Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells Mitigate Endoplasmic Reticulum Stress, Inflammation, and Lung Injury in Lipopolysaccharide-Treated Obese Mice
Abstract
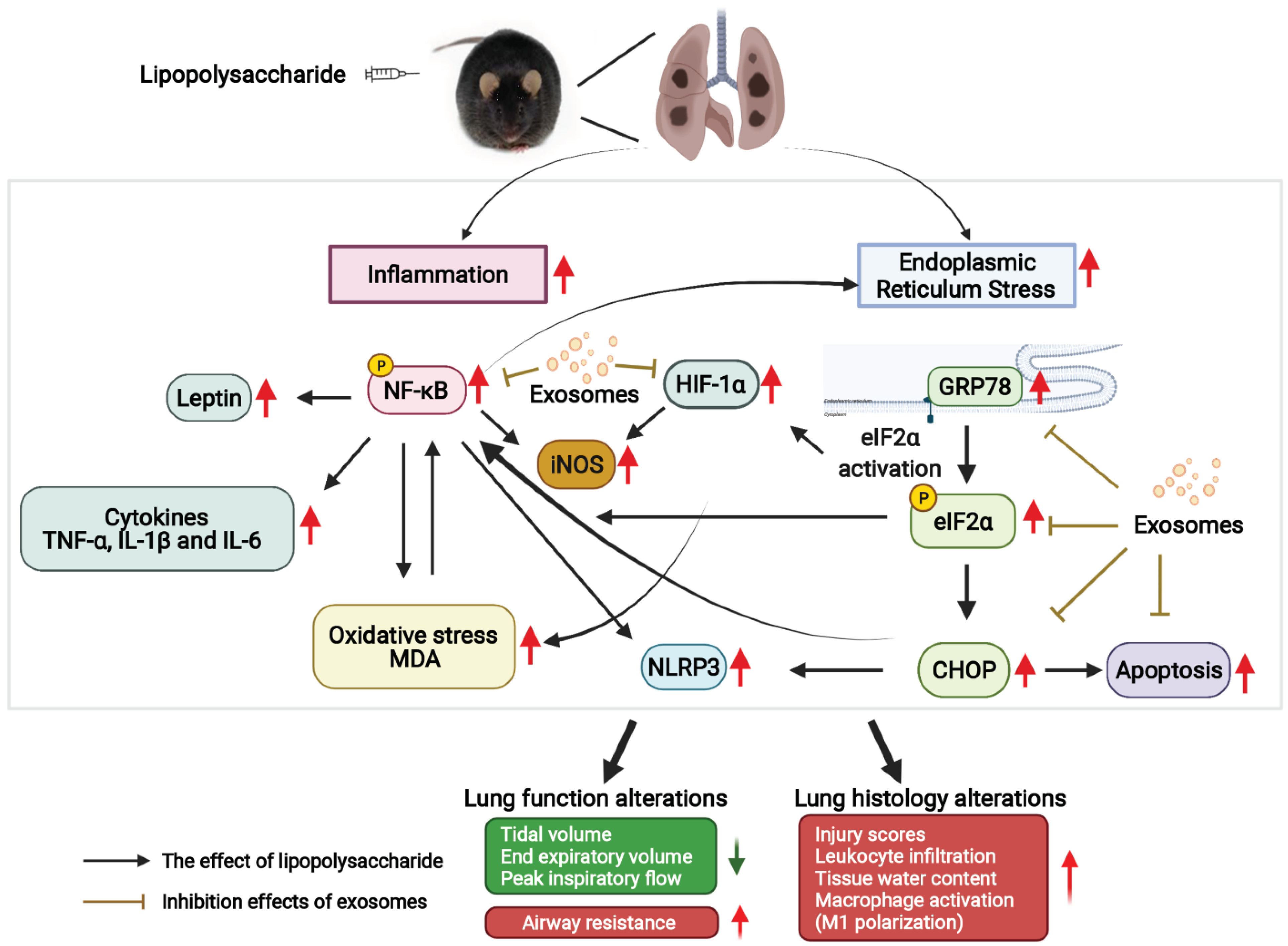
1. Introduction
2. Materials and Methods
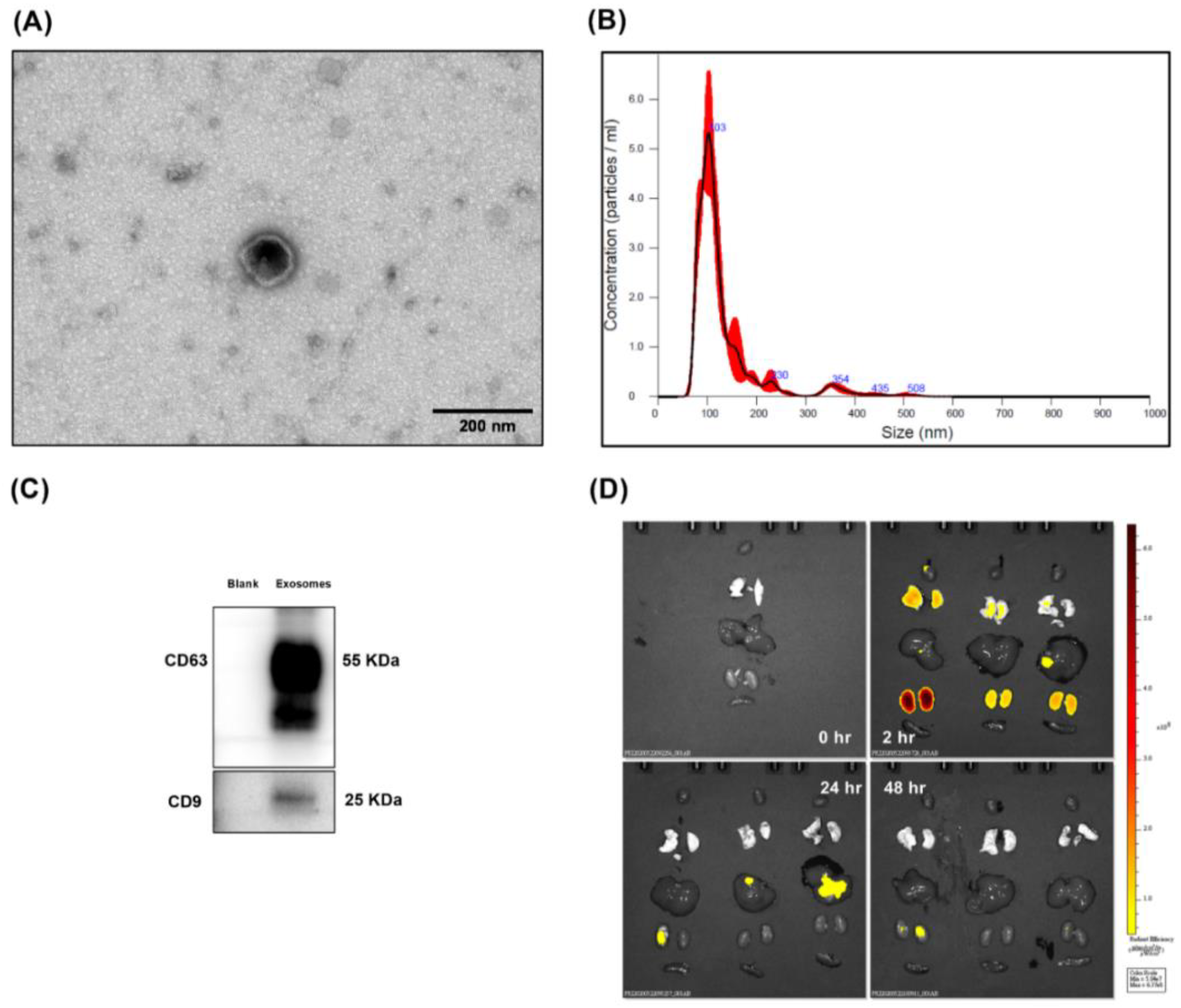
2.1. Characterization of Exosomes
2.2. TEM Analysis
2.3. Animals, Study Approval, and Diet-Induced Obesity Murine Model
2.4. Biodistribution
2.5. Experimental Design
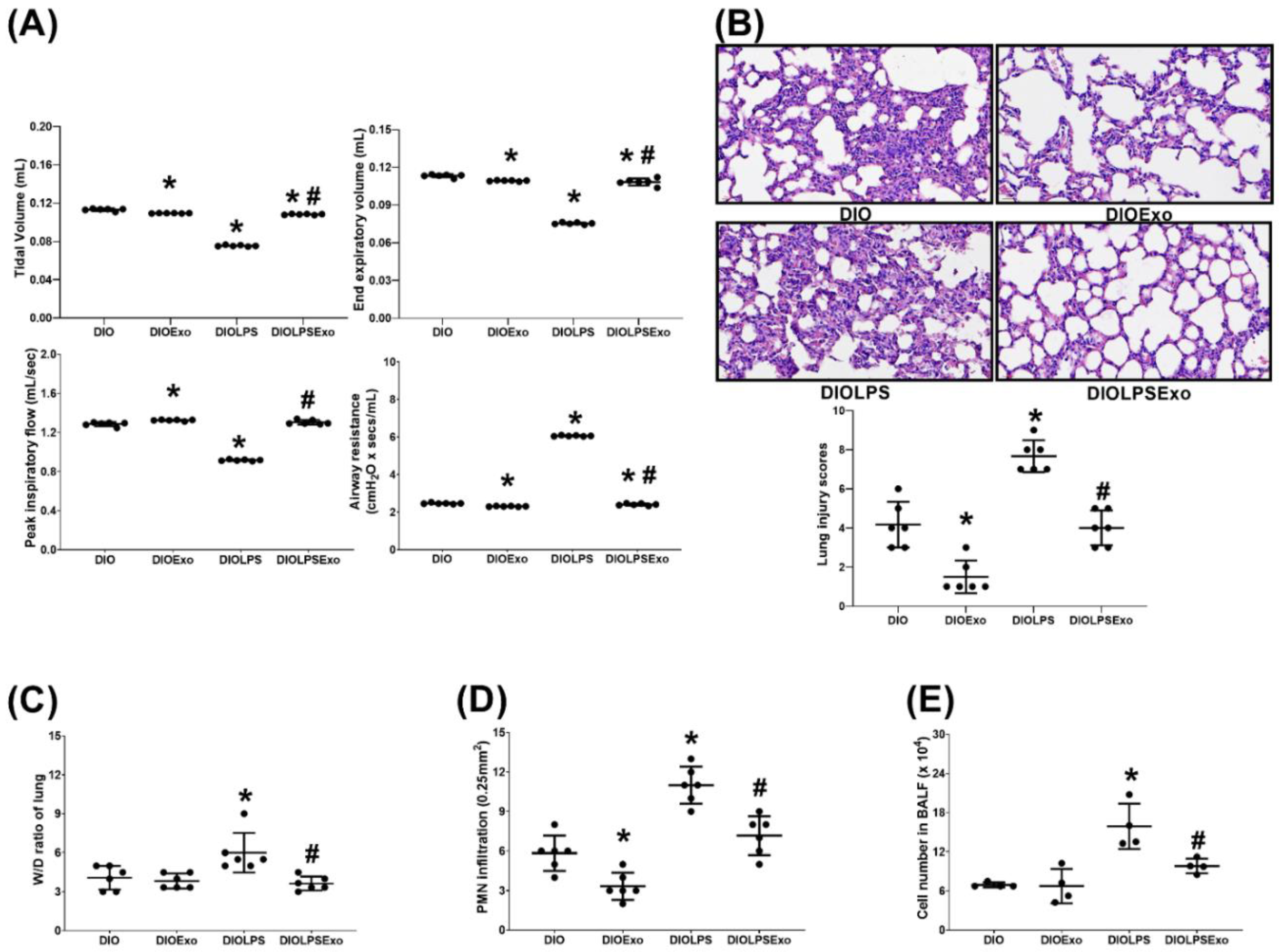
2.6. Lung Function
2.7. Bronchoalveolar Lavage, Lung Harvesting, and Wet/Dry Weight (W/D) Ratio Determination
2.8. Lung Histology
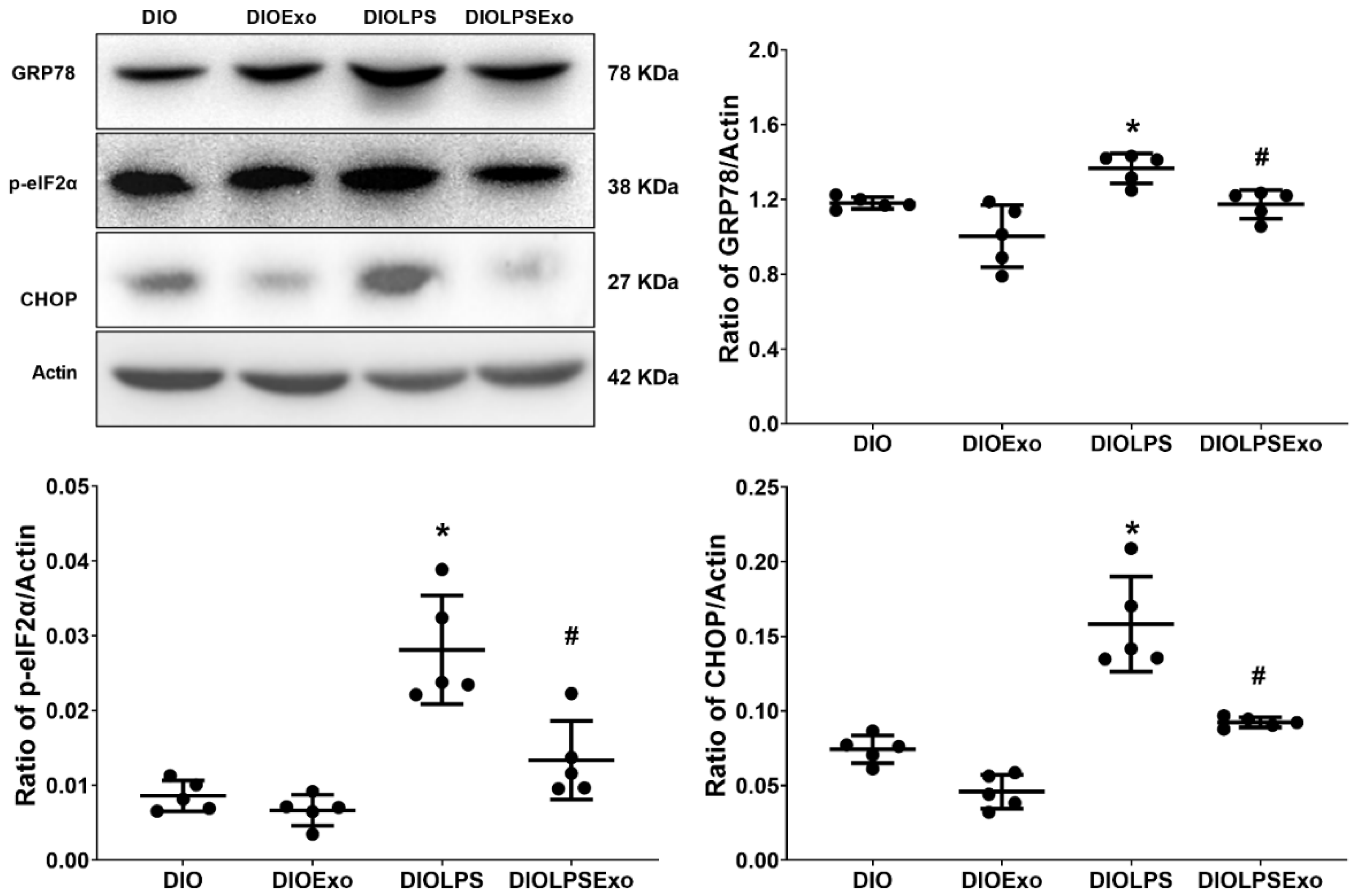
2.9. Immunoblotting Assay
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
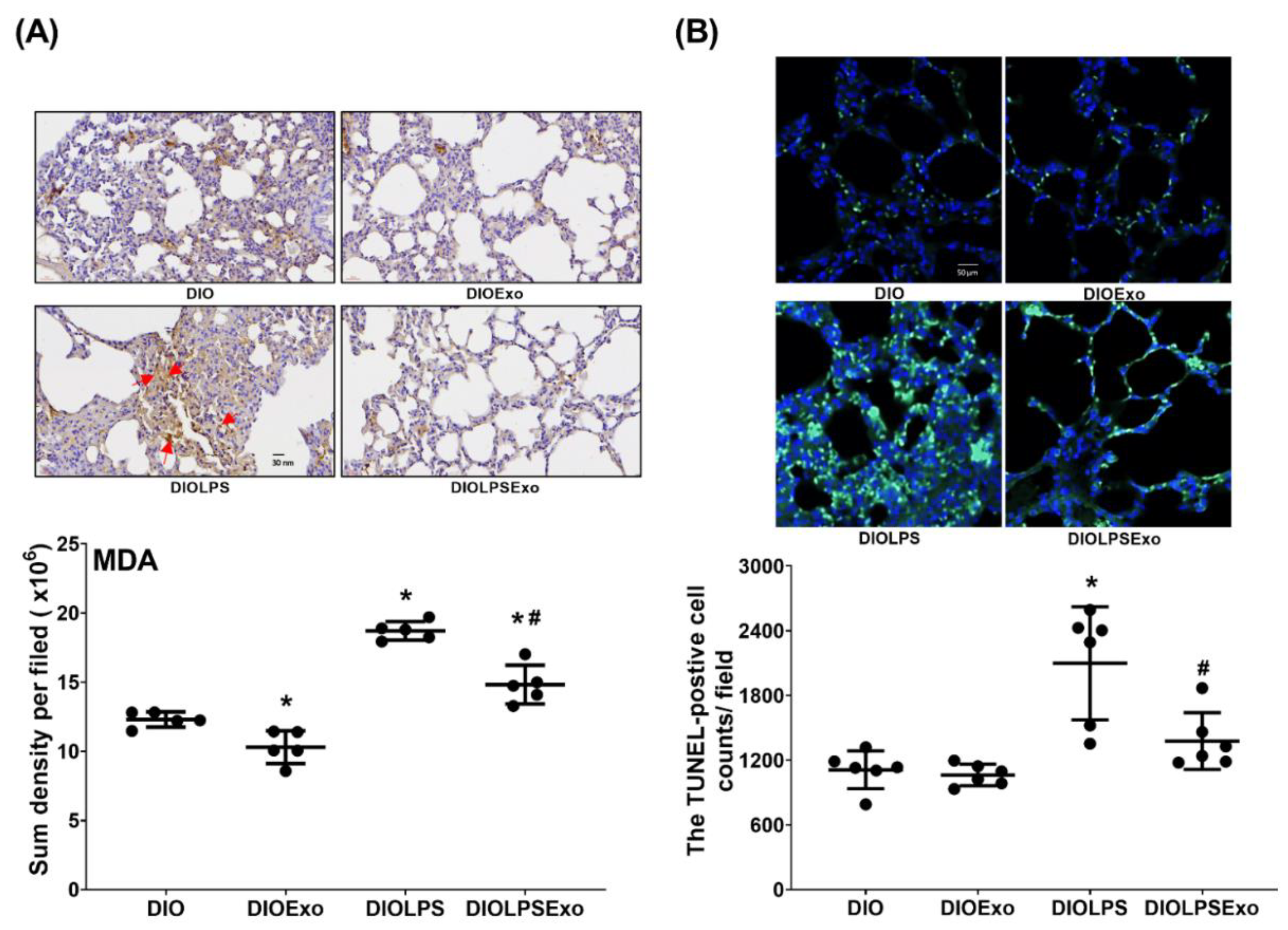
2.11. Immunohistochemistry Staining
2.12. Terminal Deoxynucleotidyl Transferase dUTP Nick End (TUNEL) Assay
2.13. Statistical Analysis
3. Results
3.1. Confirmation and Biodistribution of Exosomes
3.2. Survivorship
3.3. Lung Injury
3.4. ER Stress
3.5. Lung Inflammation
3.6. Lung Oxidation and Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, R.D.; Suratt, B.T. Obesity and nutrition in acute respiratory distress syndrome. Clin. Chest Med. 2014, 35, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zuo, Z.; Chen, K.; Fang, J.; Cui, H.; Geng, Y.; Ouyang, P.; Chen, Z.; Huang, C.; Guo, H.; et al. Diet-Induced Obesity Mice Execute Pulmonary Cell Apoptosis via Death Receptor and ER-Stress Pathways after E. coli Infection. Oxid Med. Cell Longev. 2020, 2020, 6829271. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Luo, J.; Cao, J.; Peng, L.; Ren, L.; Zhang, F. Adiponectin Protects Obese Rats from Aggravated Acute Lung Injury via Suppression of Endoplasmic Reticulum Stress. Diabetes Metab. Syndr. Obes. 2020, 13, 4179–4190. [Google Scholar] [CrossRef]
