Role of Food Hydrocolloids as Antioxidants along with Modern Processing Techniques on the Surimi Protein Gel Textural Properties, Developments, Limitation and Future Perspectives
Abstract
1. Introduction
2. Fish Protein-Based Gel Products
2.1. Heat-Induced Gel
2.2. Cold Gelation
2.3. Salt-Based Surimi Gel
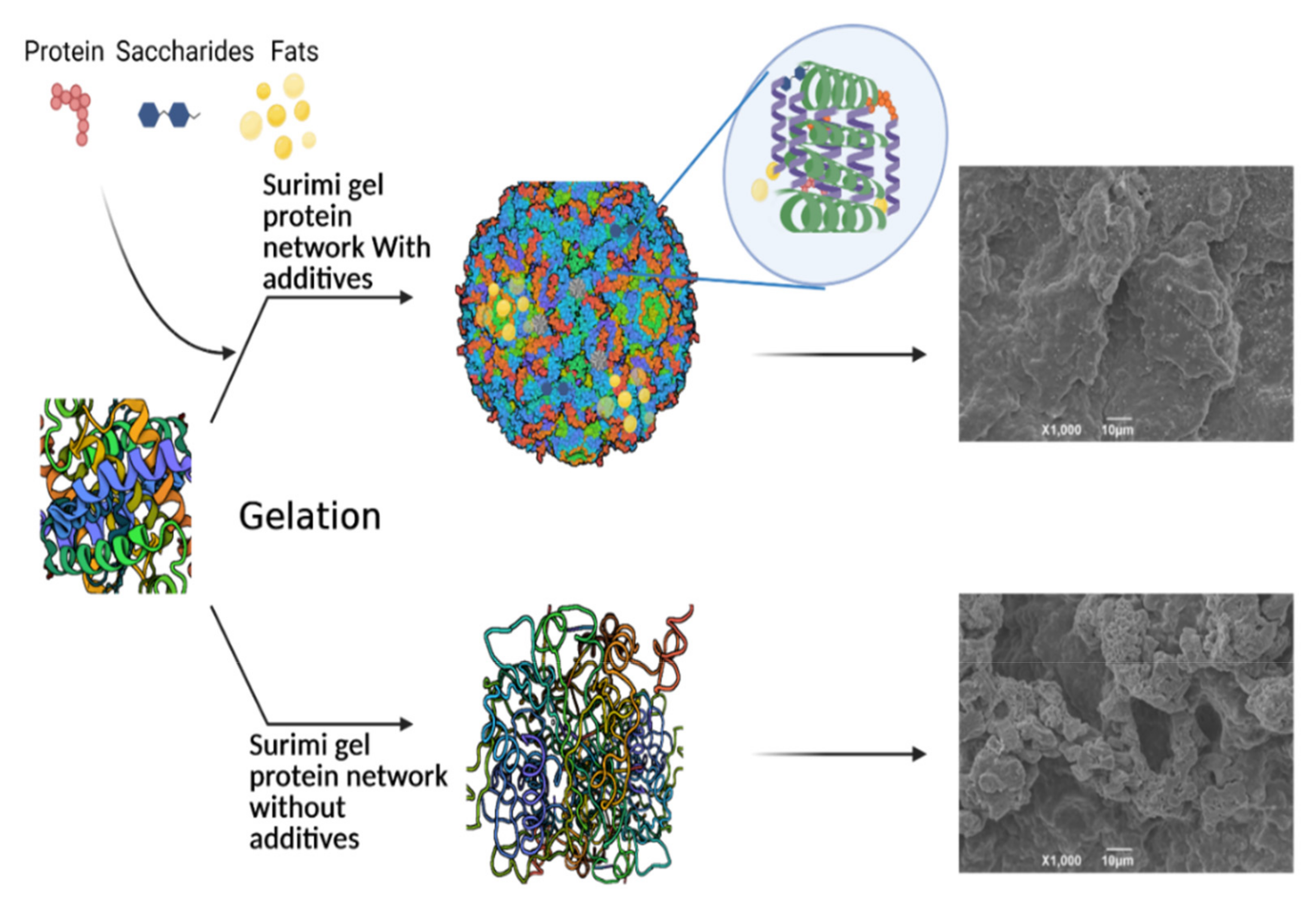
3. Role of Antioxidants in Improved Surimi Gel Textural Properties
3.1. Carbohydrate-Based Gel
3.1.1. Saccharide-Based Gel
3.1.2. Other Polysaccharide-Based Surimi Gel
3.2. Protein-Based Gel
3.3. Protein Hydrolysate-Based Gel
| Additives | Role | Results | Reference |
|---|---|---|---|
| Sucrose and sorbitol | Saccharids, cryoprotectants and functional | Improved the structural properties, conserving three dimensional and improving the protein stability in common carp surimi | [69] |
| mTGase | Microbial and protein cross-linker | Increased the surimi gel strength and viscoelastic attributes by improving the intermolecular cross-linking of protein molecules | [70] |
| Sulfated polysaccharide (SP) | Antioxidants, nutritional and functional | The addition of SP enhanced the textural and water-holding properties of silver carp surimi gel by reducing the oxidative changes | [71] |
| Fucoidan polysaccharide | Antioxidants and antibacterial | Fuoxidan polysaccharide enhanced the hardness, gumminess and water-binding characteristics of surimi gel by promoting the cross-linking of protein molecules. | [72] |
| Kappa carrageenan (KC) | Polysaccharide, antioxidant and cryoprotectant | Addition of KC in surimi gel enhanced the gel strength and textural properties. Meanwhile, improved the viscoelastic properties. | [73] |
| Konjac glucomannan (KGM) | Oligosaccharide, antioxidant and functional | Incorporation of KOG increased the textural and antioxidative properties by inhibiting the movement of free water molecules. | [52] |
| Skipjack roe protein hydrolysate (SRPH) | Antioxidant and emulsifier | SRPH restarted the protein and lipid oxidation in sausage. | [74] |
| Protein hydrolysate (PH) | Antioxidants, physiological and protein functional enhancer | PH addition in silver carp surimi gel reduced the protein oxidative changes and enhanced the gel-forming abilities. | [74] |
4. Why the Textural Properties of Surimi Gel and Oxidation Effect Are an Important Concern
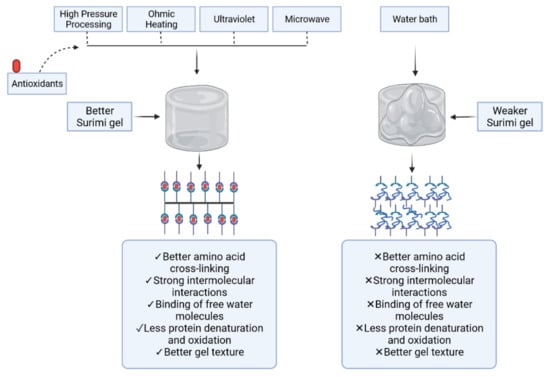
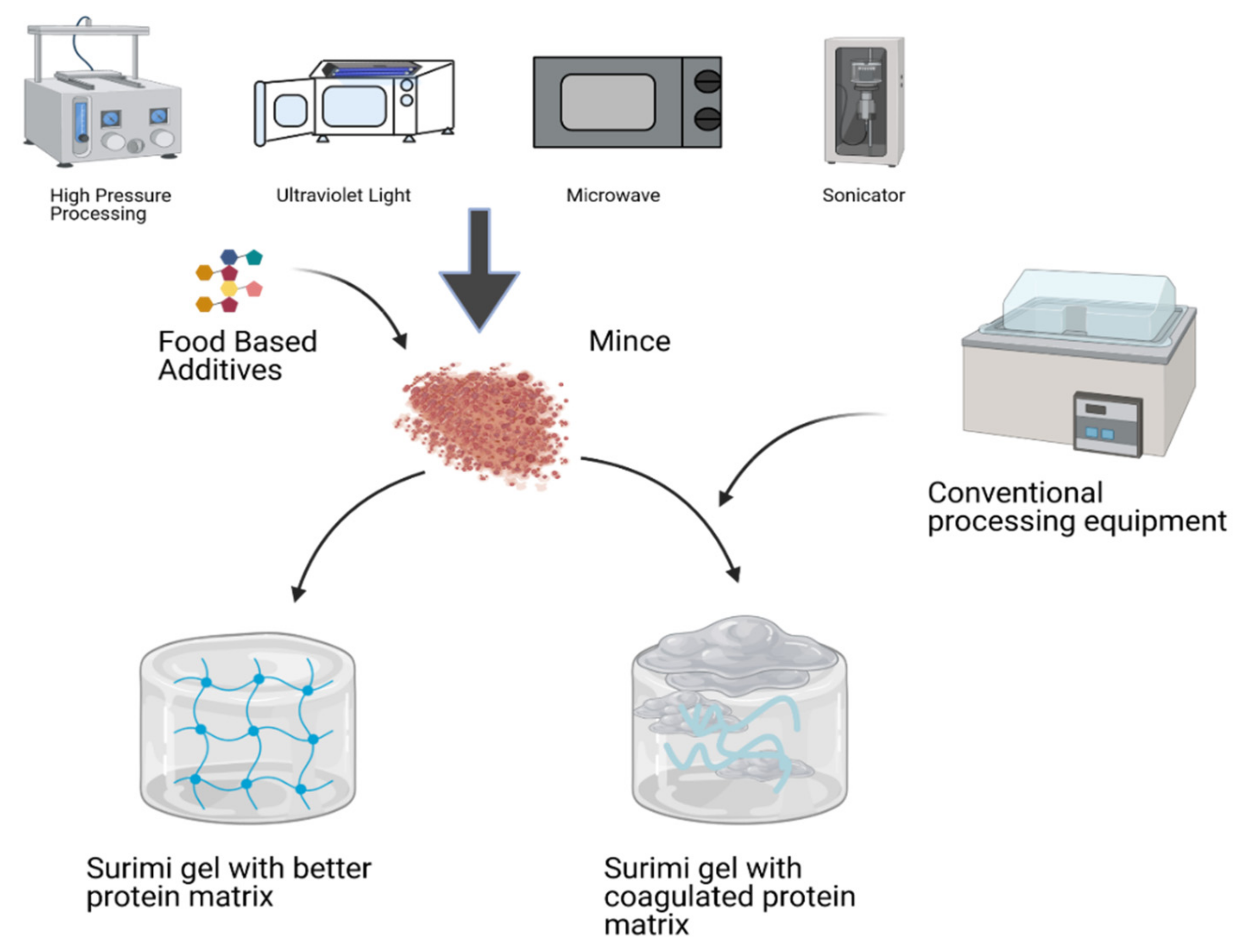
5. Role of Modern Technologies in Protein Gel-Based Products
5.1. High-Pressure Processing
5.2. Ultrasonication
5.3. Microwave (MW)
5.4. Ultraviolet
5.5. Ohmic Heating
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, H.; Xiong, Z.; Moreno, H.M.; Nawaz, A.; Niaz, N.; Randhawa, M.A. Role of Cryoprotectants in Surimi and Factors Affecting Surimi Gel Properties: A Review. Food Rev. Int. 2020. [Google Scholar] [CrossRef]
- Jiao, X.; Cao, H.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H. Effects of fish oil incorporation on the gelling properties of silver carp surimi gel subjected to microwave heating combined with conduction heating treatment. Food Hydrocoll. 2019, 94, 164–173. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Ma, X.-S.; Yi, S.-M.; Yu, Y.-M.; Li, J.-R.; Chen, J.-R. Changes in gel properties and water properties of Nemipterus virgatus surimi gel induced by high-pressure processing. LWT 2015, 61, 377–384. [Google Scholar] [CrossRef]
- Nishinari, K.; Turcanu, M.; Nakauma, M.; Fang, Y. Role of fluid cohesiveness in safe swallowing. NPJ Sci. Food 2019, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Buamard, N.; Benjakul, S. Improvement of gel properties of sardine (Sardinella albella) surimi using coconut husk extracts. Food Hydrocoll. 2015, 51, 146–155. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Li, Q.; Nawaz, A.; Zhang, Z.; Wang, P.; Niaz, N. The effectiveness of egg white protein and β-cyclodextrin during frozen storage: Functional, rheological and structural changes in the myofibrillar proteins of Culter alburnus. Food Hydrocoll. 2020, 105, 105842. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovačević, D.B.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41, e12638. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.-W.; Cheng, J.-H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Chavez, J.D.; Bruce, J.E. Chemical cross-linking with mass spectrometry: A tool for systems structural biology. Curr. Opin. Chem. Biol. 2018, 48, 8–18. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Roohinejad, S.; George, S.; Barba, F.J.; Greiner, R.; Barbosa-Cánovas, G.V.; Mallikarjunan, K. Innovative food processing technologies on the transglutaminase functionality in protein-based food products: Trends, opportunities and drawbacks. Trends Food Sci. Technol. 2018, 75, 194–205. [Google Scholar] [CrossRef]
