Jelly Fig (Ficus awkeotsang Makino) Exhibits Antioxidative and Anti-Inflammatory Activities by Regulating Reactive Oxygen Species Production via NFκB Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Cytotoxic Activity Assay
2.3. In Vitro Antioxidant Measurement
2.3.1. Ferrous Ion Chelating Activity
2.3.2. Ferric-Reducing Antioxidant Capability
2.3.3. Hydrogen Peroxide Scavenging Activity
2.3.4. DPPH Radicals Scavenging Activity
2.3.5. Hydroxyl Radical Scavenging Activity
2.4. Measurement of In Vitro Inflammatory Activity
2.5. Immunoblotting Assay
2.6. Determination of In Vivo Inflammatory Activity
2.7. Immunofluorescence Staining
2.8. Total Phenol and Flavonoids Assay
2.9. Nuclear Magnetic Resonance Spectroscopy (NMR) and High-Performance Liquid Chromatography (HPLC) Analysis
2.10. Statistical Analysis
3. Results
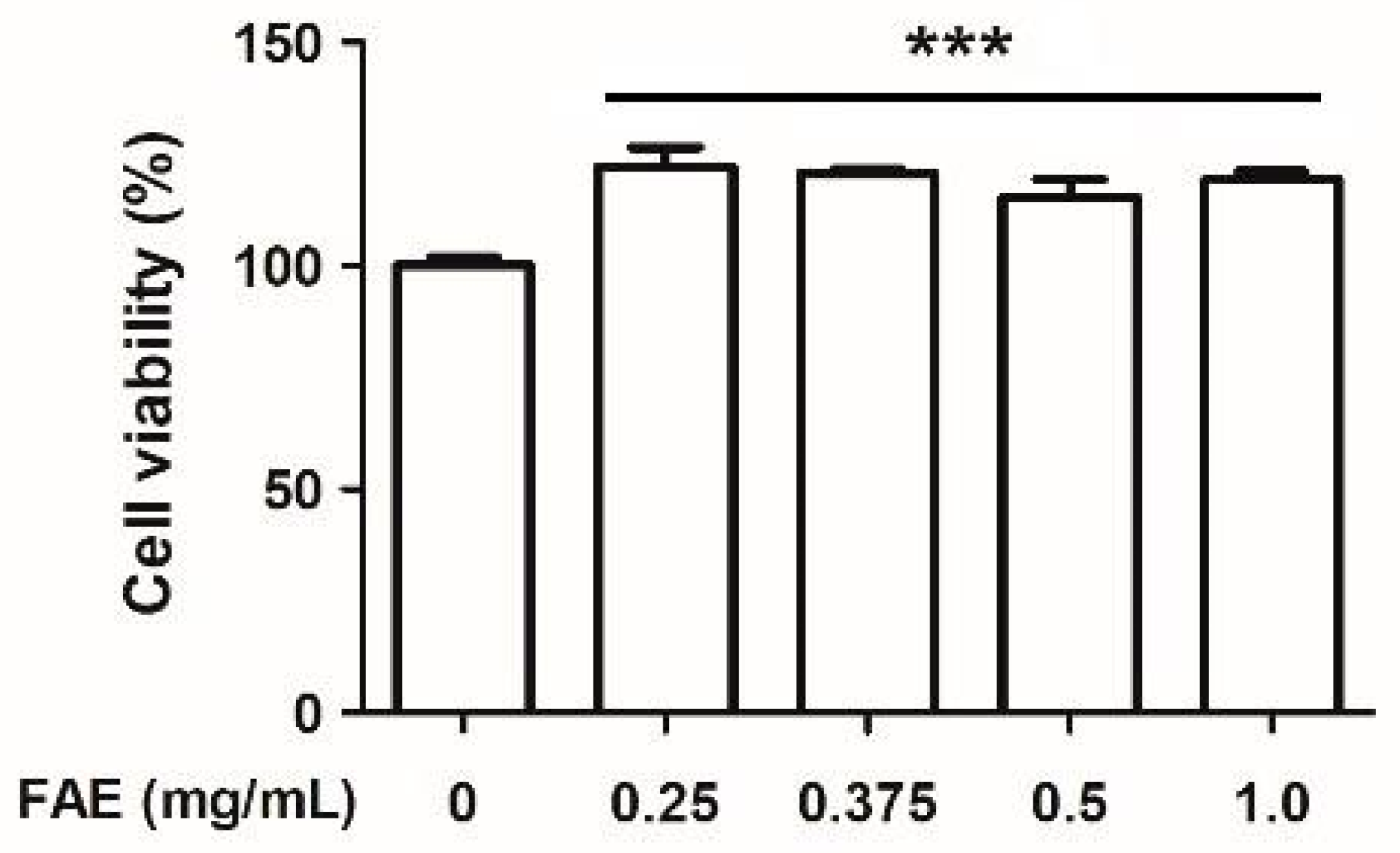
3.1. Cytotoxic Activity
3.2. Antioxidant Capacity
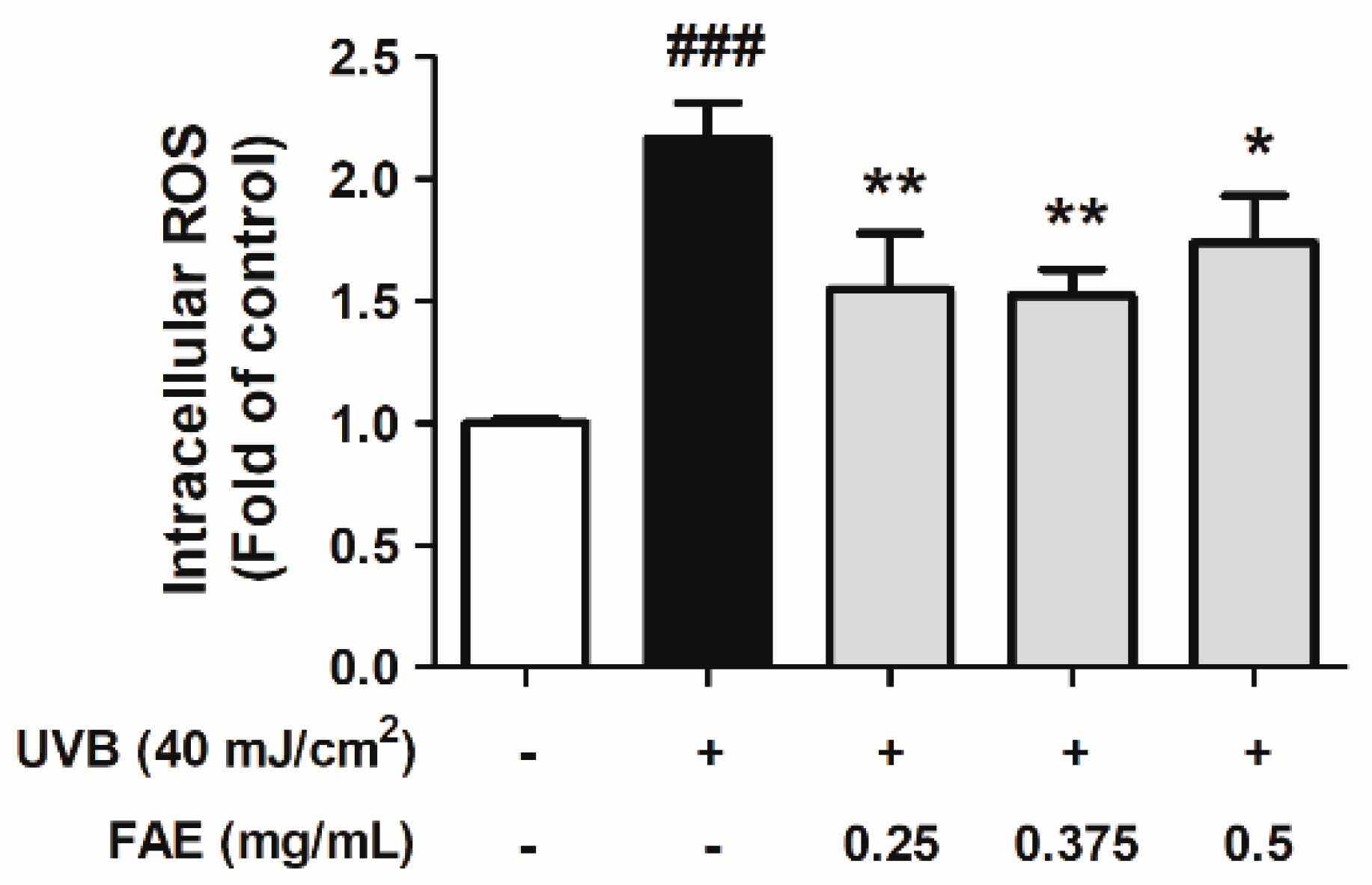
3.3. In Vitro Anti-Inflammatory Activity
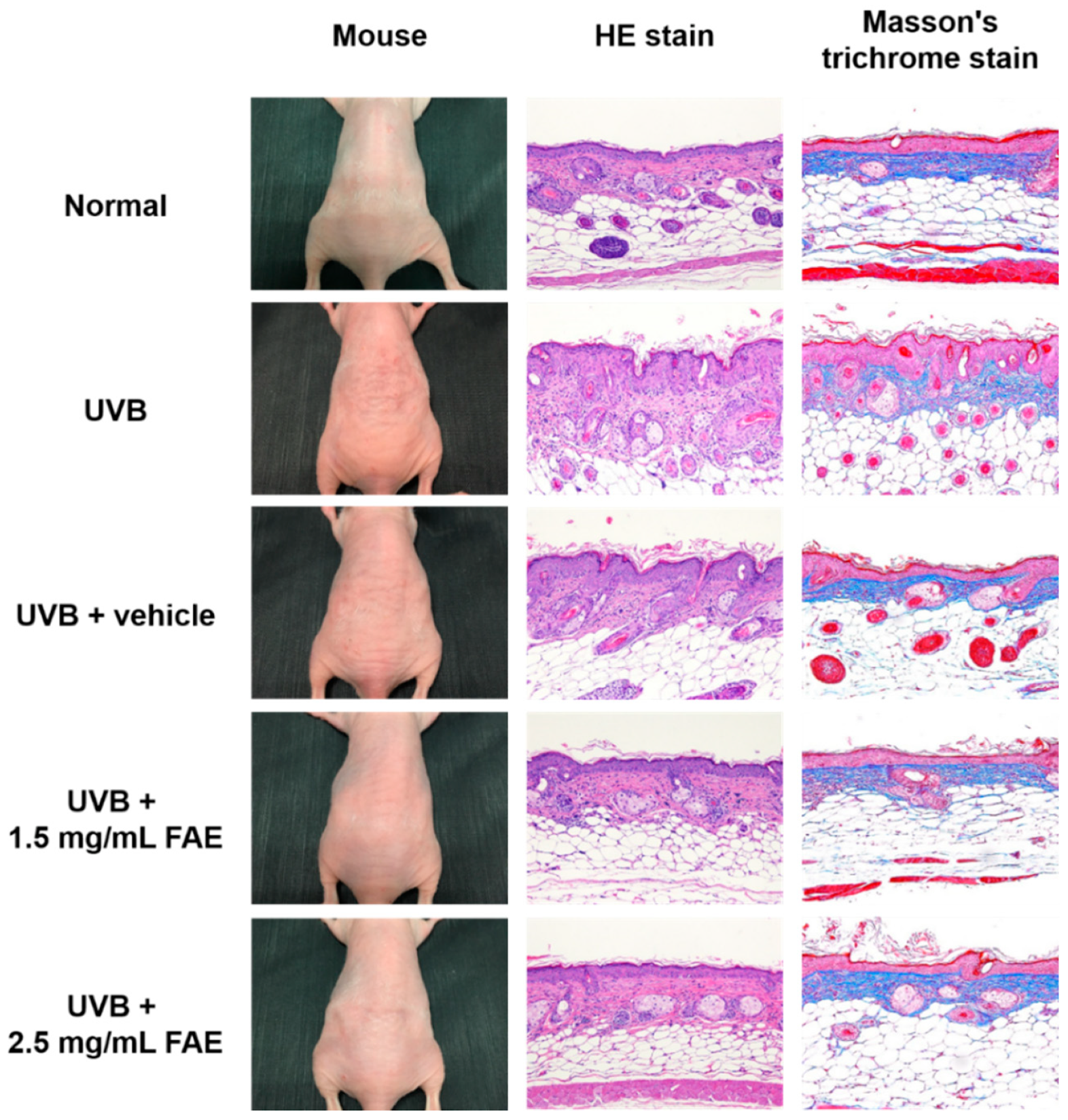
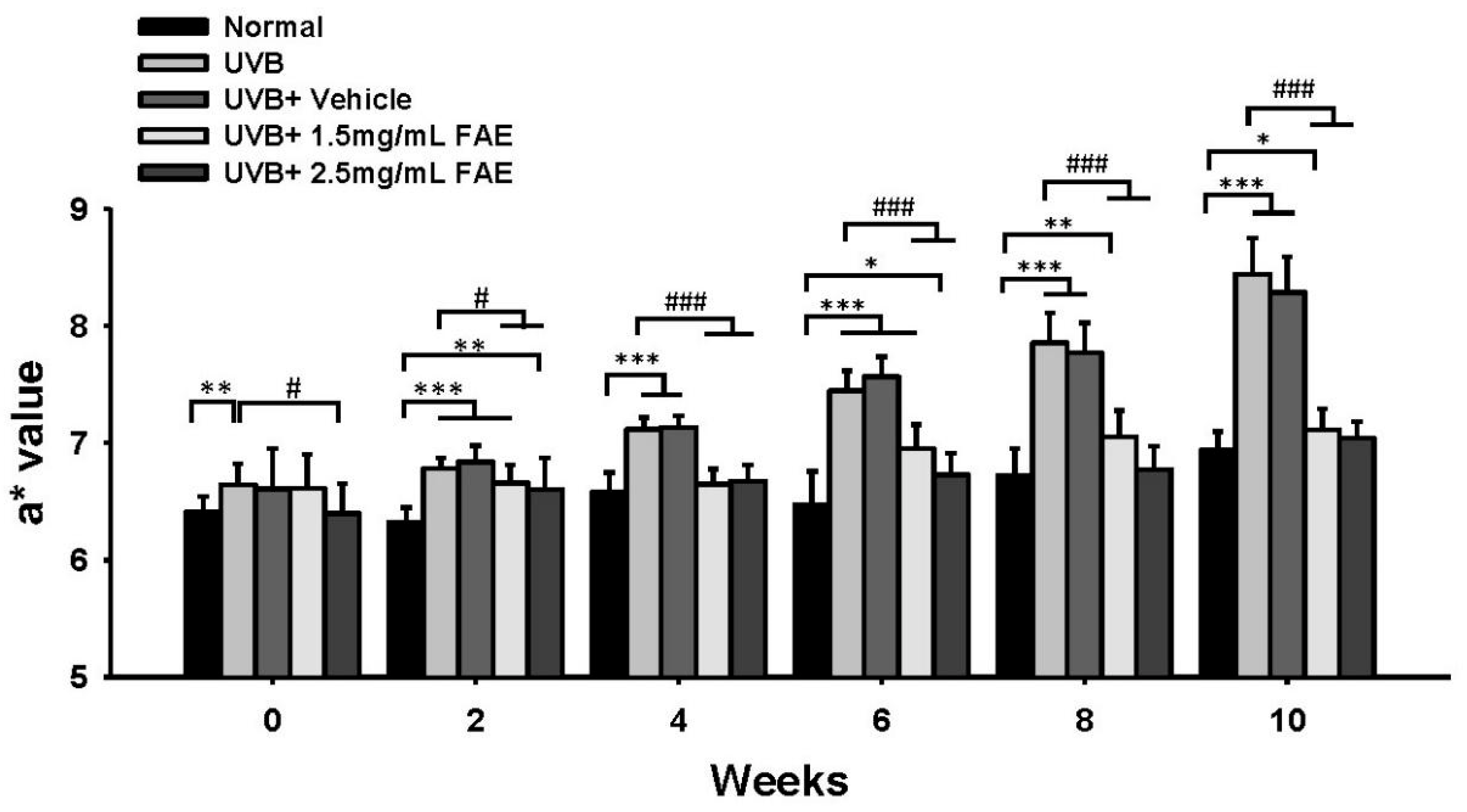
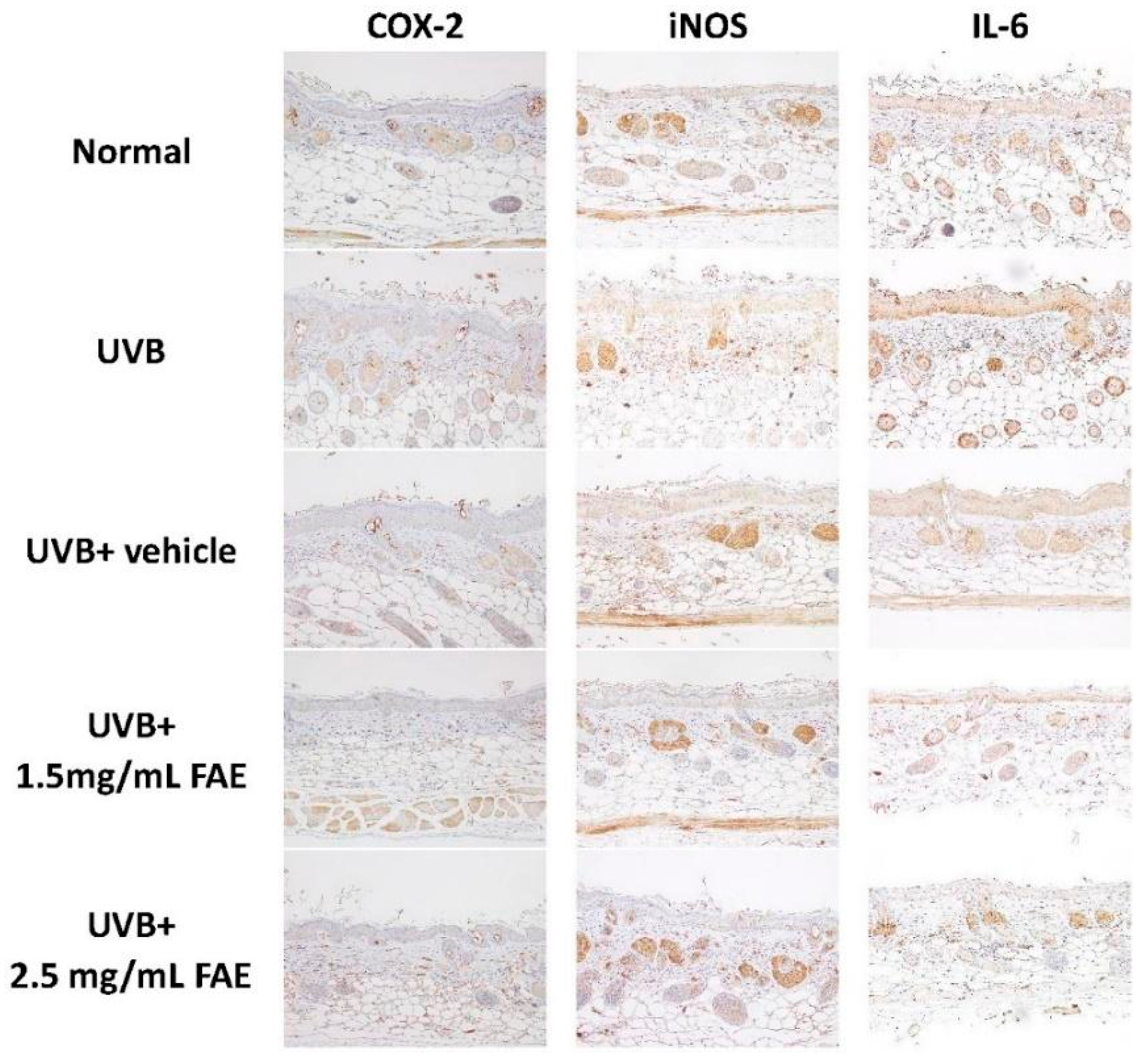
3.4. In Vivo Inflammatory Action
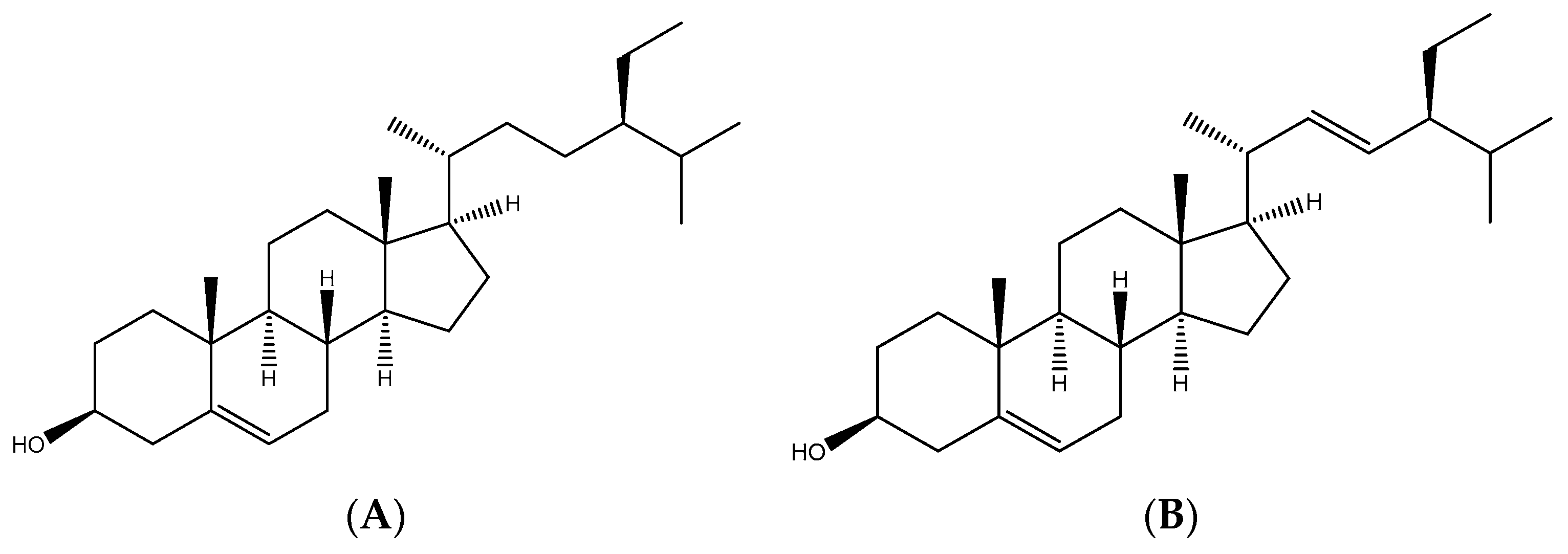
3.5. Identification of Antioxidant Compounds in FAE
4. Discussion
4.1. Antioxidant Compounds in FAE
4.2. Anti-Inflammatory Compounds in FAE
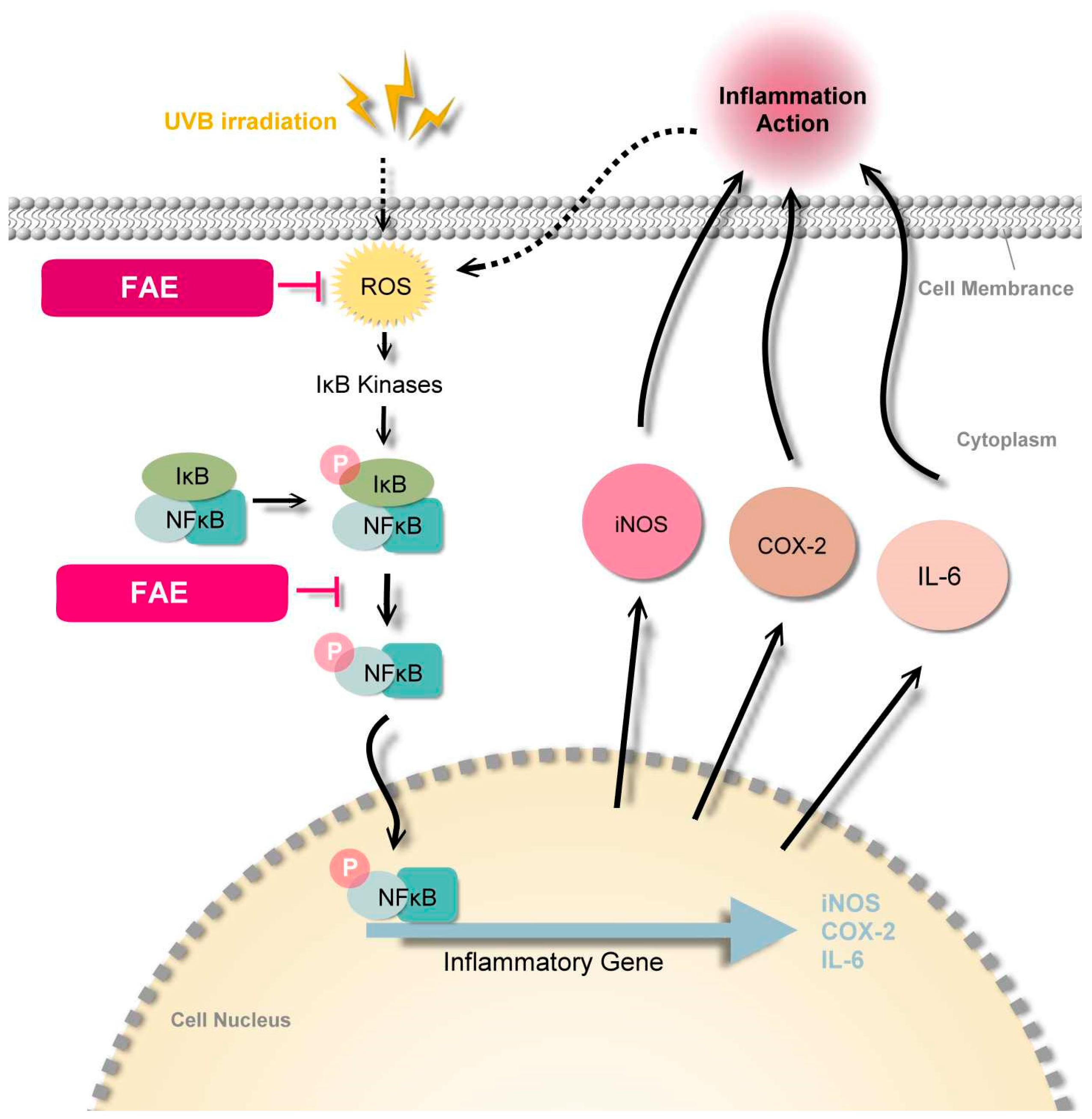
4.3. Molecular Mechanism of FAE
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef]
- Ryser, S.; Schuppli, M.; Gauthier, B.; Hernandez, D.R.; Roye, O.; Hohl, D.; German, B.; Holzwarth, J.A.; Moodycliffe, A.M. UVB-induced skin inflammation and cutaneous tissue injury is dependent on the MHC class I-like protein, CD1d. J. Investig. Dermatol. 2014, 134, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Wolfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef]
- Zaidieh, T.; Smith, J.R.; Ball, K.E.; An, Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer 2019, 19, 1224. [Google Scholar] [CrossRef] [Green Version]
- Wen, K.C.; Chiu, H.H.; Fan, P.C.; Chen, C.W.; Wu, S.M.; Chang, J.H.; Chiang, H.M. Antioxidant activity of Ixora parviflora in a cell/cell-free system and in UV-exposed human fibroblasts. Molecules 2011, 16, 5735–5752. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharmacogn. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Ceglinski, T.; Zajdel, R. Antioxidant properties of plant-derived phenolic compounds and their effect on skin fibroblast cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef]
