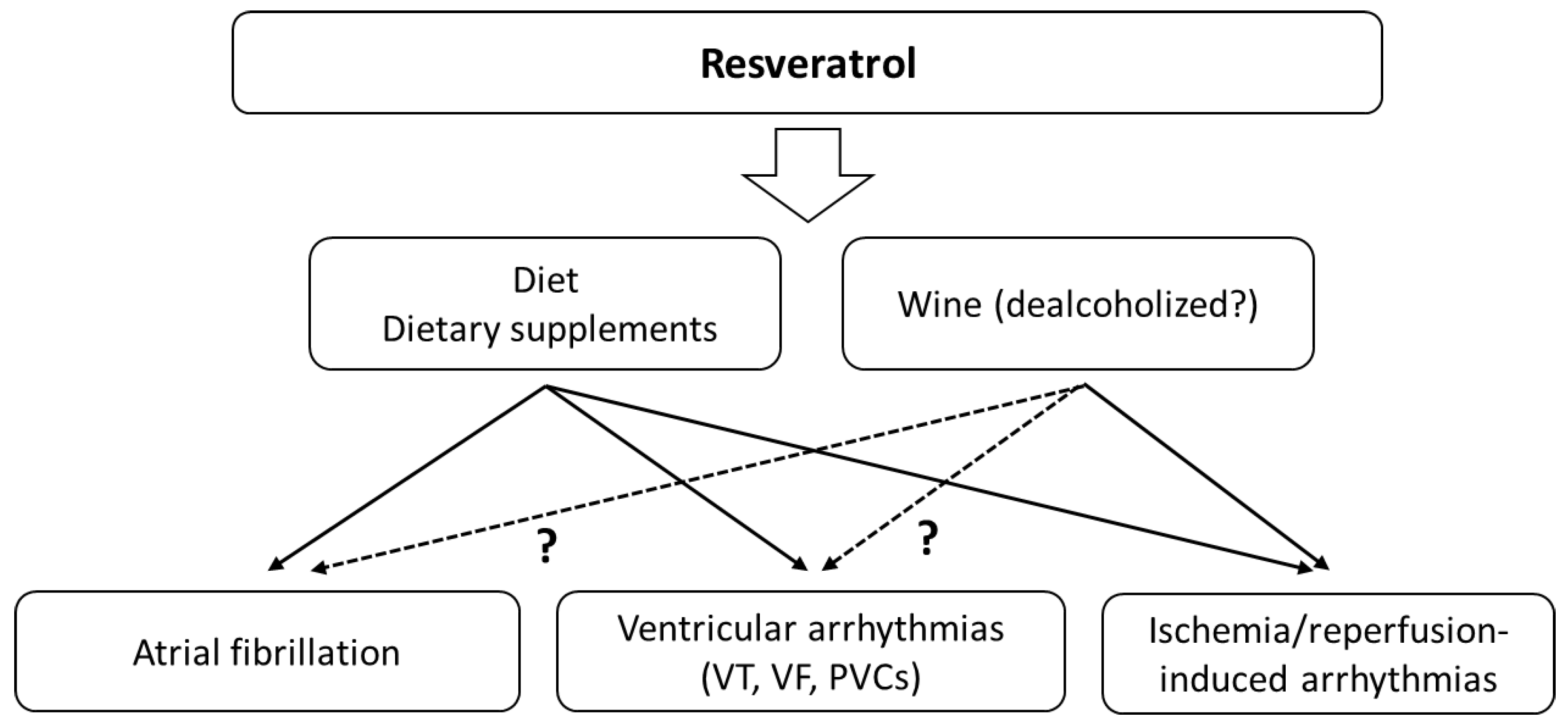
3.3. Resveratrol and Atrial Fibrillation
3.3.1. Animal Studies
3.3.2. Human Studies
3.4. Resveratrol and Ventricular Arrhythmias
Animal Studies
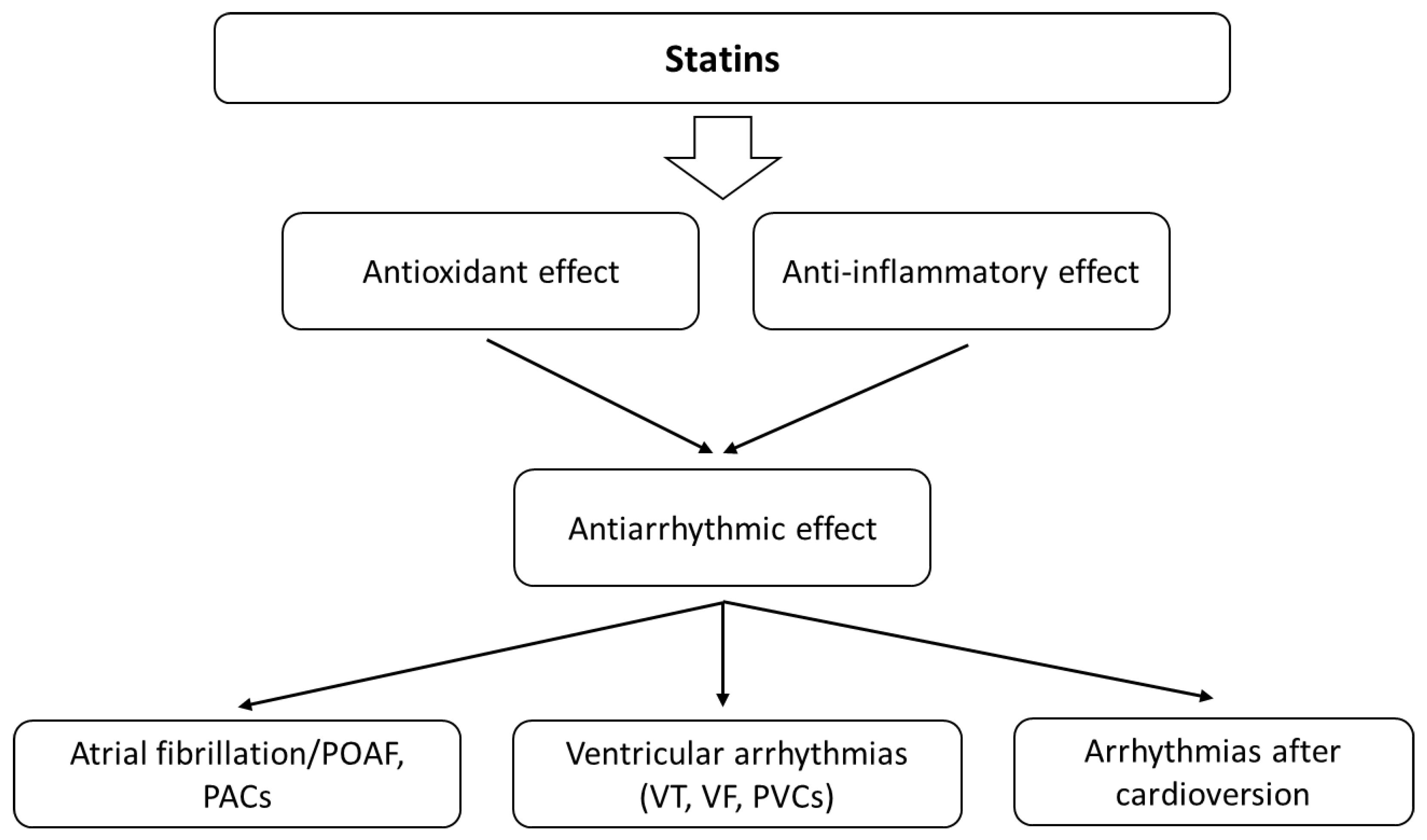
3.5. Statins and Atrial Fibrillation
3.5.1. Animal Studies
3.5.2. Human Studies
3.6. Statins and Ventricular Arrhythmias
3.6.1. Animal Studies
3.6.2. Human Studies
3.7. Other Studies with Antioxidants
4. Conclusions
5. Limitations of the Data and Their Interpretation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigo, R.; González, J.; Paoletto, F. The Role of Oxidative Stress in the Pathophysiology of Hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef]
- Korsager Larsen, M.; Matchkov, V.V. Hypertension and Physical Exercise: The Role of Oxidative Stress. Medicina 2016, 52, 19–27. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating Oxidative Stress in Heart Failure: Past, Present and Future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial Dysfunction and Oxidative Stress in Heart Disease. Exp. Mol. Med. 2019, 51, 1–3. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef]
- Olejnik, A.; Krzywonos-Zawadzka, A.; Banaszkiewicz, M.; Bil-Lula, I. Ameliorating Effect of Klotho Protein on Rat Heart during i/r Injury. Oxid. Med. Cell. Longev. 2020, 2020, 6427284. [Google Scholar] [CrossRef]
- Sovari, A.A.; Dudley, S.C. Reactive Oxygen Species-Targeted Therapeutic Interventions for Atrial Fibrillation. Front. Physiol. 2012, 3, 311. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakamura, K.; Kusano, K.F.; Morita, H.; Ohta-Ogo, K.; Miura, D.; Miura, A.; Nakagawa, K.; Tada, T.; Murakami, M.; et al. Elevated Oxidative Stress is Associated with Ventricular Fibrillation Episodes in Patients with Brugada-Type Electrocardiogram without SCN5A Mutation. Cardiovasc. Pathol. 2011, 20, e37–e42. [Google Scholar] [CrossRef]
- Dey, S.; DeMazumder, D.; Sidor, A.; Brian Foster, D.; O’Rourke, B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ. Res. 2018, 123, 356–371. [Google Scholar] [CrossRef]
- Kauppila, J.P.; Hantula, A.; Kortelainen, M.L.; Pakanen, L.; Perkiömäki, J.; Martikainen, M.; Huikuri, H.V.; Junttila, M.J. Association of Initial Recorded Rhythm and Underlying Cardiac Disease in Sudden Cardiac Arrest. Resuscitation 2018, 122, 76–78. [Google Scholar] [CrossRef]
- Romuk, E.; Wojciechowska, C.; Jacheć, W.; Nowak, J.; Niedziela, J.; Malinowska-Borowska, J.; Głogowska-Gruszka, A.; Birkner, E.; Rozentryt, P. Comparison of Oxidative Stress Parameters in Heart Failure Patients Depending on Ischaemic or Nonischaemic Aetiology. Oxid. Med. Cell. Longev. 2019, 2019, 7156038. [Google Scholar] [CrossRef]
- Clementy, N.; Bodin, A.; Bisson, A.; Teixeira-Gomes, A.P.; Roger, S.; Angoulvant, D.; Labas, V.; Babuty, D. The Defibrillation Conundrum: New Insights into the Mechanisms of Shock-Related Myocardial Injury Sustained from a Life-Saving Therapy. Int. J. Mol. Sci. 2021, 22, 5003. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Sovari, A.A.; Xie, Y.; Fishbein, M.C.; Mandel, W.J.; Garfinkel, A.; Lin, S.F.; Chen, P.S.; Xie, L.H.; Chen, F.; et al. Increased Susceptibility of Aged Hearts to Ventricular Fibrillation during Oxidative Stress. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1594–H1605. [Google Scholar] [CrossRef]
- Karagueuzian, H.S.; Nguyen, T.P.; Qu, Z.; Weiss, J.N. Oxidative Stress, Fibrosis, and Early Afterdepolarization-Mediated Cardiac Arrhythmias. Front. Physiol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Morita, N.; Lee, J.H.; Xie, Y.; Sovari, A.; Qu, Z.; Weiss, J.N.; Karagueuzian, H.S. Suppression of Re-Entrant and Multifocal Ventricular Fibrillation by the Late Sodium Current Blocker Ranolazine. J. Am. Coll. Cardiol. 2011, 57, 366–375. [Google Scholar] [CrossRef]
- Annunziato, L.; Pannaccione, A.; Cataldi, M.; Secondo, A.; Castaldo, P.; Di Renzo, G.; Taglialatela, M. Modulation of Ion Channels by Reactive Oxygen and Nitrogen Species: A Pathophysiological Role in Brain Aging? Neurobiol. Aging 2002, 23, 819–834. [Google Scholar] [CrossRef]
