Potential Mechanisms of the Improvement of Glucose Homeostasis in Type 2 Diabetes by Pomegranate Juice
Abstract
:1. Introduction
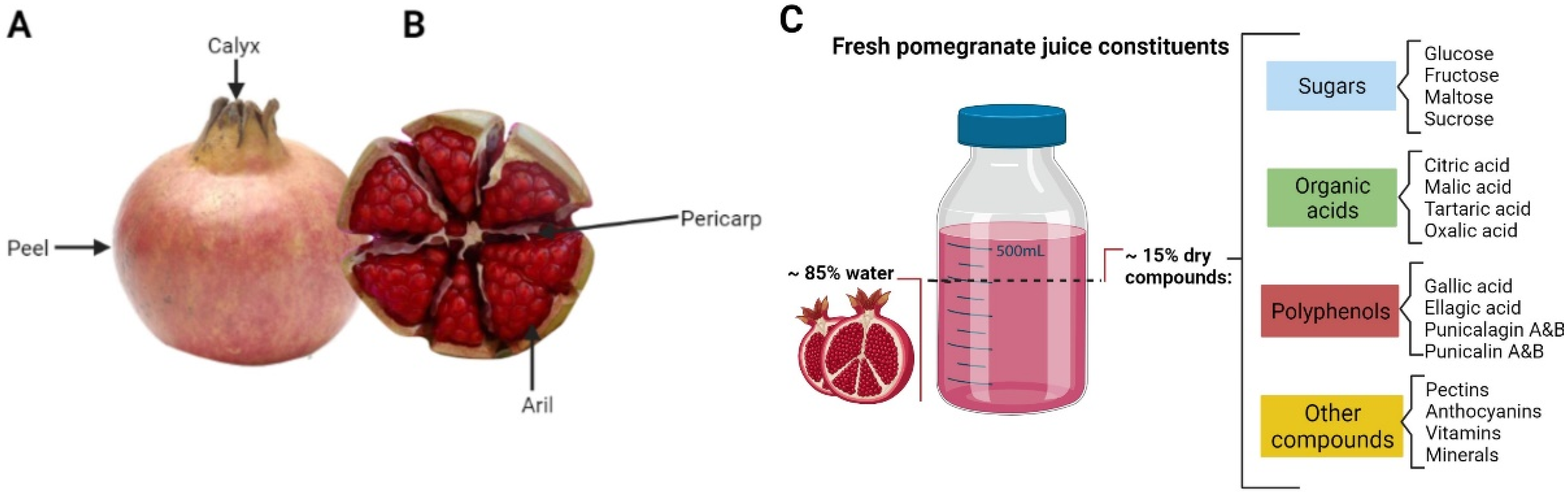
2. Pomegranate Juice Composition
3. Pharmacological Treatments against Type 2 Diabetes
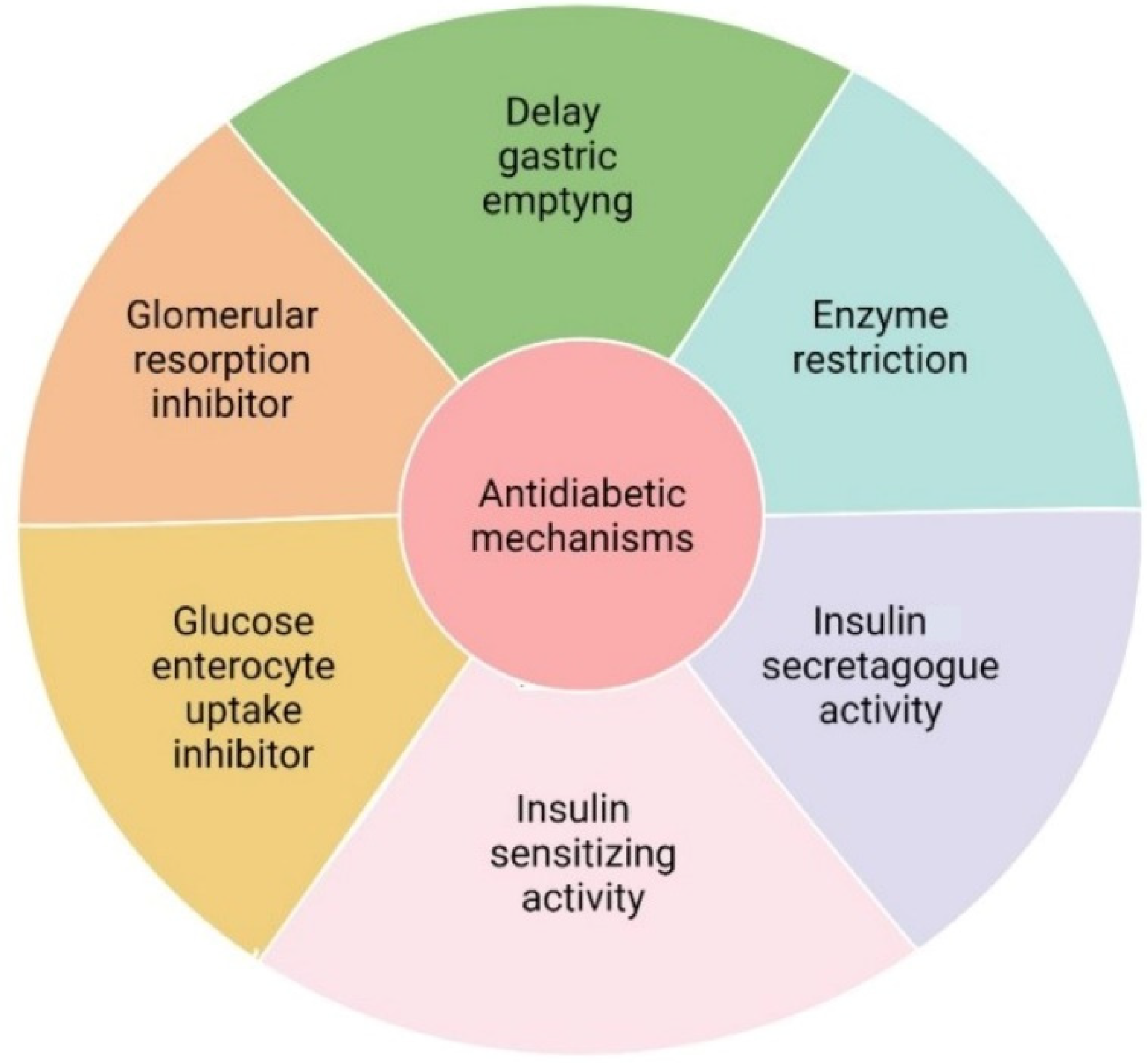
4. Fruit Derived Bioactive Compounds with Hypoglycemic Activity
- (1)
- Delayed gastric emptying through inhibition of dipeptidyl peptidase 4 (DPP-4) and glucagon-like peptide 1 (GLP-1);
- (2)
- Inhibition of α-amylase and α-glucosidase, both carbohydrate digesting enzymes, and inhibition of hepatic glucose release;
- (3)
- Insulin secretagogue activity, stimulating pancreatic β-cells function;
- (4)
- Enhancing glucose uptake by stimulating of expression of GLUT transporters in muscle, liver, and adipose cells;
- (5)
- Decrease glucose absorption by enterocytes by inhibiting sodium-glucose transport (SGLT-1) and GLUT-2 transporters;
- (6)
- Reduction of glomerular glucose reuptake followed by low glucose levels in the blood.
