Metabonomic Analysis Provides New Insights into the Response of Zhikong Scallop (Chlamys farreri) to Heat Stress by Improving Energy Metabolism and Antioxidant Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Metabolite Extraction and Profiling
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
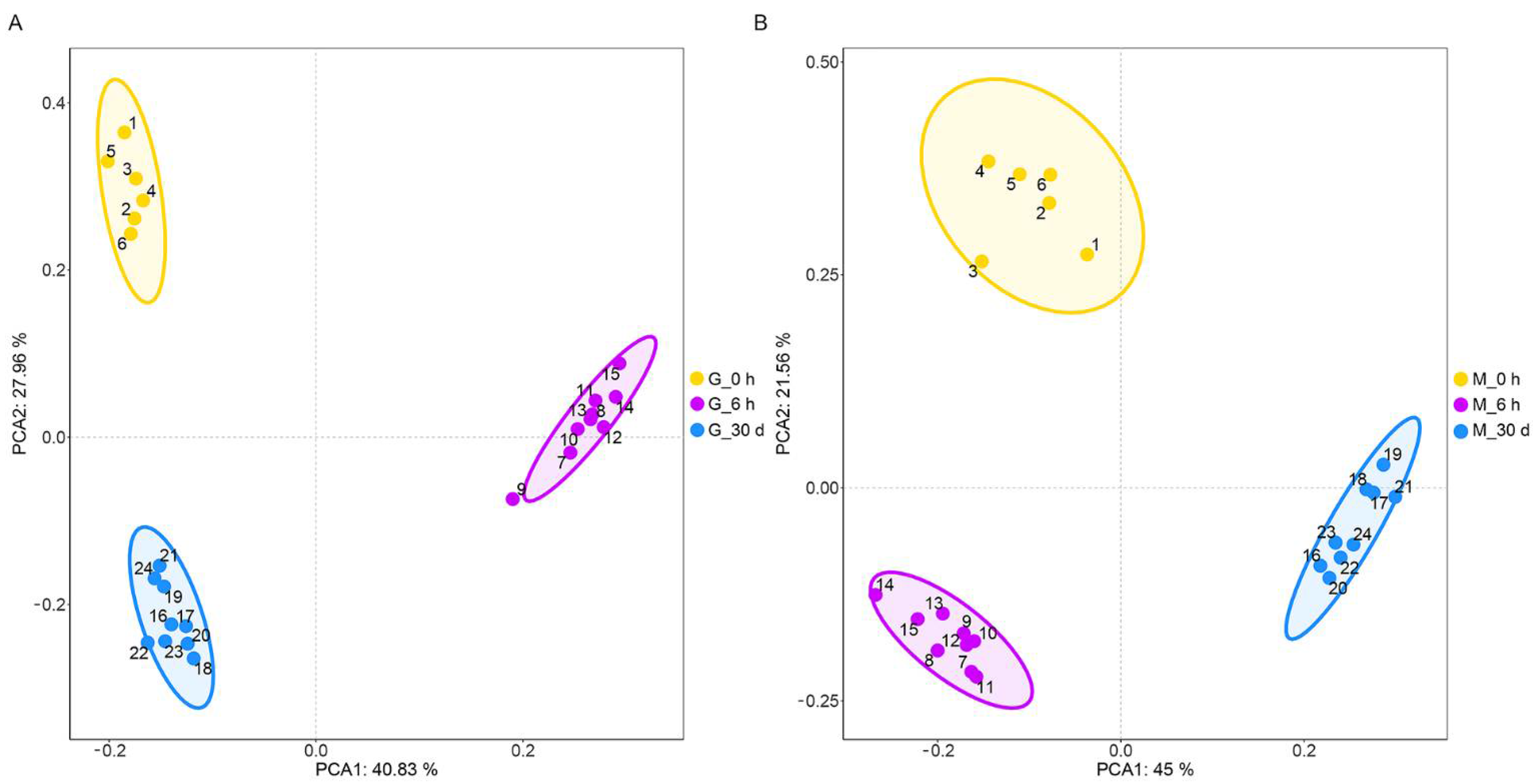
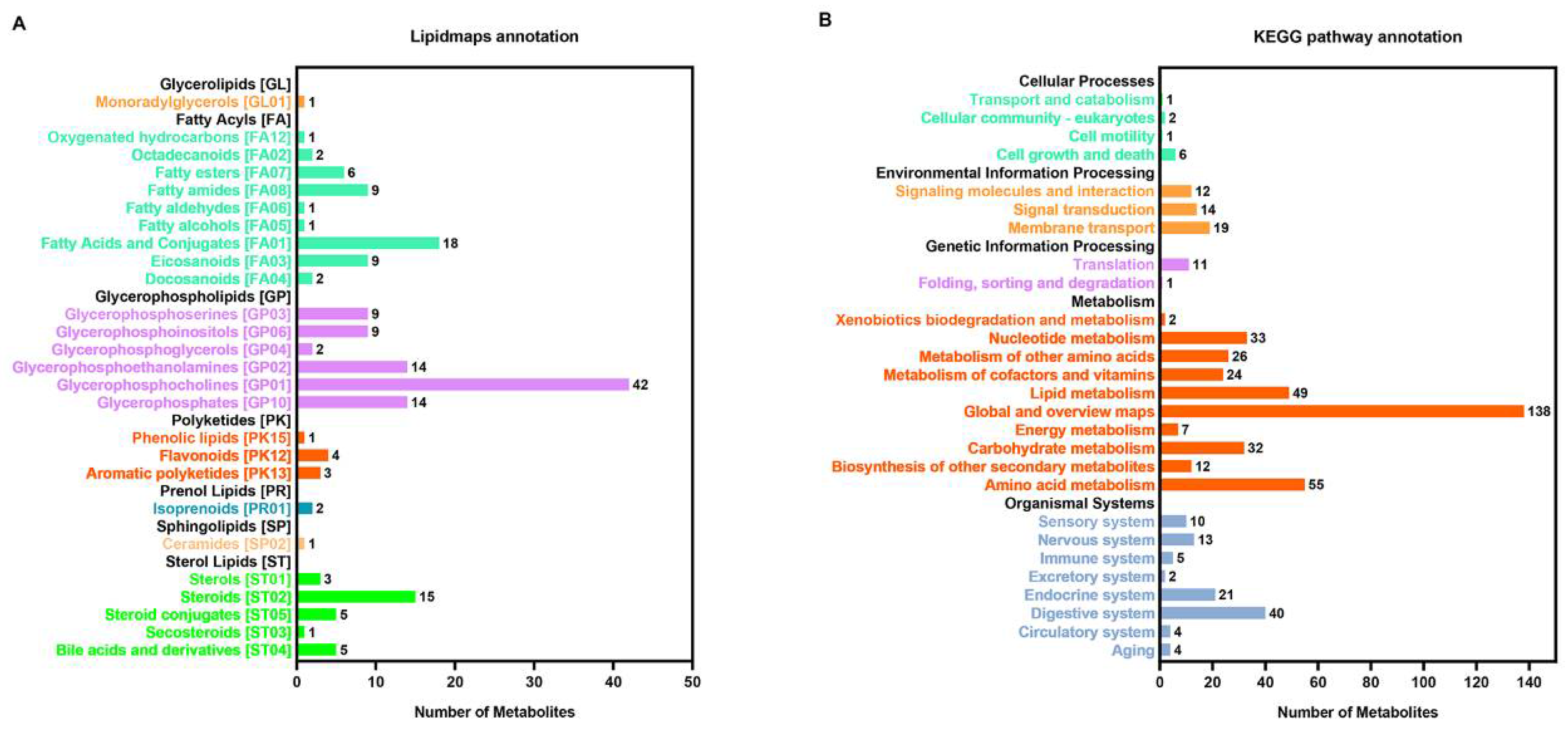
3.1. Metabolic Profile of C. farreri under Heat Stress
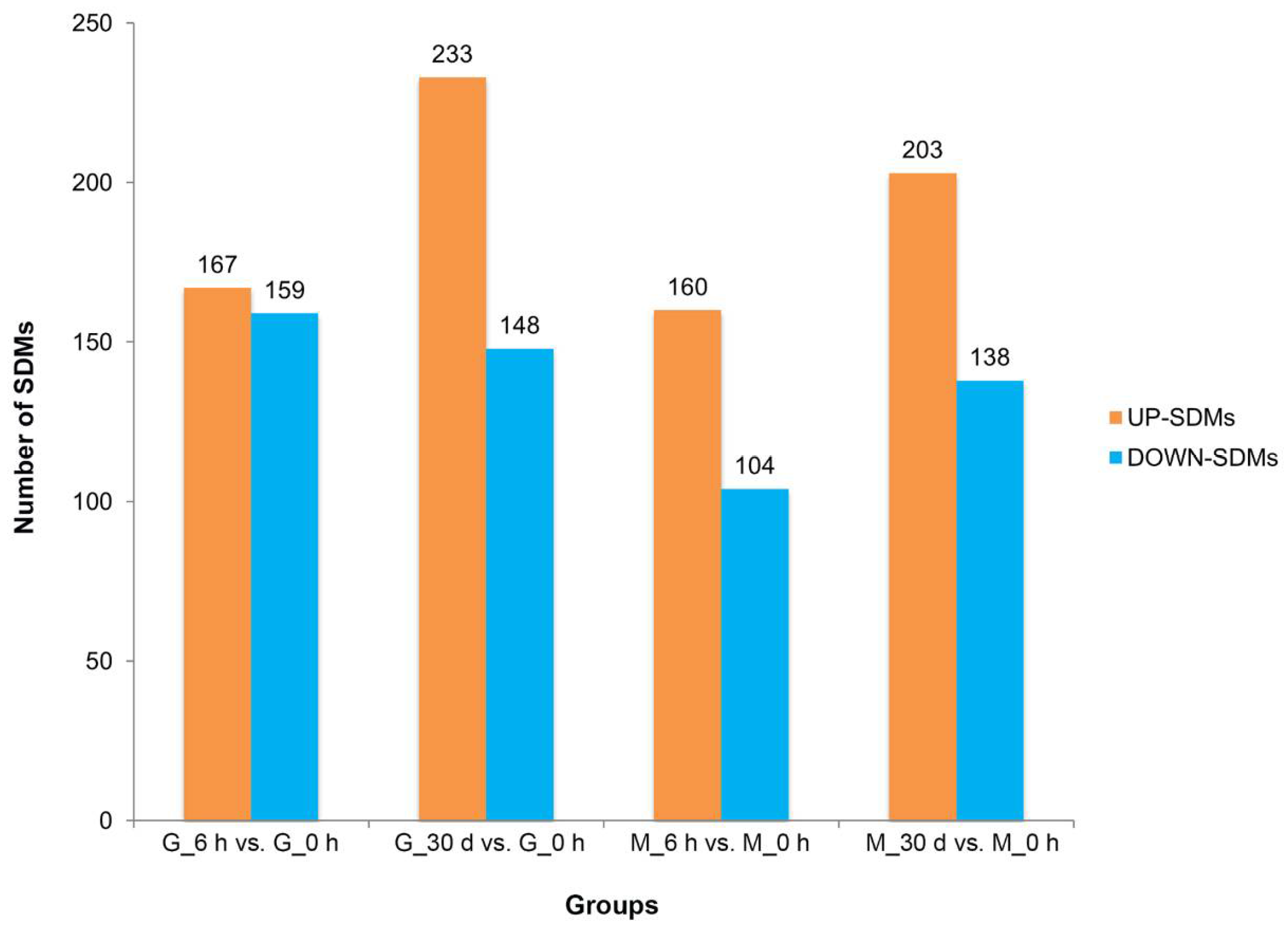
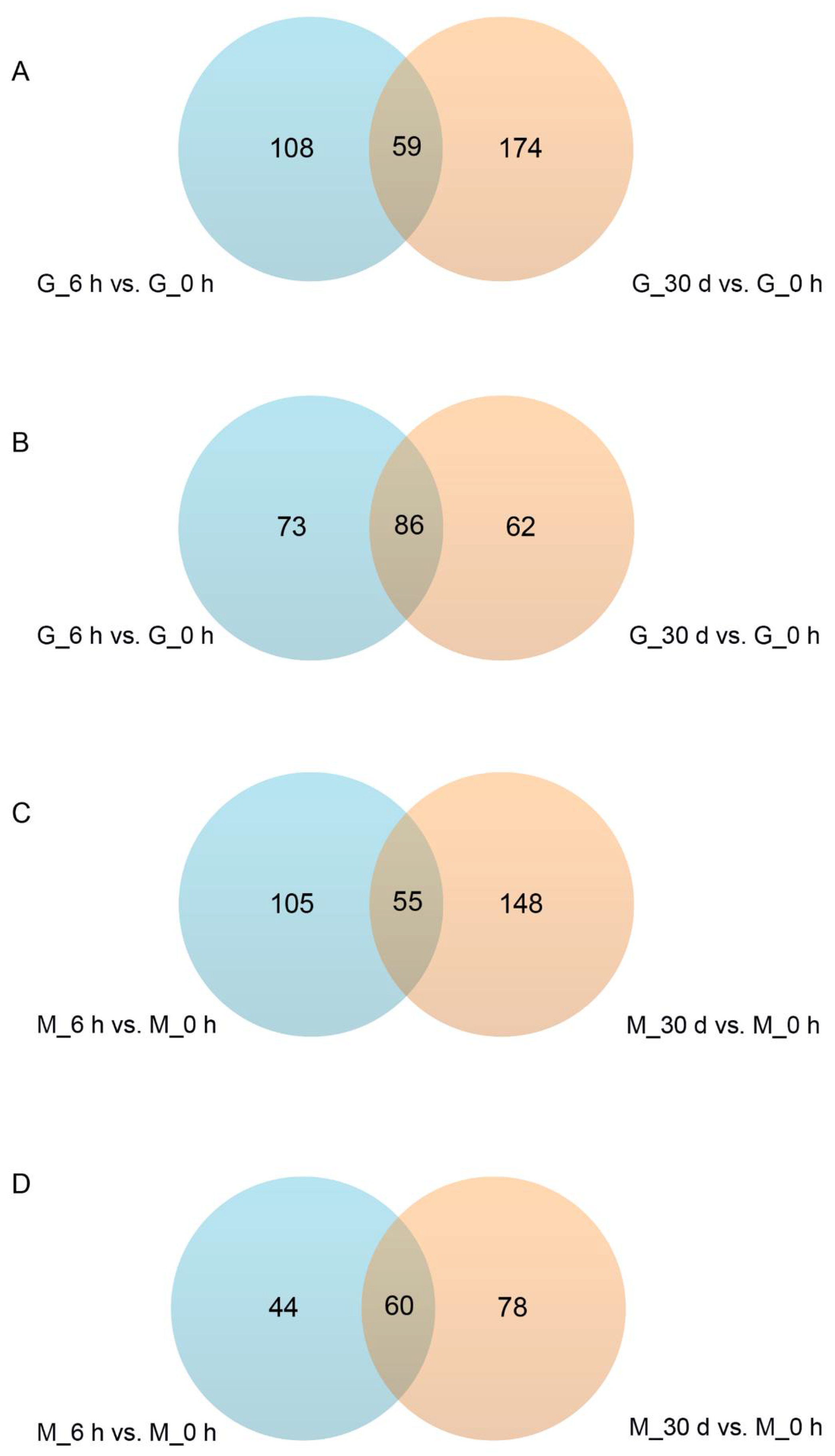
3.2. Heat Stress Response—Significantly Different Metabolites and Metabolic Pathways
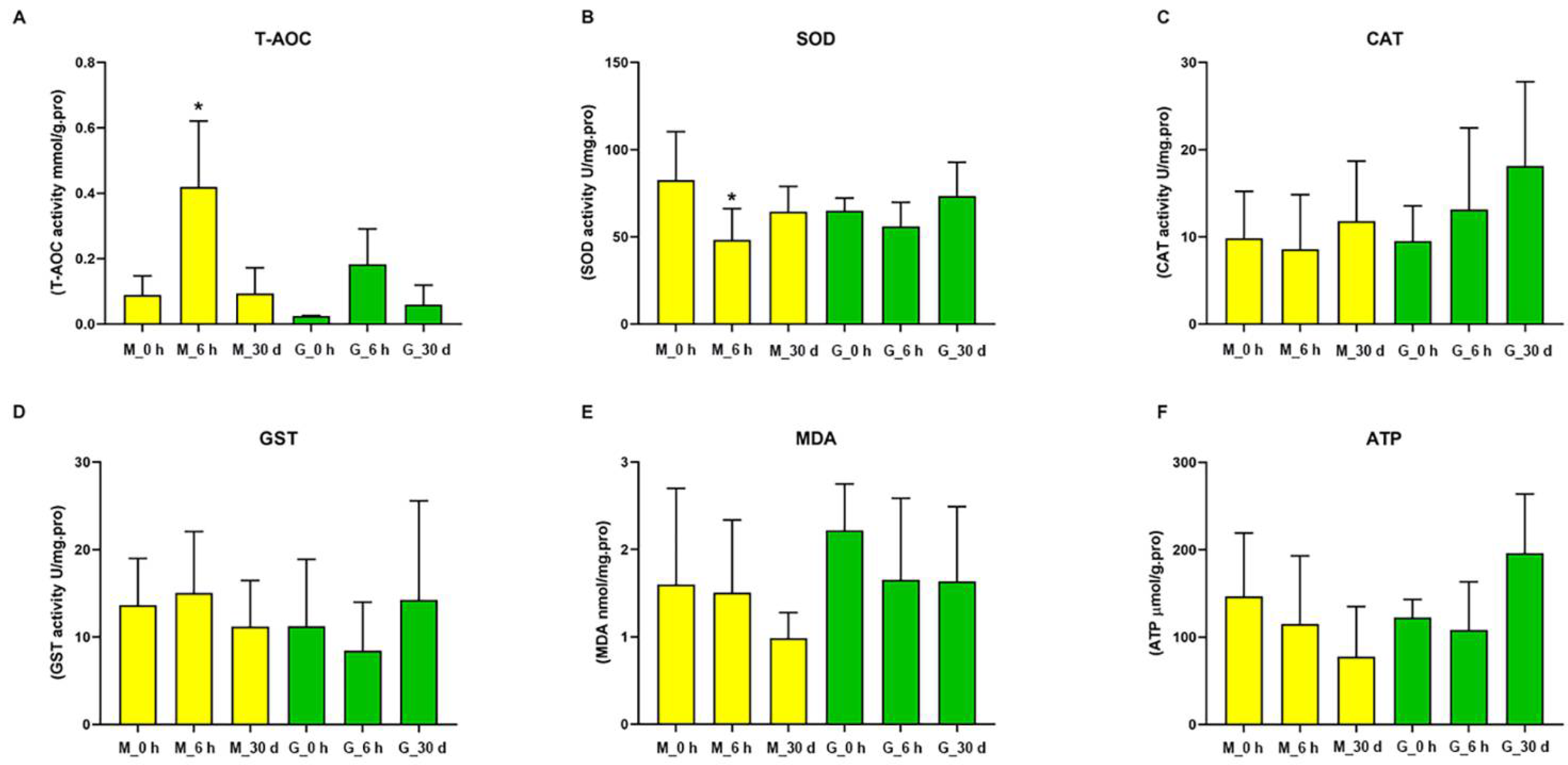
3.3. Effects of Heat on Biochemical Indexes in C. farreri
4. Discussion
4.1. Energy Metabolism
4.2. Antioxidant Ability
4.3. Metabolic States
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Said, R.M.; Nassar, S.E. Mortality, energy reserves, and oxidative stress responses of three native freshwater mussels to temperature as an indicator of potential impacts of climate change: A laboratory experimental approach. J. Therm. Biol. 2022, 104, 103154. [Google Scholar] [CrossRef] [PubMed]
- Tripp-Valdez, M.A.; Bock, C.; Lucassen, M.; Lluch-Cota, S.E.; Sicard, M.T.; Lannig, G.; Pörtner, H.-O. Metabolic response and thermal tolerance of green abalone juveniles (Haliotis fulgens: Gastropoda) under acute hypoxia and hypercapnia. J. Exp. Mar. Biol. Ecol. 2017, 497, 11–18. [Google Scholar] [CrossRef]
- Götze, S.; Bock, C.; Eymann, C.; Lannig, G.; Steffen, J.B.; Pörtner, H.-O. Single and combined effects of the “Deadly trio” hypoxia, hypercapnia and warming on the cellular metabolism of the great scallop Pecten maximus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 243, 110438. