Abstract
Melatonin is effective in modulating metabolism and regulating growth and development in many plants under biotic and abiotic stress. However, there is no systematic quantification of melatonin effects on maize growth, gas exchange, chlorophyll content, and the antioxidant defense system. A meta-analysis was conducted on thirty-two currently available published articles to evaluate the effect of stress types, study types, and maize varieties on response ratio (lnRR++) of “melatonin” to “control (no melatonin)” on plant growth, enzyme activities, gas exchange parameters, and photosynthetic pigments. Our findings revealed that melatonin application overall increased plant height, leaf area, root length, fresh and dry root weight and shoot weight, superoxide dismutase (SOD), peroxide (POD), catalase (CAT), ascorbate peroxidase (APX), soluble sugar and protein, photosynthetic rate, stomatal conductance, transpiration rate, chlorophyll, and carotenoid in maize leaf under stress conditions. In contrast, melatonin application decreased the levels of hydrogen peroxide (H2O2), superoxide anion (O2−), malondialdehyde (MDA), and electrolyte leakage. The categorical meta-analysis demonstrated that melatonin application to chilling stress resulted in higher SOD activity followed by salt stress. Melatonin application to all stress types resulted in higher POD, CAT and APX activities, except Cd stress, which had no effect on POD and decreased CAT by 38% compared to control. Compared to control, melatonin resulted in lower reactive oxygen species (ROS) and electrolyte leakage under no stress, Cd, drought, salt, lead, heat, and chilling stress in all study types (pot, growth chamber, hydroponic, and field), except O2 content which was not affected in pot and growth chamber studies. It was concluded that melatonin alleviates oxidative damage by improving stress tolerance, regulating the antioxidant defense system, and increasing leaf chlorophyll content compared to control.
1. Introduction
Maize (Zea mays L.) is one of the most important crops in the world and is cultivated extensively as a staple food in almost every part of the world under a variety of environmental conditions [1,2]. It is a multipurpose crop due to its nutritional value, and it is used as food for humans, as feed for animals and poultry, and as an important raw material for industries around the world [1,3]. Scientists are most concerned about maize because it can survive in harsh environments [4]. However, adverse environmental conditions and heavy metal accumulation caused by human activity may limit its growth and productivity [5,6]. It goes without saying that plants, with their complex physiology, will inevitably find life challenging in environments with variable stress conditions, such as varied biotic and abiotic stress factors. Salinity, heavy metal pollution, drought, heat, and chilling stress are the most serious threats to plant growth, productivity, and human health in the agro-ecosystem, particularly in areas with high anthropogenic heaviness [7,8].
Heavy metal pollution, drought, salinity, lead (Pb), heat, and chilling stress are the primary threats to plant growth and crop yield and are increasingly being recognized as a serious issue throughout the world [1,3,9,10,11]. Cadmium is one of the most hazardous metals for a variety of food crops, particularly maize, and it has been shown to limit crop growth by disrupting normal cellular activities and metabolism [1,12]. Changes in morpho-physiological development and antioxidant enzyme activities in maize caused by cadmium also led to oxidative stress in maize due to an increase in reactive oxygen species generation (ROS) [1,13]. Moreover, it was observed that Cd toxicity decreased maize photosynthetic pigments, antioxidant enzyme activity, and morphological growth [14]. Maize is an essential crop in agricultural production, but the soil in China is currently polluted by Cd in many regions, leading to the enhancement of Cd content [15]. Consequently, it is imperative that scientists figure out how to reduce the amount of Cd in crops grown in polluted areas.
The production of maize in arid and semi-arid areas of China is wholly reliant on rainfall. However, extremely limited and unpredictable precipitation during the growing period in these areas causes drought stress, which dramatically reduces maize production [16,17]. Moreover, drought is a major abiotic stressor that significantly negatively impacts corn production, resulting in a 40% decrease in maize yield globally over the last 25 years [2,18]. Even still, fertilizers and plant growth regulators are critical for increasing crop yield [16,19,20]. In agricultural production, drought or osmotic stress-induced water deprivation is one of the most severe abiotic stressors. On the other hand, melatonin has been widely reported to boost plant tolerance to water deprivation stress in a variety of plants [21,22].
Likewise, salinity is also a critical abiotic factor that affects crop growth and development [23,24]. Around the world, more than 0.8 billion hectares of farmland are severely salinized, making it a serious agricultural concern that results in low productivity [24,25]. Several essential metabolic processes in plants, including glucose metabolism, lipid metabolism, and protein synthesis, are adversely affected by salinity to a great extent because of ionic and osmotic stress [24,26,27]. Several ways have been used to maintain osmotic homeostasis and ion balance and avoid damage to plants to mitigate the detrimental effects of salinity stress [28,29,30,31]. Salinity stress also causes a decrease in chlorophyll content and stomatal conductance, which diminishes carbon dioxide assimilation, damage to photosynthetic organs, nutritional imbalance, oxidative damage, metabolic disorders, and alterations in the photosynthesis-related enzyme activity [31,32]. According to the findings of Ahanger et al. [33], salt stress decreased chlorophyll content, stomatal conductance, net photosynthesis, and transpiration in tomatoes. In recent years, several plant growth regulators have been employed to ameliorate the negative effects of stress on plants, such as salicylic acid [34], proline [35], nitric oxide [36] and melatonin [30]. External treatments are among the most appropriate and cost-effective techniques to boost germination and eventual yields under various abiotic stressors [30]. Melatonin is required for plant growth, germination, and development, and it also increases plant resistance to abiotic stresses such as salt stress, drought stress, heat stress, and cold stress conditions [30,37].
Application of melatonin enhances plant growth and photosynthetic activity, increases chlorophyll content, and decreases ROS formation and hence oxidative damage to plants [38]. Ren et al. [39] reported that melatonin application to stressed plants significantly increases leaf area, biomass, and photosynthetic efficiency. Additionally, melatonin application in saline circumstances decreased the Na+ level of the root and leaf and raised the K+ content [40]. As a result, improving maize plants’ stress tolerance is critical for increasing maize yields. Although melatonin is increasingly being used to improve plant growth, enzymatic activities, gas exchange parameters, and photosynthetic pigments [30,41,42], no meta-analysis comparing different stress types, study types, and maize varieties has been reported. In order to better understand the effects of exogenous melatonin dosages on maize seedling and growth performance, photosynthetic activity, stress sensitivity index, and enzymatic activities, a meta-analysis based on the available literature was conducted to synthesize the overall effect of melatonin on plant growth, gas exchange parameters, photosynthetic pigments, and enzymatic activities under different stress types, study types, and maize varieties. In this meta-analysis, we hypothesized that (1) melatonin application would have an overall positive effect on plant growth and enzyme activities, (2) such an effect would vary with stress types, study types, and maize varieties, and (3) optimum melatonin application would improve plant growth and enzyme activities that reduce oxidative plant damages.
2. Materials and Methods
2.1. Database Construction and Literature Search
The goal of this meta-analysis is to quantify the effect of melatonin on maize plant growth, gas exchange (net photosynthetic rate, intercellular CO2, stomatal conductance, transpiration rate), chlorophyll content, and improving enzymatic and non-enzymatic activities. A total of 32 studies across the globe were collected from the available literature on the Web of Science (www.sciencedirect.com; accessed on 18 May 2021) for this meta-analysis. Keywords, such as net photosynthetic rate, intercellular CO2, stomatal conductance, transpiration rate, enzyme activities, chlorophyll content, heavy metal stress (cadmium and lead stress), drought stress, salt stress, chilling stress, antioxidant defense system, and melatonin, were used for the search. Experiment and stress types, such as temperature, humidity, light intensity, and maize verity of the selected studies were also recorded. Meta-analyses, unlike traditional literature reviews, are led by a specific search procedure that contains defined inclusion and exclusion criteria as well as a screening process that further determines study eligibility.
The data for the above-mentioned parameter was taken from the literature. Graph digitizer software GetData 2.26 was used to extract the data presented in graphical form. All kinds of studies (field study, growth chamber, and greenhouse experiment) were included for the data collection on melatonin with control (no melatonin). The PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses, http://www.prisma-statement.org/ accessed on 21 December 2021) was employed for reporting the screening process. After removing duplicate records, all the publications were checked against a set of exclusion and inclusion criteria Studies were included having three or above replications and standard error (SE) or standard deviation (SD), while discarded with no true control of melatonin [43]. Data was collected from publications comparing melatonin with no melatonin under the maize crop on plant gas exchange parameters, chlorophyll, and enzymatic and non-enzymatic activities. The published studies were discorded from the database with the lack of mean value, SD, SE and replication. The standard deviation of the mean was calculated through the following equation:
whereas n is the number of replications, SD and SE are the standard deviations and standard error of the mean. The current meta-analysis, including 1649 pairwise comparisons was drawn from 32 published studies that met the aforementioned criteria. In order to evaluate the performance of melatonin and control, we collected the quantitative data and analyzed the difference.
2.2. Data Calculation and Analysis
The effect of melatonin on maize growth, enzymatic activities, and gas exchange parameters were estimated through response ratios (RRs) as the effect size using MetaWin software. Melatonin was tested on measured parameters using the RR [44,45], and was calculated using the following equation:
whereas XM and XNM are the means of enzymatic activities, plant growth, and gas exchange parameters in melatonin and control treatment. The natural log ratio is used to confirm that the numerator and denominator changed proportionately. Different stress types, maize varieties, and experimental types were considered distinct observations for each study. The variance of RRs for each sample was calculated as follows.
where SM and SNM are the standard deviations, nM and nNM are the replication of the studies, and XM and XNM are the mean values of melatonin and control treatments, respectively. The weight (W) for each RRs was calculated through the following equation:
The overall mean response ratio (RRE++) for individual melatonin treatment was computed using the equation as follows:
where “n” and “m” are the number of treatments and comparisons of the enzyme’s activities, maize growth, and gas exchange parameters, respectively. The SE of overall response ratio (RRE++) was calculated as:
To perform a meta-analysis and evaluate the effect of melatonin on enzymes, maize growth, and gas exchange parameters by computing the mean effect size and 95% bootstrapped confidence intervals (CIs), the metaWin 2.1 was used [43,45]. Melatonin was considered to be significant if the 95% confidence intervals did not overlap the zero line. The effect of melatonin on stress types, study types, and maize varieties were also examined among the selected parameters. The total heterogeneity of lnRR++ (95% CIs) among studies (QT) was calculated using categorical analysis in MetaWin, and then the I-square index was calculated by dividing the difference between QT and degrees of freedom (n − 1) by QT [45,46]. Table S1, Figures S1–S3 in the Supplementary Information summarizes the data heterogeneities for the target variables, with greater QT and I2 values indicating a significant amount of heterogeneity [45]. Rosenthal’s failsafe number and Spearman rank-order correlation were used for publication bias in MetaWin 2.1, with a failsafe number greater than 5n + 10 indicating no publication bias (Table S1), where n is the observation [47,48]. The heterogeneity of this meta-analysis was calculated as:
Additionally, we used Origin software 2021 to plot the kernel density estimations for enzyme activities, maize growth, and gas exchange parameters.
