Ginkgo biloba in the Aging Process: A Narrative Review
Abstract
1. Introduction
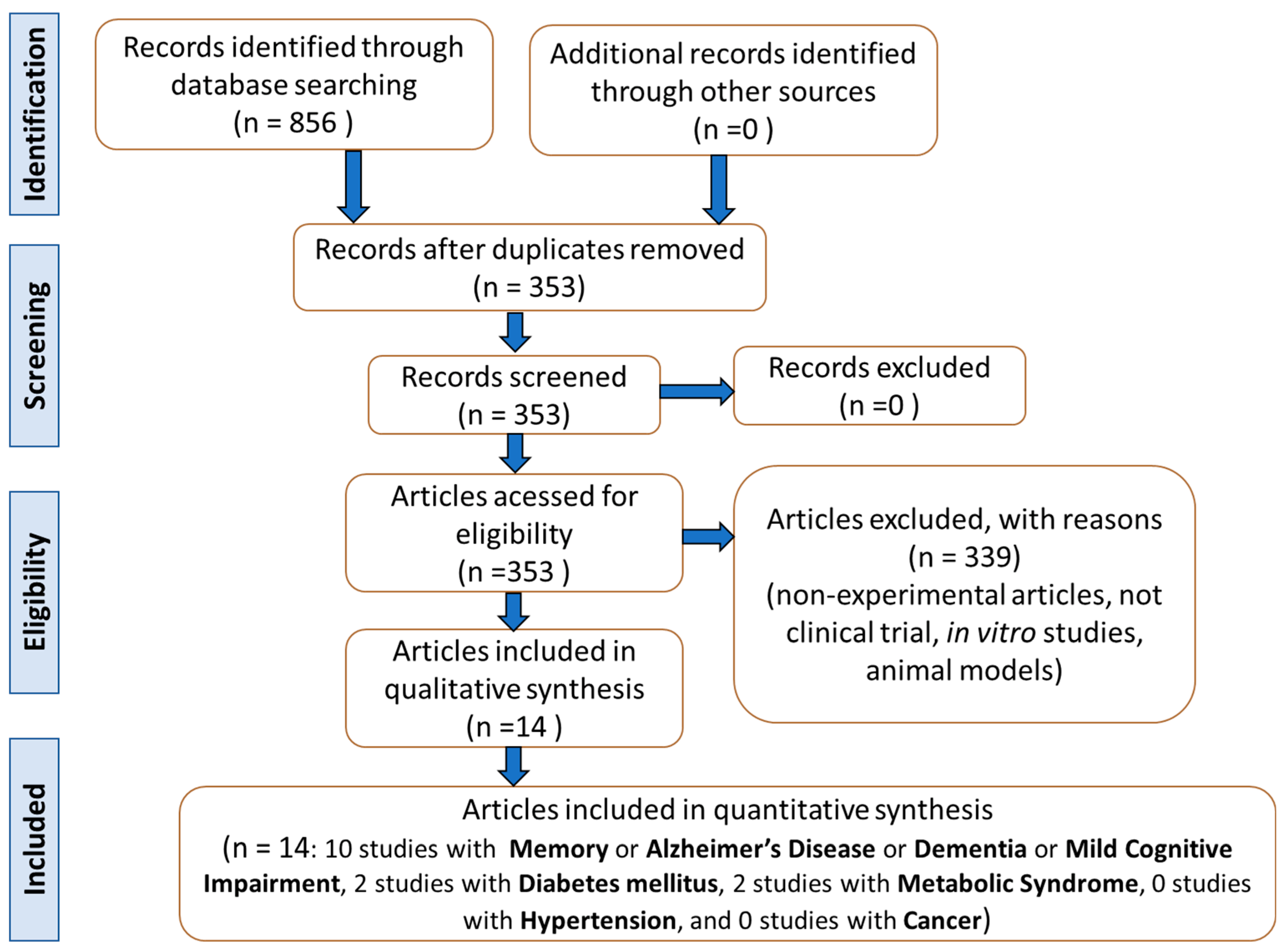
2. Materials and Methods
2.1. Focused Question
2.2. Language
2.3. Databases
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
| Reference | Local | Model and Patients | Intervention | Outcomes | Adverse Effects (AEs) |
|---|---|---|---|---|---|
| Neurodegenerative Diseases | |||||
| [19] | Germany | Randomized, double-blind, placebo-controlled, mono-center trial with 188 mentally healthy male and female subjects, 45–65 y, with higher secondary education. | Subjects received EGb 761 240 mg/day or placebo/6 w. | Subjects treated with EGb 761 significantly improved the number of appointments correctly recalled. The effects on qualitative recall performance (proportion of false to correct items) were similar. GB had no superiority in another routine memory test that required recognition of a driving route. | Seven AEs in the EGb 761 group (headache, n = 4; gastric complaints, n = 3) and five in the placebo group (gastric complaints, n = 3; conjunctivitis, nettle rash). No serious AEs occurred during the study. |
| [20] | Ukraine | Randomized, double-blind, placebo-controlled, multicenter trial with 410 outpatients (132 male and 272 female), 50 y or older with mild to moderate dementia (AD, vascular dementia, or mixed form) with scores between 9 and 23 on the SKT cognitive tests battery, at least 5 in the NPI and 3 or more in at least one item of the NPI. | Patients were allocated to receive 240 mg of EGb 761 or placebo once a day/24 w. Primary efficacy measures were the SKT, and the 12-item NPI. | EGb was found to be significantly superior to placebo in the treatment of patients with neuropsychiatric symptoms; significant improvement on the SKT and NPI total score (placebo showed deterioration on SKT). | AE rates were similar for both treatment groups (headache, respiratory tract infection, dizziness, angina pectoris, diarrhea, and tinnitus). |
| [21] | Republic of Belarus, Republic of Moldova, and Russian Federation | Multicenter, double-blind, randomized, placebo-controlled study with 410 outpatients, ≥50 y (279 female and 123 male) with mild to moderate dementia (AD or vascular dementia) related to neuropsychiatric symptoms. Participants scored 9–23 on the SKT cognitive battery and at least 6 on the NPI, with at least one of four items rated at minimum 4. | Patients were randomized to receive 240 mg of EGb 761 once a day/24 w. | Treatment with EGb 761 was safe and significantly improved functional measures, cognition, psychopathology, and quality of life of patients. | Lethal cardiac arrest due to chronic heart failure in a patient suffering from multiple illnesses; a lethal ischaemic infarction in the region of the terminal branches of the middle and posterior cerebral arteries in a patient with a history of DM, hypertension, atherosclerosis, myocardial infarction, and a previous stroke. |
| [22] | Iran | Randomized double-blind study with 56 patients (23 male and 28 female), 50–75 y, with a diagnosis of probable AD according to the DSM IV. | Patients were allocated into (1) GB (120 mg once a day) or (2) rivastigmine (4.5 mg once a day)/24 w. The SMT and the MMSE measured the severity of dementia. | There was a significant improvement of the MMSE scores in the rivastigmine group but not in the GB group. The same results were observed for the SMT. | AEs were not reported. |
| [23] | China | Randomized clinical trial with 80 patients with VCIND (60–75 y, 46 males and 34 females), disorder shown by revised mini-mental state examination. | Group 1 received 75 mg aspirin 3 times/d/3 m. Group 2 received 19.2 mg GBT 3 times/d/3 m with anti-platelet aggregation drugs. MoCA and TCD were used to observe changes in cognitive ability and cerebral blood flow in VCIND patients. | GBT can improve the therapeutic efficacy and enhance the cognitive ability and cerebral blood flow supply of patients with VCIND. | AEs were not reported. |
| [24] | EUA | Open-label phase II clinical trial with 34 patients (23 female and 11 male), symptomatic irradiated brain tumor survivors, life expectancy ≥ 30 w, partial or whole-brain radiation ≥ 6 m before enrollment, no imaging evidence of tumor progression in previous 3 m, or stable or decreasing steroid dose, and no brain tumor treatment planned while in the study. | The GB dose was 120 mg/day (40 mg t.i.d.) for 24 w, followed by a 6 w washout period. | There were significant improvements at 24 w in executive function, attention/concentration, and non-verbal memory and mood. | AEs included gastrointestinal toxicity and intracranial hemorrhage. |
| [25] | Russia | Double-blind randomized multicenter trial with 160 patients (124 female and 33 male, ≥55 y) with MCI who scored at least 6 on the 12-item NPI were enrolled. | Patients received 240 mg of EGb 761 daily or placebo/24 w. Effects on NPS were evaluated using the NPI, the state subscore of the State-Trait Anxiety Inventory and the Geriatric Depression Scale. | EGb 761 ameliorated NPS and cognitive performance in subjects with MCI. The drug was safe and well tolerated. | Headache, increased blood pressure, respiratory tract infection, and dyspepsia/epigastric discomfort. |
| [26] | China | Randomized clinical trial with 80 cerebral infarction patients (46 males and 34 females) 60–75 y. | Group 1 received aspirin 75 mg 3 times/d/3 m, and Group 2 received 40 m of GBT with aspirin 3 times/d/3 m. | GBT improved the therapeutic efficacy, cerebral blood flow supply, and cognitive ability of patients with VCIND. | AEs were not reported. |