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, B.; Ruggiero, K.; Zamora, L.; Ragg, N. Metabolomic analysis of heat-hardening in adult green-lipped mussel (Perna canaliculus): A key role for succinic acid and the GABAergic synapse pathway. J. Therm. Biol. 2018, 74, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Alfaro, A.C.; Nguyen, T.V.; Young, T.; Lulijwa, R. An integrated omics approach to investigate summer mortality of New Zealand Greenshell™ mussels. Metabolomics 2020, 16, 100. [Google Scholar] [CrossRef]
- Zhang, C.G.; Ding, W.D.; Cao, Z.M.; Bing, X.W.; Xu, C.; Li, L. Effects of acute high temperature stress on antioxidant enzymes activity, digestive enzymes activity and gene expression of heat shock proteins in mandarin fish (Siniperca chuatsi). J. South Agric. 2021, 52, 815–826. [Google Scholar]
- Huo, D.; Sun, L.; Zhang, L.; Ru, X.; Liu, S.; Yang, H. Metabolome responses of the sea cucumber Apostichopus japonicus to multiple environmental stresses: Heat and hypoxia. Mar. Pollut. Bull. 2019, 138, 407–420. [Google Scholar] [CrossRef]
- Seena, S.; Sobral, O.; Cano, A. Metabolomic, functional, and ecologic responses of the common freshwater fungus Neonectria lugdunensis to mine drainage stress. Sci. Total Environ. 2020, 718, 137359. [Google Scholar] [CrossRef]
- Gong, W.J.; Jia, J.; Zhang, B.; Mi, S.; Zhang, L.; Xie, X.; Guo, H.; Shi, J.; Tu, C. Serum Metabolomic Profiling of Piglets Infected with Virulent Classical Swine Fever Virus. Front. Microbiol. 2017, 8, 731. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yin, W.; Zhan, Y.; Jia, Y.; Cui, D.; Zhang, W.; Chang, Y. Comparative metabolome analysis provides new insights into increased larval mortality under seawater acidification in the sea urchin Strongylocentrotus intermedius. Sci. Total Environ. 2020, 747, 141206. [Google Scholar] [CrossRef]
- Jiao, S.; Nie, M.; Song, H.; Xu, D.; You, F. Physiological responses to cold and starvation stresses in the liver of yellow drum (Nibea albiflora) revealed by LC-MS metabolomics. Sci. Total Environ. 2020, 715, 136940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, C.; Fan, K.; Zhang, L.; Liu, Y.; Liu, P.