- Okura, M.; Yamashita, T.; Ishii-Osai, Y.; Yoshikawa, M.; Sumikawa, Y.; Wakamatsu, K.; Ito, S. Effects of rhododendrol and its metabolic products on melanocytic cell growth. J. Dermatol. Sci. 2015, 80, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Wakamatsu, K. Biochemical mechanism of rhododendrol-induced leukoderma. Int. J. Mol. Sci. 2018, 19, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.P.; Liu, C.C.; Chen, S.W.; Wang, W.Y. Purification and characterization of pectinmethylesterase from Ficus awkeotsang Makino achenes. Plant Physiol. 1989, 91, 1445–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.C.; Lin, J.H.; Chua, A.C.; Chung, T.Y.; Tsai, I.C.; Tzen, J.T.; Chou, W.M. Cloning and expression of pathogenesis-related protein 4 from jelly fig (Ficus awkeotsang Makino) achenes associated with ribonuclease, chitinase and anti-fungal activities. Plant Physiol. Biochem. 2012, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Wang, Y.T.; Chang, H.M. Pectinesterase inhibitor from jelly fig (Ficus awkeotsang Makino) achene induces apoptosis of human leukemic U937 cells. Ann. N. Y. Acad. Sci. 2005, 1042, 506–515. [Google Scholar] [CrossRef]
- Chou, W.M.; Chen, C.N.; Hsieh, H.T.; Lo, T.Y.; Juan, P.Y.; Mai, F.D. G2/M arrest and apoptosis of human colorectal cancer cells induced by water extract from residues of jelly fig achene. Technol. Health Care 2015, 24 (Suppl. 1), S147–S153. [Google Scholar] [CrossRef]
- Shih, Y.Z.; Huang, A.J.; Hou, C.Y.; Jiang, C.M.; Wu, M.C. The stimulating effects of polyphenol and protein fractions from jelly fig (Ficus awkeotsang Makino) achenes against proliferation of leukemia cells. J. Food Drug Anal. 2017, 25, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.C.; Jiang, C.M.; Chen, Y.J.; Chen, Y.Y. Pectinesterase inhibitor from jelly fig (Ficus awkeotsang Makino) achene inhibits surface antigen expression by human hepatitis B virus. Evid. Based Complement. Alternat. Med. 2013, 2013, 434823. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef]