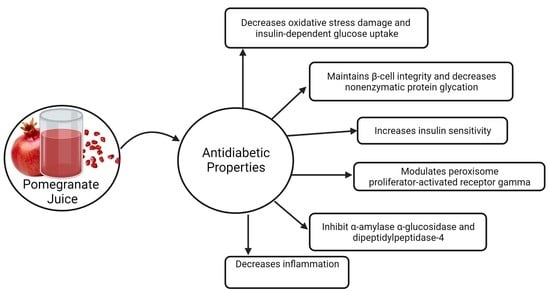
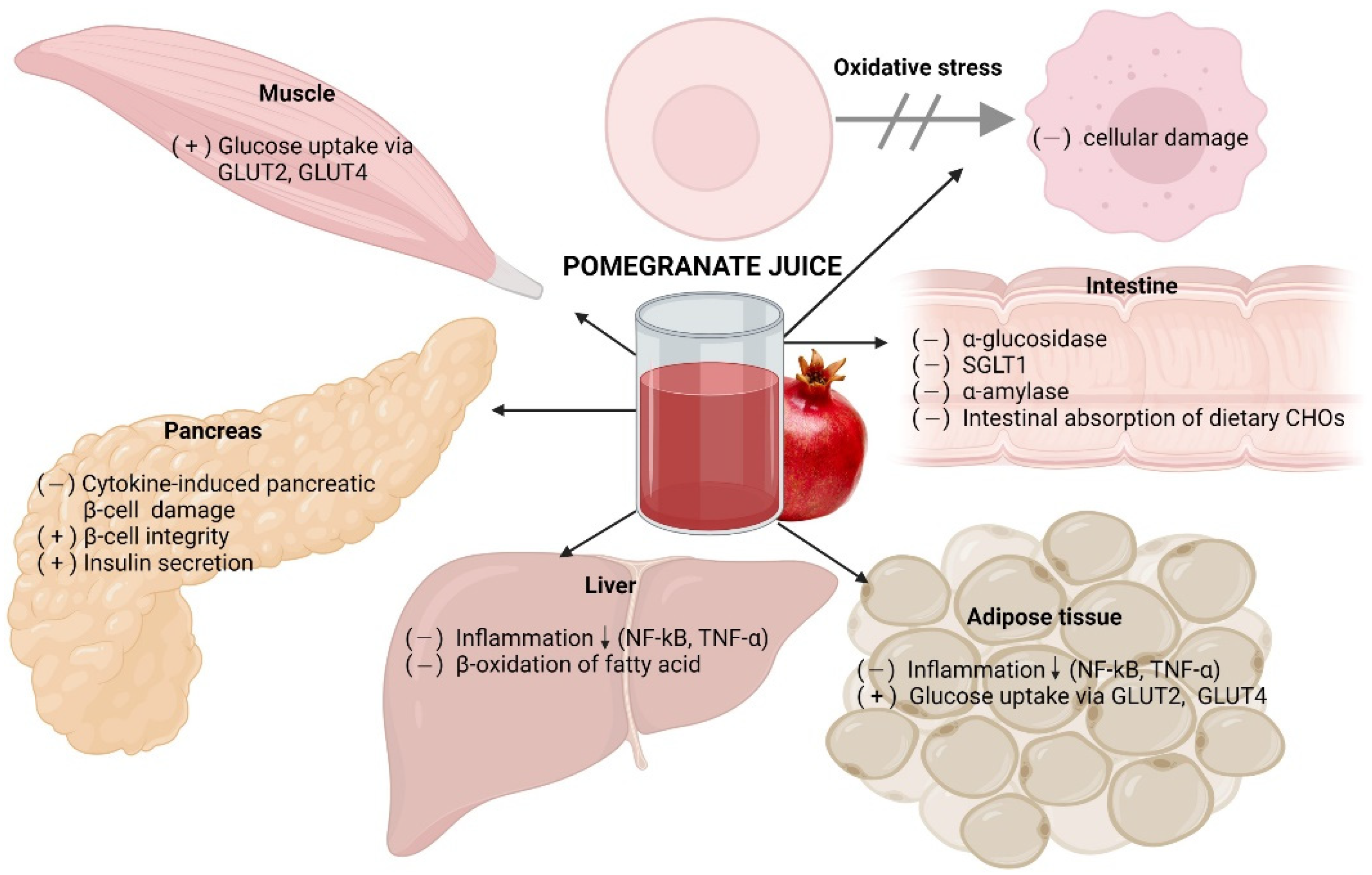
5. Antidiabetic Effects of Fresh Pomegranate Juice
5.1. Human Studies
5.2. Rodent Studies
5.3. In Vitro Studies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CYP2C9 | Cytochrome P450 2C9 |
| DPP-4 | Dipeptidyl peptidase 4 |
| GLP-1 | Glucagon-like peptide 1 |
| GLUT2 | Glucose transporter 2 |
| GLUT4 | Glucose transporter 4 |
| HDL | High-density lipoprotein |
| Il | Interleukin |
| LDL | Low-density lipoprotein |
| NF-κB | Nuclear factor-kappa B |
| •OH | Hydroxyl radical |
| PON1 | Paraoxonase 1 |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| SGLT-1 | Sodium glucose transporter 1 |
| TNF-α | Tumor Necrosis Factor alpha |
References
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A Review Study on Punica granatum L. Evid.-Based Complement. Altern. Med. 2016, 21, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, A.; Penugonda, K. Pomegranate juice: A heart-healthy fruit juice. Nutr. Rev. 2009, 67, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Naveed, M.; BiBi, J.; Kamboh, A.A.; Arain, M.A.; Shah, Q.A.; Alagawany, M.; El-Hack, M.E.A.; Abdel-Latif, M.A.; Yatoo, M.I.; et al. The Promising Pharmacological Effects and Therapeutic/Medicinal Applications of Punica Granatum L. (Pomegranate) as a Functional Food in Humans and Animals. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Babu, K.D.; Jadhav, V.T.; Jaime, A.; Silva, T. Origin, history and domestication of pomegranate. Fruit Veg. Cereal Sci. Biotechnol. 2010, 2, 1–6. [Google Scholar]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective Effects of Pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viuda-Martos, M.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Manandhar, B.; Paudel, K.R. Anticancer activity of Punica granatum (pomegranate): A review. Phytother Res. 2017, 31, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar] [PubMed]
- Kandylis, P.; Kokkinomagoulos, E. Food Applications and Potential Health Benefits of Pomegranate and its Derivatives. Foods. 2020, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumienna, M.; Szwengiel, A.; Górna, B. Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eur. Food Res. Technol. 2016, 242, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerimi, A.; Nyambe-Silavwe, H.; Gauer, J.S.; Tomás-Barberán, F.A.; Williamson, G. Pomegranate juice, but not an extract, confers a lower glycemic response on a high-glycemic index food: Randomized, crossover, controlled trials in healthy subjects. Am. J. Clin. Nutr. 2017, 106, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, K.; Rana, M.B.M.; Sultan, S. Oral Hypoglycemic Medications; Stat Pearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Rubaiy, H.N. The therapeutic agents that target ATP-sensitive potassium channels. Acta Pharm. 2016, 66, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotta, N. A new perspective on the biguanide, metformin therapy in type 2 diabetes and lactic acidosis. J. Diabetes Investig. 2019, 10, 906–908. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ma, S. Recent Advances in Synthetic α-Glucosidase Inhibitors. ChemMedChem 2017, 12, 819–829. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A. Risks Associated with SGLT2 Inhibitors: An Overview. Curr. Drug Saf. 2018, 13, 84–91. [Google Scholar] [CrossRef]
- Haidar, M.S. Comparison of Effectiveness between DPP4 inhibitors combined with Versus Other Oral Hypoglycaemic Agent(s) in Diabetic patients. Bangladesh J. Med. Sci. 2019, 30, 63–70. [Google Scholar] [CrossRef]
