Generation of Reactive Oxygen Species (ROS) by Harmful Algal Bloom (HAB)-Forming Phytoplankton and Their Potential Impact on Surrounding Living Organisms
Abstract
1. Introduction
2. Marine Microalgae Species with ROS-Producing Activities
3. Chattonella
3.1. Mechanisms of ROS Production by Chattonella
3.2. NADPH Oxidase as a Superoxide-Anion-Producing Enzyme System
3.3. Glycocalyx as a Cell Surface Structure with ROS Generation System
4. Raphidophycean Flagellates
5. Cochlodinium Polykrikoides
6. Karenia Mikimotoi
7. Nitric Oxide (NO) Production in Marine Microalgae
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Andretta, E.; Costa, C.; Longobardi, C.; Damiano, S.; Giordano, A.; Pagnini, F.; Montagnaro, S.; Quintiliani, M.; Lauritano, C.; Ciarcia, R. Potential approaches versus approved or developing chronic myeloid leukemia therapy. Front. Oncol. 2021, 11, 801779. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Zohdi, E.; Abbaspour, M. Harmful algal blooms (red tide): A review of causes, impacts and approaches to monitoring and prediction. Int. J. Environ. Sci. Technol. 2019, 16, 1789–1806. [Google Scholar] [CrossRef]
- Cho, K.; Heo, J.; Han, J.; Hong, H.D.; Jeon, H.; Hwang, H.J.; Hong, C.Y.; Kim, D.; Han, J.W.; Baek, K. Industrial applications of Dinoflagellate phycotoxins based on their modes of action: A review. Toxins 2020, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G., Jr. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Rensel, J.E.; Whyte, J.N.C. Finfish mariculture and harmful algal blooms. Man. Harmful Mar. Microalgae. Monogr. Oceanogr. Methodol. 2003, 11, 693–722. [Google Scholar]
- Smayda, T.J.; Villareal, T.A. The 1985 ‘brown-tide’and the open phytoplankton niche in Narragansett Bay during summer. In Novel Phytoplankton Blooms; Springer: Berlin/Heidelberg, Germany, 1989; pp. 159–187. [Google Scholar]
- Hallegraeff, G.M. Transport of toxic dinoflagellates via ships ballast water: Bioeconomic risk assessment and efficacy of possible ballast water management strategies. Mar. Ecol. Prog. Ser. 1998, 168, 297–309. [Google Scholar] [CrossRef]
- Honjo, T. Potential transfer of Heterocapsa circularisquama with pearl oyster consignments. Harmful Algae 1998, 224–226. [Google Scholar]
- Hégaret, H.; Wikfors, G.H. Time-dependent changes in hemocytes of eastern oysters, Crassostrea virginica, and northern bay scallops, Argopecten irradians irradians, exposed to a cultured strain of Prorocentrum minimum. Harmful Algae 2005, 4, 187–199. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Nishitani, G.; Nagai, S. Direct detection of harmful algae from the oyster spat and live fish transport trailers. In Proceedings of the XIII International Conference on Harmful Algae, Hong Kong, China, 6 November 2008; pp. 185–189. [Google Scholar]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Anderson, D.M. Red tides. Sci. Am. 1994, 271, 62–68. [Google Scholar] [CrossRef]
- Honjo, T. The biology and prediction of representative red tides associated with fish kills in Japan. Rev. Fish. Sci. 1994, 2, 225–253. [Google Scholar] [CrossRef]
- Shumway, S.E. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquacult. Soc. 1990, 21, 65–104. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Shumway, S.E. Impacts of harmful algal blooms on shellfisheries aquaculture. In New Technologies in Aquaculture; Burnell, G., Allan, G., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 580–609. [Google Scholar]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK 2016, 96, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef]
- McCabe, R.M.; Hickey, B.M.; Kudela, R.M.; Lefebvre, K.A.; Adams, N.G.; Bill, B.D.; Gulland, F.M.D.; Thomson, R.E.; Cochlan, W.P.; Trainer, V.L. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 2016, 43, 10–366. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W. Impacts of Climate Change on Cyanobacteria in Aquatic Environments; Caister Academic Press: Poole, UK, 2016; pp. 5–22. [Google Scholar]
- Granéli, E.; Haraldsson, C. Can increased leaching of trace metals from acidified areas influence phytoplankton growth in coastal waters? Ambio 1993, 22, 308–311. [Google Scholar]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic conditions trigger record harmful algal bloom in western Patagonia (summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef] [PubMed]
- Ono, C.; Takano, H. Chattonella antiqua (Hada) comb. nov., and its occurrence on the Japanese coast. Bull. Tokai Reg. Fish. Res. Lab. 1980, 102, 93–100. [Google Scholar]
- Simakova, N.V.; Orlova, T.; Selina, M.S. Red tide caused by raphidophytes Chattonella sp. Amurskii Bay, the Sea of Japan. Russ. J. Mar. Biol. 1990, 16, 77–79. [Google Scholar]
- Munday, B.L.; Hallegraeff, G.M. Mass mortality of captive southern bluefin tuna (Thunnus maccoyii) in April/May 1996 in Boston Bay, South Australia: A complex diagnostic problem. Fish Pathol. 1998, 33, 343–350. [Google Scholar] [CrossRef][Green Version]
- Smayda, T.J. Ecophysiology and bloom dynamics of Heterosigma akashiwo (Raphidophyceae), In Physiological ecology of harmful algal blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; . Springer: Berlin, Germany, 1998; pp. 113–131. [Google Scholar]
- Edvardsen, B.; Imai, I. The ecology of harmful flagellates within Prymnesiophyceae and Raphidophyceae. In Ecology of Harmful Algae; Springer: Berlin/Heigelberg, Germany, 2006; pp. 67–79. [Google Scholar]
- Imai, I.; Yamaguchi, M. Life cycle, physiology, ecology and red tide occurrences of the fish-killing raphidophyte Chattonella. Harmful Algae 2012, 14, 46–70. [Google Scholar] [CrossRef]
- García-Mendoza, E.; Cáceres-Martínez, J.; Rivas, D.; Fimbres-Martinez, M.; Sánchez-Bravo, Y.; Vásquez-Yeomans, R.; Medina-Elizalde, J. Mass mortality of cultivated northern bluefin tuna Thunnus thynnus orientalis associated with Chattonella species in Baja California, Mexico. Front. Mar. Sci. 2018, 5, 454. [Google Scholar] [CrossRef]
- Okaichi, T. Akashio no Kagaku; Koseisha-Koseikaku: Tokyo, Japan, 1987; p. 294. [Google Scholar]
- Okaichi, T. Red-Tide Phenomena; Terra Scientific Publishing Company: Tokyo, Japan, 2003; pp. 7–60. [Google Scholar]
- Hallegraeff, G.M.; Munday, B.L.; Baden, D.G.; Whitney, P.L. Chatonnella maria raphidophyte bloom associated with mortality of cultured bluefin tuna (Thunnus maccoyii) in south Australia. In Harmful Algae; Reguera, B., Blanco, J., Ferandz, M.L., Wyatt, T., Eds.; Xunta de Galacia and IOC: Santiago de Compostela, Spain, 1998; pp. 93–96. [Google Scholar]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- McKenzie, C.H.; Bates, S.S.; Martin, J.L.; Haigh, N.; Howland, K.L.; Lewis, N.I.; Locke, A.; Peña, A.; Poulin, M.; Rockon, A.; et al. Three decades of Canadian marine harmful algal events: Phytoplankton and phycotoxins of concern to human and ecosystem health. Harmful Algae 2021, 102, 101852. [Google Scholar] [CrossRef]
- Barraza-Guardado, R.; Cortés-Altamirano, R.; Sierra-Beltrán, A. Marine die-offs from Chattonella marina and Ch. cf. ovata in Kun Kaak Bay, Sonora in the Gulf of California. Harmful Algae News 2004, 25, 7–8. [Google Scholar]