- Lin, Y.K.; Lin, F.Z.; Chen, Y.C.; Cheng, C.C.; Lin, C.I.; Chen, Y.J.; Chen, S.A. Oxidative Stress on Pulmonary Vein and Left Atrium Arrhythmogenesis. Circ. J. 2010, 74, 1547–1556. [Google Scholar] [CrossRef]
- Favero, T.G.; Zable, A.C.; Abramson, J.J. Hydrogen Peroxide Stimulates the Ca2+ Release Channel from Skeletal Muscle Sarcoplasmic Reticulum. J. Biol. Chem. 1995, 270, 25557–25563. [Google Scholar] [CrossRef]
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.X.; Marks, A.R. Mitochondrial Oxidative Stress Promotes Atrial Fibrillation. Sci. Rep. 2015, 5, 11427. [Google Scholar] [CrossRef]
- Neuman, R.B.; Bloom, H.L.; Shukrullah, I.; Darrow, L.A.; Kleinbaum, D.; Jones, D.P.; Dudley, S.C. Oxidative Stress Markers Are Associated with Persistent Atrial Fibrillation. Clin. Chem. 2007, 53, 1652–1657. [Google Scholar] [CrossRef]
- Corradi, D.; Callegari, S.; Manotti, L.; Ferrara, D.; Goldoni, M.; Alinovi, R.; Pinelli, S.; Mozzoni, P.; Andreoli, R.; Asimaki, A.; et al. Persistent Lone Atrial Fibrillation: Clinicopathologic Study of 19 Cases. Heart Rhythm 2014, 11, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Guzik, T.J.; Zhang, Y.H.; Zhang, M.H.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. A Myocardial Nox2 Containing NAD(P)H Oxidase Contributes to Oxidative Stress in Human Atrial Fibrillation. Circ. Res. 2005, 97, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. Association of Atrial Nicotinamide Adenine Dinucleotide Phosphate Oxidase Activity with the Development of Atrial Fibrillation after Cardiac Surgery. J. Am. Coll. Cardiol. 2008, 51, 68–74. [Google Scholar] [CrossRef]
- Markovits, N.; Kurnik, D.; Halkin, H.; Margalit, R.; Bialik, M.; Lomnicky, Y.; Loebstein, R. Database Evaluation of the Association between Serum Magnesium Levels and the Risk of Atrial Fibrillation in the Community. Int. J. Cardiol. 2016, 205, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Lawrence, A.T.; Krishnan, K.; Kavinsky, C.J.; Trohman, R.G. Current Concepts in the Mechanisms and Management of Drug-Induced QT Prolongation and Torsade de Pointes. Am. Heart J. 2007, 153, 891–899. [Google Scholar] [CrossRef]
- Salaminia, S.; Sayehmiri, F.; Angha, P.; Sayehmiri, K.; Motedayen, M. Evaluating the Effect of Magnesium Supplementation and Cardiac Arrhythmias after Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2018, 18, 129. [Google Scholar] [CrossRef]
- Liu, M.; Jeong, E.M.; Liu, H.; Xie, A.; So, E.Y.; Shi, G.; Jeong, G.E.; Zhou, A.; Dudley, S.C. Magnesium supplementation improves diabetic mitochondrial and cardiac diastolic function. JCI Insight 2019, 4, e123182. [Google Scholar] [CrossRef]
- Liu, M.; Dudley, S.C. Magnesium, Oxidative Stress, Inflammation, and Cardiovascular Disease. Antioxidants 2020, 9, 907. [Google Scholar] [CrossRef]
- Fukuda, K.; Davies, S.S.; Nakajima, T.; Ong, B.H.; Kupershmidt, S.; Fessel, J.; Amarnath, V.; Anderson, M.E.; Boyden, P.A.; Viswanathan, P.C.; et al. Oxidative Mediated Lipid Peroxidation Recapitulates Proarrhythmic Effects on Cardiac Sodium Channels. Circ. Res. 2005, 97, 1262–1269. [Google Scholar] [CrossRef]
- Ishiguchi, H.; Kobayashi, S.; Myoren, T.; Kohno, M.; Nanno, T.; Murakami, W.; Oda, S.; Oishi, K.; Okuda, S.; Okada, M.; et al. Urinary 8-Hydroxy-2′-Deoxyguanosine as a Myocardial Oxidative Stress Marker Is Associated with Ventricular Tachycardia in Patients with Active Cardiac Sarcoidosis. Circ. Cardiovasc. Imaging 2017, 10, e006764. [Google Scholar] [CrossRef]
- Li, X.; Guo, D.; Zhou, H.; Hu, Y.; Fang, X.; Chen, Y. Interplay of Pro-Inflammatory Cytokines, pro-Inflammatory Microparticles and Oxidative Stress and Recurrent Ventricular Arrhythmias in Elderly Patients after Coronary Stent Implantations. Cytokine 2021, 137, 155345. [Google Scholar] [CrossRef]