| [27] | Germany | Randomized double-blind placebo-controlled trial with 75 volunteers (50–65 y) with subjective memory impairment evidenced by at least one answered item as “rather often” or “very often” or at least five questions answered “sometimes” in the Prospective and Retrospective Memory Questionnaire. | 240 mg EGb 761® or placebo once a day in the morning as film coated tablet/56 ± 4 days. | Baseline fMRI data evidenced BOLD responses in regions commonly activated by the specific tasks. Task-switch costs reduced with EGb761®, suggesting improvement in cognitive flexibility. Go–NoGo task reaction times corrected for error rates showed a trend of improved response inhibition. | Headache. |
| [28] | Germany | Randomized, double-blind, placebo-controlled exploratory study with 50 patients (25 female and 25 males; 50–85 y) with MCI and associated dual task-related gait impairment. | Patients received GBE (Symfona® forte 120 mg) 2 times/d/6 m or placebo capsules. A 6 m open-label phase with identical GBE dosage followed. Gait was quantified at months 0, 3, 6, and 12. | After 6 m, dual task-related cadence increased in the intervention group compared to the control. GBE-associated numerical non-significant trends were found after 6 m for dual task-related gait velocity and stride time variability. | Seven SAEs in four patients in GB group and six SAEs in five patients of control group: nasal septum surgery, diverticula, suspected coronary heart disease, pancreatitis, symptomatic cholecystolithiasis, and transient ischemic attack. |
| Gingko biloba and Diabetes Mellitus | |||||
| [29] | Lithuanian | Randomized double-blind placebo-controlled with 56 patients with T2DM (21 male and 35 females; 37–78 y) and followed up for diabetic retinopathy, nephropathy, or neuropathy. | Patients received standardized GB dry extract (80 mg) or placebo capsules. For the first 9 m, patients used one capsule 2 times/d, and for the second 9 m, one capsule 3 times/d. | The level of perceived stress was reduced significantly after 9 m and 18 m, and the psychological aspect of quality of life significantly improved after 18 m of GB use. | AEs were not reported. |
| [30] | Iraq | Randomized, placebo-controlled, double-blinded, multicenter trial with 60 T2DM patients, 25–65 y. | The patients currently using metformin were allocated to receive GB extract (120 mg/day) or placebo/3 m. | GBE significantly reduced HbA1c, glycemia and insulin levels, BMI, WC, and VAI. GB extract did not negatively impact the liver, kidneys, or hematopoietic functions. | No SAEs were observed. |
| Ginkgo biloba and Metabolic Syndrome | |||||
| [31] | Bulgaria | Randomized preventive study with 11 patients (two male, nine female, 26–48 y) with MS, smokers, and Lp (a) concentration > 30 mg/dL. | The standard therapy was EGB 761 120 mg 2 times/d/2 m. No statins, no calcium antagonists, and no nitrate compounds were given. | There was a decrease in oxidative stress biomarkers, atherosclerotic plaque formation, plaque stability and progression, and inflammation. | No AEs occurred. |
| [32] | Bulgaria | Randomized preventive study with 11 patients (two male, nine female, 26–48 y) with MS, smokers, and Lp (a) concentration > 30 mg/dL. | The standard therapy was EGB 761 120 mg 2 times/d/2 m. No statins, no calcium antagonists, and no nitrate compounds were given. | Simultaneous decreases in hs-CRP and HOMA-IR, as well as a beneficial change in arteriosclerotic, inflammatory, and oxidative stress biomarkers, were observed. IL-6 and nano-plaque formation were additionally reduced. | No AEs occurred. |
3. Results
4. Discussion
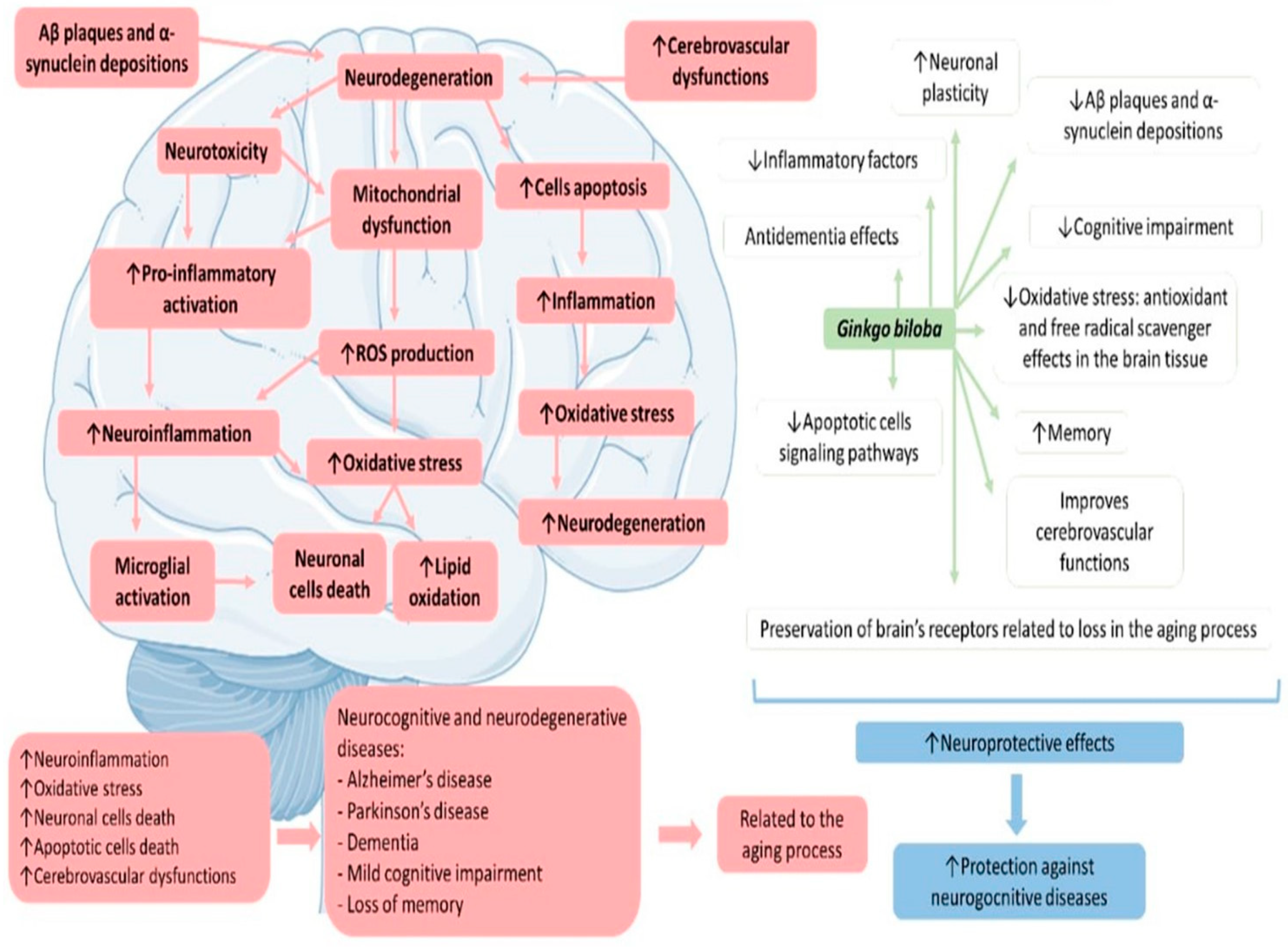
4.1. Ginkgo biloba, Inflammation, and Oxidative Stress
4.2. Gingko biloba, Mitochondrial Dysfunction, and Apoptosis
4.3. Ginkgo biloba and Neurodegenerative Diseases
4.3.1. Ginkgo biloba and Memory
4.3.2. Ginkgo biloba and Dementia
4.3.3. Ginkgo biloba and Mild Cognitive Impairment
4.3.4. Ginkgo biloba and Alzheimer’s Disease
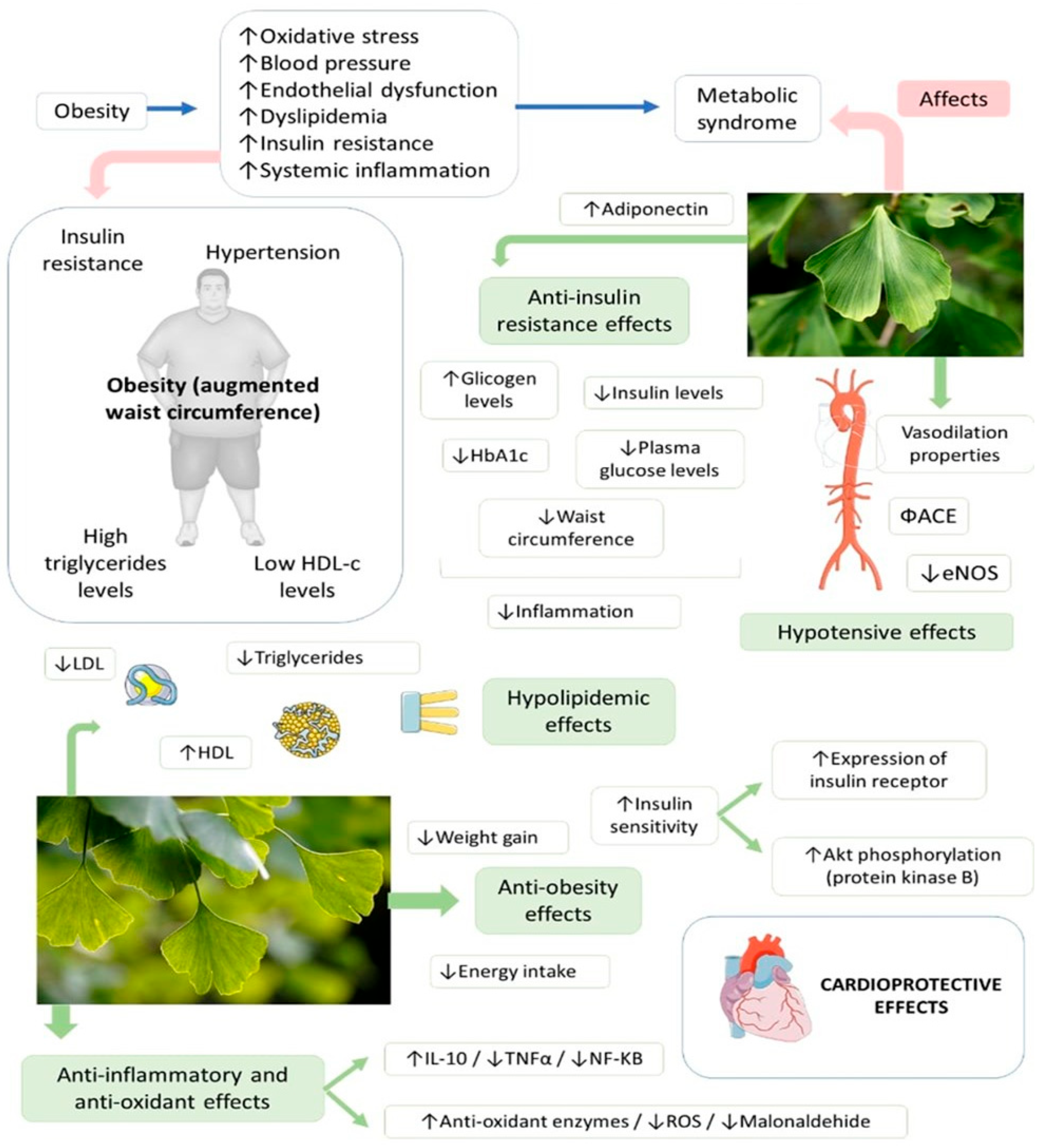
4.4. Ginkgo biloba, Metabolic Syndrome and Cardiovascular Diseases
4.5. Ginkgo biloba Bioavailability and Safety
4.6. Implication and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Zuo, X.; Ouyang, Z.; Qiao, P.; Wang, F. A Systematic Review of Antiaging Effects of 23 Traditional Chinese Medicines. Evid.-Based Complement. Altern. Med. 2021, 2021, 5591573. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Tofano, R.J.; Chagas, E.F.B.; Detregiachi, C.R.P.; de Alvares Goulart, R.; Flato, U.A.P.J.E.G. Benchside to the bedside of frailty and cardiovascular aging: Main shared cellular and molecular mechanisms. Exp. Gerontol. 2021, 148, 111302. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Fazelian, S.; Agah, S.; Khazdouz, M.; Rahimlou, M.; Agh, F.; Potter, E.; Heshmati, S.; Heshmati, J.J.C. Effect of ginger (Zingiber officinale) on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Cytokine 2020, 135, 155224. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in nfeuroprotective action of quercetin. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Soheili, M.; Karimian, M.; Hamidi, G.; Salami, M. Alzheimer’s disease treatment: The share of herbal medicines. Iran. J. Basic Med. Sci. 2021, 24, 123–135. [Google Scholar] [CrossRef]
- Banin, R.M.; Hirata, B.K.S.; Andrade, I.S.d.; Zemdegs, J.C.S.; Clemente, A.P.G.; Dornellas, A.P.S.; Boldarine, V.T.; Estadella, D.; Albuquerque, K.T.d.; Oyama, L.M.J.B.J.o.M.; et al. Beneficial effects of Ginkgo biloba extract on insulin signaling cascade, dyslipidemia, and body adiposity of diet-induced obese rats. Braz. J. Med. Biol. Res. 2014, 47, 780–788. [Google Scholar] [CrossRef]