- Okaichi, T. Red tide in the Seto Inland Sea. In Sustainable Development in the Seto Inland Sea, Japan—From the Viewpoint of Fisheries; Okaichi, T., Yanagi, T., Eds.; Terra Scientific Publishing Company: Tokyo, Japan, 1997; pp. 251–304. [Google Scholar]
- Onitsuka, G.; Aoki, K.; Shimizu, M.; Matsuyama, Y.; Kimoto, K.; Matsuo, H.; Kitadai, Y.; Nishi, H.; Tahara, Y.; Sakurada, K. Short-term dynamics of a Chattonella antiqua bloom in the Yatsushiro Sea, Japan, in summer 2010: Characteristics of its appearance in the southern area. Bull. Jpn. Soc. Fish. Oceanogr. 2011, 75, 143–153. [Google Scholar]
- Domingos, P.; Menezes, M. Taxonomic remarks on planktonic phytoflagellates in a hypertrophic tropical lagoon (Brazil). In Phytoplankton and Trophic Gradients; Springer: Dordrecht, The Netherlands, 1998; pp. 297–313. [Google Scholar]
- Hallegraeff, G.M.; Schweibold, L.; Jaffrezic, E.; Rhodes, L.; MacKenzie, L.; Hay, B.; Farrell, H. Overview of Australian and New Zealand harmful algal species occurrences and their societal impacts in the period 1985 to 2018, including a compilation of historic records. Harmful Algae 2021, 102, 101848. [Google Scholar] [CrossRef]
- Marshall, J.A. Comparative Ecophysiology, Chemotaxonomy and Ichthyotoxicity of Chattonella marina (Raphidophyceae) from Australia and Japan. Ph.D. Thesis, University of Tasmania, Tasmania, Australia, 2003. [Google Scholar]
- Stacca, D.; Satta, C.T.; Casabianca, S.; Penna, A.; Padedda, B.M.; Sechi, N.; Lugliè, A. Identification of Chattonella (Raphidophyceae) species in long-term phytoplankton samples from Santa Giusta Lagoon, Italy. Sci. Mar. 2016, 80, 17–25. [Google Scholar]
- Sakamoto, S.; Lim, W.A.; Lu, D.; Dai, X.; Orlova, T.; Iwataki, M. Harmful algal blooms and associated fisheries damage in East Asia: Current status and trends in China, Japan, Korea and Russia. Harmful Algae 2021, 102, 101787. [Google Scholar] [CrossRef]
- Sunesen, I.; Méndez, S.M.; Mancera-Pineda, J.E.; Bottein, M.Y.D.; Enevoldsen, H. The Latin America and Caribbean HAB status report based on OBIS and HAEDAT maps and databases. Harmful Algae 2021, 102, 101920. [Google Scholar] [CrossRef]
- Yñiguez, A.T.; Lim, P.T.; Leaw, C.P.; Jipanin, S.J.; Iwataki, M.; Benico, G.; Azanza, R.V. Over 30 years of HABs in the Philippines and Malaysia: What have we learned? Harmful Algae 2021, 102, 101776. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hirayama, K. Lethal effect of Gymnodinium sp. on the rotifer, Brachionus plicatilis. Bull. Fac. Fish. 1979, 46, 1–6. [Google Scholar]
- Li, X.; Yan, T.; Yu, R.; Zhou, M. A review of Karenia mikimotoi: Bloom events, physiology, toxicity and toxic mechanism. Harmful Algae 2019, 90, 101702. [Google Scholar] [CrossRef]
- Gentien, P.; Arzul, G. Exotoxin production by Gyrodinium cf. aureolum (Dinophyceae). J. Mar. Biol. Assoc. UK 1990, 70, 571–581. [Google Scholar] [CrossRef]
- Yasumoto, T. Marine microorganisms toxins—An overview. In Toxic Marine Phytoplankton; Granéli, E., Sundstrom, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 3–8. [Google Scholar]
- Arzul, G.; Bodennec, G.; Erard, E.; Gentien, P. Fish kills and Gymnodinium cf. nagasakiense in Corsica (France). Harmful Algae News 1994, 8, 7. [Google Scholar]
- Gentien, P. Bloom dynamics and ecophysiology of the Gymnodinum mikimotoi species complex. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 155–173. [Google Scholar]
- Parrish, C.C.; Bodennec, G.; Gentien, P. Haemolytic glycoglycerolipids from Gymnodinium species. Phytochemistry 1998, 47, 783–787. [Google Scholar] [CrossRef]
- Daugbjerg, N.; Hansen, G.; Larsen, J.; Moestrup, Ø. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia 2000, 39, 302–317. [Google Scholar] [CrossRef]
- Hansen, G.; Daugbjerg, N.; Henriksen, P. Comparative study of Gymnodinium mikimotoi and Gymnodinium aureolum comb. nov. (=Gyrodinium aureolum) based on morphology, pigment composition, and molecular data. J. Phycol. 2000, 36, 394–410. [Google Scholar] [CrossRef]
- Ho, M.-S.; Zubkoff, P.L. The effects of a Cochlodinium heterolobatum bloom on the survival and calcium uptake by larvae of the American oyster, Crassostrea virginica. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.H., Eds.; Elsevier: New York, NY, USA, 1979; pp. 409–412. [Google Scholar]
- Onoue, Y.; Nozawa, K. Zinc-bound PSP toxins separated from Cochlodinium red tide. In Mycotoxins and Phycotoxins ’88; Natori, S., Hashimoto, K., Ueno, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 359–366. [Google Scholar]
- Yuki, K.; Yoshimatsu, S. Two Fish-Killing Species of Cochlodinium from Harima Nada, Seto Inland Sea, Japan; Elsevier: New York, NY, USA, 1989; pp. 451–454. [Google Scholar]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar] [CrossRef]
- Lee, J.S. Bioactive components from red tide plankton, Cochlodinium polykrikoides. J. Korean Fish. Soc. 1996, 29, 165–173. [Google Scholar]
- Kim, C.S.; Lee, S.G.; Kim, H.G.; Jung, J. Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J. Plankton Res. 1999, 21, 2105–2115. [Google Scholar] [CrossRef]
- Needler, A.B. Paralytic shellfish poisoning and Gonyaulax tamarensis. J. Fish. Res. Board Can. 1949, 7, 490–504. [Google Scholar] [CrossRef]
- Prakash, A. Source of paralytic shellfish toxin in the Bay of Fundy. J. Fish. Res. Board Can. 1963, 20, 983–996. [Google Scholar] [CrossRef]
- Prakash, A. Growth and toxicity of a marine dinoflagellate, Gonyaulax tamarensis. J. Fish. Res. Board Can. 1967, 24, 1589–1606. [Google Scholar] [CrossRef]
- White, A.W.; Maranda, L. Paralytic shellfish toxins in the dinoflagellate Gonyaulax excavata and in shellfish. J. Fish. Res. Board Can. 1978, 35, 397–402. [Google Scholar] [CrossRef]
- Oshima, Y.; Yasumoto, T. Analysis of toxins in cultured Gonyaulax excavate. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.H., Eds.; Elsevier: New York, NY, USA, 1979; pp. 377–380. [Google Scholar]
- Betz, J.M.; Blogoslawski, W.J. Toxicity of Gonyaulax tamarensis var. excavata cells to the brine shrimp Artemia salina L. J. Pharm. Sci. 1981, 71, 463–465. [Google Scholar]
- White, A.W. Sensitivity of marine fishes to toxins from the red-tide dinoflagellate Gonyaulax excavata and implications for fish kills. Mar. Biol. 1981, 65, 255–260. [Google Scholar] [CrossRef]
- Anderson, D.M.; Kulis, D.M.; Orphanos, J.A.; Ceurvels, A.R. Distribution of the toxic dinoflagellate Gonyaulax tamarensis in the southern New England region. Estuar. Coast. Shelf Sci. 1982, 14, 447–458. [Google Scholar] [CrossRef]
- Schantz, E.J. Historical perspective on paralytic shellfish poisons. In Seafood Toxins; Ragelis, E.P., Ed.; American Chemical Society: Washington, DC, USA, 1984; pp. 99–111. [Google Scholar]
- Maranda, L.; Anderson, D.M.; Shimizu, Y. Comparison between populations of Gonyaulax tamarensis of eastern North America waters. Estuar. Coast. Shelf Sci. 1985, 21, 401–410. [Google Scholar] [CrossRef]
- Ogata, T.; Kodama, M. Ichthyotoxicity found in cultured media of Protogonyaulax spp. Mar. Biol. 1986, 92, 31–34. [Google Scholar] [CrossRef]