3. Results
3.1. Publication Bias and Data Heterogeneity
Thirty-two published studies with a total of 1649 pairwise observations were available for meta-analysis, and most of these studies involved multiple cases. Our meta-analysis indicated that data were normally distributed with strong heterogeneity for all moderators (fresh and dry root weight, fresh and dry shoot weight, plant height, leaf area, root length and diameter, photosynthetic rate, stomatal conductance, transpiration rate, intercellular CO2 concentration, chlorophyll a and b, carotenoid and total chlorophyll content, relative water content, SOD, POD, CAT, APX, GPX, O2, H2O2, MDA, proline, soluble sugar, soluble protein and electro leakage) among stress types, study types, and maize varieties, as characterized with greater values of QT and I-square (Figures S1–S3 and Table S1). The greater fail-safe numbers for all the above-mentioned parameters indicate no publication bias (Table S1), suggesting that the quality of the study meets the standard for meta-analysis.
3.2. Exogenous Melatonin Improved Biomass and Plant Growth
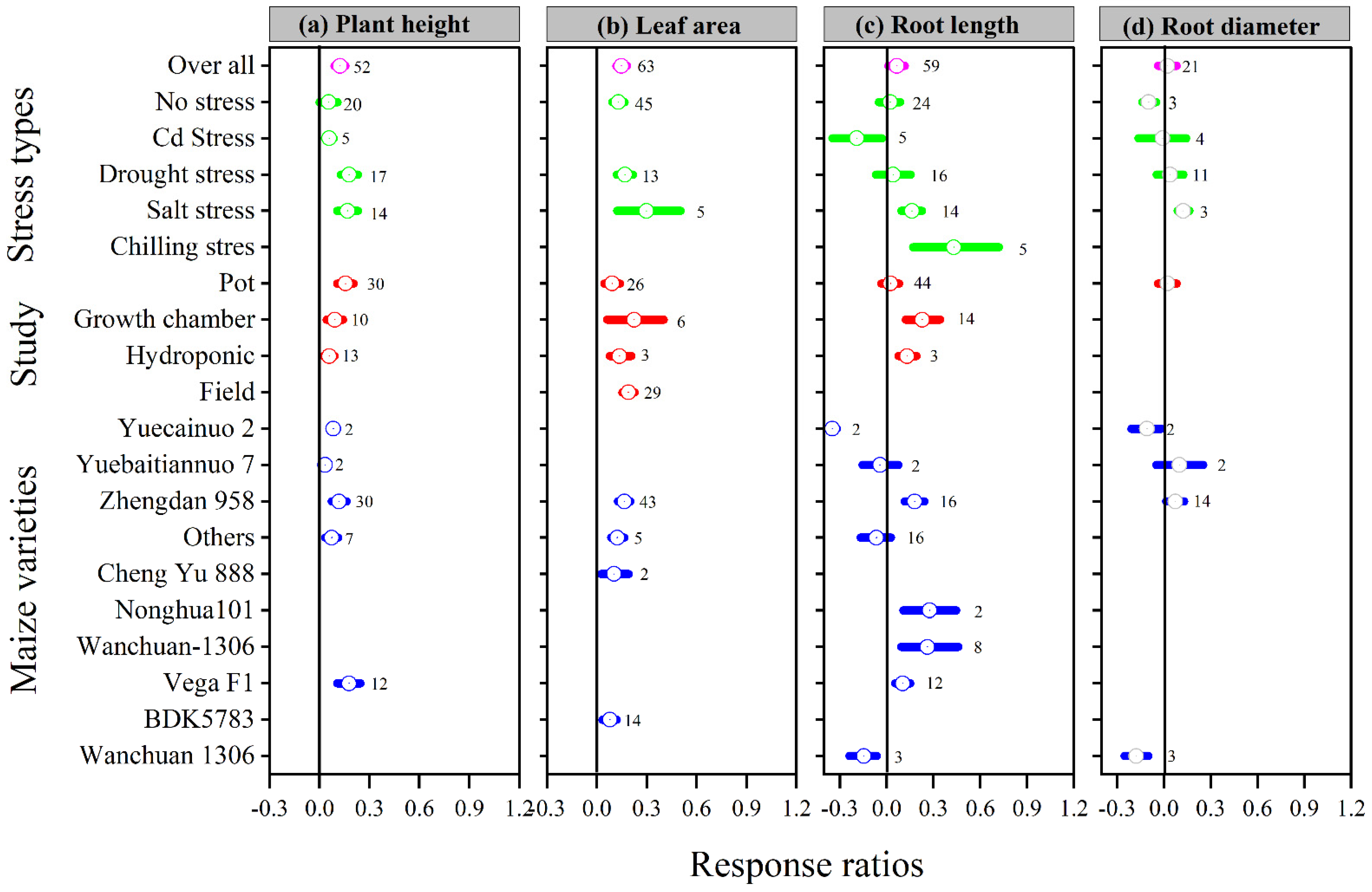
The overall lnRR++ was positive for plant height, leaf area, and root length with melatonin application; however, the overall lnRR++ for root diameter was not affected (Figure 1). These results suggest that the melatonin application increased plant height, leaf area, and root length compared to control. The categorical meta-analysis showed that different stress types, study types, and maize varieties have a variable effect on the above-mentioned parameter. Melatonin application significantly increased plant height and leaf area with all stress types, study types, and maize varieties, except Cd stress for plant height. Among the stress types, drought stress had the maximum plant height, which is not statistically different from salt stress. The categorical analysis also revealed that among the study types, the pot study had significantly higher plant height than hydroponic, while the maximum leaf area was observed for field study, which is significantly higher than the pot study (Figure 1). Among the maize varieties, Yuecainuo 2 and Wanchuan 1306 resulted in lower lnRR++ for root length and Wanchuan 1306 for root diameter, however, the Zhengdan 958 had positive and greater lnRR++ for both root length and root diameter.
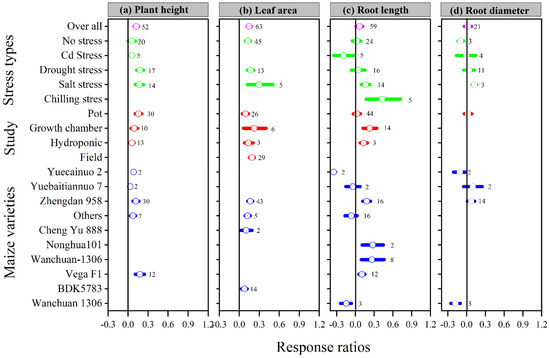
Figure 1.
Response ratio of melatonin compared to control with bootstrapped 95% confidence interval on plant height (a), leaf area (b), root length (c), and root diameter (d), for different stress types, study types, and maize varieties. The vertical line (lnRR++ = 0) indicates no difference between melatonin and control. Numbers following the box indicate the number of observations for comparison.
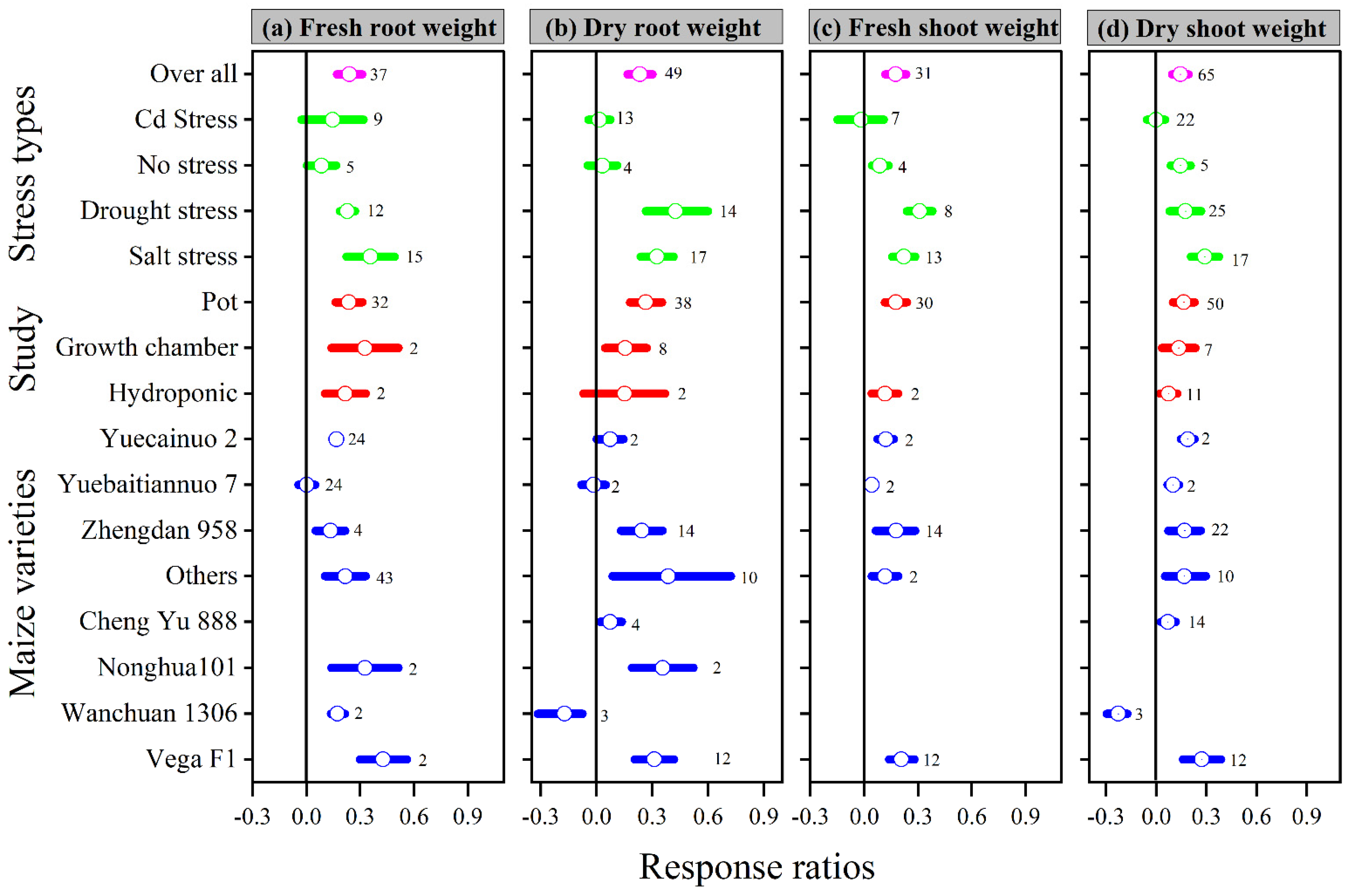
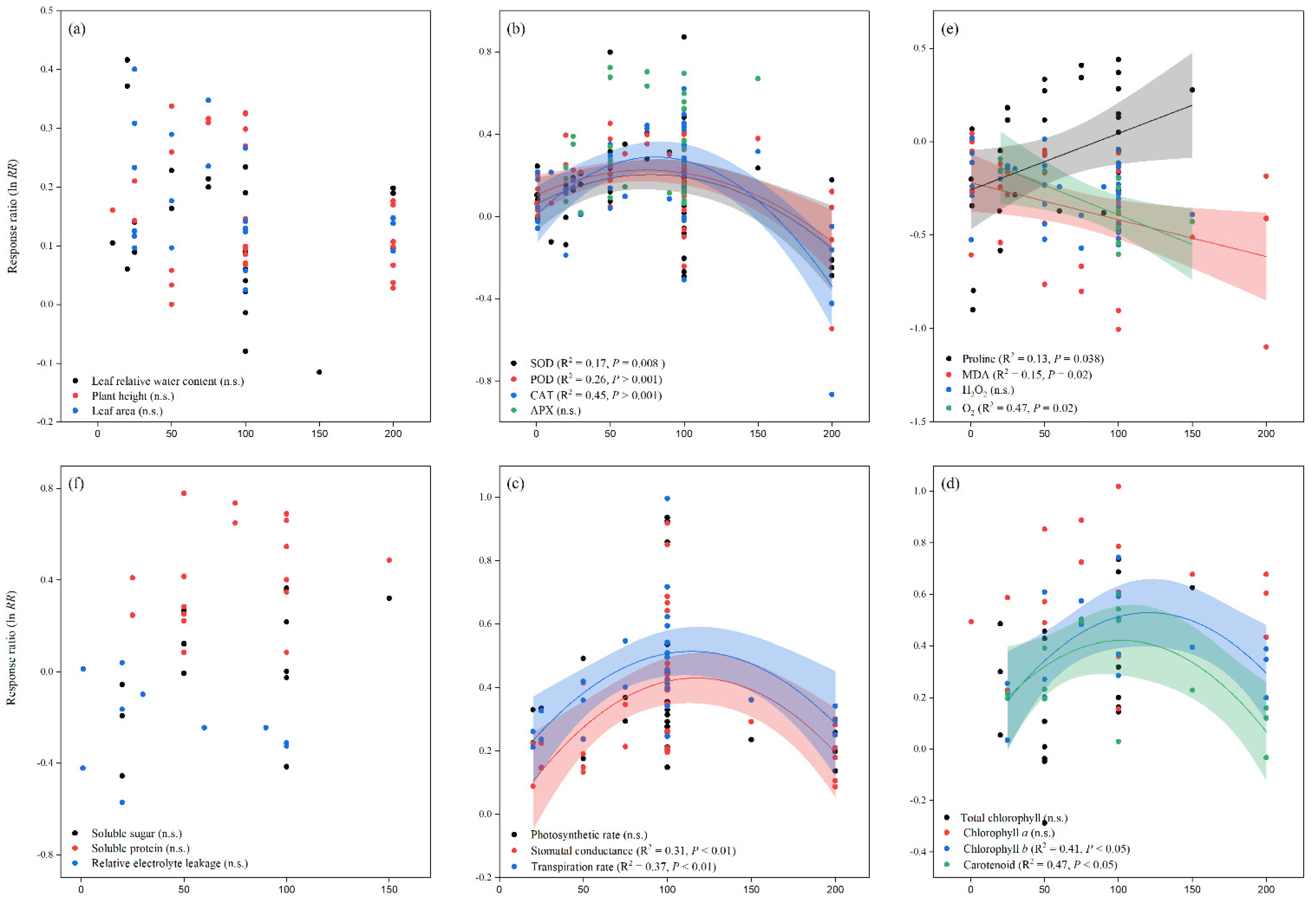
