Vitamin K2 (Menaquinone-7) Reverses Age-Related Structural and Cognitive Deterioration in Naturally Aging Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
2.2. Preparation of Tissue Homogenate
2.3. Assessment of Oxidative Stress in Frontal Cortex and Hippocampus Homogenate
2.4. Behavioral Tests
2.4.1. Anxiety Test: Crawley’s Sociability Test
2.4.2. Depressive-like Behavior Testing: Forced Swim Test (FST)
2.4.3. Memory Testing: Modified T-Maze
2.5. Histopathology and Immunohistochemistry (IHC)
2.6. Real Time Polymerase Chain Reaction for IL-6 and Nrf-2
2.7. Assessment of Caspase-1, IL-1β and NLRP3 Contents in Frontal Cortex and Hippocampus Homogenate
2.8. Statistical Analyses
3. Results
3.1. Effect of Vit. K2 on Anxiety Testing; Crawley’s Sociability Test
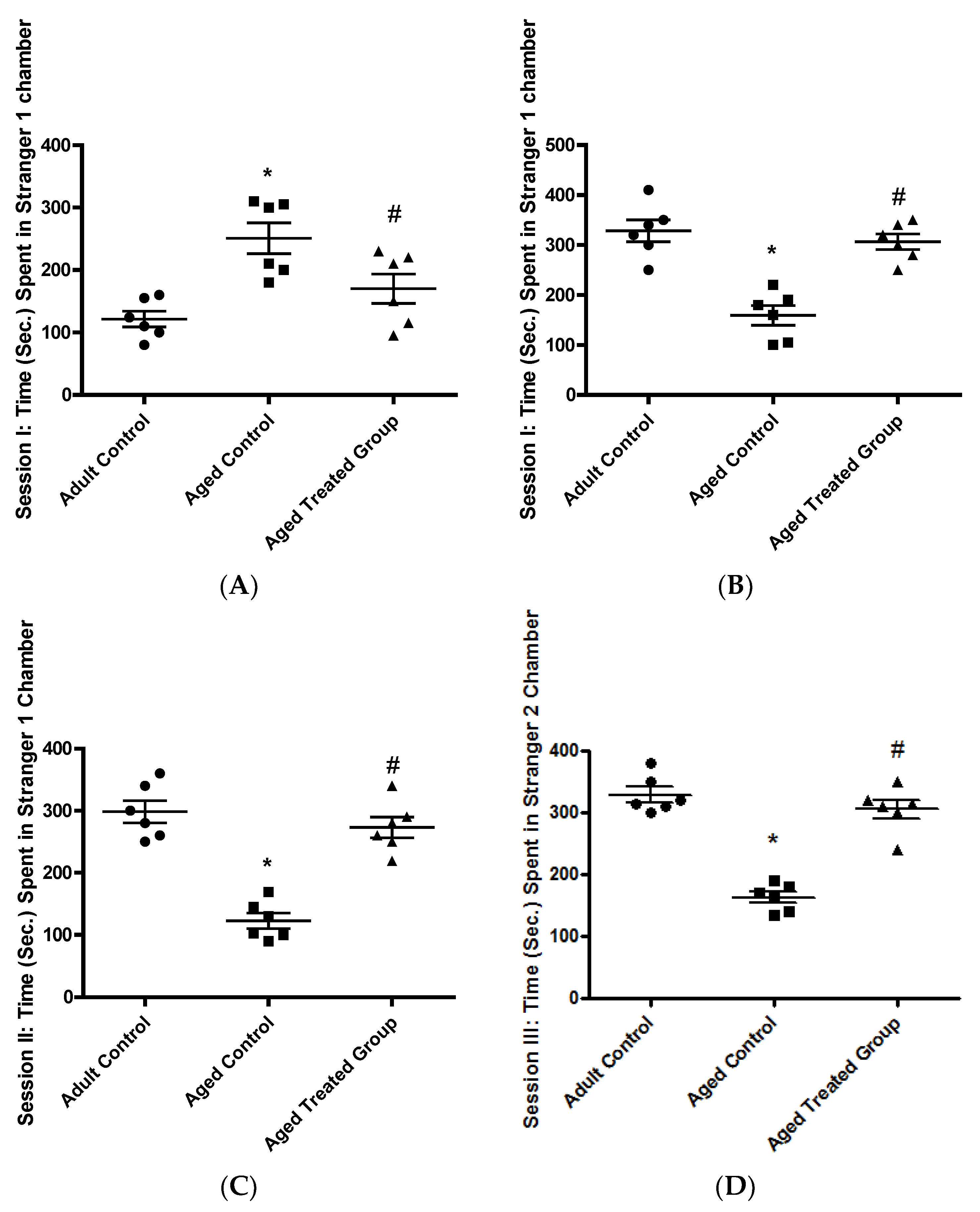
3.1.1. Time (Sec.) Spent in the Empty Chamber, Session I: Habituation
3.1.2. Time (Sec.) Spent in the Stranger 1 Chamber, Session I
3.1.3. Time (Sec.) Spent in the Stranger 1 Chamber, Session II: Social Affiliation
3.1.4. Time (Sec.) Spent in Stranger 2 Chamber, Session III: Social Novelty
3.2. Effect of Vit. K2 on Memory Testing: T-Maze Test
3.3. Effect of Vit. K2 on Depressive-like Behavior Testing: Forced Swimming Test
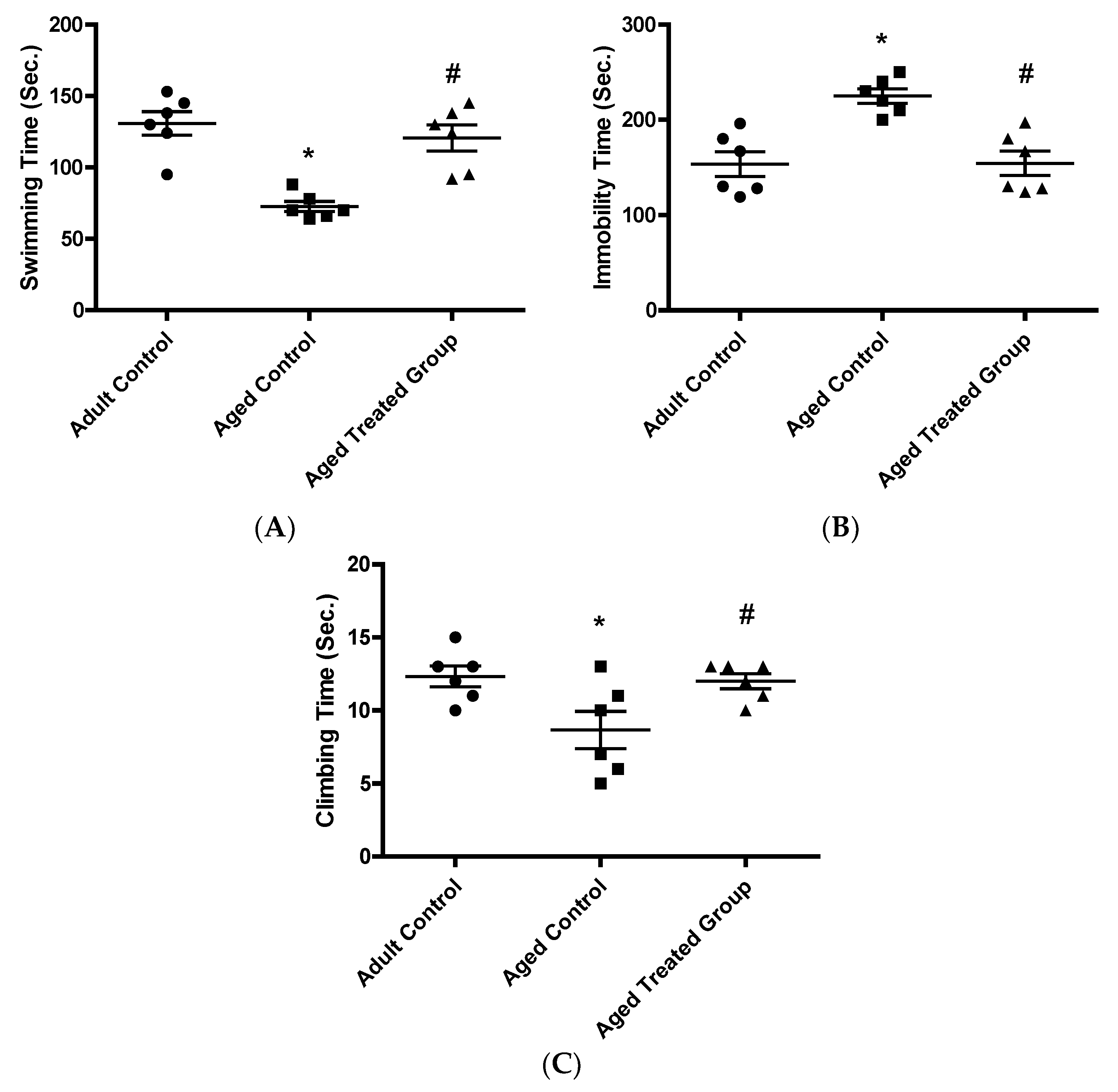
3.3.1. Swimming Time (Sec.)
3.3.2. Immobility Time (Sec.)
3.3.3. Climbing Time (Sec.)
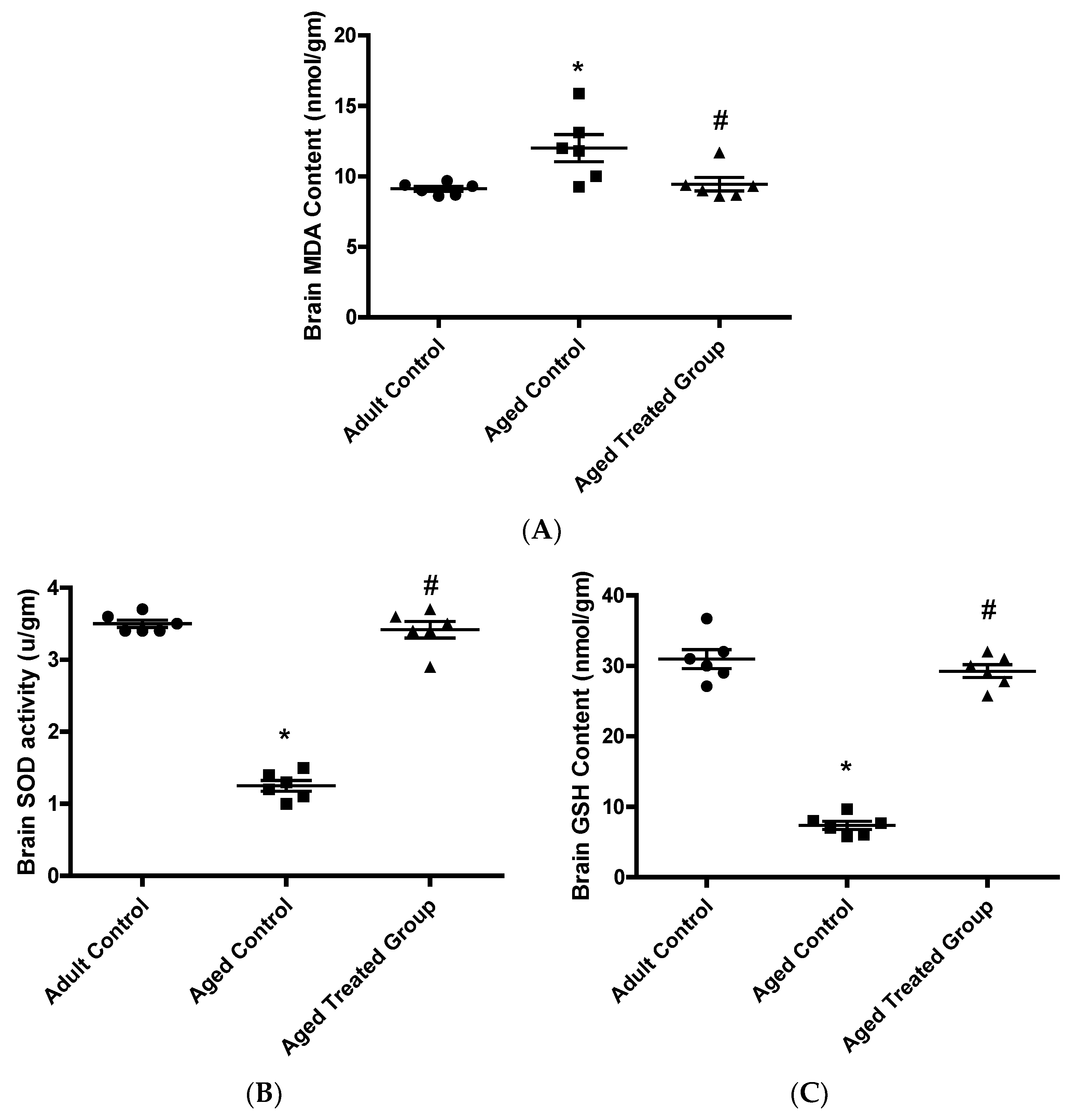
3.4. Effect of Vit. K2 on Oxidative (MDA)/Anti-Oxidative (GSH and SOD) Status in Frontal Cortex and Hippocampus
3.5. Effect of Vit. K2 on Frontal Cortex and Hippocampus Gene Expression of IL-6 and Nrf-2