- González, S. Dietary Bioactive Compounds and Human Health and Disease. Nutrients 2020, 12, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Ata, A.; Anil-Kumar, N.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, A.S.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.S.; Jun, H.S. Role of Bioactive Food Components in Diabetes Prevention: Effects on Beta-Cell Function and Preservation. Nutr. Metab. Insights 2014, 7, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thulé, P.M. Mechanisms of current therapies for diabetes mellitus type 2. Adv. Physiol. Educ. 2012, 36, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lankatillake, C.; Huynh, T.; Dias, D.A. Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods 2019, 15, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betanzos-Cabrera, G.; Guerrero-Solano, J.A.; Martínez-Pérez, M.M.; Miller, Z.G.; Miller, H.; Cancino-Díaz, J.C. Pomegranate juice increases levels of paraoxonase 1 (PON1) expression and enzymatic activity in streptozotocin-induced diabetic mice fed with a high-fat diet. Food Res. Int. 2011, 44, 1381. [Google Scholar] [CrossRef]
- Chakraborty, M.; Ahmed, M.G.; Bhattacharjee, A. The potential for interaction of tolbutamide with pomegranate juice against diabetic induced complications in rats. Integr. Med. Res. 2017, 6, 354–360. [Google Scholar] [CrossRef]
- Parsaeyan, N.; Mozaffari-Khosravi, H.; Mozayan, M.R. Effect of pomegranate juice on paraoxonase enzyme activity in patients with type 2 diabetes. J. Diabetes Metab. Disord. 2012, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Banihani, S.A.; Fashtaky, R.A.; Makahleh, S.M.; El-Akawi, Z.J.; Khabour, O.F.; Saadeh, N.A. Effect of fresh pomegranate juice on the level of melatonin, insulin, and fasting serum glucose in healthy individuals and people with impaired fasting glucose. Food Sci. Nutr. 2019, 8, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A.; Makahleh, S.M.; El-Akawi, Z.; Al-Fashtaki, R.A.; Khabour, O.F.; Gharibeh, M.Y.; Saadah, N.A.; Al-Hashimi, F.H.; Al-Khasieb, N.J. Fresh pomegranate juice ameliorates insulin resistance, enhances β-cell function, and decreases fasting serum glucose in type 2 diabetic patients. Nutr. Res. 2014, 34, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, G.; Roshan, H.; Ebrahimof, S.; Nikpayam, O.; Sotoudeh, G.; Siasi, F. Effects of pomegranate juice consumption on blood pressure and lipid profile in patients with type 2 diabetes: A single-blind randomized clinical trial. Clin. Nutr. ESPEN 2019, 29, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, G.; Angoorani, P.; Tohidi, M.; Tabibi, H.; Kimiagar, M.; Nasrollahzadeh, J. Pomegranate (Punicagranatum) juice decreases lipid peroxidation, but has no effect on plasma advanced glycated end-products in adults with type 2 diabetes: A randomized double-blind clinical trial. Food & nutrition research. Food Nutr. Res. 2015, 59, 28551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, W.; Rosenblat, M.; Miller-Lotan, R.; Levy, A.P.; Elias, M.; Aviram, M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 2008, 56, 8704–8713. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A.; Shuaibu, S.M.; Al-Husein, B.A.; Makahleh, S.S. Fresh pomegranate juice decreases fasting serum erythropoietin in patients with type 2 diabetes. Int. J. Food Sci. 2019, 2019, 1269341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shishehbor, F. Effects of concentrated pomegranate juice on subclinical inflammation and cardiometabolic risk factors for type 2 diabetes: A quasi-experimental study. Int. J. Endocrinol. Metab. 2016, 14, e33835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharib, E.; Kouhsari, S.M. Study of the antidiabetic activity of Punica granatum L. fruits aqueous extract on the alloxan-diabetic wistar rats. Iran. J. Pharm. Res. 2019, 18, 358–368. [Google Scholar] [PubMed]