Similarly, when compared to the control, melatonin application increased the overall lnRR++ of fresh and dry root weight, as well as fresh and dry shoot weight, by 24, 23, 18, and 14%, respectively. The lnRR++ of fresh and dry root weight and fresh and dry shoot weight to melatonin application differed among stress types, study types, and maize varieties (Figure 2). Melatonin application significantly increased fresh and dry root weight under drought and salt stress but had no significant effect under Cd and no stress conditions. Fresh and dry shoot weight was significantly and positively affected by melatonin application with all stress types except Cd stress. Among the stress types, drought and salt stress had greater lnRR++ for fresh shoot weight and dry shoot weight, respectively. Among the study types, the growth chamber had the highest lnRR++ for fresh root weight and pot study for dry root weight, fresh and dry shoot weight (Figure 2). In contrast, no significant effect of melatonin application on dry root weight was observed for hydroponic study. The categorical analysis for maize varieties suggests that most of the varieties resulted in positive lnRR++, however, Wanchuan 1306 had negative lnRR++ for dry root and dry shoot weight. These results suggest that melatonin application to the maize variety Wanchuan 1306 decreased the dry root weight by 32 and the dry shoot weight by 30% compared to control. The regression analysis showed that the response ratio of plant height and leaf area was not related to melatonin application (Figure 3a).
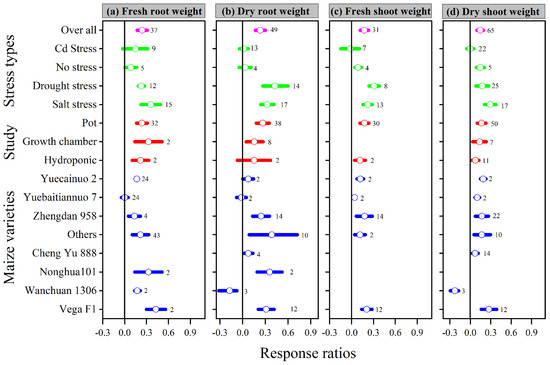
Figure 2.
Response ratio of melatonin compared to control with bootstrapped 95% confidence interval on fresh root weight (a), dry root weight (b), fresh shoot weight (c), and dry shoot weight (d), for different stress types, study types, and maize varieties. The vertical line (lnRR++ = 0) indicates no difference between melatonin and control. Numbers following the box indicate the number of observations for comparison.
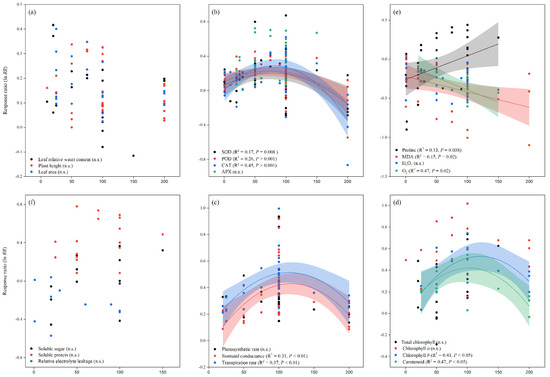
Figure 3.
Relationships between the response ratios of melatonin compared to control, leaf relative water content, plant height, and leaf area (a), SOD, POD, CAT, and APX (b), proline, MDA, H2O2 and O2 (c), soluble sugar, soluble protein, and relative electrolyte leakage (d), photosynthetic rate, stomatal conductance, and transpiration rate (e), total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid (f). The horizontal dash line (lnRR++ = 0) indicates no difference between cover cropping and no cover cropping systems.
3.3. Exogenous Melatonin Improved Enzymatic Activities
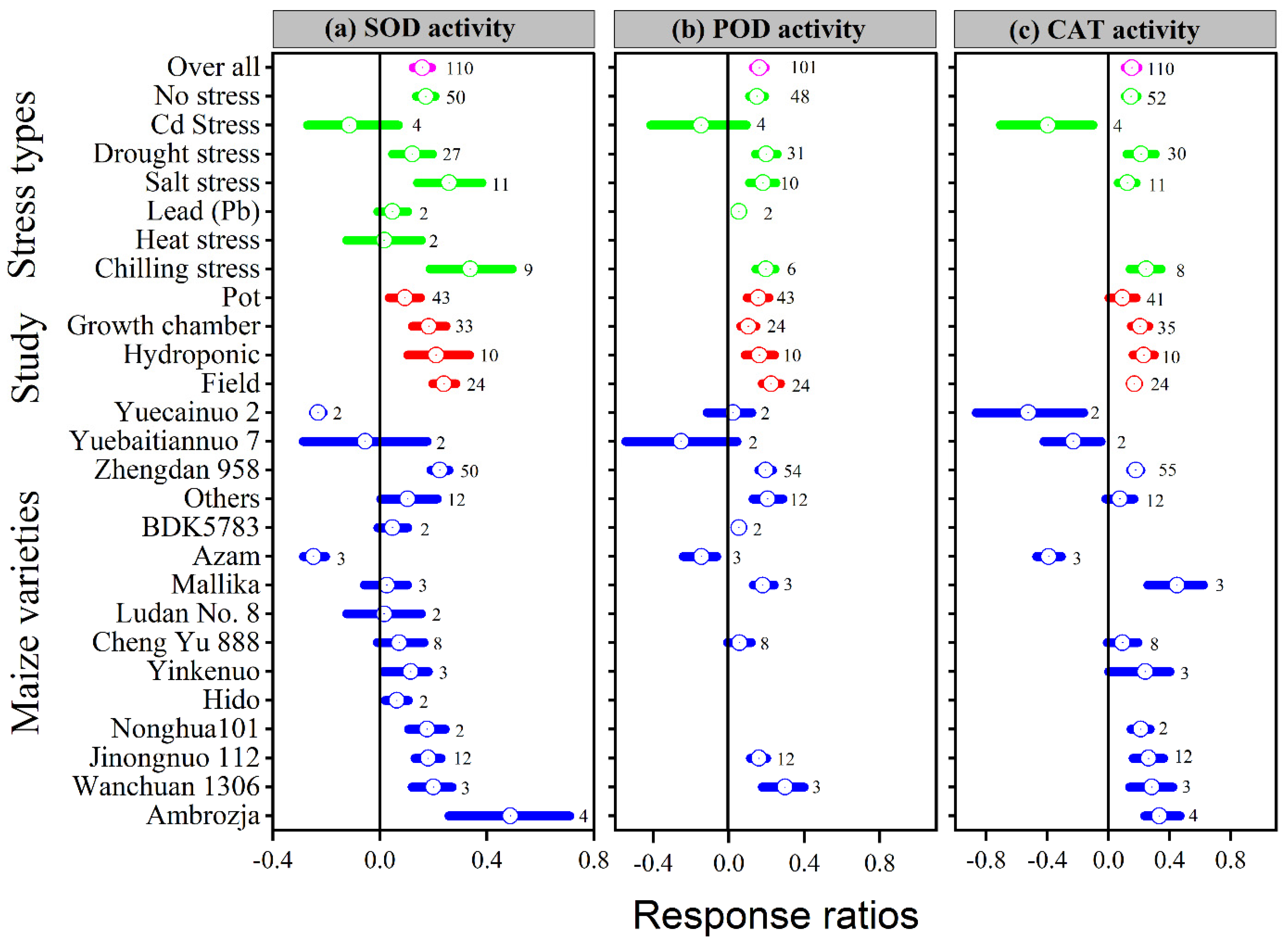
Application of melatonin increased overall antioxidant enzyme activities (SOD, POD, CAT and APX) by 16, 16, 15 and 24%, respectively, compared to control (p ≤ 0.05, Figure 4a–c and Figure 5a). In contrast, no significant effect of melatonin application on GPX activity was observed for the overall effect (Figure 5b). The categorical analysis showed that the melatonin application to Cd, lead, and heat stress had no effect on SOD activity. These results suggest that the lnRR++ for Cd, lead and heat stress overlaps the zero line, which means melatonin had no significant effect on SOD activity compared with control. Chilling stress resulted in higher SOD activity compared to no stress, drought stress, and salt stress; however, this increase was statistically similar.