- Ogata, T.; Kodama, M.; Ishimaru, T. Toxin production in the dinoflagellate Protogonyaulax tamarensis. Toxicon 1987, 25, 923–928. [Google Scholar] [CrossRef]
- Cembella, A.D.; Sullivan, J.J.; Boyer, G.L.; Taylor, F.J.R.; Andersen, R.J. Variation in paralytic shellfish toxin composition within the Protogonyaulax tamarensis/catenella species complex; red tide dinoflagellates. Biochem. Syst. Ecol. 1987, 15, 171–186. [Google Scholar] [CrossRef]
- Cembella, A.D.; Therriault, J.-C.; Beland, P. Toxicity of cultured isolates and natural populations of Protogonyaulax tamarensis from the St. Lawrence Estuary. J. Shellfish Res. 1988, 7, 611–621. [Google Scholar]
- Lassus, P.; Frémy, J.-M.; Ledoux, M.; Bardouil, M.; Bohec, M. Patterns of experimental contamination by Protogonyaulax tamarensis in some French commerical shellfish. Toxicon 1989, 27, 1313–1321. [Google Scholar] [CrossRef]
- Kodama, M.; Ogata, T.; Sato, S.; Sakamoto, S. Possible association of marine bacteria with paralytic shellfish toxicity in bivalves. Mar. Ecol. Prog. Ser. 1990, 61, 203–206. [Google Scholar] [CrossRef]
- Kodama, M.; Sato, S.; Ogata, T. Alexandrium tamarense as a source of tetrodotoxin in the scallop Patinopecten yessoensis. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 401–406. [Google Scholar]
- Kodama, M.; Sato, S.; Sakamoto, S.; Ogata, T. Occurrence of tetrodotoxin in Alexandrium tamarense: A causative dinoflagellate of paralytic shellfish poisoning. Toxicon 1996, 34, 1101–1105. [Google Scholar] [CrossRef]
- Lee, J.S.; Jeon, J.-K.; Han, M.-S.; Oshima, Y.; Yasumoto, T. Paralytic shellfish toxins in the mussel Mytilus edulis and dinoflagellate Alexandrium tamarense from Jinhae Bay, Korea. Bull. Korean Fish. Soc. 1992, 25, 144–150. [Google Scholar] [CrossRef]
- Kim, C.-H.; Sako, Y.; Ishida, Y. Comparison of toxin composition between populations of Alexandrium spp. from geographically distinct areas. Nippon Suisan Gakkaishi 1993, 59, 641–646. [Google Scholar] [CrossRef]
- Perovic, S.; Tretter, L.; Brummer, F.; Wetzler, C.; Brenner, J.; Donner, G.; Schroder, H.C.; Muller, W.E. Dinoflagellates from marine algal blooms produce neurotoxic compounds: Effects on free calcium levels in neuronal cells and synaptosomes. Environ. Toxicol. Pharmacol. 2000, 8, 83–94. [Google Scholar] [CrossRef]
- Chang, F.H.; Anderson, C.; Boustead, N.C. First record of a Heterosigma (Raphidophyceae) bloom with associated mortality of cage-reared salmon in Big Glory Bay, New Zealand. N. Z. J. Mar. Fresh. 1990, 24, 461–469. [Google Scholar] [CrossRef]
- Yang, C.Z.; Albright, L.J.; Yousif, A.N. Oxygen-radical-mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1995, 23, 101–108. [Google Scholar] [CrossRef]
- Khan, S.; Arakawa, O.; Onoue, Y. Neurotoxins in a toxic red tide of Heterosigma akashiwo (Raphidophyceae) in Kagoshima Bay, Japan. Aquac. Res. 1997, 28, 9–14. [Google Scholar] [CrossRef]
- Lush, G.J.; Hallegraeff, G.M. High toxicity of the red tide dinoflagellate Alexandrium minutum to the brine shrimp Artemia salina. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1996; pp. 389–392. [Google Scholar]
- Oda, T.S.; Nakamura, A.; Shikayama, M.; Kawano, I.; Ishimatsu, A.; Muramatsu, T. Generation of reactive oxygen species by raphidophycean phytoplankton. Biosci. Biotechnol. Biochem. 1997, 61, 1658–1662. [Google Scholar] [CrossRef]
- Twiner, M.J.; Trick, C.G. Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate Heterosigma akashiwo. J. Plankton Res. 2000, 22, 1961–1975. [Google Scholar] [CrossRef]
- Onoue, Y.; Nozawa, K. Separation of toxins from harmful red tides occurring along the coast of Kagoshima Prefecture. In Red Tides, Biology, Environmental Science and Toxicology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier: New York, NY, USA, 1989; pp. 371–374. [Google Scholar]
- Onoue, Y.; Haq, M.S.; Nozawa, K. Separation of neurotoxins from Chattonella marina. Nippon Suisan Gakkaishi 1990, 56, 695. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Arakawa, O.; Onoue, Y. Toxicity of cultured Chattonella marina. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard-Le-Denn, E., Gentien, P., Marcaillou-Le-Baut, C., Eds.; Lavoisier: New York, NY, USA, 1995; pp. 499–504. [Google Scholar]
- Khan, D.; Ahmed, M.S.; Arakawa, O.; Onoue, Y. Properties of neurotoxins separated from a harmful redtide organism Chattonella marina. Isr. J. Aquac. 1995, 47, 137–141. [Google Scholar]
- Kim, D.; Nakamura, A.; Okamoto, T.; Komatsu, N.; Oda, T.; Iida, T.; Ishimatsu, A.; Muramatsu, T. Mechanisms of superoxide anion generation in the toxic red tide phytoplankton Chattonella marina: Possible involvement of NAD(P)H oxidase. Biochim. Biophys. Acta 2000, 1524, 220–227. [Google Scholar] [CrossRef]
- Hiroishi, S.; Okada, H.; Imai, I.; Yoshida, T. High toxicity of the novel bloom-forming species Chattonella ovata (Raphidophyceae) to cultured fish. Harmful Algae 2005, 4, 783–787. [Google Scholar] [CrossRef]
- Shimada, M.; Murakami, T.H.; Imahayashi, T.; Ozaki, H.S.; Toyoshima, T.; Okaichi, T. Effects of sea bloom, Chattonella antigua, on gill primary lamellae of the young yellowtail, Seriola quinqueradiata. Acta Histochem. Cytochem. 1983, 16, 232–244. [Google Scholar] [CrossRef]
- Shimada, M.; Akagi, N.; Nakai, Y.; Goto, H.; Watanabe, M.; Watanabe, H.; Nakanishi, M.; Yoshimatsu, S.; Ono, C. Free radical production by the red tide alga, Chattonella antiqua. Histochem. J. 1991, 23, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Satake, M.; Murata, M.; Yasumoto, T. Screening of marine phytoplankton for antifungal substances. In Toxic Marine Phytoplankton; Granéli, E., Sundstrom, B., Edler, L., Anderson, D.M., Eds.; Academic Press: New York, NY, USA, 1990; pp. 385–390. [Google Scholar]
- Tanaka, K.; Muto, Y.; Shimada, M. Generation of superoxide anion radicals by the marine phytoplankton organism, Chattonella antiqua. J. Plankton Res. 1994, 16, 161–169. [Google Scholar] [CrossRef]
- Khan, S.; Arakawa, P.O.; Onoue, Y. A toxicological study of the marine phytoflagellate, Chattonella antiqua (Raphidophyceae). Phycologia 1996, 35, 239–244. [Google Scholar] [CrossRef]
- Viana, T.V.; Fistarol, G.O.; Amario, M.; Menezes, R.B.; Carneiro, B.L.; Chaves, D.M.; Hargreaves, P.I.; Silva-Lima, A.W.; Valentin, J.L.; Tenenbaum, D.R.; et al. Massive blooms of Chattonella subsalsa Biecheler (Raphidophyceae) in a hypereutrophic, tropical estuary—Guanabara Bay, Brazil. Front. Mar. Sci. 2019, 6, 85. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Liu, C.Y.; Wu, Z.Z.; Xing, L.; Li, P.F. Detection of nitric oxide in culture media and studies on nitric oxide formationby marine microalgae. Med. Sci. Monit. 2006, 12, BR75–BR85. [Google Scholar] [PubMed]
- Ianora, A.; Poulet, S.A.; Miralto, A. A comparative study of the inhibitory effect of diatoms on the reproductive biology of the copepod Acartia clausi. Mar. Biol. 1995, 121, 533–539. [Google Scholar] [CrossRef]
- Kent, M.L.; Whyte, J.N.C.; LaTrace, C. Gill lesions and mortality in seawater pen-reared Atlantic salmon Salmo salar associated with a dense bloom of Skeletonema costatum and Thalassiosira species. Dis. Aquat. Org. 1995, 22, 77–81. [Google Scholar] [CrossRef]