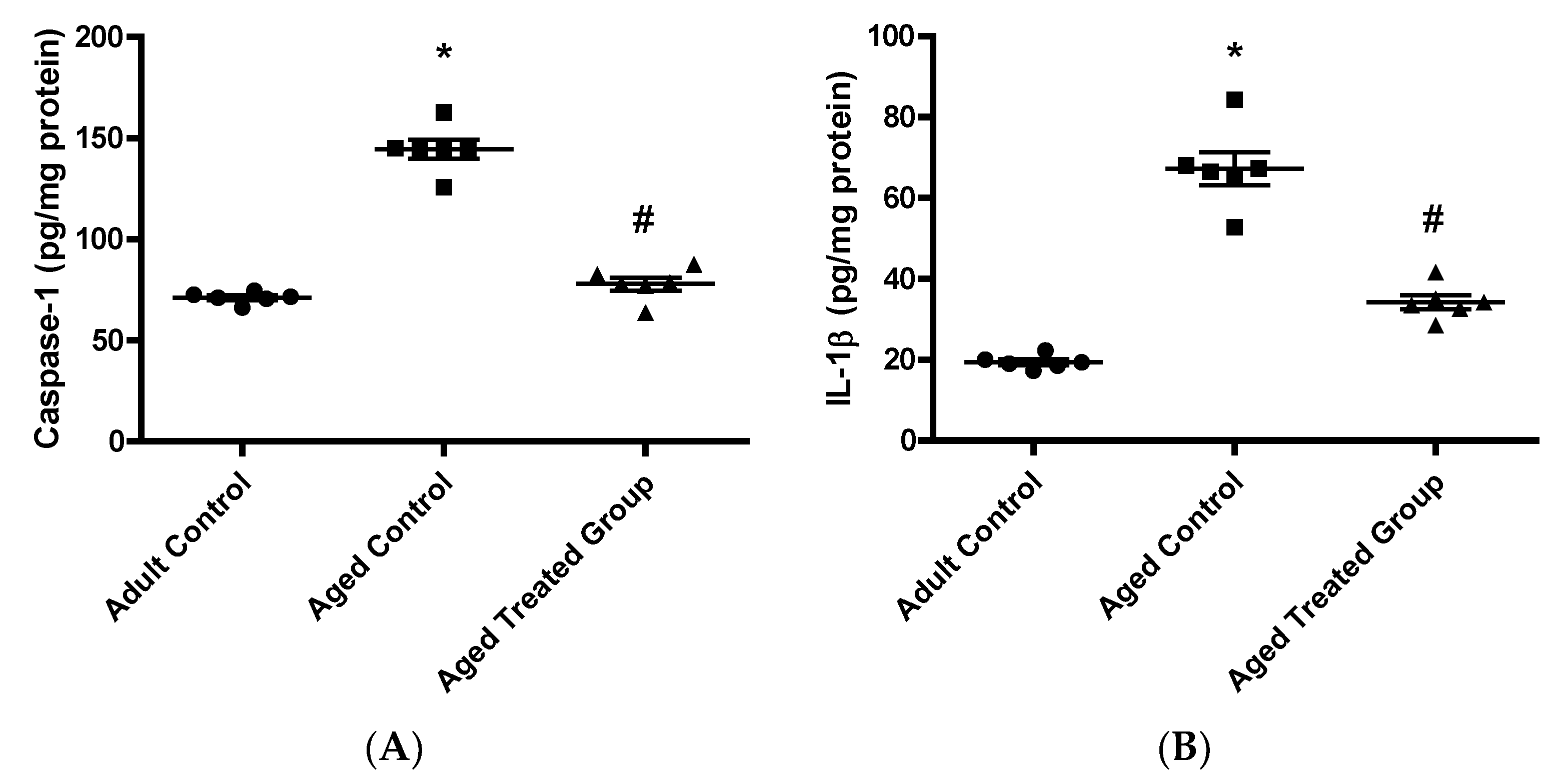
3.6. Effect of Vit. K2 on Caspase-1, Il-1β, and NLRP3 Contents in Frontal Cortex and Hippocampus
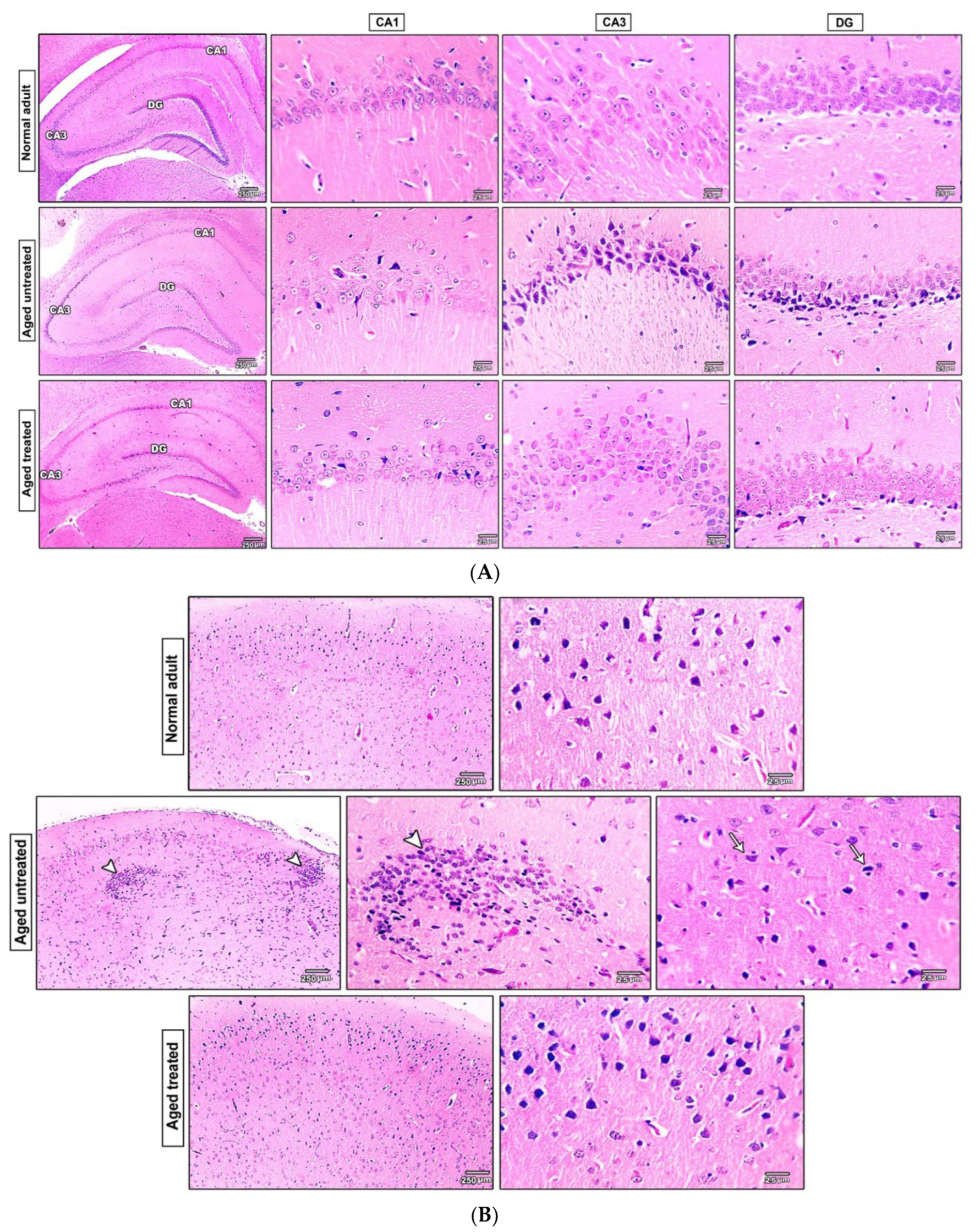
3.7. Effect of Vit. K2 on the Histopathological Changes in the Hippocampus and the Frontal Cortex of Naturally Aging Rats
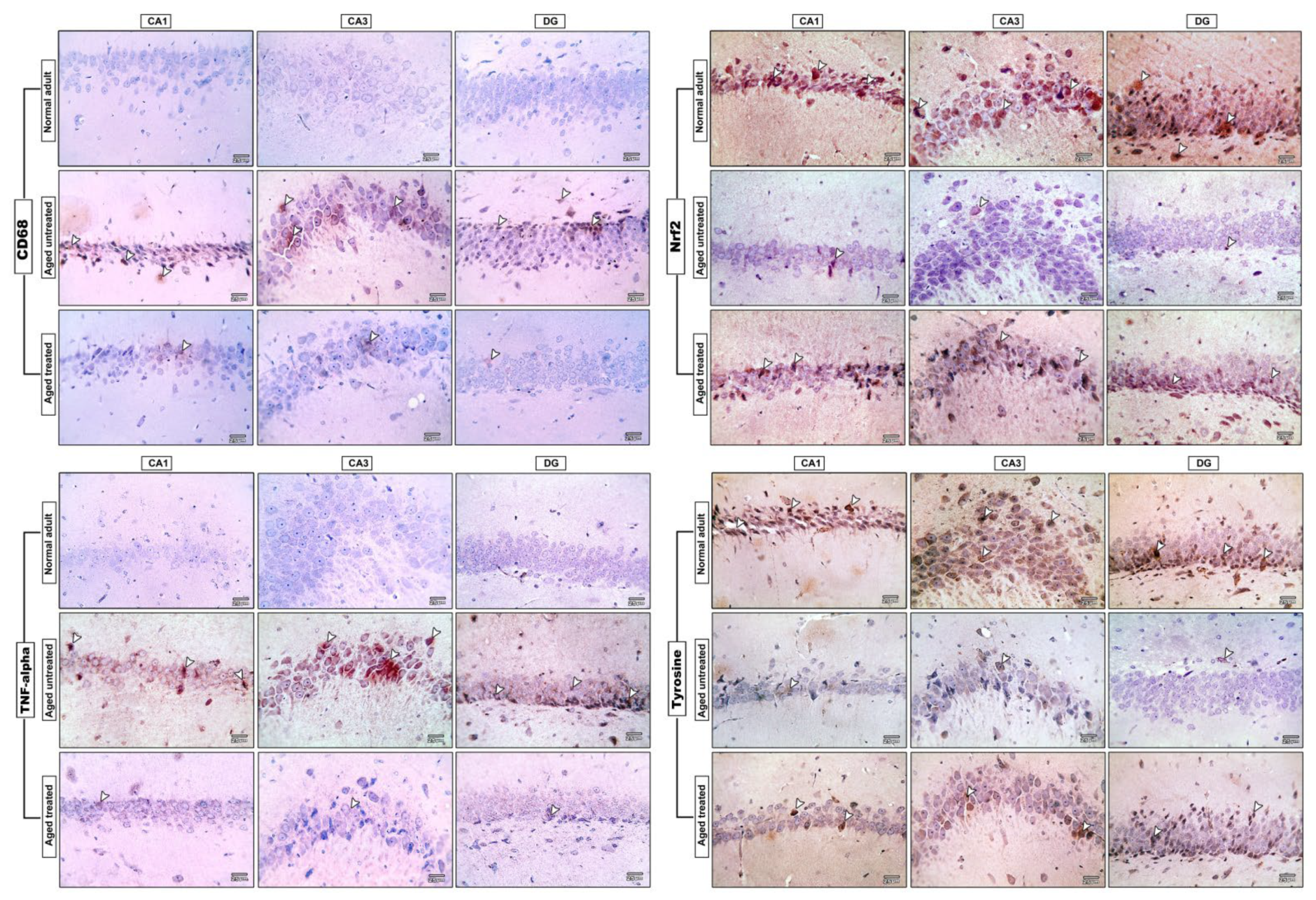
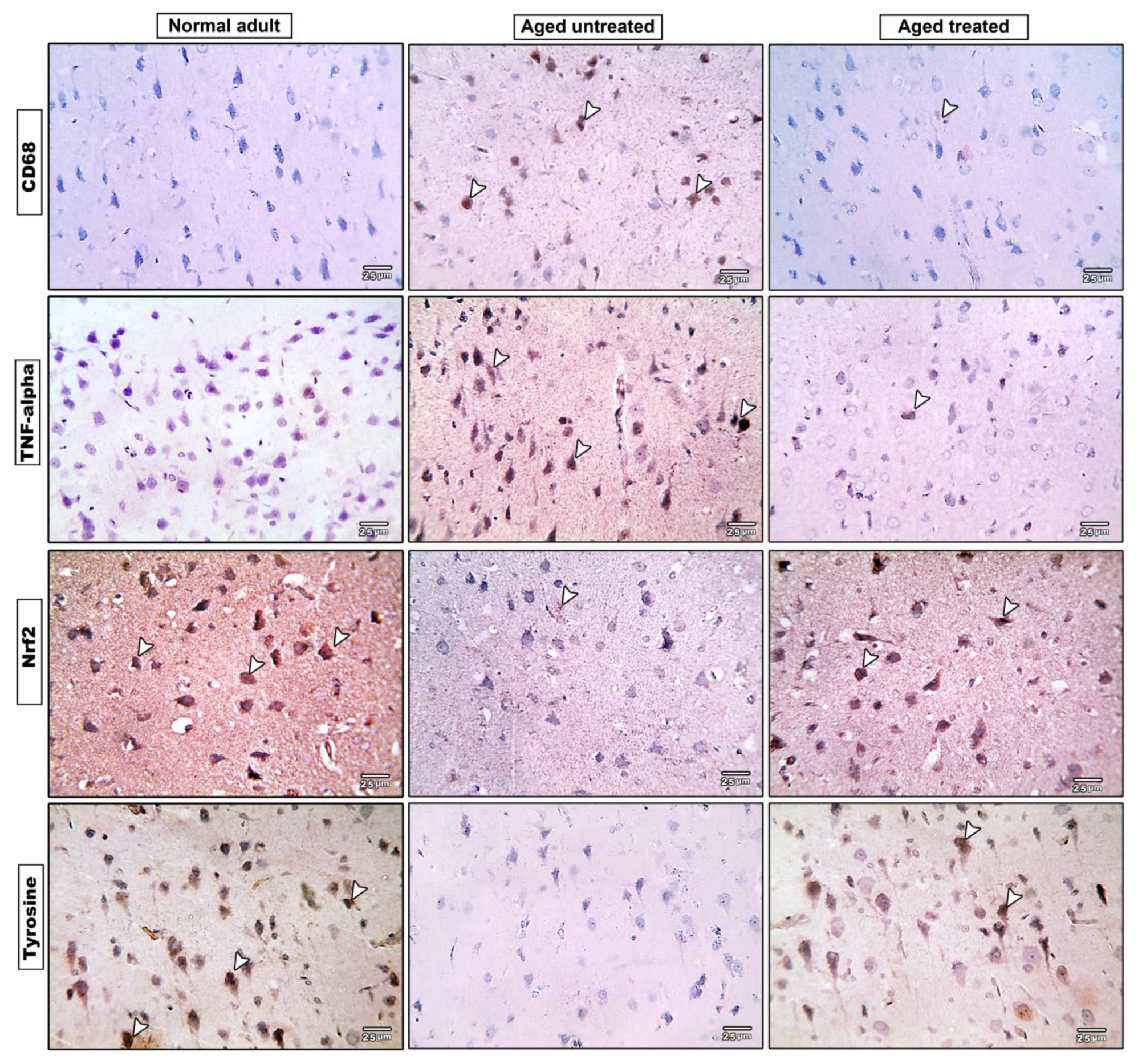
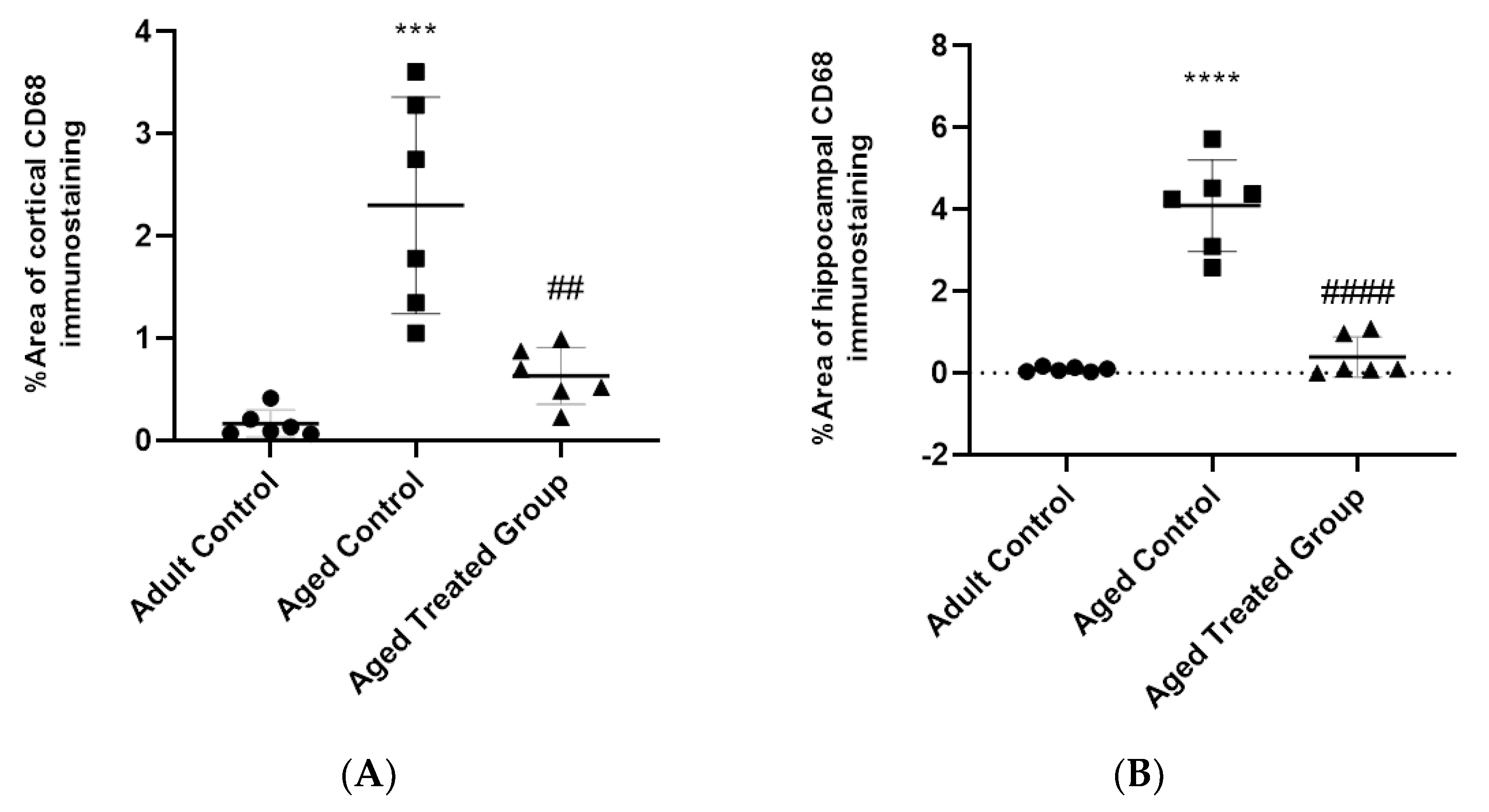
3.8. Effect of Vit. K2 on CD68 Expression in Frontal Cortex and Hippocampus
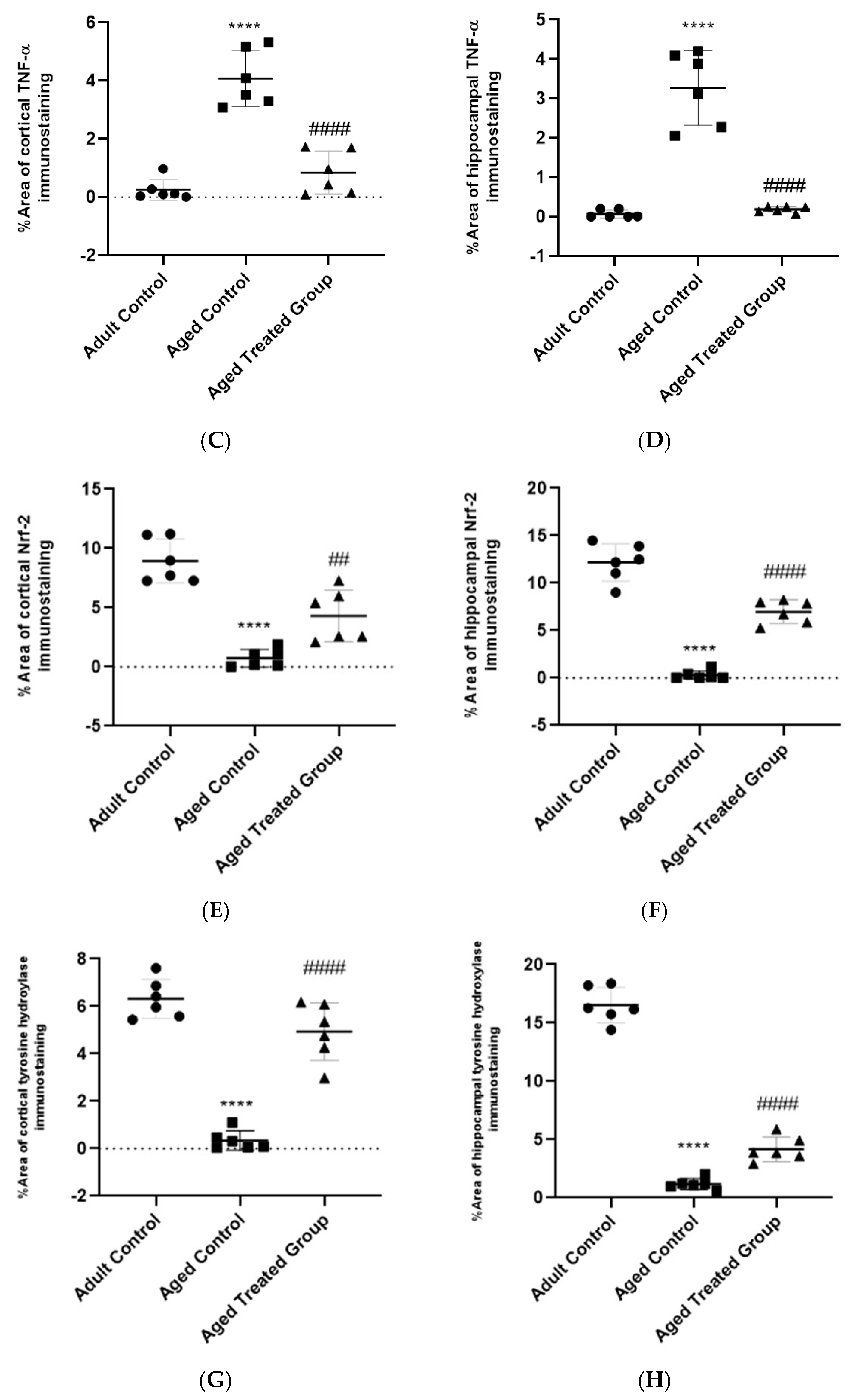
3.9. Effect of Vit. K2 on TNF-α Expression in Frontal Cortex and Hippocampus
3.10. Effect of Vit. K2 on Nrf-2 Expression in Frontal Cortex and Hippocampus
3.11. Effect of Vit. K2 on Tyrosine Hydroxylase Expression in Frontal Cortex and Hippocampus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elobeid, A.; Libard, S.; Leino, M.; Popova, S.N.; Alafuzoff, I. Altered Proteins in the Aging Brain. J. Neuropathol. Exp. Neurol. 2016, 75, 316–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, R. Ageing and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Melzer, T.M.; Manosso, L.M.; Yau, S.-Y.; Gil-Mohapel, J.; Brocardo, P.S. In Pursuit of Healthy Aging: Effects of Nutrition on Brain Function. Int. J. Mol. Sci. 2021, 22, 5026. [Google Scholar] [CrossRef]
- Ramazani, E.; Fereidoni, M.; Tayarani-Najaran, Z. Protective effects of vitamin K2 on 6-OHDA-induced apoptosis in PC12 cells through modulation bax and caspase-3 activation. Mol. Biol. Rep. 2019, 46, 5777–5783. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; Booth, S.L.; van den Heuvel, E.G.H.M.; Stoecklin, E.; Baka, A.; Vermeer, C. The role of menaquinones (vitamin K₂) in human health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Schurgers, L.J.; Uenishi, K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr. J. 2012, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Ferland, G. Vitamin K and brain function. Semin. Thromb. Hemost. 2013, 39, 849–855. [Google Scholar] [CrossRef]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef] [Green Version]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Powerful Micronutrient in Aging and Age-Related Diseases: Pros and Cons from Clinical Studies. Int. J. Mol. Sci. 2019, 20, 4150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, A.; German, M. Vitamin K2 Holds Promise for Alzheimer’s Prevention and Treatment. Nutrients 2021, 13, 2206. [Google Scholar] [CrossRef] [PubMed]
- Saputra, W.D.; Aoyama, N.; Komai, M.; Shirakawa, H. Menaquinone-4 Suppresses Lipopolysaccharide-Induced Inflammation in MG6 Mouse Microglia-Derived Cells by Inhibiting the NF-κB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, M.; Esposito, G.; Edirisinghe, J.N.; Vilain, S.; Haddad, D.M.; Slabbaert, J.R.; van Meensel, S.; Schaap, O.; de Strooper, B.; Meganathan, R.; et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science 2012, 336, 1306–1310. [Google Scholar] [CrossRef] [Green Version]
- Hadipour, E.; Tayarani-Najaran, Z.; Fereidoni, M. Vitamin K2 protects PC12 cells against Aβ (1-42) and H2O2-induced apoptosis via p38 MAP kinase pathway. Nutr. Neurosci. 2020, 23, 343–352. [Google Scholar] [CrossRef]