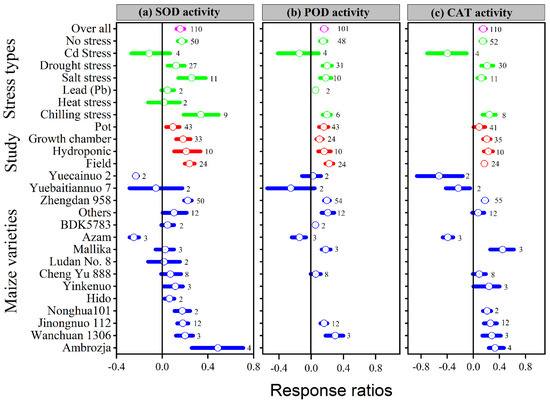
Figure 4.
Response ratio of melatonin compared to control with bootstrapped 95% confidence interval on SOD (a), POD (b), and CAT activities (c), for different stress types, study types, and maize varieties. The vertical line (lnRR++ = 0) indicates no difference between melatonin and control. Numbers following the box indicate the number of observations for comparison.
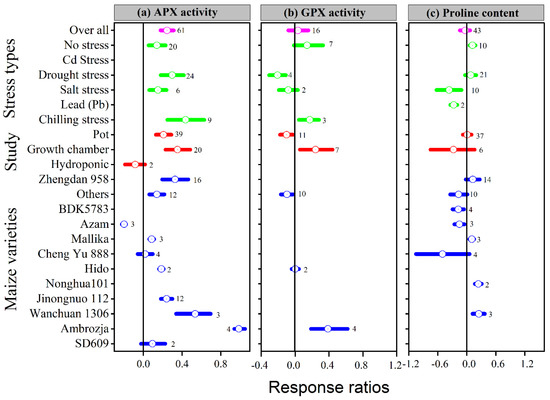
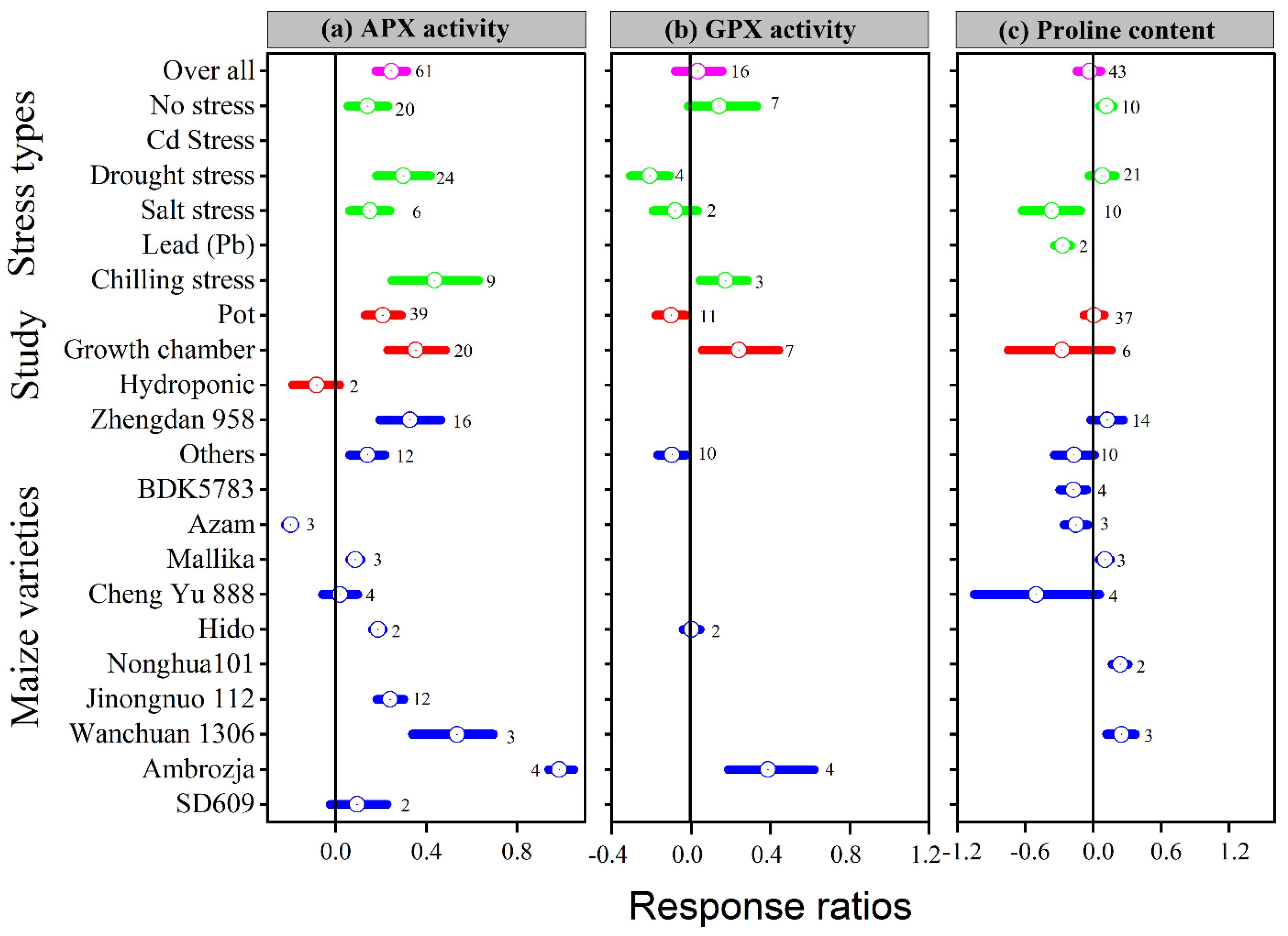
Figure 5.
Response ratio of melatonin compared to control with bootstrapped 95% confidence interval on APX (a), GPX (b), and proline content (c), for different stress types, study types, and maize varieties. The vertical line (lnRR++ = 0) indicates no difference between melatonin and control. Numbers following the box indicate the number of observations for comparison.
Our results showed that the effect of melatonin application on POD, CAT and APX activities was significantly higher and positive with all types of stresses (Figure 4b,c and Figure 5a), except Cd stress for POD and CAT activities. Similarly, the lnRR++ of melatonin for SOD and POD activities were significantly higher in all study types, with a significantly higher increase of 24 and 22% in the field study (Figure 4a,b). In contrast, there was no significant effect of melatonin on CAT activities in the pot study over control (Figure 4c), however, the pot study significantly decreased the GPX activity (Figure 5b). The lnRR++ of melatonin application on enzymatic activities also varied with different maize varieties (Figure 4 and Figure 5). The lnRR++ of SOD activity was significantly greater in the Ambrozja variety than in the Hido variety, but not significantly different from Zhengda 958, others, Yinkenuo, Nonghua 101, Jinonguo 112, and Wanchuan 1306. Among the maize varieties, the lnRR++ of Yuecainuo, Yuebaitiannuo, and Cheng Yu 888 varieties have no effect on POD activities; however, the variety Azam significantly decreases POD and CAT activities. The lnRR++ of GPX activity was significantly higher in the Ambrozja variety with chilling stress in the growth chamber study (Figure 5b). The regression analysis revealed that the lnRR++ of SOD, POD and CAT activities are polynomially affected by melatonin levels. No significant relationship was found between lnRR++ of APX activity (Figure 3d). These activities are polynomially increased with increasing melatonin level up to 100 µM, and further increasing melatonin application-level declines the SOD, POD and CAT activities. Furthermore, about 17, 26 and 45% of the variability in lnRR++ of SOD, POD and CAT activities were explained by melatonin application levels (Figure 3b).
