Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction
2.2. Analysis
2.2.1. Determination of Total Phenolic Content (TPC)
2.2.2. HPLC Analysis of Total Anthocyanin Content (TAC)
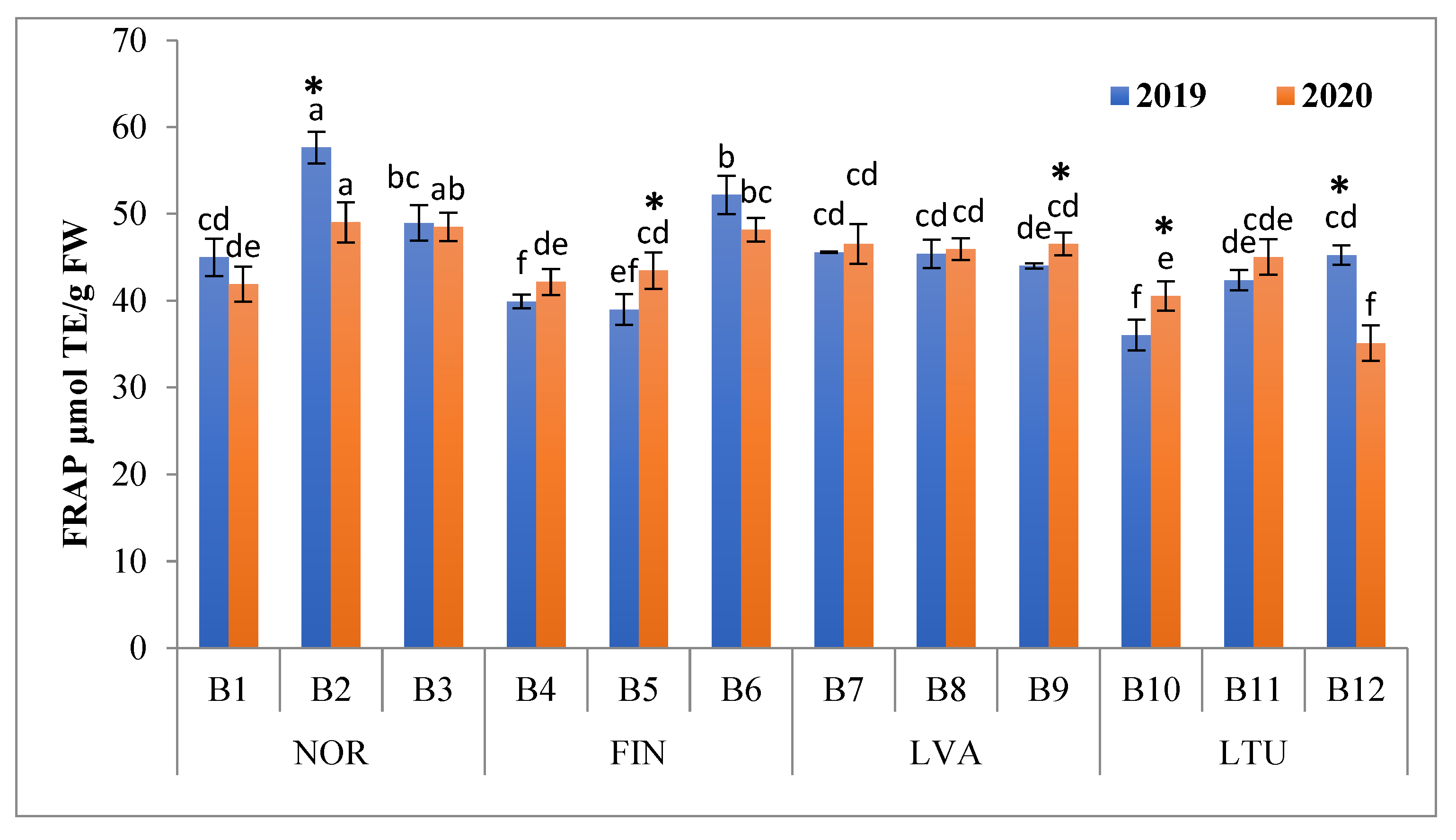
2.3. Determination of Ferric-Reducing Antioxidant Power (FRAP)
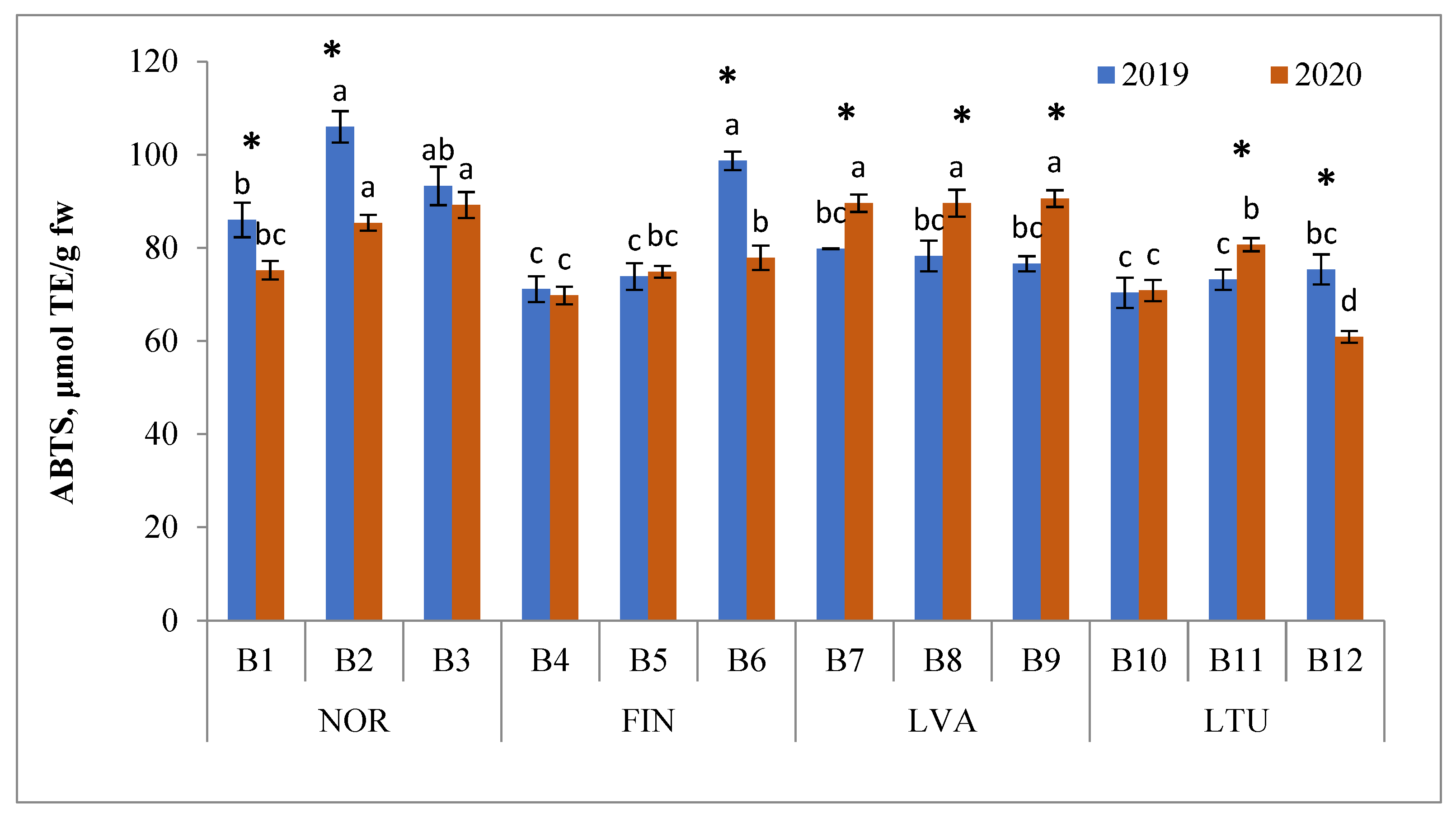
2.4. Determination of ABTS Radical Scavenging Activity (ABTS RSA)
2.5. Determination of Dry Matter (DM), Total Soluble Solids (TSS) and pH
2.6. Statistical Analysis
3. Results
3.1. Sample Locations