- Mújica-Paz, H.; Valdez-Fragoso, A.; Samson, C.T.; Welti-Chanes, J.; Torres, J.A. High-Pressure Processing Technologies for the Pasteurization and Sterilization of Foods. Food Bioprocess Technol. 2011, 4, 969–985. [Google Scholar] [CrossRef]
- Yan, B.; Jiao, X.; Zhu, H.; Wang, Q.; Huang, J.; Zhao, J.; Cao, H.; Zhou, W.; Zhang, W.; Ye, W.; et al. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]
- Gani, A.; Benjakul, S. Impact of virgin coconut oil nanoemulsion on properties of croaker surimi gel. Food Hydrocoll. 2018, 82, 34–44. [Google Scholar] [CrossRef]
- Jiang, D.; Bai, Y.; He, B.; Sui, Y.; Dong, X.; Yu, C.; Qi, H. Improvement of gel properties of mackerel mince by phlorotannin extracts from sporophyll of Undaria pinnatifidai and UVA induced cross-linking. J. Texture Stud. 2020, 51, 333–342. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Wang, H.; Ye, Q. Effects of a highly resistant rice starch and pre-incubation temperatures on the physicochemical properties of surimi gel from grass carp (Ctenopharyn Odon Idellus). Food Chem. 2013, 145, 212–219. [Google Scholar] [CrossRef]
- Moreno, H.M.; Herranz, B.; Pérez-Mateos, M.; Sánchez-Alonso, I.; Borderías, J.A. New alternatives in seafood restructured products. Crit. Rev. Food Sci. Nutr. 2016, 56, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Borderías, A.J.; Tovar, C.A.; Domínguez-Timón, F.; Díaz, M.T.; Pedrosa, M.M.; Moreno, H.M. Characterization of healthier mixed surimi gels obtained through partial substitution of myofibrillar proteins by pea protein isolates. Food Hydrocoll. 2020, 107, 105976. [Google Scholar] [CrossRef]
- Omura, F.; Takahashi, K.; Okazaki, E.; Osako, K. A novel and simple non-thermal procedure for preparing low-pH-induced surimi gel from Alaska pollock (Theragra chalcogramma) using glucose oxidase. Food Chem. 2020, 321, 126722. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Cheng, J.-H.; Sun, D.-W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends Food Sci. Technol. 2018, 74, 12–25. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Moon, J.H.; Yoon, W.B.; Park, J.W. Assessing the textural properties of Pacific whiting and Alaska pollock surimi gels prepared with carrot under various heating rates. Food Biosci. 2017, 20, 12–18. [Google Scholar] [CrossRef]
- Bakli, S.; Nath, S.; Chowdhury, S.; Pati, K. Surimi powder: Processing technology and potential application. 2020. J. Entomol. Zool. Stud. 2020, 8, 850–859. [Google Scholar]
- Gao, X.; Xie, Y.; Yin, T.; Hu, Y.; You, J.; Xiong, S.; Liu, R. Effect of high intensity ultrasound on gelation properties of silver carp surimi with different salt contents. Ultrason. Sonochem. 2020, 70, 105326. [Google Scholar] [CrossRef]
- Omarov, R.; Shlykov, S.; Khramchenko, A.; Gorbacheva, A.; Chernyavskaya, A. Modern technological solutions in the production of restructured ham. Indo Am. J. Pharm. Sci. 2019, 6, 5752–5757. [Google Scholar]
- Sampels, S. The effects of processing technologies and preparation on the final quality of fish products. Trends Food Sci. Technol. 2015, 44, 131–146. [Google Scholar] [CrossRef]
- Lee, E.-J.; Hong, G.-P. Effects of microbial transglutaminase and alginate on the water-binding, textural and oil absorption properties of soy patties. Food Sci. Biotechnol. 2019, 29, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, N.; Sarbon, N.M.; Amin, A.M. Physical properties of cobia (Rachycentron canadum) surimi: Effect of washing cycle at different salt concentrations. J. Food Sci. Technol. 2014, 52, 4773–4784. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Jamali, M.A.; Guo, X.; Peng, Z. Heat-induced gel properties of porcine myosin in a sodium chloride solution containing L-lysine and L-histidine. LWT 2017, 85, 16–21. [Google Scholar] [CrossRef]
- Tahergorabi, R.; Jaczynski, J. Physicochemical changes in surimi with salt substitute. Food Chem. 2012, 132, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Çarkcioğlu, E.; Rosenthal, A.; Candogan, K. Rheological and textural properties of sodium reduced salt soluble myofibrillar protein gels containing sodium tri-polyphosphate. J. Texture Stud. 2015, 47, 181–187. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Fan, Q.; Teng, C.; Xie, W.; Shi, Y.; Su, Y.; Yang, Y. Combination effects of NaOH and NaCl on the rheology and gel characteristics of hen egg white proteins. Food Chem. 2018, 250, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Walayat, N.; Ding, Y.; Liu, J. The role of trifunctional cryoprotectants in the frozen storage of aquatic foods: Recent developments and future recommendations. Compr. Rev. Food Sci. Food Saf. 2021, 21, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hao, G.-J.; Cao, H.-J.; Tang, H.; Zhang, Y.-Y.; Deng, S.-G. The cryoprotectant effect of xylooligosaccharides on denaturation of peeled shrimp (Litopenaeus vannamei) protein during frozen storage. Food Hydrocoll. 2018, 77, 228–237. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, C.; Walayat, N.; Nawaz, A.; Ding, Y.; Liu, J. Recent development in evaluation methods, influencing factors and control measures for freeze denaturation of food protein. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Walayat, N.; Wang, X.; Liu, J.; Nawaz, A.; Zhang, Z.; Khalifa, I.; Rincón Cervera, M.Á.; Pateiro, M.; Lorenzo, J.M.; Nikoo, M.; et al. Kappa-carrageenan as an effective cryoprotectant on water mobility and functional properties of grass carp myofibrillar protein gel during frozen storage. LWT 2021, 154, 112675. [Google Scholar] [CrossRef]