- Kaplan, J.M.; Nowell, M.; Lahni, P.; O’Connor, M.P.; Hake, P.W.; Zingarelli, B. Short-term high fat feeding increases organ injury and mortality after polymicrobial sepsis. Obesity 2012, 20, 1995–2002. [Google Scholar] [CrossRef]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Wu, J.; Kaufman, R.J. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006, 13, 374–384. [Google Scholar] [CrossRef]
- Khan, M.M.; Yang, W.L.; Wang, P. Endoplasmic reticulum stress in sepsis. Shock 2015, 44, 294–304. [Google Scholar] [CrossRef]
- Shah, D.; Romero, F.; Duong, M.; Wang, N.; Paudyal, B.; Suratt, B.T.; Kallen, C.B.; Sun, J.; Zhu, Y.; Walsh, K.; et al. Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Sci. Rep. 2015, 5, 11362. [Google Scholar] [CrossRef]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Xie, X.; Chen, H.; Zhu, Q.; Ge, Z.; Wei, H.; Deng, J.; Xia, Z.; Lian, Q. Heme Oxygenase-1 Reduces Sepsis-Induced Endoplasmic Reticulum Stress and Acute Lung Injury. Mediat. Inflamm. 2018, 2018, 9413876. [Google Scholar] [CrossRef]
- Zeng, M.; Sang, W.; Chen, S.; Chen, R.; Zhang, H.; Xue, F.; Li, Z.; Liu, Y.; Gong, Y.; Zhang, H.; et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol. Lett. 2017, 271, 26–37. [Google Scholar] [CrossRef]
- Kaplon, R.E.; Chung, E.; Reese, L.; Cox-York, K.; Seals, D.R.; Gentile, C.L. Activation of the unfolded protein response in vascular endothelial cells of nondiabetic obese adults. J. Clin. Endocrinol. Metab. 2013, 98, E1505–E1509. [Google Scholar] [CrossRef]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Mei, S.H.J.; Haitsma, J.J.; Santos, C.C.D.; Deng, Y.; Lai, P.F.H.