An Update on the Exploratory Use of Curcumin in Neuropsychiatric Disorders
Abstract
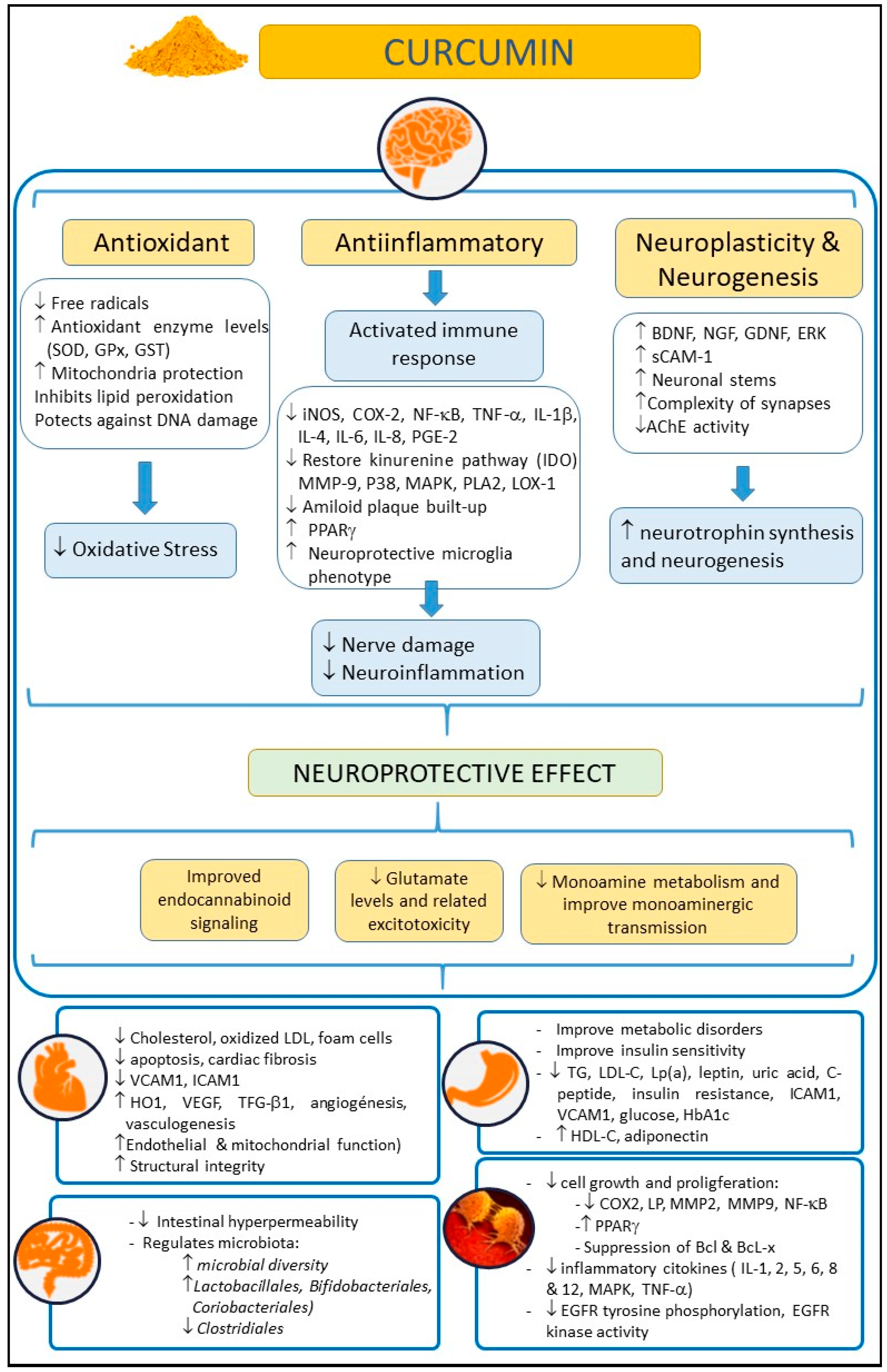
1. Introduction
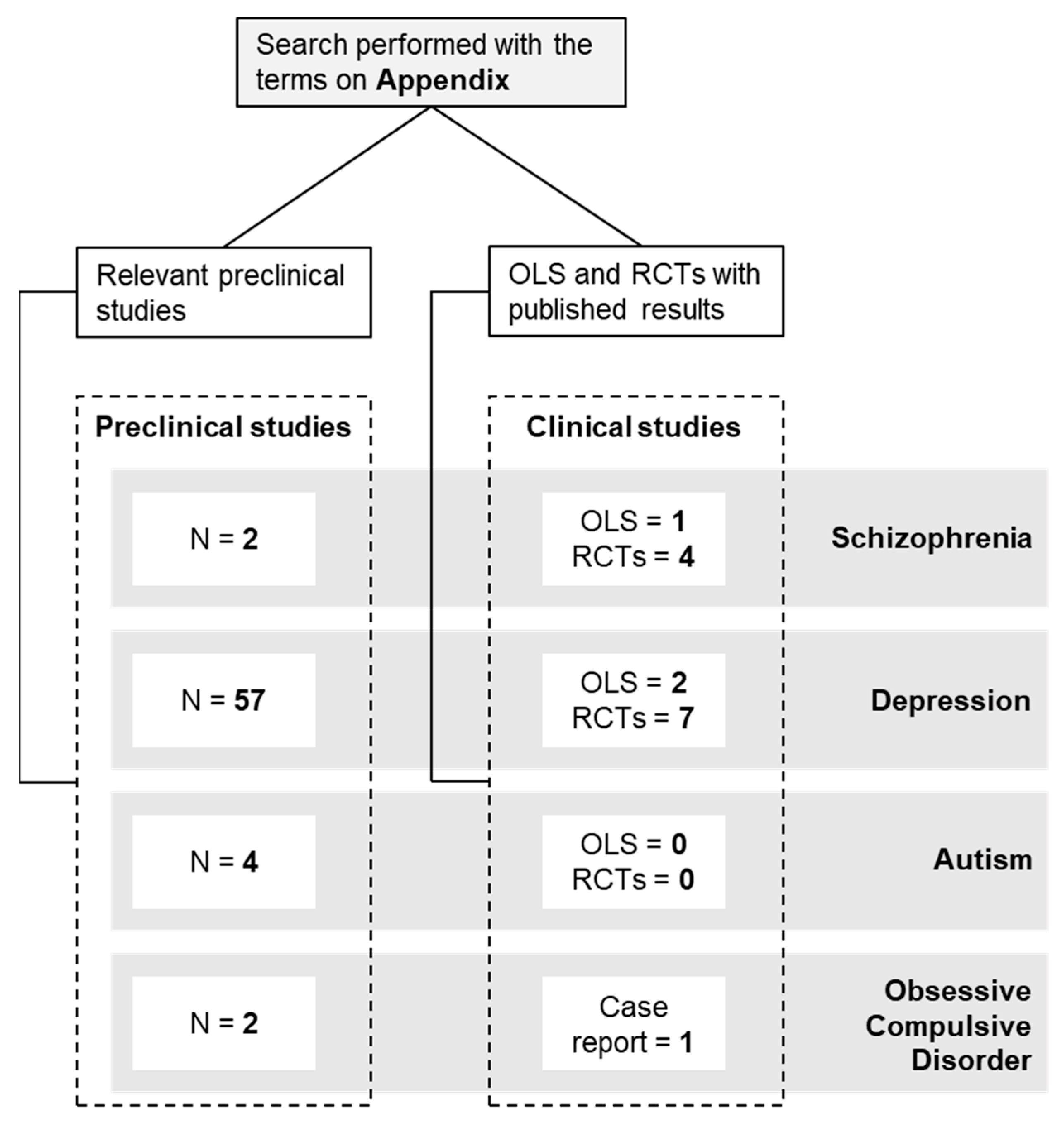
2. Materials and Methods
3. Results
3.1. Schizophrenia
3.2. Depression
3.3. Autism Spectrum Disorder (ASD)
3.4. Obsessive Compulsive Disorder (OCD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AchE | Acetylcholinesterase |
| ATP | Adenosine triphosphate |
| ASD | Autism spectrum disorders |
| Bcl | B-cell lympho MAO |
| BDI-II | Beck Depression Inventory II |
| BDNF | Brain-derived neurotrophic factor |
| BLYS | B lymphocyte stimulator |
| BZDs | Benzodiazepines |
| Ca+2 | Calcium |
| cAMP | cyclic adenosine monophosphate |
| CAT | Catalase |
| CBR | Cannabinoid receptor |
| CCI | Chronic constriction injury |
| CDSS | Clinical depression screening scale |
| CGI-I | Clinical global impressions-improvement |
| CGI-S | Clinical global impressions-severity |
| CMS | Chronic mild stress |
| COX-2 | Cyclooxygenase-2 |
| CUMS | Chronic unpredictable mild stress |
| CORT | Corticosterone |
| CREB | cAMP response element-binding protein |
| CUS | Chronic unpredictable stress |
| CUR | Curcumin |
| CY-BOCS | Children’s Yale–Brown Obsessive Compulsive Scale |
| DA | Dopamine |
| DOPAC | 4-dihydroxyphenylacetic acid |
| ECG | Electrocardiogram |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular signal-regulated kinase |
| ET-1 | Endothelin 1 |
| GABA | Gamma-aminobutyric acid |
| GD | Gestational day |
| GDNF | Glial cell line-derived neurotrophic factor |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GST | Glutathione S-transferase |
| HADS | Hospital Anxiety and Depression Scale |
| HbA1c | Glycosylated hemoglobin A1c |
| HDL-C | High density lipoprotein cholesterol |
| HDRS | Hamilton Depression Rating Scale |
| HO-1 | Heme oxygenase-1 |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IDO | Indolamine-2, 3-Dioxygenase |
| IDS-SR30 | Inventory of depressive symptomatology |
| IFN-γ | Interferon |
| IL-1β | Interleukine-1β |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10; |
| IMPS | Invalid metabolic panaceas |
| iNOS | Inducible nitric oxide synthase |
| IOS | Inflammation and oxidative stress |
| IRS-1 | Insulin receptor substrate 1 |
| LDL-C | Low density lipoprotein cholesterol |
| LOX-1 | Lectin-like oxidized low-density lipoprotein receptor |
| Lp(a) | Lipoprotein(a) |
| LP | Lipooxigenase |
| LPS | Lipopolysaccharide |
| MADRS | Montgomery–Asberg Depression Rating Scale |
| MA | Motor activity test |
| MAO | Monoamine oxidase |
| MAPK | Mitogen-activated protein kinase |
| MBB | Marble-burying behavior test |
| MCAO | Middle cerebral artery occlusion |
| MDA | Malondialdehyde |
| MDD | Major depressive disorder |
| MEK | Methyl ethyl ketone |
| MMP | Mitochondrial membrane potential |
| MMP-9 | Matrix metalloproteinase 9 |
| mRNA | Messenger ribonucleic acid |
| NA | Noradrenaline |
| NAC | N-Acetylcysteine |
| NE | Norepinephrine |
| NF-κβ | Nuclear factor κβ |
| NGF | Nerve growth factor |
| NMDA | N-Methyl-D-Aspartate |
| NQO-1 | Quinine oxidoreductase-1 |
| OB | Olfactory bulbectomy |
| OCD | Obsessive compulsive disorder |
| OLS | Open label clinical studies |
| OVX | Ovarectomy/Ovarectomized |
| P38 | P38 MAPK |
| PAINS | Pan assay interference compound |
| PANSS | Positive and negative syndrome scale |
| PGE-2 | Prostaglandin E2 |
| PET | Positron emission tomography |
| PKA | Protein kinase A |
| PKB | Protein kinase B |
| PLA2 | Phospholipase A2 |
| PPA | Propanoic acid |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PSD-95 | Postsynaptic density protein-95 |
| RCT | Randomized clinical trial |
| sCAM-1 | Soluble cell adhesion molecule 1 |
| SCZ | Schizophrenia |
| SD | Sprague-Dawley |
| SL327 | ERK inhibitor |
| SNRIs | Serotonin–norepinephrine reuptake inhibitors |
| SOD | Superoxide dismutase |
| SREBPs | Hepatic sterol regulatory element-binding proteins |
| SPS | Single prolonged stress |
| SSRIs | Selective serotonin reuptake inhibitors |
| STAI | State-Trait Anxiety Inventory |
| SUB-P | Substance P |
| TBARs | Thiobarbituric acid reactive substances |
| TBX-B2 | Thromboxane B2 |
| TCAs | Tricyclic antidepressants |
| TG | Triglycerides |
| TGF-β1 | Transforming growth factor |
| TN | Trigeminal neuralgia |
| TNF-α | Tumor necrosis factor |
| Tuj1 | Neuron-specific class III β-tubulin |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VEGF | Vascular endothelial growth factor |
| VPA | Valproic acid |
| YGTSS | Yale global tic severity scale |
| 5-HIAA | High 5-hydroxyindoleacetic acid |
| 5-HT | Serotonin |
Appendix A
References
- Zhang, H.A.; Kitts, D.D. Turmeric and its bioactive constituents trigger cell signaling mechanisms that protect against diabetes and cardiovascular diseases. Mol. Cell. Biochem. 2021, 476, 3785–3814. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef]
- Phumsuay, R.; Muangnoi, C.; Wasana, P.W.D.; Hasriadi, H.; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Molecular Insight into the Anti-Inflammatory Effects of the Curcumin Ester Prodrug Curcumin Diglutaric Acid In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 5700. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by curcumin: A review on brain delivery strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Manthiannem, E.; Padamati, J.; Chandra, R.; Chidambaram, S.B.; Sakharkar, M.K. Benefits of curcumin in brain disorders. BioFactors 2019, 45, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hang, Y.; Liu, J.; Hou, Y.; Wang, N.; Wang, M. Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncol. Lett. 2017, 13, 4825–4831. [Google Scholar] [CrossRef] [PubMed]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, L.; Sikora, E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef]
