High-Fat-Diet-Induced Oxidative Stress in Giant Freshwater Prawn (Macrobrachium rosenbergii) via NF-κB/NO Signal Pathway and the Amelioration of Vitamin E
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Ingredients and Diets
2.2. Prawns and the Feeding Trial
2.3. Sample Collection
2.4. Ultrastructure Study
2.5. Apoptosis Detection
2.6. Biochemical and Antioxidative Parameters in Hemolymph
2.7. RNA Isolation and RT-qPCR Analysis
2.8. Knock-Down of Relish and Dorsal In Vivo Expression by RNA Interference
2.9. Calculations and Statistical Analysis
- Weight gain rate (WGR, %) = (Wt − W0) × 100/W0.
- Specific growth rate (SGR, % day−1) = (LnWt − LnW0) × 100/day.
- Feed conversion ratio (FCR) = feed consumption (g)/Weight gain (g).
- Survival rate (SR, %) = initial number/final number × 100
- Where W0 and Wt are initial and final body weight.
3. Results
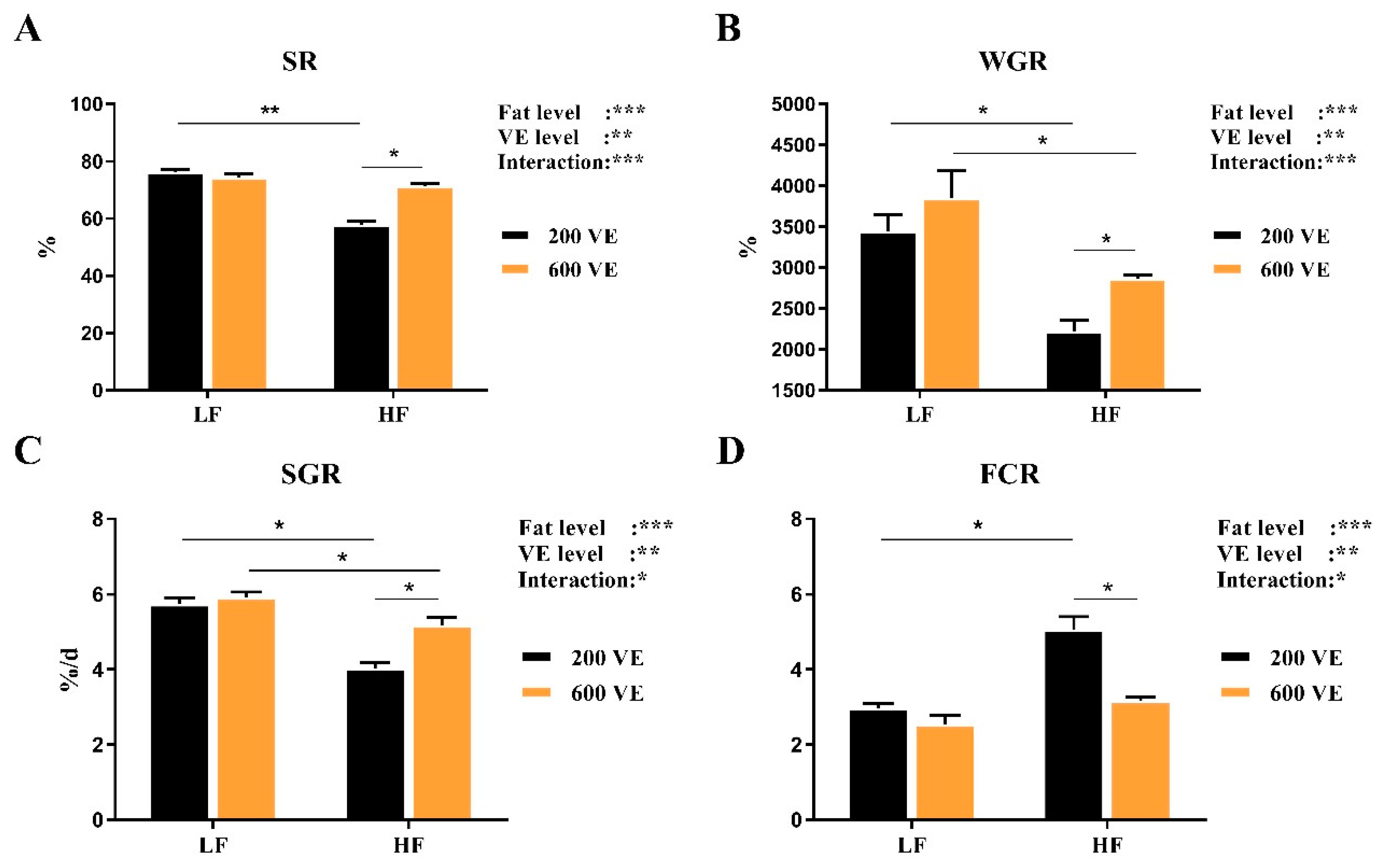
3.1. Growth Performance
3.2. Hemolymph Biochemistry Parameters
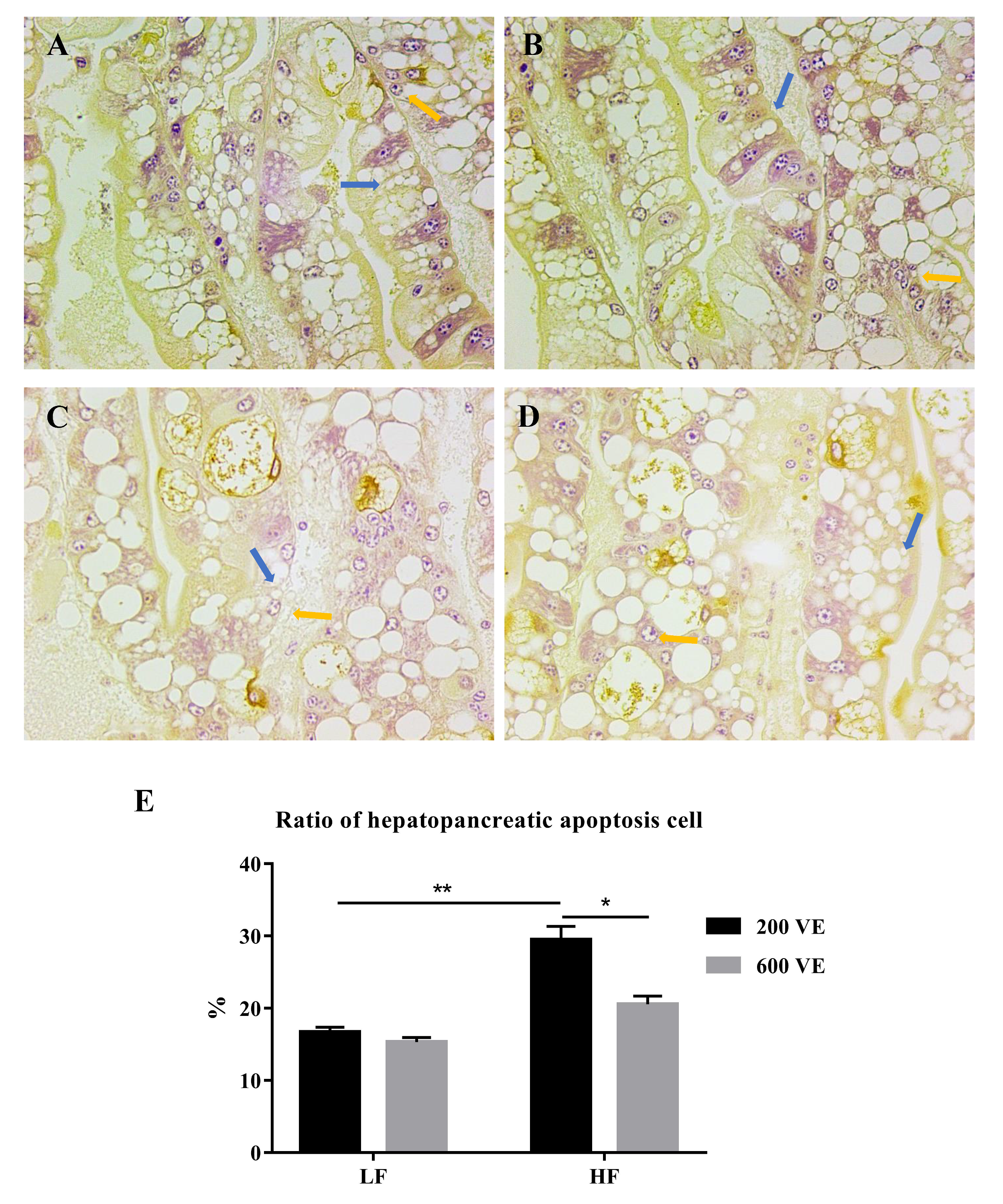
3.3. Hepatopancreas Ultrastructure and Apoptosis
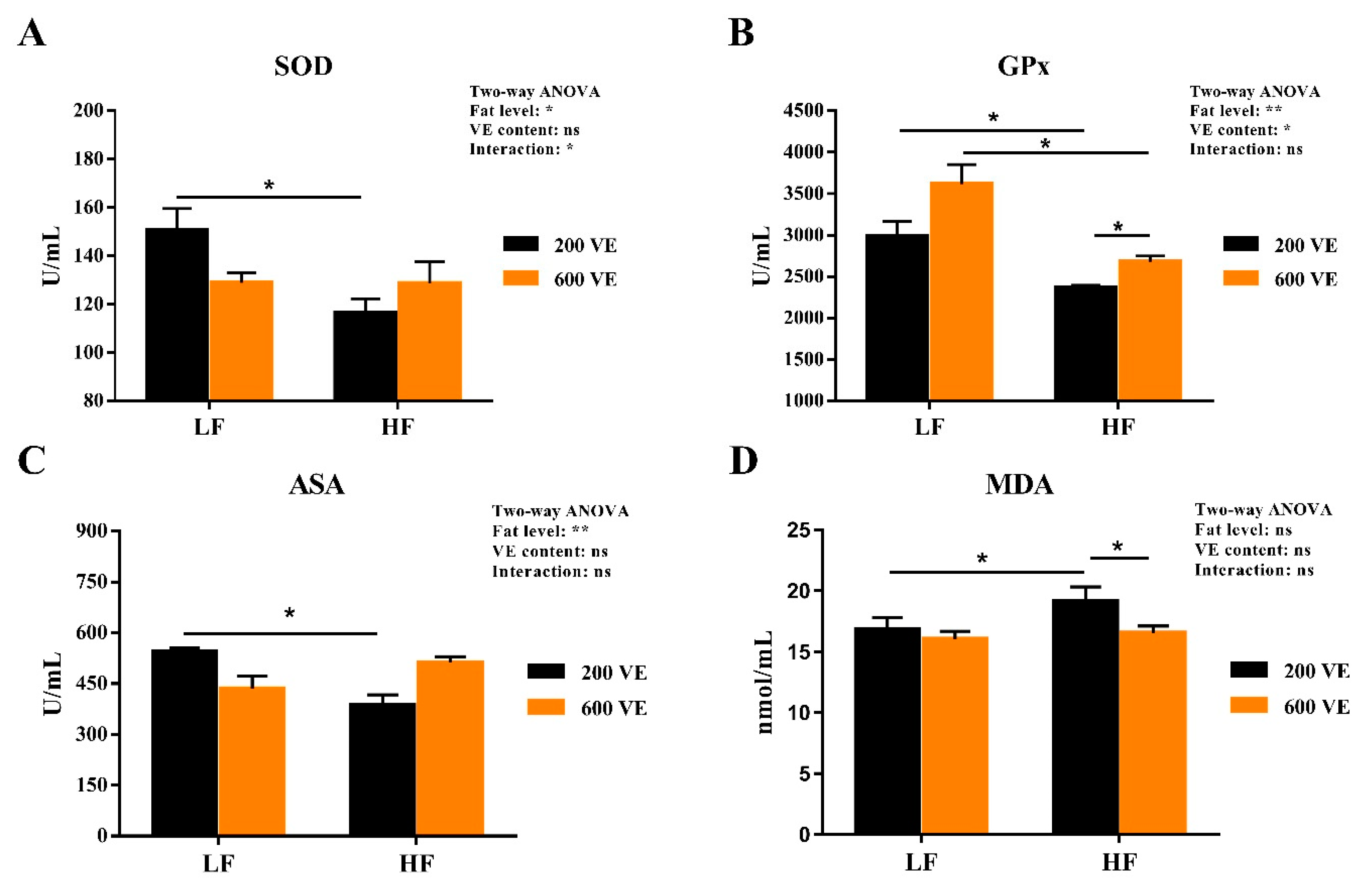
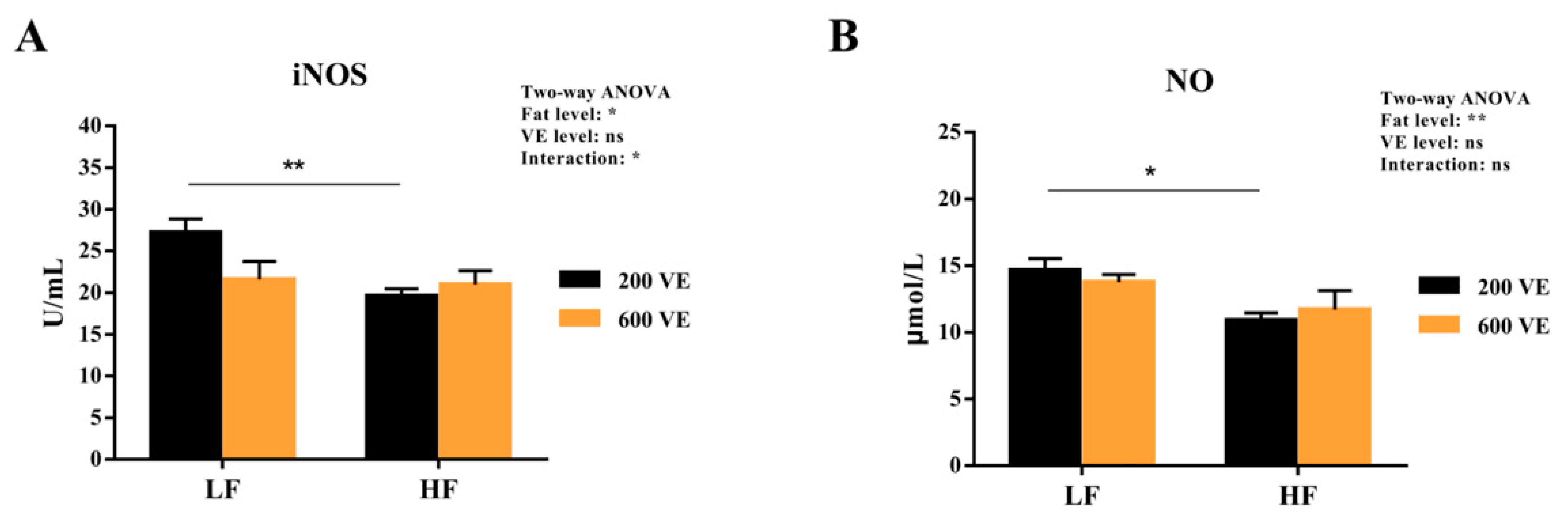
3.4. Hemolymph Antioxidant Capacity
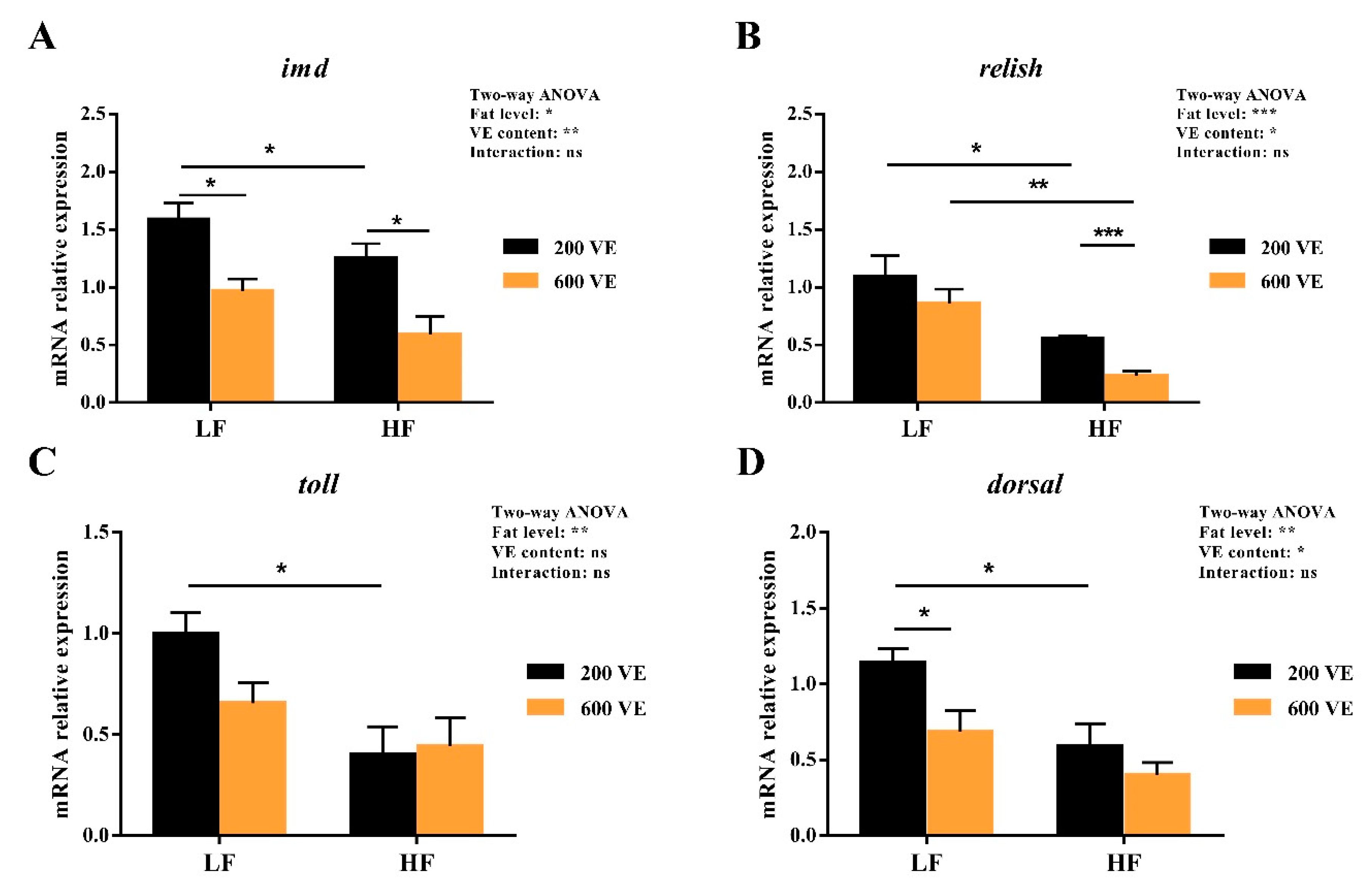
3.5. Hepatopancreas NF-κB Signal Pathway Expression
3.6. In Vivo Knock-Down of Relish and Dorsal by RNA Interference
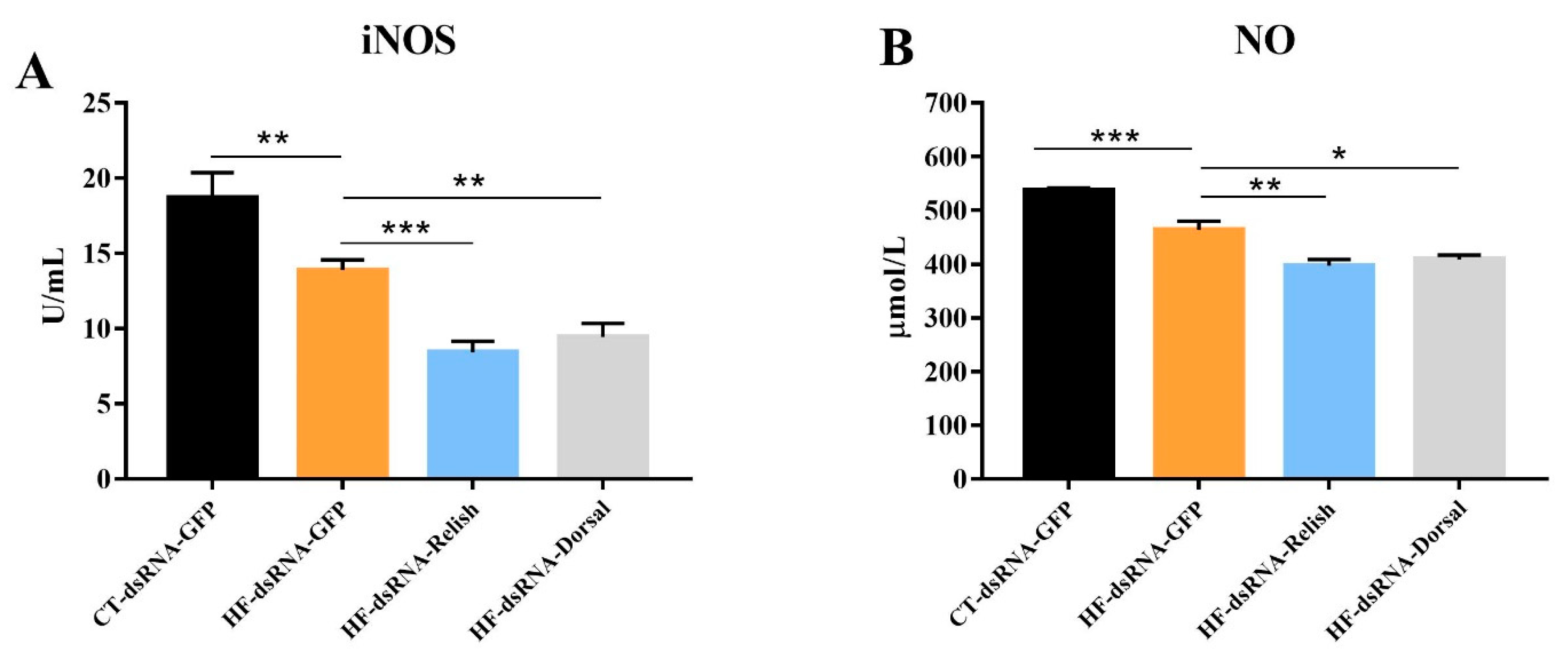
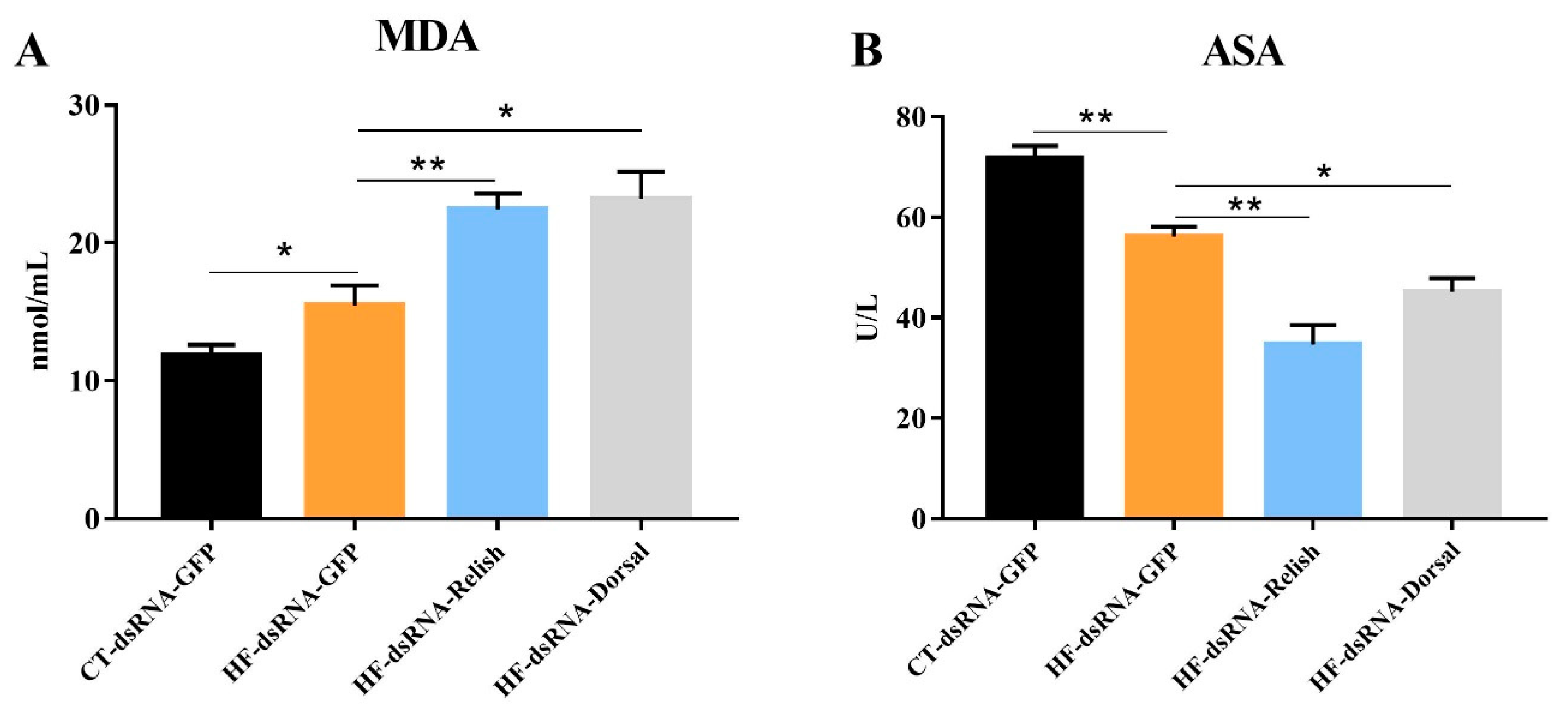
3.7. Hemolymph Antioxidant Capacity after NF-κB Suppression
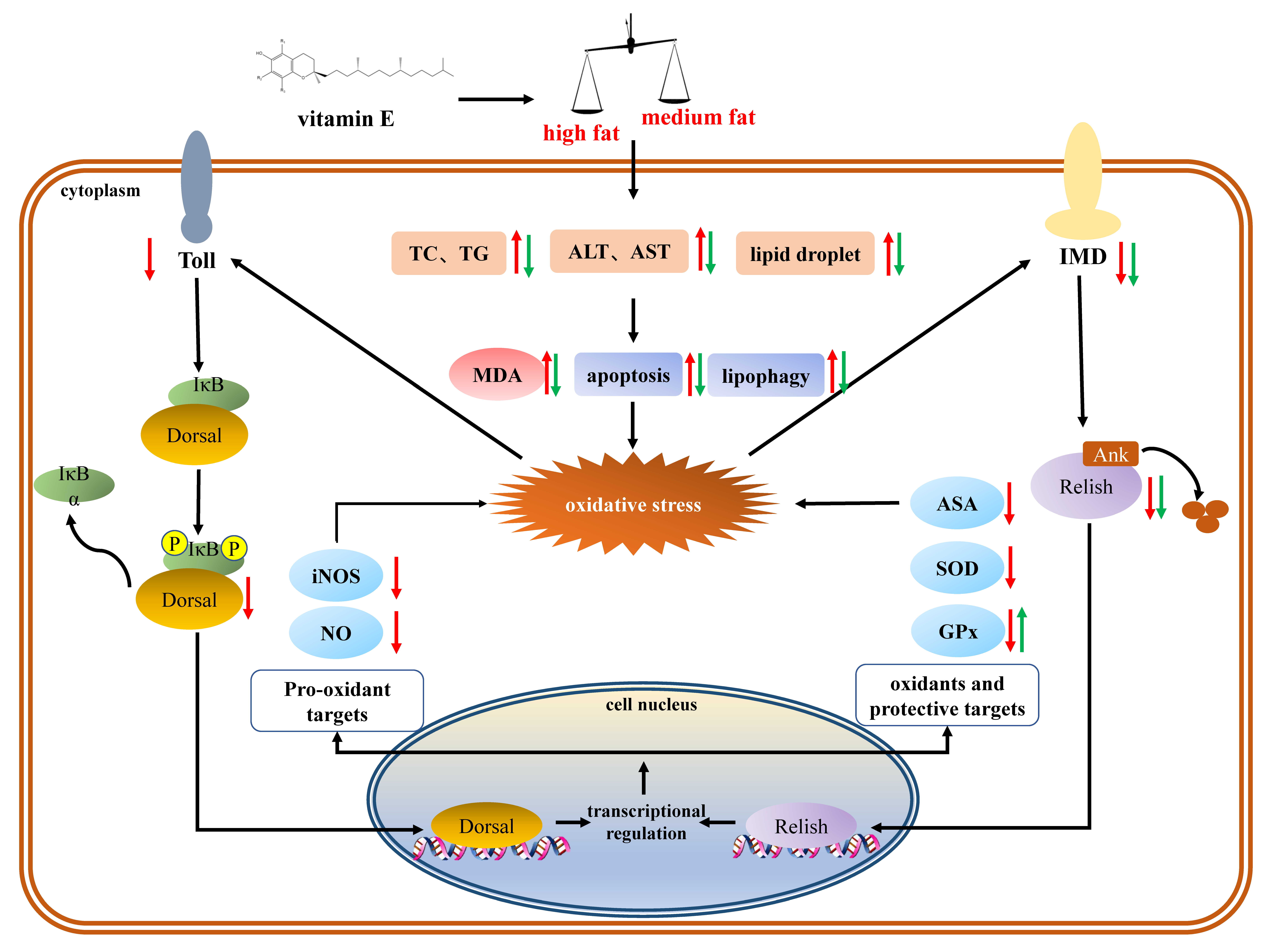
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Méndez-Martínez, Y.; García-Guerrero, M.U.; Arcos-Ortega, F.G.; Martínez-Córdova, L.R.; Yamasaki-Granados, S.; Pérez-Rodríguez, J.C.; Cortés-Jacinto, E. Effect of different ratios of dietary protein-energy on growth, body proximal composition, digestive enzyme activity, and hepatopancreas histology in Macrobrachium americanum (Bate, 1868) prawn juveniles. Aquaculture 2018, 485, 1–11. [Google Scholar] [CrossRef]
- Zhang, N.N.; Ma, Q.Q.; Fan, W.J.; Xing, Q.; Zhao, Y.L.; Chen, L.Q.; Ye, J.Y.; Zhang, M.L.; Du, Z.Y. Effects of the dietary protein to energy ratio on growth, feed utilization and body composition in Macrobrachium nipponense. Aquac. Nutr. 2017, 23, 313–321. [Google Scholar] [CrossRef]
- Jia, Y.; Jing, Q.; Niu, H.; Huang, B. Ameliorative effect of vitamin E on hepatic oxidative stress and hypoimmunity induced by high-fat diet in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2017, 67, 634–642. [Google Scholar] [CrossRef]