- Zhang, M.; Prosser, B.L.; Bamboye, M.A.; Gondim, A.N.S.; Santos, C.X.; Martin, D.; Ghigo, A.; Perino, A.; Brewer, A.C.; Ward, C.W.; et al. Contractile Function during Angiotensin-II Activation: Increased Nox2 Activity Modulates Cardiac Calcium Handling via Phospholamban Phosphorylation. J. Am. Coll. Cardiol. 2015, 66, 261–272. [Google Scholar] [CrossRef]
- Andelova, K.; Benova, T.E.; Bacova, B.S.; Sykora, M.; Prado, N.J.; Diez, E.R.; Hlivak, P.; Tribulova, N. Cardiac Connexin-43 Hemichannels and Pannexin1 Channels: Provocative Antiarrhythmic Targets. Int. J. Mol. Sci. 2021, 22, 260. [Google Scholar] [CrossRef]
- Khaper, N.; Kaur, K.; Li, T.; Farahmand, F.; Singal, P.K. Antioxidant Enzyme Gene Expression in Heart Failure. Mol. Cell. Biochem. 2003, 251, 9–15. [Google Scholar] [CrossRef]
- Adameova, A.; Shah, A.K.; Dhalla, N.S. Role of Oxidative Stress in the Genesis of Ventricular Arrhythmias. Int. J. Mol. Sci. 2020, 21, 4200. [Google Scholar] [CrossRef]
- Frolkis, V.V.; Frolkis, R.A.; Dubur, G.Y.; Khmelevsky, Y.V.; Shevchuk, V.G.; Golovchenko, S.F.; Mkhitarjan, L.S.; Voronkov, G.S.; Tsyomik, V.A.; Lysenko, I.V.; et al. Antioxidants as Antiarrhythmic Drugs. Cardiology 1987, 74, 124–132. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Kolettis, T.M.; Kountouris, E.; Dimitroula, V.; Karanikis, P.; Pappa, E.; Siogas, K.; Goudevenos, J.A. Oral Vitamin C Administration Reduces Early Recurrence Rates after Electrical Cardioversion of Persistent Atrial Fibrillation and Attenuates Associated Inflammation. Int. J. Cardiol. 2005, 102, 321–326. [Google Scholar] [CrossRef]
- Rodrigo, R.; Korantzopoulos, P.; Cereceda, M.; Asenjo, R.; Zamorano, J.; Villalabeitia, E.; Baeza, C.; Aguayo, R.; Castillo, R.; Carrasco, R.; et al. A Randomized Controlled Trial to Prevent Post-Operative Atrial Fibrillation by Antioxidant Reinforcement. J. Am. Coll. Cardiol. 2013, 62, 1457–1465. [Google Scholar] [CrossRef]
- Liu, T.; Korantzopoulos, P.; Li, G. Antioxidant Therapies for the Management of Atrial Fibrillation. Cardiovasc. Diagn. Ther. 2012, 2, 298–307. [Google Scholar] [CrossRef]
- Gasparova, I.; Kubatka, P.; Opatrilova, R.; Caprnda, M.; Filipova, S.; Rodrigo, L.; Malan, L.; Mozos, I.; Rabajdova, M.; Nosal, V.; et al. Perspectives and Challenges of Antioxidant Therapy for Atrial Fibrillation. Naunyn. Schmiedebergs. Arch. Pharmacol. 2017, 390, 1–14. [Google Scholar] [CrossRef]
- Mirhoseini, M.F.; Hamblin, S.E.; Moore, W.P.; Pouliot, J.; Jenkins, J.M.; Wang, W.; Chandrasekhar, R.; Collier, B.R.; Patel, M.B. Antioxidant Supplementation and Atrial Arrhythmias in Critically Ill Trauma Patients. J. Surg. Res. 2018, 222, 10–16. [Google Scholar] [CrossRef]
- Bhavnani, S.P.; Coleman, C.I.; White, C.M.; Clyne, C.A.; Yarlagadda, R.; Guertin, D.; Kluger, J. Association between Statin Therapy and Reductions in Atrial Fibrillation or Flutter and Inappropriate Shock Therapy. Europace 2008, 10, 854–859. [Google Scholar] [CrossRef]
- Hadjizacharia, P.; O’Keeffe, T.; Brown, C.V.R.; Inaba, K.; Salim, A.; Chan, L.S.; Demetriades, D.; Rhee, P. Incidence, Risk Factors, and Outcomes for Atrial Arrhythmias in Trauma Patients. Am. Surg. 2011, 77, 634–639. [Google Scholar] [CrossRef]
- Violi, F.; Pastori, D.; Pignatelli, P.; Loffredo, L. Antioxidants for Prevention of Atrial Fibrillation: A Potentially Useful Future Therapeutic Approach? A Review of the Literature and Meta-Analysis. Europace 2014, 16, 1107–1116. [Google Scholar] [CrossRef]
- Guo, X.Y.; Yan, X.L.; Chen, Y.W.; Tang, R.B.; Du, X.; Dong, J.Z.; Ma, C.S. Omega-3 Fatty Acids for Postoperative Atrial Fibrillation: Alone or in Combination with Antioxidant Vitamins? Heart Lung Circ. 2014, 23, 743–750. [Google Scholar] [CrossRef]
- Sethi, R.; Adameova, A.; Dhalla, K.S.; Khan, M.; Elimban, V.; Dhalla, N.S. Modification of Epinephrine-Induced Arrhythmias by N-Acetyl-L-Cysteine and Vitamin E. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 134–142. [Google Scholar] [CrossRef]
- Sethi, R.; Takeda, N.; Nagano, M.; Dhalla, N.S. Beneficial Effects of Vitamin E Treatment in Acute Myocardial Infarction. J. Cardiovasc. Pharmacol. Ther. 2000, 5, 51–58. [Google Scholar] [CrossRef]
