An In Vitro and In Silico Study of Antioxidant Properties of Curcuminoid N-alkylpyridinium Salts: Initial Assessment of Their Antitumoral Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis
2.1.1. Route A
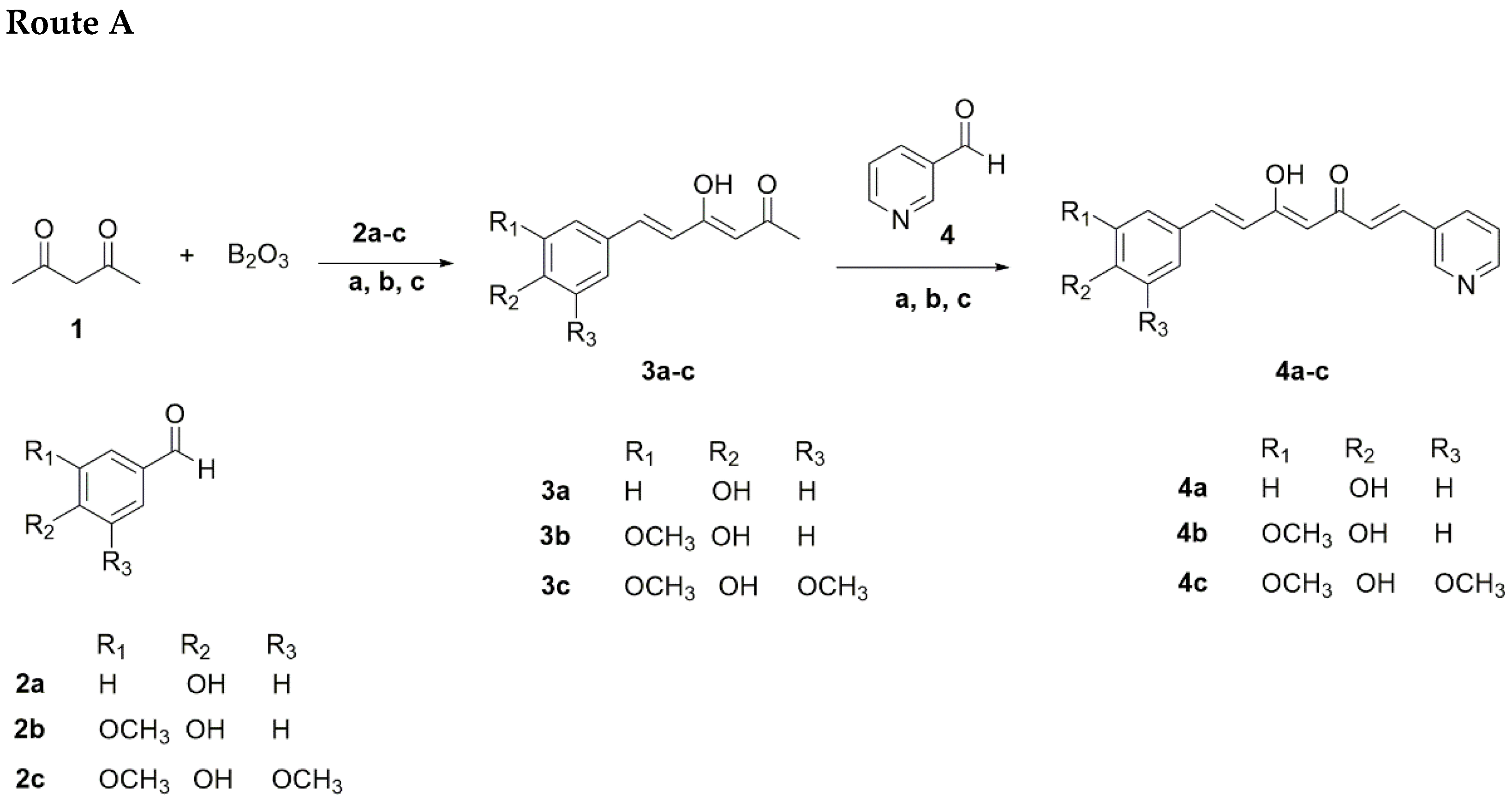
| R1 | R2 | R3 | Yield Route A | Yield Route B | |
|---|---|---|---|---|---|
| 4a | H | OH | H | <1.0 | 19.5 |
| 4b | OCH3 | OH | H | 6.8 | 20.2 |
| 4c | OCH3 | OH | OCH3 | 5.0 | 20.8 |
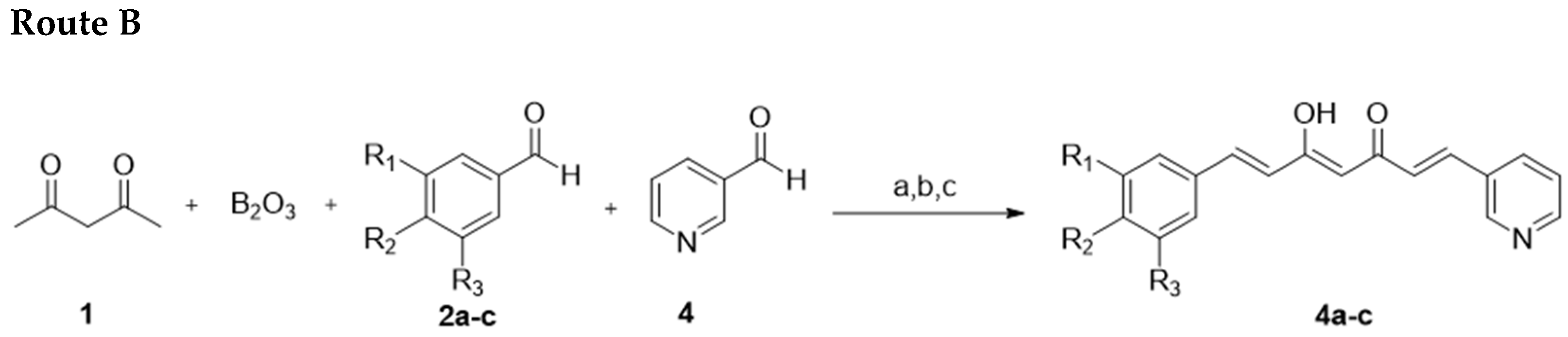
2.1.2. Route B “Mixed Pabon Reaction”
2.1.3. Curcuminoid-Derived Pyridinium Salts
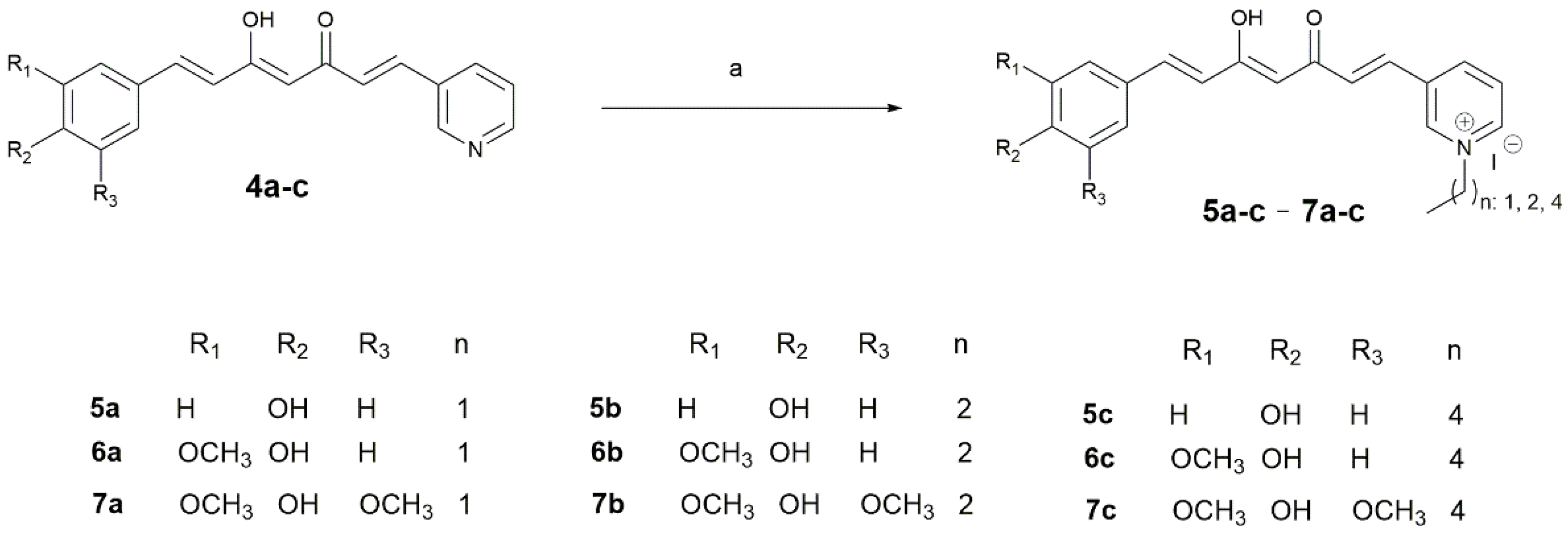
| Pyridinium Salt | Yield | |
|---|---|---|
| 5 | a, n: 1 | 11.1 |
| b, n: 2 | 19.3 | |
| c, n: 4 | 16.4 | |
| 6 | a, n: 1 | 34.0 |
| b, n: 2 | 25.1 | |
| c, n: 4 | 6.2 | |
| 7 | a, n: 1 | 34.3 |
| b, n: 2 | 30.7 | |
| c, n: 4 | 27.3 | |
2.2. GC–MS Analysis
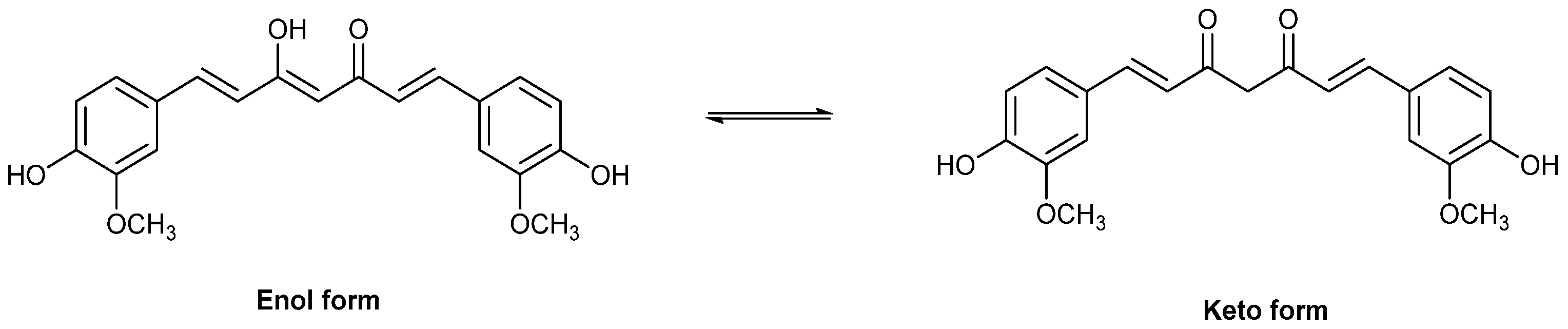
2.3. Computational Calculations
2.4. In Vitro Antioxidant Activity
2.4.1. DPPH Radical Scavenging Method
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Cell Lines and Culture
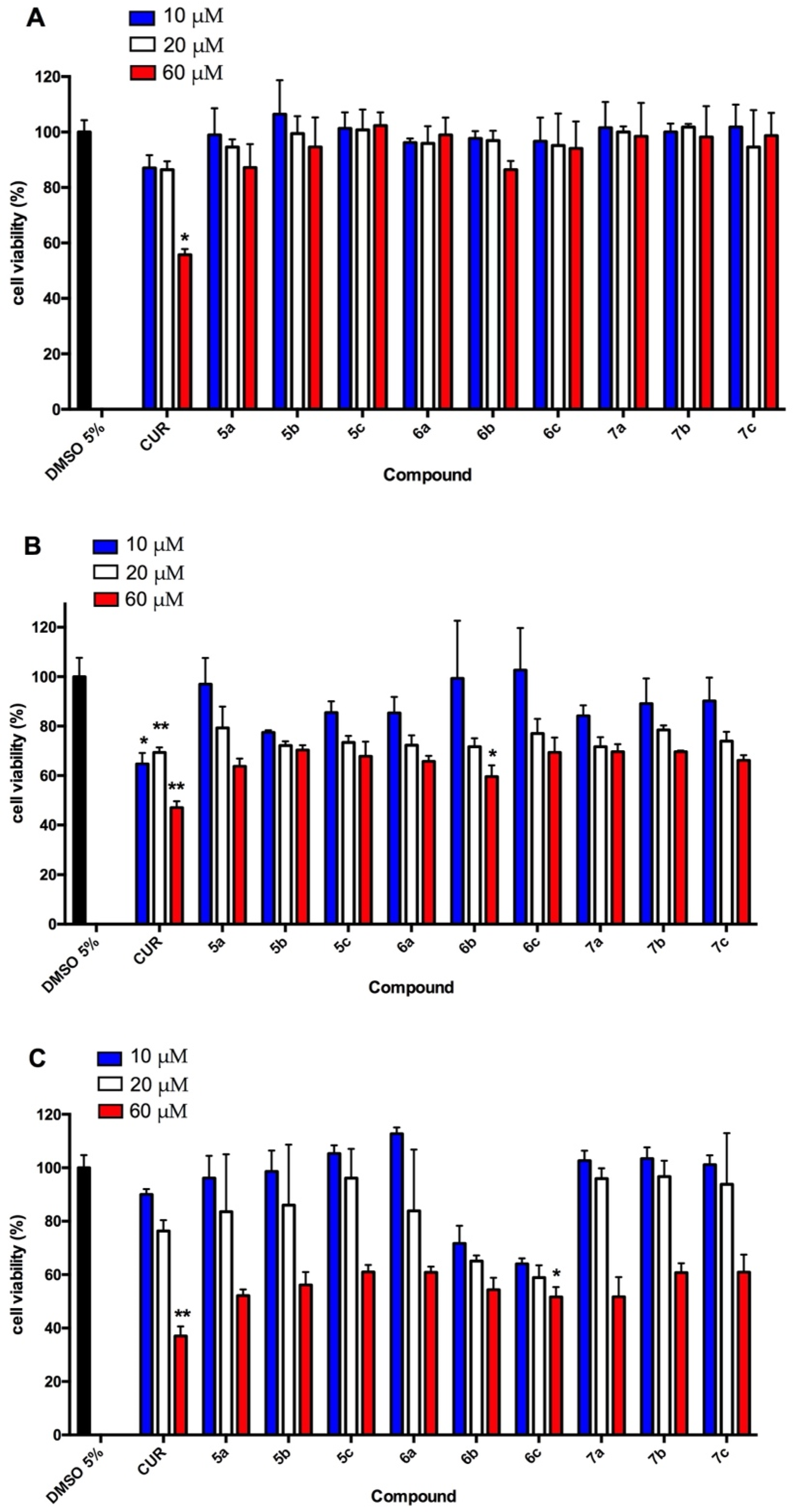
2.5.1. Viability Assay
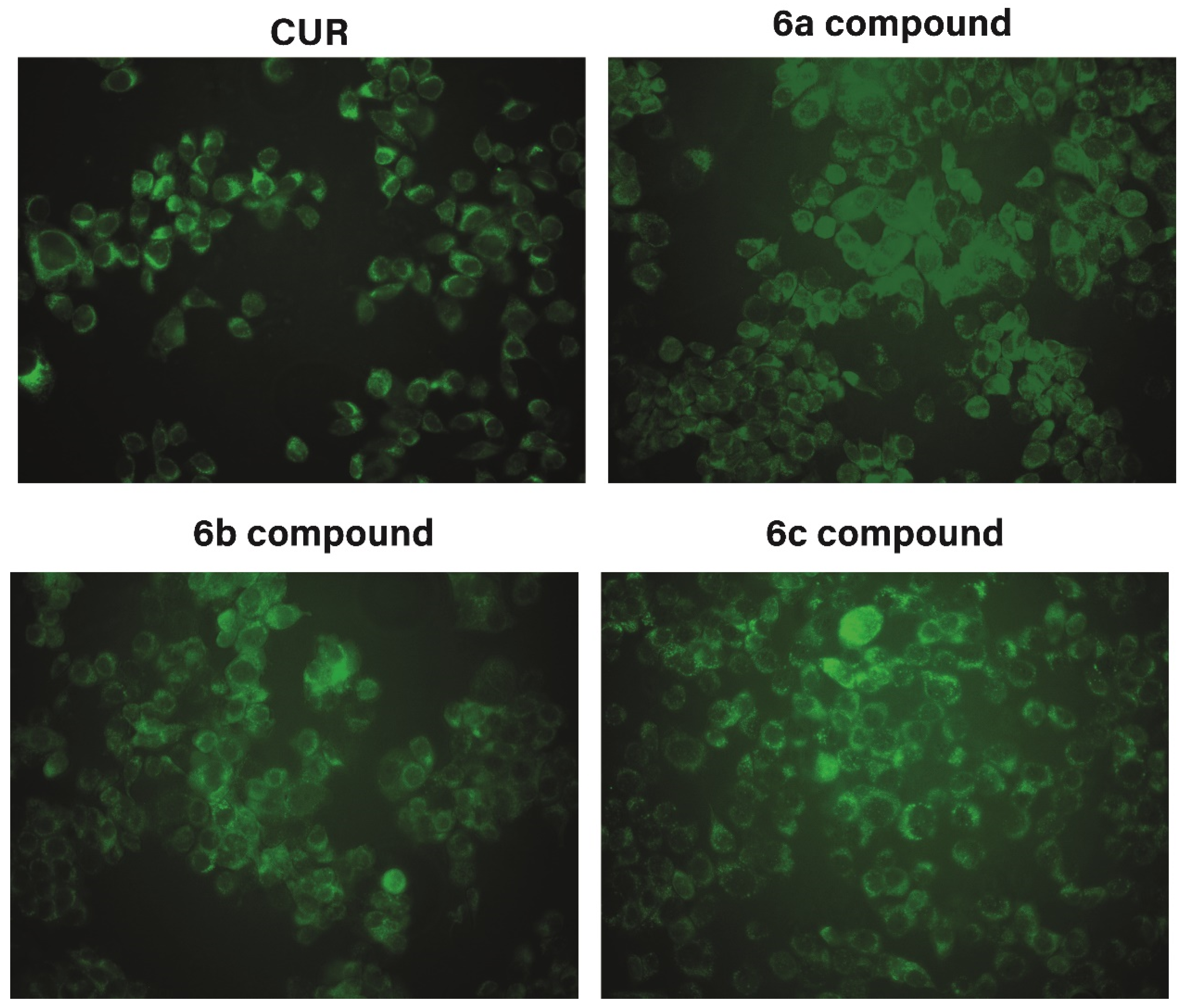
2.5.2. Fluorescence Images
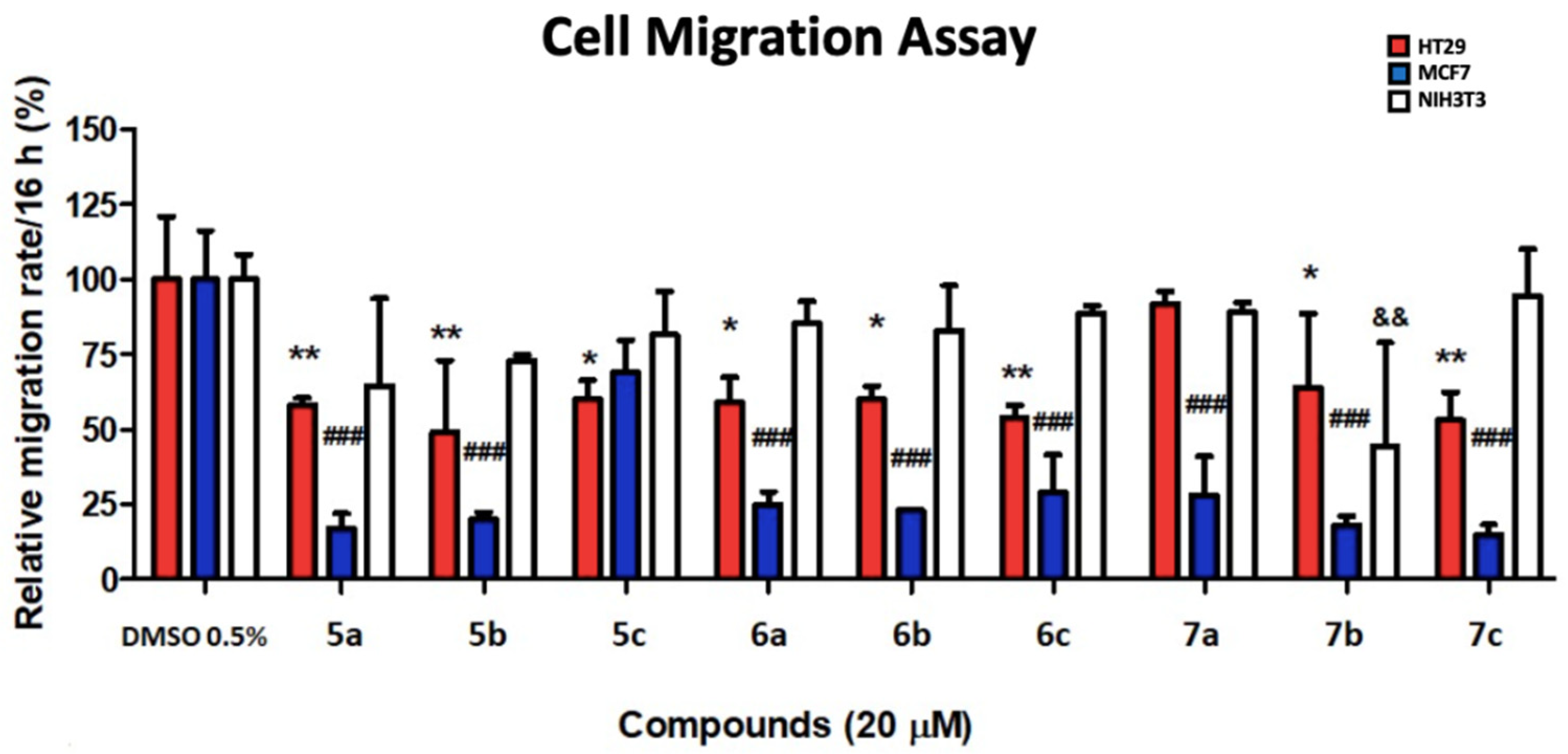
2.5.3. Cell Migration Assay
2.5.4. Statistical Analysis
3. Results and Discussion
3.1. Chemical Synthesis
3.2. In Vitro and In Silico Assessment of Antioxidant Capacity
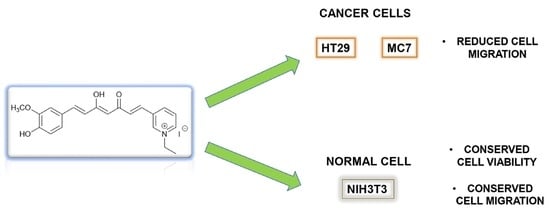