- Sun, Y.-L.; Zhou, F.-M.; Wang, H.-R. Mechanism of pomegranate ellagic polyphenols reducing insulin resistance on gestational diabetes mellitus rats. Am. J. Transl. Res. 2019, 11, 5487–5500. [Google Scholar]
- Nadia, M.; Ramadan, G.; El-Husseiny, E.A.; Hussein, A.M. Effects of pomegranate aril juice and its punicalagin on some key regulators of insulin resistance and oxidative liver injury in streptozotocin-nicotinamide type 2 diabetic rats. Mol. Biol. Rep. 2019, 46, 3701–3711. [Google Scholar] [CrossRef]
- Tugcu, B.; Nacaroglu, S.A.; Gedikbasi, A.; Uhri, M.; Acar, N.; Ozdemir, H. Protective effect of pomegranate juice on retinal oxidative stress in streptozotocin-induced diabetic rats. Int. J. Ophthalmol. 2017, 10, 1662–1668. [Google Scholar]
- Mohan, M.; Waghulde, H.; Kasture, S. Effect of pomegranate juice on Angiotensin II-induced hypertension in diabetic wistar rats. Phytother. Res. 2010, 24, S196–S203. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhang, B.; Li, Q.; Tu, J.; Zhou, B. Effect of punicalagin on multiple targets in streptozotocin/high-fat diet-induced diabetic mice. Food Funct. 2020, 11, 10617–10634. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Guh, J.Y.; Hwang, C.C.; Chiou, S.J.; Lin, T.D.; Ko, Y.M.; Huang, J.S.; Yang, Y.L.; Chuang, L.Y. Advanced glycation end-products activate extracellular signal-regulated kinase via the oxidative stress-EGF receptor pathway in renal fibroblasts. J. Cell. Biochem. 2010, 109, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, H.; Frost, L.; Yuan, T.; Dain, J.A.; Seeram, N.P. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food Funct. 2014, 5, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Sell, D.R.; Nagaraj, R.H.; Miyata, S.; Grandhee, S.; Odetti, P.; Ibrahim, S.A. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes 1992, 41, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.V.; Smith, C.C.; Wolff, S.P. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes 1990, 39, 1420–1424. [Google Scholar] [CrossRef]
- Dorsey, P.G.; Greenspan, P. Inhibition of nonenzymatic protein glycation by pomegranate and other fruit juices. J. Med. Food 2014, 17, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Bierhaus, A.; Chevion, S.; Chevion, M.; Hofmann, M.; Quehenberger, P.; Illmer, T.; Luther, T.; Berentshtein, E.; Tritschler, H.; Müller, M.; et al. Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes 1997, 46, 1481–1490. [Google Scholar] [CrossRef]
- Estrada-Luna, D.; Martínez-Hinojosa, E.; Cancino-Diaz, J.C.; Belefant-Miller, H.; Betanzos-Cabrera, G. Daily supplementation with fresh pomegranate juice increases paraoxonase 1 expression and activity in mice fed a high-fat diet. Eur. J. Nutr. 2018, 57, 383–389. [Google Scholar] [CrossRef]
- Rosenblat, M.; Hayek, T.; Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 2006, 187, 363–371. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Tahbaz, F.; Gaieni, I.; Alavi-Majd, H.; Azadbakht, L. Concentrated pomegranate juice improves lipid profiles in diabetic patients with hyperlipidemia. J. Med. Food 2004, 7, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef]