-F. Metabolomics analysis of the effects of temperature on the growth and development of juvenile European seabass (Dicentrarchus labrax). Sci. Total Environ. 2021, 769, 145155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ma, A.; Yang, S.; Huang, Z. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 102937. [Google Scholar] [CrossRef]
- Doris, A.; Pablo, V.A.J.; Tania, Z.S. Oxidative Stress in Aquatic Ecosystems; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Jiang, W.W. Effects of Temperature Variation on Physiological Activities of Scallops and Abalone. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2017. [Google Scholar]
- Coppola, F.; Henriques, B.; Soares, A.M.; Figueira, E.; Pereira, E.; Freitas, R. Influence of temperature rise on the recovery capacity of Mytilus galloprovincialis exposed to mercury pollution. Ecol. Indic. 2018, 93, 1060–1069. [Google Scholar] [CrossRef]
- Matoo, O.B.; Ivanina, A.V.; Ullstad, C.; Beniash, E.; Sokolova, I.M. Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 545–553. [Google Scholar] [CrossRef]
- Shumway, S.E.; Parsons, G.J. Scallops: Biology, Ecology, Aquaculture, and Fisheries; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Guo, X.M.; Luo, Y.S. Scallops and scallop aquaculture in China. Dev. Aquac. Fish Sci. 2016, 40, 937–952. [Google Scholar]
- Bureau of Fisheries of the Ministry of Agriculture. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2020; pp. 21–23. [Google Scholar]
- Lian, S.S. Construction of Assessment Indexes for Energy Metabolism and Antioxidant-Stress Ability of Zhikong Scallop (Chlamys farreri). Ph.D. Thesis, Ocean University of China, Qingdao, China, 2013. [Google Scholar]
- Chen, Z.; Zhang, D.; Xing, J.; Zhan, W. Effects of Temperature on Haemocyte and Granulocyte Counts and Expressions of Immunity-Related Genes in Haemocytes of Scallop Chlamys farreri after Vibrio anguillarum Infection. J. Ocean Univ. China 2019, 18, 1163–1173. [Google Scholar] [CrossRef]