- Ferland, G. Vitamin K, an emerging nutrient in brain function. BioFactors 2012, 38, 151–157. [Google Scholar] [CrossRef]
- Ferland, G. Vitamin K and the nervous system: An overview of its actions. Adv. Nutr. 2012, 3, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Alisi, L.; Cao, R.; De Angelis, C.; Cafolla, A.; Caramia, F.; Cartocci, G.; Librando, A.; Fiorelli, M. The Relationships between Vitamin K and Cognition: A Review of Current Evidence. Front. Neurol. 2019, 10, 239. [Google Scholar] [CrossRef]
- Gancheva, S.M.; Zhelyazkova-Savova, M.D. Vitamin K2 Improves Anxiety and Depression but not Cognition in Rats with Metabolic Syndrome: A Role of Blood Glucose? Folia Med. 2016, 58, 264–272. [Google Scholar] [CrossRef]
- Iwamoto, D.; Masaki, C.; Shibata, Y.; Watanabe, C.; Nodai, T.; Munemasa, T.; Mukaibo, T.; Kondo, Y.; Hosokawa, R. Microstructural and mechanical recovery of bone in ovariectomized rats: The effects of menaquinone-7. J. Mech. Behav. Biomed. Mater. 2021, 120, 104571. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment. Retard. Dev. Disabil. Res. Rev. 2004, 10, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef]
- Wu, C.Y.C.; Lerner, F.M.; e Silva, A.C.; Possoit, H.E.; Hsieh, T.-H.; Neumann, J.T.; Minagar, A.; Lin, H.W.; Lee, R.H.C. Utilizing the Modified T-Maze to Assess Functional Memory Outcomes after Cardiac Arrest. J. Vis. Exp. JoVE 2018, e56694. [Google Scholar] [CrossRef] [PubMed]