- Eisvand, F.; Razavi, B.M.; Hosseinzadeh, H. The effects of Ginkgo biloba on metabolic syndrome: A review. Phytother. Res. PTR 2020, 34, 1798–1811. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract against AD and Other Neurological Disorders. Neurother. J. Am. Soc. Exp. NeuroTher. 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Tomino, C.; Ilari, S.; Solfrizzi, V.; Malafoglia, V.; Zilio, G.; Russo, P.; Proietti, S.; Marcolongo, F.; Scapagnini, G.; Muscoli, C.; et al. Mild Cognitive Impairment and Mild Dementia: The Role of Ginkgo biloba (EGb 761®). Pharmaceuticals 2021, 14, 305. [Google Scholar] [CrossRef]
- Hirata, B.K.; Pedroso, A.P.; Machado, M.M.; Neto, N.I.; Perestrelo, B.O.; de Sá, R.D.; Alonso-Vale, M.I.C.; Nogueira, F.N.; Oyama, L.M.; Ribeiro, E.B. Ginkgo biloba extract modulates the retroperitoneal fat depot proteome and reduces oxidative stress in diet-induced obese rats. Front. Pharmacol. 2019, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Achete de Souza, G.; de Marqui, S.V.; Matias, J.N.; Guiguer, E.L.; Barbalho, S.M. Effects of Ginkgo biloba on Diseases Related to Oxidative Stress. Planta Med. 2020, 86, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Unger, M. Pharmacokinetic drug interactions involving Ginkgo biloba. Drug Metab. Rev. 2013, 45, 353–385. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Schlaefke, S. Efficacy and tolerability of Ginkgo biloba extract EGb 761® in dementia: A systematic review and meta-analysis of randomized placebo-controlled trials. Clin. Interv. Aging 2014, 9, 2065. [Google Scholar] [CrossRef]
- Meng, M.; Ai, D.; Sun, L.; Xu, X.; Cao, X. EGb 761 inhibits Aβ1–42-induced neuroinflammatory response by suppressing P38 MAPK signaling pathway in BV-2 microglial cells. Neuroreport 2019, 30, 434–440. [Google Scholar] [CrossRef]
- Abdul-Latif, R.; Stupans, I.; Allahham, A.; Adhikari, B.; Thrimawithana, T. Natural antioxidants in the management of Parkinson’s disease: Review of evidence from cell line and animal models. J. Integr. Med. 2021, 19, 300–310. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Kaschel, R. Specific memory effects of Ginkgo biloba extract EGb 761 in middle-aged healthy volunteers. Phytomed. Int. J. Phytother. Phytopharm. 2011, 18, 1202–1207. [Google Scholar] [CrossRef]
- Ihl, R.; Bachinskaya, N.; Korczyn, A.D.; Vakhapova, V.; Tribanek, M.; Hoerr, R.; Napryeyenko, O. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2011, 26, 1186–1194. [Google Scholar] [CrossRef]
- Herrschaft, H.; Nacu, A.; Likhachev, S.; Sholomov, I.; Hoerr, R.; Schlaefke, S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J. Psychiatr. Res. 2012, 46, 716–723. [Google Scholar] [CrossRef]
- Nasab, N.M.; Bahrammi, M.A.; Nikpour, M.R.; Rahim, F.; Naghibis, S.N. Efficacy of rivastigmine in comparison to ginkgo for treating Alzheimer’s dementia. J. Pak. Med. Assoc. 2012, 62, 677–680. [Google Scholar] [PubMed]
- Zhang, S.J.; Xue, Z.Y. Effect of Western medicine therapy assisted by Ginkgo biloba tablet on vascular cognitive impairment of none dementia. Asian Pac. J. Trop. Med. 2012, 5, 661–664. [Google Scholar] [CrossRef]
- Attia, A.; Rapp, S.R.; Case, L.D.; D’Agostino, R.; Lesser, G.; Naughton, M.; McMullen, K.; Rosdhal, R.; Shaw, E.G. Phase II study of Ginkgo biloba in irradiated brain tumor patients: Effect on cognitive function, quality of life, and mood. J. Neuro-Oncol. 2012, 109, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, S.I.; Preuss, U.W.; Wong, J.W.; Hoerr, R.; Kaschel, R.; Bachinskaya, N. Efficacy and safety of Ginkgo biloba extract EGb 761 in mild cognitive impairment with neuropsychiatric symptoms: A randomized, placebo-controlled, double-blind, multi-center trial. Int. J. Geriatr. Psychiatry 2014, 29, 1087–1095. [Google Scholar] [CrossRef]
- Wang, L.P.; Zhang, X.Y.; Liu, N.; Ma, Z.Z.; Fang, D.S. Comparison of integrated traditional Chinese and western medicine therapy on vascular cognitive impairment with no dementia. Genet. Mol. Res. GMR 2015, 14, 4896–4902. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.M.; Ruge, H.; Schindler, C.; Burkart, M.; Miller, R.; Kirschbaum, C.; Goschke, T. Effects of Ginkgo biloba extract EGb 761® on cognitive control functions, mental activity of the prefrontal cortex and stress reactivity in elderly adults with subjective memory impairment—A randomized double-blind placebo-controlled trial. Hum. Psychopharmacol. 2016, 31, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Gschwind, Y.J.; Bridenbaugh, S.A.; Reinhard, S.; Granacher, U.; Monsch, A.U.; Kressig, R.W. Ginkgo biloba special extract LI 1370 improves dual-task walking in patients with MCI: A randomised, double-blind, placebo-controlled exploratory study. Aging Clin. Exp. Res. 2017, 29, 609–619. [Google Scholar] [CrossRef]
- Lasaite, L.; Spadiene, A.; Savickiene, N.; Skesters, A.; Silova, A. The effect of Ginkgo biloba and Camellia sinensis extracts on psychological state and glycemic control in patients with type 2 diabetes mellitus. Nat. Prod. Commun. 2014, 9, 1345–1350. [Google Scholar] [CrossRef]
- Aziz, T.A.; Hussain, S.A.; Mahwi, T.O.; Ahmed, Z.A.; Rahman, H.S.; Rasedee, A. The efficacy and safety of Ginkgo biloba extract as an adjuvant in type 2 diabetes mellitus patients ineffectively managed with metformin: A double-blind, randomized, placebo-controlled trial. Drug Des. Dev. Ther. 2018, 12, 735–742. [Google Scholar] [CrossRef]