- Jimenez-Alvarez, D.; Giuffrida, F.; Vanrobaeys, F.; Golay, P.A.; Cotting, C.; Lardeau, A.; Keely, B.J. High-Throughput Methods To Assess Lipophilic and Hydrophilic Antioxidant Capacity of Food Extracts in Vitro. J. Agric. Food Chem. 2008, 56, 3470–3477. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta. 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, C. Contect of ATP in RBC detect by CPK. J. Pract. Med. Tech. 2002, 9, 908–909. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Song, M.; Zhao, J.; Wen, H.-S.; Li, Y.; Li, J.-F.; Li, L.-M.; Tao, Y.-X. The impact of acute thermal stress on the metabolome of the black rockfish (Sebastes schlegelii). PLoS ONE 2019, 14, e0217133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.; Chen, Z.-G.; Zheng, J.; Peng, B. Metabolites-Enabled Survival of Crucian Carps Infected by Edwardsiella tarda in High Water Temperature. Front. Immunol. 2019, 10, 1991. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Metabolism Mechanism of Agasicles hygrophila in Response to High Temperature. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- Liao, G.Z.; Chai, S.M. Research advance of branced-chain amino acids. Feed Rev. 2005, 04, 7–10. [Google Scholar]
- Fan, X.Q.; Hu, J.P. Physiological function of glycine and its role in metabolism: Research advances. J. Int. Pharm. Res. 2018, 45, 102–107. [Google Scholar]
- Pogoda, B.; Buck, B.; Saborowski, R.; Hagen, W. Biochemical and elemental composition of the offshore-cultivated oysters Ostrea edulis and Crassostrea gigas. Aquaculture 2013, 400, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Han, G.; Pham, C.V.; Koyanagi, K.; Song, Y.; Sudo, R.; Lauwereyns, J.; Cockrem, J.F.; Furuse, M.; Chowdhury, V.S. An acute increase in water temperature can increase free amino acid concentrations in the blood, brain, liver, and muscle in goldfish (Carassius auratus). Fish Physiol. Biochem. 