- Maresz, K. Growing Evidence of a Proven Mechanism Shows Vitamin K2 Can Impact Health Conditions Beyond Bone and Cardiovascular. Integr. Med. Clin. J. 2021, 20, 34–38. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sá-Nakanishi, A.B.; Soares, A.A.; de Oliveira, A.L.; Comar, J.F.; Peralta, R.M.; Bracht, A. Effects of treating old rats with an aqueous Agaricus blazei extract on oxidative and functional parameters of the brain tissue and brain mitochondria. Oxidative Med. Cell. Longev. 2014, 2014, 563179. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Chen, F.; Zhou, B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci. Rep. 2018, 8, 1465. [Google Scholar] [CrossRef] [Green Version]
- Presse, N.; Belleville, S.; Gaudreau, P.; Greenwood, C.E.; Kergoat, M.-J.; Morais, J.A.; Payette, H.; Shatenstein, B.; Ferland, G. Vitamin K status and cognitive function in healthy older adults. Neurobiol. Aging 2013, 34, 2777–2783. [Google Scholar] [CrossRef]
- Carrié, I.; Portoukalian, J.; Vicaretti, R.; Rochford, J.; Potvin, S.; Ferland, G. Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J. Nutr. 2004, 134, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Allison, A.C. The possible role of vitamin K deficiency in the pathogenesis of Alzheimer’s disease and in augmenting brain damage associated with cardiovascular disease. Med. Hypotheses 2001, 57, 151–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamadon-Nejad, S.; Ouliass, B.; Rochford, J.; Ferland, G. Vitamin K Deficiency Induced by Warfarin Is Associated With Cognitive and Behavioral Perturbations, and Alterations in Brain Sphingolipids in Rats. Front. Aging Neurosci. 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Su, S.; Cai, W.; Cao, J.; Miao, X.; Zang, W.; Gao, S.; Xu, Y.; Yang, J.; Tao, Y.-X.; et al. Differentially Expressed Genes in the Brain of Aging Mice with Cognitive Alteration and Depression- and Anxiety-Like Behaviors. Front. Cell Dev. Biol. 2020, 8, 814. [Google Scholar] [CrossRef] [PubMed]