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Giebel, B.; Kordelas, L.; Börger, V. Clinical potential of mesenchymal stem/stromal cell derived extracellular vesicles. Stem Cell Investig. 2017, 4, 84. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Zhang, X.; Yang, L.; Xia, Z.; Zhang, Q. Exosomes from adipose-derived mesenchymal stem cells alleviate sepsis-induced lung injury in mice by inhibiting the secretion of IL-27 in macrophages. Cell Death Discov. 2022, 8, 18. [Google Scholar] [CrossRef]
- Liu, X.; Gao, C.; Wang, Y.; Niu, L.; Jiang, S.; Pan, S. BMSC-Derived Exosomes Ameliorate LPS-Induced Acute Lung Injury by miR-384-5p-Controlled Alveolar Macrophage Autophagy. Oxid Med. Cell Longev. 2021, 2021, 9973457. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, K.Y.; Shih, H.J.; Chiang, M.; Huang, I.; Huang, Y.H.; Huang, J.C. Let-7i-5p Mediates the Therapeutic Effects of Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells on Mitigating Endotoxin-Induced Mortality and Liver Injury in High-Fat Diet-Induced Obese Mice. Pharmaceuticals 2022, 15, 36. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Deng, J.S.; Pan, H.P.; Liao, J.C.; Huang, S.S.; Huang, G.J. Sclareol ameliorate lipopolysaccharide-induced acute lung injury through inhibition of MAPK and induction of HO-1 signaling. Int. Immunopharmacol. 2017, 44, 16–25. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Tong, W.; Cui, Y.; Li, X.; Sun, H. Umbelliferone alleviates lipopolysaccharide-induced inflammatory responses in acute lung injury by down-regulating TLR4/MyD88/NF-κB signaling. Inflammation 2019, 42, 440. [Google Scholar] [CrossRef]