- Ban, S.; Burns, C.; Castel, J.; Chaudron, Y.; Christou, E.; Escribano, R.; Fonda Umani, S.; Gasparini, S.; Guerrero Ruiz, F.; Hoffmeyer, M.; et al. The paradox of diatom–copepod interactions. Mar. Ecol. Prog. Ser. 1997, 157, 287–293. [Google Scholar] [CrossRef]
- Naviner, M.; Berge, J.-P.; Durand, P.; Le Bris, H. Antibacterial activity of the marine diatom Skeletonema costatum against aquacultural pathogens. Aquaculture 1999, 174, 15–24. [Google Scholar] [CrossRef]
- Uye, S.; Takamatsu, K. Feeding interactions between planktonic copepods and red-tide flagellates from Japanese coastal waters. Mar. Ecol. Prog. Ser. 1990, 59, 97–107. [Google Scholar] [CrossRef]
- Kim, D.; Nakamura, A.; Okamoto, T.; Komatsu, N.; Oda, T.; Ishimatsu, A.; Muramatsu, T. Toxic potential of the raphidophyte Olisthodiscus luteus: Mediation by reactive oxygen species. J. Plankton Res. 1999, 21, 1017–1027. [Google Scholar] [CrossRef]
- Khan, S.; Arakawa, P.O.; Onoue, Y. Neurotoxin production by a chloromonad, Fibrocapsa japonica (Raphidophyceae). J. World Aquac. Soc. 1996, 27, 254–263. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Nagai, K.; Mizuguchi, T.; Fujiwara, M.; Ishimura, M.; Yamaguchi, M.; Uchida, T.; Honjo, T. Ecological features and mass mortality of pearl oysters during the red tide of Heterocapsa sp. in Ago Bay in 1992. Nippon Suisan Gakkaishi 1995, 61, 35–41. [Google Scholar] [CrossRef]
- Uchida, T.; Yamaguchi, M.; Matsuyama, Y.; Honjo, T. The red-tide dinoflagellate Heterocapsa sp. kills Gyrodinium instriatum by cell contact. Mar. Ecol. Prog. Ser. 1995, 118, 301–303. [Google Scholar] [CrossRef]
- Kamiyama, T. Growth and grazing responses of tintinnid ciliates feeding on the toxic dinoflagellate Heterocapsa circularisquama. Mar. Biol. 1997, 128, 509–515. [Google Scholar] [CrossRef]
- Kamiyama, T.; Arima, S. Lethal effect of the dinoflagellate Heterocapsa circularisquama upon the tintinnid ciliate Favella taraikaensis. Mar. Ecol. Prog. Ser. 1997, 160, 27–33. [Google Scholar] [CrossRef]
- Kim, D.; Sato, Y.; Oda, T.; Muramatsu, T.; Matsuyama, Y.; Honjo, T. Specific toxic effect of dinoflagellate Heterocapsa circularisquama on the rotifer Brachionus plicatilis. Biosci. Biotechnol. Biochem. 2000, 64, 2719–2722. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.S.; Sato, Y.; Kim, D.; Muramatsu, T.; Matsuyama, Y.; Honjo, T. Hemolytic activity of Heterocapsa circularisquama (Dinophyceae) and its possible involvement in shellfish toxicity. J. Phycol. 2001, 37, 509–516. [Google Scholar] [CrossRef]
- Cardwell, R.D.; Olsen, S.; Carr, M.I.; Sanborn, E.W. Causes of Oyster Mortality in South Puget Sound; Washington Department of Fisheries, Salmon Research and Development: Brinnan: Washington, DC, USA, 1979. [Google Scholar]
- Tindall, D.R.; Dickey, R.W.; Carlson, R.D.; Morey-Gaines, G. Ciguatoxigenic dinoflagellates from the Caribbean Sea. In Seafood Toxins; Ragelis, E.P., Ed.; American Chemical Society Symposium Series: Washington, DC, USA, 1984; pp. 225–240. [Google Scholar]
- Marshall, S.M.; Orr, A.P. On the biology of Calanus finmarchicus. VIII. Food uptake, assimilation and excretion in adult and stage V Calanus. J. Mar. Biol. Assoc. UK 1953, 34, 495–529. [Google Scholar] [CrossRef]
- Abbott, B.C.; Ballantine, D. The toxin from Gymnodinium veneficum Ballantine. J. Mar. Biol. Assoc. UK 1957, 36, 169–189. [Google Scholar] [CrossRef]
- Schantz, E.J.; Lynch, J.M.; Vayvada, G.; Matsumoto, K.; Rapoport, H. The purification and characterization of the poison produced by Gonyaulax catenella in axenic culture. Biochemistry 1966, 5, 1191–1195. [Google Scholar] [CrossRef]
- Proctor, N.H.; Chan, S.L.; Trevor, A.J. Production of saxitoxin by cultures of Gonyaulax catenella. Toxicon 1975, 13, 1–9. [Google Scholar] [CrossRef]
- Onoue, P.; Noguchi, T.; Hashimoto, K. Studies on paralytic shellfish poison from the oyster cultured in Senzaki Bay, Yamaguchi Prefecture. Nippon Suisan Gakkaishi 1980, 46, 1031–1034. [Google Scholar] [CrossRef]
- Onoue, P.; Noguchi, T.; Maruyama, J.; Hashimoto, K.; Ikeda, T. New toxins separated from oysters and Protogonyaulax catenella from Senzaki Bay, Yamaguchi Prefecture. Nippon Suisan Gakkaishi 1981, 47, 1643. [Google Scholar] [CrossRef]
- Onoue, P.; Noguchi, T.; Maruyama, J.; Uneda, Y.; Hashimoto, K.; Ikeda, T. Comparison of PSP compositions between toxic oysters and Protogonyaulax catenella from Senzaki Bay, Yamaguchi Prefecture. Nippon Suisan Gakkaishi 1981, 47, 1347–1350. [Google Scholar] [CrossRef]
- Boyer, G.L.; Sullivan, J.J.; Andersen, R.J.; Harrison, P.J.; Taylor, F.J.R. Toxin production in three isolates of Protogonyaulax sp. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: New York, NY, USA, 1985; pp. 281–286. [Google Scholar]
- Hallegraeff, G.M.; Bolch, C.J.; Blackburn, S.I.; Oshima, Y. Species of the toxigenic dinoflagellate genus Alexandrium in southeastern Australian waters. Bot. Mar. 1991, 34, 575–587. [Google Scholar] [CrossRef]
- Nakazima, M. Studies on the source of shellfish poison in Lake Hamana. I. Relation of the abundance of a species of the dinoflagellate, Prorocentrum sp. to shellfish toxicity. Nippon Suisan Gakkaishi 1965, 31, 198–203. [Google Scholar] [CrossRef]
- Nakazima, M. Studies on the source of shellfish poison in Lake Hamana. II. Shellfish toxicity during the “Red-Tide”. Nippon Suisan Gakkaishi 1965, 31, 204–207. [Google Scholar] [CrossRef]
- Nakazima, M. Studies on the source of shellfish poison in Lake Hamana. III. Poisonous effects of shellfishes feeding on Prorocentrum sp. Nippon Suisan Gakkaishi 1965, 31, 281–285. [Google Scholar] [CrossRef]
- Nakazima, M. Studies on the source of shellfish poison in Lake Hamana. IV. Identification and collection of the noxious dinoflagellate. Nippon Suisan Gakkaishi 1968, 34, 130–131. [Google Scholar] [CrossRef]
- Okaichi, T.; Imatomi, Y. Toxicity of Prorocentrum minimum var. mariae-lebouriae assumed to be a causative agent of short-necked clam poisoning. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.H., Eds.; Elsevier: New York, NY, USA, 1979; pp. 385–388. [Google Scholar]
- Andersen, R.J.; LeBlanc, J.J.; Sum, F.W. 1–(2,6,6–trimethyl-4–hydroxy-cyclo-hexenyl)-1,3–butanedione, an extracellular metabolite from Prorocentrum minimum. J. Org. Chem. 1980, 45, 1169–1170. [Google Scholar] [CrossRef]
- Trick, C.G.; Andersen, R.J.; Gillam, A.; Harrison, J.P. Prorocentrin: An extracellular siderophore produced by the marine dinoflagellate Prorocentrum minimum. Science 1983, 219, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Luckenbach, M.W.; Sellner, K.G.; Shumway, S.E.; Greene, K. Effects of two bloom-forming dinoflagellates, Prorocentrum minimum and Gyrodinium uncatenatum, on the growth and survival of the eastern oyster (Gmelin, 1791). J. Shellfish Res. 1993, 12, 411–415. [Google Scholar]
- Wikfors, G.H.; Smolowitz, R.M. Detrimental effects of a Prorocentrum isolate upon hard clams and bay scallops in laboratory feeding studies. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 447–452. [Google Scholar]