2019, 45, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Tripp-Valdez, M.A.; Bock, C.; Lannig, G.; Koschnick, N.; Pörtner, H.O.; Lucassen, M. Assessment of muscular energy metabolism and heat shock response of the green abalone Haliotis fulgens (Gastropoda: Philipi) at extreme temperatures combined with acute hypoxia and hypercapnia. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 227, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Shen, S.Q.; Yang, X.Q. Effects of short-time high temperature stress on protein and amino acid content in Eupolyphaga sinensis Walker. J. Environ. Entomo. 2020, 42, 979–984. [Google Scholar]
- Fan, X.P. Stress Response and Mechanism of Waterless Preservation after Low-Temperature Induced Dormancy on Pearl Gentian Grouper (♀Epinephelusfuscoguttatus × ♂Epinepheluslanceolatus). Ph.D. Thesis, Guangdong Ocean University, Zhanjiang, China, 2019. [Google Scholar]
- Keller, M.; Sommer, A.M.; Pörtner, H.O.; Abele, D. Seasonality of energetic functioning and production of reactive oxygen species by lugworm (Arenicola marina) mitochondria exposed to acute temperature changes. J. Exp. Biol. 2004, 207, 2529–2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.S. Effects of elevated temperature on prooxidant-antioxidant homeostasis and redox status in the American oyster: Signaling pathways of cellular apoptosis during heat stress. Environ. Res. 2021, 196, 110428. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Dubey, R.S. Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul. 2009, 59, 37–49. [Google Scholar] [CrossRef]
- Srivastava, S.; Dubey, R.S. Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regul. 2011, 64, 1–16. [Google Scholar] [CrossRef]
- Zeng, T.; Li, J.-J.; Wang, D.-Q.; Li, G.-Q.; Wang, G.-L.; Lu, L.-Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: Evidence for differential thermal sensitivities. Cell Stress Chaperones 2014, 19, 895–901. [Google Scholar] [CrossRef]