- Virgen-Carrillo, C.A.; Martínez Moreno, A.G.; Valdés Miramontes, E.H. Potential Hypoglycemic Effect of Pomegranate Juice and Its Mechanism of Action: A Systematic Review. J. Med. Food 2020, 23, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Aboonabi, A.; Rahmat, A.; Othman, F. Antioxidant effect of pomegranate against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Toxicol. Rep. 2014, 1, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Virgen-Carrillo, C.A.; Miramontes, E.H.V.; Moreno, A.G.M.; Mojica, L.; Castañeda-Saucedo, M.C. Consumo de jugo de granada (Punica granatum) y su efecto sobre la glucemia, perfil lipídico e histología del páncreas en un modelo de hiperglucemia inducida mediante estreptozotocina. Arch. Latinoam. Nutr. 2018, 68, 29–40. [Google Scholar]
- Taheri Rouhi, S.Z.; Sarker, M.M.R.; Rahmat, A.; Alkahtani, S.A.; Othman, F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague-Dawley rats. BMC Complement. Altern. Med. 2017, 17, 156. [Google Scholar] [CrossRef] [Green Version]
- Rozenberg, O.; Howell, A.; Aviram, M. Pomegranate juice sugar fraction reduces macrophage oxidative state, whereas white grape juice sugar fraction increases it. Atherosclerosis 2006, 188, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- McTernan, P.G.; Fisher, F.M.; Valsamakis, G.; Chetty, R.; Harte, A.; McTernan, C.L.; Clark, P.M.S.; Smith, S.A.; Barnett, A.H.; Kumar, S. Resistin and type 2 diabetes: Regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J. Clin. Endocrinol. Metab. 2003, 88, 6098–6106. [Google Scholar] [CrossRef] [Green Version]
- Shabbir, M.A.; Khan, M.R.; Saeed, M.; Pasha, I.; Khalil, A.A.; Siraj, N. Punicic acid: A striking health substance to combat metabolic syndromes in humans. Lipids Health Dis. 2017, 16, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassaganya-Riera, J.; DiGuardo, M.; Climent, M.; Vives, C.; Carbo, A.; Jouni, Z.E.; Einerhand, A.W.C.; O’Shea, M.; Hontecillas, R. Activation of PPARγ and δ by dietary punicic acid ameliorates intestinal inflammation in mice. Br. J. Nutr. 2011, 106, 878–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit αglucosidase activity in vitro and reduce starch digestibility under simulated gastrointestinal conditions. Int. J. Food Sci. Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino-Wakagi, Y.; Yoshimura, Y.; Uzawa, Y.; Zaima, N.; Moriyama, T.; Kawamura, Y. Ellagic acid in pomegranate suppresses resistin secretion by a novel regulatory mechanism involving the degradation of intracellular resistin protein in adipocytes. Biochem. Biophys. Res. Commun. 2012, 417, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, Q.; Cai, H.; Wang, H.; Ma, Z.; Zhang, X. miR-218 regulates diabetic nephropathy via targeting IKK-β and modulating NK-κB-mediated inflammation. J. Cell. Physiol. 2020, 235, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.Y.; Neeman, I.; Resnick, N. A novel mechanism for the inhibition of NF-kappaB activation in vascular endothelial cells by natural antioxidants. FASEB J. 2002, 16, 1931–1933. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Suppression of the nuclear factorkappaB activation pathway by spice-derived phytochemicals: Reasoning for seasoning. Ann. N. Y. Acad. Sci. 2004, 1030, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.; Jungbauer, A. Culinary plants, herbs and spices–A rich source of PPARγ ligands. Food Chem. 2009, 117, 660–667. [Google Scholar] [CrossRef]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Peng, G.; Kota, B.P.