- Lu, K.; Wang, L.; Zhang, D.; Liu, W.; Xu, W. Berberine attenuates oxidative stress and hepatocytes apoptosis via protecting mitochondria in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Physiol. Biochem. 2017, 43, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, B.; Huang, Y.; Wu, D.; Zhang, M.; Zhao, Y. Effects of dietary vitamin E on reproductive performance and antioxidant capacity of Macrobrachium nipponense female shrimp. Aquac. Nutr. 2018, 24, 1698–1708. [Google Scholar] [CrossRef]
- Griboff, J.; Morales, D.; Bertrand, L.; Bonansea, R.I.; Monferrán, M.V.; Asis, R.; Wunderlin, D.A.; Amé, M.V. Oxidative stress response induced by atrazine in Palaemonetes argentinus: The protective effect of vitamin E. Ecotoxicol. Environ. Saf. 2014, 108, 1–8. [Google Scholar] [CrossRef]
- Mourente, G.; Dıaz-Salvago, E.; Bell, J.G.; Tocher, D.R. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: Attenuation by dietary vitamin E. Aquaculture 2002, 214, 343–361. [Google Scholar] [CrossRef]
- Lu, K.L.; Xu, W.N.; Liu, W.B.; Wang, L.N.; Zhang, C.N.; Li, X.F. Association of mitochondrial dysfunction with oxidative stress and immune suppression in blunt snout bream Megalobrama amblycephala fed a high-fat diet. J. Aquat. Anim. Health 2014, 26, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Essick, E.E.; Sam, F. Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid. Med. Cell. Longev. 2010, 3, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Du, P.; An, L.; Yuan, G.; Sun, Z. Anti-diabetic effect of Coptis Chinensis polysaccharide in high-fat diet with STZ-induced diabetic mice. Int. J. Biol. Macromol. 2013, 55, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Cherif, S.; Fendri, A.; Miled, N.; Trabelsi, H.; Mejdoub, H.; Gargouri, Y. Crab digestive lipase acting at high temperature: Purification and biochemical characterization. Biochimie 2007, 89, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Nutrient Requirements of Fish and Shrimp; National Academies Press: Pittsburgh, PA, USA, 2011. [Google Scholar]