- Cando, D.; Herranz, B.; Borderías, A.J.; Moreno, H.M. Different additives to enhance the gelation of surimi gel with reduced sodium content. Food Chem. 2016, 196, 791–799. [Google Scholar] [CrossRef]
- Veena, N.; Nath, S.; Arora, S. Polydextrose as a functional ingredient and its food applications: A review. Indian J. Dairy Sci. 2016, 69, 239–251. [Google Scholar]
- Santana, P.; Zilda, D.; Huda, N. Physicochemical properties of surimi powder made from threadfin bream (Nemipterus japonicus) with various dryoprotectants added. J. Fundam. Appl. Sci. 2018, 9, 866. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Enteshari, M.; Metzger, L. Lactitol: Production, properties, and applications. Trends Food Sci. Technol. 2019, 83, 181–191. [Google Scholar] [CrossRef]
- Kulkarni, A.K.; Relekar, S.S.; Joshi, S.A.; Gore, S.B.; Pathan, J.G.K. Cryoprotective Effect of Maltodextrins on Frozen Storage of Bleached Horse Mackerel (Megalapsis cordyla) Minced Meat. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1666–1677. [Google Scholar] [CrossRef]
- Blikra, M.J.; Jessen, F.; Feyissa, A.H.; Vaka, M.R.; Skipnes, D. Low-concentration salting of cod loins: The effect on biochemical properties and predicted water retention during heating. LWT 2019, 118, 108702. [Google Scholar] [CrossRef]
- Wu, S. Effect of Trehalose on the State of Water, Protein Denaturation and Gel-Forming Ability of Weever Surimi. Int. J. Food Prop. 2015, 19, 521–525. [Google Scholar] [CrossRef]
- Lee, J.; Yuan, P.; Heidolph, B.B.; Park, J.W. Physicochemical properties of frozen Alaska pollock fillets and surimi as affected by various sodium phosphates. J. Food Process. Preserv. 2017, 42, e13530. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H.; et al. Intervention of transglutaminase in surimi gel under microwave irradiation. Food Chem. 2018, 268, 378–385. [Google Scholar] [CrossRef]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2020, 112, 106290. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, T.; Feng, D.; Xue, Y.; Wang, Y.; Li, Z.; Yang, W.; Xue, C. Effects of modified starches on the gel properties of Alaska Pollock surimi subjected to different temperature treatments. Food Hydrocoll. 2015, 56, 20–28. [Google Scholar] [CrossRef]
- Mi, H.; Wang, C.; Su, Q.; Li, X.; Yi, S.; Li, J. The effect of modified starches on the gel properties and protein conformation of Nemipterus virgatus surimi. J. Texture Stud. 2019, 50, 571–581. [Google Scholar] [CrossRef]
- Hasanpour, F.; Hoseini, E.; Motalebi, A.A.; Darvish, F. Effects of Soy protein concentrate and Xanthan gum on physical properties of Silver carp (Hypophthalmichthys molitrix) surimi. Iran. J. Fish. Sci. 2012, 11, 518–530. [Google Scholar] [CrossRef]
- Liu, J.; Fang, C.; Luo, Y.; Ding, Y.; Liu, S. Effects of konjac oligo-glucomannan on the physicochemical properties of frozen surimi from red gurnard (Aspitrigla cuculus). Food Hydrocoll. 2018, 89, 668–673. [Google Scholar] [CrossRef]
- Ye, T.; Dai, H.; Lin, L.; Lu, J. Employment of κ-carrageenan and high pressure processing for quality improvement of reduced NaCl surimi gels. J. Food Process. Preserv. 2019, 43, e14074. [Google Scholar] [CrossRef]
- Buda, U.; Priyadarshini, M.B.; Majumdar, R.; Mahanand, S.; Patel, A.; Mehta, N. Quality characteristics of fortified silver carp surimi with soluble dietary fiber: Effect of apple pectin and konjac glucomannan. Int. J. Biol. Macromol. 2021, 175, 123–130. [Google Scholar] [CrossRef]
- Alakhrash, F.; Anyanwu, U.; Tahergorabi, R. Physicochemical properties of Alaska pollock (Theragra chalcograma) surimi gels with oat bran. LWT 2016, 66, 41–47. [Google Scholar] [CrossRef]
- Yaguchi, S.; Shimoda, M.; Fukushima, H.; Maeda, T. Comparison of gel strength of kamaboko containing powders from nine different vegetbales and fruits. J. Natl. Fish. Univ. 2017, 65, 1–8. [Google Scholar]
- Lin, Y.; Chen, K.; Tu, D.; Yu, X.; Dai, Z.; Shen, Q. Characterization of dietary fiber from wheat bran (Triticum aestivum L.) and its effect on the digestion of surimi protein. LWT 2018, 102, 106–112. [Google Scholar] [CrossRef]