- Siegel, G.; Ermilov, E. Hs-CRP may be associated with white blood cell count in metabolic syndrome patients treated with Ginkgo biloba. Atherosclerosis 2011, 218, 250–252. [Google Scholar] [CrossRef]
- Siegel, G.; Ermilov, E.; Knes, O.; Rodríguez, M. Combined lowering of low grade systemic inflammation and insulin resistance in metabolic syndrome patients treated with Ginkgo biloba. Atherosclerosis 2014, 237, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.R.; Almeida, P.P.; de Oliveira Santos, L.; Rodrigues, L.P.; de Carvalho, J.L.; Boroni, M. Hallmarks of Aging in Macrophages: Consequences to Skin Inflammaging. Cells 2021, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Hammock, B.D.; Hwang, I.K.; Li, Q.; Moustaid-Moussa, N.; Park, Y.; Safe, S.; Suh, N.; Yi, S.S.; Zeldin, D.C.; et al. Natural Products in the Prevention of Metabolic Diseases: Lessons Learned from the 20th KAST Frontier Scientists Workshop. Nutrients 2021, 13, 1881. [Google Scholar] [CrossRef] [PubMed]
- Edler, M.K.; Mhatre-Winters, I.; Richardson, J.R. Microglia in Aging and Alzheimer’s Disease: A Comparative Species Review. Cells 2021, 10, 1138. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, H.; Wang, J. Metabolism and Chronic Inflammation: The Links between Chronic Heart Failure and Comorbidities. Front. Cardiovasc. Med. 2021, 8, 650278. [Google Scholar] [CrossRef] [PubMed]
- Rabilloud, T.; Heller, M.; Rigobello, M.P.; Bindoli, A.; Aebersold, R.; Lunardi, J. The mitochondrial antioxidant defence system and its response to oxidative stress. Proteomics 2001, 1, 1105–1110. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Strømgaard, K.; Nakanishi, K. Chemistry and biology of terpene trilactones from Ginkgo biloba. Angew. Chem. (Int. Ed.) 2004, 43, 1640–1658. [Google Scholar] [CrossRef]
- Tao, Z.; Jin, W.; Ao, M.; Zhai, S.; Xu, H.; Yu, L. Evaluation of the anti-inflammatory properties of the active constituents in Ginkgo biloba for the treatment of pulmonary diseases. Food Funct. 2019, 10, 2209–2220. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Y.; Liu, Y.; Chen, K.; Lyu, S. Ginkgo biloba leaf extract protects against myocardial injury via attenuation of endoplasmic reticulum stress in streptozotocin-induced diabetic ApoE−/− mice. Oxid. Med. Cell. Longev. 2018, 2018, 2370617. [Google Scholar] [CrossRef]
- Sarkar, C.; Quispe, C.; Jamaddar, S.; Hossain, R.; Ray, P.; Mondal, M.; Abdulwanis Mohamed, Z.; Sani Jaafaru, M.; Salehi, B.; Islam, M.T.; et al. Therapeutic promises of ginkgolide A: A literature-based review. Biomed. Pharmacother. 2020, 132, 110908. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Giri, L.; Bahukhandi, A.; Tariq, M.; Kewlani, P.; Bhatt, I.D.; Rawal, R.S. Chapter 3.19—Ginkgo biloba. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 241–250. [Google Scholar]
- Kuribara, H.; Weintraub, S.T.; Yoshihama, T.; Maruyama, Y. An Anxiolytic-Like Effect of Ginkgo biloba Extract and Its Constituent, Ginkgolide-A, in Mice. J. Nat. Prod. 2003, 66, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-H.; Ge, J.-B.; Li, M.; Wu, F.; Zhang, W.; Qin, Z.-H. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur. J. Pharm. Sci. 2012, 47, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wang, C.; Li, J.; Hou, Q.; Li, J.; Ma, J.; Wang, W.; Wang, Z. Ginkgolide B protects hippocampal neurons from apoptosis induced by beta-amyloid 25–35 partly via up-regulation of brain-derived neurotrophic factor. Eur. J. Pharmacol. 2010, 647, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, L.; Zhang, D.; Hu, B.; Luo, Q.; Han, D.; Li, J.; Shen, C. Cardioprotection of Ginkgolide B on Myocardial Ischemia/Reperfusion-Induced Inflammatory Injury via Regulation of A20-NF-κB Pathway. Front. Immunol. 2018, 9, 2844. [Google Scholar] [CrossRef]
- Liu, J.; Wu, P.; Xu, Z.; Zhang, J.; Liu, J.; Yang, Z. Ginkgolide B inhibits hydrogen peroxide-induced apoptosis and attenuates cytotoxicity via activating the PI3K/Akt/mTOR signaling pathway in H9c2 cells. Mol. Med. Rep. 2020, 22, 310–316. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Q.-H.; Zhou, H.; Wu, J.-L.; Quan, W.-Q.; Ji, P.; Yao, Y.-W.; Li, D.; Sun, Z.-J. Ginkgolide B inhibits lung cancer cells promotion via beclin-1-dependent autophagy. BMC Complement. Med. Ther. 2020, 20, 194. [Google Scholar] [CrossRef]
- Zhi, Y.; Pan, J.; Shen, W.; He, P.; Zheng, J.; Zhou, X.; Lu, G.; Chen, Z.; Zhou, Z. Ginkgolide B Inhibits Human Bladder Cancer Cell Migration and Invasion through MicroRNA-223-3p. Cell. Physiol. Biochem. 2016, 39, 1787–1794. [Google Scholar] [CrossRef]
- Chan, W.-H. The Signaling Cascades of Ginkgolide B-Induced Apoptosis in MCF-7 Breast Cancer Cells. Int. J. Mol. Sci. 2007, 8, 1177–1195. [Google Scholar] [CrossRef]
- Liou, C.-J.; Lai, X.-Y.; Chen, Y.-L.; Wang, C.-L.; Wei, C.-H.; Huang, W.-C. Ginkgolide C Suppresses Adipogenesis in 3T3-L1 Adipocytes via the AMPK Signaling Pathway. Evid.-Based Complement. Altern. Med. 2015, 2015, 298635. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chen, Y.-L.; Liu, H.-C.; Wu, S.-J.; Liou, C.-J. Ginkgolide C reduced oleic acid-induced lipid accumulation in HepG2 cells. Saudi Pharm. J. 2018, 26, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, D.; Li, Z.; Shen, C.; Zhang, Y.; Li, J.; Yan, G.; Li, S.; Hu, B.; Li, J.; et al. Ginkgolide C Alleviates Myocardial Ischemia/Reperfusion-Induced Inflammatory Injury via Inhibition of CD40-NF-κB Pathway. Front. Pharmacol. 2018, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Ha, I.J.; Lee, S.-G.; Lee, J.; Um, J.-Y.; Ahn, K.S. Ginkgolide C promotes apoptosis and abrogates metastasis of colorectal carcinoma cells by targeting Wnt/β-catenin signaling pathway. IUBMB Life 2021, 73, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Baek, S.H.; Um, J.