- Xu, C.; Li, E.; Liu, Y.; Wang, S.; Wang, X.; Chen, K.; Qin, J.G.; Chen, L. Effect of dietary lipid level on growth, lipid metabolism and health status of the Pacific white shrimp Litopenaeus vannamei at two salinities. Aquac. Nutr. 2018, 24, 204–214. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, X.; Li, S.; Zhu, D.; Li, Y. Effects of dietary lipid levels on growth, feed utilization, body composition and antioxidants of juvenile mud crab Scylla paramamosain (Estampador). Aquaculture 2015, 435, 200–206. [Google Scholar] [CrossRef]
- D’Ignazio, L.; Bandarra, D.; Rocha, S. NF-κB and HIF crosstalk in immune responses. FEBS J. 2016, 283, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Meyerovich, K.; Fukaya, M.; Terra, L.F.; Ortis, F.; Eizirik, D.L.; Cardozo, A.K. The non-canonical NF-κB pathway is induced by cytokines in pancreatic beta cells and contributes to cell death and proinflammatory responses in vitro. Diabetologia 2016, 59, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.S.; Wu, X.Z. Characterization of a Rel\NF-κB homologue in a gastropod abalone, Haliotis diversicolor supertexta. Dev. Comp. Immunol. 2007, 31, 121–131. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Zhang, H.; Zheng, P.; Zhao, J.; Qiu, L.; Zhang, Y.; Song, L. Molecular cloning and expression of a Relish gene in Chinese mitten crab Eriocheir sinensis. Int. J. Immunogenet. 2010, 37, 499–508. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Arockiaraj, J.; Avin, F.A.; Vanaraja, P.; Easwvaran, S.; Singh, A.; Othman, R.Y.; Bhassu, S. Immune role of MrNFκBI-α, an IκB family member characterized in prawn M. rosenbergii. Fish Shellfish Immunol. 2012, 33, 619–625. [Google Scholar] [CrossRef]
- Huang, X.; Wang, W.; Ren, Q. Dorsal transcription factor is involved in regulating expression of crustin genes during white spot syndrome virus infection. Dev. Comp. Immunol. 2016, 63, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Srisuk, C.; Longyant, S.; Senapin, S.; Sithigorngul, P.; Chaivisuthangkura, P. Molecular cloning and characterization of a Toll receptor gene from Macrobrachium rosenbergii. Fish Shellfish Immunol. 2014, 36, 552–562. [Google Scholar] [CrossRef]
- Shi, Y.R. Study on Sequences Analysis and Function of IMD and Relish in IMD Signal Pathway in Macrobrachium rosenbergii. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2016. [Google Scholar]
- Fishery Bureau of Ministry of Agriculture of the People’s Republic of China. China Fishery Statistics Yearbook 2021; China Agriculture Press: Beijing, China, 2021.
