Physical-Exercise-Induced Antioxidant Effects on the Brain and Skeletal Muscle
Abstract
1. Introduction
2. The Antioxidant System
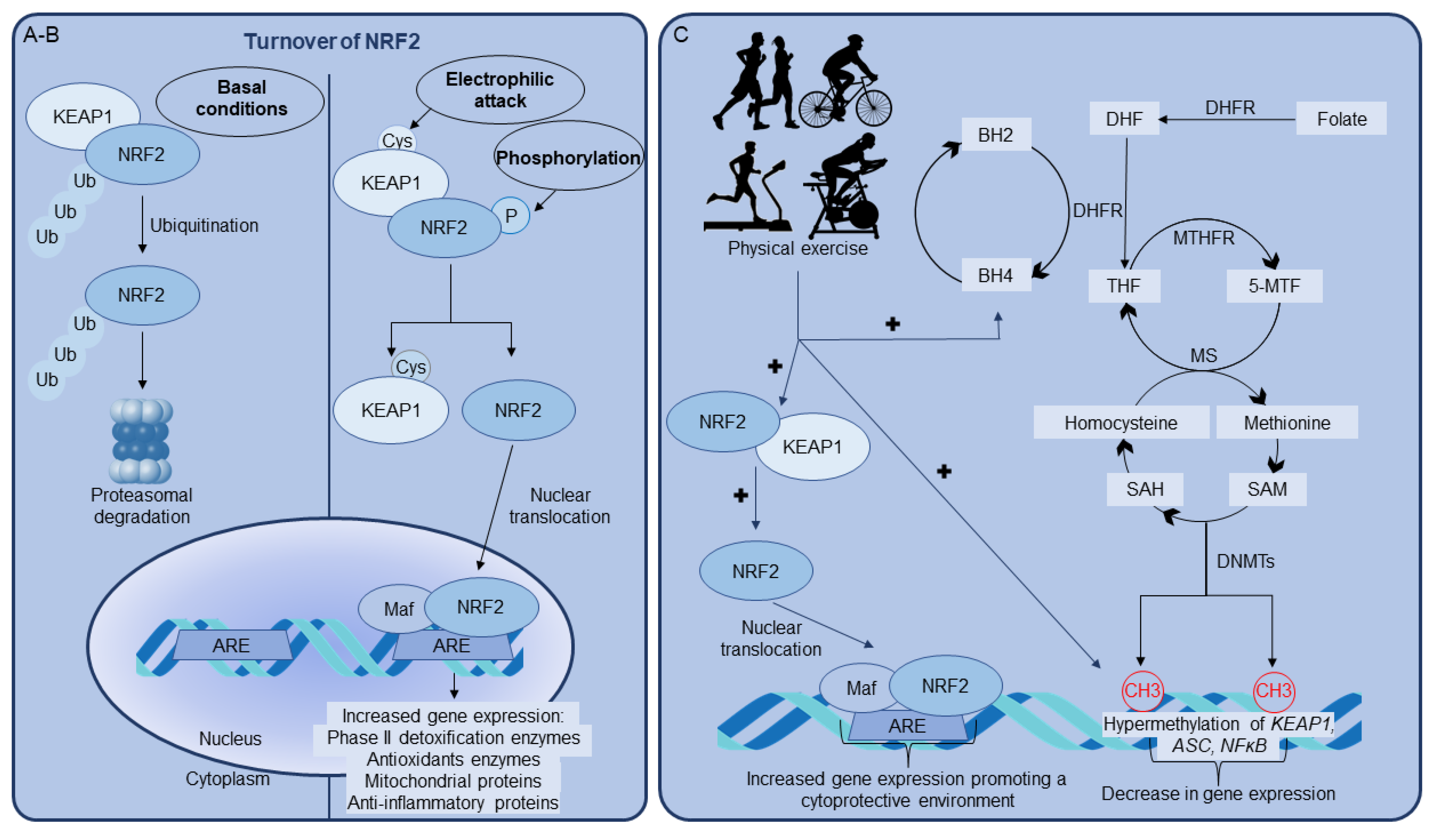
2.1. NRF2 and Central Nervous System
2.2. NRF2 Activation by Physical Exercise
3. Role of BH4 on NRF2/ARE Pathway Activated by Physical Exercise
4. Epigenetics as a Key Player in NRF2 Upregulation Induced by Physical Exercise
4.1. DNA Methylation
4.2. Histone Modifications
4.3. Post-Transcriptional Regulation
5. Effects of BH4 on Epigenetic Modulation Induced by Physical Exercise
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamada, M.; Iwata, M.; Warabi, E.; Oishi, H.; Lira, V.A.; Okutsu, M. p62/SQSTM1 and Nrf2 are essential for exercise-mediated enhancement of antioxidant protein expression in oxidative muscle. FASEB J. 2019, 33, 8022–8032. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.R.; Kannan, S.; Sadhaasivam, K.; Gounder, S.S.; Davidson, C.J.; Boeheme, C.; Hoidal, J.R.; Wang, L.; Rajasekaran, N.S. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic. Biol. Med. 2012, 52, 366–376. [Google Scholar] [CrossRef] [PubMed]
- da Luz Scheffer, D.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta - Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997. [Google Scholar] [CrossRef]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA 2000, 97, 12475–12480. [Google Scholar] [CrossRef]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef]
- Lee, J.M.; Hanson, J.M.; Chu, W.A.; Johnson, J.A. Phosphatidylinositol 3-Kinase, Not Extracellular Signal-regulated Kinase, Regulates Activation of the Antioxidant-Responsive Element in IMR-32 Human Neuroblastoma Cells. J. Biol. Chem. 2001. [Google Scholar] [CrossRef]
- Strom, J.; Xu, B.; Tian, X.; Chen, Q.M. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016, 30, 66–80. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Chen, K.; Gunter, K.; Maines, M.D. Neurons Overexpressing Heme Oxygenase-1 Resist Oxidative Stress-Mediated Cell Death. J. Neurochem. 2001, 75, 304–313. [Google Scholar] [CrossRef]
- Satoh, T.; Okamoto, S.-I.; Cui, J.; Watanabe, Y.; Furuta, K.; Suzuki, M.; Tohyama, K.; Lipton, S.A. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophillic phase II inducers. Proc. Natl. Acad. Sci. USA 2006, 103, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; White, C.C.; Mohar, I.; Kavanagh, T.J.; Costa, L.G. Glutathione Levels Modulate Domoic Acid–Induced Apoptosis in Mouse Cerebellar Granule Cells. Toxicol. Sci. 2007, 100, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, K.-M.; Kim, S.W.; Hwang, O.; Choi, H.J. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: Novel cytoprotective mechanism against oxidative damage. Pharmacol. Res. 2008, 57, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Agbaga, M.-P.; Anderson, R.E. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic. Biol. Med. 2007, 42, 1838–1850. [Google Scholar] [CrossRef]
- Monir, D.M.; Mahmoud, M.E.; Ahmed, O.G.; Rehan, I.F.; Abdelrahman, A. Forced exercise activates the NrF2 pathway in the striatum and ameliorates motor and behavioral manifestations of Parkinson’s disease in rotenone-treated rats. Behav. Brain Funct. 2020, 16, 9. [Google Scholar] [CrossRef]
- Tsou, Y.-H.; Shih, C.-T.; Ching, C.-H.; Huang, J.-Y.; Jen, C.J.; Yu, L.; Kuo, Y.-M.; Wu, F.-S.; Chuang, J.-I. Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP+ toxicity. Exp. Neurol. 2015, 263, 50–62. [Google Scholar] [CrossRef]
- WU, C.; YANG, L.; TUCKER, D.; DONG, Y.; ZHU, L.; DUAN, R.; LIU, T.C.-Y.; ZHANG, Q. Beneficial Effects of Exercise Pretreatment in a Sporadic Alzheimer’s Rat Model. Med. Sci. Sport. Exerc. 2018, 50, 945–956. [Google Scholar] [CrossRef]
- Rushmore, T.H.; Pickett, C.B. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 1990, 265, 14648–14653. [Google Scholar] [CrossRef]
- Przedborski, S.; Kostic, V.; Jackson-Lewis, V.; Naini, A.B.; Simonetti, S.; Fahn, S.; Carlson, E.; Epstein, C.J. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. 1992, 12, 1658–1667. [Google Scholar] [CrossRef]
- Kinouchi, H.; Epstein, C.J.; Mizui, T.; Carlson, E.; Chen, S.F.; Chan, P.H. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc. Natl. Acad. Sci. USA 1991, 88, 11158–11162. [Google Scholar] [CrossRef]
- Ripps, M.E.; Huntley, G.W.; Hof, P.R.; Morrison, J.H.; Gordon, J.W. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 1995, 92, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Call, J.A.; Donet, J.; Martin, K.S.; Sharma, A.K.; Chen, X.; Zhang, J.; Cai, J.; Galarreta, C.A.; Okutsu, M.; Du, Z.; et al. Muscle-derived extracellular superoxide dismutase inhibits endothelial activation and protects against multiple organ dysfunction syndrome in mice. Free Radic. Biol. Med. 2017, 113, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Call, J.A.; Chain, K.H.; Martin, K.S.; Lira, V.A.; Okutsu, M.; Zhang, M.; Yan, Z. Enhanced skeletal muscle expression of extracellular superoxide dismutase mitigates streptozotocin-induced diabetic cardiomyopathy by reducing oxidative stress and aberrant cell signaling. Circ. Hear. Fail. 2015, 8, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Weisbrot-Lefkowitz, M.; Reuhl, K.; Perry, B.; Chan, P.H.; Inouye, M.; Mirochnitchenko, O. Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Mol. Brain Res. 1998, 53, 333–338. [Google Scholar] [CrossRef]