- Belovicova, K.; Bogi, E.; Csatlosova, K.; Dubovicky, M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017, 10, 40–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, A.S.; Shah, V.D.; Baker, S.L.; Vogel, J.W.; O’Neil, J.P.; Janabi, M.; Schwimmer, H.D.; Marks, S.M.; Jagust, W.J. Aging Affects Dopaminergic Neural Mechanisms of Cognitive Flexibility. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 12559–12569. [Google Scholar] [CrossRef]
- Morcom, A.M.; Bullmore, E.T.; Huppert, F.A.; Lennox, B.; Praseedom, A.; Linnington, H.; Fletcher, P.C. Memory encoding and dopamine in the aging brain: A psychopharmacological neuroimaging study. Cereb. Cortex 2010, 20, 743–757. [Google Scholar] [CrossRef]
- De La Cruz, C.P.; Revilla, E.; Venero, J.L.; Ayala, A.; Cano, J.; Machado, A. Oxidative inactivation of tyrosine hydroxylase in substantia nigra of aged rat. Free Radic. Biol. Med. 1996, 20, 53–61. [Google Scholar] [CrossRef]
- Porcher, L.; Bruckmeier, S.; Burbano, S.D.; Finnell, J.E.; Gorny, N.; Klett, J.; Wood, S.K.; Kelly, M.P. Aging triggers an upregulation of a multitude of cytokines in the male and especially the female rodent hippocampus but more discrete changes in other brain regions. J. Neuroinflamm. 2021, 18, 219. [Google Scholar] [CrossRef]
