Formulation of Gels and Emulgels with Malus domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Solvents
2.3. Preparation of the Apple Lyophilizate
2.4. Production of Dry Apple Extracts
2.5. HPLC-PDA Analysis for the Characterization of Phenols
2.6. Formulation of Semi-Solid Pharmaceutical Forms and Their Biopharmaceutical Evaluation
2.6.1. Preparation of Gels, Emulsions and Emulgels
2.6.2. Evaluation of Physiochemical Properties of Gels, Emulsions and Emulgels
2.6.3. In Vitro Studies of Release from Gel and Emulgel Formulations
2.7. Evaluation of Antioxidant Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Content of Apple Extracts
3.2. Formulation and Physicochemical Characteristics of Semi-Dolid Forms
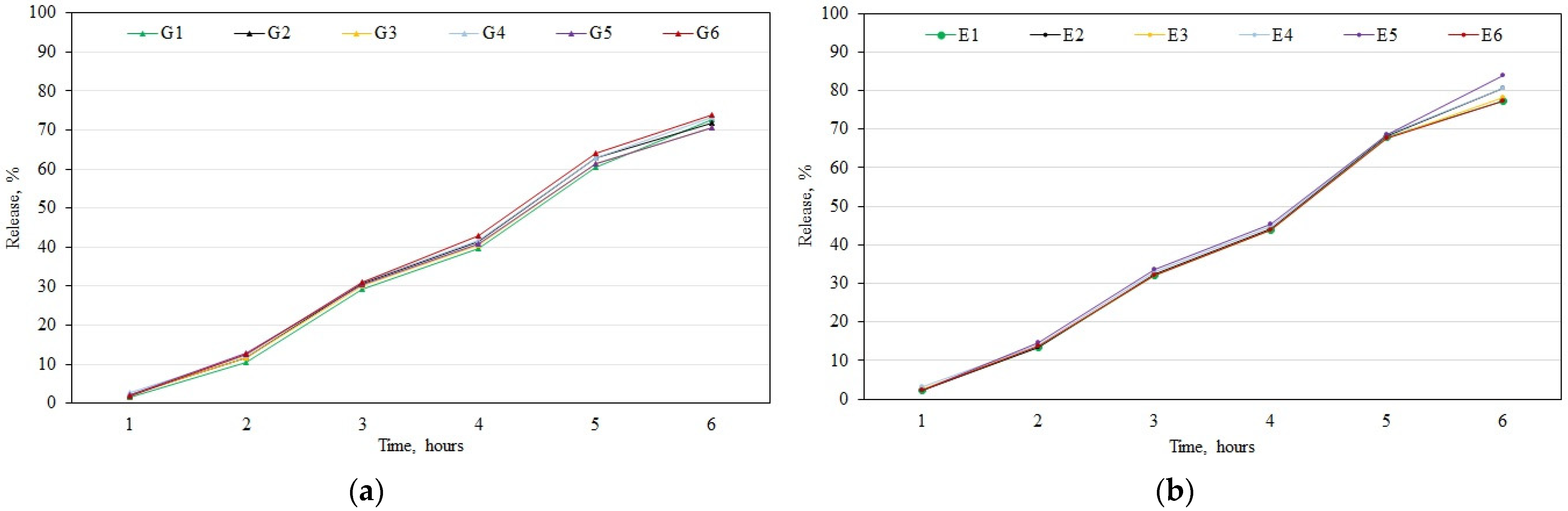
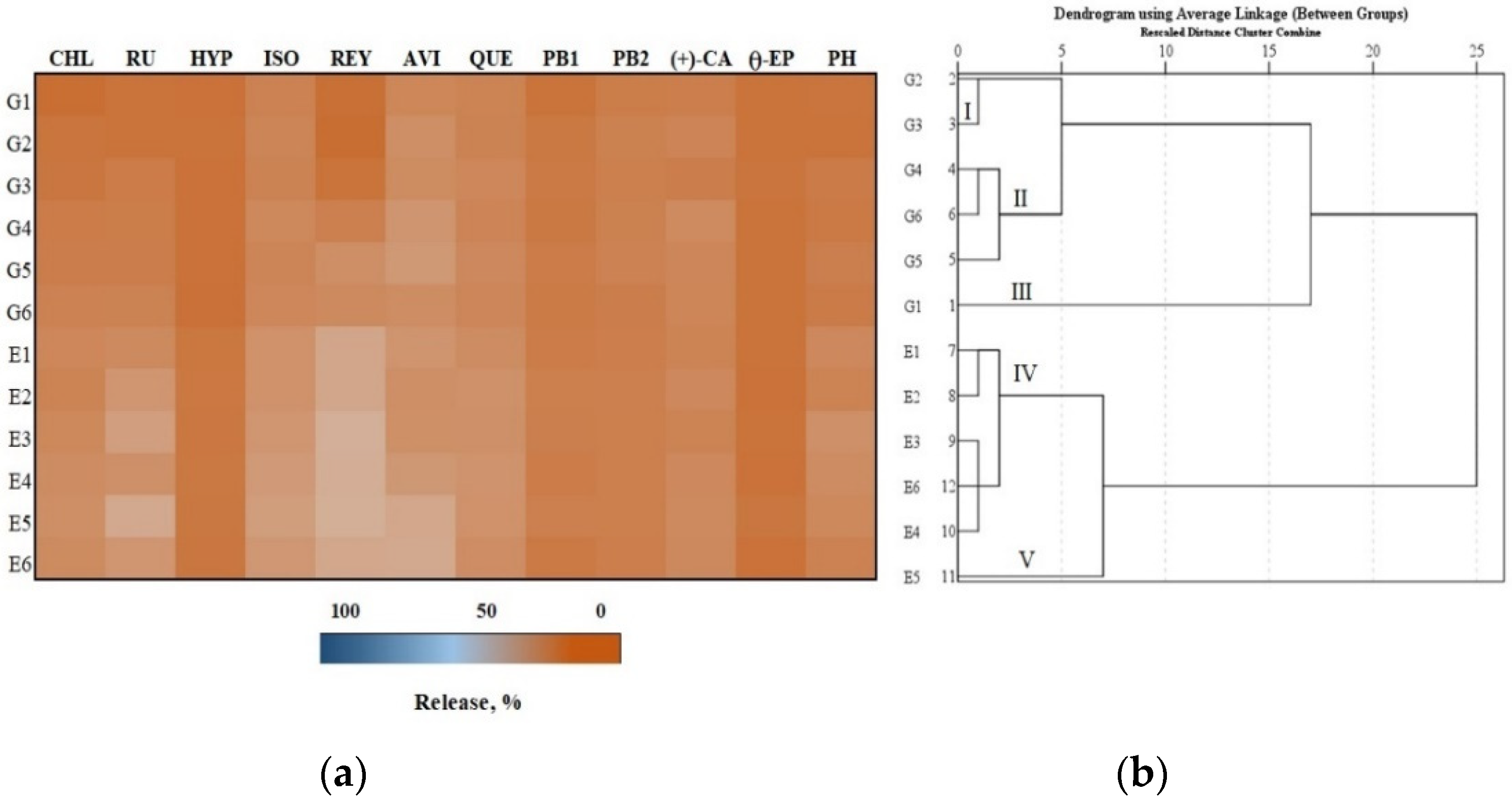
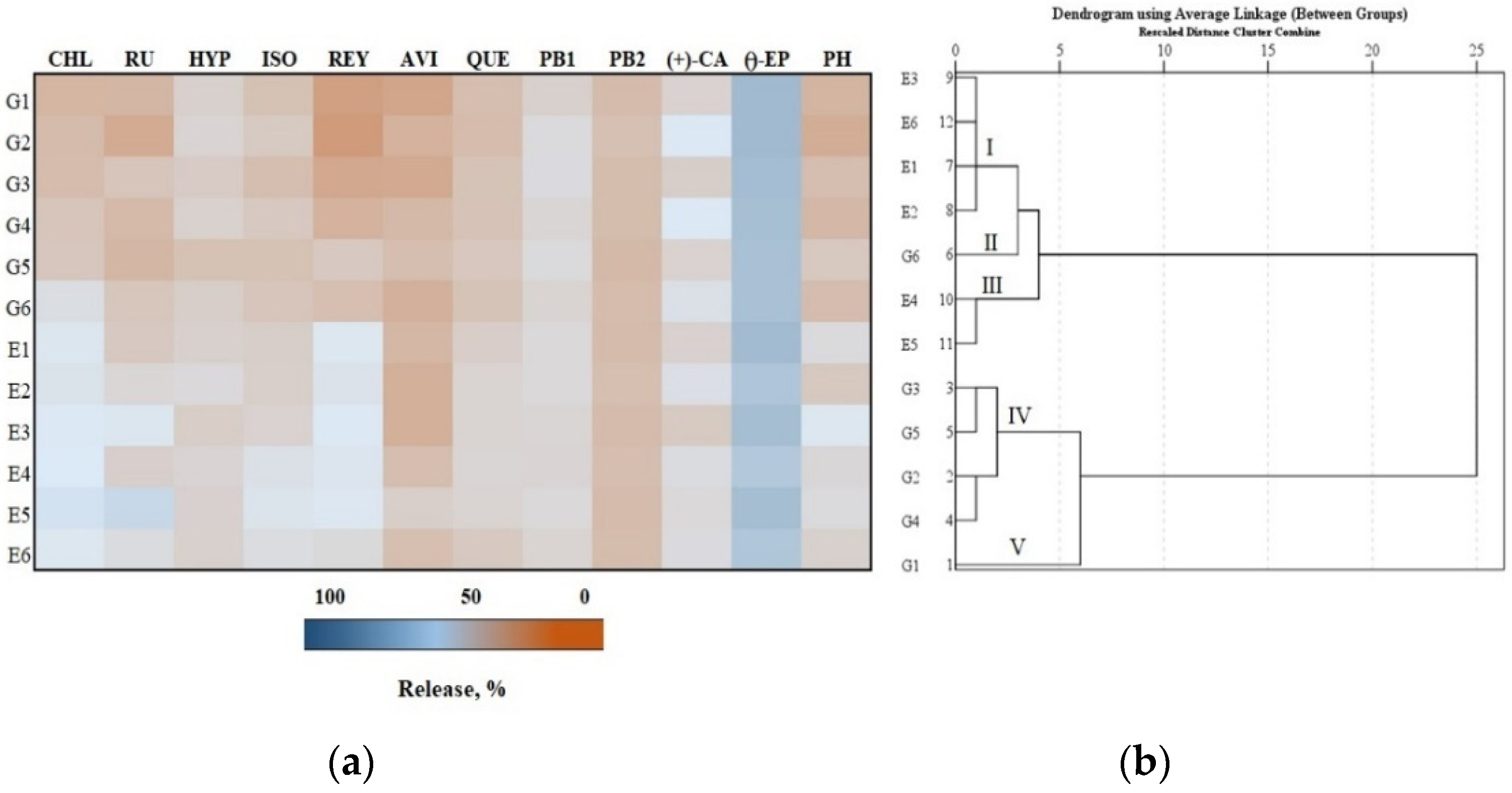
3.3. In Vitro Testing of the Release of Phenolic Compounds from Gels and Emulgels
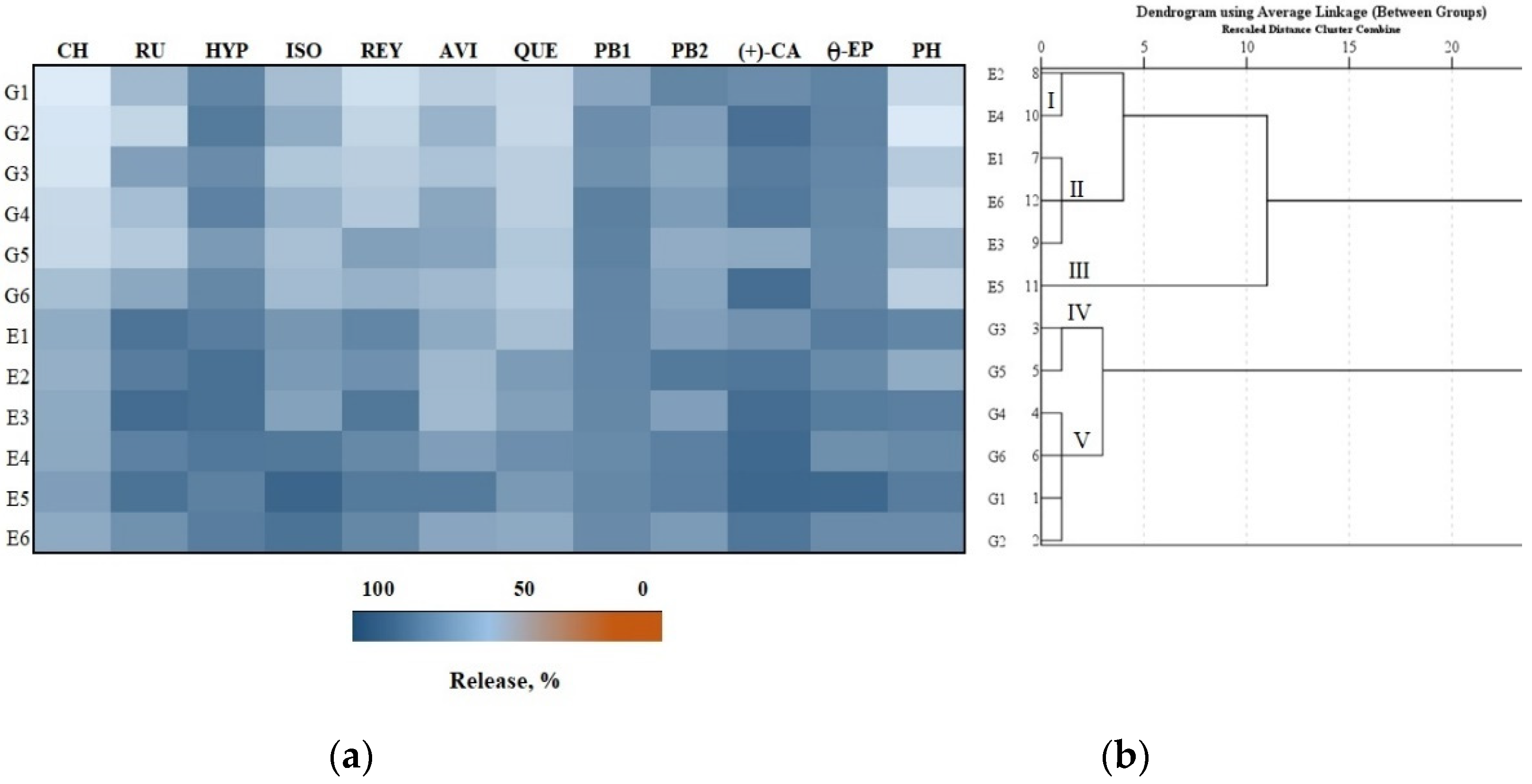
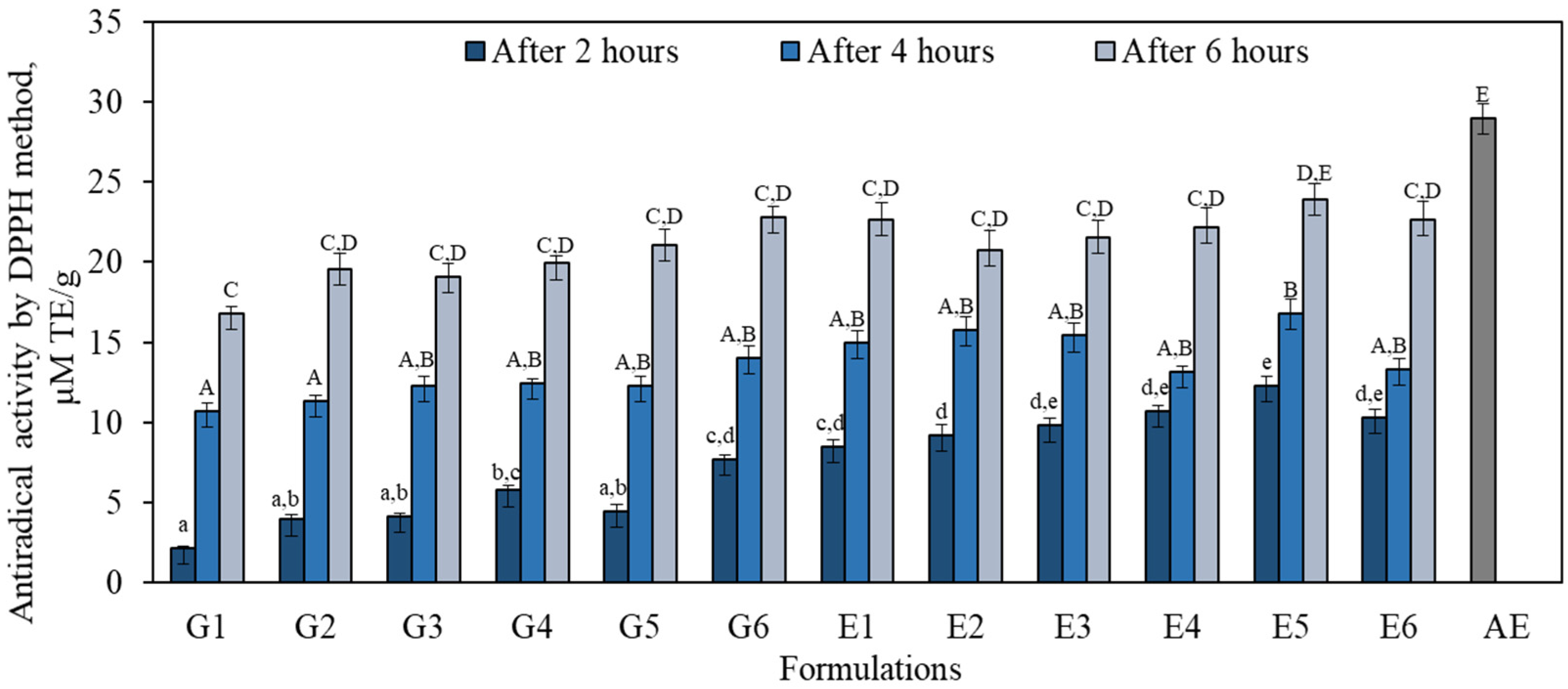
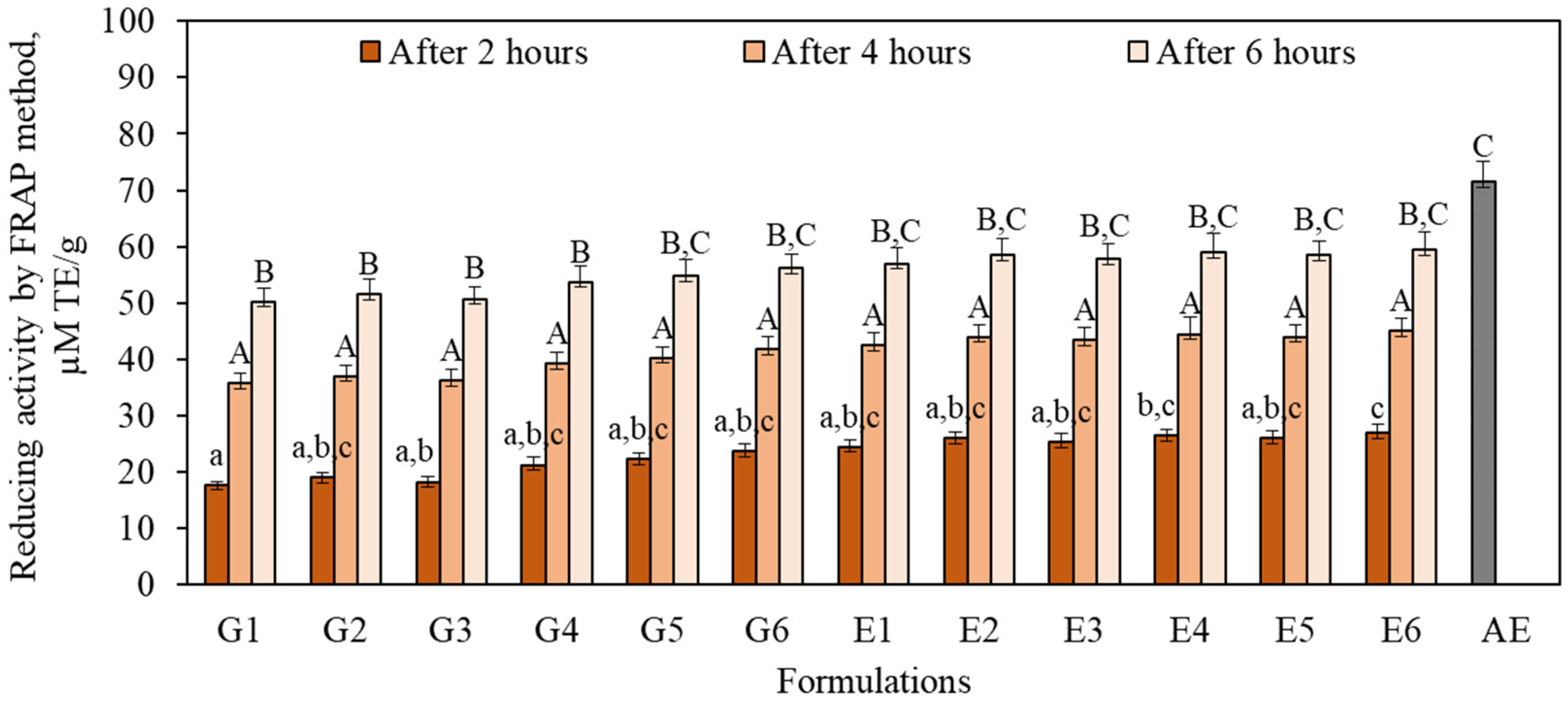
3.4. Antioxidant Activity In Vitro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 24 February 2021).
- Preti, R.; Tarola, A.M. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. Eur. Food Res. Technol. 2021, 247, 273–283. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Maragò, E.; Iacopini, P.; Camangi, F.; Scattino, C.; Ranieri, A.; Stefani, A.; Sebastiani, L. Phenolic profile and antioxidant activity in apple juice and pomace: Effects of different storage conditions. Fruits 2015, 70, 213–223. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.; La Porta, R.; Napolitano, M.; Galletti, P.; Quagliuolo, L.; Boccellino, M. Effect of Annurca apple polyphenols on human HaCaT keratinocytes proliferation. J. Med. Food 2012, 15, 1024–1031. [Google Scholar] [CrossRef]
- Muniandy, K.; Gothai, S.; Tan, W.S.; Kumar, S.; Esa, N.M.; Chandramohan, G.; Al-Numair, K.S.; Arulselvan, P. In vitro wound healing potential of stem extract of Alternanthera sessilis. Evid. Based Complement. Altern. Med. 2018, 2018, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Landete, J.M. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Starowicz, M.; Achrem–Achremowicz, B.; Piskuła, M.L.; Zieliński, H. Phenolic compounds from apples: Reviewing their occurrence, absorption, bioavailability, processing, and antioxidant activity—A review. Pol. J. Food Nutr. Sci. 2020, 70, 321–336. [Google Scholar] [CrossRef]
- Porrini, M.; Riso, P. Factors influencing the bioavailability of antioxidants in foods: A critical appraisal. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 647–650. [Google Scholar] [CrossRef]
- Kikwai, L.; Babu, R.J.; Prado, R.; Kolot, A.; Armstrong, C.A.; Ansel, J.C.; Singh, M. In vitro and in vivo evaluation of topical formulations of spantide. Pharm. Sci. Tech. 2005, 6, e565–e572. [Google Scholar] [CrossRef] [Green Version]
- Moshfeghi, A.A.; Peyman, G.A. Micro- and nanoparticulates. Adv. Drug Deliv. Rev. 2005, 57, 2047–2052. [Google Scholar] [CrossRef]
- Rosen, H.; Abribat, T. The rise and rise of drug delivery. Nat. Rev. Drug Discov. 2005, 4, 381–385. [Google Scholar] [CrossRef]