- Wikfors, G.H.; Smolowitz, R.M. Experimental and histological studies of four life-history stages of the eastern oyster, Crassostrea virginica exposed to a cultured strain of the dinoflagellate Prorocentrum minimum. Biol. Bull. 1995, 188, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Wikfors, G.H.; Smolowitz, R.M.; Smith, B.C. Effects of a Prorocentrum isolate upon the oyster, Crassostrea virginica: A study of three life-history stages. J. Shellfish Res. 1993, 12, 114–115. [Google Scholar]
- Grzebyk, D.; Denardou, A.; Berland, B.; Pouchus, Y.F. Evidence of a new toxin in the red-tide dinoflagellate Prorocentrum minimum. J. Plankton Res. 1997, 19, 1111–1114. [Google Scholar] [CrossRef]
- Denardou-Quenehervea, A.; Grzebyk, D.; Pouchus, Y.-F.; Sauviat, M.P.; Alliot, E.; Biard, J.-F.; Berland, B.; Verbist, J.-F. Toxicity of French strains of the dinoflagellate Prorocentrum minimum experimental and natural contaminations of mussels. Toxicon 1999, 37, 1711–1719. [Google Scholar] [CrossRef]
- Shilo, M. The toxic principles of Prymnesium parvum. In The Water Environment: Algal Toxins and Health; Carmichael, W.W., Ed.; Plenum Press: New York, NY, USA, 1981; pp. 37–47. [Google Scholar]
- Kozakai, H.; Oshima, Y.; Yasumoto, T. Isolation and structural elucidation of hemolysin from the phytoflagellate Prymnesium parvum. Agric. Biol. Chem. 1982, 46, 233–236. [Google Scholar] [CrossRef]
- Igarashi, T.; Oshima, Y.; Murata, M.; Yasumoto, T. Chemical studies on prymnesins isolated from Prymnesium parvum. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard-Le-Denn, E., Gentien, P., Marcaillou-Le-Baut, C., Eds.; Lavoisier: Paris, France, 1995; pp. 303–308. [Google Scholar]
- Igarashi, T.; Satake, M.; Yasumoto, T. Prymnesin-2: A potent ichthyotoxic and hemolytic glycoside isolated from the red tide alga Prymnesium parvum. J. Am. Chem. Soc. 1996, 118, 479–480. [Google Scholar] [CrossRef]
- Meldahl, A.-S.; Edvardsen, B.; Fonnum, F. Toxicity of four potentially ichthyotoxic marine phytoflagellates determined by four different test methods. J. Toxicol. Environ. Health 1994, 42, 289–301. [Google Scholar] [CrossRef]
- Moestrup, Ø. Economic aspects: ‘Blooms’, nuisance species, and toxins. In The Haptophyte Algae; Green, J.C., Leadbeater, B.S.C., Eds.; University Press: New York, NY, USA, 1994; pp. 265–285. [Google Scholar]
- Shaw, B.A.; Andersen, R.J.; Harrison, P.J. Feeding deterrence properties of apo-fucoxanthinoids from marine diatoms. I. Chemical structures of apo-fucoxanthinoids produced by Phaeodactylum tricornutum. Mar. Biol. 1995, 124, 467–472. [Google Scholar] [CrossRef]
- Shaw, B.A.; Andersen, R.J.; Harrison, P.J. Feeding deterrence properties of apo-fucoxanthinoids from marine diatoms. II. Physiology of production of apo-fucoxanthinoids by the marine diatoms Phaeodactylum tricornutum and Thalassiosira pseudonana, and their feeding deterrent effects on the copepod Tigriopus californicus. Mar. Biol. 1995, 124, 473–481. [Google Scholar]
- Hansel, C.M.; Buchwald, C.; Diaz, J.M.; Ossolinski, J.E.; Dyhrman, S.T.; Van Mooy, B.A.S.; Polyviou, D. Dynamics of extracellular superoxide production by Trichodesmium colonies from the Sargasso Sea. Limnol. Oceanogr. 2016, 61, 1188–1200. [Google Scholar] [CrossRef]
- Houdan, A.; Bonnard, A.; Fresnel, J.; Fouchard, S.; Billard, C.; Probert, I. Toxicity of coastal coccolithophores (Prymnesiophyceae, Haptophyta). J. Plankton Res. 2004, 26, 875–883. [Google Scholar] [CrossRef]
- Saragosti, E.; Tchernov, D.; Katsir, A.; Shaked, Y. Extracellular production and degradation of superoxide in the coral Stylophora pistillata and cultured Symbiodinium. PLoS ONE 2010, 5, e12508. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, V.D.; Krishnamurthy, S. The antibacterial properties of marine blue-green alga Trichodesmium erythraeum. Curr. Sci. 1967, 19, 524–525. [Google Scholar]
- Ramamurthy, V.D. Antibacterial activity of the marine blue-green alga Trichodesmium erythraeum in the gastro-intestinal contents of the sea gull Larus brunicephalus. Mar. Biol. 1970, 6, 74–76. [Google Scholar] [CrossRef]
- Hahn, S.T.; Capra, M.F. The cyanobacterium Oscillatoria erythraea—A potential source of toxin in the ciguatera food chain. Food Addit. Contam. 1992, 9, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Endean, R.; Griffith, J.K.; Robbins, J.J.; Monks, S.A. Multiple toxins in a specimen of the narrow-barred Spanish mackerel, Scomberomorus commersoni. Toxicon 1993, 31, 195–204. [Google Scholar] [CrossRef]
- Pinto, J.S.; Silva, E.S. The toxicity of Cardium edulum L. and its possible relation to the dinoflagellate Prorocentrum micans Ehr. Notas Estudos Instituto Biologia Maritima 1956, 12, 1–20. [Google Scholar]
- Uchida, T. Excretion of a diatom inhibiting substance by Prorocentrum micans Ehrenberg. Jpn. J. Ecol. 1977, 27, 1–4. [Google Scholar]
- Lembeye, G.; Campodonico, I. First recorded bloom of the dinoflagellate Prorocentrum micans Her in South-Central Chile. Bot. Mar. 1984, 27, 491–493. [Google Scholar]
- Clement, A.; Lembeye, G. Phytoplankton monitoring program in the fish farming region of south Chile. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 223–228. [Google Scholar]
- Oda, T.; Akaike, T.; Sato, K.; Ishimatsu, A.; Takeshita, S.; Muramatsu, T.; Maeda, H. Hydroxyl radical generation by red tide algae. Arch. Biochem. Biophys. 1992, 294, 38–43. [Google Scholar] [CrossRef]
- Oda, T.; Ishimatsu, A.; Shimada, M.; Takeshita, S.; Muramatsu, T. Oxygen-radical-mediated toxic effects of the red tide flagellate Chattonella marina on Vibrio alginolyticus. Mar. Biol. 1992, 112, 505–509. [Google Scholar] [CrossRef]
- Oda, T.; Ishimatsu, A.; Takeshita, S.; Muramatsu, T. Hydrogen peroxide production by the red-tide flagellate Chattonella marina. Biosci. Biotechnol. Biochem. 1994, 58, 957–958. [Google Scholar] [CrossRef]
- Kim, D.; Nakashima, T.; Matsuyama, Y.; Niwano, Y.; Yamaguchi, K.; Oda, T. Presence of the distinct systems responsible for superoxide anion and hydrogen peroxide generation in red tide phytoplankton Chattonella marina and Chattonella ovata. J. Plankton Res. 2007, 29, 241–247. [Google Scholar] [CrossRef]
- Shimada, M.; Kawamoto, S.; Nakatsuka, Y.; Watanabe, M. Localization of superoxide anion in the red tide alga Chattonella antiqua. J. Histochem. Cytochem. 1993, 41, 507–511. [Google Scholar] [CrossRef]
- Cho, K.; Sakamoto, J.; Noda, T.; Nishiguchi, T.; Ueno, M.; Yamasaki, Y.; Yagi, M.; Kim, D.; Oda, T. Comparative studies on the fish-killing activities of Chattonella marina isolated in 1985 and Chattonella antiqua isolated in 2010, and their possible toxic factors. Biosci. Biotechnol. Biochem. 2016, 80, 811–817. [Google Scholar] [CrossRef]
- Mahean Haque, S.; Onoue, Y. Variation in toxin compositions of two harmful raphidophytes, Chattonella antiqua and Chattonella marina, at different salinities. Environ. Toxicol. 2002, 17, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Nichols, P.D.; Hamilton, B.; Lewis, R.J.; Hallegraeff, G.M. Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): The synergistic role of reactive oxygen species and free fatty acids. Harmful Algae 2003, 2, 273–281. [Google Scholar] [CrossRef]