- Karahaliou, A.; Katsouras, C.; Koulouras, V.; Nikas, D.; Niokou, D.; Papadopoulos, G.; Nakos, G.; Sideris, D.; Michalis, L. Ventricular Arrhythmias and Antioxidative Medication: Experimental Study. Hell. J. Cardiol. 2008, 49, 320–328. [Google Scholar]
- Chen, Y.; Yin, C.; Yang, Y.; Fan, Z.; Shang, J.; Tan, W. Inhibition of Rapid Delayed Rectifier Potassium Current (IKr) by Ischemia/Reperfusion and Its Recovery by Vitamin E in Ventricular Myocytes. J. Electrocardiol. 2017, 50, 437–443. [Google Scholar] [CrossRef]
- Barbosa, J.L.; Thiers, C.A.; Pereira, B.D.B.; Do Nascimento, E.M.; Ribeiro Frazon, C.M.; Budni, P.; Filho, D.W.; Pedrosa, R.C. Impact of the Use of Benznidazole Followed by Antioxidant Supplementation in the Prevalence of Ventricular Arrhythmias in Patients with Chronic Chagas Disease: Pilot Study. Am. J. Ther. 2016, 23, e1474–e1483. [Google Scholar] [CrossRef]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant Effects of Resveratrol in the Cardiovascular System. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Cho, S.; Namkoong, K.; Shin, M.; Park, J.; Yang, E.; Ihm, J.; Thu, V.T.; Kim, H.K.; Han, J. Cardiovascular Protective Effects and Clinical Applications of Resveratrol. J. Med. Food 2017, 20, 323–334. [Google Scholar] [CrossRef]
- Chong, E.; Chang, S.L.; Hsiao, Y.W.; Singhal, R.; Liu, S.H.; Leha, T.; Lin, W.Y.; Hsu, C.P.; Chen, Y.C.; Chen, Y.J.; et al. Resveratrol, a Red Wine Antioxidant, Reduces Atrial Fibrillation Susceptibility in the Failing Heart by PI3K/AKT/ENOS Signaling Pathway Activation; Elsevier: Amsterdam, The Netherlands, 2015; Volume 12, ISBN 8862287356. [Google Scholar]
- Zhang, Y.; Zhang, S.; Liu, Z.; Zhao, X.; Yuan, Y.; Sheng, L.; Li, Y. Resveratrol Prevents Atrial Fibrillation by Inhibiting Atrial Structural and Metabolic Remodeling in Collagen-Induced Arthritis Rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2018, 391, 1179–1190. [Google Scholar] [CrossRef]
- McCalley, A.E.; Kaja, S.; Payne, A.J.; Koulen, P. Resveratrol and Calcium Signaling: Molecular Mechanisms and Clinical Relevance. Molecules 2014, 19, 7327–7340. [Google Scholar] [CrossRef]
- Chen, W.P.; Su, M.J.; Hung, L.M. In Vitro Electrophysiological Mechanisms for Antiarrhythmic Efficacy of Resveratrol, a Red Wine Antioxidant. Eur. J. Pharmacol. 2007, 554, 196–204. [Google Scholar] [CrossRef]
- Frommeyer, G.; Wolfes, J.; Ellermann, C.; Kochhäuser, S.; Dechering, D.G.; Eckardt, L. Acute Electrophysiologic Effects of the Polyphenols Resveratrol and Piceatannol in Rabbit Atria. Clin. Exp. Pharmacol. Physiol. 2019, 46, 94–98. [Google Scholar] [CrossRef]
- Larsson, S.C.; Drca, N.; Wolk, A. Alcohol Consumption and Risk of Atrial Fibrillation: A Prospective Study and Dose-Response Meta-Analysis. J. Am. Coll. Cardiol. 2014, 64, 281–289. [Google Scholar] [CrossRef]
- Hung, L.M.; Chen, J.K.; Huang, S.S.; Lee, R.S.; Su, M.J. Cardioprotective Effect of Resveratrol, a Natural Antioxidant Derived from Grapes. Cardiovasc. Res. 2000, 47, 549–555. [Google Scholar] [CrossRef]
- Kaya, S.T.; Bozdogan, O.; Ozarslan, T.O.; Taskin, E.; Eksioglu, D.; Erim, F.; Firat, T.; Yasar, S. The Protection of Resveratrol and Its Combination with Glibenclamide, but Not Berberine on the Diabetic Hearts against Reperfusion-Induced Arrhythmias: The Role of Myocardial K ATP Channel. Arch. Physiol. Biochem. 2019, 125, 114–121. [Google Scholar] [CrossRef]
- Menezes-Rodrigues, F.S.; Errante, P.R.; Araújo, E.A.; Fernandes, M.P.P.; da Silva, M.M.; Pires-Oliveira, M.; Scorza, C.A.; Scorza, F.A.; Taha, M.O.; Caricati-Neto, A. Cardioprotection Stimulated by Resveratrol and Grape Products Prevents Lethal Cardiac Arrhythmias in an Animal Model of Ischemia and Reperfusion. Acta Cir. Bras. 2021, 36, e360306. [Google Scholar] [CrossRef]
- Kazemirad, H.; Kazerani, H.R. Cardioprotective Effects of Resveratrol Following Myocardial Ischemia and Reperfusion. Mol. Biol. Rep. 2020, 47, 5843–5850. [Google Scholar] [CrossRef]