3.4. Exogenous Melatonin Effect Proline and Reduced the Malonaldehyde, Superoxide, and Hydrogen Peroxide
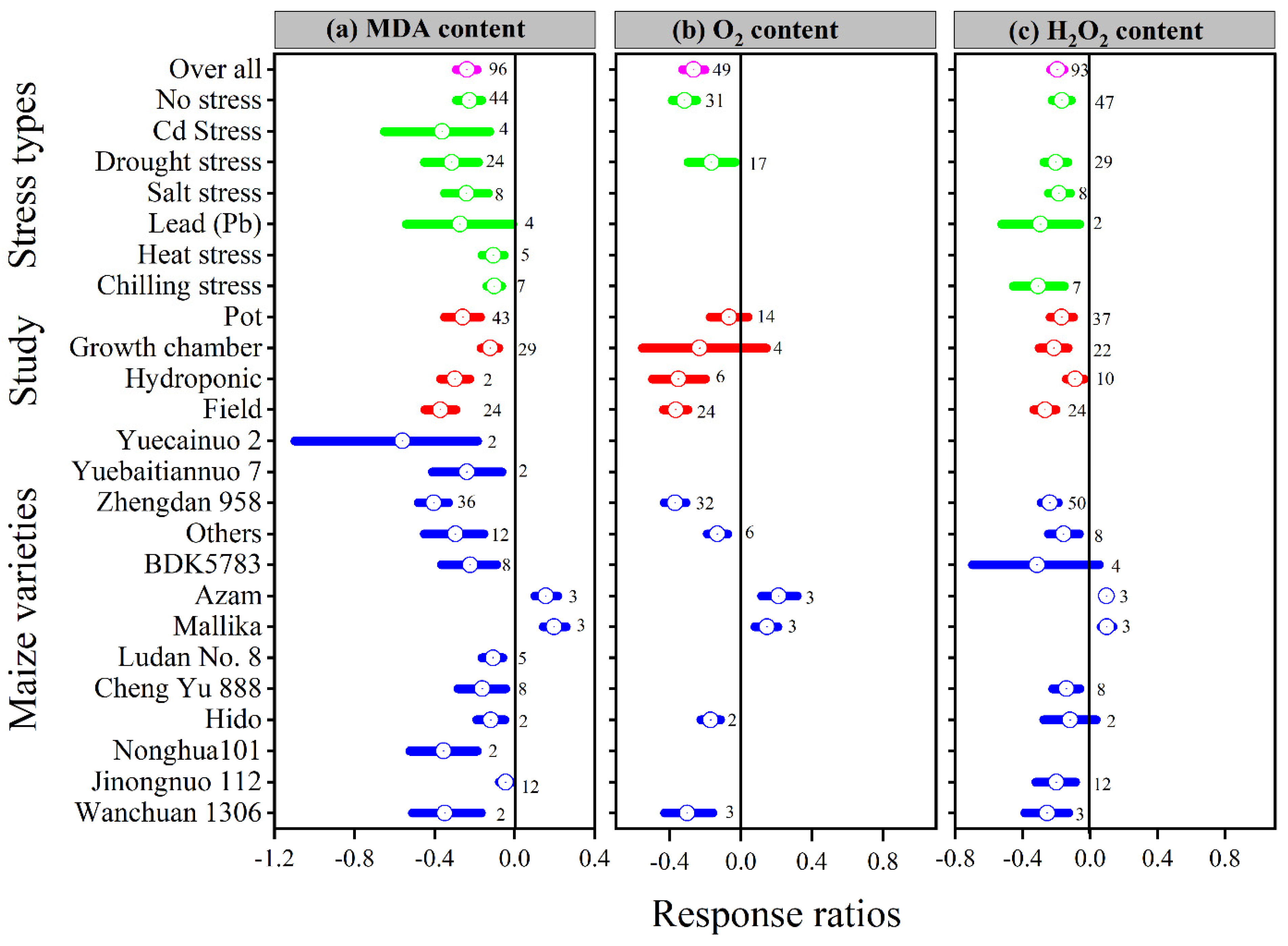
Melatonin application had a significant effect on the lnRR++ of proline, malonaldehyde, superoxide, and hydrogen peroxide under different stress types, study types, and maize varieties (Figure 5a and Figure 6a–c). Compared with the control, melatonin application had no significant effect on the overall lnRR++ of proline content (Figure 5c). However, significantly decreased the overall lnRR++ of MDA by 24%, O2 by 27% and H2O2 content by 19% (Figure 6a–c). The results of the current meta-analysis revealed that the melatonin application significantly increased proline by 12% in no stress compared to control. Similarly, among different maize varieties, Nonghua 101 and Wanchuan 1306 increased proline by 24 and 25%, respectively (Figure 5c). Generally, melatonin application mitigated the MDA, O2 and H2O2 content, and the decrease was significant for all types of stresses (Figure 6a–c). Moreover, the lnRR++ of O2 content to melatonin application was not significantly affected with pot and growth chamber studies. Among the maize varieties, Azam and Mallika significantly increased the MDA, O2 and H2O2 content, vice versa. However, melatonin application had no significant effect on H2O2 content when applied to BDK5783, and Hido maize varieties (Figure 6c). The lnRR++ of H2O2 content was not related to melatonin levels, while MDA (R2 = 0.15, p = 0.02) and O2 (R2 = 0.47, p = 0.02) content decreased linearly with increasing melatonin application levels (Figure 3c).
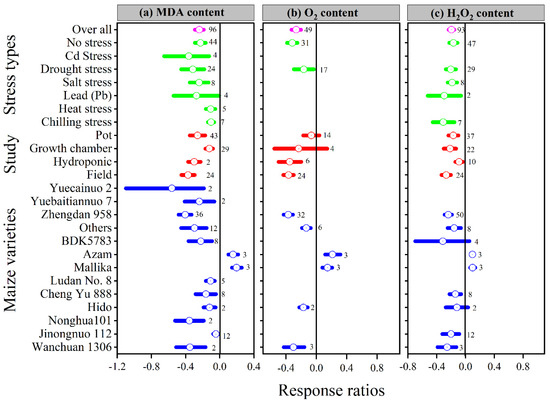
Figure 6.
Response ratio of melatonin compared to control with bootstrapped 95% confidence interval on MDA (a), O2 (b) and H2O2 (c), for different stress types, study types, and maize varieties. The vertical line (lnRR++ = 0) indicates no difference between melatonin and control. Numbers following the box indicate the number of observations for comparison.
3.5. Exogenous Melatonin Stimulated Soluble Sugar and Protein, and Reduced Electrolyte Leakage
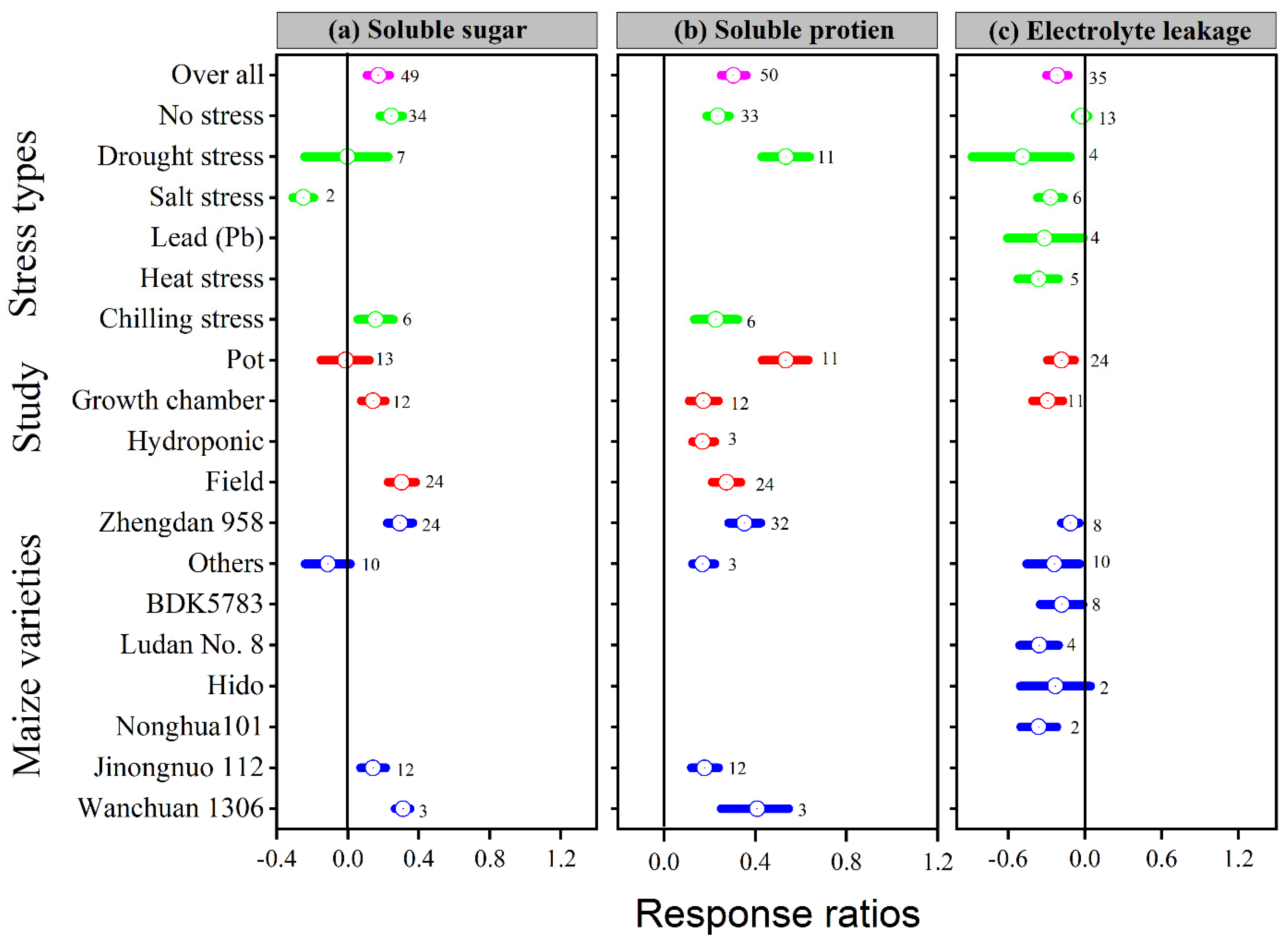
The lnRR++ of soluble sugar, soluble protein, and electrolyte leakage to melatonin application significantly differed among different stress types, study types, and maize varieties (Figure 7a–c). Overall, melatonin application significantly stimulated soluble sugar by 17%; p < 0.01, with a significantly higher increase of 24, 30 and 31% in the no stress, field study, and Wanchuan 1306, respectively (Figure 7a). Among the stress types, salt stress resulted in lower soluble sugar; in contrast, no stress and chilling stress increased the soluble sugar of the maize crop, whereas the pot study had no significant effect on soluble sugar, it significantly increased within the field study followed by growth chamber study. Similarly, melatonin application also increased soluble protein by 30%; p < 0.01, with a significantly higher increase of 54, 54 and 43% in the drought stress, pot study, and Wanchuan 1306 variety, respectively (Figure 7b). The categorical analysis of soluble protein was highly significant in stress types (p = 0.001), study types (p = 0.001), and maize varieties (p = 0.02). These results further demonstrated that the lnRR++ of drought stress was significantly higher than other stress types in the pot study with the Wanchuan 1306 maize variety and Zhengdan 958. The overall lnRR++ of electro leakage was significantly decreased with melatonin application compared to control (Figure 7c). Furthermore, the categorical analysis suggested that stress types significantly affected electrolyte leakage (p = 0.007), but study types and maize varieties decreased the electro leakage compared to control. The proline content was significantly and linearly increased with increasing melatonin application levels; however, the lnRR++ of soluble sugar, soluble protein, and relative electrolyte leakage were not significant with application of melatonin concentrations (Figure 3d).
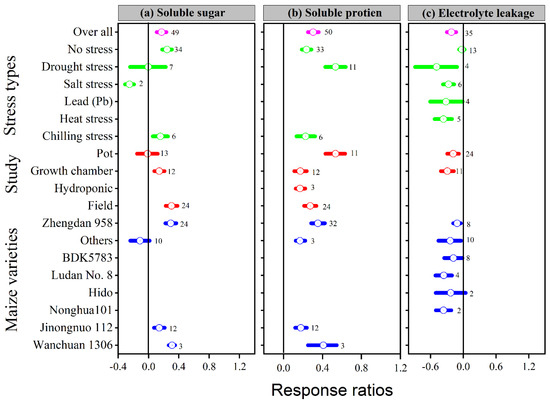
Figure 7.
Response ratio of melatonin compared to control with bootstrapped 95% confidence interval on soluble sugar (a), soluble protein (b), and electrolyte leakage (c), for different stress types, study types, and maize varieties. The vertical line (lnRR++ = 0) indicates no difference between melatonin and control. Numbers following the box indicate the number of observations for comparison.