- Endo, M.; Foscarini, R.; Kuroki, A. Electrocardiogram of a marine fish, Pagrus major, exposed to red tide plankton, Chattonella marina. Mar. Biol. 1988, 97, 477–481. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Maruta, H.; Tsuchiyama, T.; Ozaki, M. Respiratory, ionoregulatory and cardiovascular responses of the yellowtail Seriola quinqueradiata to exposure to the red tide plankton Chattonella. Nippon Suisan Gakkaishi 1990, 56, 189–199. [Google Scholar] [CrossRef][Green Version]
- Ishimatsu, A.; Sameshima, M.; Tamura, A.; Oda, T. Histological analysis of the mechanisms of Chattonella-induced hypoxemia in yellowtail. Fish. Sci. 1996, 62, 50–58. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Maruta, H.; Oda, T.; Ozaki, M. A comparison of physiological responses in yellowtail to fatal environmental hypoxia and exposure to Chattonella marina. Fish. Sci. 1997, 63, 557–562. [Google Scholar] [CrossRef]
- Matsusato, T.; Kobayashi, H. Studies on death of fish caused by red tide. Bull. Nansei Reg. Fish. Res. Lab. 1974, 7, 43–67. [Google Scholar]
- Ishimatsu, A.; Oda, T.; Yoshida, M.; Ozaki, M. Oxygen radicals are probably involved in the mortality of yellowtail by Chattonella marina. Fish. Sci. 1996, 62, 836–837. [Google Scholar] [CrossRef]
- Marshall, J.A.; de Salas, M.; Oda, T.; Hallegraeff, G. Superoxide production by marine microalgae: I. Survey of 37 species from 6 classes. Mar. Biol. 2005, 147, 533–540. [Google Scholar] [CrossRef]
- Portune, K.J.; Cary, S.C.; Warner, M.E. Antioxidant enzyme response and reactive oxygen species production in marine raphidophytes. J. Phycol. 2010, 46, 1161–1171. [Google Scholar] [CrossRef]
- Mooney, B.D.; Dorantes-Aranda, J.J.; Place, A.R.; Hallegraeff, G.M. Ichthyotoxicity of gymnodinioid dinoflagellates: PUFA and superoxide effects in sheepshead minnow larvae and rainbow trout gill cells. Mar. Ecol. Prog. Ser. 2011, 426, 213–224. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; Seger, A.; Mardones, J.I.; Nichols, P.D.; Hallegraeff, G.M. Progress in understanding algal bloom-mediated fish kills: The role of superoxide radicals, phycotoxins and fatty acids. PLoS ONE 2015, 10, e0133549. [Google Scholar] [CrossRef]
- Mardones, J.I.; Dorantes-Aranda, J.J.; Nichols, P.D.; Hallegraeff, G.M. Fish gill damage by the dinoflagellate Alexandrium catenella from Chilean fjords: Synergistic action of ROS and PUFA. Harmful Algae 2015, 49, 40–49. [Google Scholar] [CrossRef]
- Kim, D.; Oda, T.; Muramatsu, T.; Kim, D.; Matsuyama, Y.; Honjo, T. Possible factors responsible for the toxicity of Cochlodinium polykrikoides, a red tide phytoplankton. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 415–423. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. Cochlodinium polykrikoides blooms and clonal isolates from the northwest Atlantic coast cause rapid mortality in larvae of multiple bivalve species. Mar. Biol. 2009, 156, 2601–2611. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Temperature controls the toxicity of the ichthyotoxic dinoflagellate Cochlodinium polykrikoides. Mar. Ecol. Prog. Ser. 2016, 545, 63–76. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Kim, D.I.; Matsuyama, Y.; Oda, T.; Honjo, T. Production of superoxide anion and hydrogen peroxide by the red tide dinoflagellate Karenia mikimotoi. J. Biosci. Bioeng. 2004, 97, 212–215. [Google Scholar] [CrossRef]
- Cho, K.; Kasaoka, T.; Ueno, M.; Basti, L.; Yamasaki, Y.; Kim, D.; Oda, T. Haemolytic activity and reactive oxygen species production of four harmful algal bloom species. Eur. J. Phycol. 2017, 52, 311–319. [Google Scholar] [CrossRef]
- Kim, D.; Wencheng, L.; Matsuyama, Y.; Cho, K.; Yamasaki, Y.; Takeshita, S.; Yamaguchi, K.; Oda, T. Extremely high level of reactive oxygen species (ROS) production in a newly isolated strain of the dinoflagellate Karenia mikimotoi. Eur. J. Phycol. 2019, 54, 632–640. [Google Scholar] [CrossRef]
- Diaz, J.M.; Plummer, S. Production of extracellular reactive oxygen species by phytoplankton: Past and future directions. J. Plankton Res. 2018, 40, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yamaguchi, K.; Oda, T. Nitric oxide synthase-like enzyme mediated nitric oxide generation by harmful red tide phytoplankton, Chattonella marina. J. Plankton Res. 2006, 28, 613–620. [Google Scholar] [CrossRef]
- Stamler, J.S. Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell 1994, 78, 931–936. [Google Scholar] [CrossRef]
- Lamattina, L.; García-Mata, C.; Graziano, M.; Pagnussat, G. Nitric oxide: The versatility of an extensive signal molecule. Annu. Rev. Plant Biol. 2003, 54, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Czymmek, K.J.; Shapiro, A.D. Nitric oxide does not trigger early programmed cell death events but may contribute to cell-to-cell signaling governing progression of the Arabidopsis hypersensitive response. Mol. Plant Microbe Interact. 2003, 16, 962–972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamamoto, A.; Katou, S.; Yoshioka, H.; Doke, N.; Kawakita, K. Involvement of nitric oxide generation in hypersensitive cell death induced by elicitin in tobacco cell suspension culture. J. Gen. Plant Pathol. 2004, 70, 85–92. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Gotoh, N.; Niki, E.; Yokoyama, K.; Tsuzuki, M.; Takeuchi, T.; Karube, I. Chemiluminescence detection of red tide phytoplankton Chattonella marina. Anal. Chem. 1995, 67, 225–228. [Google Scholar] [CrossRef]
- Oda, T.; Nakamura, A.; Okamoto, T.; Ishimatsu, A.; Muramatsu, T. Lectin-induced enhancement of superoxide anion production by red tide phytoplankton. Mar. Biol. 1998, 131, 383–390. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; Nichols, P.D.; Waite, T.D.; Hallegraeff, G.M. Strain variability in fatty acid composition of Chattonella marina (Raphidophyceae) and its relation to differing ichthyotoxicity toward rainbow trout gill cells. J. Phycol. 2013, 49, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, K.; Shikata, T.; Kitatsuji, S.; Yamasaki, Y.; Nishiyama, Y. Extracellular secretion of superoxide is regulated by photosynthetic electron transport in the noxious red-tide-forming raphidophyte Chattonella antiqua. J. Photochem. Photobiol. B Biol. 2020, 205, 111839. [Google Scholar] [CrossRef]
- Kim, D.; Yamasaki, Y.; Yamatogi, T.; Yamaguchi, K.; Matsuyama, Y.; Kang, Y.S.; Lee, Y.; Oda, T. The possibility of reactive oxygen species (ROS)-indipendent toxic effects of Cochlodinium polykrikoides on damselfish (Chromis caerulea). Biosci. Biotechnol. Biochem. 2009, 73, 613–618. [Google Scholar] [CrossRef]
- Sato, E.; Niwano, Y.; Matsuyama, Y.; Kim, D.; Nakashima, T.; Oda, T.; Kohno, M. Some dinophycean red tide plankton species generate a superoxide scavenging substance. Biosci. Biotechnol. Biochem. 2007, 71, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Kustka, A.B.; Shaked, Y.; Milligan, A.J.; King, D.W.; Morel, F.M. Extracellular production of superoxide by marine diatoms: Contrasting effects on iron redox chemistry and bioavailability. Limnol. Oceanogr. 2005, 50, 1172–1180. [Google Scholar] [CrossRef]