- Paul, P.; Rahman, M.A.; Mer, M.; Hossain, M.; Islam, M.S.; Mondal, S.; Haq, M.M. Effect of stocking density on the growth and production of freshwater prawn (Macrobrachium rosenbergii). Int. J. Fish. Aquac. Sci. 2016, 6, 77–86. [Google Scholar]
- Lu, K.L.; Xu, W.N.; Li, J.Y.; Li, X.F.; Huang, G.Q.; Liu, W.B. Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish. Sci. 2013, 79, 661–671. [Google Scholar] [CrossRef]
- Wangari, M.R.; Gao, Q.; Sun, C.; Liu, B.; Song, C.; Tadese, D.A.; Zhou, Q.; Zhang, H.; Liu, B. Effect of dietary Clostridium butyricum and different feeding patterns on growth performance, antioxidant and immune capacity in freshwater prawn (Macrobrachium rosenbergii). Aquac. Res. 2021, 52, 12–22. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Xia, T.; Lin, J.B.; Wang, L.; Song, K.; Zhang, C.X. Quercetin attenuates high-fat diet-induced excessive fat deposition of spotted seabass (Lateolabrax maculatus) through the regulatory for mitochondria and endoplasmic reticulum. Front. Mar. Sci. 2021, 8, 746811. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Zhuang, Z.; He, X.; Tang, X.; Tian, L.; Liu, Y.; Niu, J. Dietary supplementation of bile acid attenuate adverse effects of high-fat diet on growth performance, antioxidant ability, lipid accumulation and intestinal health in juvenile largemouth bass (Micropterus salmoides). Aquaculture 2021, 531, 735864. [Google Scholar] [CrossRef]
- Zhou, W.; Rahimnejad, S.; Lu, K.; Wang, L.; Liu, W. Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol. Biochem. 2019, 45, 83–91. [Google Scholar] [CrossRef]
- Limbu, S.M.; Ma, Q.; Zhang, M.L.; Du, Z.Y. High fat diet worsens the adverse effects of antibiotic on intestinal health in juvenile Nile tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 680, 169–180. [Google Scholar] [CrossRef]
- Sink, T.D.; Lochmann, R.T.; Goodwin, A.E.; Marecaux, E. Mortality rates in golden shiners fed high-fat diets with or without a dairy-yeast prebiotic before challenge with Flavobacterium columnare. N. Am. J. Aquac. 2007, 69, 305–308. [Google Scholar] [CrossRef]
- Cao, X.F.; Dai, Y.J.; Liu, M.Y.; Yuan, X.Y.; Wang, C.C.; Huang, Y.Y.; Liu, W.B.; Jiang, G.Z. High-fat diet induces aberrant hepatic lipid secretion in blunt snout bream by activating endoplasmic reticulum stress-associated IRE1/XBP1 pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Wasipe, A.; He, J.; Tao, Y.F.; Xu, P.; Bao, J.W.; Chen, D.J.; Zhu, J.H. Dietary vitamin E deficiency inhibits fat metabolism, antioxidant capacity, and immune regulation of inflammatory response in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) fingerlings following Streptococcus iniae infection. Fish Shellfish Immunol. 