- Ao, C.; Li, A.; Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control 2008, 19, 940–948. [Google Scholar] [CrossRef]
- Çalişkan, O.; Aytekin Polat, A. Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Sci. Hortic. 2011, 128, 473–478. [Google Scholar] [CrossRef]
- Sultana, J.; Kabir, A.S.; Hakim, M.A.; Abdullah, M.; Islam, N.; Reza, M.A. Evaluation of the antioxidant activity of Ficus Racemosa plant extracts from north-western district of Bangladesh. J. Life Earth Sci. 2013, 8, 93–99. [Google Scholar] [CrossRef]
- Soni, N.; Mehta, S.; Satpathy, G.; Gupta, R.K. Estimation of nutritional, phytochemical, antioxidant and antibacterial activity of dried fig (Ficus carica). J. Pharmacogn. Phytochem. 2014, 3, 158–165. [Google Scholar]
- Harzallah, A.; Bhouri, A.M.; Amri, Z.; Soltana, H.; Hammami, M. Phytochemical content and antioxidant activity of different fruit parts juices of three figs (Ficus carica L.) varieties grown in Tunisia. Ind. Crops Prod. 2016, 83, 255–267. [Google Scholar] [CrossRef]
- Lin, M.-J.; Wu, D.-J.; Wu, H.-H.; Peng, S.-C.; Lu, M.-C. Breeding of new jelly fig (Ficus awkeotsang Makino) cultivars Miaoli No. 1 and Miaoli No. 2. J. Taiwan Soc. Hort. Sci. 2017, 63, 83–97. [Google Scholar]
- Chiang, H.M.; Chiu, H.H.; Liao, S.T.; Chen, Y.T.; Chang, H.C.; Wen, K.C. Isoflavonoid-rich flemingia macrophylla extract attenuates UVB-induced skin damage by scavenging reactive oxygen species and inhibiting MAP kinase and MMP expression. Evid. Based Complement. Alternat. Med. 2013, 2013, 696879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tundis, R.; Menichini, F.; Bonesi, M.; Conforti, F.; Statti, G.; Menichini, F.; Loizzo, M.R. Antioxidant and hypoglycaemic activities and their relationship to phytochemicals in Capsicum annuum cultivars during fruit development. LWT-Food Sci. Technol. 2013, 53, 370–377. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Wu, P.-Y.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Protective effects and mechanisms of Terminalia catappa L. methenolic extract on hydrogen-peroxide-induced oxidative stress in human skin fibroblasts. BMC Complement Altern. Med. 2018, 18, 266. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Lyu, J.-L.; Kuo, Y.-H.; Chiu, C.-Y.; Wen, K.-C.; Chiang, H.