- Tilton, S.C.; Waters, K.M.; Karin, N.J.; Webb-Robertson, B.M.; Zangar, R.C.; Lee, K.M.; Bigelow, D.J.; Pounds, J.G.; Corley, R.A. Diet-induced obesity reprograms the inflammatory response of the murine lung to inhaled endotoxin. Toxicol. Appl. Pharmacol. 2013, 267, 137–148. [Google Scholar] [CrossRef][Green Version]
- Su, L.J.; Wu, M.S.; Hui, Y.Y.; Chang, B.M.; Pan, L.; Hsu, P.C.; Chen, Y.T.; Ho, H.N.; Huang, Y.H.; Ling, T.Y.; et al. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. Sci. Rep. 2017, 7, 45607. [Google Scholar] [CrossRef] [PubMed]
- Soares Martins, T.; Catita, J.; Martins Rosa, I.; da Cruz, E.A.B.; Silva, O.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Bonnardel, E.; Prevel, R.; Campagnac, M.; Dubreuil, M.; Marthan, R.; Berger, P.; Dupin, I. Determination of reliable lung function parameters in intubated mice. Respir. Res. 2019, 20, 211. [Google Scholar] [CrossRef]
- Chen, C.H.; Tsai, P.S.; Huang, C.J. Minocycline ameliorates lung and liver dysfunction in a rodent model of hemorrhagic shock/resuscitation plus abdominal compartment syndrome. J. Surg. Res. 2013, 180, 301–309. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M.; Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]
- Kao, M.; Chung, C.Y.; Chang, Y.Y.; Lin, C.K.; Sheu, J.R.; Huang, C.J. Salutary Effects of Cepharanthine against Skeletal Muscle and Kidney Injuries following Limb Ischemia/Reperfusion. Evid. Based Complement Alternat. Med. 2015, 2015, 504061. [Google Scholar] [CrossRef]
- Vincent, J.L.; Nelson, D.R.; Williams, M.D. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit. Care Med. 2011, 39, 1050–1055. [Google Scholar] [CrossRef]
- Rahman, A.; Fazal, F. Blocking NF-κB: An inflammatory issue. Proc. Am. Thorac. Soc. 2011, 8, 497–503. [Google Scholar] [CrossRef]