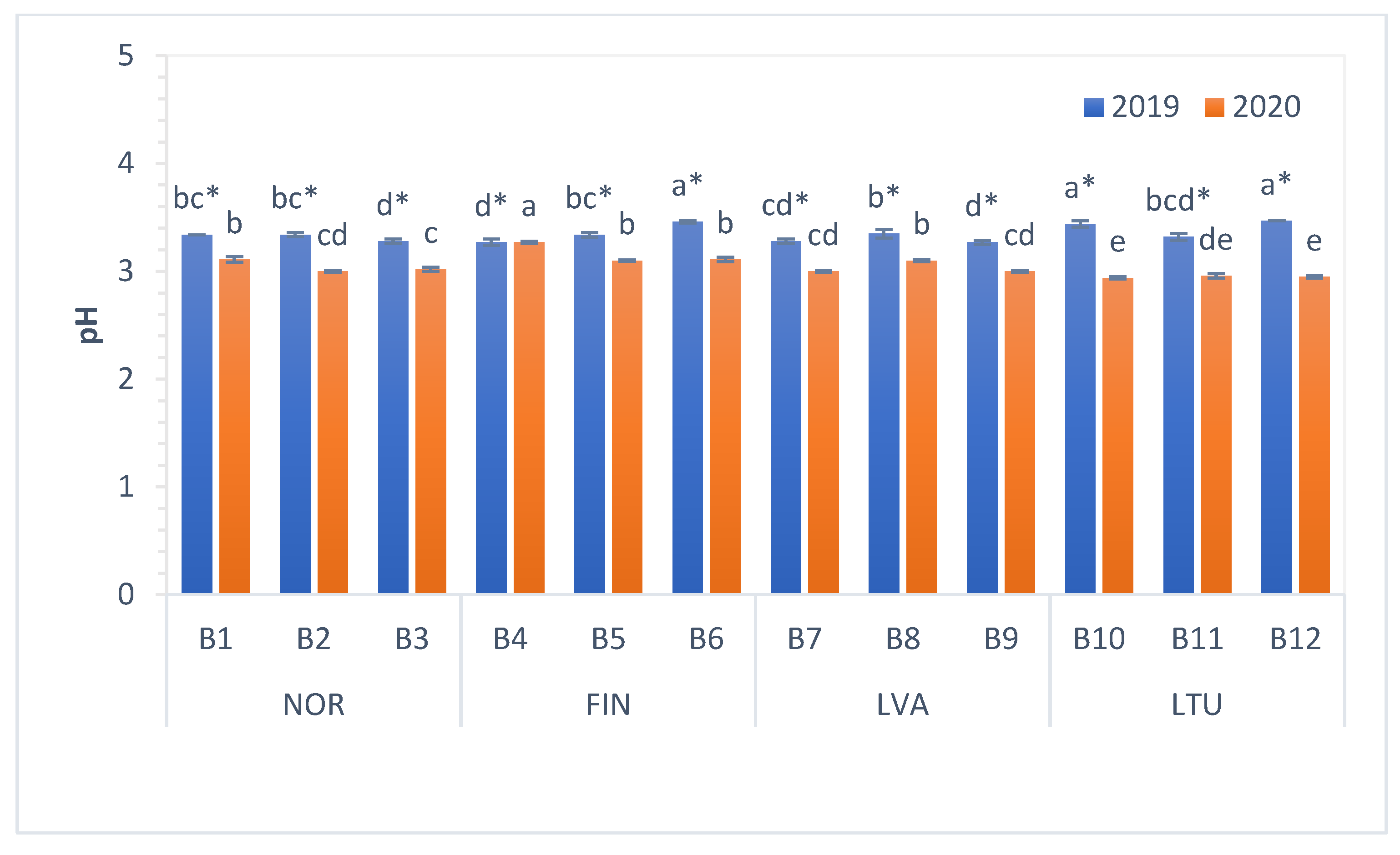
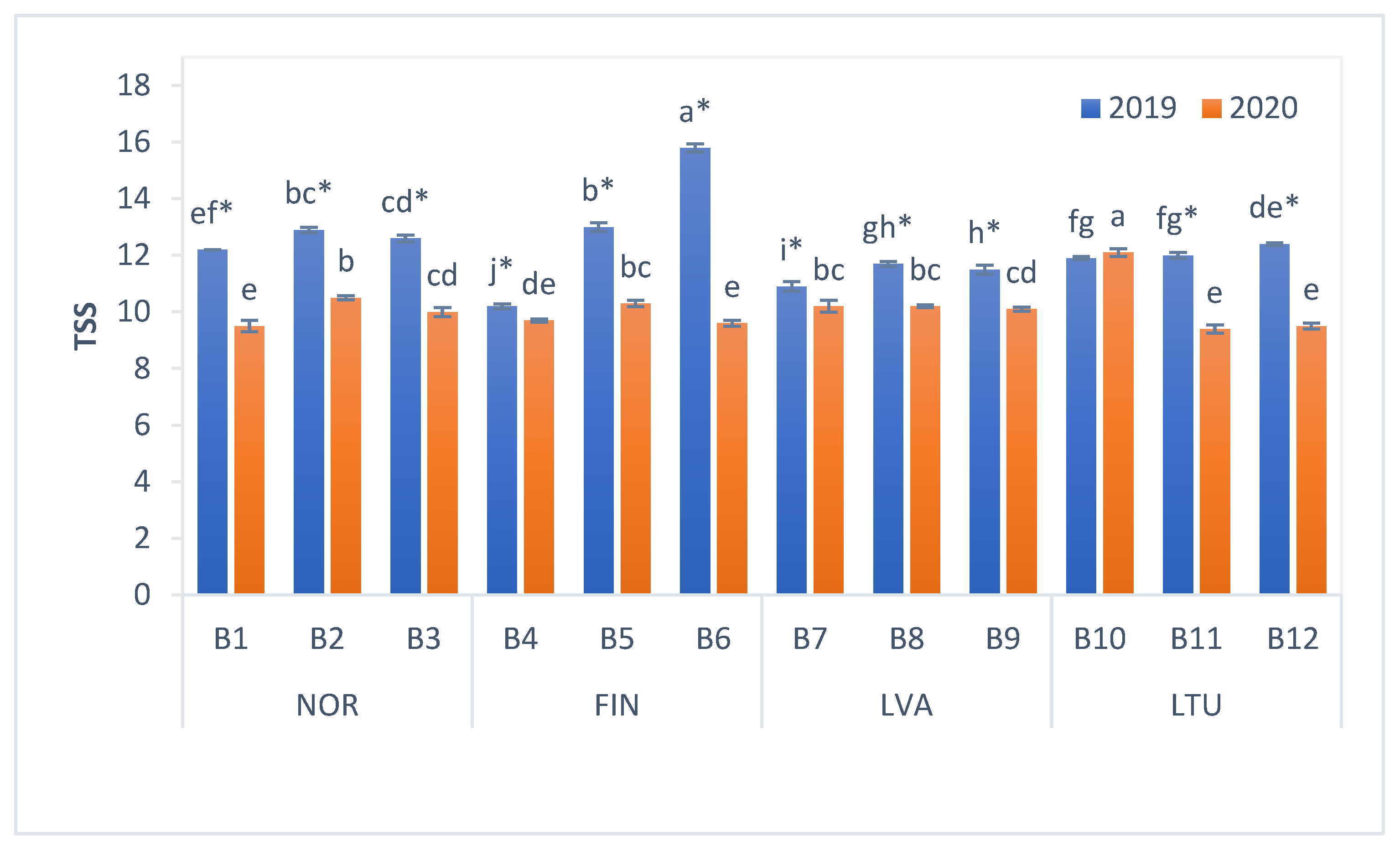
3.2. The pH and Total Soluble Solids (TSS) Content
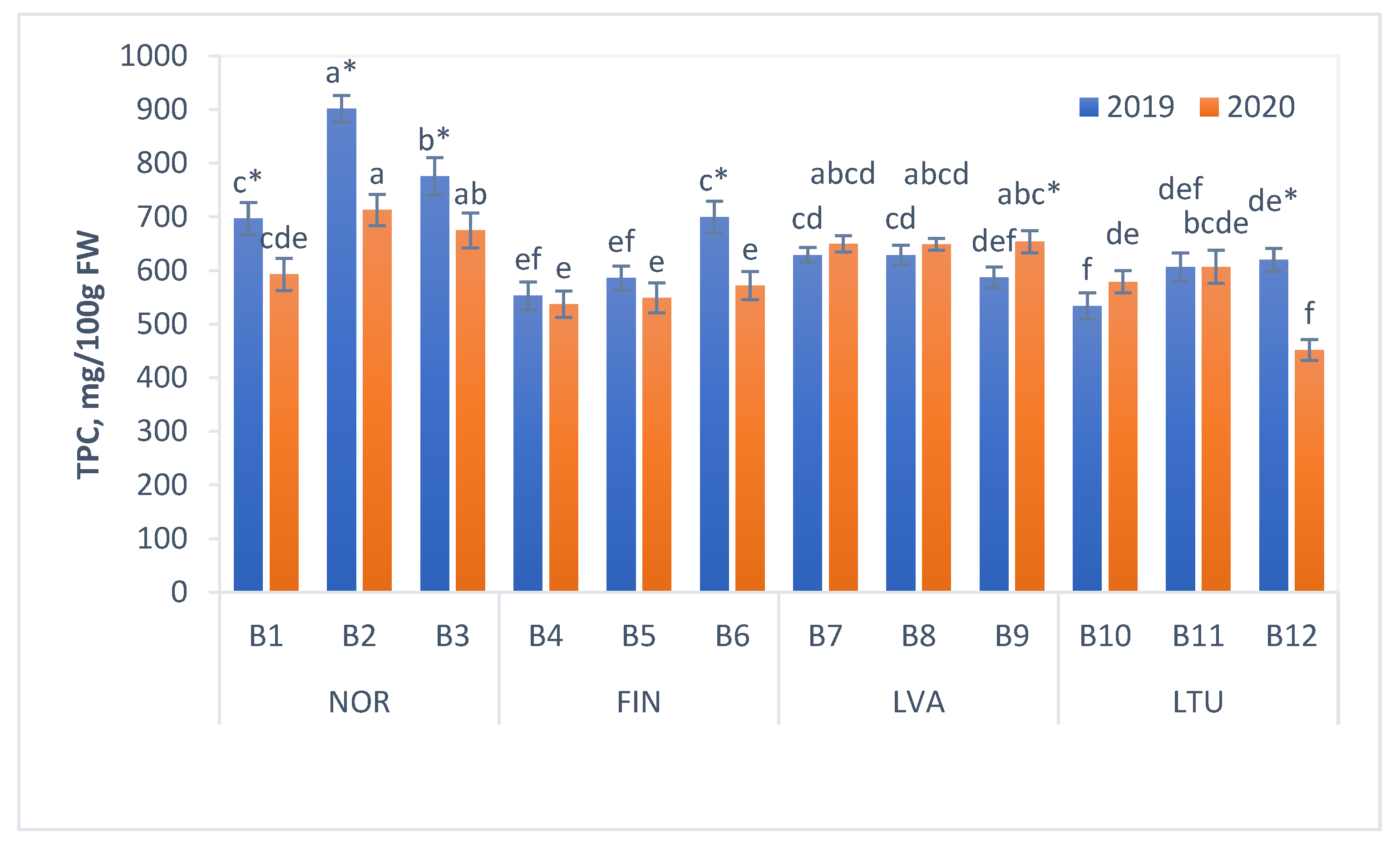
3.3. Total Phenolic Content (TPC)
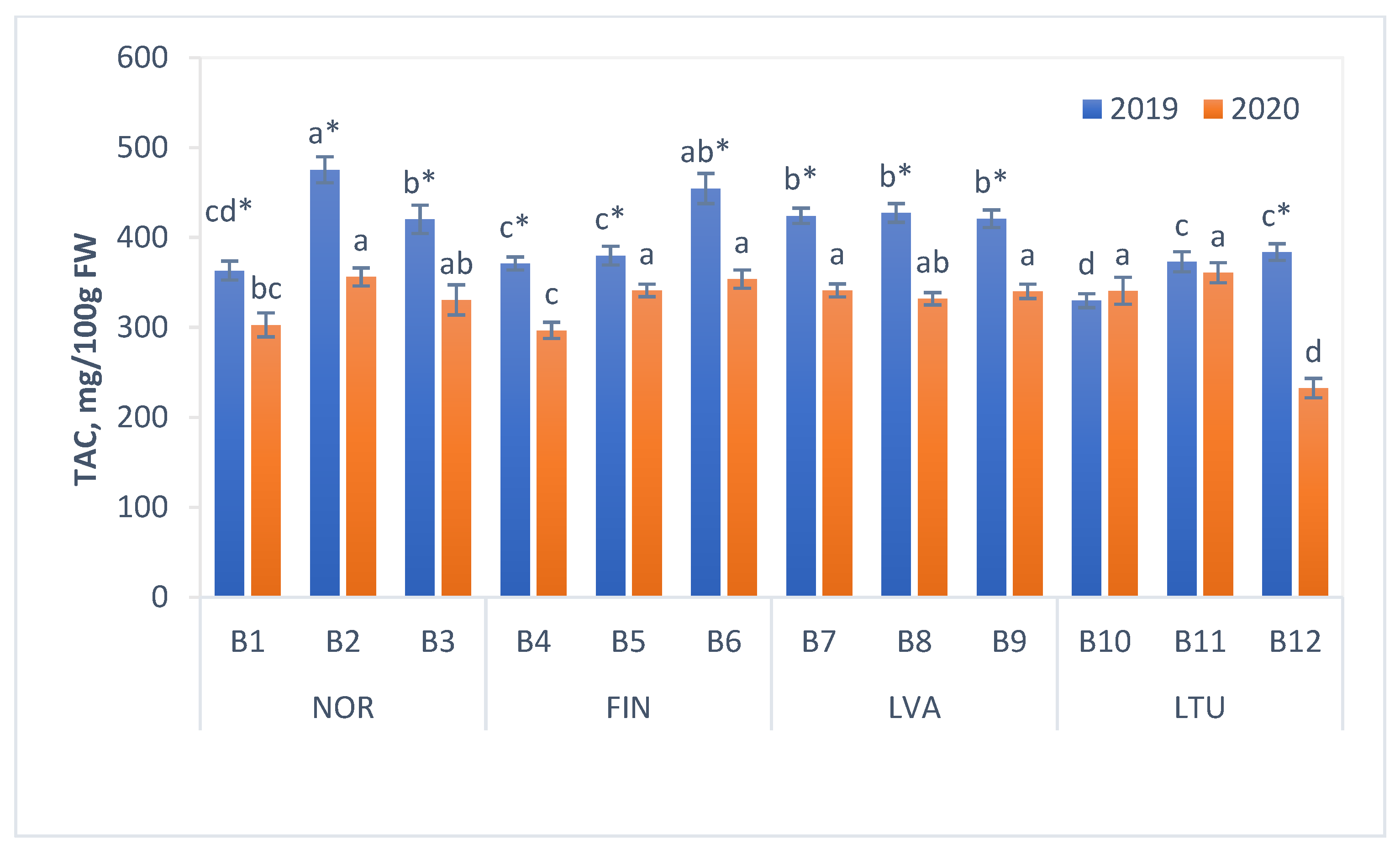
3.4. Total Anthocyanin Content (TAC)
3.5. Antioxidant Activity (AA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Josuttis, M.; Verrall, S.; Stewart, D.; Krüger, E.; McDougall, G.J. Genetic and environmental effects on tannin composition in strawberry (Fragaria× ananassa) cultivars grown in different European locations. J. Agric. Food Chem. 2013, 61, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žvikas, V.; Viškelis, J.; Viškelis, P. Phenolic profiles, antioxidant activity and phenotypic characterization of Lonicera caerulea L. berries, cultivated in Lithuania. Antioxidants 2021, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Szajdek, A.; Borowska, E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008, 63, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Jaakola, L.; Häggman, H.; Giongo, L. Anthocyanin profile in berries of wild and cultivated Vaccinium spp. along altitudinal gradients in the Alps. J. Agric. Food Chem. 2015, 63, 8641–8650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plessi, M.; Bertelli, D.; Albasini, A. Distribution of metals and phenolic compounds as a criterion to evaluate variety of berries and related jams. Food Chem. 2007, 100, 419–427. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Q.; Cheng, H.; Ge, Y.; Liu, H.; Ye, X.; Chen, Y. Metabolomic approach for the authentication of berry fruit juice by liquid chromatography quadrupole time-of-flight mass spectrometry coupled to chemometrics. J. Agric. Food Chem. 2018, 66, 8199–8208. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 168–187. [Google Scholar] [CrossRef]
- Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2007, 7, 281–299. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Primetta, A.K.; Jaakola, L.; Ayaz, F.A.; Inceer, H.; Riihinen, K.R. Anthocyanin fingerprinting for authenticity studies of bilberry (Vaccinium myrtillus L.). Food Control 2013, 30, 662–667. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.; Wrolstad, R. AOAC 2005.02: Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines—pH Differential Method. In Official Methods of Analysis of AOAC International; AOAC: Washington, DC, USA, 2005; pp. 37–39. [Google Scholar]
- Council of Europe. European Pharmacopoeia. Strasbourg: Council of Europe, 4th ed.; p. 1099. Available online: https://www.edqm.eu/en (accessed on 10 January 2022).