3.3. Preliminary Antitumor Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin Analogues and Their Hybrid Molecules as Multifunctional Drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef] [PubMed]
- Mahran, R.I.; Hagras, M.M.; Sun, D.; Brenner, D.E. Bringing Curcumin to the Clinic in Cancer Prevention: A Review of Strategies to Enhance Bioavailability and Efficacy. AAPS J. 2017, 19, 54–81. [Google Scholar] [CrossRef]
- Flory, S.; Sus, N.; Haas, K.; Jehle, S.; Kienhöfer, E.; Waehler, R.; Adler, G.; Venturelli, S.; Frank, J. Increasing Post-Digestive Solubility of Curcumin Is the Most Successful Strategy to Improve Its Oral Bioavailability: A Randomized Cross-Over Trial in Healthy Adults and In Vitro Bioaccessibility Experiments. Mol. Nutr. Food Res. 2021, 65, 2100613. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Ito, Y.; Takashima, M.; Yagyu, K.; Oh-Hashi, K.; Suzuki, H.; Ono, K.; Furuta, K.; Sawada, M. Novel Oxindole–Curcumin Hybrid Compound for Antioxidative Stress and Neuroprotection. ACS Chem. Neurosci. 2019, 11, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tavaf, Z.; Dangolani, S.K.; Yousefi, R.; Panahi, F.; Shahsavani, M.B.; Khalafi-Nezhad, A. Synthesis of New Curcumin Derivatives as Influential Antidiabetic α-Glucosidase and α-Amylase Inhibitors with Anti-Oxidant Activity. Carbohydr. Res. 2020, 494, 108069. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.; Giacomini, D. Antibacterial and Antioxidant Activities for Natural and Synthetic Dual-Active Compounds. Eur. J. Med. Chem. 2018, 158, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.C.; Kumar, N.V.A.; Thakur, G. Developments in the Anticancer Activity of Structurally Modified Curcumin: An up-to-Date Review. Eur. J. Med. Chem. 2019, 177, 76–104. [Google Scholar] [CrossRef]
- Bonaccorsi, P.M.; Labbozzetta, M.; Barattucci, A.; Salerno, T.M.G.; Poma, P.; Notarbartolo, M. Synthesis of Curcumin Derivatives and Analysis of Their Antitumor Effects in Triple Negative Breast Cancer (TNBC) Cell Lines. Pharmaceuticals 2019, 12, 161. [Google Scholar] [CrossRef] [Green Version]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics—Anti-Metastatic and Anti-Invasion Drugs: Promises and Challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef] [Green Version]
- Riggi, N.; Aguet, M.; Stamenkovic, I. Cancer Metastasis: A Reappraisal of Its Underlying Mechanisms and Their Relevance to Treatment. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 117–140. [Google Scholar] [CrossRef]
- Urra, F.A.; Muñoz, F.; Córdova-Delgado, M.; Ramírez, M.P.; Peña-Ahumada, B.; Rios, M.; Cruz, P.; Ahumada-Castro, U.; Bustos, G.; Silva-Pavez, E. FR58P1a; a New Uncoupler of OXPHOS That Inhibits Migration in Triple-Negative Breast Cancer Cells via Sirt1/AMPK/Β1-Integrin Pathway. Sci. Rep. 2018, 8, 13190. [Google Scholar] [CrossRef] [PubMed]