- Ahsan, A.; Miana, G.A.; Naureen, H.; Rehman, M.U.; Anum, K.; Malik, I. Formulation, characterization and wound-healing potential of emulgel and in-situ gel containing root extract of Saussurea lappa Clarke (Asteraceae). Trop. J. Pharm. Res. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef]
- Ashara, K.; Soniwala, M.; Shah, K. Emulgel: A novel drug delivery system. J. Pak. Assoc. Dermatol. 2016, 26, 244–249. [Google Scholar]
- Ajazuddin; Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Petrikaite, V.; Jurgaityte, V.; Liaudanskas, M.; Janulis, V. Antioxidant, anti-inflammatory, and cytotoxic activity of extracts from some commercial apple cultivars in two colorectal and glioblastoma human cell lines. Antioxidants 2021, 10, 1098. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viskelis, P.; Kviklys, D.; Raudonis, R.; Janulis, V. A comparative study of phenolic content in apple fruits. Int. J. Food Prop. 2015, 18, 945–953. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Ivanauskas, L.; Perminaite, K.; Ramanauskiene, K. Ophthalmic in situ gels with balsam poplar buds extract: Formulation, rheological characterization, and quality evaluation. Pharmaceutics 2021, 13, 953. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1998, 299, 15–27. [Google Scholar]
- Belviso, S.; Scursatone, B.; Re, G.; Zeppa, G. Novel data on the polyphenol composition of Italian ancient apple cultivars. Int. J. Food Prop. 2013, 16, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which polyphenolic compounds contribute to the total antioxidant activities of apple. J. Agric. Food Chem. 2005, 53, 4989–4995. [Google Scholar] [CrossRef]