-M. The anti-melanogenesis effect of 3, 4-Dihydroxybenzalacetone through downregulation of melanosome maturation and transportation in B16F10 and human epidermal melanocytes. Int. J. Mol. Sci. 2021, 22, 2823. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar] [PubMed]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedula, V.S.P.; Prakash, I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Azman, N.A.N.; Alhawarri, M.B.; Rawa, M.S.A.; Dianita, R.; Gazzali, A.M.; Nogawa, T.; Wahab, H.A. Potential anti-acetylcholinesterase activity of cassia timorensis DC. Molecules 2020, 25, 4545. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Van den Ende, W.; Peshev, D.; De Gara, L. Disease prevention by natural antioxidants and prebiotics acting as ROS scavengers in the gastrointestinal tract. Trends Food Sci. Technol. 2011, 22, 689–697. [Google Scholar] [CrossRef]
- Kahl, R. Synthetic antioxidants: Biochemical actions and interference with radiation, toxic compounds, chemical mutagens and chemical carcinogens. Toxicology 1984, 33, 185–228. [Google Scholar] [CrossRef]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm. Unters. Forsch. 1993, 196, 329–338. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, S.C.; Moldao-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Jemli, M.; Kamal, R.; Marmouzi, I.; Zerrouki, A.; Cherrah, Y.; Alaoui, K. Radical-scavenging activity and ferric reducing ability of Juniperus thurifera (L.), J. oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv. Pharmacol. Sci. 2016, 2016, 6392656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.J.; Chen, J.N.; Wu, J.S.B. Antibacterial activity and antioxidant properties of water extract from the residue of jelly fig (Ficus awkeotsang Makino) achenes. J. Food Drug Anal. 2008, 16, 31–38. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agr. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Aboul-Enein, A.M.; Baz, F.K.E.; El-Baroty, G.S.; Youssef, A.M.; El-Baky, H.H.A. Antioxidant activity of algal extracts on lipid peroxidation. J. Med. Sci. 2003, 3, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Gulcin, I.; Buyukokuroglu, M.E.; Kufrevioglu, O.I. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal Res. 2003, 34, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdeltaif, S.; SirElkhatim, K.; Hassan, A. Estimation of phenolic and flavonoid compounds and antioxidant activity of spent coffee and black tea (processing) waste for potential recovery and reuse in Sudan. Recycling 2018, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.; Fry, S.C. Phenolic components of the plant cell wall. In International Review of Cytology; Jeon, K.W., Jarvik, J., Eds.; Academic Press: Cambridge, MA, USA, 1994; Volume 151, pp. 229–267. [Google Scholar]
- Binns, A.N.; Chen, R.H.; Wood, H.N.; Lynn, D.G. Cell division promoting activity of naturally occurring dehydrodiconiferyl glucosides: Do cell wall components control cell division? Proc. Natl. Acad. Sci. USA 1987, 84, 980–984. [Google Scholar] [CrossRef] [Green Version]
- Kamiloglu, S.; Capanoglu, E. Polyphenol content in figs (Ficus carica L.): Effect of sun-drying. Int. J. Food Prop. 2014, 18, 521–535. [Google Scholar] [CrossRef]
- Sumi, S.A.; Siraj, M.A.; Hossain, A.; Mia, M.S.; Afrin, S.; Rahman, M.M. Investigation of the key pharmacological activities of Ficus Racemosa and analysis of Its major bioactive polyphenols by HPLC-DAD. Evid. Based Complement. Alternat. Med. 2016, 2016, 3874516. [Google Scholar] [CrossRef] [PubMed]
- Issa-Issa, H.; Cano-Lamadrid, M.; Calin-Sanchez, A.; Wojdylo, A.; Carbonell-Barrachina, A.A. Volatile composition and sensory attributes of smoothies based on pomegranate juice and mediterranean fruit purees (fig, jujube and quince). Foods 2020, 9, 926. [Google Scholar] [CrossRef]
- Bölek, S. Effects of waste fig seed powder on quality as an innovative ingredient in biscuit formulation. J. Food Sci. 2020, 86, 55–60. [Google Scholar] [CrossRef]
- Teruel-Andreu, C.; Andreu-Coll, L.; López-Lluch, D.; Sendra, E.; Hernández, F.; Cano-Lamadrid, M. Ficus carica fruits, by-products and based products as potential sources of bioactive compounds: A review. Agronomy 2021, 11, 1834. [Google Scholar] [CrossRef]
- Khodarahmi, G.A.; Ghasemi, N.; Hassanzadeh, F.; Safaie, M. Cytotoxic effects of different extracts and latex of Ficus carica L. on heLa cell line. Iran J. Pharm. Res. 2011, 10, 273–277. [Google Scholar] [PubMed]