- Hovatta, I.; Tennant, R.S.; Helton, R.; Marr, R.A.; Singer, O.; Redwine, J.M.; Ellison, J.A.; Schadt, E.E.; Verma, I.M.; Lockhart, D.J.; et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature 2005, 438, 662–666. [Google Scholar] [CrossRef]
- Hwang, P.; Morales Marroquín, F.E.; Gann, J.; Andre, T.; McKinley-Barnard, S.; Kim, C.; Morita, M.; Willoughby, D.S. Eight weeks of resistance training in conjunction with glutathione and L-Citrulline supplementation increases lean mass and has no adverse effects on blood clinical safety markers in resistance-trained males. J. Int. Soc. Sports Nutr. 2018, 15, 30. [Google Scholar] [CrossRef]
- Wen, Y.T.; Liu, T.T.; Lin, Y.F.; Chen, C.C.; Kung, W.M.; Huang, C.C.; Lin, T.J.; Wang, Y.H.; Wei, L. Heatstroke effect on brain heme oxygenase-1 in rats. Int. J. Med. Sci. 2015, 12, 737–741. [Google Scholar] [CrossRef]
- Panahian, N.; Yoshiura, M.; Maines, M.D. Overexpression of Heme Oxygenase-1 Is Neuroprotective in a Model of Permanent Middle Cerebral Artery Occlusion in Transgenic Mice. J. Neurochem. 2008, 72, 1187–1203. [Google Scholar] [CrossRef]
- Yu, X.; Han, W.; Wang, C.; Sui, D.; Bian, J.; Bo, L.; Deng, X. Upregulation of Heme Oxygenase-1 by Hemin Alleviates Sepsis-Induced Muscle Wasting in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Chan, M.C.; Ziegler, O.; Liu, L.; Rowe, G.C.; Das, S.; Otterbein, L.E.; Arany, Z. Heme oxygenase and carbon monoxide protect from muscle dystrophy. Skelet. Muscle 2016, 6, 41. [Google Scholar] [CrossRef]
- Lubec, J.; Smidak, R.; Malikovic, J.; Feyissa, D.D.; Korz, V.; Höger, H.; Lubec, G. Dentate Gyrus Peroxiredoxin 6 Levels Discriminate Aged Unimpaired From Impaired Rats in a Spatial Memory Task. Front. Aging Neurosci. 2019, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Olthoff, J.T.; Lindsay, A.; Abo-Zahrah, R.; Baltgalvis, K.A.; Patrinostro, X.; Belanto, J.J.; Yu, D.Y.; Perrin, B.J.; Garry, D.J.; Rodney, G.G.; et al. Loss of peroxiredoxin-2 exacerbates eccentric contraction-induced force loss in dystrophin-deficient muscle. Nat. Commun. 2018, 9, 5104. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.D.; Huang, T.; Sheng, W.H.; Guan, Y.F.; Du, Y.F.; Lin, Y.T.; Ruan, X.Y. Neuroprotective effect of recombinant adeno-associated virus human thioredoxin-PR39 on acute cerebral infarction in rats. Exp. Ther. Med. 2018, 16, 2633–2638. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.H.; Mattox, T.A.; Thayne, K.; Katunga, L.A.; La Favor, J.D.; Neufer, P.D.; Hickner, R.C.; Wingard, C.J.; Anderson, E.J. Novel role for thioredoxin reductase-2 in mitochondrial redox adaptations to obesogenic diet and exercise in heart and skeletal muscle. J. Physiol. 2013, 591, 3471–3486. [Google Scholar] [CrossRef]
- Matsushima, Y.; Nanri, H.; Nara, S.; Okufuji, T.; Ohta, M.; Hachisuka, K.; Ikeda, M. Hindlimb unloading decreases thioredoxin-related antioxidant proteins and increases thioredoxin-binding protein-2 in rat skeletal muscle. Free Radic. Res. 2006, 40, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, R.; Cigliano, L.; Verderame, M. Age-related changes of metallothionein 1/2 and metallothionein 3 expression in rat brain. C. R. Biol. 2017, 340, 13–17. [Google Scholar] [CrossRef]
- Smith, H.K.; Omura, S.; Vital, S.A.; Becker, F.; Senchenkova, E.Y.; Kaur, G.; Tsunoda, I.; Peirce, S.M.; Gavins, F.N.E. Metallothionein I as a direct link between therapeutic hematopoietic stem/progenitor cells and cerebral protection in stroke. FASEB J. 2018, 32, 2381–2394. [Google Scholar] [CrossRef] [PubMed]
- Di Foggia, V.; Zhang, X.; Licastro, D.; Gerli, M.F.M.; Phadke, R.; Muntoni, F.; Mourikis, P.; Tajbakhsh, S.; Ellis, M.; Greaves, L.C.; et al. Bmi1 enhances skeletal muscle regeneration through MT1-mediated oxidative stress protection in a mouse model of dystrophinopathy. J. Exp. Med. 2014, 211, 2617–2633. [Google Scholar] [CrossRef]