- Rana, S.; Rana, A.; Gupta, S.; Bhushan, S. Varietal influence on phenolic constituents and nutritive characteristics of pomace obtained from apples grown in western Himalayas. J. Food Sci. Technol. 2021, 58, 166–174. [Google Scholar] [CrossRef]
- Piccolo, E.L.; Landi, M.; Massai, R.; Remorini, D.; Conte, G.; Guidi, L. Ancient apple cultivars from Garfagnana (Tuscany, Italy): A potential source for ’nutrafruit’ production. Food Chem. 2019, 294, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical profiles of new red-fleshed apple varieties compared with old and new white-fleshed varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szo, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Light, K.; Karboune, S. Emulsion, hydrogel and emulgel systems and novel applications in cannabinoid delivery: A review. Crit. Rev. Food Sci. Nutr. 2021, 22, 1–31. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.M.; Hafez, S.A.; Mahdy, M.M. Organogels, hydrogels and bigels as transdermal delivery systems for diltiazem hydrochloride. Asian J. Pharm. Sci. 2013, 8, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E. PH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Gray, M.; Black, J.M.; Baharestani, M.M.; Bliss, D.Z.; Colwell, J.C.; Goldberg, M.; Kennedy–Evans, K.L.; Ratliff Mois, S.L.C. Moisture–associated skin damage. J. Wound Ostomy Cont. Nurs. 2011, 38, 233–241. [Google Scholar] [CrossRef]