- McKenna, S.; Wright, C.J. Inhibiting IκBβ-NFκB signaling attenuates the expression of select pro-inflammatory genes. J. Cell Sci. 2015, 128, 2143–2155. [Google Scholar] [CrossRef]
- Lee, I.T.; Yang, C.M. Inflammatory signalings involved in airway and pulmonary diseases. Mediat. Inflamm. 2013, 2013, 7912312013. [Google Scholar] [CrossRef] [PubMed]
- Gaestel, M.; Kotlyarov, A.; Kracht, M. Targeting innate immunity protein kinase signalling in inflammation. Nat. Rev. Drug Discov. 2009, 8, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1: Master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998, 8, 588–594. [Google Scholar] [CrossRef]
- Jung, Y.J.; Isaacs, J.S.; Lee, S.; Trepel, J.; Neckers, L. I IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003, 17, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xia, J.; Huang, L.; Li, Y. HIF-1α promotes NLRP3 inflammasome activation in bleomycin-induced acute lung injury. Mol. Med. Rep. 2019, 20, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Oyadomari, S.; Suga, M.; Mori, M.; Gotoh, T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J. Biochem. 2005, 138, 501–507. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, J.S.; Kim, S.R.; Park, S.Y.; Chae, H.J.; Lee, Y.C. Inhibition of endoplasmic reticulum stress alleviates lipopolysaccharide-induced lung inflammation through modulation of NF-κB/HIF-1α signaling pathway. Sci. Rep. 2013, 3, 1142. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, H.J.; Kim, D.I.; Lee, K.B.; Park, H.J.; Jeong, J.S.; Cho, S.H.; Lee, Y.C. Blockade of Interplay between IL-17A and Endoplasmic Reticulum Stress Attenuates LPS-Induced Lung Injury. Theranostics 2015, 5, 1343–1362. [Google Scholar] [CrossRef]