- Concetta Scuto, M.; Mancuso, C.; Tomasello, B.; Laura Ontario, M.; Cavallaro, A.; Frasca, F.; Maiolino, L.; Trovato Salinaro, A.; Calabrese, E.J.; Calabrese, V. Curcumin, Hormesis and the Nervous System. Nutrients 2019, 11, 2417. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell. Physiol. 2018, 233, 830–848. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanovic-Radic, Z.; Matejic, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Barbalho, S.M.; de Sousa Gonzaga, H.F.; de Souza, G.A.; de Alvares Goulart, R.; de Sousa Gonzaga, M.L.; de Alvarez Rezende, B. Dermatological effects of Curcuma species: A systematic review. Clin. Exp. Dermatol. 2021, 46, 825–833. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Tran, T.H.; Mattheolabakis, G.; Aldawsari, H.; Amiji, M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immunol. 2015, 160, 46–58. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Gigliobianco, M.R.; Pellitteri, R.; Santonocito, D.; Carbone, C.; Di Martino, P.; Puglisi, G.; Musumeci, T. Optimization of Curcumin Nanocrystals as Promising Strategy for Nose-to-Brain Delivery Application. Pharmaceutics 2020, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Don, T.M.; Chang, W.J.; Jheng, P.R.; Huang, Y.C.; Chuang, E.Y. Curcumin-laden dual-targeting fucoidan/chitosan nanocarriers for inhibiting brain inflammation via intranasal delivery. Int. J. Biol. Macromol. 2021, 181, 835–846. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef] [PubMed]
- Shireen, E. Experimental treatment of antipsychotic-induced movement disorders. J. Exp. Pharmacol. 2016, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Parikh, V.; Terry, A.V., Jr.; Mahadik, S.P. Long-term antipsychotic treatments and crossover studies in rats: Differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 2007, 41, 372–386. [Google Scholar] [CrossRef]
- Naserzadeh, P.; Hafez, A.A.; Abdorahim, M.; Abdollahifar, M.A.; Shabani, R.; Peirovi, H.; Simchi, A.; Ashtari, K. Curcumin loading potentiates the neuroprotective efficacy of Fe3O4 magnetic nanoparticles in cerebellum cells of schizophrenic rats. Biomed. Pharmacother. 2018, 108, 1244–1252. [Google Scholar] [CrossRef]
- Moghaddam, A.H.; Maboudi, K.; Bavaghar, B.; Sangdehi, S.R.M.; Zare, M. Neuroprotective effects of curcumin-loaded nanophytosome on ketamine-induced schizophrenia-like behaviors and oxidative damage in male mice. Neurosci. Lett. 2021, 765, 136249. [Google Scholar] [CrossRef]
- Wynn, J.K.; Green, M.F.; Hellemann, G.; Karunaratne, K.; Davis, M.C.; Marder, S.R. The effects of curcumin on brain-derived neurotrophic factor and cognition in schizophrenia: A randomized controlled study. Schizophr. Res. 2018, 195, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Hosseininasab, M.; Zarghami, M.; Mazhari, S.; Salehifar, E.; Moosazadeh, M.; Fariborzifar, A.; Babaeirad, S.; Hendouei, N. Nanocurcumin as an Add-on to Antipsychotic Drugs for Treatment of Negative Symptoms in Patients With Chronic Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Psychopharmacol. 2021, 41, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kucukgoncu, S.; Guloksuz, S.; Tek, C. Effects of Curcumin on Cognitive Functioning and Inflammatory State in Schizophrenia: A Double-Blind, Placebo-Controlled Pilot Trial. J. Clin. Psychopharmacol. 2019, 39, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Miodownik, C.; Lerner, V.; Kudkaeva, N.; Lerner, P.P.; Pashinian, A.; Bersudsky, Y.; Eliyahu, R.; Kreinin, A.; Bergman, J. Curcumin as Add-On to Antipsychotic Treatment in Patients With Chronic Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. Clin. Neuropharmacol. 2019, 42, 117–122. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Wu, H.L.; Li, Y.B.; Li, Y.H.; Guo, J.B.; Li, X.J. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur. J. Pharmacol. 2008, 578, 43–50. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Wang, S.; Yu, L.; Liu, D.; Zhan, R.; Yu, S.Y. Curcumin produces antidepressant effects via activating MAPK/ERK-dependent brain-derived neurotrophic factor expression in the amygdala of mice. Behav. Brain Res. 2012, 235, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, T.; Wang, S.; Yu, L.; Liu, D.; Zhan, R.; Yu, S.Y. NMDA GluN2B receptors involved in the antidepressant effects of curcumin in the forced swim test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 12–17. [Google Scholar] [CrossRef]
- He, X.; Yang, L.; Wang, M.; Zhuang, X.; Huang, R.; Zhu, R.; Wang, S. Targeting the Endocannabinoid/CB1 Receptor System For Treating Major Depression Through Antidepressant Activities of Curcumin and Dexanabinol-Loaded Solid Lipid Nanoparticles. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 42, 2281–2294. [Google Scholar] [CrossRef]
- Choi, G.Y.; Kim, H.B.; Hwang, E.S.; Lee, S.; Kim, M.J.; Choi, J.Y.; Lee, S.O.; Kim, S.S.; Park, J.H. Curcumin Alters Neural Plasticity and Viability of Intact Hippocampal Circuits and Attenuates Behavioral Despair and COX-2 Expression in Chronically Stressed Rats. Mediat. Inflamm. 2017, 2017, 6280925. [Google Scholar] [CrossRef]
- Abd-Rabo, M.M.; Georgy, G.S.; Saied, N.M.; Hassan, W.A. Involvement of the serotonergic system and neuroplasticity in the antidepressant effect of curcumin in ovariectomized rats: Comparison with oestradiol and fluoxetine. Phytother. Res. PTR 2019, 33, 387–396. [Google Scholar] [CrossRef]
- Mohammed, H.S.; Khadrawy, Y.A.; El-Sherbini, T.M.; Amer, H.M. Electrocortical and Biochemical Evaluation of Antidepressant Efficacy of Formulated Nanocurcumin. Appl. Biochem. Biotechnol. 2019, 187, 1096–1112. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Yang, L.; Wang, Z.J.; Huang, R.Q.; Zhu, R.R.; Cheng, L.M. Solid lipid nanoparticles loading with curcumin and dexanabinol to treat major depressive disorder. Neural Regen. Res. 2021, 16, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Khadrawy, Y.A.; Hosny, E.N.; Magdy, M.; Mohammed, H.S. Antidepressant effects of curcumin-coated iron oxide nanoparticles in a rat model of depression. Eur. J. Pharmacol. 2021, 908, 174384. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.K.; Bhutani, M.K.; Bishnoi, M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology 2008, 201, 435–442. [Google Scholar] [CrossRef]
- Bhutani, M.K.; Bishnoi, M.; Kulkarni, S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009, 92, 39–43. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Wang, M.; Jing, G.; Zhu, R.; Wang, S. Antidepressant effects of curcumin and HU-211 coencapsulated solid lipid nanoparticles against corticosterone-induced cellular and animal models of major depression. Int. J. Nanomed. 2016, 11, 4975–4990. [Google Scholar] [CrossRef]
- Hurley, L.L.; Akinfiresoye, L.; Nwulia, E.; Kamiya, A.; Kulkarni, A.A.; Tizabi, Y. Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behav. Brain Res. 2013, 239, 27–30. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Akula, K.K.; Deshpande, J. Evaluation of antidepressant-like activity of novel water-soluble curcumin formulations and St. John’s wort in behavioral paradigms of despair. Pharmacology 2012, 89, 83–90. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.S.; Yao, H.Y.; Lin, Y.H.; Ma, X.; Zhang, Y.H.; Li, X.J. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol. Biochem. Behav. 2005, 82, 200–206. [Google Scholar] [CrossRef]