2019, 92, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yu, H.; Wang, C.; Li, P.; Liu, S.; Zhang, X.; Zhang, C.; Qi, M.; Ji, H. Nano-selenium supplements in high-fat diets relieve hepatopancreas injury and improve survival of grass carp Ctenopharyngodon Idella by reducing lipid deposition. Aquaculture 2021, 538, 736580. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.F.; Tan, Q.; Yuan, X.; Liu, L.; Zhou, Y.; Li, B. Effects of vitamin E on growth performance and antioxidant status in juvenile grass carp Ctenopharyngodon idellus. Aquaculture 2014, 430, 21–27. [Google Scholar] [CrossRef]
- Lin, Y.H.; Shiau, S.Y. Dietary vitamin E requirement of grouper, Epinephelus malabaricus, at two lipid levels, and their effects on immune responses. Aquaculture 2005, 248, 235–244. [Google Scholar] [CrossRef]
- Wang, L.; Xu, B.; Sagada, G.; Ng, W.K.; Chen, K.; Zhang, J.; Shao, Q. Dietary berberine regulates lipid metabolism in muscle and liver of black sea bream (Acanthopagrus schlegelii) fed normal or high-lipid diets. Br. J. Nutr. 2021, 125, 481–493. [Google Scholar] [CrossRef]
- Liu, C.; Liao, J.Z.; Li, P.Y. Traditional Chinese herbal extracts inducing autophagy as a novel approach in therapy of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 1964–1973. [Google Scholar] [CrossRef]
- Ling, S.C.; Wu, K.; Zhang, D.G.; Luo, Z. Endoplasmic reticulum stress-mediated autophagy and apoptosis alleviate dietary Fat-induced triglyceride accumulation in the intestine and in isolated intestinal epithelial cells of yellow catfish. J. Nutr. 2019, 149, 1732–1741. [Google Scholar] [CrossRef]
- Uetake, Y.; Ikeda, H.; Irie, R.; Tejima, K.; Matsui, H.; Ogura, S.; Wang, H.; Mu, S.; Hirohama, D.; Ando, K.; et al. High-salt in addition to high-fat diet may enhance inflammation and fibrosis in liver steatosis induced by oxidative stress and dyslipidemia in mice. Lipids Health Dis. 2015, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Sarna, L.K.; Sid, V.; Wang, P.; Siow, Y.L.; House, J.D.; Karmin, O. Tyrosol attenuates high fat diet-induced hepatic oxidative stress: Potential involvement of cystathionine β-synthase and cystathionine γ-lyase. Lipids 2016, 51, 583–590. [Google Scholar] [CrossRef]