- Garg, A.D.; Kaczmarek, A.; Krysko, O.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. ER stress-induced inflammation: Does it aid or impede disease progression? Trends Mol. Med. 2012, 18, 589–598. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Lourie, R.; Das, I.; Chen, A.C.; McGuckin, M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 2012, 90, 260–270. [Google Scholar] [CrossRef]
- Shenderov, K.; Riteau, N.; Yip, R.; Mayer-Barber, K.D.; Oland, S.; Hieny, S.; Fitzgerald, P.; Oberst, A.; Dillon, C.P.; Green, D.R.; et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J. Immunol. 2014, 192, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.W.; Kutzler, L.; Kimball, S.R.; Tabas, I. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat. Cell Biol. 2012, 14, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Bouaziz, J.D.; Matsushita, T.; Tsubata, T.; Tedder, T.F. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 2009, 182, 7459–7472. [Google Scholar] [CrossRef] [PubMed]
- Ayaub, E.A.; Kolb, P.S.; Mohammed-Ali, Z.; Tat, V.; Murphy, J.; Bellaye, P.; Shimbori, C.; Boivin, F.J.; Lai, R.; Lynn, E.G.; et al. GRP78 and CHOP modulate macrophage apoptosis and the development of bleomycin-induced pulmonary fibrosis. J. Pathol. 2016, 239, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.S.; Liu, C.C.; Lin, J.H.; Hsu, T.W.; Hsu, J.W.; Su, K.; Hung, S.C. Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Sci. Rep. 2017, 7, 14272. [Google Scholar] [CrossRef]