- Hirata, K.; Yoshitomi, S.; Dwi, S.; Iwabe, O.; Mahakant, A.; Polchai, J.; Miyamoto, K. Generation of reactive oxygen species undergoing redox cycle of nostocine A: A cytotoxic violet pigment produced by freshwater cyanobacterium Nostoc spongiaeforme. J. Biotechnol. 2004, 110, 29–35. [Google Scholar] [CrossRef]
- Kim, D.; Oda, T. Possible factors responsible for the fish-killing mechanisms of the red tide phytoplankton, Chattonella marina and Cochlodinium polykrikoides. Coast. Environ. Ecosyst. Issues East China Sea 2010, 245–268. [Google Scholar]
- Godrant, A.; Rose, A.L.; Sarthou, G.; Waite, T.D. New method for the determination of extracellular production of superoxide by marine phytoplankton using the chemiluminescence probes MCLA and red-CLA. Limnol. Oceanogr. Methods 2009, 7, 682–692. [Google Scholar] [CrossRef]
- Hyslop, P.A.; Sklar, L.A. A quantitative fluorimetric assay for the determination of oxidant production by polymorphonuclear leukocytes: Its use in the simultaneous fluorimetric assay of cellular activation processes. Anal. Biochem. 1984, 141, 280–286. [Google Scholar] [CrossRef]
- Nathan, C.F.; Root, R.K. Hydrogen peroxide release from mouse peritoneal macrophages: Dependence on sequential activation and triggering. J. Exp. Med. 1977, 146, 1648–1662. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement ofhydr ogen peroxide produced by cells in culture. J. Immunol. Meth. 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Kim, D.; Oda, T. Production of nitric oxide by marine unicellular red tide phytoplankton, Chattonella marina. In Nitric Oxide in Plants: Metabolism and Role in Stress Physiolog; Springer: Cham, Switzerland, 2014; pp. 75–84. [Google Scholar]
- Kim, D.; Kang, Y.S.; Lee, Y.; Yamaguchi, K.; Matsuoka, K.; Lee, K.W.; Choi, K.S.; Oda, T. Detection of nitric oxide (NO) in marine phytoplankters. J. Biosci. Bioeng. 2008, 105, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Shimono, R.; Murakami, T.H.; Yoshimatsu, S.; Ono, C. Red tide, Chattonella antiqua reduces cytochrome c from horse heart. In Red tides: Biology, Environmental Science, and Toxicology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier: New York, NY, USA, 1989; pp. 443–446. [Google Scholar]
- Vrieling, E.G.; Koeman, R.P.T.; Nagasaki, K.; Ishida, Y.; Pererzak, L.; Gieskes, W.W.C.; Veenhuis, M. Chattonella and Fibrocapsa (Raphidophyceae): First observation of, potentially harmful, red tide organisms in Dutch coastal waters. Neth. J. Sea Res. 1995, 33, 183–191. [Google Scholar] [CrossRef]
- Odebrecht, C.; Abreu, P.C. Raphidophycean in southern Brazil. Harmful Algae News 1995, 12, 4. [Google Scholar]
- Oda, T.; Moritomi, J.; Kawano, I.; Hamaguchi, S.; Ishimatsu, A.; Muramatsu, T. Catalase-and superoxide dismutase-induced morphological changes and growth inhibition in the red tide phytoplankton Chattonella marina. Biosci. Biotechnol. Biochem. 1995, 59, 2044–2048. [Google Scholar] [CrossRef]
- Kawano, I.; Oda, T.; Ishimatsu, A.; Muramatsu, T. Inhibitory effect of the iron chelator Desferrioxamine (Desferal) on the generation of activated oxygen species by Chattonella marina. Mar. Biol. 1996, 126, 765–771. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Khan, S.; Arakawa, O.; Onoue, Y. Properties of hemagglutinins newly separated from toxic phytoplankton. Biochim. Biophys. Acta Gen. Subj. 1995, 1243, 509–512. [Google Scholar] [CrossRef]
- Furey, A.; Garcia, J.; O’Callaghan, K.; Lehane, M.; Amandi, M.J.; James, K.J. Brevetoxins: Structure, toxicology and origin. In Phycotoxins: Chemistry and Biochemistry; Botana, L.M., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 19–46. ISBN 9780813827001. [Google Scholar]
- Okaichi, T. Red tide problems in the Seto Inland Sea, Japan. In Red Tides, Biology, Environmental Science, and Toxicology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 137–142. [Google Scholar]
- Shikata, T.; Yuasa, K.; Kitatsuji, S.; Sakamoto, S.; Akita, K.; Fujinami, Y.; Nishiyama, Y.; Kotake, T.; Tanaka, R.; Yamasaki, Y. Superoxide production by the red tide-producing Chattonella marina complex (Raphidophyceae) correlates with toxicity to aquacultured fishes. Antioxidants 2021, 10, 1635. [Google Scholar] [CrossRef]
- Rose, A.L.; Salmon, T.P.; Lukondeh, T.; Neilan, B.A.; Waite, T.D. Use of superoxide as an electron shuttle for iron acquisition by the marine cyanobacterium Lyngbya majuscula. Environ. Sci. Technol. 2005, 39, 3708–3715. [Google Scholar] [CrossRef]
- Rose, A.L.; Moffett, J.W.; Waite, T.D. Determination of superoxide in seawater using 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo[1,2-a]pyrazin-3(7H)-one chemiluminescence. Anal. Chem. 2008, 80, 1215–1227. [Google Scholar] [CrossRef]
- Fujii, M.; Dang, T.C.; Rose, A.L.; Omura, T.; Waite, T.D. Effect of light on iron uptake by the freshwater cyanobacterium Microcystis aeruginosa. Environ. Sci. Technol. 2011, 45, 1391–1398. [Google Scholar] [CrossRef]
- Aquino-Cruz, A.; Band-Schmidt, C.J.; Zenteno-Savín, T. Superoxide production rates and hemolytic activity linked to cellular growth phases in Chattonella species (Raphidophyceae) and Margalefidinium polykrikoides (Dinophyceae). J. Appl. Phycol. 2020, 32, 4029–4046. [Google Scholar] [CrossRef]
- Nakamura, A.; Okamoto, T.; Komatsu, N.; Ooka, S.; Oda, T.; Ishimatsu, A.; Muramatsu, T. Fish mucus stimurates the generation of superoxide anion by Chattonella marina and Heterosigma akashiwo. Fish. Sci. 1998, 64, 866–869. [Google Scholar] [CrossRef]
- Kim, D.; Watanabe, M.; Nakayasu, Y.; Kohata, K. Production of superoxide anion and hydrogen peroxide associated with cell growth of Chattonella antiqua. Aquat. Microb. Ecol. 2004, 35, 57–64. [Google Scholar] [CrossRef]
- Milne, A.; Davey, M.S.; Worsfold, P.J.; Achterberg, E.P.; Taylor, A.R. Real-time detection of reactive oxygen species generation by marine phytoplankton using flow injection—Chemiluminescence. Limnol. Oceanogr. Methods 2009, 7, 706–715. [Google Scholar] [CrossRef]
- Diaz, J.M.; Plummer, S.; Hansel, C.M.; Andeer, P.F.; Saito, M.A.; McIlvin, M.R. NADPH-dependent extracellular superoxide production is vital to photophysiology in the marine diatom Thalassiosira oceanica. Proc. Natl. Acad. Sci. USA 2019, 116, 16448–16453. [Google Scholar] [CrossRef] [PubMed]
- Palenik, B.; Zafiriou, O.C.; Morel, F.M. Hydrogen peroxide production by a marine phytoplankter 1. Limnol. Oceanogr. 1987, 32, 1365–1369. [Google Scholar] [CrossRef]
- Zhang, T.; Diaz, J.M.; Brighi, C.; Parsons, R.J.; McNally, S.; Apprill, A.; Hansel, C.M. Dark production of extracellular superoxide by the coral Porites astreoides and representative symbionts. Front. Mar. Sci. 2016, 3, 232. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Akaike, T.; Hamamoto, T.; Suzuki, F.; Hirano, T.; Maeda, H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science 1989, 244, 974–976. [Google Scholar] [CrossRef]
