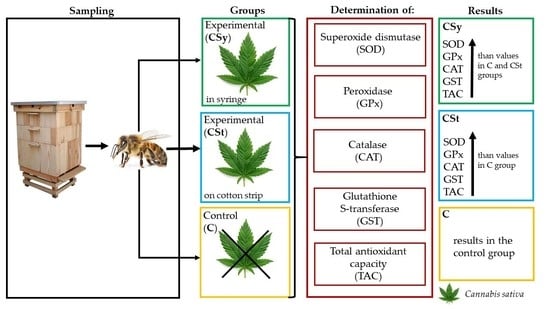
Impressive Impact of Hemp Extract on Antioxidant System in Honey Bee (Apis mellifera) Organism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Hemolymph Extraction
2.3. Determination of Antioxiant Activities
- −
- SOD according to Podczasy and Wei (1988)
- −
- GPx according to the methods described by Chance and Maehly (1955)
- −
- CAT according to Aebi (1983)
- −
- GST according to Warholm et al. (1985)
- −
- TAC according to the protocol included in the Assay Kit produced by Sigma Aldrich. The reaction was carried out at a temperature of 30 °C. Absorbances were measured at 570 nm.
2.4. Statistical Analysis
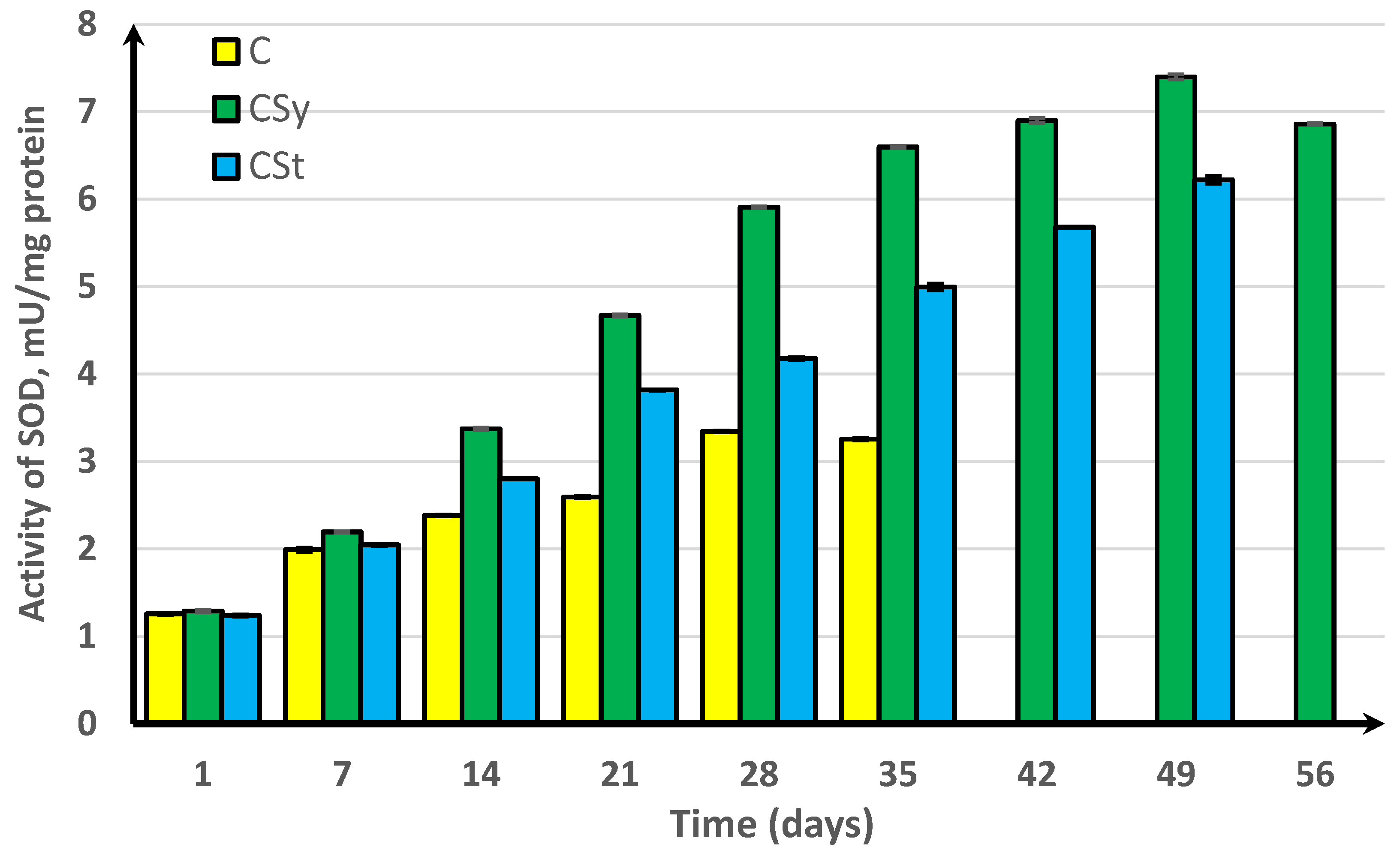
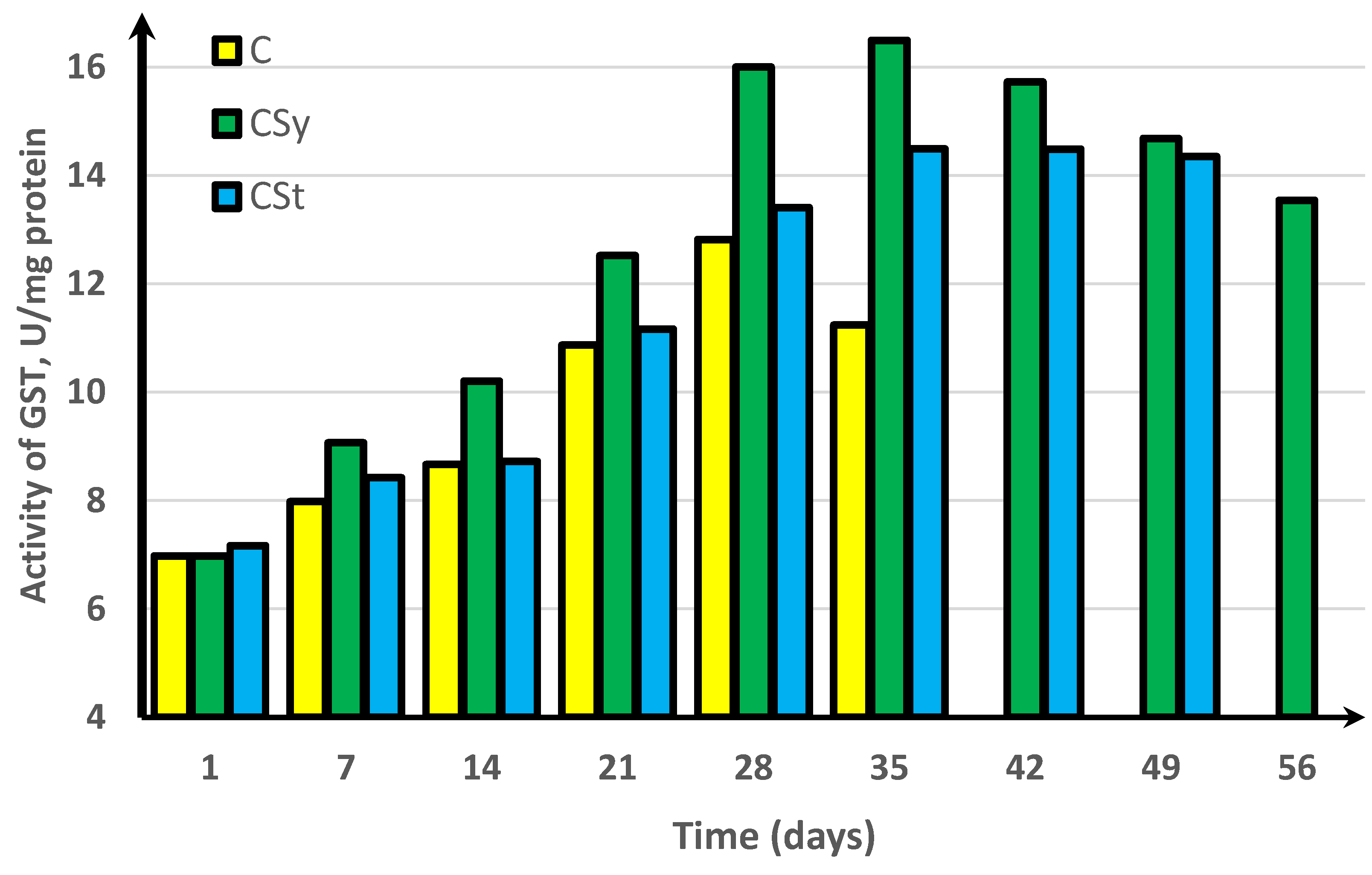
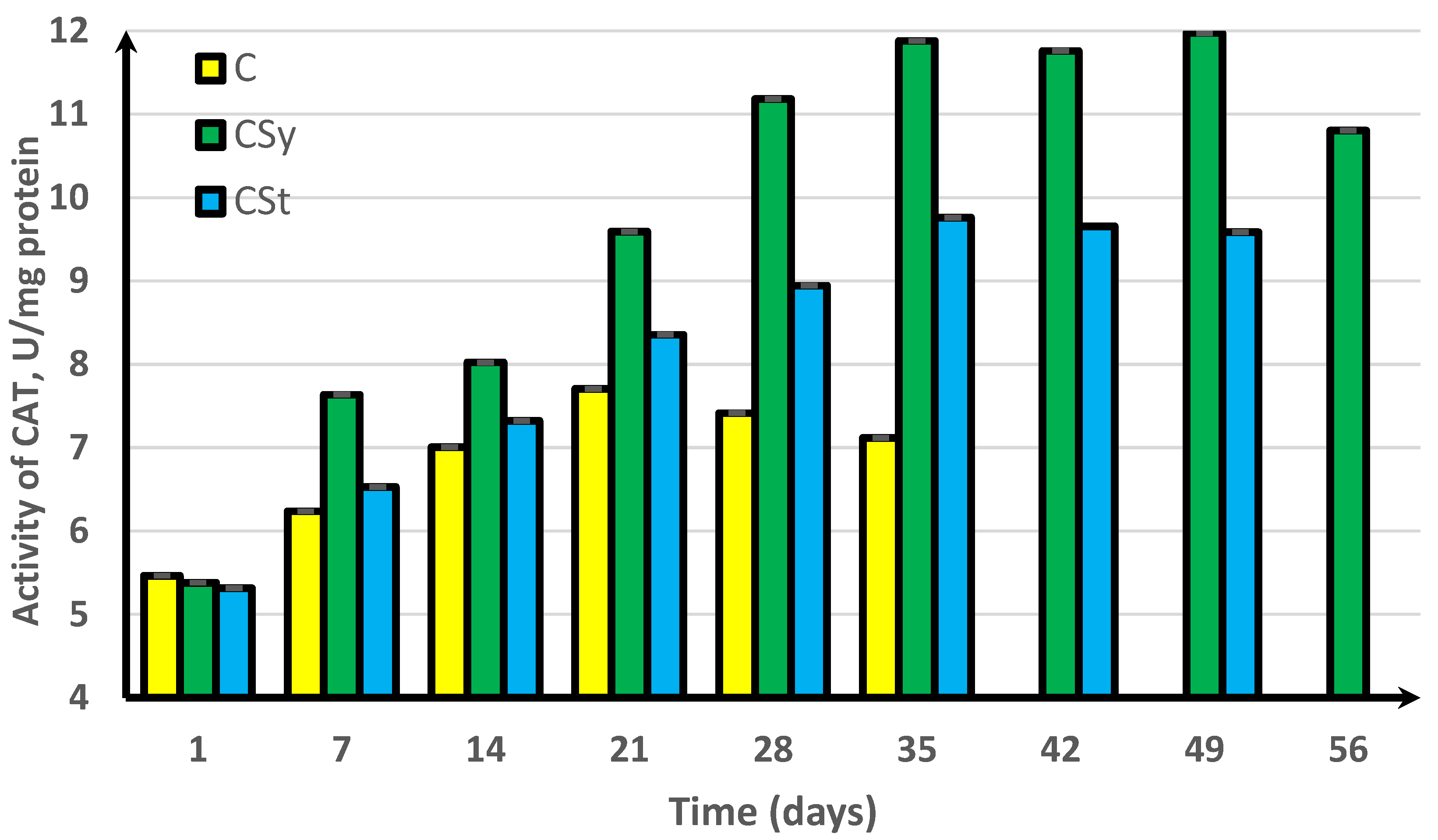
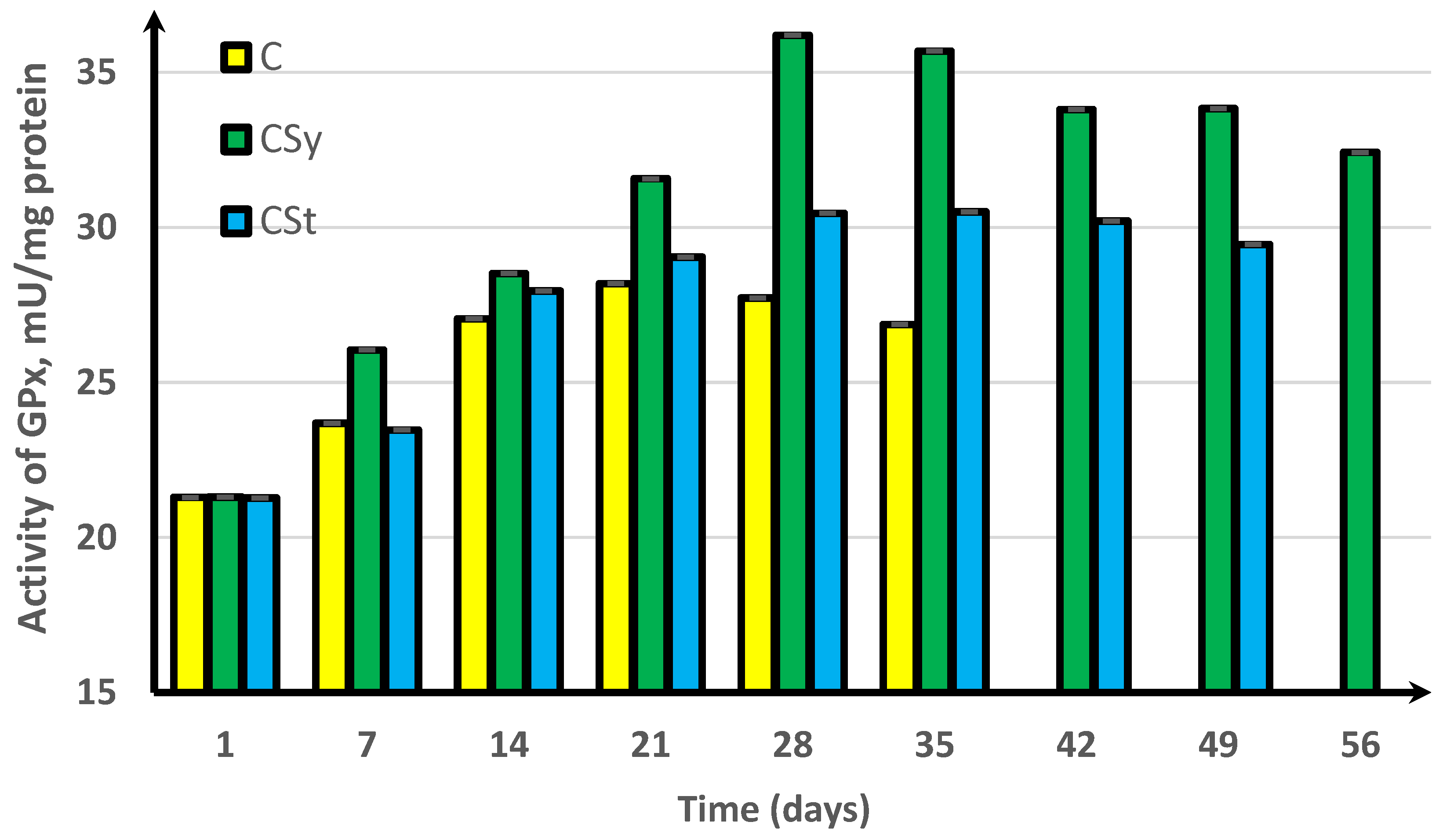
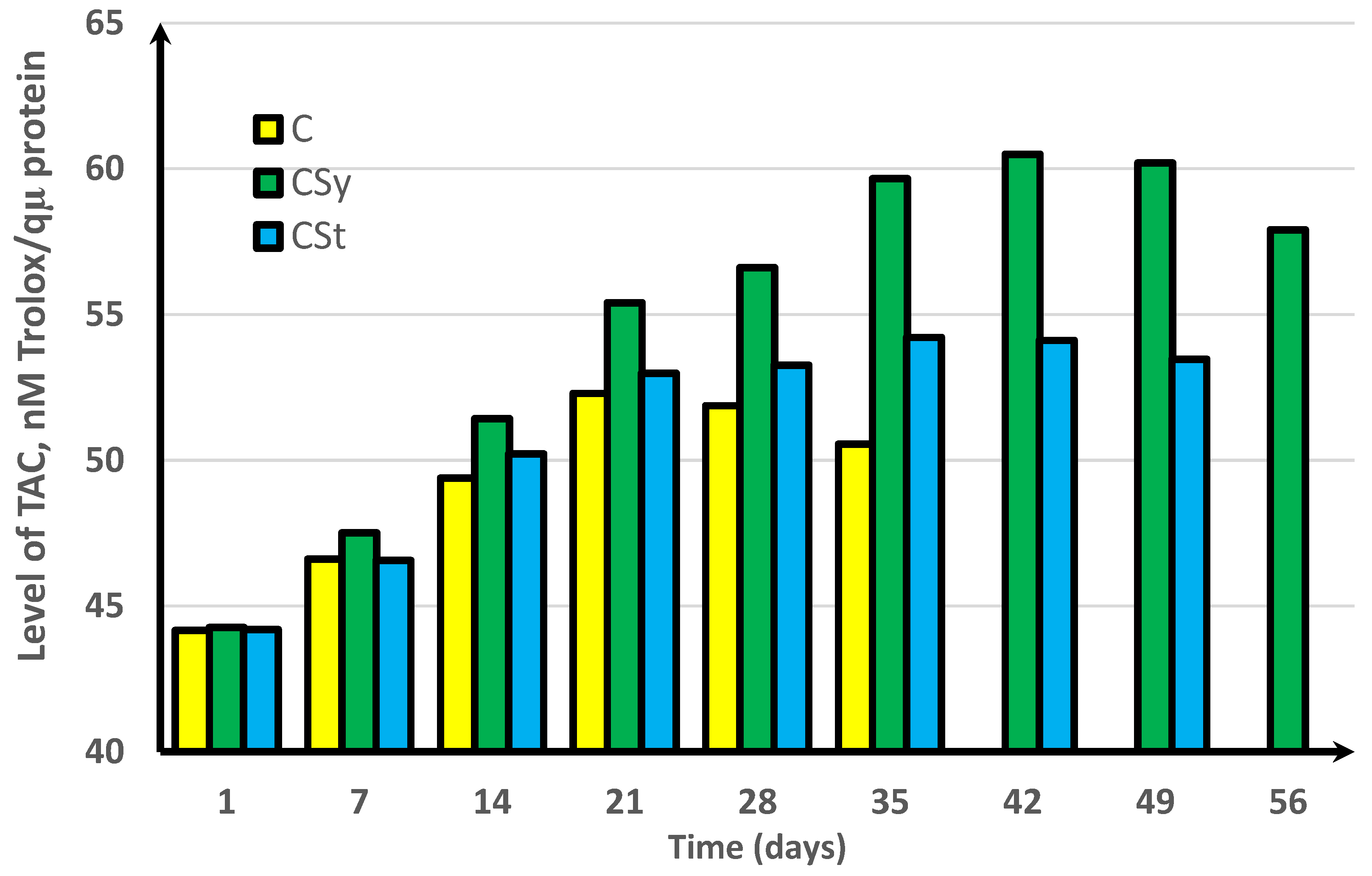
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Conte, Y.; Navajas, M. Climate change: Impact on honey bee populations and diseases. OIE Rev. Sci. Tech. 2008, 27, 485–510. [Google Scholar] [CrossRef]
- Lacetera, N. Impact of climate change on animal health and welfare. Anim. Front. 2019, 9, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Varroa treatment with bromfenvinphos markedly suppresses honeybee biochemical defence levels. Entomol. Exp. Appl. 2016, 160, 57–71. [Google Scholar] [CrossRef]