- Jufri, M.; Rachmadiva; Gozan, M.; Suyono, E.A. Formulation, stability test and in vitro penetration test of emulgel from tobacco leaves extract. J. Young Pharm. 2018, 10, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.K.; Raw, A.; Lionberger, R.; Yu, L. Generic development of topical dermatologic products: Formulation development, process development, and testing of topical dermatologic products. AAPS J. 2012, 15, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayati, F.; Ghamsari, S.M.; Dehghan, M.M.; Oryan, A. Effects of carbomer 940 hydrogel on burn wounds: An in vitro and in vivo study. J. Dermatol. Treat. 2018, 29, 593–599. [Google Scholar] [CrossRef]
- Vilasau, J.; Solans, C.; Gómez, M.J.; Dabrio, J.; Mújika-Garai, R.; Esquena, J. Phase behaviour of a mixed ionic/nonionic surfactant system used to prepare stable oil-in-water paraffin emulsions. Colloids Surf. A Physicochem. Eng. 2011, 384, 473–481. [Google Scholar] [CrossRef]
- Bhosale, M.S.; Khade, S.; Patil, A.S.; Raut, I.D.; Nitalikar, M.M.; Jadhav, K.K. Emulgel: A novel approach to topical drug delivery. Levant 2021, 20, 394–406. [Google Scholar]
- Yassin, G.E. Formulation and evaluation of optimized clotrimazole emulgel formulations. Brit. J. Pharma Res. 2014, 4, 1014–1030. [Google Scholar] [CrossRef]
- Kregar, M.L.; Dürrigl, M.; Rožman, A.; Jelcic, Ž.; Cetina- Cižmek, B.; Filipovic-Grcic, J. Development and validation of an in vitro release method for topical particulate delivery systems. Int. J. Pharm. 2015, 485, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Haneefa, K.P.M.; Easo, S.; Hafsa, P.V.; Mohanta, G.P.; Nayar, C. Emulgel: An advanced review. J. Pharm. Sci. Res. 2013, 5, 254–258. [Google Scholar]