- Daneshyar, M. The effect of dietary turmeric on antioxidant properties of thigh meat in broiler chickens after slaughter. Anim. Sci. J. 2012, 83, 599–604. [Google Scholar] [CrossRef]
- Long, S.; Dong, X.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Xie, S.; Deng, J.; Yang, Y. The antioxidant ability, histology, proximate, amino acid and fatty acid compositions, and transcriptome analysis of muscle in juvenile hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatus) fed with oxidized fish oil. Aquaculture 2021, 547, 737510. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Y.; Lu, C.; Ahmad, H.; Zhang, H.; He, J.; Zhang, L.; Wang, T. Influence of Butyrate Loaded Clinoptilolite Dietary Supplementation on Growth Performance, Development of Intestine and Antioxidant Capacity in Broiler Chickens. PLoS ONE 2016, 11, e0154410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, K.P.; Dong, S.L.; Zhou, Y.G.; Gao, Q.F.; Sun, D.J. Antioxidant responses of rainbow trout with different ploidies to acute temperature stress. J. Appl. Ecol. 2018, 29, 3102–3110. [Google Scholar]
- Xu, Y.Q.; Yi, B.; Ding, Z.K. Effect of glutamine on the antioxidation of aquatic animal. Feed Ind. 2013, 34, 22–24. [Google Scholar]
- Qi, B.; Wu, S.G.; Wang, J.; Qi, G.H.; Zhang, H.J. Physiological Function and Its Application in Animal Feeding of β-Alanine. Chin. J. Anim. Nutr. 2016, 28, 1027–1034. [Google Scholar]
- Ding, Y.Q. The Protective Effect of Pantothenic Calcium on Starved Rats and Mice and Its Probable Mechanisms. Master Thesis, Naval Medical University, Shanghai, China, 2001. [Google Scholar]
- Currie, S.; Ahmady, E.; Watters, M.; Perry, S.; Gilmour, K. Fish in hot water: Hypoxaemia does not trigger catecholamine mobilization during heat shock in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, Z.; Song, Z.; Xiao, P.; Liu, Y.; Zhang, P.; You, F. Insight into the heat resistance of fish via blood: Effects of heat stress on metabolism, oxidative stress and antioxidant response of olive flounder Paralichthys olivaceus and turbot Scophthalmus maximus. Fish Shellfish Immunol. 2016, 58, 125–135. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, Y.Y.; Liu, Y.; Liu, J.R. Research on Opine dehydrogenase in commercial marine mollusks: A review. J. Dalian Ocean Univ. 2019, 34, 296–302. [Google Scholar]