- Higes, M.; Martín-Hernández, R.; Garrido-Bailón, E.; González-Porto, A.V.; García-Palencia, P.; Meana, A.; Del Nozal, M.J.; Mayo, R.; Bernal, J.L. Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ. Microbiol. Rep. 2009, 1, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Harder, L.D. The Global Stock of Domesticated Honey Bees Is Growing Slower than Agricultural Demand for Pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, J.A. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual. Atmos. Health 2011, 4, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birch-Machin, M.A.; Bowman, A. Oxidative stress and ageing. Br. J. Dermatol. 2016, 175, 26–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Łoś, A.; Filipczuk, J.; Schulz, M. Individual and social immune mechanisms of the honey bee. Med. Weter. 2018, 74, 426–433. [Google Scholar]
- Strachecka, A.; Olszewski, K.; Paleolog, J.; Borsuk, G.; Bajda, M.; Krauze, M.; Merska, M.; Chobotow, J. Coenzyme Q10 treatments influence the lifespan and key biochemical resistance systems in the honeybee, Apis mellifera. Arch. Insect Biochem. Physiol. 2014, 86, 165–179. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Simone-Finstrom, M. Nutritional and prebiotic efficacy of the microalga Arthrospira platensis (spirulina) in honey bees. Apidologie 2020, 51, 898–910. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Curcumin stimulates biochemical mechanisms of Apis mellifera resistance and extends the apian life-span. J. Apic. Sci. 2015, 59, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Rascón, B.; Hubbard, B.P.; Sinclair, D.A.; Amdam, G.V. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging 2012, 4, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Farjan, M.; Dmitryjuk, M.; LipiŃski, Z.; Biernat-łOpieŃska, E.; Zółtowska, K. Supplementation of the honey bee diet with vitamin C: The effect on the antioxidative system of Apis mellifera carnica brood at different stages. J. Apic. Res. 2012, 51, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Hacke, A.C.M.; Lima, D.; de Costa, F.; Deshmukh, K.; Li, N.; Chow, A.M.; Marques, J.A.; Pereira, R.P.; Kerman, K. Probing the antioxidant activity of Δ9-tetrahydrocannabinol and cannabidiol in Cannabis sativa extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef]
- Yadav, M.; Jain, S.; Tomar, R.; Prasad, G.B.K.S.; Yadav, H. Medicinal and biological potential of pumpkin: An updated review. Nutr. Res. Rev. 2010, 23, 184–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagne, A.M.; Fotio, Y.; Lin, L.; Squire, E.; Ahmed, F.; Rashid, T.I.; Azari, E.K.; Piomelli, D. Palmitoylethanolamide and hemp oil extract exert synergistic anti-nociceptive effects in mouse models of acute and chronic pain. Pharmacol. Res. 2021, 167, 105545. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.J.; Abelev, S.V.; Connor, S.J.; Corte, C.J.; Martin, L.J.; Gold, L.K.; Suraev, A.S.; McGregor, I.S. Medicinal Cannabis for inflammatory bowel disease: A survey of perspectives, experiences, and current use in australian patients. Crohn’s Colitis 360 2020, 2, otaa015. [Google Scholar] [CrossRef] [Green Version]