- Prado, N.J.; Perdicaro, D.J.; Parra, M.; Carrión, A.M.; Miatello, R.M.; Renna, N.F.; Ponce, Z.A.Z.; Vazquez, P.M.A.; Diez, E.R. Antiarrhythmic Mechanisms of Malbec Wine and Resveratrol in Isolated Rat Heart. Cardiovasc. Disord. Med. 2017, 3, 1–7. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Liu, Y.; Wang, T.; Li, B.; Li, H.; Wang, Z.; Yang, B. Resveratrol, a Natural Ingredient of Grape Skin: Antiarrhythmic Efficacy and Ionic Mechanisms. Biochem. Biophys. Res. Commun. 2006, 340, 1192–1199. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. A Systematic Review and Meta-Analysis of the Effect of Statins on Glutathione Peroxidase, Superoxide Dismutase, and Catalase. Antioxidants 2021, 10, 1841. [Google Scholar] [CrossRef]
- De Sutter, J.; Firsovaite, V.; Tavernier, R. Prevention of Sudden Death in Patients with Coronary Artery Disease: Do Lipid-Lowering Drugs Play a Role? Prev. Cardiol. 2002, 5, 177–182. [Google Scholar] [CrossRef]
- Costa, S.; Reina-Couto, M.; Albino-Teixeira, A.; Sousa, T. Statins and Oxidative Stress in Chronic Heart Failure. Rev. Port. Cardiol. 2016, 35, 41–57. [Google Scholar] [CrossRef]
- Davignon, J.; Jacob, R.F.; Mason, R.P. The Antioxidant Effects of Statins. Coron. Artery Dis. 2004, 15, 251–258. [Google Scholar] [CrossRef]
- Ota, H.; Eto, M.; Kano, M.R.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Induction of Endothelial Nitric Oxide Synthase, SIRT1, and Catalase by Statins Inhibits Endothelial Senescence through the Akt Pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2205–2211. [Google Scholar] [CrossRef]
- Aoki, C.; Nakano, A.; Tanaka, S.; Yanagi, K.; Ohta, S.; Jojima, T.; Kasai, K.; Takekawa, H.; Hirata, K.; Hattori, Y. Fluvastatin Upregulates Endothelial Nitric Oxide Synthase Activity via Enhancement of Its Phosphorylation and Expression and via an Increase in Tetrahydrobiopterin in Vascular Endothelial Cells. Int. J. Cardiol. 2012, 156, 55–61. [Google Scholar] [CrossRef]
- Vonderlin, N.; Siebermair, J.; Kaya, E.; Köhler, M.; Rassaf, T.; Wakili, R. Critical Inflammatory Mechanisms Underlying Arrhythmias. Herz 2019, 44, 121–129. [Google Scholar] [CrossRef]
- Sezer, E.D.; Sozmen, E.Y.; Nart, D.; Onat, T. Effect of Atorvastatin Therapy on Oxidantantioxidant Status and Atherosclerotic Plaque Formation. Vasc. Health Risk Manag. 2011, 7, 333–343. [Google Scholar] [CrossRef]
- Hadi, N.R.; Abdelhussein, M.A.; Rudha, A.R.M.; Jamil, D.A.; Al-Aubaidy, H.A. Simvastatin Use in Patients with Type 2 Diabetes Mellitus: The Effects on Oxidative Stress. Oman Med. J. 2015, 30, 237–240. [Google Scholar] [CrossRef]
- Mohamadin, A.M.; Elberry, A.A.; Abdel Gawad, H.S.; Morsy, G.M.; Al-Abbasi, F.A. Protective Effects of Simvastatin, a Lipid Lowering Agent, against Oxidative Damage in Experimental Diabetic Rats. J. Lipids 2011, 2011, 167958. [Google Scholar] [CrossRef]
- Najjari, M.; Vaezi, G.; Hojati, V.; Mousavi, Z.; Bakhtiarian, A.; Nikoui, V. Involvement of IL-1β and IL-6 in Antiarrhythmic Properties of Atorvastatin in Ouabain-Induced Arrhythmia in Rats. Immunopharmacol. Immunotoxicol. 2018, 40, 256–261. [Google Scholar] [CrossRef]
- Allah, E.A.; Kamel, E.Z.; Osman, H.M.; Abd-Elshafy, S.K.; Nabil, F.; Elmelegy, T.T.H.; Elkhayat, H.; Ibrahim, A.S.; Minshawy, A. Al Could Short-Term Perioperative High-Dose Atorvastatin Offer Antiarrhythmic and Cardio-Protective Effects in Rheumatic Valve Replacement Surgery? J. Cardiothorac. Vasc. Anesth. 2019, 33, 3340–3347. [Google Scholar] [CrossRef]
- Rezaei, Y.; Gholami-Fesharaki, M.; Dehghani, M.R.; Arya, A.; Haghjoo, M.; Arjmand, N. Statin Antiarrhythmic Effect on Atrial Fibrillation in Statin-Naive Patients Undergoing Cardiac Surgery. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 167–176. [Google Scholar] [CrossRef]
- Jayaram, R.; Jones, M.; Reilly, S.; Crabtree, M.J.; Pal, N.; Goodfellow, N.; Nahar, K.; Simon, J.; Carnicer, R.; DeSilva, R.; et al. Atrial Nitroso-Redox Balance and Refractoriness Following on-Pump Cardiac Surgery: A Randomized Trial of Atorvastatin. Cardiovasc. Res. 2020, 118, 184–195. [Google Scholar] [CrossRef]