3.6. Exogenous Melatonin Impacts Gas Exchange Parameters
The photosynthetic pigments were significantly affected with melatonin application under different stress types, study types, and maize varieties (Table 1). The overall lnRR++ of photosynthetic rate (p = 0.001), stomatal conductance (p = 0.001), and transpiration rate (p = 0.001) were positively affected by melatonin application. In contrast, the overall and categorical lnRR++ of intercellular CO2 concentration (p > 0.05) was not significantly affected by melatonin application, these results suggest that the lower CI is negative (−0.0285) and the upper CI is positive (0.1324), which means the lnRR++ of intercellular CO2 concentration overlaps the zero line. According to our categorical analysis, photosynthetic rate, stomatal conductance, and transpiration rate were positively increased by melatonin application compared to control; however, hydroponic studies have no effect on photosynthetic rate (Table 1). Moreover, this positive increase in photosynthetic rate, stomatal conductance, and transpiration rate was not statistically different among the different study types. The categorical analysis for maize varieties showed that Azam variety significantly decreased the photosynthetic rate and stomatal conductance (p < 0.05), however, had no effect on transpiration rate. The relationship between stomatal conductance and transpiration rate was found to be significant for melatonin levels. The stomatal conductance and transpiration rate were polynomially increased and showed 31 and 37% variability, respectively (Figure 3e). The stomatal conductance (R2 = 0.31, p < 0.01) and transpiration rate (R2 = 0.37, p < 0.01) were maximized at 100 µM melatonin application.

Table 1.
Response ratio of melatonin compared to control with bootstrapped 95% confidence interval on photosynthetic rate, stomatal conductance, transpiration rate, and intercellular CO2 concentration for different stress types, study types, and maize varieties. The negative bootstrap values indicate no difference between melatonin and control.
3.7. Exogenous Melatonin Improved Leaf Chlorophyll Content
Table 2 shows that leaf chlorophyll and carotenoid content, demonstrating that melatonin application significantly increased the overall lnRR++ of total chlorophyll, chlorophyll a and b, and carotenoid compared to control. In addition, most of the stress types resulted in positive and greater leaf chlorophyll content with melatonin application than without melatonin. However, this positive increase was not statistically different among the stress types (p > 0.05). Both study types (p = 0.001) and maize varieties (p = 0.002) significantly increased the leaf chlorophyll content, with a greater increase of 28% in the field study and 59% when using the Wanchuan 1306 maize variety (Table 2). With melatonin application, total chlorophyll, chlorophyll a and b, and carotenoid levels were significantly increased and significantly different among different stress types. Furthermore, when compared to the control, melatonin application into different study types and maize varieties resulted in higher chlorophyll a, b, and carotenoid levels, but these levels were not significantly different in the corresponding groups (study types and maize varieties). The lnRR++ of chlorophyll b (R2 = 0.41, p < 0.05) and carotenoid (R2 = 0.47, p < 0.05) increased polynomially with increased melatonin application levels (Figure 3f).

Table 2.
Response ratio of melatonin compared to control with bootstrapped 95% confidence interval on leaf chlorophyll content, chlorophyll a content, chlorophyll b content, and carotenoid for different stress types, study types, and maize varieties.
3.8. Effect of Exogenous Melatonin on Leaf Relative Water Content, Leaf Relative Water Potential, and Water Use Efficiency
The leaf relative water content is shown in Table 3, these results confirm that melatonin application significantly increased the leaf relative content compared to control. Among the different stress types, drought stress had a higher leaf relative content (11.4%), which is statistically similar to salt stress (11.1%) and significantly higher than no stress (p = 0.002; Table 3). Compared to control, melatonin application resulted in higher lnRR++ for both pot and growth chamber studies, but both of the study types are statistically similar (p > 0.05). Leaf relative water content was also significantly affected by maize varieties. Our categorical analysis revealed that Wanchuan 1306 significantly decreased the leaf relative water content (p = 0.02); however, the rest of the varieties resulted in higher leaf relative water content Table 3. The overall lnRR++ of leaf water potential and water use efficiency were not affected by melatonin application (Table 3). The leaf water potential was also significantly affected by stress types (p = 0.03), but not by study types and maize varieties (p > 0.05). Lead stress significantly increased leaf water potential compared to other stress in the group. The categorical analysis for water use efficiency revealed that there is no significant effect among the stress types, study types, and maize varieties (p > 0.05; Table 3).

Table 3.
Response ratio of melatonin compared to control with bootstrapped 95% confidence interval on relative water content, leaf water potential, and water use efficiency for different stress types, study types, and maize varieties.
4. Discussion
It is well known that drought, salt, heavy metals, chilling, and heat stress are the major environmental factors in the soil that inhibit and negatively affect plant growth, development, and production [11,49]. Under stressful situations, plants commonly respond by reducing biomass production, photosynthetic efficiency, enzymatic activity, and producing more ROS [50]. Melatonin is a newly discovered plant growth regulator that has a remarkable growth-promoting influence at all stages of plant development, from seed germination to vegetative and reproductive development [51,52,53]. This meta-analysis evaluated the protective effects of melatonin application on different maize varieties and stress types. Our meta-analysis showed that melatonin had a positive effect on plant growth parameters compared to control, which confirmed our first hypothesis that melatonin significantly increased plant height, leaf area, root length, fresh and dry root weight, and fresh and shoot weight. The increase in the above parameters with melatonin was probably due to its remarkable antioxidant effects against oxidative stress, and it is involved in numerous physiological systems in plants [1,52]. Our meta-analysis showed that the melatonin application significantly increased the morphological attributes of maize by modulating its plant height, leaf area, root length, fresh and dry root weight, and fresh and dry shoot weight compared to control (Figure 1 and Figure 2). Recently, it was noted that compared to control, melatonin application at 100 µM had significantly greater root and shoot biomass and root length of tobacco under Cd stress [54], and greater root and shoot biomass, leaf area, root and shoot length, and primary root length of maize crop were reported under drought stress [1,55]. In addition, the melatonin had a strong and positive effect on the lnRR++ of plant height, leaf area, fresh and dry root weight, fresh and dry shoot weight under drought and salt stress, root length under chilling stress, and root diameter under salt stress. Similarly, melatonin application increased plant height and leaf area for all maize varieties, but for Yuecainuo 2 and Wanchuan 1306, the melatonin application resulted in short root length, and Wanchuan 1306 had thinner root diameter, lower dry root and shoot weight (Figure 1 and Figure 2). Conversely, stress-reduced root length, plant height, leaf surface area, and chlorophyll content while increasing ROS such as O2 and H2O2, as well as oxidative damage such as MDA content and electrolyte leakage level. However, melatonin application significantly increased plant growth parameters while reducing ROS content and oxidative damage compared to control [1,10].
Melatonin has also been shown to boost antioxidant enzyme activities by maintaining higher antioxidant enzyme activity to reduce oxidation damage in plants caused by abiotic stress, such as cold, drought, Cd, lead, heat, and salinity [1,56,57]. Antioxidant enzyme activity regulation is an intrinsic plant response to counteract oxidative stress generated by diverse biotic and abiotic environmental factors [33,58]. The overall positive lnRR++ of SOD, POD, CAT, and APX revealed that melatonin application had a significant effect on the antioxidant defense systems of the maize crop compared with control but had no effect on GPX activity (p < 0.05; Figure 4 and Figure 5). The melatonin application significantly increased the SOD activity of maize leaf under drought, salt, and chilling stress, but had no effect under Cd, lead, and heat stress. In addition, a significant positive effect of melatonin on POD and CAT activities under all stress types was detected, except that Cd stress had no effect on SOD and significantly decreased CAT activity compared to control (Figure 4b,c). Likewise, melatonin application to different maize varieties had effects on the antioxidant defense system, suggesting that Yuecainuo 2 and Azam significantly decreased SOD activity, Azam decreased POD and APX activities, and Yuecainuo 2, Yuebaitiannuo 7 and Azam had lower CAT activity. Melatonin application drastically decreased oxidative damage by modulating antioxidant activities such as SOD, POD, CAT, and APX activities, resulting in lower MDA content in wheat, maize, and Bermuda grass [1,59,60]. Our findings are in agreement with the previous findings of Lin et al. [61], who reported that increasing melatonin application up to 100 µM improved antioxidant enzyme activities, but further increasing melatonin from 150 to 200 µM had a deleterious impact on Tamarillo (tree tomato). In contrast, Ma, Huang, Li, Ashraf, Yang, Liu, Xu, Li and Mo [1] detected that melatonin application at 200 μM could increase the antioxidant defense mechanism in maize. Thus, melatonin application at lower rates could be ascribed to a modulation in antioxidant activities, which can also result in a significant reduction in oxidative plant damage [1,62]. In order to protect cells from damage, the antioxidative defense system includes SOD, POD, CAT and APX. This system helps to maintain the equilibrium of ROS in cells, slows down membrane lipid peroxidation, and resists stress [31]. Melatonin can directly scavenge ROS, maintain steady H2O2 concentrations [63], and indirectly scavenge H2O2 by stimulating CAT and POD activities in plants under environmental stress conditions [31].
The results of this meta-analysis showed that the melatonin application significantly decreased the overall lnRR++ of MDA, O2, H2O2, and electrolyte leakage by 24, 27, 19 and 21% compared to control (Figure 6a–c and Figure 7c). Additionally, the toxicity of Pb damaged membrane integrity, resulting in enhanced membrane deterioration and the formation of ROS, such as H2O2 and O2, in plant leaves [8,64]. Heavy metals and environmental stress are the key issues that disturb virtually all features of the biochemistry and physiology of plants. Okant and Kaya [8] reported that the universal antioxidant melatonin is able to easily pass through the plasma membrane and go into the subcellular sections, and thus the use of melatonin in plants under stress could be justified. Furthermore, the melatonin supplementation reduced Pb-induced oxidative stress by lowering H2O2 and MDA levels and lowering the electrolyte leakage rate [65]. Melatonin application significantly decreases the accumulation of H2O2, O2, and MDA in maize under drought stress [66] and cucumber seedlings under salinity stress [67]. Likewise, our results also suggest that melatonin application to all kinds of stress significantly decreases MDA, O2, H2O2 and electrolyte leakage compared to control (p < 0.05; Figure 6a–c and Figure 7c). It has been demonstrated that exogenous melatonin has a protective effect against membrane damage when subjected to salt stress [31] and Cd stress [1], owing to a decrease in H2O2 and MDA levels in leaves. Guo, Li, Zhao, Xue and Zhang [2] demonstrated that MDA levels increased under drought stress; however, melatonin application reduced the accumulation of H2O2, O2, and MDA levels in both cultivars SD609 and SD902 under drought stress. Increased SOD, POD, and APX activities were found to be associated with lower electrolyte leakage and MDA concentration in melatonin-treated maize leaves [24], and cucumber leaves [56], under salt stress, showing that salinity-induced oxidative damage is susceptible to mitigation through the application of melatonin. Under salinity stress, plants enhance their osmotic adaptation capacity by synthesizing and accumulating organic osmolytes; soluble sugar is one of the essential osmolytes for osmotic adjustment [39,68]. The categorical analysis revealed that the soluble sugar was dramatically increased by melatonin application under no and chilling stress, and protein under all stress types, but salt stress resulted in lower soluble sugar than control (Figure 7a,b). It has been demonstrated that drought stress can affect protein production [69]. However, melatonin has been found to repair PSII by maintaining protein availability in tomato under salt stress [70], and to protect PSII proteins in maize under drought stress [71].