- Delbrel, E.; Soumare, A.; Naguez, A.; Label, R.; Bernard, O.; Bruhat, A.; Fafournoux, P.; Tremblais, G.; Marchant, D.; Gille, T.; et al. HIF-1α triggers ER stress and CHOP-mediated apoptosis in alveolar epithelial cells, a key event in pulmonary fibrosis. Sci. Rep. 2018, 8, 17939. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, X.; Liu, F. Silencing TTTY15 mitigates hypoxia-induced mitochondrial energy metabolism dysfunction and cardiomyocytes apoptosis via TTTY15/let-7i-5p and TLR3/NF-κB pathways. Cell Signal. 2020, 76, 109779. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Galluzzi, L.; Kepp, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, W.; Liu, J.; Tian, J.; Wang, S.; Rui, K. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front. Immunol. 2021, 12, 749192. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.K.; Kant, S. Targeting inflammation in diabetes: Newer therapeutic options. World J. Diabetes. 2014, 5, 697–710. [Google Scholar] [CrossRef]
- Albuquerque, D.; Stice, E.; Rodríguez-López, R.; Manco, L.; Nóbrega, C. Current review of genetics of human obesity: From molecular mechanisms to an evolutionary perspective. Mol. Genet. Genom. 2015, 290, 1191–1221. [Google Scholar] [CrossRef]
- Burhans, M.G.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of AT inflammation to the development of T2D mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [CrossRef]
- Chawla, A. Control of macrophage activation and function by PPARs. Circ. Res. 2010, 106, 1559–1569. [Google Scholar] [CrossRef]
- Sharma, N.K.; Das, S.K.; Mondal, A.K.; Hackney, O.G.; Chu, W.S.; Kern, P.A.; Rasouli, N.; Spencer, H.J.; Yao-Borengasser, A.; Elbein, S.C. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J. Clin. Endocrinol. Metab. 2008, 93, 4532–4541. [Google Scholar] [CrossRef]