- Luo, S.; Lei, K.; Xiang, D.; Ye, K. NQO1 is regulated by PTEN in glioblastoma, mediating cell proliferation and oxidative stress. Oxid. Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Miller, C.J.; Gounder, S.S.; Kannan, S.; Goutam, K.; Muthusamy, V.R.; Firpo, M.A.; Symons, J.D.; Paine, R.; Hoidal, J.R.; Rajasekaran, N.S. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim. Biophys. Acta - Mol. Basis Dis. 2012, 1822, 1038–1050. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Zhang, L.; Li, J.; Wang, R.; Wang, M. Effects of troxerutin on cognitive deficits and glutamate cysteine ligase subunits in the hippocampus of streptozotocin-induced type 1 diabetes mellitus rats. Brain Res. 2017, 1657, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Brown-Borg, H.M.; Rakoczy, S.G.; Ferrington, D.A.; Thompson, L.V. Aging impairs the expression of the catalytic subunit of glutamate cysteine ligase in soleus muscle under stress. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2010, 65, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Kwong, M.; Hou, S.; Lee, C.; Chan, J.Y. Deficiency of the Nrf1 and Nrf2 Transcription Factors Results in Early Embryonic Lethality and Severe Oxidative Stress. J. Biol. Chem. 2003, 278, 48021–48029. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct Cysteine Residues in Keap1 Are Required for Keap1-Dependent Ubiquitination of Nrf2 and for Stabilization of Nrf2 by Chemopreventive Agents and Oxidative Stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Noda, M.; Nishizawa, M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol. Cell. Biol. 1994. [Google Scholar] [CrossRef]
- Kurokawa, H.; Motohashi, H.; Sueno, S.; Kimura, M.; Takagawa, H.; Kanno, Y.; Yamamoto, M.; Tanaka, T. Structural Basis of Alternative DNA Recognition by Maf Transcription Factors. Mol. Cell. Biol. 2009, 29, 6232–6244. [Google Scholar] [CrossRef]
- Quiles, J.M.; Pepin, M.E.; Sunny, S.; Shelar, S.B.; Challa, A.K.; Dalley, B.; Hoidal, J.R.; Pogwizd, S.M.; Wende, A.R.; Rajasekaran, N.S. Identification of Nrf2-responsive microRNA networks as putative mediators of myocardial reductive stress. Sci. Rep. 2021, 11, 11977. [Google Scholar] [CrossRef]
- Bellezza, I.; Riuzzi, F.; Chiappalupi, S.; Arcuri, C.; Giambanco, I.; Sorci, G.; Donato, R. Reductive stress in striated muscle cells. Cell. Mol. Life Sci. 2020, 77, 3547–3565. [Google Scholar] [CrossRef] [PubMed]
- de Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone Methyl in Type 2 Diabetes and Stage 4 Chronic Kidney Disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Hubbs, A.F.; Benkovic, S.A.; Miller, D.B.; O’Callaghan, J.P.; Battelli, L.; Schwegler-Berry, D.; Ma, Q. Vacuolar Leukoencephalopathy with Widespread Astrogliosis in Mice Lacking Transcription Factor Nrf2. Am. J. Pathol. 2007, 170, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; Innamorato, N.G.; Martín-Moreno, A.M.; De Ceballos, M.L.; Yamamoto, M.; Cuadrado, A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia 2010, 58, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Innamorato, N.G.; Rojo, A.I.; García-Yagüe, Á.J.; Yamamoto, M.; de Ceballos, M.L.; Cuadrado, A. The Transcription Factor Nrf2 Is a Therapeutic Target against Brain Inflammation. J. Immunol. 2008, 181, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J. Physiol. 2016, 594, 5195–5207. [Google Scholar] [CrossRef]
- Tutakhail, A.; Nazary, Q.A.; Lebsir, D.; Kerdine-Romer, S.; Coudore, F. Induction of brain Nrf2-HO-1 pathway and antinociception after different physical training paradigms in mice. Life Sci. 2018, 209, 149–156. [Google Scholar] [CrossRef]
- Scheffer, D.L.; Silva, L.A.; Tromm, C.B.; da Rosa, G.L.; Silveira, P.C.L.; de Souza, C.T.; Latini, A.; Pinho, R.A. Impact of different resistance training protocols on muscular oxidative stress parameters. Appl. Physiol. Nutr. Metab. 2012, 37. [Google Scholar] [CrossRef]
- Done, A.J.; Gage, M.J.; Nieto, N.C.; Traustadóttir, T. Exercise-induced Nrf2-signaling is impaired in aging. Free Radic. Biol. Med. 2016, 96, 130–138. [Google Scholar] [CrossRef]
- Done, A.J.; Newell, M.J.; Traustadóttir, T. Effect of exercise intensity on Nrf2 signalling in young men. Free Radic. Res. 2017, 51, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Gounder, S.S.; Kannan, S.; Devadoss, D.; Miller, C.J.; Whitehead, K.J.; Whitehead, K.S.; Odelberg, S.J.; Firpo, M.A.; Paine, R.; Hoidal, J.R.; et al. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS ONE 2012, 7, e45697. [Google Scholar] [CrossRef]
- Li, T.; He, S.; Liu, S.; Kong, Z.; Wang, J.; Zhang, Y. Effects of different exercise durations on Keap1-Nrf2-ARE pathway activation in mouse skeletal muscle. Free Radic. Res. 2015, 49, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S.; Duzzioni, M.; Remor, A.P.; Tristão, F.S.M.; Matheus, F.C.; Raisman-Vozari, R.; Latini, A.; Prediger, R.D. Moderate-intensity physical exercise protects against experimental 6-hydroxydopamine-induced hemiparkinsonism through Nrf2-antioxidant response element pathway. Neurochem. Res. 2016, 41, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.L.; Lv, C.N.; Xing, Y.; Geng, Y.; Zhang, F.; Bu, W.; Wang, M.W. Downregulated nuclear factor E2-related factor 2 (Nrf2) aggravates cognitive impairments via neuroinflammation and synaptic plasticity in the senescence-accelerated mouse prone 8 (SAMP8) mouse: A model of accelerated senescence. Med. Sci. Monit. 2018, 24, 1132–1144. [Google Scholar] [CrossRef]
- Gores, G.J.; Flarsheim, C.E.; Dawson, T.L.; Nieminen, A.L.; Herman, B.; Lemasters, J.J. Swelling, reductive stress, and cell death during chemical hypoxia in hepatocytes. Am. J. Physiol. Physiol. 1989, 257, C347–C354. [Google Scholar] [CrossRef]