- Vandghanooni, S.; Forouharmehr, A.; Eskandani, M.; Barzegari, A.; Kafil, V.; Kashanian, S.; Ezzati Nazhad Dolatabadi, J. Cytotoxicity and DNA fragmentation properties of butylated hydroxyanisole. DNA Cell Biol. 2013, 32, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Arsianti, A.; Bahtiar, A.; Fadilah, F.; Wangsaputra, V.K.; Paramita, R.I.; Azizah, N.N.; Nadapdap, L.D.; Fajrin, A.M.; Tanimoto, H.; Kakiuchi, K. Synthesis, characterization, and cytotoxicity evaluation of gallic acid nanoparticles towards breast T47D cancer cells. Pharmacogn. J. 2020, 12, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Deng, H.D.; Li, Y.; Cui, H.; Wang, X.; Fang, J.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, L.C.; Artis, D. Beyond host defense: Emerging functions of the immune system in regulating complex tissue physiology. Cell 2018, 173, 554–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, M.F.; Robinson, M.K.; Baron, E.D.; Cooper, K.D. Skin immune systems and inflammation: Protector of the skin or promoter of aging? J. Investig. Dermatol. Symp. Proc. 2008, 13, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruza, L.L.; Pentland, A.P. Mechanisms of UV-induced inflammation. J. Investig. Dermatol. 1993, 100, S35–S41. [Google Scholar] [CrossRef] [Green Version]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [Green Version]
- Ciazynska, M.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibinska, M.; Lesiak, A. Ultraviolet radiation and chronic inflammation-molecules and mechanisms involved in skin carcinogenesis: A narrative review. Life 2021, 11, 326. [Google Scholar] [CrossRef]
- Wang, P.W.; Cheng, Y.C.; Hung, Y.C.; Lee, C.H.; Fang, J.Y.; Li, W.T.; Wu, Y.R.; Pan, T.L. Red raspberry extract protects the skin against UVB-induced damage with antioxidative and anti-inflammatory properties. Oxid. Med. Cell Longev. 2019, 2019, 9529676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Kim, J.E.; Choi, Y.J.; Gong, J.E.; Park, S.H.; Douangdeuane, B.; Souliya, O.; Park, J.M.; Lee, H.S.; Kim, B.H.; et al. Therapeutic effects of dipterocarpus tuberculatus with high antioxidative activity against UV-induced photoaging of NHDF cells and nude mice. Antioxidants 2021, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Perez, R.; Flores-Mondragon, G.; Reyes-Legorreta, C.; Herrera-Lopez, B.; Cervantes-Hernandez, I.; Madrigal-Santillan, O.; Morales-Gonzalez, J.A.; Alvarez-Gonzalez, I.; Madrigal-Bujaidar, E. Evaluation of the anti-inflammatory capacity of beta-sitosterol in rodent assays. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.E.; Woo, J.S.; Yun, C.-Y.; Lee, J.-S.; Kim, J.-H.; Song, G.-Y.; Yang, E.J.; Hur, I.K.; Kim, I.S. Effects of lactose-β-sitosterol and β-sitosterol on ovalbumin-induced lung inflammation in actively sensitized mice. Int. Immunopharmacol. 2007, 7, 1517–1527. [Google Scholar] [CrossRef]
- Loizou, S.; Lekakis, I.; Chrousos, G.P.; Moutsatsou, P. Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2010, 54, 551–558. [Google Scholar] [CrossRef]
- Mahajan, S.G.; Mehta, A.A. Suppression of ovalbumin-induced Th2-driven airway inflammation by β-sitosterol in a guinea pig model of asthma. Eur. J. Pharmacol. 2011, 650, 458–464. [Google Scholar] [CrossRef]
- Liu, R.; Hao, D.; Xu, W.; Li, J.; Li, X.; Shen, D.; Sheng, K.; Zhao, L.; Xu, W.; Gao, Z.; et al. β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm. Biol. 2019, 57, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.K.; Veeraraghavan, V.P.; Palanisamy, C.P.; Cui, B.; Periyasamy, V.; Chandrasekar, K. beta-Sitosterol circumvents obesity induced inflammation and insulin resistance by down-regulating IKKbeta/NF-kappaB and JNK signaling pathway in adipocytes of type 2 diabetic rats. Molecules 2021, 26, 2101. [Google Scholar] [CrossRef]
- Antwi, A.O.; Obiri, D.D.; Osafo, N. Stigmasterol modulates allergic airway inflammation in guinea pig model of ovalbumin-Induced asthma. Mediat. Inflamm. 2017, 2017, 2953930. [Google Scholar] [CrossRef]
- Antwi, A.O.; Obiri, D.D.; Osafo, N.; Forkuo, A.D.; Essel, L.B. Stigmasterol inhibits lipopolysaccharide-induced innate immune responses in murine models. Int. Immunopharmacol. 2017, 53, 105–113. [Google Scholar] [CrossRef]
- Ahmad Khan, M.; Sarwar, A.; Rahat, R.; Ahmed, R.S.; Umar, S. Stigmasterol protects rats from collagen induced arthritis by inhibiting proinflammatory cytokines. Int. Immunopharmacol. 2020, 85, 106642. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, L.; Hou, W.; Wu, J. beta-Sitosterol alleviates inflammatory response via inhibiting the activation of ERK/p38 and NF-kappaB pathways in LPS-exposed BV2 cells. Biomed. Res. Int. 2020, 2020, 7532306. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karin, M. How NF-κB is activated: The role of the IκB kinase (IKK) complex. Oncogene 1999, 18, 6867–6874. [Google Scholar] [CrossRef] [Green Version]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB light stimulates production of reactive oxygen species: Unexpected role for catalase. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar] [CrossRef] [Green Version]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-enriched rice attenuates UVB-ROS-induced skin aging via downregulation of inflammatory cascades. Oxid. Med. Cell Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Umesalma, S.; Sudhandiran, G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-κB, iNOS, COX-2, TNF-α, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2010, 107, 650–655. [Google Scholar] [CrossRef]




| Total Phenol and Flavonoids Contents | Jelly Fig Extract |
|---|---|
| Total phenol content (mg GAE g−1 DW) | 107.622 ± 0.176 z |
| Total flavonoids content (mg QE g−1 DW) | 10.251 ± 0.020 |
| Flavonoids/phenol ratio | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-J.; Lin, P.; Wen, K.-C.; Chiang, H.-M.; Lu, M.-C. Jelly Fig (Ficus awkeotsang Makino) Exhibits Antioxidative and Anti-Inflammatory Activities by Regulating Reactive Oxygen Species Production via NFκB Signaling Pathway. Antioxidants 2022, 11, 981. https://doi.org/10.3390/antiox11050981
Lin M-J, Lin P, Wen K-C, Chiang H-M, Lu M-C. Jelly Fig (Ficus awkeotsang Makino) Exhibits Antioxidative and Anti-Inflammatory Activities by Regulating Reactive Oxygen Species Production via NFκB Signaling Pathway. Antioxidants. 2022; 11(5):981. https://doi.org/10.3390/antiox11050981
Chicago/Turabian StyleLin, Meng-Jin, Ping Lin, Kuo-Ching Wen, Hsiu-Mei Chiang, and Mei-Chun Lu. 2022. "Jelly Fig (Ficus awkeotsang Makino) Exhibits Antioxidative and Anti-Inflammatory Activities by Regulating Reactive Oxygen Species Production via NFκB Signaling Pathway" Antioxidants 11, no. 5: 981. https://doi.org/10.3390/antiox11050981
APA StyleLin, M.-J., Lin, P., Wen, K.-C., Chiang, H.-M., & Lu, M.-C. (2022). Jelly Fig (Ficus awkeotsang Makino) Exhibits Antioxidative and Anti-Inflammatory Activities by Regulating Reactive Oxygen Species Production via NFκB Signaling Pathway. Antioxidants, 11(5), 981. https://doi.org/10.3390/antiox11050981