- Sjaastad, F.V.; Jensen, I.J.; Berton, R.R.; Badovinac, V.P.; Griffith, T.S. Inducing Experimental Polymicrobial Sepsis by Cecal Ligation and Puncture. Curr. Protoc. Immunol. 2020, 131, e110. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Choudhury, S. Experimental Protocol for Cecal Ligation and Puncture Model of Polymicrobial Sepsis and Assessment of Vascular Functions in Mice. Methods Mol. Biol. 2018, 1717, 161–187. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, M.D.; Chang, C.-Y.; Shih, H.-J.; Le, V.L.; Huang, Y.-H.; Huang, C.-J. Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells Mitigate Endoplasmic Reticulum Stress, Inflammation, and Lung Injury in Lipopolysaccharide-Treated Obese Mice. Antioxidants 2022, 11, 615. https://doi.org/10.3390/antiox11040615
Chiang MD, Chang C-Y, Shih H-J, Le VL, Huang Y-H, Huang C-J. Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells Mitigate Endoplasmic Reticulum Stress, Inflammation, and Lung Injury in Lipopolysaccharide-Treated Obese Mice. Antioxidants. 2022; 11(4):615. https://doi.org/10.3390/antiox11040615
Chicago/Turabian StyleChiang, Milton D., Chao-Yuan Chang, Hung-Jen Shih, Van Long Le, Yen-Hua Huang, and Chun-Jen Huang. 2022. "Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells Mitigate Endoplasmic Reticulum Stress, Inflammation, and Lung Injury in Lipopolysaccharide-Treated Obese Mice" Antioxidants 11, no. 4: 615. https://doi.org/10.3390/antiox11040615
APA StyleChiang, M. D., Chang, C.-Y., Shih, H.-J., Le, V. L., Huang, Y.-H., & Huang, C.-J. (2022). Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells Mitigate Endoplasmic Reticulum Stress, Inflammation, and Lung Injury in Lipopolysaccharide-Treated Obese Mice. Antioxidants, 11(4), 615. https://doi.org/10.3390/antiox11040615