- Xiao, W.; Loscalzo, J. Metabolic Responses to Reductive Stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial Complex III ROS Regulate Adipocyte Differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Jedrychowski, M.P.; Lu, G.Z.; Erickson, B.K.; Szpyt, J.; Pierce, K.A.; Laznik-Bogoslavski, D.; Vetrivelan, R.; Clish, C.B.; et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 2016, 532, 112–116. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Manford, A.G.; Rodríguez-Pérez, F.; Shih, K.Y.; Shi, Z.; Berdan, C.A.; Choe, M.; Titov, D.V.; Nomura, D.K.; Rape, M. A Cellular Mechanism to Detect and Alleviate Reductive Stress. Cell 2020, 183, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta - Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Thony, B.; Auerbach, G.; Blau, N.; Thöny, B.; Auerbach, G.; Blau, N.; Thöny, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000, 347 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Werner, E.R.E.R.; Blau, N.; Thöny, B. Tetrahydrobiopterin: Biochemistry and pathophysiology. Biochem. J. 2011, 438, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.R.; Werner-Felmayer, G.; Fuchs, D.; Hausen, A.; Reibnegger, G.; Yim, J.J.; Pfleiderer, W.; Wachter, H. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J. Biol. Chem. 1990, 265, 3189–3192. [Google Scholar] [CrossRef]
- Ghisoni, K.; de Paula Martins, R.; Barbeito, L.; Latini, A. Neopterin as a potential cytoprotective brain molecule. J. Psychiatr. Res. 2015, 71, 134–139. [Google Scholar] [CrossRef]
- Cronin, S.J.F.; Seehus, C.; Weidinger, A.; Talbot, S.; Reissig, S.; Seifert, M.; Pierson, Y.; McNeill, E.; Longhi, M.S.; Turnes, B.L.; et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature 2018, 563, 564–568. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.Y.; Cho, Y.; No, H.; Kim, S.W.; Hwang, O. Tetrahydrobiopterin causes mitochondrial dysfunction in dopaminergic cells: Implications for Parkinson’s disease. Neurochem. Int. 2006, 48, 255–262. [Google Scholar] [CrossRef]
- Bailey, J.; Shaw, A.; Fischer, R.; Ryan, B.J.; Kessler, B.M.; McCullagh, J.; Wade-Martins, R.; Channon, K.M.; Crabtree, M.J. A novel role for endothelial tetrahydrobiopterin in mitochondrial redox balance. Free Radic. Biol. Med. 2017, 104, 214–225. [Google Scholar] [CrossRef]
- Ghisoni, K.; Latini, A. Kuehne LK, Reiber H, Bechter K, Hagberg L, Fuchs D., Cerebrospinal fluid neopterin is brain-derived and not associated with blood-CSF barrier dysfunction in non-inflammatory affective and schizophrenic spectrum disorders. Letter to the Editor. J. Psychiatr. Res. 2015, 63, 141–142. [Google Scholar] [CrossRef]
- Martins, R.D.P.; Ghisoni, K.; Lim, C.K.; Aguiar, A.S.; Guillemin, G.J.; Latini, A. Neopterin preconditioning prevents inflammasome activation in mammalian astrocytes. Free Radic. Biol. Med. 2018, 115, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Ghisoni, K.; Aguiar, A.S.; de Oliveira, P.A.; Matheus, F.C.; Gabach, L.; Perez, M.; Carlini, V.P.; Barbeito, L.; Mongeau, R.; Lanfumey, L.; et al. Neopterin acts as an endogenous cognitive enhancer. Brain. Behav. Immun. 2016, 56, 156–164. [Google Scholar] [CrossRef]
- da Luz Scheffer, D.; Ghisoni, K.; Aguiar, A.S.; Latini, A. Moderate running exercise prevents excessive immune system activation. Physiol. Behav. 2019, 204, 248–255. [Google Scholar] [CrossRef] [PubMed]
- HASHIMOTO, R.; NAGATSU, T.; OHTA, T.; MIZUTANI, M.; OMURA, I. Changes in the Concentrations of Tetrahydrobiopterin, the Cofactor of Tyrosine Hydroxylase, in Blood under Physical Stress and in Depression. Ann. N. Y. Acad. Sci. 2004, 1018, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Hashimoto, R.; Ohta, T.; Nakazawa, K.; Nagatsu, T. The Effect of Exercise on Plasma Biopterin Levels. Neuropsychobiology 1994, 29, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, H.; Jacobs, C.; Nain, M.; Gressner, A.M.; Prinz, H.; Wesemann, W.; Gemsa, D. Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clin. Immunol. Immunopathol. 1992, 63, 188–195. [Google Scholar] [CrossRef]
- Baxter-Parker, G.; Chu, A.; Petocz, P.; Samman, S.; Gieseg, S.P. Simultaneous analysis of neopterin, kynurenine and tryptophan by amine-HPLC shows minor oxidative stress from short-term exhaustion exercise. Pteridines 2019, 30, 21–32. [Google Scholar] [CrossRef]
- Strasser, B.; Geiger, D.; Schauer, M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Effects of Exhaustive Aerobic Exercise on Tryptophan-Kynurenine Metabolism in Trained Athletes. PLoS ONE 2016, 11, e0153617. [Google Scholar] [CrossRef]
- Dantas de Lucas, R.; Caputo, F.; Mendes de Souza, K.; Sigwalt, A.R.; Ghisoni, K.; Lock Silveira, P.C.; Remor, A.P.; da Luz Scheffer, D.; Antonacci Guglielmo, L.G.; Latini, A. Increased platelet oxidative metabolism, blood oxidative stress and neopterin levels after ultra-endurance exercise. J. Sports Sci. 2014, 32, 22–30. [Google Scholar] [CrossRef]