- Koroth, J.; Nirgude, S.; Tiwari, S.; Gopalakrishnan, V.; Mahadeva, R.; Kumar, S.; Karki, S.S.; Choudhary, B. Investigation of Anti-Cancer and Migrastatic Properties of Novel Curcumin Derivatives on Breast and Ovarian Cancer Cell Lines. BMC Complement. Altern. Med. 2019, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-Z.; Li, A.-F.; Sun, Y.-H.; Sun, G.-C. A Novel Synthetic Curcumin Derivative MHMM-41 Induces ROS-Mediated Apoptosis and Migration Blocking of Human Lung Cancer Cells A549. Biomed. Pharmacother. 2018, 103, 391–398. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of Ionic Liquids in the Chemical Industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Ranke, J.; Mölter, K.; Stock, F.; Bottin-Weber, U.; Poczobutt, J.; Hoffmann, J.; Ondruschka, B.; Filser, J.; Jastorff, B. Biological Effects of Imidazolium Ionic Liquids with Varying Chain Lengths in Acute Vibrio Fischeri and WST-1 Cell Viability Assays. Ecotoxicol. Environ. Saf. 2004, 58, 396–404. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V. A Profile of the in Vitro Anti-Tumor Activity of Imidazolium-Based Ionic Liquids. Bioorg. Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef]
- Fahs, S.; Rowther, F.B.; Dennison, S.R.; Patil-Sen, Y.; Warr, T.; Snape, T.J. Development of a Novel, Multifunctional, Membrane-Interactive Pyridinium Salt with Potent Anticancer Activity. Bioorg. Med. Chem. Lett. 2014, 24, 3430–3433. [Google Scholar] [CrossRef]
- Martinez-Cifuentes, M.; Weiss-Lopez, B.; Santos, S.L.; Araya-Maturana, R. Heterocyclic Curcumin Derivatives of Pharmacological Interest: Recent Progress. Curr. Top. Med. Chem. 2015, 15, 1663–1672. [Google Scholar] [CrossRef]