- Pradhan, M.; Singh, D.; Singh, M.R. Novel colloidal carriers for psoriasis: Current issues, mechanistic insight and novel delivery approaches. J. Control Release 2013, 170, 380–395. [Google Scholar] [CrossRef]
- Elbayoumi, T.A.; Torchilin, V.P. Liposomes for targeted delivery of antithrombotic drugs. Expert Opin. Drug Deliv. 2008, 5, 1185–1198. [Google Scholar] [CrossRef]
- Faramarzi, S.; Pacifico, S.; Yadollahi, A.; Lettieri, A.; Nocera, P.; Piccolella, S. Red-fleshed apples: Old autochthonous fruits as a novel source of anthocyanin antioxidants. Plant Foods Hum. Nutr. 2015, 70, 324–330. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, e1901502. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Joshi, A.; Arora, B.; Bhowmik, A.; Sharma, R.R.; Kumar, P. Significance of FRAP, DPPH, and CUPRAC assays for antioxidant activity determination in apple fruit extracts. Eur. Food Res. Technol. 2020, 246, 591–598. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. J. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Phenolic profiling of five different Australian grown apples. Appl. Sci. 2021, 11, 2421. [Google Scholar] [CrossRef]
- Chen, W.C.; Liou, S.S.; Tzeng, T.F.; Lee, S.L.; Liu, I.M. Effect of topical application of chlorogenic acid on excision wound healing in rats. Planta Med. 2013, 79, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Khan, H.M.S.; Anwar, Z.; Talbot, B.; Walsh, J.J. HPLC profiling of Mimosa pudica polyphenols and their non-invasive biophysical investigations for anti-dermatoheliotic and skin reinstating potential. Biomed. Pharmacother. 2019, 109, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]

| Composition No. | Carbomer, g | NaOH, mL | Glycerin, g | Castor Oil, g | Span 20, g | Tween 20, g | Water, g | Content, g |
|---|---|---|---|---|---|---|---|---|
| Gels | ||||||||
| G1 | 0.5 | 3–4 drops | - | - | - | - | ad 100 | 100 ± 0.5 |
| G2 | 1.0 | 3–4 drops | - | - | - | - | ad 100 | 100 ± 0.5 |
| G3 | 2.0 | 3–4 drops | - | - | - | - | ad 100 | 100 ± 0.5 |
| G4 | 2.5 | 3–4 drops | - | - | - | - | ad 100 | 100 ± 0.5 |
| G5 | 3.0 | 3–4 drops | - | - | - | - | ad 100 | 100 ± 0.5 |
| G6 | 3.5 | 3–4 drops | - | - | - | - | ad 100 | 100 ± 0.5 |
| Emulsion | ||||||||
| N8 | - | - | 10 | 10 | 6 | 6 | ad 100 | 100 ± 0.5 |
| Emulgels | ||||||||
| E1 | Mixed G1 and N8 (1:1) | 100 ± 0.5 | ||||||
| E2 | Mixed G2 and N8 (1:1) | 100 ± 0.5 | ||||||
| E3 | Mixed G3 and N8 (1:1) | 100 ± 0.5 | ||||||
| E4 | Mixed G4 and N8 (1:1) | 100 ± 0.5 | ||||||
| E5 | Mixed G5 and N8 (1:1) | 100 ± 0.5 | ||||||
| E6 | Mixed G6 and N8 (1:1) | 100 ± 0.5 | ||||||
| Formulations | ||||||
|---|---|---|---|---|---|---|
| Gel | ||||||
| Composition No. | G1 | G2 | G3 | G4 | G5 | G6 |
| Organoleptic characteristics |  |  |  |  |  |  |
| Yellow homogeneous gel with base odor | Dark yellow homogeneous gel with base odor | |||||