- United States Pharmacopeia (USP). 31–NF 26 Second Supplement; Powdered Bilberry Extract (Identification and Assay); United States Pharmacopeia (USP): Rockville, MD, USA, 2008; p. 685. [Google Scholar]
- Ongkowijoyo, P.; Luna-Vital, D.A.; de Mejia, E.G. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, N.M.; Yaman, M. Determination of anthocyanins in cherry cranberry by high-performance liquid chromatography-electrospray ionization-mass spectrometry. Eur. Food Res. Technol. 2016, 242, 127–135. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinämäki, J.; Ollilainen, V.; Heinonen, M. Berry anthocyanins: Isolation, identification and antioxidant activities. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar] [CrossRef]
- Valls, J.; Millán, S.; Martí, M.P.; Borràs, E.; Arola, L. Advanced separation methods of food anthocyanins, isoflavones and flavanols. J. Chromatogr. A 2009, 1216, 7143–7172. [Google Scholar] [CrossRef] [PubMed]
- Willemse, C.M.; Stander, M.A.; Tredoux, A.G.J.; Villiers, A. Comprehensive two-dimensional liquid chromatography analysis of anthocyanins. J. Chromatogr. A 2014, 1359, 189–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Kou, X.; Fugal, K.; McLaughlin, J. Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J. Agric. Food Chem. 2004, 52, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Burdulis, D.; Sarkinas, A.; Jasutiene, I.; Stackevicené, E.; Nikolajevas, L.; Janulis, V. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar] [PubMed]
- Cassinese, C.; Combarieu, E.D.; Falzoni, M.; Fuzzati, N.; Pace, R.; Sardone, N. New liquid chromatography method with ultraviolet detection for analysis of anthocyanins and anthocyanidins in Vaccinium myrtillus fruit dry extracts and commercial preparations. J. AOAC Int. 2007, 90, 911–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lätti, A.K.; Riihinen, K.R.; Kainulainen, P.S. Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 2008, 56, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Može, Š.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Ulrih, N.P.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Pataro, G.; Bobinaitė, R.; Bobinas, Č.; Šatkauskas, S.; Raudonis, R.; Visockis, M.; Ferrari, G.; Viškelis, P. Improving the extraction of juice and anthocyanins from blueberry fruits and their by-products by application of pulsed electric fields. Food Bioprocess Technol. 2017, 10, 1595–1605. [Google Scholar] [CrossRef]
- Koswig, S. Determination of foreign fruit types and fruit varieties—Analyses, evaluation and practical problems. Fruit Processing. 2006, 6, 401–412. [Google Scholar]
- Filip, M.; Vlassa, M.; Copaciu, F.; Coman, V. Identification of anthocyanins and anthocyanidins from berry fruits by chromatographic and spectroscopic techniques to establish the juice authenticity from market. JPC-J. Planar Chromatogr.-Mod. TLC 2012, 25, 534–541. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Viljakainen, S.; Visti, A.; Laakso, S. Concentrations of organic acids and soluble sugars in juices from Nordic berries. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2002, 52, 101–109. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries. Crop Production Science in Horticulture Series; CAB International: Wallingford, UK, 2012; pp. 1–336. [Google Scholar]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 112, 903–908. [Google Scholar] [CrossRef]
- Turkben, C.; Barut, E.; Incedayi, B. Investigations on population of blueberry (Vaccinium myrtillus L.) in Uludag (Mount Olympus) in Bursa, Turkey. Akdeniz Üniversitesi Ziraat Fakültesi Derg. 2008, 21, 41–44. [Google Scholar]
- Oancea, S.; Moiseenco, F.; Traldi, P. Total phenolics and anthocyanin profiles of Romanian wild and cultivated blueberries by direct infusion ESI-IT-MS/MS. Rom. Biotechnol. Lett. 2013, 18, 8351. [Google Scholar]
- Ochmian, I.; Oszmianski, J.; Skupien, K. Chemical composition, phenolics, and firmness of small black fruits. J. Appl. Bot. Food Qual. 2009, 83, 64–69. [Google Scholar]