- Bairwa, K.; Grover, J.; Kania, M.; Jachak, S.M. Recent Developments in Chemistry and Biology of Curcumin Analogues. RSC Adv. 2014, 4, 13946–13978. [Google Scholar] [CrossRef]
- Takahashi, T.; Hijikuro, I.; Sugimoto, H.; Kihara, T.; Shimmyo, Y.; Niidome, T. Preparation of Novel Curcumin Derivatives as βsecretase Inhibitors. PCT International Patent WO 2008066151 A1, 5 June 2008. [Google Scholar]
- Kim, B.R.; Park, J.-Y.; Jeong, H.J.; Kwon, H.-J.; Park, S.-J.; Lee, I.-C.; Ryu, Y.B.; Lee, W.S. Design, Synthesis, and Evaluation of Curcumin Analogues as Potential Inhibitors of Bacterial Sialidase. J. Enzyme Inhib. Med. Chem. 2018, 33, 1256–1265. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009; Volume 32, pp. 5648–5652. [Google Scholar]
- Lipparini, F.; Mennucci, B. Perspective: Polarizable Continuum Models for Quantum-Mechanical Descriptions. J. Chem. Phys. 2016, 144, 160901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational Strategies for Predicting Free Radical Scavengers’ Protection against Oxidative Stress: Where Are We and What Might Follow? Int. J. Quantum Chem. 2019, 119, e25665. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell Migration in Tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef]