| pH value (n = 3) | 6.60 ± 0.34 | 6.67 ± 0.34 | 6.72 ± 0.35 | 6.71 ± 0.35 | 6.76 ± 0.35 | 6.76 ± 0.35 |
| Viscosity (Pa·s, n = 3) | 2.92 ± 0.15 | 7.74 ± 0.39 | 7.96 ± 0.40 | 8.25 ± 0.41 | 9.03 ± 0.55 | 9.18 ± 0.46 |
| Emulgel | ||||||
| Composition No. | E1 | E2 | E3 | E4 | E5 | E6 |
| Organoleptic characteristics |  |  |  |  |  |  |
| Homogeneous emulgel with slightly yellow color, base odor, non-oily. | ||||||
| pH value (n = 3) | 6.04 ± 0.30 | 6.03 ± 0.30 | 6.14 ± 0.30 | 6.16 ± 0.30 | 6.20 ± 0.30 | 6.27 ± 0.30 |
| Viscosity (Pa·s, n = 3) | 2.94 ± 0.15 | 7.51 ± 0.38 | 7.68 ± 0.38 | 8.01 ± 0.40 | 8.97 ± 0.45 | 8.98 ± 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkeviciute, A.; Ramanauskiene, K.; Janulis, V. Formulation of Gels and Emulgels with Malus domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants 2022, 11, 373. https://doi.org/10.3390/antiox11020373
Butkeviciute A, Ramanauskiene K, Janulis V. Formulation of Gels and Emulgels with Malus domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants. 2022; 11(2):373. https://doi.org/10.3390/antiox11020373
Chicago/Turabian StyleButkeviciute, Aurita, Kristina Ramanauskiene, and Valdimaras Janulis. 2022. "Formulation of Gels and Emulgels with Malus domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro" Antioxidants 11, no. 2: 373. https://doi.org/10.3390/antiox11020373
APA StyleButkeviciute, A., Ramanauskiene, K., & Janulis, V. (2022). Formulation of Gels and Emulgels with Malus domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants, 11(2), 373. https://doi.org/10.3390/antiox11020373