- Moser, B.; Schroecksnadel, K.; Hörtnagl, H.; Rieder, J.; Fuchs, D.; Gottardis, M. Influence of Extreme Long Endurance Sports Activity on Neopterin Excretion. Pteridines 2008, 19, 114–119. [Google Scholar] [CrossRef]
- Lindsay, A.; Lewis, J.; Scarrott, C.; Draper, N.; Gieseg, S.P. Changes in acute biochemical markers of inflammatory and structural stress in rugby union. J. Sports Sci. 2015, 33, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.; Janmale, T.; Draper, N.; Gieseg, S.P. Measurement of changes in urinary neopterin and total neopterin in body builders using SCX HPLC. Pteridines 2014, 25, 53–63. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Vezzoli, A.; Dellanoce, C.; Comassi, M.; Giardini, G.; Bruno, R.M.; Montorsi, M.; Corciu, A.; Greco, F.; et al. Acute Effects of Triathlon Race on Oxidative Stress Biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; et al. Effects of Mountain Ultra-Marathon Running on ROS Production and Oxidative Damage by Micro-Invasive Analytic Techniques. PLoS ONE 2015, 10, e0141780. [Google Scholar] [CrossRef] [PubMed]
- McNeill, E.; Crabtree, M.J.; Sahgal, N.; Patel, J.; Chuaiphichai, S.; Iqbal, A.J.; Hale, A.B.; Greaves, D.R.; Channon, K.M. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic. Biol. Med. 2015, 79, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.; Costello, J.T. Realising the Potential of Urine and Saliva as Diagnostic Tools in Sport and Exercise Medicine. Sport. Med. 2017, 47, 11–31. [Google Scholar] [CrossRef]
- Gieseg, S.; Baxter-Parker, G.; Lindsay, A. Neopterin, Inflammation, and Oxidative Stress: What Could We Be Missing? Antioxidants 2018, 7, 80. [Google Scholar] [CrossRef]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef]
- Waddington, C.H. The epigenotype. 1942. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Tost, J. DNA methylation: An introduction to the biology and the disease-associated changes of a promising biomarker. Mol. Biotechnol. 2010, 44, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Baulcombe, D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science (80-. ). 1999, 286, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity (Edinb). 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Sawan, C.; Vaissiere, T.; Murr, R.; Herceg, Z. Epigenetic drivers and genetic passengers on the road to cancer. Mutat. Res. 2008, 642, 1–13. [Google Scholar] [CrossRef]
- Loenarz, C.; Schofield, C.J. Oxygenase catalyzed 5-methylcytosine hydroxylation. Chem. Biol. 2009, 16, 580–583. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, S.; Zhang, C.; Kong, A.N.T. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 337–349. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Zhao, X.-Z.; Zhang, X.-S.; Zhang, M. Promoter DNA demethylation of Keap1 gene in diabetic cardiomyopathy. Int. J. Clin. Exp. Pathol. 2014, 7, 8756–8762. [Google Scholar]
- Ferrari, L.; Vicenzi, M.; Tarantini, L.; Barretta, F.; Sironi, S.; Baccarelli, A.A.; Guazzi, M.; Bollati, V. Effects of Physical Exercise on Endothelial Function and DNA Methylation. Int. J. Environ. Res. Public Health 2019, 16, 2530. [Google Scholar] [CrossRef]
- Dimauro, I.; Paronetto, M.P.; Caporossi, D. Exercise, redox homeostasis and the epigenetic landscape. Redox Biol. 2020, 35, 101477. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, Y.; Yang, X.; Xu, X.; Zhao, Y.; Zhu, Z.; Wang, X.; Deng, H.; Li, C.; Gao, F.; et al. Hypermethylation of the Keap1 gene inactivates its function, promotes Nrf2 nuclear accumulation, and is involved in arsenite-induced human keratinocyte transformation. Free Radic. Biol. Med. 2015, 89, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; An, J.; Ji, F.; Jiao, H.; Sun, H.; Zhou, D. Hypermethylation of the Keap1 gene in human lung cancer cell lines and lung cancer tissues. Biochem. Biophys. Res. Commun. 2008, 373, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, X.; Wei, A.; Chen, F.; Gao, Q.; Lu, K.; Jiang, Q.; Cao, W. Nrf2 epigenetic derepression induced by running exercise protects against osteoporosis. Bone Res. 2021, 9, 15. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Kim, K.C.; Kang, H.K.; Chang, W.Y.; Park, I.C.; Keum, Y.S.; Surh, Y.J.; Hyun, J.W. Epigenetic modification of Nrf2 in 5-fluorouracil-resistant colon cancer cells: Involvement of TET-dependent DNA demethylation. Cell Death Dis. 2014, 5, e1183. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Kang, H.K.; Chang, W.Y.; Keum, Y.S.; Hyun, J.W. Interaction of DNA demethylase and histone methyltransferase upregulates Nrf2 in 5-fluorouracil-resistant colon cancer cells. Oncotarget 2016, 7, 40594–40620. [Google Scholar] [CrossRef]
- Garcea, R.L.; Alberts, B.M. Comparative Studies of Histone Acetylation in Nucleosomes, Nuclei, and Intact Cells. Biol. Chem. 1980, 255, 11454–11463. [Google Scholar] [CrossRef]
- Fowler, E.; Farb, R.; El-Saidy, S. Distribution of the core histones H2A H2B, H3 and H4 during cell replication. Nucleic Acids Res. 1982, 10, 735–748. [Google Scholar] [CrossRef][Green Version]