- Pabon, H.J.J. A Synthesis of Curcumin and Related Compounds. Recl. Des Trav. Chim. Pays-Bas 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Ito, Y. Golden Rules and Pitfalls in Selecting Optimum Conditions for High-Speed Counter-Current Chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Patel, K.; Krishna, G.; Sokoloski, E.; Ito, Y. Preparative Separation of Curcuminoids from Crude Curcumin and Turmeric Powder by PH-Zone-Refining Countercurrent Chromatography; Taylor & Francis: Milton Park, UK, 2000. [Google Scholar]
- Forero-Doria, O.; Araya-Maturana, R.; Barrientos-Retamal, A.; Morales-Quintana, L.; Guzmán, L. N-Alkylimidazolium Salts Functionalized with p-Coumaric and Cinnamic Acid: A Study of Their Antimicrobial and Antibiofilm Effects. Molecules 2019, 24, 3484. [Google Scholar] [CrossRef] [Green Version]
- Doria, O.F.; Castro, R.; Gutierrez, M.; Valenzuela, D.G.; Santos, L.; Ramirez, D.; Guzman, L. Novel Alkylimidazolium Ionic Liquids as an Antibacterial Alternative to Pathogens of the Skin and Soft Tissue Infections. Molecules 2018, 23, 2354. [Google Scholar] [CrossRef] [Green Version]
- Dorai, T.; Gehani, N.; Katz, A. Therapeutic potential of curcumin in human prostate cancer— I. curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000, 3, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Termini, D.; Den Hartogh, D.J.; Jaglanian, A.; Tsiani, E. Curcumin against Prostate Cancer: Current Evidence. Biomolecules 2020, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Belluti, S.; Orteca, G.; Semeghini, V.; Rigillo, G.; Parenti, F.; Ferrari, E.; Imbriano, C. Potent Anti-Cancer Properties of Phthalimide-Based Curcumin Derivatives