Reductions in photosynthetic pigments and antioxidant enzyme activities, as well as morphological growth, were found in maize exposed to salt stress [24], Cd stress [1,6], and drought stress [21]. Drought stress triggers stomatal closure or destruction of photosynthetic reaction in plants, which can result in a significant decrease in photosynthetic rate [72,73]. Previous studies demonstrated that melatonin application had significantly higher photosynthetic efficiency, transpiration rate, and stomatal conductance than control in tomato [70], wheat [74] and Medicago sativa [75]. Similarly, our results showed that the overall lnRR++ of melatonin-treated maize resulted in a higher photosynthetic rate (17%), stomatal conductance (17%), and transpiration rate (21%) than those of the control (Table 1). However, melatonin had a nonsignificant effect on the overall and categorical lnRR++ of intercellular CO2 concentration. Furthermore, melatonin application to drought and salt stress significantly increased the photosynthetic rates, stomatal conductance, and transpiratory rates compared to control. In contrast, the negative bootstrap value suggests that the melatonin application to no-stress maize is not statistically significant (Table 1). Campos, Avila, de Souza, Azevedo and Alves [73] demonstrated that enhanced tomato tolerance to drought stress by stimulating cuticle production, which in turn reduced water loss. In drought stress conditions, those plants treated with 300 M of melatonin showed higher stomatal conductance and photosynthetic and transpiration rates, providing a greater supply of assimilates for growing tissues. A previous study reported that melatonin treatment enhanced gas exchange parameters and thus biochemical reactions by regulating leaf water potential [73]. Previous studies have shown that tomato seedlings treated with melatonin provide greater stomatal conductance and contribute to the maintenance of photosynthetic rates under water deficit conditions [70], Arabian coffee [73], and maize under salt stress conditions [15].
Melatonin regulates flowering, photosynthesis, chlorophyll synthesis, callus formation, root regeneration, and plant photosynthetic properties under varied environmental stressors. [56,76]. Melatonin is also an antioxidant that protects plants from biotic and abiotic stresses [38,77], such as salt [41,78], drought [79,80,81], cold [59,82], and heavy metal stress [7]. Melatonin application reduced stress-induced photosynthetic inhibition (Table 2), suggesting that the overall lnRR++ of total chlorophyll, chlorophyll a and b, and carotenoid were 18, 38, 24, and 18% higher than control, respectively. These results are in line with the findings of Zhou, Zhao, Cao, Hu, Du, Baluška and Zou [70] and Chen, Mao, Sun, Huang, Ding, Gu, Liao, Hu, Zhang and Yuan [3], they found that the increased biomass in melatonin-treated plants could be attributed to the plants’ ability to retain a high photosynthetic capacity. However, melatonin had no effect on total chlorophyll under Cu stress, chlorophyll a and b under no stress, and carotenoid under no and salt stress conditions (Table 2). Melatonin at the rate of 10 µM was reported to improve nitrogen metabolism and proline stability in drought-stressed alfalfa, resulting in greater levels of chlorophyll content [75,83]. It is known that salt stress can limit the synthesis of chlorophyll and speed up its decomposition because of chlorophyll fragility and susceptibility to ROS [7,84]. There is some evidence that melatonin treatment delays leaf senescence and improves tolerance to salinity stress in rice leaves [85], as well as improving the photosynthetic pigment-synthesis pathway and slowing the decomposition rate of chlorophyll in maize leaves under salinity stress [31]. The overall relative water content in the leaf was significantly increased with melatonin under stress conditions compared to control, but melatonin application had no effect on relative water content under no stress conditions (Table 2). These results are supported by Qiao, Ren, Yin, Liu, Deng, Liu and Wang [21], who demonstrated that under control conditions, whether with or without melatonin application, the leaf relative water content did not alter, although melatonin effectively reduced the stress-induced reduction in leaf relative water content. According to Jiang, Li and Song [24], who reported that plants exposed to salinity stress had lower leaf water content, this was alleviated by 1 µM melatonin application. The categorical meta-analysis revealed that the melatonin application had no significant effect on leaf water potential under no stress, drought, and salt stress conditions. However, lead stress resulted in 11% higher water potential compared to control (Table 2). In contrast, a recent study reported that leaf relative water potential significantly increased by 15.9% in melatonin-treated plants under drought stress conditions compared to control [21]. Sharma and Dubey [86] and Okant and Kaya [8] reported that lead toxicity disrupted water status in plants, which could be an explanation of low water potential under Pb stress. Melatonin application resulted in higher leaf water potential than those of the water deficit [73].
5. Conclusions
The meta-analysis identified that melatonin has a varied effect on plant growth, antioxidant defense system, gas exchange parameters, photosynthetic pigments, and soluble sugar and protein content under different stress types, study types, and maize varieties. Melatonin application increased plant growth, antioxidant enzyme activities, gas exchange parameters, photosynthetic pigments, and soluble sugar and protein compared to control. The greater QT and I-square values showed the total heterogeneity among the studies in terms of stress types, study types, and maize varieties. Drought stress with melatonin had the highest photosynthetic rate and stomatal conductance followed by salt stress, but the transpiration rate was greater in salt stress. Melatonin had no effect on the overall and category lnRR++ for intercellular CO2 concentration. However, melatonin had an overall negative impact on MDA, O2, H2O2 and electrolyte leakage under all stress types, but no stress had any effect on electrolyte leakage compared to control. The categorical analysis showed that all the maize varieties decreased the MDA, O2 and H2O2 content but increased with Azam and Mallika, whereas BDK5783 and Hido had no effect on H2O2 content. We concluded from our results that melatonin performs various functions under stress and that tailored changes can increase maize’s stress tolerance through decreasing ROS and improving the antioxidant defense system. However, further research is needed to determine the effect of melatonin in combination with synthetic fertilizers and other signaling molecules in response to various biotic and abiotic stress, as well as the optimal dose of melatonin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11030512/s1, Figure S1: Kernel density estimates (smoothed version of the histogram) for plant growth parameters; Figure S2: Kernel density estimates (smoothed version of the histogram) for gas exchange parameters, and chlorophyll pigments; Figure S3: Kernel density estimates (smoothed version of the histogram) for antioxidant enzymes, soluble sugar, soluble protein, proline, and relative electro leakage; Table S1 Observations’ number (n) and results of testing publication bias and heterogeneity for each target variable;
Author Contributions
Conceptualization, I.M.; Methodology, I.M. and L.Y.; Formal Analysis, I.M., L.Y. and S.A.; Investigation, I.S.M.M., A.A.A.-G. and A.M.A.; Resources, X.-B.Z. Data Curation, I.M. and L.Y.; Writing—Original Draft Preparation: I.M.; Writing—Review and Editing, S.A.; Supervision, X.-B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Natural Science Foundation of Guangxi Province (Grant No. 2019GXNSFAA185028) and Bama County Program for Talents in Science and Technology, Guangxi, China (Grant No. 20210049). The authors extend their appreciation to the Researchers Supporting Project number (Grant No. RSP2022R483), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Acknowledgments
The authors express their special gratitude to all the funding sources and especially to Guangxi University for the financial assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, L.; Huang, Z.; Li, S.; Ashraf, U.; Yang, W.; Liu, H.; Xu, D.; Li, W.; Mo, Z. Melatonin and nitrogen applications modulate early growth and related physio-biochemical attributes in maize under Cd stress. J. Soil Sci. Plant Nutr. 2021, 21, 978–990. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Zhao, C.; Xue, J.; Zhang, R. Exogenous melatonin improves drought tolerance in maize seedlings by regulating photosynthesis and the ascorbate–glutathione cycle. Russ. J. Plant Physiol. 2020, 67, 809–821. [Google Scholar] [CrossRef]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Ali, S.; Rizwan, M.; Hussain, M.B.; Hussain, A.; Alam, P.; Alqarawi, A.A.; Hashem, A.; AbdAllah, E.F. The ameliorative role of 5-aminolevulinic acid (ALA) under Cr stress in two maize cultivars showing differential sensitivity to Cr stress tolerance. J. Plant Growth Regul. 2019, 38, 788–798. [Google Scholar] [CrossRef]
- Lv, S.; Yang, X.; Lin, X.; Liu, Z.; Zhao, J.; Li, K.; Mu, C.; Chen, X.; Chen, F.; Mi, G. Yield gap simulations using ten maize cultivars commonly planted in Northeast China during the past five decades. Agric. For. Meteorol. 2015, 205, 1–10. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Zia-ur-Rehman, M.; Abbas, Z.; Hannan, F. Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: A critical review. Environ. Geochem. Health 2017, 39, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Sürücü, A.; Mohammad, D.M.; Günal, E.; Budak, M. Concentration of heavy metals in soils along three major roads of Sulaimani, Northeast Iraq. Carpathian J. Earth Environ. Sci. 2018, 13, 523–538. [Google Scholar] [CrossRef]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef]
- Cao, Q.; Li, G.; Cui, Z.; Yang, F.; Jiang, X.; Diallo, L.; Kong, F. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci. Rep. 2019, 9, 15044. [Google Scholar] [CrossRef] [Green Version]
- Turk, H.; Genisel, M. Melatonin-related mitochondrial respiration responses are associated with growth promotion and cold tolerance in plants. Cryobiology 2020, 92, 76–85. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamran, M.; Ding, R.; Meng, X.; Wang, H.; Ahmad, I.; Fahad, S.; Han, Q. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 2019, 7, e7793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pál, M.; Horváth, E.; Janda, T.; Páldi, E.; Szalai, G. Physiological changes and defense mechanisms induced by cadmium stress in maize. J. Plant Nutr. Soil Sci. 2006, 169, 239–246. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.; Zia-ur-Rehman, M.; Naeem, A.; Nawaz, R.; Ali, S.; Murtaza, G.; Maqsood, M.A.; Azhar, M.; Khalid, H.; Rizwan, M. Photosynthesis and growth response of maize (Zea mays L.) hybrids exposed to cadmium stress. Environ. Sci. Pollut. Res. 2017, 24, 5521–5529. [Google Scholar] [CrossRef]