-Y.; Ahn, K.S. Anti-neoplastic Effect of Ginkgolide C through Modulating c-Met Phosphorylation in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 8303. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Yang, F.; Zhu, W.; Cai, M.; Li, X.T.; Zhang, J.S.; Yu, Z.H.; Zhang, W.; Cai, D.F. Bilobalide inhibits inflammation and promotes the expression of Aβ degrading enzymes in astrocytes to rescue neuronal deficiency in AD models. Transl. Psychiatry 2021, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Qin, J.; Shen, W.; Wu, A. Bilobalide Enhances AMPK Activity to Improve Liver Injury and Metabolic Disorders in STZ-Induced Diabetes in Immature Rats via Regulating HMGB1/TLR4/NF-κB Signaling Pathway. BioMed Res. Int. 2021, 2021, 8835408. [Google Scholar] [CrossRef]
- Maerz, S.; Liu, C.-H.; Guo, W.; Zhu, Y.-Z. Anti-ischaemic effects of bilobalide on neonatal rat cardiomyocytes and the involvement of the platelet-activating factor receptor. Biosci. Rep. 2011, 31, 439–447. [Google Scholar] [CrossRef][Green Version]
- Hua, Z.; Wu, C.; Fan, G.; Tang, Z.; Cao, F. The antibacterial activity and mechanism of ginkgolic acid C15:1. BMC Biotechnol. 2017, 17, 5. [Google Scholar] [CrossRef]
- Borenstein, R.; Hanson, B.A.; Markosyan, R.M.; Gallo, E.S.; Narasipura, S.D.; Bhutta, M.; Shechter, O.; Lurain, N.S.; Cohen, F.S.; Al-Harthi, L.; et al. Ginkgolic acid inhibits fusion of enveloped viruses. Sci. Rep. 2020, 10, 4746. [Google Scholar] [CrossRef]
- Baek, S.H.; Ko, J.-H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.-G.; Yang, W.M.; Um, J.-Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-β-Induced EMT of Lung Cancer Cells through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef]
- Ku, S.-K.; Kim, T.H.; Bae, J.-S. Anticoagulant activities of persicarin and isorhamnetin. Vasc. Pharmacol. 2013, 58, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, S.; Lopez, S.; Varela, L.M.; Rodriguez-Arcos, R.; Jimenez, A.; Abia, R.; Guillen, R.; Muriana, F.J.G. The Flavonol Isorhamnetin Exhibits Cytotoxic Effects on Human Colon Cancer Cells. J. Agric. Food Chem. 2010, 58, 10869–10875. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.-s.; Lu, Y.-H.; Wang, Z.-T.; Tao, X.-Y.; Wei, D.-Z. In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharmacol. Res. 2006, 54, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Shin, B.Y.; Han, J.Y.; Kim, M.G.; Wi, J.E.; Kim, Y.W.; Cho, I.J.; Kim, S.C.; Shin, S.M.; Ki, S.H. Isorhamnetin protects against oxidative stress by activating Nrf2 and inducing the expression of its target genes. Toxicol. Appl. Pharmacol. 2014, 274, 293–301. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Milanezi, F.G.; Meireles, L.M.; de Christo Scherer, M.M.; de Oliveira, J.P.; da Silva, A.R.; de Araujo, M.L.; Endringer, D.C.; Fronza, M.; Guimarães, M.C.C.; Scherer, R. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with quercetin. Saudi Pharm. J. 2019, 27, 968–974. [Google Scholar] [CrossRef]
- Ferenczyova, K.; Kalocayova, B.; Bartekova, M. Potential Implications of Quercetin and its Derivatives in Cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, 2000746. [Google Scholar] [CrossRef]
- Boots, A.W.; Drent, M.; de Boer, V.C.J.; Bast, A.; Haenen, G.R.M.M. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011, 30, 506–512. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, A.Y.; Li, M.; Chen, C.; Yao, Q. Ginkgo biloba Extract Kaempferol Inhibits Cell Proliferation and Induces Apoptosis in Pancreatic Cancer Cells. J. Surg. Res. 2008, 148, 17–23. [Google Scholar] [CrossRef] [PubMed]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, X.; Zhao, H.; Wu, B.; Liu, G. Antioxidant, anti-inflammatory and neuroprotective effect of kaempferol on rotenone-induced Parkinson’s disease model of rats and SH-S5Y5 cells by preventing loss of tyrosine hydroxylase. J. Funct. Foods 2020, 74, 104140. [Google Scholar] [CrossRef]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Ojha, S.; Arya, D.S. Molecular Pathways Involved in the Amelioration of Myocardial Injury in Diabetic Rats by Kaempferol. Int. J. Mol. Sci. 2017, 18, 1001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ren, H.; Han, J.; Wang, W.; Zheng, Q.; Wang, D. Protective Effects of Kaempferol against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart via Antioxidant Activity and Inhibition of Glycogen Synthase Kinase-3. Oxid. Med. Cell. Longev. 2015, 2015, 481405. [Google Scholar] [CrossRef]
- Shukla, R.; Pandey, V.; Vadnere, G.P.; Lodhi, S. Chapter 18—Role of Flavonoids in Management of Inflammatory Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 293–322. [Google Scholar]
- Sathya, S.; Pandima Devi, K. Chapter 15—The Use of Polyphenols for the Treatment of Alzheimer’s Disease. In Role of the Mediterranean Diet in the Brain and Neurodegenerative Diseases; Farooqui, T., Farooqui, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 239–252. [Google Scholar]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid that Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef]
- Trebaticka, J.; Ďuračková, Z. Psychiatric disorders and polyphenols: Can they be helpful in therapy? Oxid. Med. Cell. Longev. 2015, 2015, 248529. [Google Scholar] [CrossRef]
- Choudhary, S.; Kumar, P.; Malik, J.J.P.R. Plants and phytochemicals for Huntington’s disease. Pharmacogn. Rev. 2013, 7, 81. [Google Scholar]
- Saini, A.S.; Taliyan, R.; Sharma, P.L. Protective effect and mechanism of Ginkgo biloba extract-EGb 761 on STZ-induced diabetic cardiomyopathy in rats. Pharmacogn. Mag. 2014, 10, 172. [Google Scholar] [CrossRef]