- Reithmeier, D.; Tang-Wai, R.; Seifert, B.; Lyon, A.W.; Alcorn, J.; Acton, B.; Corley, S.; Prosser-Loose, E.; Mousseau, D.D.; Lim, H.J.; et al. The protocol for the Cannabidiol in children with refractory epileptic encephalopathy (CARE-E) study: A phase 1 dosage escalation study. BMC Pediatr. 2018, 18, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.R.; Ali, D.W. Pharmacology of Medical Cannabis. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1162, pp. 151–165. [Google Scholar]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Ashton, C.H. Pharmacology and effects of cannabis: A brief review. Br. J. Psychiatry 2001, 178, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A.; et al. Metabolomic profile and antioxidant/anti-inflammatory effects of industrial hemp water extract in fibroblasts, keratinocytes and isolated mouse skin specimens. Antioxidants 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Magi, G.; Ferretti, G.; Bacchetti, T.; Giuliani, A.; Pugnaloni, A.; Rippo, M.R.; Facinelli, B. Attenuation of Listeria monocytogenes virulence by Cannabis sativa L. Essential oil. Front. Cell. Infect. Microbiol. 2018, 8, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.L. Biochemistry; Third edition (Stryer, Lubert). J. Chem. Educ. 1988, 65, A337. [Google Scholar] [CrossRef] [Green Version]