- Ren, S.; Deng, Q.; Peng, J.; Lin, L.; Zhang, H. Effects of exogenous melatonin on growth and cadmium content of Zizyphus acidojujuba seedlings. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 042006. [Google Scholar]
- Ahmad, S.; Kamran, M.; Zhou, X.; Ahmad, I.; Meng, X.; Javed, T.; Iqbal, A.; Wang, G.; Su, W.; Wu, X. Melatonin improves the seed filling rate and endogenous hormonal mechanism in grains of summer maize. Physiol. Plant. 2021, 172, 1059–1072. [Google Scholar] [CrossRef]
- Khan, A.; Zahir Afridi, M.; Airf, M.; Ali, S.; Muhammad, I. A sustainable approach toward maize production: Effectiveness of farm yard manure and urea N. Ann. Biol. Sci. 2017, 5, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Lv, X.; Li, T.; Wen, X.; Liao, Y.; Liu, Y. Effect of potassium foliage application post-anthesis on grain filling of wheat under drought stress. Field Crops Res. 2017, 206, 95–105. [Google Scholar] [CrossRef]
- Kamran, M.; Wennan, S.; Ahmad, I.; Xiangping, M.; Wenwen, C.; Xudong, Z.; Siwei, M.; Khan, A.; Qingfang, H.; Tiening, L. Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Sci. Rep. 2018, 8, 4818. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Ren, J.; Yin, L.; Liu, Y.; Deng, X.; Liu, P.; Wang, S. Exogenous melatonin alleviates PEG-induced short-term water deficiency in maize by increasing hydraulic conductance. BMC Plant Biol. 2020, 20, 218. [Google Scholar] [CrossRef]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin improves drought resistance in maize seedlings by enhancing the antioxidant system and regulating abscisic acid metabolism to maintain stomatal opening under PEG-induced drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Dubey, S.; Bhargava, A.; Fuentes, F.; Shukla, S.; Srivastava, S. Effect of salinity stress on yield and quality parameters in flax (Linum usitatissimum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 954–966. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Song, X. Seed priming with melatonin effects on seed germination and seedling growth in maize under salinity stress. Pak. J. Bot. 2016, 48, 1345–1352. [Google Scholar]
- Velmurugan, A.; Swarnam, P.; Subramani, T.; Meena, B.; Kaledhonkar, M. Water demand and salinity. In Desalination-Challenges and Opportunities; IntechOpen: London, UK, 2020. [Google Scholar]
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Chi, Y.X.; Gao, F.; Muhammad, I.; Huang, J.H.; Zhou, X.B. Effect of water conditions and nitrogen application on maize growth, carbon accumulation and metabolism of maize plant in subtropical regions. Arch. Agron. Soil Sci. 2022, 1–15. [Google Scholar] [CrossRef]
- Ghosh, N.; Adak, M.; Ghosh, P.; Gupta, S.; Gupta, D.S.; Mandal, C. Differential responses of two rice varieties to salt stress. Plant Biotechnol. Rep. 2011, 5, 89–103. [Google Scholar] [CrossRef]
- Abbasi, G.H.; Akhtar, J.; Ahmad, R.; Jamil, M.; Anwar-ul-Haq, M.; Ali, S.; Ijaz, M. Potassium application mitigates salt stress differentially at different growth stages in tolerant and sensitive maize hybrids. Plant Growth Regul. 2015, 76, 111–125. [Google Scholar] [CrossRef]
- Sezer, İ.; Kiremit, M.S.; Öztürk, E.; Subrata, B.A.G.; Osman, H.M.; Akay, H.; Arslan, H. Role of melatonin in improving leaf mineral content and growth of sweet corn seedlings under different soil salinity levels. Sci. Hortic. 2021, 288, 110376. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wang, J.; Shi, S.H.; Huang, L.X.; Zhu, M.; Li, F.H. Exogenous melatonin ameliorates salinity-induced oxidative stress and improves photosynthetic capacity in sweet corn seedlings. Photosynthetica 2021, 59, 327–336. [Google Scholar] [CrossRef]
- Kurunc, A.; Aslan, G.E.; Karaca, C.; Tezcan, A.; Turgut, K.; Karhan, M.; Kaplan, B. Effects of salt source and irrigation water salinity on growth, yield and quality parameters of Stevia rebaudiana Bertoni. Sci. Hortic. 2020, 270, 109458. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, N.; Ghassemi-Golezani, K. Physiological changes of Mentha pulegium in response to exogenous salicylic acid under salinity. Sci. Hortic. 2020, 267, 109325. [Google Scholar] [CrossRef]
- Youssef, N.M.; Hashish, K.I.; Taha, L.S. Salinity tolerance improvement of in vitro propagated Paulownia tomentosa using proline. Bull. Natl. Res. Cent. 2020, 44, 90. [Google Scholar] [CrossRef]
- Bowes, K.; Mercuri, A.M.; Rattighieri, E.; Rinaldi, R.; Arnoldus-Huyzendveld, A.; Ghisleni, M.; Grey, C.; Mackinnon, M.; Vaccaro, E. Palaeoenvironment and land use of Roman peasant farmhouses in southern Tuscany. Plant Biosyst. 2015, 149, 174–184. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, Y.; He, H.; Xi, Q.; Fu, J.; Zhao, Y. Exogenous melatonin improves growth in hulless barley seedlings under cold stress by influencing the expression rhythms of circadian clock genes. PeerJ 2021, 9, e10740. [Google Scholar] [CrossRef]
- Kul, R.; Esringü, A.; Dadasoglu, E.; Sahin, Ü.; Turan, M.; Örs, S.; Ekinci, M.; Agar, G.; Yildirim, E. Melatonin: Role in increasing plant tolerance in abiotic stress conditions. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019; pp. 109–128. [Google Scholar]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Li, W.; Liu, Z.; Tang, S.; Chen, L.; Ding, C.; Jiang, Y.; Ding, Y.; Li, G. Melatonin improves K+ and Na+ homeostasis in rice under salt stress by mediated nitric oxide. Ecotoxicol. Environ. Saf. 2020, 206, 111358. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z.; et al. Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J. Plant Growth Regul. 2021, 40, 1270–1283. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, X.; Niu, J. Effects of melatonin on morphological characteristics, mineral nutrition, nitrogen metabolism, and energy status in alfalfa under high-nitrate stress. Front. Plant Sci. 2021, 12, 694179. [Google Scholar] [CrossRef]
- Muhammad, I.; Sainju, U.M.; Zhao, F.; Khan, A.; Ghimire, R.; Fu, X.; Wang, J. Regulation of soil CO2 and N2O emissions by cover crops: A meta-analysis. Soil Till. Res. 2019, 192, 103–112. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, J.; Sainju, U.M.; Zhang, S.; Zhao, F.; Khan, A. Cover cropping enhances soil microbial biomass and affects microbial community structure: A meta-analysis. Geoderma 2021, 381, 114696. [Google Scholar] [CrossRef]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marin-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychol. Methods 2006, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Dieleman, W.I.; Janssens, I.A. Can publication bias affect ecological research? A case study on soil respiration under elevated CO2. New Phytol. 2011, 190, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Wang, J.; Khan, A.; Ahmad, S.; Yang, L.; Ali, I.; Zeeshan, M.; Ullah, S.; Fahad, S.; Ali, S.; et al. Impact of the mixture verses solo residue management and climatic conditions on soil microbial biomass carbon to nitrogen ratio: A systematic review. Environ. Sci. Pollut. Res. 2021, 28, 64241–64252. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, R.; Meysam, H.S. Effect of different levels of drought stress (PEG 6000 concentrations) on seed germination and inorganic elements content in purslane (Portulaca oleraceae L.) leaves. J. Stress Physiol. Biochem. 2012, 8, 51–61. [Google Scholar]
- Deng, B.L.; Yang, K.J.; Zhang, Y.F.; Li, Z.T. Can antioxidant’s reactive oxygen species (ROS) scavenging capacity contribute to aged seed recovery? Contrasting effect of melatonin, ascorbate and glutathione on germination ability of aged maize seeds. Free Radic. Res. 2017, 51, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Wang, M.; Duan, S.; Zhou, Z.; Chen, S.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in Nicotiana tabacum L. Ecotoxicol. Environ. Saf. 2019, 170, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (C-ucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Bhatla, S.C. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Islam, F.; Yang, C.; Nawaz, A.; Athar, H.-u.-R.; Gill, R.A.; Ali, B.; Song, W.; Zhou, W. Methyl jasmonate alleviates arsenic-induced oxidative damage and modulates the ascorbate–glutathione cycle in oilseed rape roots. Plant Growth Regul. 2018, 84, 135–148. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Chen, F.; Liao, M.a.; Tang, Y.; Liang, D.; Xia, H.; Lai, Y.; Wang, X.; Chen, C. Effects of melatonin on the growth and cadmium characteristics of Cyphomandra betacea seedlings. Environ. Monit. Assess. 2018, 190, 119. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 2020, 156, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Marta, B.; Szafrańska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Wang, W.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Lee, G.; Carrow, R.N.; Duncan, R.R.; Eiteman, M.A.; Rieger, M.W. Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ. Exp. Bot. 2008, 63, 19–27. [Google Scholar] [CrossRef]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant. 2016, 158, 225–235. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front. Plant Sci. 2016, 7, 1823. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem ii in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [Green Version]
- Gleason, S.M.; Wiggans, D.R.; Bliss, C.A.; Comas, L.H.; Cooper, M.; DeJonge, K.C.; Young, J.S.; Zhang, H. Coordinated decline in photosynthesis and hydraulic conductance during drought stress in Zea mays. Flora 2017, 227, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.N.; Avila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Ahmadi, J.; Mehrabi, A.A.; Etminan, A.; Moghaddam, M.; Siddique, K.H. Physiological responses to drought stress in wild relatives of wheat: Implications for wheat improvement. Acta Physiol. Plant. 2017, 39, 106. [Google Scholar] [CrossRef]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in M edicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Wang, T.; Wang, P. Mitigation effect and mechanism of exogenous melatonin on maize seedling under salt stress. Chin. J. Grassl. 2020, 42, 14–21. [Google Scholar]
- Cao, L.; Kou, F.; Zhang, M.; Jin, X.; Ren, C.; Yu, G.; Zhang, Y.; Wang, M. Effect of exogenous melatonin on the quality of soybean and natto products under drought stress. J. Chem. 2021, 2021, 8847698. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Zeeshan, M.; Zhou, X.B. Melatonin and KNO3 Application improves growth, physiological and biochemical characteristics of maize seedlings under waterlogging stress conditions. Biology 2022, 11, 99. [Google Scholar] [CrossRef]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Chi, Y.X.; Zeeshan, M.; Nasar, J.; Zhou, X.B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in A rabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef]
- Chi, Y.X.; Yang, L.; Zhao, C.J.; Muhammad, I.; Zhou, X.B.; De Zhu, H. Effects of soaking seeds in exogenous vitamins on active oxygen metabolism and seedling growth under low-temperature stress. Saudi J. Biol. Sci. 2021, 28, 3254–3261. [Google Scholar]
- Moghadam, N.K.; Motesharezadeh, B.; Maali-Amiri, R.; Lajayer, B.A.; Astatkie, T. Effects of potassium and zinc on physiology and chlorophyll fluorescence of two cultivars of canola grown under salinity stress. Arab. J. Geosci. 2020, 13, 771. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).