- Wang, L.; Bai, Y.; Wang, B.; Cui, H.; Wu, H.; Lv, J.-R.; Mei, Y.; Zhang, J.-S.; Liu, S.; Qi, L.-W. Suppression of experimental abdominal aortic aneurysms in the mice by treatment with Ginkgo biloba extract (EGb 761). J. Ethnopharmacol. 2013, 150, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yang, Q.; Li, Q.; Wang, X.; Hao, S.; Wang, J.; Ren, M. Ginkgo biloba L. extract reduces H2O2-induced bone marrow mesenchymal stem cells cytotoxicity by regulating mitogen-activated protein kinase (MAPK) signaling pathways and oxidative stress. Med. Sci. Monit. 2018, 24, 3159. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, B. Ginkgo biloba extract attenuates oxidative stress and apoptosis in mouse cochlear neural stem cells. Phytotherapy Res. 2016, 30, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sharma, N.; Nehru, B. Anti-inflammatory effects of Ginkgo biloba extract against trimethyltin-induced hippocampal neuronal injury. Inflammopharmacology 2018, 26, 87–104. [Google Scholar] [CrossRef]
- Zuo, W.; Yan, F.; Zhang, B.; Li, J.; Mei, D. Advances in the Studies of Ginkgo biloba Leaves Extract on Aging-Related Diseases. Aging Dis. 2017, 8, 812–826. [Google Scholar] [CrossRef]
- Rhein, V.; Giese, M.; Baysang, G.; Meier, F.; Rao, S.; Schulz, K.L.; Hamburger, M.; Eckert, A. Ginkgo biloba extract ameliorates oxidative phosphorylation performance and rescues Aβ-induced failure. PLoS ONE 2010, 5, e12359. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Scherping, I.; Hauptmann, S.; Müller, W.E. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann. N. Y. Acad. Sci. 2005, 1056, 474–485. [Google Scholar] [CrossRef]
- Shi, C.; Xiao, S.; Liu, J.; Guo, K.; Wu, F.; Yew, D.T.; Xu, J. Ginkgo biloba extract EGb761 protects against aging-associated mitochondrial dysfunction in platelets and hippocampi of SAMP8 mice. Platelets 2010, 21, 373–379. [Google Scholar] [CrossRef]
- Schindowski, K.; Leutner, S.; Kressmann, S.; Eckert, A.; Müller, W.E. Age-related increase of oxidative stress-induced apoptosis in micePrevention by Ginkgo biloba extract (EGb761). J. Neural Transm. 2001, 108, 969–978. [Google Scholar] [CrossRef]
- Longpré, F.; Garneau, P.; Ramassamy, C. Protection by EGb 761 against β-amyloid-induced neurotoxicity: Involvement of NF-κB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic. Biol. Med. 2006, 41, 1781–1794. [Google Scholar] [CrossRef]
- You, O.H.; Kim, S.-H.; Kim, B.; Sohn, E.J.; Lee, H.-J.; Shim, B.-S.; Yun, M.; Kwon, B.-M.; Kim, S.-H. Ginkgetin induces apoptosis via activation of caspase and inhibition of survival genes in PC-3 prostate cancer cells. Bioorg. Med. Chem. Lett. 2013, 23, 2692–2695. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Bai, W.; Wang, Z.; Xiao, W.; Zhu, J. Study on Mechanism of Ginkgo biloba L. Leaves for the Treatment of Neurodegenerative Diseases Based on Network Pharmacology. Neurochem. Res. 2021, 46, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Suliman, N.A.; Mat Taib, C.N.; Mohd Moklas, M.A.; Adenan, M.I.; Hidayat Baharuldin, M.T.; Basir, R. Establishing Natural Nootropics: Recent Molecular Enhancement Influenced by Natural Nootropic. Evid.-Based Complement. Altern. Med. Ecam 2016, 2016, 4391375. [Google Scholar] [CrossRef]
- Rendeiro, C.; Guerreiro, J.D.; Williams, C.M.; Spencer, J.P. Flavonoids as modulators of memory and learning: Molecular interactions resulting in behavioural effects. Proc. Nutr. Soc. 2012, 71, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.R. Memory dysfunction. Continuum 2015, 21, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.; Radhakrishnan, R. Dementia. BMJ Clin. Evid. 2012, 2012, 1001. [Google Scholar]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef]
- Weinmann, S.; Roll, S.; Schwarzbach, C.; Vauth, C.; Willich, S.N. Effects of Ginkgo biloba in dementia: Systematic review and meta-analysis. BMC Geriatr. 2010, 10, 14. [Google Scholar] [CrossRef]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra, E.O.T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef]
- Lopez, O.L.; Chang, Y.; Ives, D.G.; Snitz, B.E.; Fitzpatrick, A.L.; Carlson, M.C.; Rapp, S.R.; Williamson, J.D.; Tracy, R.P.; DeKosky, S.T.; et al. Blood amyloid levels and risk of dementia in the Ginkgo Evaluation of Memory Study (GEMS): A longitudinal analysis. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2019, 15, 1029–1038. [Google Scholar] [CrossRef]
- Li, F.; Harmer, P.; Voit, J.; Chou, L.S. Implementing an Online Virtual Falls Prevention Intervention during a Public Health Pandemic for Older Adults with Mild Cognitive Impairment: A Feasibility Trial. Clin. Interv. Aging 2021, 16, 973–983. [Google Scholar] [CrossRef] [PubMed]
- do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Paterson, A.; Roodenrys, S.; Thomas, S.; Bliokas, V.; Potter, J.; Walton, K.; Weston-Green, K.; et al. Food anthocyanins decrease concentrations of TNF-α in older adults with mild cognitive impairment: A randomized, controlled, double blind clinical trial. Nutr. Metab. Cardiovasc. Dis. NMCD 2021, 31, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, N.; Ong, P.A.; Yuda, T.; Ng, L.L.; Mamun, K.; Merchant, R.A.; Chen, C.; Dominguez, J.; Marasigan, S.; Ampil, J.; et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: Expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci. Ther. 2019, 25, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Huang, L.-B.; Zhong, Y.-B.; Zhou, Q.-H.; Wang, H.-L.; Zheng, G.-Q.; Lin, Y. An overview of systematic reviews of Ginkgo biloba extracts for mild cognitive impairment and dementia. Front. Aging Neurosci. 2016, 8, 276. [Google Scholar] [CrossRef]