- Yang, X.-J.; Seto, E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef]
- Jiang, Y.; Jakovcevski, M.; Bharadwaj, R.; Connor, C.; Schroeder, F.A.; Lin, C.L.; Straubhaar, J.; Martin, G.; Akbarian, S. Setdb1 histone methyltransferase regulates mood-related behaviors and expression of the NMDA receptor subunit NR2B. J. Neurosci. 2010, 30, 7152–7167. [Google Scholar] [CrossRef]
- Zhong, T.; Ren, F.; Huang, C.S.; Zou, W.Y.; Yang, Y.; Pan, Y.D.; Sun, B.; Wang, E.; Guo, Q.L. Swimming exercise ameliorates neurocognitive impairment induced by neonatal exposure to isoflurane and enhances hippocampal histone acetylation in mice. Neuroscience 2016, 316, 378–388. [Google Scholar] [CrossRef] [PubMed]
- de Meireles, L.C.F.; Bertoldi, K.; Cechinel, L.R.; Schallenberger, B.L.; da Silva, V.K.; Schröder, N.; Siqueira, I.R. Treadmill exercise induces selective changes in hippocampal histone acetylation during the aging process in rats. Neurosci. Lett. 2016, 634, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Huang, J.; Ding, P.; Zang, H.; Kou, Z.; Li, T.; Fan, J.; Peng, Z.; Yan, W. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav. Brain Res. 2016, 309, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Park, S.-H.; Chang, H.-C.; Shapiro, J.S.; Vassilopoulos, A.; Sawicki, K.T.; Chen, C.; Shang, M.; Burridge, P.W.; Epting, C.L.; et al. Sirtuin 2 regulates cellular iron homeostasis via deacetylation of transcription factor NRF2. J. Clin. Invest. 2017, 127, 1505–1516. [Google Scholar] [CrossRef]
- Cao, W.; Hong, Y.; Chen, H.; Wu, F.; Wei, X.; Ying, W. SIRT2 mediates NADH-induced increases in Nrf2, GCL, and glutathione by modulating Akt phosphorylation in PC12 cells. FEBS Lett. 2016, 590, 2241–2255. [Google Scholar] [CrossRef]
- Chen, W.-K.; Tsai, Y.-L.; Shibu, M.A.; Shen, C.-Y.; Chang-Lee, S.N.; Chen, R.-J.; Yao, C.-H.; Ban, B.; Kuo, W.-W.; Huang, C.-Y. Exercise training augments Sirt1-signaling and attenuates cardiac inflammation in D-galactose induced-aging rats. Aging (Albany. NY). 2018, 10, 4166–4174. [Google Scholar] [CrossRef]
- Tunca, U.; Saygin, M.; Ozmen, O.; Aslankoc, R.; Yalcin, A. The impact of moderate-intensity swimming exercise on learning and memory in aged rats: The role of Sirtuin-1. Iran. J. Basic Med. Sci. 2021, 24, 1413–1420. [Google Scholar] [CrossRef]
- Vargas-Ortiz, K.; Pérez-Vázquez, V.; Macías-Cervantes, M.H. Exercise and Sirtuins: A Way to Mitochondrial Health in Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2717. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, L.; Bei, Y.; Wu, X.; Wang, J.; Zhou, Q.; Tao, L.; Das, S.; Li, X.; Xiao, J. Long Noncoding RNA Cardiac Physiological Hypertrophy–Associated Regulator Induces Cardiac Physiological Hypertrophy and Promotes Functional Recovery After Myocardial Ischemia-Reperfusion Injury. Circulation 2021, 144, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhu, Y.; Zheng, C.; Hu, D.; Ma, S.; Chen, L.; Wang, Q.; Chen, Z.; Xie, J.; Yan, Y.; et al. Antihypertrophic Memory After Regression of Exercise-Induced Physiological Myocardial Hypertrophy Is Mediated by the Long Noncoding RNA Mhrt779. Circulation 2021, 143, 2277–2292. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhu, G.; Zeng, C.; Yuan, S.; Qian, Y.; Ye, Z.; Zhao, S.; Li, R. Next-generation sequencing of miRNAs and lncRNAs from rat femur and tibia under mechanical stress. Mol. Med. Rep. 2021, 24, 561. [Google Scholar] [CrossRef] [PubMed]
- Wohlwend, M.; Laurila, P.-P.; Williams, K.; Romani, M.; Lima, T.; Pattawaran, P.; Benegiamo, G.; Salonen, M.; Schneider, B.L.; Lahti, J.; et al. The exercise-induced long noncoding RNA CYTOR promotes fast-twitch myogenesis in aging. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Zhao, W.; Yin, Y.; Cao, H.; Wang, Y. Exercise Improves Endothelial Function via the lncRNA MALAT1/miR-320a Axis in Obese Children and Adolescents. Cardiol. Res. Pract. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhao, Z.; Chen, X.; Li, W.; Li, X. Transcriptome sequencing reveals aerobic exercise training-associated lncRNAs for improving Parkinson’s disease. 3 Biotech 2020, 10, 498. [Google Scholar] [CrossRef]
- Wang, J.; Niu, Y.; Tao, H.; Xue, M.; Wan, C. Knockdown of lncRNA TUG1 inhibits hippocampal neuronal apoptosis and participates in aerobic exercise-alleviated vascular cognitive impairment. Biol. Res. 2020, 53, 53. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Marandi, S.M.; Baharlooie, M.; Safaeinejad, Z.; Nasr-Esfahani, M.H.; Ghaedi, K. Aerobic exercise modulates noncoding RNA network upstream of FNDC5 in the Gastrocnemius muscle of high-fat-diet-induced obese mice. J. Physiol. Biochem. 2021, 77, 589–600. [Google Scholar] [CrossRef]
- He, Y.; Qiang, Y. Mechanism of Autonomic Exercise Improving Cognitive Function of Alzheimer’s Disease by Regulating lncRNA SNHG14. Am. J. Alzheimer’s Dis. Other Dement. 2021, 36, 36. [Google Scholar] [CrossRef]