- Norden, D.M.; Godbout, J.P. Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013, 39, 19–34. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, S.; Cao, L.; Chen, Y.; Zuo, Z.; Peng, S. Critical role of NLRP3-caspase-1 pathway in age-dependent isoflurane-induced microglial inflammatory response and cognitive impairment. J. Neuroinflamm. 2018, 15, 109. [Google Scholar] [CrossRef] [Green Version]
- Eisa, N.H.; Khodir, A.E.; El-Sherbiny, M.; Elsherbiny, N.M.; Said, E. Phenethyl isothiocyanate attenuates diabetic nephropathy via modulation of glycative/oxidative/inflammatory signaling in diabetic rats. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 142, 111666. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Deretic, V.; Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 2018, 14, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vago, J.P.; Amaral, F.A.; van de Loo, F.A.J. Resolving inflammation by TAM receptor activation. Pharmacol. Ther. 2021, 227, 107893. [Google Scholar] [CrossRef]
- Jian, Z.; Li, K.; Song, P.; Zhu, G.; Zhu, L.; Cui, T.; Liu, B.; Tang, L.; Wang, X.; Wang, G.; et al. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: A possible mechanism for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2014, 134, 2221–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Xia, T.; Liu, S. Mechanisms of nanosilver-induced toxicological effects: More attention should be paid to its sublethal effects. Nanoscale 2015, 7, 7470–7481. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Gene Symbol | Primer Sequence from 5′-3′ | Gene Bank Accession Number |
|---|---|---|
| β-actin | F: TCCGTCGCCGGTCCACACCC R: TCACCAACTGGGACGATATG | NM_031144.3 |