- Dong, Z.H.; Zhang, C.Y.; Pu, B.H. Effects of Ginkgo biloba tablet in treating mild cognitive impairment. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi Chin. J. Integr. Tradit. West. Med. 2012, 32, 1208–1211. [Google Scholar]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- Liu, J.; Hlávka, J.; Hillestad, R.J.; Mattke, S. Assessing the Preparedness of the US Health Care System Infrastructure for an Alzheimer’s Treatment; RAND: Santa Monica, CA, USA, 2017. [Google Scholar]
- Kim, E.; Otgontenger, U.; Jamsranjav, A.; Kim, S.S. Deleterious Alteration of Glia in the Brain of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6676. [Google Scholar] [CrossRef]
- Zuin, M.; Cervellati, C.; Trentini, A.; Passaro, A.; Rosta, V.; Zimetti, F.; Zuliani, G. Association between Serum Concentrations of Apolipoprotein A-I (ApoA-I) and Alzheimer’s Disease: Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 984. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- D’Mello, S.R. When Good Kinases Go Rogue: GSK3, p38 MAPK and CDKs as Therapeutic Targets for Alzheimer’s and Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 5911. [Google Scholar] [CrossRef]
- Gallardo, G.; Holtzman, D.M. Amyloid-β and Tau at the Crossroads of Alzheimer’s Disease. Tau Biol. 2019, 1184, 187–203. [Google Scholar]
- De Strooper, B.; Karran, E. The cellular phase of Alzheimer’s disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.G.; Lana, D.; Traini, C.; Vannucchi, M.G. The Microbiota-Gut-Brain Axis and Alzheimer Disease. From Dysbiosis to Neurodegeneration: Focus on the Central Nervous System Glial Cells. J. Clin. Med. 2021, 10, 2358. [Google Scholar] [CrossRef]
- Onyango, I.G.; Jauregui, G.V.; Čarná, M.; Bennett, J.P., Jr.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Arslan, J.; Jamshed, H.; Qureshi, H. Early Detection and Prevention of Alzheimer’s Disease: Role of Oxidative Markers and Natural Antioxidants. Front. Aging Neurosci. 2020, 12, 231. [Google Scholar] [CrossRef]
- Smith, J.; Luo, Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004, 64, 465–472. [Google Scholar]
- Yao, Z.-x.; Drieu, K.; Papadopoulos, V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from β-amyloid-induced cell death by inhibiting the formation of β-amyloid-derived diffusible neurotoxic ligands. Brain Res. 2001, 889, 181–190. [Google Scholar] [CrossRef]
- Gong, Q.-H.; Wu, Q.; Huang, X.-N.; Sun, A.-S.; Nie, J.; Shi, J.-S. Protective effect of Ginkgo biloba leaf extract on learning and memory deficit induced by aluminum in model rats. Chin. J. Integr. Med. 2006, 12, 37–41. [Google Scholar]
- Liao, Z.; Cheng, L.; Li, X.; Zhang, M.; Wang, S.; Huo, R. Meta-analysis of Ginkgo biloba Preparation for the Treatment of Alzheimer’s Disease. Clin. Neuropharmacol. 2020, 43, 93–99. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, L.; Zhu, B.; Li, Q.; Yew, D.T.; Yao, Z.; Xu, J. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against β-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem. Interact. 2009, 181, 115–123. [Google Scholar] [CrossRef]
- Yao, Z.-X.; Han, Z.; Drieu, K.; Papadopoulos, V. Ginkgo biloba extract (Egb 761) inhibits β-amyloid production by lowering free cholesterol levels. J. Nutr. Biochem. 2004, 15, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Moltedo, O.; Damato, A.; Martinello, K.; Di Pietro, P.; Oliveti, M.; Acernese, F.; Giugliano, G.; Izzo, R.; Sommella, E.; et al. New Nutraceutical Combination Reduces Blood Pressure and Improves Exercise Capacity in Hypertensive Patients via a Nitric Oxide-Dependent Mechanism. J. Am. Heart Assoc. 2020, 9, e014923. [Google Scholar] [CrossRef] [PubMed]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Tofano, R.J.; Pescinni-Salzedas, L.M.; Chagas, E.F.B.; Detregiachi, C.R.P.; Guiguer, E.L.; Araujo, A.C.; Bechara, M.D.; Rubira, C.J.; Barbalho, S.M. Association of Metabolic Syndrome and Hyperferritinemia in Patients at Cardiovascular Risk. Diabetes Metab. Syndr. Obesity Targets Ther. 2020, 13, 3239. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Effect of eggplant (Solanum melongena) on the metabolic syndrome: A review. Iran. J. Basic Med. Sci. 2021, 24, 420–427. [Google Scholar] [CrossRef]
- Priyanka, A.; Sindhu, G.; Shyni, G.; Rani, M.P.; Nisha, V.; Raghu, K. Bilobalide abates inflammation, insulin resistance and secretion of angiogenic factors induced by hypoxia in 3T3-L1 adipocytes by controlling NF-κB and JNK activation. Int. Immunopharmacol. 2017, 42, 209–217. [Google Scholar] [CrossRef]
- An, X.F.; Zhao, Y.; Yu, J.Y. Treatment of Early Diabetic Retinopathy by Liuwei Dihuang Pill Combined Ginkao Leaf Tablet. Chin. J. Integr. Tradit. West. Med. 2016, 36, 674–677. [Google Scholar]
- Tanaka, S.; Han, L.-K.; Zheng, Y.-N.; Okuda, H. Effects of the flavonoid fraction from Ginkgo biloba extract on the postprandial blood glucose elevation in rats. Yakugaku Zasshi 2004, 124, 605–611. [Google Scholar] [CrossRef]
- Hussein, A.A.; Assad, H.C.; Rabeea, I.S. Research. Antihyperlipidemic, Antioxidant and Anti-Inflammatory Effects of Ginkgo biloba in High Cholesterol Fed Rabbits. J. Pharm. Sci. Res. 2017, 9, 2163–2167. [Google Scholar]
- Hirata, B.K.S.; Banin, R.M.; Dornellas, A.P.S.; de Andrade, I.S.; Zemdegs, J.C.S.; Caperuto, L.C.; Oyama, L.M.; Ribeiro, E.B.; Telles, M.M. Ginkgo biloba extract improves insulin signaling and attenuates inflammation in retroperitoneal adipose tissue depot of obese rats. Mediat. Inflamm. 2015, 2015, 419106. [Google Scholar] [CrossRef]