- Borre, Y.E.; Panagaki, T.; Koelink, P.J.; Morgan, M.E.; Hendriksen, H.; Garssen, J.; Kraneveld, A.D.; Olivier, B.; Oosting, R.S. Neuroprotective and cognitive enhancing effects of a multi-targeted food intervention in an animal model of neurodegeneration and depression. Neuropharmacology 2014, 79, 738–749. [Google Scholar] [CrossRef]
- Rinwa, P.; Kumar, A.; Garg, S. Suppression of neuroinflammatory and apoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression. PLoS ONE 2013, 8, e61052. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Wang, Y.; Xie, K.; Zhang, Q.; Luan, Q.; Chen, W.; Liu, D. Antidepressant-like effects of curcumin in chronic mild stress of rats: Involvement of its anti-inflammatory action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 47, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Shi, L.; Lu, J.; Li, J.; Hu, H.; Zuo, C.; Tang, W.; Lu, Y.; Bao, A.; Xu, L. Effects of curcumin on glucose metabolism in the brains of rats subjected to chronic unpredictable stress: A 18 F-FDG micro-PET study. BMC Complementary Altern. Med. 2013, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Z.; Gao, Z.; Xie, K.; Zhang, Q.; Jiang, H.; Pang, Q. Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav. Brain Res. 2014, 271, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.R.; Wang, L.; Li, J.; Wu, D.S. Analysis of anti-depressant potential of curcumin against depression induced male albino wistar rats. Brain Res. 2016, 1642, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Li, Q.; Zhang, M.; Zhao, Y.J.; Huang, F.; Chen, Y.J. Long-term curcumin treatment antagonizes masseter muscle alterations induced by chronic unpredictable mild stress in rats. Arch. Oral Biol. 2014, 59, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Naqvi, F.; Batool, Z.; Tabassum, S.; Sadir, S.; Liaquat, L.; Naqvi, F.; Zuberi, N.A.; Shakeel, H.; Perveen, T. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res. Bull. 2015, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, J.; Zhang, M.; Yao, W.; Ma, X.; Yu, S.Y. Effects of curcumin on chronic, unpredictable, mild, stress-induced depressive-like behaviour and structural plasticity in the lateral amygdala of rats. Int. J. Neuropsychopharmacol. 2014, 17, 793–806. [Google Scholar] [CrossRef]
- Demir, E.A.; Oz, M.; Alp, M.I.; Gergerlioglu, H.S.; Nurullahoglu, K.E.; Yerlikaya, F.H. Co-administration of cisplatin and curcumin does not alter mood-associated behaviors. Bratisl. Lek. Listy 2016, 117, 106–111. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Saracheva, K.E.; Ivanovska, M.V.; Petrova, A.P.; Marchev, A.S.; Georgiev, M.I.; Murdjeva, M.A.; Getova, D.P. Antidepressant-like effect of salidroside and curcumin on the immunoreactivity of rats subjected to a chronic mild stress model. Food Chem. Toxicol. 2018, 121, 604–611. [Google Scholar] [CrossRef]
- Lee, B.; Lee, H. Systemic Administration of Curcumin Affect Anxiety-Related Behaviors in a Rat Model of Posttraumatic Stress Disorder via Activation of Serotonergic Systems. Evid.-Based Complementary Altern. Med. 2018, 2018, 9041309. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Lv, C.; Cao, L.; Yao, D.; Wu, Y.; Long, M.; Liu, N.; Jiang, P. Curcumin Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behaviors via Restoring Changes in Oxidative Stress and the Activation of Nrf2 Signaling Pathway in Rats. Oxidative Med. Cell. Longev. 2020, 2020, 9268083. [Google Scholar] [CrossRef]
- Saied, N.M.; Georgy, G.S.; Hussien, R.M.; Hassan, W.A. Neuromodulatory effect of curcumin on catecholamine systems and inflammatory cytokines in ovariectomized female rats. Clin. Exp. Pharmacol. Physiol. 2021, 48, 337–346. [Google Scholar] [CrossRef]
- Mohammad Abu-Taweel, G.; Al-Fifi, Z. Protective effects of curcumin towards anxiety and depression-like behaviors induced mercury chloride. Saudi J. Biol. Sci. 2021, 28, 125–134. [Google Scholar] [CrossRef] [PubMed]
- da Silva Marques, J.G.; Antunes, F.T.T.; da Silva Brum, L.F.; Pedron, C.; de Oliveira, I.B.; de Barros Falcao Ferraz, A.; Martins, M.I.M.; Dallegrave, E.; de Souza, A.H. Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav. Brain Res. 2021, 399, 113002. [Google Scholar] [CrossRef] [PubMed]
- Rubab, S.; Naeem, K.; Rana, I.; Khan, N.; Afridi, M.; Ullah, I.; Shah, F.A.; Sarwar, S.; Din, F.U.; Choi, H.I.; et al. Enhanced neuroprotective and antidepressant activity of curcumin-loaded nanostructured lipid carriers in lipopolysaccharide-induced depression and anxiety rat model. Int. J. Pharm. 2021, 603, 120670. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, X.M.; Li, Z.Y.; Feng, C.R.; Pan, A.J.; Mao, Q.Q. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci. Lett. 2011, 493, 145–148. [Google Scholar] [CrossRef]
- Li, Y.C.; Wang, F.M.; Pan, Y.; Qiang, L.Q.; Cheng, G.; Zhang, W.Y.; Kong, L.D. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 435–449. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Yuan, L.; Wang, S.; Liu, L.; Yang, X.; Li, G.; Liu, D. The effects of curcumin on depressive-like behavior in mice after lipopolysaccharide administration. Behav. Brain Res. 2014, 274, 282–290. [Google Scholar] [CrossRef]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Liu, B.; Yu, S.Y. Curcumin Protects Against Chronic Stress-induced Dysregulation of Neuroplasticity and Depression-like Behaviors via Suppressing IL-1beta Pathway in Rats. Neuroscience 2018, 392, 92–106. [Google Scholar] [CrossRef]
- Fan, C.; Song, Q.; Wang, P.; Li, Y.; Yang, M.; Yu, S.Y. Neuroprotective Effects of Curcumin on IL-1beta-Induced Neuronal Apoptosis and Depression-Like Behaviors Caused by Chronic Stress in Rats. Front. Cell. Neurosci. 2018, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Madiha, S.; Haider, S. Curcumin restores rotenone induced depressive-like symptoms in animal model of neurotoxicity: Assessment by social interaction test and sucrose preference test. Metab. Brain Dis. 2019, 34, 297–308. [Google Scholar] [CrossRef]