- Reilly, S.N.; Jayaram, R.; Nahar, K.; Antoniades, C.; Verheule, S.; Channon, K.M.; Alp, N.J.; Schotten, U.; Casadei, B. Atrial Sources of Reactive Oxygen Species Vary with the Duration and Substrate of Atrial Fibrillation: Implications for the Antiarrhythmic Effect of Statins. Circulation 2011, 124, 1107–1117. [Google Scholar] [CrossRef]
- Pinho-Gomes, A.C.; Reilly, S.; Brandes, R.P.; Casadei, B. Targeting Inflammation and Oxidative Stress in Atrial Fibrillation: Role of 3-Hydroxy-3-Methylglutaryl-Coenzyme a Reductase Inhibition with Statins. Antioxid. Redox Signal. 2014, 20, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, L.; Hallsson, L.; Tscharre, M.; Oebel, S.; Pfeffer, M.; Schönbauer, R.; Tokarska, L.; Stix, L.; Haiden, A.; Kraus, J.; et al. Upstream Statin Therapy and Long-Term Recurrence of Atrial Fibrillation after Cardioversion: A Propensity-Matched Analysis. J. Clin. Med. 2021, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, E.; Navarro-García, J.A.; González-Lafuente, L.; Aceves-Ripoll, J.; Vázquez-Sánchez, S.; Poveda, J.; Mercado-García, E.; Corbacho-Alonso, N.; Calvo-Bonacho, E.; Fernández-Velasco, M.; et al. Oxidized Low-Density Lipoprotein Associates with Ventricular Stress in Young Adults and Triggers Intracellular Ca2+ Alterations in Adult Ventricular Cardiomyocytes. Antioxidants 2020, 9, 1213. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kong, L.; Qi, S.; Wang, D. Atorvastatin Blocks Increased L-Type Ca2+ Current and Cell Injury Elicited by Angiotensin II via Inhibiting Oxide Stress. Acta Biochim. Biophys. Sin. 2016, 48, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Bloom, H.L.; Shukrullah, I.; Veledar, E.; Gutmann, R.; London, B.; Dudley, S.C. Statins Decrease Oxidative Stress and ICD Therapies. Cardiol. Res. Pract. 2010, 1, 253803. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Liu, H.; Bin; Sha, Y.; Shi, Y.; Wang, H.; Yin, D.W.; Chen, Y.D.; Shi, X.M. Effects of Statin on Arrhythmia and Heart Rate Variability in Healthy Persons with 48-Hour Sleep Deprivation. J. Am. Heart Assoc. 2016, 5, e003833. [Google Scholar] [CrossRef] [PubMed]
- Sovari, A.A.; Rutledge, C.A.; Jeong, E.M.; Dolmatova, E.; Arasu, D.; Liu, H.; Vahdani, N.; Gu, L.; Zandieh, S.; Xiao, L.; et al. Mitochondria Oxidative Stress, Connexin43 Remodeling, and Sudden Arrhythmic Death. Circ. Arrhythmia Electrophysiol. 2013, 6, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Van Norstrand, D.W.; Asimaki, A.; Rubinos, C.; Dolmatova, E.; Srinivas, M.; Tester, D.J.; Saffitz, J.E.; Duffy, H.S.; Ackerman, M.J. Connexin43 Mutation Causes Heterogeneous Gap Junction Loss and Sudden Infant Death. Circulation 2012, 125, 474–481. [Google Scholar] [CrossRef]
- Kukushkina, O.I.; Fedotkina, L.K.; Balashov, V.P.; Balykova, L.A.; Sosunov, A.A. Effect of NO-Synthase Inhibitor L-NAME on Occlusion and Reperfusion Arrhythmias in Cats. Bull. Exp. Biol. Med. 1999, 127, 460–462. [Google Scholar] [CrossRef]
- Dallas, M.L.; Yang, Z.; Boyle, J.P.; Boycott, H.E.; Scragg, J.L.; Milligan, C.J.; Elies, J.; Duke, A.; Thireau, J.; Reboul, C.; et al. Carbon Monoxide Induces Cardiac Arrhythmia via Induction of the Late Na+ Current. Am. J. Respir. Crit. Care Med. 2012, 186, 648–656. [Google Scholar] [CrossRef]
- Pabla, R.; Curtis, M.J. Effects of NO Modulation on Cardiac Arrhythmias in the Rat Isolated Heart. Circ. Res. 1995, 77, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Y.; Huang, S.S.; Tsai, S.K. Magnolol Reduces Infarct Size and Suppresses Ventricular Arrhythmia in Rats Subjected to Coronary Ligation. Clin. Exp. Pharmacol. Physiol. 1996, 23, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.K.; Huang, C.H.; Huang, S.S.; Hung, L.M.; Hong, C.Y. Antiarrhythmic Effect of Magnolol and Honokiol during Acute Phase of Coronary Occlusion in Anesthetized Rats: Influence of L-NAME and Aspirin. Pharmacology 1999, 59, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kazemirad, H.; Kazerani, H.R. The Anti-Arrhythmic Effects of Pomegranate (Punica Granatum) are Mainly Mediated by Nitric Oxide. J. Berry Res. 2020, 10, 573–584. [Google Scholar] [CrossRef]