- Yin, T.; Yao, R.; Ullah, I.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Shi, L. Effects of nanosized okara dietary fiber on gelation properties of silver carp surimi. LWT 2019, 111, 111–116. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, T.; Lin, H.; Chen, H.; Liu, J.; Lyu, F.; Ding, Y. Physicochemical properties and microstructure of surimi treated with egg white modified by tea polyphenols. Food Hydrocoll. 2018, 90, 82–89. [Google Scholar] [CrossRef]
- Xiong, Y. Muscle proteins. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 127–148. [Google Scholar]
- Henriques, M.H.F.; Gomes, D.M.G.S.; Borges, A.R.; Pereira, C.J.D. Liquid whey protein concentrates as primary raw material for acid dairy gels. Food Sci. Technol. 2020, 40, 361–369. [Google Scholar] [CrossRef]
- Benjakul, S.; Yarnpakdee, S.; Visessanguan, W.; Phatcharat, S. Combination effects of whey protein concentrate and calcium chloride on the properties of goatfish surimi gel. J. Texture Stud. 2010, 41, 341–357. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Benjakul, S. Whey protein concentrate: Autolysis inhibition and effects on the gel properties of surimi prepared from tropical fish. Food Chem. 2008, 106, 1077–1084. [Google Scholar] [CrossRef]
- Lin, J.; Hong, H.; Zhang, L.; Zhang, C.; Luo, Y. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Hong, H.; Luo, Y. Prevention of protein oxidation and enhancement of gel properties of silver carp (Hypophthalmichthys molitrix) surimi by addition of protein hydrolysates derived from surimi processing by-products. Food Chem. 2020, 316, 126343. [Google Scholar] [CrossRef] [PubMed]
- Karnjanapratum, S.; Benjakul, S. Cryoprotective and antioxidative effects of gelatin hydrolysate from unicorn leatherjacket skin. Int. J. Refrig. 2015, 49, 69–78. [Google Scholar] [CrossRef]
- Limpisophon, K.; Iguchi, H.; Tanaka, M.; Suzuki, T.; Okazaki, E.; Saito, T.; Takahashi, K.; Osako, K. Cryoprotective effect of gelatin hydrolysate from shark skin on denaturation of frozen surimi compared with that from bovine skin. Fish. Sci. 2014, 81, 383–392. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Rahmanifarah, K. Hydrolysates from marine sources as cryoprotective substances in seafoods and seafood products. Trends Food Sci. Technol. 2016, 57, 40–51. [Google Scholar] [CrossRef]
- Techaratanakrai, B.; Nishida, M.; Igarashi, Y.; Watanabe, M.; Okazaki, E.; Osako, K. Effect of setting conditions on mechanical properties of acid-induced Kamaboko gel from squid Todarodes pacificus mantle muscle meat. Fish. Sci. 2011, 77, 439–446. [Google Scholar] [CrossRef]
- Fang, M.; Xiong, S.; Hu, Y.; Yin, T.; You, J. In vitro pepsin digestion of silver carp (Hypophthalmichthys molitrix) surimi gels after cross-linking by Microbial Transglutaminase (MTGase). Food Hydrocoll. 2019, 95, 152–160. [Google Scholar] [CrossRef]
- Alipour, H.J.; Rezaei, M.; Shabanpour, B.; Tabarsa, M. Effects of sulfated polysaccharides from green alga Ulva intestinalis on physicochemical properties and microstructure of silver carp surimi. Food Hydrocoll. 2018, 74, 87–96. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, X.; Chuai, P.; Jiang, Z.; Zhu, Y.; Zhang, B.; Ni, H.; Li, Q. Effects of crude fucoidan on physicochemical properties, antioxidation and bacteriostasis of surimi products. Food Control 2020, 122, 107806. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, X.; Ji, L.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Phase behaviors involved in surimi gel system: Effects of phase separation on gelation of myofibrillar protein and kappa-carrageenan. Food Res. Int. 2017, 100, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W.; Wu, J. Effects of skipjack roe protein hydrolysate on properties and oxidative stability of fish emulsion sausage. LWT 2014, 58, 280–286. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, Z.; Walayat, N.; Lorenzo, J.M.; Liu, J.; Nawaz, A.; Xiong, H. Influence of the Mixture of Carrageenan Oligosaccharides and Egg White Protein on the Gelation Properties of Culter alburnus Myofibrillar Protein under Repeated Freezing–Thawing Cycles. Antioxidants 2021, 11, 32. [Google Scholar] [CrossRef]
- Eom, S.-H.; Kim, J.-A.; Son, B.-Y.; You, D.H.; Han, J.M.; Oh, J.-H.; Kim, B.-Y.; Kong, C.-S. Effects of Carrageenan on the Gelatinization of Salt-Based Surimi Gels. Fish. Aquat. Sci. 2013, 16, 143–147. [Google Scholar] [CrossRef][Green Version]
- Quan, T.H.; Benjakul, S. Impact of salted duck egg albumen powder on proteolysis and gelling properties of sardine surimi. J. Texture Stud. 2019, 50, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Nawaz, A.; Niaz, N.; Hu, C.; Taj, M.I.; Mushtaq, B.S.; Khalifa, I. The effect of egg white protein and β-cyclodextrin mixture on structural and functional properties of silver carp myofibrillar proteins during frozen storage. LWT 2020, 135, 109975. [Google Scholar] [CrossRef]