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Anti-diabetic action of Punica granatum flower extract: Activation of PPAR-gamma and identification of an active component. Toxicol. Appl. Pharmacol. 2005, 207, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Suzuki, R.; Yasui, Y.; Hosokawa, M.; Miyashita, K.; Tanaka, T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004, 95, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Shiner, M.; Fuhrman, B.; Aviram, M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPARγ and AP-1 pathway activation. Atherosclerosis 2007, 195, 313–321. [Google Scholar] [CrossRef] [PubMed]
| Oral Hypoglycemic Medications | Mechanism of Action | Main Adverse Effects | Reference |
|---|---|---|---|
| Sulfonylureas | Binds to K-ATP channels leading to the influx of Ca++ and thus increasing insulin secretion by pancreatic beta cells. | Dizziness, nervousness, diarrhea, dyspepsia, and headache. | [17] |
| Meglitinides | Binds at different pancreatic beta-cell receptors increasing influx of Ca++ promoting the releasing of insulin. | Hypoglycemia, headache, and respiratory infections. | |
| Biguanides | Reduces hepatic gluconeogenesis, decreases intestinal absorption of glucose, and increases insulin sensitivity of muscle cells. | Diarrhea, nausea and vomiting, flatulence, and headache. | [18] |
| Thiazolidinediones | Promotes genic expression at different levels by binding to PPAR-γ receptors which improves insulin sensitivity, decreases hepatic glucose production, and mediates the inflammatory response. | Edema, hypoglycemia, cardiac failure, and headache. | [19] |
| α-Glucosidase inhibitors | Decreases glucose absorption at the intestinal level by competitively inhibiting α -glucosidase. | Flatulence, diarrhea, and abdominal pain. | [20] |
| SGLT2 inhibitors | Prevents glomerular glucose reabsorption by inhibiting the sodium-glucose cotransporter 2 (SGLT-2). | Urinary tract infections, glycosuria, dyslipidemia, and hyperphosphatemia. | [21] |
| DPP-4 inhibitors | Inhibits protease activity of DPP-4 by prolonging the release of glucagon; furthermore, it reduces gastric emptying and improves insulin secretion. | Hypoglycemia, headache, and urinary tract infections. | [22] |
| Study | Derivative | Model | Dose | Effect | Reference |
|---|---|---|---|---|---|
| Pomegranate juice, but not an extract, confers a lower glycemic response on a high glycemic index | Pomegranate juice | Healthy human | 200 mL in 4 days | Reduced blood glucose | [14] |
| Effect of pomegranate juice on paraoxonase enzyme activity in patients with type 2 diabetes | Pomegranate juice | Humans with type 2 diabetes mellitus | 200 mL for 6 weeks | Reduced concentration of fasting blood sugar and paraoxonase and increased arylesterase activity of PON1 | [31] |
| Fresh pomegranate juice decreases fasting serum erythropoietin in patient with type 2 diabetes | Pomegranate juice | Humans with type 2 diabetes mellitus | 3 h afer administration of 1.5 mL per kg afer a 12-h fast | Reduced serum erythropoietin level | [37] |
| Effects of concentrated pomegranate juice on subclinical inflammation and cardiometabolic risk facto for type 2 diabetes: | Concentrated pomegranate juice | Humans with type 2 diabetes mellitus | 50 g for 4 weeks | Caused significant reduction in serum interleukin-6 (IL-6). However, fasting blood glucose changes were not statistically significant. | [38] |