- Mei, T.; Liu, Y.; Wang, J.; Zhang, Y. miR-340-5p: A potential direct regulator of Nrf2 expression in the post-exercise skeletal muscle of mice. Mol. Med. Rep. 2018, 19, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, A.S.; Appling, D.R. Compartmentalization of Mammalian Folate-Mediated One-Carbon Metabolism. Annu. Rev. Nutr. 2010, 30, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Chuang, S.-C.; Vaissière, T.; Cuenin, C.; Ricceri, F.; Collaborators, G.; Johansson, M.; Ueland, P.; Brennan, P.; Herceg, Z. DNA methylation changes associated with cancer risk factors and blood levels of vitamin metabolites in a prospective study. Epigenetics 2011, 6, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, L.; Boukouvala, S.; Sim, E. Characterisation of CpG methylation in the upstream control region of mouse Nat2: Evidence for a gene–environment interaction in a polymorphic gene implicated in folate metabolism. Gene 2010, 452, 16–21. [Google Scholar] [CrossRef]
- McKay, J.A.; Xie, L.; Harris, S.; Wong, Y.K.; Ford, D.; Mathers, J.C. Blood as a surrogate marker for tissue-specific DNA methylation and changes due to folate depletion in post-partum female mice. Mol. Nutr. Food Res. 2011, 55, 1026–1035. [Google Scholar] [CrossRef]
- McKay, J.A.; Wong, Y.K.; Relton, C.L.; Ford, D.; Mathers, J.C. Maternal folate supply and sex influence gene-specific DNA methylation in the fetal gut. Mol. Nutr. Food Res. 2011, 55, 1717–1723. [Google Scholar] [CrossRef]
- Hirsch, S.; Ronco, A.M.; Guerrero-Bosagna, C.; de la Maza, M.P.; Leiva, L.; Barrera, G.; Llanos, M.; Alliende, M.A.; Silva, F.; Bunout, D. Methylation status in healthy subjects with normal and high serum folate concentration. Nutrition 2008, 24, 1103–1109. [Google Scholar] [CrossRef]
- Chen, M.J.; Shimada, T.; Moulton, A.D.; Cline, A.; Humphries, R.K.; Maizel, J.; Nienhuis, A.W. The functional human dihydrofolate reductase gene. J. Biol. Chem. 1984, 259, 3933–3943. [Google Scholar] [CrossRef]
- Holmquist, C.; Larsson, S.; Wolk, A.; de Faire, U. Multivitamin Supplements Are Inversely Associated with Risk of Myocardial Infarction in Men and Women—Stockholm Heart Epidemiology Program (SHEEP). J. Nutr. 2003, 133, 2650–2654. [Google Scholar] [CrossRef]
- Lamprecht, S.A.; Lipkin, M. Chemoprevention of colon cancer by calcium, vitamin D and folate: Molecular mechanisms. Nat. Rev. Cancer 2003, 3, 601–614. [Google Scholar] [CrossRef]
- Smithells, R.W.; Sheppard, S.; Schorah, C.J. Vitamin dificiencies and neural tube defects. Arch. Dis. Child. 1976, 51, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Laurence, K.M.; James, N.; Miller, M.H.; Tennant, G.B.; Campbell, H. Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. BMJ 1981, 282, 1509–1511. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.-I.; Eda, S.; Masada, M. Alterations of tetrahydrobiopterin biosynthesis and pteridine levels in mouse tissues during growth and aging. Brain Dev. 2000, 22, 45–49. [Google Scholar] [CrossRef]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in Cardiovascular Health and Disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef]
- Crabtree, M.J.; Tatham, A.L.; Hale, A.B.; Alp, N.J.; Channon, K.M. Critical Role for Tetrahydrobiopterin Recycling by Dihydrofolate Reductase in Regulation of Endothelial Nitric-oxide Synthase Coupling. J. Biol. Chem. 2009, 284, 28128–28136. [Google Scholar] [CrossRef]
- Bagley, J.R.; Burghardt, K.J.; McManus, R.; Howlett, B.; Costa, P.B.; Coburn, J.W.; Arevalo, J.A.; Malek, M.H.; Galpin, A.J. Epigenetic Responses to Acute Resistance Exercise in Trained vs. Sedentary Men. J. Strength Cond. Res. 2020, 34, 1574–1580. [Google Scholar] [CrossRef]
- Thöny, B. Tetrahydrobiopterin and its functions. In PKU and BH4: Advances in Phenylketonuria and Tetrahydrobiopterin Research; SPS Publications: Heilbronn, Germany, 2006; pp. 503–504. [Google Scholar]
- Nishida, Y.; Hara, M.; Higaki, Y.; Taguchi, N.; Nakamura, K.; Nanri, H.; Horita, M.; Shimanoe, C.; Yasukata, J.; Miyoshi, N.; et al. Habitual Light-intensity Physical Activity and ASC Methylation in a Middle-aged Population. Int. J. Sports Med. 2019, 40, 670–677. [Google Scholar] [CrossRef]
- Mariathasan, S.; Newton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Hashimoto, S.; Fujii, C.; Hida, S.; Ito, K.; Matsumura, T.; Sakaizawa, T.; Morikawa, M.; Masuki, S.; Nose, H.; et al. NFκB2 Gene as a Novel Candidate that Epigenetically Responds to Interval Walking Training. Int. J. Sports Med. 2015, 36, 769–775. [Google Scholar] [CrossRef]
| Antioxidant/Detoxifying Enzyme | Reported Effect | |
|---|---|---|
| Brain | Skeletal Muscle | |
| Superoxide dismutase | ↑ Resistance to neurotoxicity [19] ↓ Level of ischemic damage [20] ↓ Motoneuron degeneration [21] | ↑ Protection against multiple organ dysfunction [22] ↑ Protection against diabetic cardiomyopathy [23] |
| Glutathione peroxidase | ↑ Protection against stroke damage [24] | ↑ Muscle damage recovery |
| Glutathione reductase | ↓ Anxiety-like behavior [25] | ↑ Lean mass and muscle strength [26] |