- Shen, J.D.; Wei, Y.; Li, Y.J.; Qiao, J.Y.; Li, Y.C. Curcumin reverses the depressive-like behavior and insulin resistance induced by chronic mild stress. Metab. Brain Dis. 2017, 32, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, W.; Zhao, F.; Han, Y.; Liu, C.; Jia, K. Curcumin amends Ca(2+) dysregulation in microglia by suppressing the activation of P2 × 7 receptor. Mol. Cell. Biochem. 2020, 465, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, Z.; Wu, Z.; Jin, M.; An, L.; Xue, F. Curcumin Improves Chronic Pain Induced Depression Through Regulating Serum Metabolomics in a Rat Model of Trigeminal Neuralgia. J. Pain Res. 2020, 13, 3479–3492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Guo, Y.J.; Han, W.X.; Yang, M.Q.; Wen, L.P.; Wang, K.Y.; Jiang, P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int. Immunopharmacol. 2019, 67, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Batool, Z.; Sadir, S.; Liaquat, L.; Shahzad, S.; Tabassum, S.; Ahmad, S.; Kamil, N.; Perveen, T.; Haider, S. Therapeutic Potential of Curcumin in Reversing the Depression and Associated Pseudodementia via Modulating Stress Hormone, Hippocampal Neurotransmitters, and BDNF Levels in Rats. Neurochem. Res. 2021, 46, 3273–3285. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Tie, L.; Yao, H.; Jiang, W.; Ma, X.; Li, X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006, 1122, 56–64. [Google Scholar] [CrossRef]
- Yohn, S.E.; Gorka, D.; Mistry, A.; Collins, S.; Qian, E.; Correa, M.; Manchanda, A.; Bogner, R.H.; Salamone, J.D. Oral Ingestion and Intraventricular Injection of Curcumin Attenuates the Effort-Related Effects of the VMAT-2 Inhibitor Tetrabenazine: Implications for Motivational Symptoms of Depression. J. Nat. Prod. 2017, 80, 2839–2844. [Google Scholar] [CrossRef]
- Lian, L.; Xu, Y.; Zhang, J.; Yu, Y.; Zhu, N.; Guan, X.; Huang, H.; Chen, R.; Chen, J.; Shi, G.; et al. Antidepressant-like effects of a novel curcumin derivative J147: Involvement of 5-HT1A receptor. Neuropharmacology 2018, 135, 506–513. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.S.; Yao, H.Y.; Lin, Y.H.; Ma, X.; Zhang, Y.H.; Li, X.J. The effects of curcumin on depressive-like behaviors in mice. Eur. J. Pharmacol. 2005, 518, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, M.; Khan, R.A.; Maghrabi, I.A.; Ahmed, B. Polysorbate-80-coated, polymeric curcumin nanoparticles for in vivo anti-depressant activity across BBB and envisaged biomolecular mechanism of action through a proposed pharmacophore model. J. Microencapsul. 2016, 33, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, C.; Zhang, J.F.; Liu, L.; Liu, A.M.; Ma, Q.; Zhou, W.H.; Xu, Y. Chronic curcumin treatment normalizes depression-like behaviors in mice with mononeuropathy: Involvement of supraspinal serotonergic system and GABAA receptor. Psychopharmacology 2014, 231, 2171–2187. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, E.M.; Savall, A.S.P.; da Luz Abreu, E.; Carvalho, F.; Teixeira, F.E.G.; Haas, S.E.; Bazanella Sampaio, T.; Pinton, S. Curcumin-Loaded Nanocapsules Reverses the Depressant-Like Behavior and Oxidative Stress Induced by beta-Amyloid in Mice. Neuroscience 2019, 423, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, L.; Li, G.; Chen, X.; Hu, S.; Zheng, L.; Luria, V.; Lv, J.; Sun, Y.; Xu, Y.; et al. Sub-Acute Treatment of Curcumin Derivative J147 Ameliorates Depression-Like Behavior Through 5-HT1A-Mediated cAMP Signaling. Front. Neurosci. 2020, 14, 701. [Google Scholar] [CrossRef]
- Pan, X.; Chen, L.; Xu, W.; Bao, S.; Wang, J.; Cui, X.; Gao, S.; Liu, K.; Avasthi, S.; Zhang, M.; et al. Activation of monoaminergic system contributes to the antidepressant- and anxiolytic-like effects of J147. Behav. Brain Res. 2021, 411, 113374. [Google Scholar] [CrossRef]
- Qi, X.J.; Liu, X.Y.; Tang, L.M.; Li, P.F.; Qiu, F.; Yang, A.H. Anti-depressant effect of curcumin-loaded guanidine-chitosan thermo-sensitive hydrogel by nasal delivery. Pharm. Dev. Technol. 2020, 25, 316–325. [Google Scholar] [CrossRef]
- Choudhary, K.M.; Mishra, A.; Poroikov, V.V.; Goel, R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur. J. Pharmacol. 2013, 704, 33–40. [Google Scholar] [CrossRef]
- Ceremuga, T.E.; Helmrick, K.; Kufahl, Z.; Kelley, J.; Keller, B.; Philippe, F.; Golder, J.; Padron, G. Investigation of the Anxiolytic and Antidepressant Effects of Curcumin, a Compound From Turmeric (Curcuma longa), in the Adult Male Sprague-Dawley Rat. Holist. Nurs. Pract. 2017, 31, 193–203. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Cui, L.; Li, X.; Barish, P.A.; Foster, T.C.; Ogle, W.O. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007, 1162, 9–18. [Google Scholar] [CrossRef]
- Arora, V.; Kuhad, A.; Tiwari, V.; Chopra, K. Curcumin ameliorates reserpine-induced pain-depression dyad: Behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology 2011, 36, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Sanmukhani, J.; Satodia, V.; Trivedi, J.; Patel, T.; Tiwari, D.; Panchal, B.; Goel, A.; Tripathi, C.B. Efficacy and safety of curcumin in major depressive disorder: A randomized controlled trial. Phytother. Res.: PTR 2014, 28, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; Miodownik, C.; Bersudsky, Y.; Sokolik, S.; Lerner, P.P.; Kreinin, A.; Polakiewicz, J.; Lerner, V. Curcumin as an add-on to antidepressive treatment: A randomized, double-blind, placebo-controlled, pilot clinical study. Clin. Neuropharmacol. 2013, 36, 73–77. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maes, M.; Maker, G.L.; Hood, S.D.; Drummond, P.D. Curcumin for the treatment of major depression: A randomised, double-blind, placebo controlled study. J. Affect. Disord. 2014, 167, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Maes, M.; Meddens, M.J.; Maker, G.L.; Arnoldussen, E.; Drummond, P.D. Curcumin and major depression: A randomised, double-blind, placebo-controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2015, 25, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Badeli, R.; Karami, G.R.; Sahebkar, A. Investigation of the efficacy of adjunctive therapy with bioavailability-boosted curcuminoids in major depressive disorder. Phytother. Res. 2015, 29, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Pei, L.B.; Zhang, Y.; Wen, Z.Y.; Yang, J.L. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin. Psychopharmacol. 2015, 35, 406–410. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2017, 207, 188–196. [Google Scholar] [CrossRef]
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox. Res. 2018, 33, 621–633. [Google Scholar] [CrossRef]
- Bhandari, R.; Kuhad, A. Neuropsychopharmacotherapeutic efficacy of curcumin in experimental paradigm of autism spectrum disorders. Life Sci. 2015, 141, 156–169. [Google Scholar] [CrossRef]