- Shinozuka, K.; Umegaki, K.; Kubota, Y.; Tanaka, N.; Mizuno, H.; Yamauchi, J.; Nakamura, K.; Kunitomo, M. Feeding of Ginkgo biloba extract (GBE) enhances gene expression of hepatic cytochrome P-450 and attenuates the hypotensive effect of nicardipine in rats. Life Sci. 2002, 70, 2783–2792. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Wang, H.; Liu, X.; Jiang, L.; Liang, S.; Wu, Z.; Wang, Z.; Zhou, J.; Xiao, W.; et al. Systems pharmacology reveals the multi-level synergetic mechanism of action of Ginkgo biloba L. leaves for cardiomyopathy treatment. J. Ethnopharmacol. 2021, 264, 113279. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wu, J.; Duan, X.; Cui, Y.; Liu, S.; Jing, Z. Efficacy and safety of ginkgo injections in the treatment of angina pectoris caused by coronary heart disease in China: A network Meta-analysis and systematic review. J. Tradit. Chin. Med. Chung I Tsa Chih Ying Wen Pan 2019, 39, 285–296. [Google Scholar] [PubMed]
- Li, X.; Lu, L.; Chen, J.; Zhang, C.; Chen, H.; Huang, H. New Insight into the Mechanisms of Ginkgo biloba Extract in Vascular Aging Prevention. Curr. Vasc. Pharmacol. 2020, 18, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weng, W.; Gao, R.; Liu, Y. New Insights for Cellular and Molecular Mechanisms of Aging and Aging-Related Diseases: Herbal Medicine as Potential Therapeutic Approach. Oxid. Med. Cell. Longev. 2019, 2019, 4598167. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.-P.; Cao, Z.-Y.; Zhang, X.-Z.; Cao, L.; Wang, Z.-Z.; Xiao, W. Effect of compatibility of ginkgolide A, ginkgolide B and ginkgolide K. J. Pharm. Biomed. Anal. 2018, 43, 1410–1415. [Google Scholar]
- Lu, X.; Chen, L.; Liu, T.; Ke, H.; Gong, X.; Wang, Q.; Zhang, J.; Fan, X. Chemical analysis, pharmacological activity and process optimization of the proportion of bilobalide and ginkgolides in Ginkgo biloba extract. J. Pharm. Biomed. Anal. 2018, 160, 46–54. [Google Scholar]
- Mei, N.; Guo, X.; Ren, Z.; Kobayashi, D.; Wada, K.; Guo, L. Review of Ginkgo biloba-induced toxicity, from experimental studies to human case reports. J. Environ. Sci. Health Part C 2017, 35, 1–28. [Google Scholar] [CrossRef]
- Mahady, G.B. Ginkgo biloba: A review of quality, safety, and efficacy. Nutr. Clin. Care 2001, 4, 140–147. [Google Scholar] [CrossRef]
- McKenna, D.J.; Jones, K.; Hughes, K. Efficacy, safety, and use of Ginkgo biloba in clinical and preclinical applications. Altern. Ther. Health Med. 2001, 7, 70. [Google Scholar]
| Study | Question Focus | Appropriate Randomization | Allocation Blinding | Double-Blind | Losses (<20%) | Prognostics or Demographic Characteristics | Outcomes | Intention to Treat Analysis | Sample Calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| [19] | Yes | Yes | Yes | Yes | Yes | No | Yes | NR | Yes | Yes |
| [20] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [22] | Yes | NR | Yes | Yes | Yes | Yes | Yes | No | NR | Yes |
| [23] | Yes | NR | NR | No | NR | No | Yes | No | NR | Yes |
| [24] | Yes | NR | No | No | No | Yes | Yes | No | Yes | Yes |
| [25] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| [26] | Yes | NR | NR | No | NR | No | Yes | No | NR | Yes |
| [27] | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes |
| [28] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
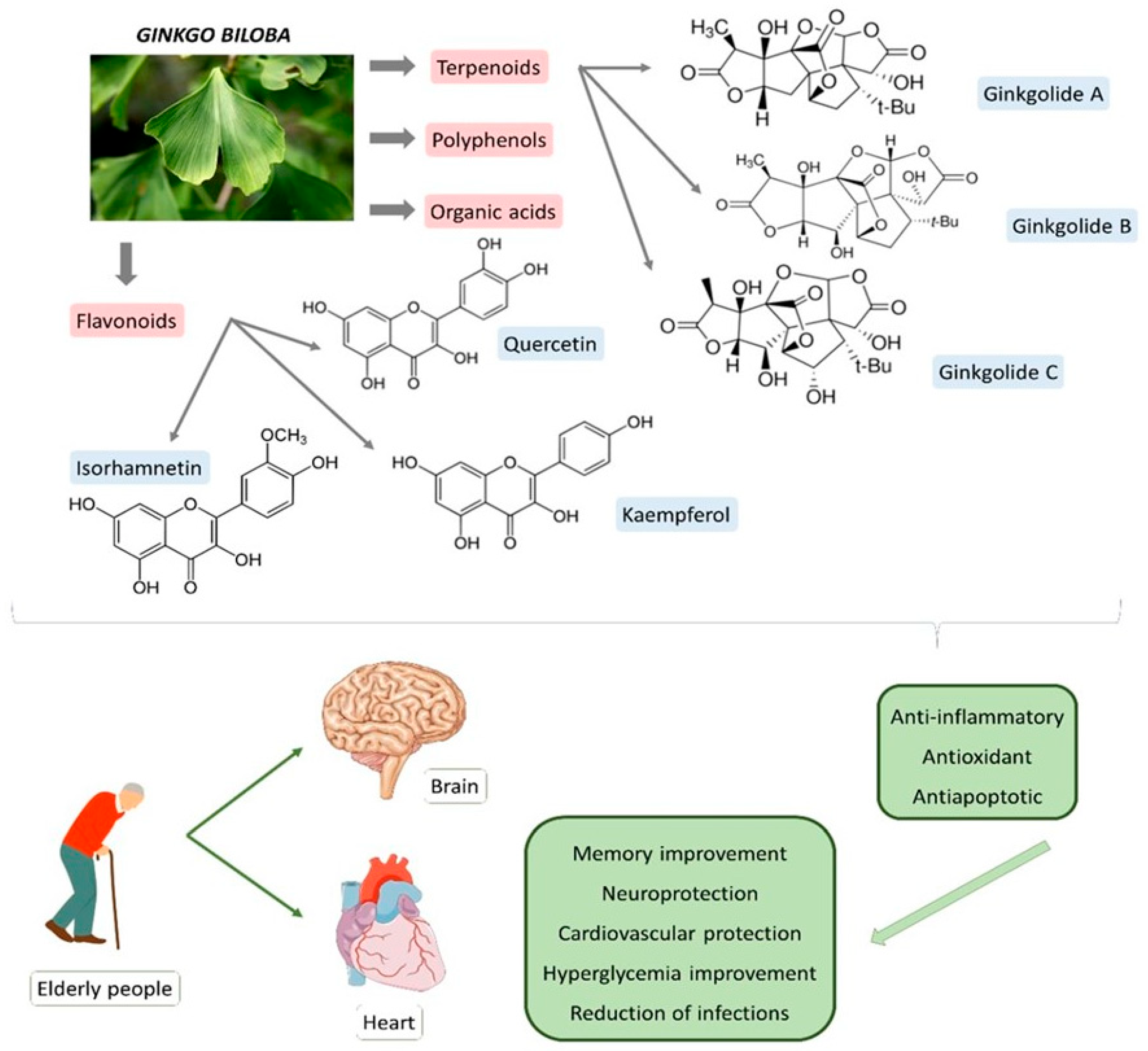
| Bioactive Compound | Sources | Molecular Structure | Functions | References |
|---|---|---|---|---|
| Ginkgolide A (terpenoid) | Leaves, root, and bark. |  |
| [12,42,43,44] |
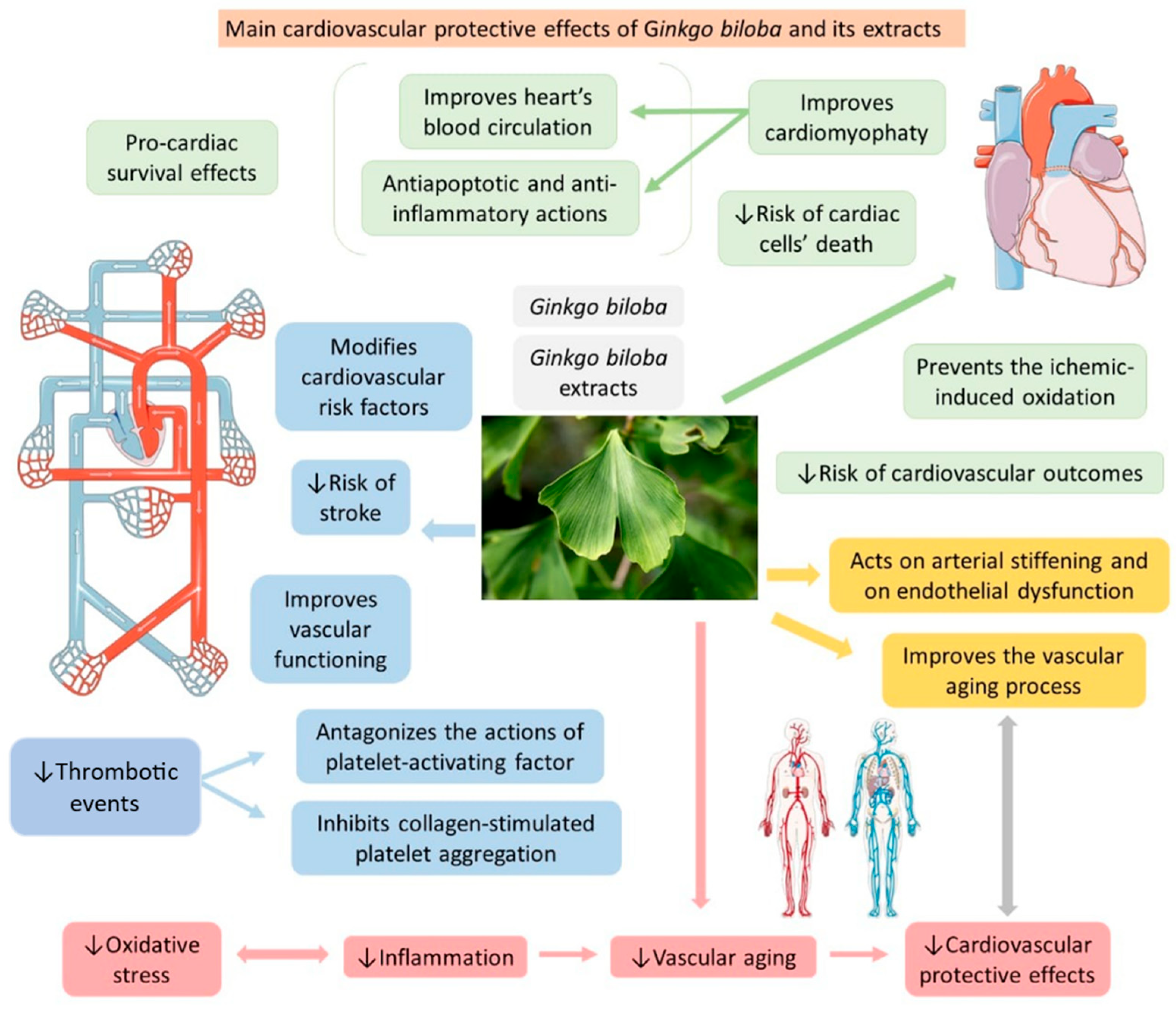
| Ginkgolide B (terpenoid) | Leaves, root, and bark. | 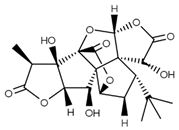 |
| [12,43,45,46,47,48,49,50,51] |
| Ginkgolide C (terpenoids) | Leaves, root, and bark. | 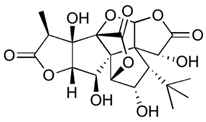 |
| [12,43,52,53,54,55,56] |
| Bilobalide (terpenoid) | Leaves and bark. | 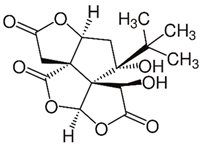 |
| [12,43,57,58,59] |
| Ginkgolic acid (organic acid) | Leaves. | 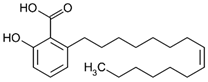 |
| [43,60,61,62] |
| Isorhamnetin (flavonoid) | Leaves. | 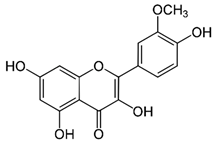 |
| [43,63,64,65,66,67] |
| Quercetin (flavonoid) | Leaves. | 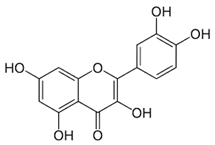 |
| [43,67,68,69,70,71,72,73] |
| Kaempferol (flavonoid) | Leaves. | 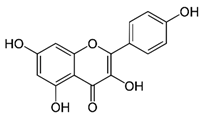 |
| [12,43,74,75,76,77,78] |
| Luteolin (flavonoid) | Leaves. | 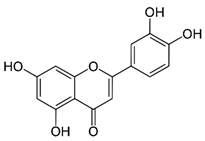 |
| [43,79,80,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.d.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. https://doi.org/10.3390/antiox11030525
Barbalho SM, Direito R, Laurindo LF, Marton LT, Guiguer EL, Goulart RdA, Tofano RJ, Carvalho ACA, Flato UAP, Capelluppi Tofano VA, et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants. 2022; 11(3):525. https://doi.org/10.3390/antiox11030525
Chicago/Turabian StyleBarbalho, Sandra Maria, Rosa Direito, Lucas Fornari Laurindo, Ledyane Taynara Marton, Elen Landgraf Guiguer, Ricardo de Alvares Goulart, Ricardo José Tofano, Antonely C. A. Carvalho, Uri Adrian Prync Flato, Viviane Alessandra Capelluppi Tofano, and et al. 2022. "Ginkgo biloba in the Aging Process: A Narrative Review" Antioxidants 11, no. 3: 525. https://doi.org/10.3390/antiox11030525
APA StyleBarbalho, S. M., Direito, R., Laurindo, L. F., Marton, L. T., Guiguer, E. L., Goulart, R. d. A., Tofano, R. J., Carvalho, A. C. A., Flato, U. A. P., Capelluppi Tofano, V. A., Detregiachi, C. R. P., Bueno, P. C. S., Girio, R. S. J., & Araújo, A. C. (2022). Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants, 11(3), 525. https://doi.org/10.3390/antiox11030525