| Hemeoxygenase-1 | ↑ Protection against heat-induced brain damage [27] ↑ Improvement of ischemic injury during acute stroke [28] | ↓ Sepsis-induced skeletal muscle atrophy [29] ↓ Muscle damage in Duchenne muscular dystrophy [30] |
| Peroxiredoxin | ↑ Memory performance [31] | ↑ Eccentric contraction-induced force [32] |
| Thioredoxin | ↑ Ameliorate ischemic brain damage [33] | ↑ Preservation of mitochondrial redox status [34] ↓ Muscle atrophy [35] |
| Metallothionein | ↑ Brain aging [36] ↑ Neuroprotection after stroke [37] | ↑ Regeneration in conditions of muscle wasting [38] |
| NAD(P)H: quinone oxidoreductase | ↓ ROS and ↑cell proliferation of glioblastoma multiforme in vitro [39] | ↑ Muscle degradation upon aging [40] |
| Glutamate cysteine ligase | ↑ Learning performance [41] | ↓ Susceptibility to oxidative damage in muscle aging [42] |
| Physical Exercise | Population and Duration of Exercise | Sample | Neopterin and BH4 Synthesis | References |
|---|---|---|---|---|
| Ergometer | Normal volunteers consist of young subjects (15 to 29 y) and middle-aged subjects (40 to 59 y) undergoing strong exercise (80% VO2max) for 10 min | Plasma | BH4 increased by up to 150% after exercise when compared to pre-training, then rapidly returned to basal levels after 30 min | [85] |
| Ergometer | Normal volunteers undergoing strong exercise (80% VO2max) for 10 min | Plasma | BH4 increased after strong exercise and decreased after 2 h | [86] |
| Running | Well-trained runners covering a distance of 20 km within 2 h | Plasma | Neopterin increased 1 h after exercise for 24 h | [87] |
| Cycle ergometer | Healthy adults—continuous progression protocol | Plasma | Neopterin increased post-exercise and returned to basal values after 60 min | [88] |
| Ergometer | Healthy and trained athletes performed a 20 min maximal pedaling | Plasma | Neopterin increased post-exercise | [89] |
| Ultra-endurance Multi-Sport Brazil race | Well-trained male athletes undergoing 90 km alternating exercise of off-road running, mountain biking, and canoeing | Plasma | Neopterin increased post-exercise | [90] |
| Running | An athlete competing in the Race Across America | Urine | Neopterin increased right after the race started until day four | [91] |
| Rugby | Rugby match | Urine | Neopterin increased post-match and 17 h later returned to basal levels | [92] |
| Bodybuilding | Competitive bodybuilders who trained for 5 d in a row and 2 d off and healthy controls | Urine | Neopterin was elevated over 1 week | [93] |
| Triathlon | Athletes during competition | Urine | Neopterin increased post-competition | [94] |
| Extreme mountain ultra-marathon | Ultra-marathon runners | Urine | Neopterin increased post-race | [95] |
| Physical Exercise | LncRNA | Reported Effect | References |
|---|---|---|---|
| Swimming | CPhar | Prevention of myocardial ischemia-reperfusion injury and cardiac dysfunction | [132] |
| Swimming | Mhrt779 | Heart antihypertrophic effect | [133] |
| Treadmill | MSTRG.2625 MSTRG.1557 MSTRG.691 MSTRG.7497 | Promotion of osteogenic differentiation | [134] |
| Treadmill | CYTOR | Regulation of fast-twitch myogenesis in aging | [135] |
| Aerobic exercise (single jump rope, double jump rope, round-trip running, and gymnastics) | MALAT1 | Improvement of endothelial dysfunction | [136] |
| Swimming | LOC102633466 LOC102637865 LOC102638670 | Improved motor performance | [137] |
| Treadmill | TUG1 | Reduction of hippocampal neuronal apoptosis | [138] |
| Treadmill | Neat1 Meg3 Malat1 Kcnq1ot1 | Possible involvement in insulin resistance and glucose homeostasis pathways | [139] |
| Running wheels | SNHG14 | Improvement of cognitive disorder and inflammation | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, J.; da Silva, R.A.; da Luz Scheffer, D.; Penteado, R.; Solano, A.; Barros, L.; Budde, H.; Trostchansky, A.; Latini, A. Physical-Exercise-Induced Antioxidant Effects on the Brain and Skeletal Muscle. Antioxidants 2022, 11, 826. https://doi.org/10.3390/antiox11050826
Souza J, da Silva RA, da Luz Scheffer D, Penteado R, Solano A, Barros L, Budde H, Trostchansky A, Latini A. Physical-Exercise-Induced Antioxidant Effects on the Brain and Skeletal Muscle. Antioxidants. 2022; 11(5):826. https://doi.org/10.3390/antiox11050826
Chicago/Turabian StyleSouza, Jennyffer, Rodrigo Augusto da Silva, Débora da Luz Scheffer, Rafael Penteado, Alexandre Solano, Leonardo Barros, Henning Budde, Andrés Trostchansky, and Alexandra Latini. 2022. "Physical-Exercise-Induced Antioxidant Effects on the Brain and Skeletal Muscle" Antioxidants 11, no. 5: 826. https://doi.org/10.3390/antiox11050826
APA StyleSouza, J., da Silva, R. A., da Luz Scheffer, D., Penteado, R., Solano, A., Barros, L., Budde, H., Trostchansky, A., & Latini, A. (2022). Physical-Exercise-Induced Antioxidant Effects on the Brain and Skeletal Muscle. Antioxidants, 11(5), 826. https://doi.org/10.3390/antiox11050826