- Al-Askar, M.; Bhat, R.S.; Selim, M.; Al-Ayadhi, L.; El-Ansary, A. Postnatal treatment using curcumin supplements to amend the damage in VPA-induced rodent models of autism. BMC Complementary Altern. Med. 2017, 17, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xiao, R.; Ruan, R.; Liu, H.; Li, X.; Cai, Y.; Zhao, J.; Fan, X. Neonatal curcumin treatment restores hippocampal neurogenesis and improves autism-related behaviors in a mouse model of autism. Psychopharmacology 2020, 237, 3539–3552. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, P.; Isaev, D.; Shabbir, W.; Lorke, D.E.; Sadek, B.; Oz, M. Curcumin Potentiates alpha7 Nicotinic Acetylcholine Receptors and Alleviates Autistic-Like Social Deficits and Brain Oxidative Stress Status in Mice. Int. J. Mol. Sci. 2021, 22, 7251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cui, C.; Xu, P.; Dang, R.; Cai, H.; Liao, D.; Yang, M.; Feng, Q.; Yan, X.; Jiang, P. Curcumin Activates AMPK Pathway and Regulates Lipid Metabolism in Rats Following Prolonged Clozapine Exposure. Front. Neurosci. 2017, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; Farooqui, T.; Kokotos, G.; Farooqui, A.A. Synthetic and natural inhibitors of phospholipases A2: Their importance for understanding and treatment of neurological disorders. ACS Chem. Neurosci. 2015, 6, 814–831. [Google Scholar] [CrossRef]
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence-A Narrative Review. Front. Psychiatry 2020, 11, 572533. [Google Scholar] [CrossRef]
| Disease | Authors | Type | Phase | N Patients | Type Patients | Country | Duration | Dosage | Other Treatment | Biological Effects | Clinical Efficacy | Safety and Tolerability |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia | NCT01875822 | Open-label study | 1–2 | 17 | Schizophrenia patients | Puerto Rico | 16 weeks | 1000 or 4000 mg CUR | 5 mg Bioperine + Antipsychotic | - | - | - |
| Wynn and Green, 2017 (NCT02104752) | Randomized, double-blind, placebo-controlled study | 1–2 | 36 (17 CUR, 19 placebo) | Schizophrenia patients and inpatients | United States | 8 weeks | 360 mg/day of Theracurcumin | - | Increase in plasma levels of BDNF in CUR patients | No effect on clinical symptoms | No significant adverse events | |
| Kucukgoncu et al., 2019 (NCT02476708) | Randomized, double-blind, placebo-controlled, add-on study | - | 12 (6 CUR, 6 placebo) | Schizophrenia outpatients | United States | 8 weeks | 180 mg/day of Theracurcumin | Usual antipsychotic medication | Reduction in IL-6 in CUR SCZ patients | Significant improvement in working memory. No significant improvement in cognitive domains, negative symptoms and total PANSS | No significant adverse events | |
| Miodownik et al., 2019 (NCT02298985) | Randomized, double-blind, placebo-controlled, add-on | 4 | 38 (20 CUR, 18 placebo) | Schizophrenia outpatients | Israel | 24 weeks | 3000 mg/day | Usual antipsychotic medication | - | Improvement in total PANSS and in the negative symptoms subscale. No changes in positive and general PANSS subscales nor the CDSS | No significant adverse events | |
| Hosseininasab et al., 2021 | Randomized, double-blind, placebo-controlled, add-on trial | - | 56 (28 CUR, 28 placebo) | Chronic and stable schizophrenia inpatients | Iran | 16 weeks | 160 mg/day | Usual antipsychotic medication | - | Improvement in total PANSS and in the negative symptoms subscale, general psychopathology subscale, positive subscale, total PNSS, CGI-S, and CGI-I. No changes in Extrapiramidal symptom rating scales nor CDSS | No significant adverse events | |
| Depression | Sanmukhani (2013) NCT01022632 | Randomized control trial | - | 51 (17 fluoxentine, 16 CUR, 18 fluoxentine + CUR) | MDD patients (>18 years) | India | 6 weeks | 1000 mg/day of CUR; 20 mg/day of Fluoxentine | Paracetamol and diazepam | Not described | No significant effects produced by curcumin or its combination with fluoxentine (HDRS) | Curcumine was well tolerated |
| Bergman (2013) NCT01750359 | Randomized, double-blind, placebo-controlled, pilot clinical trial | 4 | 39 (19 CUR, 20 plecebo) | MDD patients (20–81 years) | Israel | 5 weeks | 500 mg/day | Escitalopram and venlafaxine XR | Not described | No effects of curcumin (MADRS and HDRS) | No adverse effects during the treatment | |
| Lopresti (2014) | Randomized, double-blind, placebo-controlled trial | - | 25 (curcumine 1000 mg/day), 27 (placebo) | MDD patients (20–65 years) | Australia | 8 weeks | 1000 mg/day | SSRIs and SNRIs | Not described | Long-term improvement in IDS-SR30 total scores. Long-term improvement in STAI anxiety scores | Minor severity side effects | |
| Lopresti (2014) | Randomized, double-blind, placebo-controlled trial | - | 25 (curcumine 1000 mg/day), 25 (placebo) | MDD patients (20–65 years) | Australia | 8 weeks | 1000 mg/day | Non-specified antidepressant medication | Increased urinary TBX-B2 and SUB-P. Higher plasma ET-1 and leptin levels | Improvement in IDS-SR30 total score | Minor severity side effects | |
| Panahi (2015) | Open-label study | - | 111 (61 CUR + piperine, 50 placebo) | MDD patients (18–65 years) | Iran | 6 weeks | 1000 mg/day of CUR + 10 mg/day of piperine | TCAs, BZDs, SSRIs and SNRIs | Not described | Improvements in HADS total score. Reductions in BDI-II total score | Not described | |
| Yu (2015) | Randomized, double-blind, placebo-controlled pilot study | - | 100 (50 CUR, 50 placebo) | MDD patients (31–59 years) | China | 6 weeks | 500 or 1000 mg/day of CUR; 30 mg/day of Saffron | Escitalopram | Decrease in IL-1β, TNF-α and salive cortisol concentrations. Increase in NF levels in plasma | Improvement in HDRS and MADR total scores | No adverse effects | |
| Lopresti and Drummond (2017) | Randomized, double-blind, placebo-controlled study | - | 123 (28 CUR, 33 CURX2, 26 CUR +Saffron, 36 placebo) | MDD patients (18–65) | Australia | 12 weeks | 1000 mg/day or 500 mg/day | Non-specified antidepressant medication | Not described | Improvements in IDS-SR30 and STAI total scores after the combination of treatments. No differences between different doses of curcumin | Minor severity side effects | |
| Kanchanatawan (2018) | Randomized, double-blind, placebo-controlled study | - | 61 (30 CUR, 31 placebo) | MDD patients (18–63) | Thailand | 12 weeks | 500 mg/day to 1500 mg/day with an increment of 250 mg/week | Fluoxentine, SSRis, mianserin, trazodone, sodium valproate, propanolol and enalapril | No significant effects on blood chemistry and ECG measurements | Improvement in MADRS total score. | No adverse effects | |