- Tang, S.; Feng, G.; Gao, R.; Ren, J.; Zhou, X.; Wang, H.; Xu, H.; Zhao, Y.; Zeng, M. Thermal Gel Degradation (Modori) in Sturgeon (Acipenseridae) Surimi Gels. J. Food Sci. 2019, 84, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.A.; Santos, T.C.L. Improving quality and shelf-life of whole chilled Pacific white shrimp (Litopenaeus vannamei) by ozone technology combined with modified atmosphere packaging. LWT 2018, 99, 568–575. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Ehsani, A.; Li, J.; Wu, F.; Yang, N.; Xu, B.; Jin, Z.; Xu, X. Antioxidant and cryoprotective effects of a tetrapeptide isolated from Amur sturgeon skin gelatin. J. Funct. Foods 2014, 7, 609–620. [Google Scholar] [CrossRef]
- Walayat, N.; Wang, X.; Nawaz, A.; Zhang, Z.; Abdullah, A.; Khalifa, I.; Saleem, M.H.; Mushtaq, B.S.; Pateiro, M.; Lorenzo, J.M.; et al. Ovalbumin and Kappa-Carrageenan Mixture Suppresses the Oxidative and Structural Changes in the Myofibrillar Proteins of Grass Carp (Ctenopharyngodon idella) during Frozen Storage. Antioxidants 2021, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Wang, X.; Walayat, N.; Zhang, Z.; Saleem, M.H.; Nawaz, A.; Aadil, R.M.; Ahmed, S.; Simirgiotis, M.J.; Lorenzo, J.M.; et al. Role of Ovalbumin/β-Cyclodextrin in Improving Structural and Gelling Properties of Culter alburnus Myofibrillar Proteins during Frozen Storage. Appl. Sci. 2021, 11, 11815. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.W. Roles of TMAOase in muscle and drips of Alaska pollock fillets at various freeze/thaw cycles. J. Food Process. Preserv. 2017, 42, e13427. [Google Scholar] [CrossRef]
- Slámová, T.; Fraňková, A.; Hubáčková, A.; Banout, J. Polycyclic aromatic hydrocarbons in Cambodian smoked fish. Food Addit. Contam. Part B 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Santos, C.; Roseiro, C.; Gonçalves, H.; Aleixo, C.; Moniz, C.; da Ponte, D.J. Susceptibility of dry-cured tuna to oxidation and biogenic amines generation related to microbial status and salting/curing technology. LWT 2019, 115, 108420. [Google Scholar] [CrossRef]
- Yu, N.; Xu, Y.; Jiang, Q.; Xia, W. Textural and physicochemical properties of surimi gels prepared with potassium and calcium chloride as substitutes for sodium chloride. Int. J. Food Prop. 2016. [Google Scholar] [CrossRef]
- Marszałek, K.; Doesburg, P.; Starzonek, S.; Szczepańska, J.; Woźniak, Ł.; Lorenzo, J.M.; Skąpska, S.; Rzoska, S.; Barba, F.J. Comparative effect of supercritical carbon dioxide and high pressure processing on structural changes and activity loss of oxidoreductive enzymes. J. CO2 Util. 2019, 29, 46–56. [Google Scholar] [CrossRef]
- Nor Hasni, H.; Koh, P.C.; Noranizan, M.A.; Megat Mohd Tahir, P.N.F.; Mohamad, A.; Limpot, N.; Hamid, N.; Aadil, R.M. High-pressure processing treatment for ready-to-drink Sabah Snake Grass juice. J. Food Process. Preserv. 2020, 44, e14508. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Arshad, R.N.; Radicetti, E.; Tedeschi, P.; Shahbaz, M.U.; Walayat, N.; Nawaz, A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-Pressure Processing for Sustainable Food Supply. Sustainability 2021, 13, 13908. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Zhang, J.; Xu, D.; Yang, W. Myofibrillar Protein Structure and Gel Properties of Trichiurus Haumela Surimi Subjected to High Pressure or High Pressure Synergistic Heat. Food Bioprocess Technol. 2020, 13, 589–598. [Google Scholar] [CrossRef]
- Morton, J.D.; Pearson, R.G.; Lee, H.Y.-Y.; Smithson, S.; Mason, S.L.; Bickerstaffe, R. High pressure processing improves the tenderness and quality of hot-boned beef. Meat Sci. 2017, 133, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Cava, R.; Higuero, N.; Ladero, L. High-pressure processing and storage temperature on Listeria monocytogenes, microbial counts and oxidative changes of two traditional dry-cured meat products. Meat Sci. 2020, 171, 108273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Lanier, T.C.; Farkas, B.E.; Li, B. Transglutaminase and high pressure effects on heat-induced gelation of Alaska pollock (Theragra chalcogramma) surimi. J. Food Eng. 2014, 131, 154–160. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, A.; Liu, G.; Ying, D.; Xiao, J.; Miao, J. Changes of physicochemical properties of greater lizardfish (Saurida tumbil) surimi gels treated with high pressure combined with microbial transglutaminase. J. Food Process. Preserv. 2019, 43, e14150. [Google Scholar] [CrossRef]