- Rasool, M.; Malik, A.; Saleem, S.; Ashraf, M.A.B.; Khan, A.Q.; Waquar, S.; Zahid, A.; Shaheen, S.; Abu-Elmagd, M.; Gauthaman, K.; et al. Role of oxidative stress and the identification of biomarkers associated with thyroid dysfunction in schizophrenics. Front. Pharmacol. 2021, 12, 646287. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bansal, M.P. Protective role of dietary-supplemented selenium and vitamin E in heat-induced apoptosis and oxidative stress in mice testes. Andrologia 2015, 47, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Khorramabadi, K.M.; Talebi, A.R.; Sarcheshmeh, A.A.; Mirjalili, A. Protective effect of vitamin E on oxidative stress and sperm apoptosis in diabetic mice. Int. J. Reprod. Biomed. 2019, 17, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, W.; Jia, Q.; Feng, Z.; Guo, J.; Han, X.; Liu, Y.; Shang, H.; Wang, Y.; Liu, W.J. High dose vitamin E attenuates diabetic nephropathy via alleviation of autophagic stress. Front. Physiol. 2019, 10, 1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asrullah, M.; Arsanti Lestari, L.; Helmyati, S.; Farmawati, A. Effect supplementation of mung bean sprouts (Phaseolus radiatus L.) and vitamin E in rats fed high fat diet. KnE Life Sci. 2019, 4, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Yin, P.; Tian, L.; Yu, Y.; Liu, Y.; Niu, J. Dietary supplementation of astaxanthin improved the growth performance, antioxidant ability and immune response of juvenile largemouth bass (Micropterus salmoides) fed high-fat diet. Mar. Drugs 2020, 18, 642. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Guo, Z.X.; Wang, A.L. Growth performance and protective effect of vitamin E on oxidative stress pufferfish (Takifugu obscurus) following by ammonia stress. Fish Physiol. Biochem. 2018, 44, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Mamauag, R.E.P.; Han, Y. Effects of dietary oxidized fish oil with vitamin E supplementation on growth performance and reduction of lipid peroxidation in tissues and blood of red sea bream Pagrus major. Aquaculture 2012, 356–357, 73–79. [Google Scholar] [CrossRef]
- Sun, C.; Liu, B.; Zhou, Q.; Xiong, Z.; Shan, F.; Zhang, H. Response of Macrobrachium rosenbergii to vegetable oils replacing dietary fish oil: Insights from antioxidant defense. Front. Physiol. 2020, 11, 218. [Google Scholar] [CrossRef] [Green Version]