| NCT04744545 (2021) | Randomized, placebo-controlled trial | - | 60 (estimated) | MDD patients (>18 years) | Canada | 12 weeks | 1500 mg/Kg | Integrative treatment program based on several evidence-based practices and overseen by licensed clinical therapists that is delivered via a Smartphone app | - | - | - | |
| Obsessive Compulsive Disorder | Moore and Nat (2018) | Case report | - | 1 | One case report | United States | 3 weeks | 90 mg/day og CUR; 600–1800 mg/day of NAC | Not specified | Not described | Reduction in CY-BOCS and YGTSS total scores | Not described |
| Pathology | Reference | N Animals | Animal Model | Treatment | Biological Effects | Behavioral Effects |
|---|---|---|---|---|---|---|
| Schizophrenia | Naserzadeh et al., (2018) | 24 (6/group) | Ketamine-treated Wistar male rats | CUR-loaded magnetic nanoparticles (17 mg/300 ul PBS), i.v. | Reduction in MMP, ATP and mitochondrial complex II activity in mitochondria of the cerebellum | Reduction in the over-increased locomotor activities (side-to-side rocking and arcing of neck) in the CUR-treated ketamine rats, without reaching the control values. |
| Moghaddam et al., (2021) | 35 (7/group) | Ketamine-treated, during the last 15 days of CUR administration mice | Curcumin-loaded nanophytosomes (20 mg/kg) during 30 days | Reduction in biomarkers of oxidative stress in cortical and subcortical regions | Reduction in anxiety in CUR-treated ketamine mice. Reduction in depressive-like behaviors in ketamine mice treated with CUR | |
| Xu et al., 2005 | 60 (6/group) | Regular male ICR mice | 1.25, 2.5, 5, or 10 mg/Kg 30 min before tests, p.o. | Increment of 5-HT and DA in the frontal cortex and striatum at high doses. Inhibition of monoamine oxidase activity | High doses improved the forced swimming and tail suspension tests. No effect on locomotor activities | |
| Xu et al., 2005 | 72–84 (6–7/group) | OB male SP rats | 1.25, 2.5, 5, or 10 mg/Kg, 14 days, p.o. | Reversion of the deficits of 5-HT and NA in hippocampus and frontal cortex; 5-HIAA and DOPAC in hippocampus and DA in frontal cortex | Improvement in forced swimming, open field, and passive avoidance tests | |
| Xu et al., 2006 | 36 (6/group) | Chronically stressed male SP rats | 2.5, 5, or 10 mg/Kg, 21 days, p.o. | Reversed the effects on adrenal gland size and weight. Blocked the stress-induced decreases in BDNF and pCREB/CREB | High doses improved the effects in shuttle-box test | |
| Xu et al., 2007 | 30–35 (5–6/group) | Chronically stressed male SP rats | 5, 10, or 20 mg/Kg, 21 days, p.o. | Improved hippocampal neurogenesis and blocked the decrease in 5-HT1A mRNA and BDNF protein levels in the hippocampal subfields | - | |
| Depression | Kulkarni et al., 2008 | 30 (6/group) | Reserpine treated male Laca rats | 10–80 mg/Kg, 60 min before tests, i.p. | Increment of 5-HT, DA levels, MAO-A and MAO-B at higher doses | Dose dependent improvement in forced swimming test |
| Wang et al., 2008 | 40–48 (10–12/group) | PCPA male ICR mice | 2.5, 5, or 10 mg/Kg, 45 min before tests, p.o. | Interaction with 5-HT1A/1B and 5-HT2C receptors | Improvement in forced swimming test | |
| Li et al., 2009 | 56 (7/group) | CUMS male Wistar rats | 15 or 30 mg/Kg, 4 weeks, i.g. | Reduced serum corticosterone levels. Enhanced AC activity and cAMP levels and upregulated several AC subtypes in the hippocampus, cortex, and hypothalamus. Increased 5-HT levels | Improvement in sucrose preference test | |
| Bhutani et al., 2009 | 36 (6/group) | Chronically stressed female Wistar rats | 20 or 40 mg/Kg, 21 days, i.p. | Dose dependent reduction in MAO-A and MAO-B. Reversed the effects on NE, DA, and 5-HT levels | Dose dependent improvement in forced swimming test | |
| Arora et al., 2011 | 48 (8/group) | Reserpine treated male Wistar rats | 100, 200, or 300 mg/Kg, 2 days, i.p. | Dose dependent reversion of NE, DA and 5-HT reduced levels. Increment of SUB-P concentration, nitrodative stress, inflammatory cytokines, NF-κβ and caspase-3 levels in hippocampus and cortex | Reduced the deficits in Randall Sellitto and von-Frey hair tests. Improvement in forced swimming test | |
| Huang et al., 2011 | 18 (6/group) | CORT-treated male SP rats | 20 mg/Kg, 21 days, p.o. | Increment of BDNF levels induced by CORT treatment in hippocampus and frontal cortex | Improvement in forced swimming and sucrose preference tests | |
| Kulkarni et al., 2011 | Not specified | Regular male Laca mice | 50–200 mg/Kg, 30 min before tests, p.o. | Increment of 5-HT at low doses and DA at high doses | Dose dependent improvement in forced swimming test | |
| Zhang et al., 2012 | 60–75 (10–12/group) | SL327 male C57BL/6 mice | 40 mg/Kg, 21 days, i.p. | Improvement of ERK deregulation on BDNF expression in the amygdala | Improvement in forced swimming test | |
| Borre et al., 2013 | 40–48 (10–12/group) | OB or ZnSO4 anosmia-induced male SP rats | 20 g/day of 0.25 mg/Kg curcumine diet, 42 days | Reduced hippocampal atrophy and decreased the peripheral immune activation | Attenuation of cognitive and behavioral deficits in open field, tail suspension, passive avoidance, T-maze and holeboard tests | |
| Rinwa et al., 2013 | 50 (5/group) | OB male Wistar rats | 100, 200, or 400 mg/Kg, 2 weeks, p.o. | Dose dependent reversion of TNF-α, caspase-3 and BDNF levels | Dose dependent improvement of forced swimming, sucrose preference and open field tests | |
| Lin et al., 2013 | 40 (6–14/group) | CUS male SP rats | 40 mg/Kg, 30 days, p.o. | Strong deactivation of the left primary auditory cortex and activation of the amygdalohippocampal cortex | Improvement in sucrose preference and open field tests | |
| Hurley et al., 2013 | 32 (8/group) | Male Wistar Kyoto rats | 50, 100, or 200 mg/Kg, 10 days, i.p. | Dose dependent increase in hippocampal BDNF levels | Improvement in forced swimming test but no effects on open field test | |
| Jiang et al., 2013 | 40 (10/group) | CMS male Wistar rats | 10 mg/Kg, 3 weeks, i.g. | Inhibited cytokine gene expression at mRNA and protein level and reduced the activation of NF-κβ | Reduced sucrose preference and decreased locomotor activity in open field test | |
| Zhang et al., 2013 | 40–48 (10–12/group) | NMDA receptor antagonists treated male Kun-Ming mice | 10, 20, or 40 mg/Kg, 45 min before tests, i.p. | Interaction with glutamate-NMDA-receptors | Improvement in forced swimming test | |
| Zhao et al., 2013 | 24–36 (8–12/group) | CCI in male ICR mice | 5, 15, or 45 mg/Kg, 3 weeks, p.o. | Interaction with 5-HT1A and GABA receptors | Dose dependent improvement in forced swimming and tail suspension tests | |