| Study of the antidiabetic activity of Punica granatum L. fruits aqueous extract on the alloxan-diabetic wistar rats | Aqueous extract of pomegranate arils | Alloxan induced diabetic rats | 100, 200, 350 mg/kg for 21 days | Reduced modulation of hyperglycemia | [39] |
| Mechanism of pomegranate ellagic polyphenols reducing insulin resistance on gestational diabetes mellitus rats | Pomegranate ellagic polyphenols | STZ induced diabetic rats with gestational diabetes mellitus | 50, 150, 300 mg/(kg/day) for 14 days. | Reduced of blood glucose levels, blood biochemical index, and insulin resistance. | [40] |
| Effects of pomegranate aril juice and its punicalagin on some key regulators of insulin resistance and oxidative liver injury in streptozotocin-nicotinamide type 2 diabetic rats | Pomegranate juice and its punicalagin | STZ induced diabetic rats | 100 or 300 mg/kg and 2.6 or 7.8 mg/kg respectively for 6 weeks. | Reduced insulin resistance and hyperglycemia. Low dose of punicalagin induced some modulation, nevertheless, the high dose of punicalagin did not show any antidiabetic activity | [41] |
| Protective effect of pomegranate juice on retinal oxidative stress in streptozotocin-induced diabetic rats | Pomegranate juice | STZ induced diabetic rats | 100 µL for 10 weeks | Reduced the levels of 8-hydroxy-2′-deoxyguano-sine (8OHdG) and malondialdehyde | [42] |
| Effect of pomegranate juice on angiotensin II-induced hypertension in diabetic wistar rats | Pomegranate seed extract | STZ induced diabetic rats | 100, 300 mg/kg for 4 weeks | Reversed the biochemical changes induced by diabetes | [43] |
| Effect of punicalagin on multiple targets in streptozotocin/high-fat diet induced diabetic mice | Punicalagin | STZ induced diabetic mice | 100, 150, 200 mg/kg for 4 weeks | Reduced of blood glucose levels and had a protective effect on diabetes mellitus | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olvera-Sandoval, C.; Fabela-Illescas, H.E.; Fernández-Martínez, E.; Ortiz-Rodríguez, M.A.; Cariño-Cortés, R.; Ariza-Ortega, J.A.; Hernández-González, J.C.; Olivo, D.; Valadez-Vega, C.; Belefant-Miller, H.; et al. Potential Mechanisms of the Improvement of Glucose Homeostasis in Type 2 Diabetes by Pomegranate Juice. Antioxidants 2022, 11, 553. https://doi.org/10.3390/antiox11030553
Olvera-Sandoval C, Fabela-Illescas HE, Fernández-Martínez E, Ortiz-Rodríguez MA, Cariño-Cortés R, Ariza-Ortega JA, Hernández-González JC, Olivo D, Valadez-Vega C, Belefant-Miller H, et al. Potential Mechanisms of the Improvement of Glucose Homeostasis in Type 2 Diabetes by Pomegranate Juice. Antioxidants. 2022; 11(3):553. https://doi.org/10.3390/antiox11030553
Chicago/Turabian StyleOlvera-Sandoval, Carlos, Héctor Enrique Fabela-Illescas, Eduardo Fernández-Martínez, María Araceli Ortiz-Rodríguez, Raquel Cariño-Cortés, José Alberto Ariza-Ortega, Juan Carlos Hernández-González, Diana Olivo, Carmen Valadez-Vega, Helen Belefant-Miller, and et al. 2022. "Potential Mechanisms of the Improvement of Glucose Homeostasis in Type 2 Diabetes by Pomegranate Juice" Antioxidants 11, no. 3: 553. https://doi.org/10.3390/antiox11030553
APA StyleOlvera-Sandoval, C., Fabela-Illescas, H. E., Fernández-Martínez, E., Ortiz-Rodríguez, M. A., Cariño-Cortés, R., Ariza-Ortega, J. A., Hernández-González, J. C., Olivo, D., Valadez-Vega, C., Belefant-Miller, H., & Betanzos-Cabrera, G. (2022). Potential Mechanisms of the Improvement of Glucose Homeostasis in Type 2 Diabetes by Pomegranate Juice. Antioxidants, 11(3), 553. https://doi.org/10.3390/antiox11030553