on Prostate Tumor Cells. Int. J. Mol. Sci. 2019, 20, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flory, S.; Benz, A.; Frank, J. Uptake and Time-dependent Subcellular Localization of Native and Micellar Curcumin in Intestinal Cells. BioFactors 2022, 1. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

| Compound | DPPH (IC50, μM) | Mole Fe2+/Mole of Compound |
|---|---|---|
| 5a | 128.9 ± 0.11 | 0.6 ± 0.010 |
| 5b | 184.2 ± 1.36 | 0.6 ± 0.004 |
| 5c | >190 | 0.6 ± 0.008 |
| 6a | >190 | 0.7 ± 0.004 |
| 6b | 102.6 ± 0.45 | 0.9 ± 0.009 |
| 6c | >190 | 0.8 ± 0.056 |
| 7a | 26.5 ± 0.59 | 2.3 ± 0.097 |
| 7b | 5.7 ± 0.36 | 3.8 ± 0.012 |
| 7c | 1.8 ± 0.22 | 7.3 ± 0.242 |
| 4a | 1.33 ± 0.10 | 1.0 ± 0.037 |
| 4b | 14.2 ± 0.34 | 2.1 ± 0.103 |
| 4c | 0.90 ± 0.07 | 1.6 ± 0.008 |
| CUR | 7.0 ± 0.60 | 2.7 ± 0.050 |
| Tautomer | Compound | 4a | 4b | 4c | 5a | 6a | 7a |
|---|---|---|---|---|---|---|---|
| I | BDE1 (g) | 109.34 | 100.32 | 100.34 | 100.16 | 100.16 | 100.17 |
| BDE1 (w) | 96.9 | 97.18 | 97.09 | 109.47 | 96.93 | 96.91 | |
| BDE2 (g) | 109.37 | 100.2 | 100.38 | 100.16 | 100.14 | 100.17 | |
| BDE2 (w) | 96.84 | 97.07 | 97.08 | 109.26 | 96.81 | 96.7 | |
| II | BDE3 (g) | 84.74 | 84.5 | 80.55 | 269.77 | 296.41 | 316.61 |
| BDE3 (w) | 85.97 | 82.9 | 80.88 | 86.23 | 83.11 | 81.29 | |
| BDE4 (g) | 84.19 | 84.1 | 80.22 | 269.26 | 296.08 | 316.61 | |