- Hardewig, I.; Pörtner, H.O.; Dijk, P.V. How does the cold stenothermal gadoid Lota lota survive high water temperatures during summer? Comp. Physiol B. 2004, 174, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Morley, S.A.; Hirse, T.; Thorne, M.A.S.; Pörtner, H.O.; Peck, L.S. Physiological plasticity, long term resistance or acclimation to temperature, in the Antarctic bivalve, Laternula elliptica. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 162, 16–21. [Google Scholar] [CrossRef]
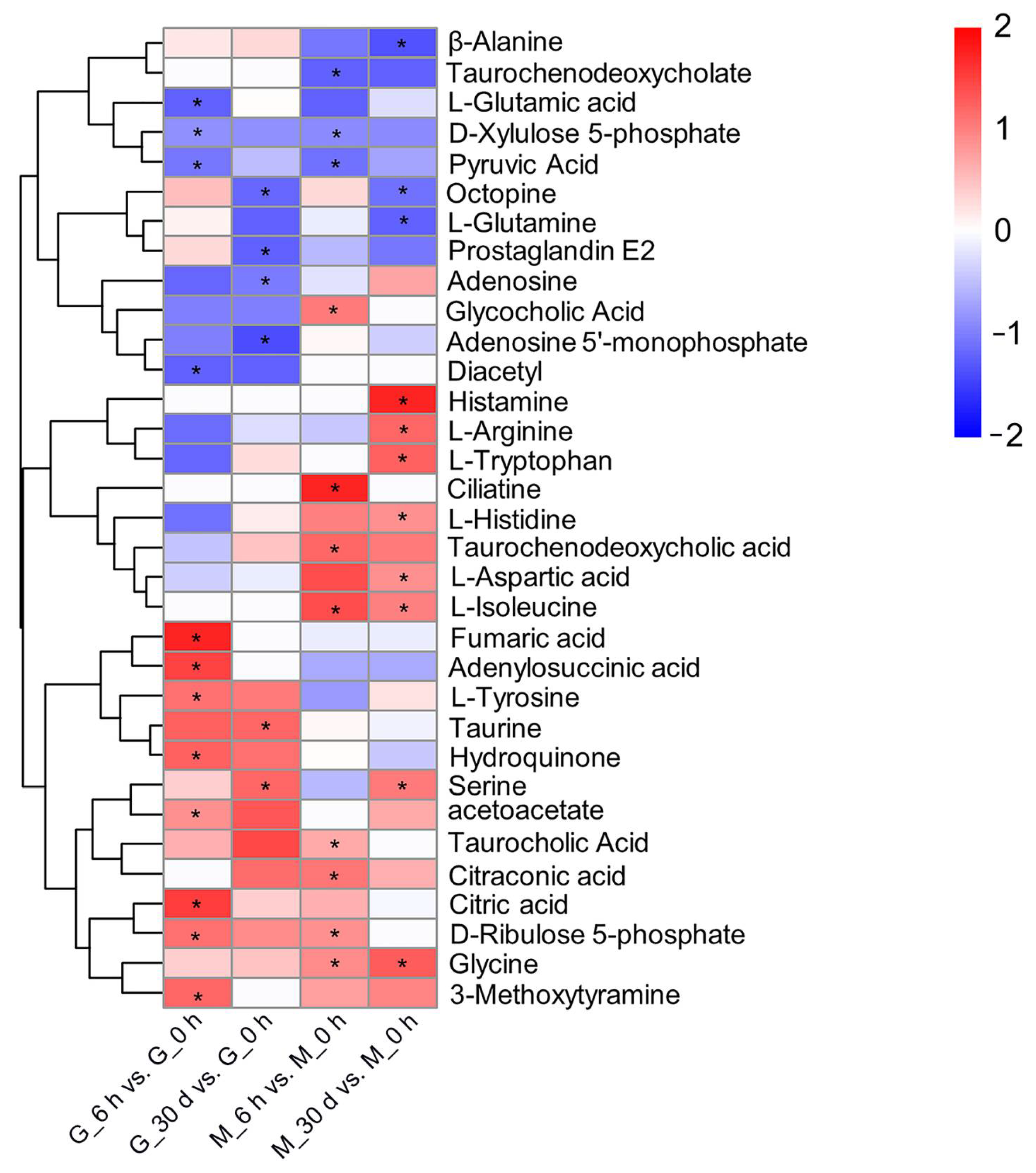
| Groups | Level 1 | Level 2 | Map Title | Metabolites | p Value |
|---|---|---|---|---|---|
| G_6 h vs. G_0 h | Metabolism | Carbohydrate metabolism | Citrate cycle (TCA cycle), Pentose and glucuronate interconversions | Pyruvic Acid, Fumaric acid, Citric acid, D-Xylulose 5-phosphate, Pyruvic acid, D-Ribulose 5-phosphate | 0.031399 |
| Amino acid metabolism | Butanoate metabolism | L-Glutamic acid, Diacetyl, Fumaric acid, acetoacetate | 0.009674 | ||
| Alanine, aspartate and glutamate metabolism | L-Glutamic acid, Adenylosuccinic acid, Fumaric acid, Pyruvic Acid | 0.013789 | |||
| Tyrosine metabolism | L-Tyrosine, Pyruvic acid, 3-Methoxytyramine, Hydroquinone, Fumaric acid, acetoacetate | 0.013789 | |||
| G_30 d vs. G_0 h | Metabolism | Energy metabolism | Sulfur metabolism | Taurine, Serine | 0.024072 |
| M_6 h vs. M_0 h | Metabolism | Lipid metabolism | Primary bile acid biosynthesis | Glycine, Glycocholic Acid, Taurochenodeoxycholic acid, Taurocholic Acid, Taurochenodeoxycholate | 0.025934 |
| Carbohydrate metabolism | Pentose and glucuronate interconversions | D-Xylulose 5-phosphate, Pyruvic Acid, D-Ribulose 5-phosphate | 0.028313 | ||