- Belavgeni, A.; Dailianis, S. The role of phosphatidylinositol-3-OH-kinase (PI3-kinase) and respiratory burst enzymes in the [omim][BF4]-mediated toxic mode of action in mussel hemocytes. Fish Shellfish Immunol. 2017, 68, 144–153. [Google Scholar] [CrossRef]
- Duan, Y.; Dong, H.; Wang, Y.; Li, H.; Liu, Q.; Zhang, Y.; Zhang, J. Intestine oxidative stress and immune response to sulfide stress in Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2017, 63, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide Biol. Chem. 2010, 23, 75–93. [Google Scholar] [CrossRef]
- Shi, Y.R.; Jin, M.; Ma, F.T.; Huang, Y.; Huang, X.; Feng, J.L.; Zhao, L.L.; Chen, Y.H.; Ren, Q. Involvement of relish gene from Macrobrachium rosenbergii in the expression of anti-microbial peptides. Dev. Comp. Immunol. 2015, 52, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yan, Y.; Xiao, Z.; Wang, M.; Xu, M.; Wang, Z.; Wang, Y.; Zhuang, Z.; Yang, D.; Chen, G.; et al. Bicyclol ameliorates nonalcoholic fatty liver disease in mice via inhibiting MAPKs and NF-κB signaling pathways. Biomed. Pharmacother. 2021, 141, 111874. [Google Scholar] [CrossRef]
- Liu, P.; Wu, P.; Yang, B.; Wang, T.; Li, J.; Song, X.; Sun, W. Kaempferol prevents the progression from simple steatosis to non-alcoholic steatohepatitis by inhibiting the NF-κB pathway in oleic acid-induced HepG2 cells and high-fat diet-induced rats. J. Funct. Foods 2021, 85, 104655. [Google Scholar] [CrossRef]



| LF/200VE | LF/600VE | HF/200VE | HF/600VE | |
|---|---|---|---|---|
| Ingredients% | ||||
| Fish meal 1 | 22.00 | 22.00 | 22.00 | 22.00 |
| Casein 2 | 24.00 | 24.00 | 24.00 | 24.00 |
| Gelatin 1 | 6.00 | 6.00 | 6.00 | 6.00 |
| α-starch 3 | 20.00 | 20.00 | 20.00 | 20.00 |
| Dextrin 3 | 5.00 | 5.00 | 5.00 | 5.00 |
| Fish oil 1 | 6.00 | 6.00 | 10.00 | 10.00 |
| Soybean oil 1 | 0.00 | 0.00 | 0.00 | 0.00 |
| Rapeseed oil 1 | 0.00 | 0.00 | 0.00 | 0.00 |
| Microcystalline cellulose 4 | 5.00 | 5.00 | 1.00 | 1.00 |
| Carboxymethyl cellulose 4 | 3.00 | 3.00 | 3.00 | 3.00 |
| Bentonite 1 | 1.53 | 1.49 | 1.53 | 1.49 |
| Soybean lecithin (50%) 1 | 2.00 | 2.00 | 2.00 | 2.00 |
| Cholesterol 1 | 0.30 | 0.30 | 0.30 | 0.30 |
| Ecdysone (2%) 5 | 0.10 | 0.10 | 0.10 | 0.10 |
| DMPT 5 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline chloride 5 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin premix 5 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin E 1 | 0.02 | 0.06 | 0.02 | 0.06 |