- Tsevdou, M.S.; Eleftheriou, E.G.; Taoukis, P.S. Transglutaminase treatment of thermally and high pressure processed milk: Effects on the properties and storage stability of set yoghurt. Innov. Food Sci. Emerg. Technol. 2013, 17, 144–152. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G.V. Pressurized and heat-treated surimi gels as affected by potato starch and egg white: Microstructure and water-holding capacity. LWT 2005, 38, 47–57. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Zhang, J.; Xu, D.; Yang, W. Chemical interactions and rheological properties of hairtail (Trichiurus haumela) surimi: Effects of chopping and pressure. Food Biosci. 2020, 38, 100781. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G.V. Ultra High Pressure Technology and its Use in Surimi Manufacture: An Overview. Food Sci. Technol. Int. 2004, 10, 207–222. [Google Scholar] [CrossRef]
- Ma, F.; Chen, C.; Zheng, L.; Zhou, C.; Cai, K.; Han, Z. Effect of high pressure processing on the gel properties of salt-soluble meat protein containing CaCl2 and κ-carrageenan. Meat Sci. 2013, 95, 22–26. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Wang, M.-S.; Liu, Z.-W.; Han, Z.; Zhang, Z.-H.; Hong, J.; Jabbar, S. A potential of ultrasound on minerals, micro-organisms, phenolic compounds and colouring pigments of grapefruit juice. Int. J. Food Sci. Technol. 2015, 50, 1144–1150. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.; Han, Z.; Sahar, A.; Khalil, A.A.; Rahman, U.U.; Khan, M.; Mehmood, T. Combined effects of pulsed electric field and ultrasound on bioactive compounds and microbial quality of grapefruit juice. J. Food Process. Preserv. 2017, 42, e13507. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, Y.; Zhao, H.; He, X.; Li, X.; Yi, S.; Li, J. Diacylglycerol pre-emulsion prepared through ultrasound improves the gel properties of golden thread surimi. Ultrason. Sonochem. 2022, 82, 105915. [Google Scholar] [CrossRef]
- Gülseren, I.; Güzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007, 14, 173–183. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lv, Y.; Li, X.; Yi, S.; Zhao, H.; Li, J.; Xu, Y. Improvement of gelation properties of silver carp surimi through ultrasound-assisted water bath heating. Ultrason. Sonochem. 2022, 83, 105942. [Google Scholar] [CrossRef]
- Pan, J.; Lian, H.; Jia, H.; Li, S.; Hao, R.; Wang, Y.; Zhang, X.; Dong, X. Ultrasound treatment modified the functional mode of gallic acid on properties of fish myofibrillar protein. Food Chem. 2020, 320, 126637. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-S.; Luo, S.-Z.; Cai, J.; Zhong, X.-Y.; Jiang, S.-T.; Zhao, Y.-Y.; Zheng, Z. Transglutaminase-induced gelation properties of soy protein isolate and wheat gluten mixtures with high intensity ultrasonic pretreatment. Ultrason. Sonochem. 2016, 31, 590–597. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Fan, X.; Zhou, Z.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S.; Zhu, L. Acid-induced gelation behavior of soybean protein isolate with high intensity ultrasonic pre-treatments. Ultrason. Sonochem. 2012, 20, 187–195. [Google Scholar] [CrossRef]
- Hu, H.; Li-Chan, E.C.; Wan, L.; Tian, M.; Pan, S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013, 32, 303–311. [Google Scholar] [CrossRef]
- Gao, W.; Hou, R.; Zeng, X.-A. Synergistic effects of ultrasound and soluble soybean polysaccharide on frozen surimi from grass carp. J. Food Eng. 2018, 240, 1–8. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, X.; Hu, T.; Cheung, I.W.; Pan, S.; Li-Chan, E.C. Effect of ultrasound pre-treatment on formation of transglutaminase-catalysed soy protein hydrogel as a riboflavin vehicle for functional foods. J. Funct. Foods 2015, 19, 182–193. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, X.; Wang, G.; Li, R.; Wang, X.; Chen, S.; Liu, J.; Jiang, L. Effects of ultrasonic treatment on the gel properties of microbial transglutaminase crosslinked soy, whey and soy–whey proteins. Food Sci. Biotechnol. 2019, 28, 1455–1464. [Google Scholar] [CrossRef]
- Pan, J.; Jia, H.; Shang, M.; Xu, C.; Lian, H.; Li, H.; Dong, X. Physiochemical properties and tastes of gels from Japanese Spanish mackerel (Scomberomorus niphonius) surimi by different washing processes. J. Texture Stud. 2018, 49, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Mendes, R.; Vaz-Pires, P.; Nunes, M.L. Effect of MTGase, Dietary Fiber and UV Irradiation Upon Heat-Induced Gilthead Seabream (Sparus aurata) gels. Food Sci. Technol. Int. 2011, 17, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-S.; Sun, Q.-Q.; Zhao, Y.-Y.; Zhong, X.-Y.; Mu, D.-D.; Jiang, S.-T.; Luo, S.-Z.; Zheng, Z. Transglutaminase-set colloidal properties of wheat gluten with ultrasound pretreatments. Ultrason. Sonochem. 2017, 39, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Nasyiruddin, R.L.; Navicha, W.B.; Ramadhan, A.H.; Yang, F.; Jiang, Q.; Xu, Y.; Yu, P.; Xia, W. Development of reduced-salt gel of silver carp meat batter using low frequency ultrasound: Effect on color, texture, cooking loss and microstructure. Trop. J. Pharm. Res. 2021, 18, 773–780. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Feng, D.; Xue, Y.; Li, Z.; Wang, Y.; Yang, W.; Xue, C. Dielectric properties of myofibrillar protein dispersions from Alaska Pollock (Theragra chalcogramma) as a function of concentration, temperature, and NaCl concentration. J. Food Eng. 2015, 166, 342–348. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Effects of microwave combined with conduction heating on surimi quality and morphology. J. Food Eng. 2018, 228, 1–11. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, H.; Wang, Q.; Fan, D.; Huang, J.; Zhao, J.; Jiao, X.; Yan, B.; Zhou, W.; Zhang, H. Intervention on activity and structure of cathepsin L during surimi gel degradation under microwave irradiation. Food Hydrocoll. 2020, 103, 105705. [Google Scholar] [CrossRef]
- Liu, X.; Feng, D.; Ji, L.; Zhang, T.; Xue, Y.; Xue, C. Effects of microwave heating on the gelation properties of heat-induced Alaska Pollock (Theragra chalcogramma) surimi. Food Sci. Technol. Int. 2018, 24, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Xue, Y.; Zhang, T.; Li, Z.; Xue, C. The effects of microwave processing on the structure and various quality parameters of Alaska pollock surimi protein-polysaccharide gels. Food Hydrocoll. 2017, 63, 77–84. [Google Scholar] [CrossRef]
- Cao, H.; Jiao, X.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, H.; Wang, M. Microwave irradiation promotes aggregation behavior of myosin through conformation changes. Food Hydrocoll. 2019, 96, 11–19. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Zhu, J.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Sun, Y.; Tan, H.; Zhang, Y. Improved solubility and interface properties of pigskin gelatin by microwave irradiation. Int. J. Biol. Macromol. 2021, 171, 1–9. [Google Scholar] [CrossRef]
- Yang, D.; Yuan, Y.; Wang, L.; Wang, X.; Mu, R.; Pang, J.; Xiao, J.; Zheng, Y. A Review on Konjac Glucomannan Gels: Microstructure and Application. Int. J. Mol. Sci. 2017, 18, 2250. [Google Scholar] [CrossRef]
- Díaz, O.; Candia, D.; Cobos, Á. Effects of ultraviolet radiation on properties of films from whey protein concentrate treated before or after film formation. Food Hydrocoll. 2016, 55, 189–199. [Google Scholar] [CrossRef]
- Lin, X.; Yang, W.; Xu, D.; Jie, Z.; Liu, W. Improving gel properties of hairtail surimi by electron irradiation. Radiat. Phys. Chem. 2014, 110, 1–5. [Google Scholar] [CrossRef]
- Lin, X.; Yang, W.; Xu, D.; Wang, L. Effect of electron irradiation and heat on the structure of hairtail surimi. Radiat. Phys. Chem. 2015, 114, 50–54. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Rodrigues, B.L.; Bernardes, P.C.; Conte-Junior, C.A. Principles and applications of non-thermal technologies and alternative chemical compounds in meat and fish. Crit. Rev. Food Sci. Nutr. 2020, 61, 1163–1183. [Google Scholar] [CrossRef]
- Jiang, S.-T.; Leu, A.S.-Z.; Tsai, G.-J. Cross-Linking of Mackerel Surimi Actomyosin by Microbial Transglutaminase and Ultraviolet Irradiation. J. Agric. Food Chem. 1998, 46, 5278–5282. [Google Scholar] [CrossRef]
- Ramaswamy, H.S.; Marcotte, M.; Sastry, S.; Abdelrahim, K. Ohmic Heating in Food Processing; CRC press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Sakr, M.; Liu, S. A comprehensive review on applications of ohmic heating (OH). Renew. Sustain. Energy Rev. 2014, 39, 262–269. [Google Scholar] [CrossRef]
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Gelation characteristics of tropical surimi under water bath and ohmic heating. LWT 2012, 46, 97–103. [Google Scholar] [CrossRef]
- Gavahian, M.; Tiwari, B.K.; Chu, Y.-H.; Ting, Y.-W.; Farahnaky, A. Food texture as affected by ohmic heating: Mechanisms involved, recent findings, benefits, and limitations. Trends Food Sci. Technol. 2019, 86, 328–339. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.-H.; Sastry, S. Extraction from Food and Natural Products by Moderate Electric Field: Mechanisms, Benefits, and Potential Industrial Applications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, P. Other Innovative Technologies in Seafood Processing. In Technologies in Seafood Processing; CRC Press: Boca Raton, FL, USA, 2019; pp. 351–368. [Google Scholar] [CrossRef]
- Fowler, M.R.; Park, J.W. Effect of salmon plasma protein on Pacific whiting surimi gelation under various ohmic heating conditions. LWT 2015, 61, 309–315. [Google Scholar] [CrossRef]
- Boonpupiphat, P.; Khukutapan, D.; Jittanit, W. Effect of thawing Nile Tilapia fish by ohmic heating method on the characteristic of fish meat and thawing time. In Agricultural Sciences: Leading Thailand to World Class Standards, Proceedings of the 52nd Kasetsart University Annual Conference, Bangkok, Thailand, 4–7 February 2014; Kasetsart University: Bangkok, Thailand; Volume 6, pp. 363–370.