- Karihtala, P.; Soini, Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS 2007, 115, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Simões, M.I.; Navarro, J.C.; Castro, B.B. First ecotoxicological characterization of paraffin microparticles: A biomarker approach in a marine suspension-feeder, Mytilus sp. Environ. Sci. Pollut. Res. 2020, 27, 41946–41960. [Google Scholar] [CrossRef]
- Nakagawa, Y. Role of Mitochondrial Phospholipid Hydroperoxide Glutathione Peroxidase (PHGPx) as a Antiapoptotic Factor. Biol. Pharm. Bull. 2004, 27, 956–960. [Google Scholar] [CrossRef] [Green Version]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Cannabis Extract Has a Positive–Immunostimulating Effect through Proteolytic System and Metabolic Compounds of Honey Bee (Apis mellifera) Workers. Animals 2021, 11, 2190. [Google Scholar] [CrossRef]
- Łoś, A.; Strachecka, A. Fast and cost-effective biochemical spectrophotometric analysis of solution of insect “blood” and body surface elution. Sensors 2018, 18, 1494. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Poovaiah, B.W.; Ryan, C.A. Hydrogen peroxide homeostasis: Activation of plant catalase by calciumcalmodulin. Proc. Natl. Acad. Sci. USA 2002, 99, 4097–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, A.R. Cannabinoid interactions with ion channels and receptors. Channels 2019, 13, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corona, M.; Hughes, K.A.; Weaver, D.B.; Robinson, G.E. Gene expression patterns