| Mineral premix 5 | 1.00 | 1.00 | 1.00 | 1.00 |
| Calcium dihydrogen phosphate 1 | 2.00 | 2.00 | 2.00 | 2.00 |
| Proximate composition % | ||||
| Crude protein | 40.90 | 40.90 | 40.90 | 40.90 |
| Crude lipid | 9.58 | 9.58 | 13.28 | 13.28 |
| Gross energy (MJ/kg) | 15.80 | 15.80 | 17.38 | 17.38 |
| Gene | GenBank Acc. No. | Primer Sequences (5′–3′) | Length (bp) | Purpose | Reference |
|---|---|---|---|---|---|
| T7-relish | KR827675.1 | TAATACGACTCACTATAGGGATCATCATGAGGCGGAAAAG | 40 | RNAi | |
| TAATACGACTCACTATAGGGTGGCATGTAGGTGAAATCCA | 40 | ||||
| T7-dorsal | KX219631.1 | TAATACGACTCACTATAGGGCAAGTGTTCCTCGAAGGCTC | 40 | RNAi | |
| TAATACGACTCACTATAGGGAACTTCACCAATTTGTCCGC | 40 | ||||
| relish | KR827675.1 | GATGAGCCTTCAGTGCCAGA | 20 | RT-qPCR | |
| CCAGGTGACGCCATGTATCA | 20 | ||||
| dorsal | KX219631.1 | TCAGTAGCGACACCATGCAG | 20 | RT-qPCR | |
| CGAGCCTTCGAGGAACACTT | 20 | ||||
| imd | CGACCACATTCTCCTCCTCCC | 21 | RT-qPCR | [24] | |
| TTCAGTGCATCCACGTCCCTC | 21 | ||||
| toll | KX610955.1 | TTCGTGACTTGTCGGCTCTC | 20 | RT-qPCR | |
| GCAGTTGTTGAAGGCATCGG | 20 | ||||
| β-actin | AY651918.2 | TCCGTAAGGACCTGTATGCC | 20 | RT-qPCR | |
| TCGGGAGGTGCGATGATTTT | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Shan, F.; Liu, M.; Liu, B.; Zhou, Q.; Zheng, X.; Xu, X. High-Fat-Diet-Induced Oxidative Stress in Giant Freshwater Prawn (Macrobrachium rosenbergii) via NF-κB/NO Signal Pathway and the Amelioration of Vitamin E. Antioxidants 2022, 11, 228. https://doi.org/10.3390/antiox11020228
Sun C, Shan F, Liu M, Liu B, Zhou Q, Zheng X, Xu X. High-Fat-Diet-Induced Oxidative Stress in Giant Freshwater Prawn (Macrobrachium rosenbergii) via NF-κB/NO Signal Pathway and the Amelioration of Vitamin E. Antioxidants. 2022; 11(2):228. https://doi.org/10.3390/antiox11020228
Chicago/Turabian StyleSun, Cunxin, Fan Shan, Mingyang Liu, Bo Liu, Qunlan Zhou, Xiaochuan Zheng, and Xiaodi Xu. 2022. "High-Fat-Diet-Induced Oxidative Stress in Giant Freshwater Prawn (Macrobrachium rosenbergii) via NF-κB/NO Signal Pathway and the Amelioration of Vitamin E" Antioxidants 11, no. 2: 228. https://doi.org/10.3390/antiox11020228
APA StyleSun, C., Shan, F., Liu, M., Liu, B., Zhou, Q., Zheng, X., & Xu, X. (2022). High-Fat-Diet-Induced Oxidative Stress in Giant Freshwater Prawn (Macrobrachium rosenbergii) via NF-κB/NO Signal Pathway and the Amelioration of Vitamin E. Antioxidants, 11(2), 228. https://doi.org/10.3390/antiox11020228