- Stanoeva, J.P.; Stefova, M.; Andonovska, K.B.; Vankova, A.; Stafilov, T. Phenolics and mineral content in bilberry and bog bilberry from Macedonia. Int. J. Food Prop. 2017, 20, S863–S883. [Google Scholar] [CrossRef]
- Šavikin, K.; Zdunić, G.; Janković, T.; Tasić, S.; Menković, N.; Stević, T.; Đorđević, B. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum. Nutr. 2009, 64, 212–217. [Google Scholar] [CrossRef]
- Milivojević, J.; Rakonjac, V.; Pristov, J.B.; Maksimović, V. Classification and fingerprinting of different berries based on biochemical profiling and antioxidant capacity. Pesqui. Agropecuária Bras. 2013, 48, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Skrede, G.; Martinsen, B.K.; Wold, A.B.; Birkeland, S.E.; Aaby, K. Variation in quality parameters between and within 14 Nordic tree fruit and berry species. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2012, 62, 193–208. [Google Scholar] [CrossRef]
- Rohloff, J.; Uleberg, E.; Nes, A.; Krogstad, T.; Nestby, R.; Martinussen, I. Nutritional composition of bilberries (Vaccinium myrtillus L.) from forest fields in Norway–Effects of geographic origin, climate, fertilization and soil properties. J. Appl. Bot. Food Qual. 2015, 88, 274–287. [Google Scholar] [CrossRef]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Sci. Food Agric. 2015, 95, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Ștefănescu, B.E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical composition and biological activities of the nord-west romanian wild bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.) leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, A.; Jaakola, L.; Bång, U.; Jaderlund, A. Effects of latitude-related factors and geographical origin on anthocyanidin concentrations in fruits of Vaccinium myrtillus L. (bilberries). J. Agric. Food Chem. 2010, 58, 11939–11945. [Google Scholar] [CrossRef] [PubMed]
- Nestby, R.; Percival, D.; Martinussen, I.; Opstad, N.; Rohloff, J. The European blueberry (Vaccinium myrtillus L.) and the potential for cultivation. Eur. J. Plant Sci. Biotechnol. 2011, 5, 5–16. [Google Scholar]
- Dincheva, I.; Badjakov, I. Assesment of the Anthocyanin Variation in Bulgarian Bilberry (Vaccinium Myrtillus L.) and Lingonberry (Vaccinium Vitis-Idaea L.). Int. J. Med. Pharm. Sci. (IJMPS) 2016, 6, 39–50. [Google Scholar]
| Analysis Method | Assay | Unit |
|---|---|---|
| Polyphenol content (TPC) | Folin–Ciocalteu | mg GAE/100 g FW |
| Total anthocyanin content (TAC) | Liquid chromatography | mg C3G/100 g FW |
| Antioxidant capacity | FRAP, ABTS | μmol TE/g FW |
| Total soluble solids (TSS) | Refractometric | Brix° |
| pH | Potentiometric | - |
| Sample No. (Collection Location) | Country Code | Coordinates | |
|---|---|---|---|
| Latitude | Longitude | ||
| B1 | NOR | 69°41.66521′ | 18°59.46854′ |
| B2 | NOR | 69°45.07693′ | 19°01.54336′ |
| B3 | NOR | 69°40.25058′ | 18°37.08972′ |
| B4 | FIN | 64°51.69040′ | 26°42.26600′ |
| B5 | FIN | 64°59.17020′ | 25°54.21950′ |
| B6 | FIN | 65°13.75280′ | 25°33.59240′ |
| B7 | LVA | 57°08.55168′ | 21°51.95172′ |
| B8 | LVA | 57°09.05976′ | 21°51.08952′ |
| B9 | LVA | 57°08.79108′ | 21°52.32660′ |
| B10 | LTU | 54°07.37150′ | 24°43.01752′ |
| B11 | LTU | 54°43.29541′ | 23°30.53200′ |
| B12 | LTU | 55°04.48091′ | 22°28.23829′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants 2022, 11, 588. https://doi.org/10.3390/antiox11030588
Urbonaviciene D, Bobinaite R, Viskelis P, Bobinas C, Petruskevicius A, Klavins L, Viskelis J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants. 2022; 11(3):588. https://doi.org/10.3390/antiox11030588
Chicago/Turabian StyleUrbonaviciene, Dalia, Ramune Bobinaite, Pranas Viskelis, Ceslovas Bobinas, Aistis Petruskevicius, Linards Klavins, and Jonas Viskelis. 2022. "Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.)" Antioxidants 11, no. 3: 588. https://doi.org/10.3390/antiox11030588
APA StyleUrbonaviciene, D., Bobinaite, R., Viskelis, P., Bobinas, C., Petruskevicius, A., Klavins, L., & Viskelis, J. (2022). Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants, 11(3), 588. https://doi.org/10.3390/antiox11030588