associated with queen honey bee longevity. Mech. Ageing Dev. 2005, 126, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, O.; Neal, J.J.; Shukle, R.H. Antioxidant defense response in a galling insect. Proc. Natl. Acad. Sci. USA 2007, 104, 1889–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Girgih, A.T.; Udenigwe, C.C.; Aluko, R.E. In Vitro Antioxidant Properties of Hemp Seed (Cannabis sativa L.) Protein Hydrolysate Fractions. J. Am. Oil Chem. Soc. 2011, 88, 381–389. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Gruschow, A. A Comparison of Antioxidant Potential, Total Phenolic Content, and Cannabidiol (CBD) Content of Cannabis Infused Hemp, MCT, and Olive Oils. Master’s Thesis, Montclair State University, Montclair, NJ, USA, 2020. [Google Scholar]
- Ziaja, P. Aktywność Antyoksydacyjna Kaliks[4]rezorcynarenów i Kaliks[4]pirogallolarenów. UW Repository. Available online: http://www.depotuw.ceon.pl/handle/item/419 (accessed on 3 February 2022).
- Strachecka, A.; Krauze, M.; Olszewski, K.; Borsuk, G.; Paleolog, J.; Merska, M.; Chobotow, J.; Bajda, M.; Grzywnowicz, K. Unexpectedly strong effect of caffeine on the vitality of western honeybees (Apis mellifera). Biochemistry 2014, 79, 1192–1201. [Google Scholar] [CrossRef]
- Schulz, M.; Łoś, A.; Grzybek, M.; Ścibior, R.; Strachecka, A. Piperine as a new natural supplement with beneficial effects on the life-span and defence system of honeybees. J. Agric. Sci. 2019, 157, 140–149. [Google Scholar] [CrossRef]