| Wang et al., 2014 | 40(10/group) | LPS treated male Kun-Ming mice | 50 mg/Kg, 7 days, i.p. | Attenuated LPS induced microglial activation and over production of pro-inflammatory cytokines, levels of inductible nitric oxide synthase and cyclooxygenase-2 mRNA in the hippocampus and prefrontal cortex | Improvement in forced swimming, tail suspension, and sucrose preference tests | |
| Liu et al., 2014 | 40 (10/group) | CUS male Wistar rats | 10 mg/Kg, 5 weeks, i.g. | Increased hippocampal BDNF and ERK levels | Reduced sucrose preference and impaired learning and memory function in open field and Morris water maze tests | |
| Cui et al., 2014 | 48 (8/group) | CUMS male SP rats | 10, 40, or 80 mg/Kg, 30 min before tests, i.g. | Improved the activity of anti-oxidant enzymes and energy metabolism enzymes | Improvement in open field and sucrose preference tests | |
| Zhang et al., 2014 | 64 (16/group) | CUMS male Wistar rats | 40 mg/Kg, 6 weeks, i.p. | Reverted the effects on the expression of BDNF, PSD-95 and synaptophysin in the lateral amygdala | Improvement in open field, forced swimming and sucrose preference tests | |
| Haider et al., 2015 | 24 (6/group) | Stressed male Wistar rats | 200 mg/Kg, 1 week, p.o. | Improved the levels of MDA, CAT, GPx, SOD and AChE | Improvement in elevated plus maze, open field and forced swimming tests | |
| He et al., 2016 | Not specified | CORT-treated female C57BL/6 mice | 20 mg/Kg, 2 weeks, i.p. | Improvement of DA levels in blood. Increase in neurotransmitters in hippocampus and striatum. Increased expression of CBR1, p-MEK1, and p-ERK1/2 | Improvement in forced swimming and rotarod tests | |
| Chang et al., 2016 | 30 (6/group) | OB male Wistar rats | 10, 20, or 40 mg/Kg, 45 days, p.o. | Reversed the effects on NA, 5-HT, 3, DOPAC acid and 5-HIAA in the hippocampus. Normalized the levels of DA, NA and 5-hydroxyindoleaceticacid in the prefrontal cortex | Improvement in passive avoidance and open field tests | |
| Yusuf et al., 2016 | 42 (6/group) | Stressed albino mice | 2.5, 5, 10, or 20 mg/Kg, 60 min before tests, i.p. | Increase in SOD catalase activity | Improvement in force despair, forced swimming and tail suspension tests | |
| Demir et al., 2016 | 34(7–10/group) | Cisplatin treated male Wistar rats | 300 mg/Kg, 5 weeks, p.o. | - | Improvement in forced swimming, open field and elevated plus maze tests | |
| Shen et al., 2017 | 48 (6/group) | CMS male SP rats | 15, 30, or 60 mg/Kg, 33 days, p.o. | Upregulation of IRS-1, Akt in the liver and reversed metabolic abnormalities | Improvement in glucose preference test | |
| Yohn et al., 2017 | 45 (9/group) | Tetrabenzamine treated male SP rats | 80–160 mg/Kg, p.o. or 2–8 ul/Kg infusions into ventricles | - | Attenuated the effort-related abnormalities in a choice procedure test | |
| He et al., 2017 | Not specified | CORT administration in C57BL/6 mice | 20 mg/Kg, 3 weeks | Increased DA/5-HT levels, CB1 mRNA levels and CB1, p-MEK1, and p-ERK1/2 protein expression levels in the hippocampus and striatum. Increment on CBR1 expression and proliferation of astrocytes in the hippocampus and striatum | Improvement in forced swimming test | |
| Choi et al., 2017 | 16 (4/group) | Chronically stressed male SP rats | 50 or 100 mg/Kg, 18 days, p.o. | Rescued the attenuated BDNF expression and inhibited the enhancement of COX-2 expression | Improvement in forced swimming test | |
| Ceremuga et al., 2017 | 55 (11/group) | Flumazenil treated male SP rats | 20 mg/Kg, 10 min before tests, i.p. | No interaction between curcumin and benzodiazepine site of the GABAs receptor was observed | No effects on forced swimming, open field and elevated plus maze tests | |
| Vasileva et al., 2018 | 48 (6–7/group) | CMS-LPS treated male Wistar rats | 20 mg/Kg, 8 days, i.g. | Reversion of the increase in cytokine levels | Improvement in open field and water maze tests | |
| Lee and Lee, 2018 | 42–49 (6–7/group) | SPS male SP rats | 20, 50, or 100 mg/Kg, 14 days, i.p. | Recover of neurochemical abnormalities and decreases of 5-HT in the hippocampus, amygdala, and striatum | Improvement in elevated pluz maze, fear conditioning and open field tests | |
| Fan et al., 2018 | 24 (8/group) | CUMS male Wistar rats | 40 mg/Kg, 5 weeks, i.p. | Repression of the inflammatory response and neuronal structural abnormalities produced by CUMS | Improvement in forced swimming and sucrose preference tests | |
| Lian et al., 2018 | 36 (6/group) | Regular male ICR mice | 2, 5, or 10 mg/Kg, 1–24 hs before tests, i.g. | Activation of 5-HT1A/cAMP/PKA/CREB/BDNF-signaling pathway | Improvement in forced swimming and tail suspension tests. No alteration in open field test | |
| Fidelis et al., 2018 | 35–40 (7–8/group) | β-amyloid treated Swiss male mice | 10 mg/mL, 12 days, i.g. | Reduced Aβ-oxidative stress via SOD and CAT in the prefrontal cortex | Improvement in forced swimming and tail suspension tests. No changes in open field test | |
| Abd-Rabo et al., 2019 | 70 (14/group) | Ovariectomized female Wistar rats | 100 mg/Kg, 30 days, p.o. | Improvement of serotonin content by upregulating 5-HT1A and down regulating monoamine oxidase | Improvement of forced swimming test | |
| Fan et al., 2019 | 72 (18/group) | CUMS male Wistar rats | 40 mg/Kg, 5 weeks, i.p. | Reduced the expression of IL-1β and inhibited neuronal apoptosis within neurons of the ventromedial prefrontal cortex | Improvement in forced swimming and sucrose preference tests | |
| Mohammed et al., 2019 | 65 (11–15/group | Reserpine treated male Wistar rats | 20 mg/Kg, 7 or 15 days, i.p. | Restored DA and 5-HT levels, but not NE levels after 7 days of treatment. Increase in alpha and beta 2-waves, tetha and beta 1, and decrease in delta waves | Improvement in forced swimming test | |
| Madiha and Haider, 2019 | 30 (6/group) | Rotenone treated Wistar rats | 100 mg/Kg, 2 weeks, p.o., pre- and post-Rotenone | Reverted DA and 5-HT levels in striatum and hippocampus | Improvement in social interaction and sucrose preference test | |
| Zhang et al., 2019 | 18–21 (6–7/group) | CUMS male SP rats | 100 mg/Kg, 4 weeks, i.g. | Reduced the expression of IL-1β, IL-6, and TNF-α and suppressed activation of NF-κβ. Inhibited the P2 × 7R/NLRP3 inflammasome axis activation, and reduced the synthesis of IL-1β. Ammeliorated the activation of IDO and increased kynurenine/tryptophan ratio | Improvement in forced swimming, elevated pluz maze and sucrose preference tests | |