| Amino acid metabolism | Valine, leucine and isoleucine biosynthesis | Pyruvic Acid, Citraconic acid, L-Isoleucine | 0.028313 | ||
| Metabolism of other amino acids | Phosphonate and phosphinate metabolism | Ciliatine, Glycine, Pyruvic Acid | 0.028313 | ||
| M_30 d vs. M_0 h | Genetic Information Processing | Translation | Aminoacyl-tRNA biosynthesis | L-Arginine, L-Histidine, L-Glutamine, L-Tryptophan, Serine, L-Isoleucine, Glycine, L-Aspartic acid, β-Alanine | 0.018137 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Yang, Z.; Liu, Z.; Wang, X.; Yu, H.; Peng, C.; Hou, X.; Lu, W.; Xing, Q.; Hu, J.; et al. Metabonomic Analysis Provides New Insights into the Response of Zhikong Scallop (Chlamys farreri) to Heat Stress by Improving Energy Metabolism and Antioxidant Capacity. Antioxidants 2022, 11, 1084. https://doi.org/10.3390/antiox11061084
Dong X, Yang Z, Liu Z, Wang X, Yu H, Peng C, Hou X, Lu W, Xing Q, Hu J, et al. Metabonomic Analysis Provides New Insights into the Response of Zhikong Scallop (Chlamys farreri) to Heat Stress by Improving Energy Metabolism and Antioxidant Capacity. Antioxidants. 2022; 11(6):1084. https://doi.org/10.3390/antiox11061084
Chicago/Turabian StyleDong, Xixi, Zujing Yang, Zhi Liu, Xuefeng Wang, Haitao Yu, Cheng Peng, Xiujiang Hou, Wei Lu, Qiang Xing, Jingjie Hu, and et al. 2022. "Metabonomic Analysis Provides New Insights into the Response of Zhikong Scallop (Chlamys farreri) to Heat Stress by Improving Energy Metabolism and Antioxidant Capacity" Antioxidants 11, no. 6: 1084. https://doi.org/10.3390/antiox11061084
APA StyleDong, X., Yang, Z., Liu, Z., Wang, X., Yu, H., Peng, C., Hou, X., Lu, W., Xing, Q., Hu, J., Huang, X., & Bao, Z. (2022). Metabonomic Analysis Provides New Insights into the Response of Zhikong Scallop (Chlamys farreri) to Heat Stress by Improving Energy Metabolism and Antioxidant Capacity. Antioxidants, 11(6), 1084. https://doi.org/10.3390/antiox11061084