| BDE4 (w) | 85.24 | 82.41 | 80.63 | 85.49 | 82.68 | 80.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forero-Doria, O.; Guzmán, L.; Jiménez-Aspee, F.; Echeverría, J.; Wehinger, S.; Valenzuela, C.; Araya-Maturana, R.; Martínez-Cifuentes, M. An In Vitro and In Silico Study of Antioxidant Properties of Curcuminoid N-alkylpyridinium Salts: Initial Assessment of Their Antitumoral Properties. Antioxidants 2022, 11, 1104. https://doi.org/10.3390/antiox11061104
Forero-Doria O, Guzmán L, Jiménez-Aspee F, Echeverría J, Wehinger S, Valenzuela C, Araya-Maturana R, Martínez-Cifuentes M. An In Vitro and In Silico Study of Antioxidant Properties of Curcuminoid N-alkylpyridinium Salts: Initial Assessment of Their Antitumoral Properties. Antioxidants. 2022; 11(6):1104. https://doi.org/10.3390/antiox11061104
Chicago/Turabian StyleForero-Doria, Oscar, Luis Guzmán, Felipe Jiménez-Aspee, Javier Echeverría, Sergio Wehinger, Claudio Valenzuela, Ramiro Araya-Maturana, and Maximiliano Martínez-Cifuentes. 2022. "An In Vitro and In Silico Study of Antioxidant Properties of Curcuminoid N-alkylpyridinium Salts: Initial Assessment of Their Antitumoral Properties" Antioxidants 11, no. 6: 1104. https://doi.org/10.3390/antiox11061104
APA StyleForero-Doria, O., Guzmán, L., Jiménez-Aspee, F., Echeverría, J., Wehinger, S., Valenzuela, C., Araya-Maturana, R., & Martínez-Cifuentes, M. (2022). An In Vitro and In Silico Study of Antioxidant Properties of Curcuminoid N-alkylpyridinium Salts: Initial Assessment of Their Antitumoral Properties. Antioxidants, 11(6), 1104. https://doi.org/10.3390/antiox11061104