- Lascorz, D.; Torella, E.; Lyng, J.G.; Arroyo, C. The potential of ohmic heating as an alternative to steam for heat processing shrimps. Innov. Food Sci. Emerg. Technol. 2016, 37, 329–335. [Google Scholar] [CrossRef]
- Jung, H.; Moon, J.H.; Park, J.W.; Yoon, W.B. Texture of surimi-canned corn mixed gels with conventional water bath cooking and ohmic heating. Food Biosci. 2020, 35, 100580. [Google Scholar] [CrossRef]
- Chai, P.P.; Park, J.W. Physical properties of fish proteins cooked with starches or protein additives under ohmic heating. J. Food Qual. 2007, 30, 783–796. [Google Scholar] [CrossRef]
- Herranz, B.; Tovar, C.A.; Borderias, A.J.; Moreno, H.M. Effect of high-pressure and/or microbial transglutaminase on physicochemical, rheological and microstructural properties of flying fish surimi. Innov. Food Sci. Emerg. Technol. 2013, 20, 24–33. [Google Scholar] [CrossRef]
- Fu, X.; Hayat, K.; Li, Z.; Lin, Q.; Xu, S.; Wang, S. Effect of microwave heating on the low-salt gel from silver carp (Hypophthalmichthys molitrix) surimi. Food Hydrocoll. 2012, 27, 301–308. [Google Scholar] [CrossRef]
| Processing Technique | Additives | Role | Results | Reference |
|---|---|---|---|---|
| HPP | Kappa-carrageenan (KC) | Oligosaccharids, antioxidants and cryoprotectants | Surimi gel treated with KC showed better WHC and gel strength on HPP (300 MPa), by improving the water state and structural properties. | [53] |
| HPP | mTGase | Microbial | The mTGase treated surimi gel showed increased fracture stress, strain and gel strength when cooked at 300 MPa processing. | [144] |
| Ultrasonication | Soybean polysaccharide (SSPS) | Polysaccharide, antioxidants, funtional and gelling | The SSPS added surimi gel revealed enhanced whiteness and gelling properties during frozen storage combined with ultrasonication. | [112] |
| Ultrasonication | Wheat gluten (WG) | Protien additive, functional and gelling | Wheat gluten-SPI gels with ultrasonication led to increase in textural properties by improving β-sheets, decreased α-helices and β-turns. | [13] |
| Microwave | NaCl | Functional and mechanical | The mechanical, structural and textural characteristics of NaCl-treated surimi gel improved after 80 s heating of MW (15 W/g). | [145] |
| Microwave | Konjac glucomannan (KGM) | Oligosaccharide, antioxidant and functional | Microwave heated KGM surimi gel displayed better starching of protein molecules and dense KGM-protein network. | [124] |
| Ultraviolet | Konjac flour (KF) | Dietary fiber, gelling | KF (1%) and 250 nm UV for 40 min increased the gel hardness (63.2 N) and springiness (0.84). | [116] |
| Ohmic heating | Corn starch (CS) | Carbohydrate, functional and thermo-stable | CS-surimi gel displayed inferior gel network due to starch gelatinization. But the control surimi gel exhibited improved hardness and gel strength when processed with ohmic technique as compared to water bath cooked gel. | [142] |
| Ohmic heating | Diced carrot (DC) | Sensory and functional | DC added surimi of Pacific whiting (PW) and Alaska Pollock (AP) reported increased hardness and cohesiveness when ohmically heated at 90 °C. | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walayat, N.; Liu, J.; Nawaz, A.; Aadil, R.M.; López-Pedrouso, M.; Lorenzo, J.M. Role of Food Hydrocolloids as Antioxidants along with Modern Processing Techniques on the Surimi Protein Gel Textural Properties, Developments, Limitation and Future Perspectives. Antioxidants 2022, 11, 486. https://doi.org/10.3390/antiox11030486
Walayat N, Liu J, Nawaz A, Aadil RM, López-Pedrouso M, Lorenzo JM. Role of Food Hydrocolloids as Antioxidants along with Modern Processing Techniques on the Surimi Protein Gel Textural Properties, Developments, Limitation and Future Perspectives. Antioxidants. 2022; 11(3):486. https://doi.org/10.3390/antiox11030486
Chicago/Turabian StyleWalayat, Noman, Jianhua Liu, Asad Nawaz, Rana Muhammad Aadil, María López-Pedrouso, and José M. Lorenzo. 2022. "Role of Food Hydrocolloids as Antioxidants along with Modern Processing Techniques on the Surimi Protein Gel Textural Properties, Developments, Limitation and Future Perspectives" Antioxidants 11, no. 3: 486. https://doi.org/10.3390/antiox11030486
APA StyleWalayat, N., Liu, J., Nawaz, A., Aadil, R. M., López-Pedrouso, M., & Lorenzo, J. M. (2022). Role of Food Hydrocolloids as Antioxidants along with Modern Processing Techniques on the Surimi Protein Gel Textural Properties, Developments, Limitation and Future Perspectives. Antioxidants, 11(3), 486. https://doi.org/10.3390/antiox11030486