- Yang, J.; Yin, H.S.; Cao, Y.J.; Jiang, Z.A.; Li, Y.J.; Song, M.C.; Wang, Y.F.; Wang, Z.H.; Yang, R.; Jiang, Y.F.; et al. Arctigenin Attenuates Ischemia/Reperfusion Induced Ventricular Arrhythmias by Decreasing Oxidative Stress in Rats. Cell. Physiol. Biochem. 2018, 49, 728–742. [Google Scholar] [CrossRef]
- Ding, K.; Wang, H.; Xu, J.; Li, T.; Zhang, L.; Ding, Y.; Zhu, L.; He, J.; Zhou, M. Melatonin Stimulates Antioxidant Enzymes and Reduces Oxidative Stress in Experimental Traumatic Brain Injury: The Nrf2-ARE Signaling Pathway as a Potential Mechanism. Free Radic. Biol. Med. 2014, 73, 1–11. [Google Scholar] [CrossRef]
- Prado, N.J.; Casarotto, M.; Calvo, J.P.; Mazzei, L.; Ponce Zumino, A.Z.; García, I.M.; Cuello-Carrión, F.D.; Fornés, M.W.; Ferder, L.; Diez, E.R.; et al. Antiarrhythmic Effect Linked to Melatonin Cardiorenal Protection Involves AT1 Reduction and Hsp70-VDR Increase. J. Pineal Res. 2018, 65, e12513. [Google Scholar] [CrossRef]
- Benova, T.; Viczenczova, C.; Radosinska, J.; Bacova, B.; Knezl, V.; Dosenko, V.; Weismann, P.; Zeman, M.; Navarova, J.; Tribulova, N. Maladaptation of Connexin-43 and Propensity of the Heart to Lethal. Can. J. Physiol. Pharmacol. 2013, 91, 633–639. [Google Scholar] [CrossRef]
- Segovia-Roldan, M.; Diez, E.R.; Pueyo, E. Melatonin to Rescue the Aged Heart: Antiarrhythmic and Antioxidant Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 8876792. [Google Scholar] [CrossRef]
- Tsvetkova, A.S.; Bernikova, O.G.; Mikhaleva, N.J.; Khramova, D.S.; Ovechkin, A.O.; Demidova, M.M.; Platonov, P.G.; Azarov, J.E. Melatonin Prevents Early but Not Delayed Ventricular Fibrillation in the Experimental Porcine Model of Acute Ischemia. Int. J. Mol. Sci. 2021, 22, 328. [Google Scholar] [CrossRef]
- Xu, N.W.; Chen, Y.; Liu, W.; Chen, Y.J.; Fan, Z.M.; Liu, M.; Li, L.J. Inhibition of JAK2/STAT3 Signaling Pathway Suppresses Proliferation of Burkitt’s Lymphoma Raji Cells via Cell Cycle Progression, Apoptosis, and Oxidative Stress by Modulating HSP70. Med. Sci. Monit. 2018, 24, 6255–6263. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Masuda, T.; Ogano, M.; Hotta, K.; Takagi, H.; Tanaka, S.; Kamada, Y.; Akiyama, A.; Kamekawa, D.; Shimizu, R.; et al. Stretching Exercises Improve Vascular Endothelial Dysfunction through Attenuation of Oxidative Stress in Chronic Heart Failure Patients with an Implantable Cardioverter Defibrillator. J. Cardiopulm. Rehabil. Prev. 2017, 37, 130–138. [Google Scholar] [CrossRef] [PubMed]

| Study | Used Antioxidant/Drug | Study Model | End-Point | Primary Findings |
|---|---|---|---|---|
| Frolkis et al. [36] | Vitamin E | Animal | SVA | Animals treated with vitamin E had lower incidence rate of supraventricular arrhythmias |
| Korantzopoulos et al. [37] | Vitamin C | Human | AF | Vitamin C reduces recurrence rates of AF after electrical cardioversion of AF |
| Rodrigo et al. [38] | Vitamin C, E, PUFAs | Human | POAF | Antioxidants significantly reduce the risk of POAFs |
| Mirhoseini et al. [41] | Vitamin C, E, selenium | Human | Arrhythmias in critically ill trauma patients | The use of antioxidants was associated with a longer expected survival time but did not decrease the incidence of atrial arrhythmias |
| Violi et al. [44] | Vitamin C, E, PUFAs | Human | POAF | 13% reduction in POAF after CABG |
| Guo et al. [45] | Vitamin C, E, PUFAs | Human | POAF | PUFAs without antioxidants did not reduce the incidence of POAF but combination therapy with vitamins C and E reduced the incidence of POAF by 68% |
| Sethi et al. [46] | Vitamin E | Animal | VT | Vitamin E reduced the incidence of VT by approx. 50% |