| Liao et al., 2020 | 24 (8/group) | CUMS male SP rats | 100 mg/Kg, 4 weeks, i.g. | Decrease in protein expression of stress markers and increase in CAT. Reversed the inhibition of Nrf2-ARE signaling pathway and increased mRNA expression of NQO-1 and HO-1. Increased the ratio ofpCREB/CREB and BDNF, PSD-95 and synaptophysin | Improvement in forced swimming, open field, novelty-suppressed feeding, and sucrose preference tests | |
| Qi et al., 2020 | 35 (6/group) | Reserpine treated male ICR mice | 5 mg/Kg, i.g. or 14.6, 29.2, 58.4 ug/Kg, nasal, 1 h before tests | Increase in NE, DA, 5-HT and their metabolites in hippocampus and striatum | Improvement in forced swimming and tail suspension tests | |
| Wang et al., 2020 | 18 (6/group) | MCAO and CMS male SP rats | 100 mg/Kg, 4 weeks, i.g. | Blocked Ca+2 accumulation, inhibited the activation Ca+2 channels | Improvement in forced swimming and sucrose preference tests | |
| Li et al., 2020 | 50(10/group) | Regular male ICR mice | 1, 3, or 9 mg/Kg, 3 days, i.g. | Modulated 5-HT1A-dependent cAMP/PKA/pCREB/BDNF signaling pathway | Improvement in forced swimming and tail suspension tests in a dose dependent manner | |
| Abu-Taweel and Al-Fifi, 2020 | 60 (6/group) | Mercury chloride treated male Swiss mice | 150 or 300 ppm, 36 days, p.o. | Dose dependent improvements of corticosterol and cortisone levels in plasma | Dose dependent improvements in forced swimming, tail suspension, open field and plus maze tests | |
| He et al., 2020 | 24 (3/group) | CORT treated CBR1+/+ and CBR1-/- mice | 20 mg/Kg, 2 weeks, i.p. | Increased mRNA and protein expression levels of neuronal markers, MEK and Tuj1. Increase in released DA and NE and the mRNA expression of CBR1 and the downstream of genes Rasgef1c and Egr1 | Improvement in forced swimming test | |
| Zhang et al., 2020 | 24–27 (8–9/group) | TN male SD rats | 45 mg/Kg, 27 alternative days, i.g. | Altered ether lipid metabolism and glycerophospholipid metabolism | Improvement in forced swimming and sucrose preference tests | |
| Da Silva-Marques et al., 2020 | 40–52 (10–13/group) | CUMS male Swiss mice | 50 mg/Kg, 28 days, p.o. | Increase in CAT levels in the brain. No potential renal and hepatic damage | Improvement in forced swimming and elevated plus maze tests | |
| Saied et al., 2021 | 50 (7–10/group) | OVX female albino rats | 100 mg/Kg, 30 days, p.o. | Modulated DA and NE levels, downregulated MAO-B and upregulated tyrosine hydroxylase and DA receptors in the limbic region. Reduced the production of corticosterone, IL-1β, IL-6, and nitric oxide. Normalized the levels of MDA | Improvement in the open field test | |
| Afzal et al., 2021 | 24 (8/group) | CRS male Wistar rats | 200 mg/Kg, 1 week | Reverted the effects on hippocampal BDNF, 5-HT, DA, and Ach levels | Improvement in Morris water maze and pattern separation tests | |
| Rubab et al., 2021 | 40 (5/group) | LPS administration in male SP rats | 40 mg/Kg, 8 days, i.p. | Suppressed the expression of BDNF, TNF-α, p-NF-κβ, and COX-2 | Improvement in forced swimming, tail suspension, elevated plus maze, and light-dark box tests | |
| Pan et al., 2021 | 45 (9/group) | Regular ICR male mice | 10 mg/Kg, 3 days, p.o. | Increased levels of 5-HT and NA in the hippocampus and frontal cortex. Inhibition of MAO-A activity | Improvement in forced swimming, tail suspension tests. No effects on sucrose preference and novelty suppressed feeding tests | |
| Khadrawy et al., 2021 | 21 (7/group) | Reserpine treated male Wistar rats | 5 mg/Kg, 14 days, i.p. | Reversion of the levels of MAO, AchE, Na+, K+, and ATPase | Improvement in forced swimming test | |
| Autism | Bhandari and Kuhad, 2015 | 40 (5/group) | Intracerebroventricular injection of PPA in male SP rats | 50, 100, or 200 mg/kg/day, during 4 weeks, p.o. | Reduction in the (TBARS) in CUR animals. Increase in glutathione, superoxide dismutase and catalase levels in CUR rats’ brains. Restoration of mitochondrial enzyme complex I activities in CUR rats. Dose-dependent reduction in MMP-9 in PPA rats treated with CUR | Dose-dependent improvements of social skills in CUR-treated PPA animal. Improvement in locomotor activity, rotarod, elevated plus maze and open field tests, especially at 200 mg/kg/day. |
| Al-Askar et al., 2017 | 40(10/group) | Fetal exposition (GD12.5) to VPA in Wistar rats | 1 mL, oral, for 7 days after birth | Increase in brain and body weight in CUR-treated VPA animals. Depletion of IFN-γ in VPA rats with curcumin treatment. Partial restoration of IL-6 and glutamate normal levels in VPA rats with CUR | - | |
| Zhong et al., 2020 | 48 (12/group) | BTBRT+ltpr3tf/J mice | 20 mg/kg, from PND 6 to PND 8, i.p. | Enhancement of neural stem cell proliferation in BTBRT mice treated with CUR | Improvement in 3-chambered social approach and novel object recognition tests. No effect of CUR in male–female reciprocal social interaction. No changes in anxiety nor locomotor activity caused by CUR | |
| Jayaprakash et al., 2021 | 54 (7/group) | BTBRT+ltpr3tf/J mice | 25, 50, or 100 mg/kg, 1 week before tests, i.p. | Restoration of catalase and superoxide dismutase in hippocampus and cerebellum of CUR-treated BTBRT mice | Dose-dependent increase in sociability in CUR-treated mice | |
| Obsessive Compulsive Disorder | Jithendra and Murthy, 2010 | 30 (6/group) | Quinpirol treated Wistar rats | 5 or 10 mg/Kg, 35 days, p.o. | Increased 5-HT and DA levels | Improvement in open field and water maze tests |
| Mishra et al., 2021 | 42 (6/group) | Male Swiss mice | 10, 15, 25, or 40 mg/Kg, i.p. | - | Dose dependent improvement in marble-burying behavior. No effects in motor activity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamanna-Rama, N.; Romero-Miguel, D.; Desco, M.; Soto-Montenegro, M.L. An Update on the Exploratory Use of Curcumin in Neuropsychiatric Disorders. Antioxidants 2022, 11, 353. https://doi.org/10.3390/antiox11020353
Lamanna-Rama N, Romero-Miguel D, Desco M, Soto-Montenegro ML. An Update on the Exploratory Use of Curcumin in Neuropsychiatric Disorders. Antioxidants. 2022; 11(2):353. https://doi.org/10.3390/antiox11020353
Chicago/Turabian StyleLamanna-Rama, Nicolás, Diego Romero-Miguel, Manuel Desco, and Maria Luisa Soto-Montenegro. 2022. "An Update on the Exploratory Use of Curcumin in Neuropsychiatric Disorders" Antioxidants 11, no. 2: 353. https://doi.org/10.3390/antiox11020353
APA StyleLamanna-Rama, N., Romero-Miguel, D., Desco, M., & Soto-Montenegro, M. L. (2022). An Update on the Exploratory Use of Curcumin in Neuropsychiatric Disorders. Antioxidants, 11(2), 353. https://doi.org/10.3390/antiox11020353