| Nrf2 | F: AGCAGGACATGGATTTGATT R: CTTCTCCTGTTCCTTCTGGA | XM_032903520.1 |
| Il-6 | F: TCCTACCCCAACTTCCAATGCTC R: TTGGATGGTCTTGGTCCTTAGCC | M26745 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkattawy, H.A.; Ghoneim, F.M.; Eladl, M.A.; Said, E.; Ebrahim, H.A.; El-Shafey, M.; Asseri, S.M.; El-Sherbiny, M.; Alsalamah, R.H.; Elsherbiny, N.M.; et al. Vitamin K2 (Menaquinone-7) Reverses Age-Related Structural and Cognitive Deterioration in Naturally Aging Rats. Antioxidants 2022, 11, 514. https://doi.org/10.3390/antiox11030514
Elkattawy HA, Ghoneim FM, Eladl MA, Said E, Ebrahim HA, El-Shafey M, Asseri SM, El-Sherbiny M, Alsalamah RH, Elsherbiny NM, et al. Vitamin K2 (Menaquinone-7) Reverses Age-Related Structural and Cognitive Deterioration in Naturally Aging Rats. Antioxidants. 2022; 11(3):514. https://doi.org/10.3390/antiox11030514
Chicago/Turabian StyleElkattawy, Hany A., Fatma M. Ghoneim, Mohamed Ahmed Eladl, Eman Said, Hasnaa Ali Ebrahim, Mohamed El-Shafey, Saad Mohamed Asseri, Mohamed El-Sherbiny, Reem Hamoud Alsalamah, Nehal M. Elsherbiny, and et al. 2022. "Vitamin K2 (Menaquinone-7) Reverses Age-Related Structural and Cognitive Deterioration in Naturally Aging Rats" Antioxidants 11, no. 3: 514. https://doi.org/10.3390/antiox11030514
APA StyleElkattawy, H. A., Ghoneim, F. M., Eladl, M. A., Said, E., Ebrahim, H. A., El-Shafey, M., Asseri, S. M., El-Sherbiny, M., Alsalamah, R. H., Elsherbiny, N. M., & Hadhod, S. (2022). Vitamin K2 (Menaquinone-7) Reverses Age-Related Structural and Cognitive Deterioration in Naturally Aging Rats. Antioxidants, 11(3), 514. https://doi.org/10.3390/antiox11030514