| Sethi et al. [47] | Vitamin E | Animal | Arrhythmia after ischemia–reperfusion | Decrease in PVCs 21 days after coronary artery occlusion |
| Karahaliou et al. [48] | Vitamin C, deferoxamine | Animal | VT/VF | Over 60% risk reduction of VT/VF in reperfusion phase after ischemia |
| Chen et al. [49] | Vitamin E | Animal | QT interval prolongation | Treatment with vitamin E shortens QTc interval |
| Barbosa et al. [50] | Vitamin C, E | Human | Arrhythmia in patients with Chagas disease | Significant reduction in PVCs |
| Chong et al. [55] | Resveratrol | Animal | AF | Reduction in AF and incidence of triggered activity |
| Zhang et al. [56] | Resveratrol | Animal | AF | Reduction in inducibility and duration of AF |
| Frommeyer et al. [59] | Resveratrol, piceatannol | Animal | AF | Significant reduction in AF incidence |
| Hung et al. [61] | Resveratrol | Animal | VT/VF | Reduction in the incidence and duration of VT/VF during reperfusion phase |
| Kaya et al. [62] | Resveratrol | Animal | VT/VF | Reduction in frequency and duration of VT/VF during reperfusion phase |
| Rodriguez et al. [63] | Resveratrol | Animal | Ventricular arrhythmia | Reduction in the incidence of atrioventricular block, lethality and VAs |
| Kazemirad et al. [64] | Resveratrol | Animal | VF | Lower incidence of VF and single arrhythmias |
| Prado et al. [65] | Resveratrol (wine) | Animal | Arrhythmias after ischemia–reperfusion | Prevention of arrhythmias in the reperfusion phase, faster recovery of sinus rhythm |
| Najjari et al. [77] | Atorwastatin | Animal | AF | Delayed time of onset of ouabaine-induced atrial arrhythmia |
| Allah et al. [78] | Atorwastatin | Human | POAF | Reduction in POAF incidence |
| Fiedler et al. [83] | Atorwastatin | Human | AF | Reduction in AF recurrence rate after successful cardioversion |
| Bloom et al. [86] | Various statins | Human | VAs, ICD events | Use of statins is associated with reduced DROMs and fewer ICD events |
| Chen et al. [87] | Atorwastatin | Human | SVA, VAs | Significantly decreased PACs and PVCs frequency |
| Sovari et al. [88] | MitoTEMPO | Animal | PVCs, VT, SCD | Decreased spontaneous PVCs, decreased VT inducibility and reduction SCD |
| Dey et al. [9] | MitoTEMPO | Animal | SCD | Decreased risk of VAs and SCD |
| Kukushkina et al. [90] | L-NAME | Animal | Arrhythmia after ischemia–reperfusion | Decreased incidence of ventricular arrhythmias and eliminated of reperfusion-induced VT/VF |
| Pabla et al. [92] | L-NAME | Animal | VF | L-NAME had no significant effect on the incidence of ischemia-induced VF, but increased the incidence of reperfusion-induced VF |
| Hong et al. [93] | Magnolol | Animal | VT/VF | Significantly reduced incidence and duration of I/R-induced VT/VF |
| Kazemirad et al. [95] | Punica granatum L. polyphenols | Animal | VF | Lower incidence and reduced duration of VF during ischemia and reperfusion phase |
| Yang et al. [96] | Arctigenin | Animal | VT/VF | Significantly reduced incidence and duration of VT/VF and ventricular ectopic beats |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyller, J.; Jagielski, D.; Bil-Lula, I. Antioxidants in Arrhythmia Treatment—Still a Controversy? A Review of Selected Clinical and Laboratory Research. Antioxidants 2022, 11, 1109. https://doi.org/10.3390/antiox11061109
Szyller J, Jagielski D, Bil-Lula I. Antioxidants in Arrhythmia Treatment—Still a Controversy? A Review of Selected Clinical and Laboratory Research. Antioxidants. 2022; 11(6):1109. https://doi.org/10.3390/antiox11061109
Chicago/Turabian StyleSzyller, Jakub, Dariusz Jagielski, and Iwona Bil-Lula. 2022. "Antioxidants in Arrhythmia Treatment—Still a Controversy? A Review of Selected Clinical and Laboratory Research" Antioxidants 11, no. 6: 1109. https://doi.org/10.3390/antiox11061109
APA StyleSzyller, J., Jagielski, D., & Bil-Lula, I. (2022). Antioxidants in Arrhythmia Treatment—Still a Controversy? A Review of Selected Clinical and Laboratory Research. Antioxidants, 11(6), 1109. https://doi.org/10.3390/antiox11061109