| Type of Group | Feeding | Method of Supplementation |
|---|---|---|
| (1) CSt | sugar syrup (v:v, 1:1) ad libitum and inside with cotton strips soaked with 3 mL hemp extract (0.25 g hemp paste extract + 3 mL water–glycerine solution) | extract on a cotton strip |
| (2) CSy | a mixture of sugar syrup (v:v, 1:1) with hemp extract ad libitum (500 mL water–glycerine solution with 4.38 g hemp paste extract) | extract in a syringe |
| (3) C | mixture of sugar and a water–glycerine solution (v:v, 1:1) | syrup in a syringe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowronek, P.; Wójcik, Ł.; Strachecka, A. Impressive Impact of Hemp Extract on Antioxidant System in Honey Bee (Apis mellifera) Organism. Antioxidants 2022, 11, 707. https://doi.org/10.3390/antiox11040707
Skowronek P, Wójcik Ł, Strachecka A. Impressive Impact of Hemp Extract on Antioxidant System in Honey Bee (Apis mellifera) Organism. Antioxidants. 2022; 11(4):707. https://doi.org/10.3390/antiox11040707
Chicago/Turabian StyleSkowronek, Patrycja, Łukasz Wójcik, and Aneta Strachecka. 2022. "Impressive Impact of Hemp Extract on Antioxidant System in Honey Bee (Apis mellifera) Organism" Antioxidants 11, no. 4: 707. https://doi.org/10.3390/antiox11040707
APA StyleSkowronek, P., Wójcik, Ł., & Strachecka, A. (2022). Impressive Impact of Hemp Extract on Antioxidant System in Honey Bee (Apis mellifera) Organism. Antioxidants, 11(4), 707. https://doi.org/10.3390/antiox11040707