- Kim, D.; Oda, T.; Ishimatsu, A.; Muramatsu, T. Isolation and characterization of a mutant strain of Chattonella marina with decreased production of superoxide anion. Biosci. Biotechnol. Biochem. 1999, 63, 1947–1952. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Oda, T. Toxic effects of harmful algal blooms on finfish and shellfish. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 543–560. [Google Scholar]
- Lee, K.; Ishimatsu, A.; Sakaguchi, H.; Oda, T. Cardiac output during exposure to Chattonella marina and environmental hypoxia in yellowtail (Seriola quinqueradiata). Mar. Biol. 2003, 142, 391–397. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Tsuchiyama, M.; Yoshida, M.; Sameshima, M.; Pawluk, M.; Oda, T. Effect of Chattonella exposure on acid-base status of the yellowtail. Nippon Suisan Gakkaishi 1991, 57, 2115–2120. [Google Scholar] [CrossRef]
- Tsuchiyam, T.; Ishimatsu, A.; Oda, T.; Uchida, S.; Ozaki, M. Effect of Chattonella exposure on plasma catecholamine levels in the yellowtail. Nippon Suisan Gakkaishi 1992, 58, 207–211. [Google Scholar] [CrossRef]
- Hishida, Y.; Ishimatsu, A.; Oda, T. Mucus blockade of lamellar water channels in yellowtail exposed to Chattonella marina. Fish. Sci. 1997, 63, 315–316. [Google Scholar] [CrossRef]
- Kim, D.; Okamoto, T.; Oda, T.; Tachibana, K.; Lee, K.S.; Ishimatsu, A.; Matsuyama, Y.; Muramatsu, T. Possible involvement of the glycocalyx in the ichthyotoxicity of Chattonella marina (Raphidophyceae): Immunological approach using antiserum against cell surface structures of the flagellate. Mar. Biol. 2001, 139, 625–632. [Google Scholar]
- Lee, K.S.; Ishimatsu, A.; Oda, T. Direct evidence for the reduction of water flow across the gills of jack mackerel (Trachurus japonicus) by a harmful alga Chattonella marina. Fish. Aquat. Sci. 2004, 7, 96–98. [Google Scholar] [CrossRef]
- Hale, W.B.; Turner, B.; LaMont, J.T. Oxygen radicals stimulate guinea pig gallbladder glycoprotein secretion in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 1987, 253, G627–G630. [Google Scholar] [CrossRef]
- LaMont, J.T.; Chantler, E. Oxygen radicals stimulate gallbladder glycoprotein secretion. In Mucus and Related Topics; Chantler, E.N., Ratcliffe, N.A., Eds.; The Company of Biologists Ltd: London, UK, 1989; pp. 273–278. [Google Scholar]
- Hiraishi, H.; Terano, A.; Ota, S.; Mutoh, H.; Sugimoto, T.; Razandi, M.; Ivey, K.J. Oxygen metabolites stimulate mucous glycoprotein secretion from cultured rat gastric mucous cells. Am. J. Physiol. 1991, 261, G662–G668. [Google Scholar] [CrossRef] [PubMed]
- Adler, K.B.; Holden-Stauffer, W.J.; Repine, J.E. Oxygen metabolites stimulate release of high-molecular-weight glycoconjugates by cell and organ cultures of rodent respiratory epithelium via an arachidonic acid-dependent mechanism. J. Clin. Investig. 1990, 85, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.T.; Fischer, B.M.; Li, C.; Rochelle, L.G.; Akley, N.J.; Adler, K.B. Oxidant stress stimulates mucin secretion and PLC in airway epithelium via a nitric oxide-dependent mechanism. Am. J. Physiol.—Lung Cell. 1996, 271, L854–L861. [Google Scholar] [CrossRef] [PubMed]
- Shephard, K.L. Functions for fish mucus. Rev. Fish Biol. Fish. 1994, 4, 401–429. [Google Scholar] [CrossRef]
- Holmgren, S.; Olsson, C. Autonomic control of glands and secretion: A comparative view. Auton. Neurosci. 2011, 165, 102–112. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Imlay, J.A. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of Gram-negative bacteria. Mol. Microbiol. 2002, 43, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef]
- Simpson, J.A.; Cheeseman, K.H.; Smith, S.E.; Dean, R.T. Free-radical generation by copper ions and hydrogen peroxide. Stimulation by Hepes buffer. Biochem. J. 1988, 254, 519–523. [Google Scholar] [CrossRef]
- Sunda, W.G.; Swift, D.G.; Huntsman, S.A. Low iron requirement for growth in oceanic phytoplankton. Nature 1991, 351, 55–57. [Google Scholar] [CrossRef]
- Brewer, P.G. Minor elements in sea water. In Chemical Oceanography; Riley, J.P., Skirrow, G., Eds.; Academic Press: New York, NY, USA, 1975; pp. 416–490. [Google Scholar]
- Tang, J.Y.; Anderson, D.M.; Au, D.W. Hydrogen peroxide is not the cause of fish kills associated with Chattonella marina: Cytological and physiological evidence. Aquat. Toxicol. 2005, 72, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Oxygen toxicity: A radical explanation. J. Exp. Biol. 1998, 201, 1203–1209. [Google Scholar] [CrossRef]
- Liochev, S.I.; Fridovich, I. The Haber-Weiss cycle—70 years later: An alternative view. Redox Rep. 2002, 7, 55–57. [Google Scholar] [CrossRef]
- Kim, K.C.; McCracken, K.; Lee, B.; Shin, C.Y.; Jo, M.J.; Lee, C.J.; Ko, K.H. Airway goblet cell mucin: Its structure and regulation of secretion. Eur. Respir. J. 1997, 10, 2644–2649. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W. The electron transport chain of the microbicidal oxidase of phagocytic cells and its involvement in the molecular pathology of chronic granulomatous disease. J. Clin. Investig. 1989, 83, 1785–1793. [Google Scholar] [CrossRef][Green Version]
- Vianello, A.; Macri, F. Generation of superoxide anion and hydrogen peroxide at the surface of plant cells. J. Bioenerg. Biomembr. 1991, 23, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Yokote, M.; Honjo, T. Morphological and histochemical demonstration of a glycocalyx on the cell surface of Chattonella antiqua, a ‘naked flagellate’. Experientia 1985, 41, 1143–1145. [Google Scholar] [CrossRef]
- Yokote, M.; Honjo, T.; Asakawa, M. Histochemical demonstration of a glycocalyx on the cell surface of Heterosigma akashiwo. Mar. Biol. 1985, 88, 295–299. [Google Scholar] [CrossRef]
- Okamoto, T.; Kim, D.; Oda, T.; Matsuoka, K.; Ishimatsu, A.; Muramatsu, T. Concanavalin A-induced discharge of the glycocalyx of raphidophycean flagellates, Chattonella marina and Heterosigma akashiwo. Biosci. Biotechnol. Biochem. 2000, 64, 1767–1770. [Google Scholar] [CrossRef]
- Suh, Y.A.; Arnold, R.S.; Lassegue, B.; Shi, J.; Xu, X.; Sorescu, D.; Chung, A.B.; Griendling, K.K.; Lambeth, J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999, 401, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Kopp, J.B.; Várnai, P.; Leto, T.L. Identification of renox, an NAD (P) H oxidase in kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 8010–8014. [Google Scholar] [CrossRef]
- Deken, X.D.; Wang, D.; Many, M.-C.; Costagliola, S.; Libert, F.; Vassart, G.; Dumont, J.E.; Miot, F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000, 275, 23227–23233. [Google Scholar] [CrossRef]
- Torres, M.A.; Onouchi, H.; Hamada, S.; Machida, C.; Hammond-Kosack, K.E.; Jones, J.D. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J. 1998, 14, 365–370. [Google Scholar] [CrossRef]
- Shikata, T.; Takahashi, F.; Nishide, H.; Shigenobu, S.; Kamei, Y.; Sakamoto, S.; Yuasa, K.; Nishiyama, Y.; Yamasaki, Y.; Uchiyama, I. RNA-seq analysis reveals genes related to photoreception, nutrient uptake, and toxicity in a noxious red-tide raphidophyte Chattonella antiqua. Front. Microbiol. 2019, 10, 1764. [Google Scholar] [CrossRef]
- Tang, L.B. Neuroprotection by glucose-6-phosphate dehydrogenase and the pentose phosphate pathway. J. Cell. Biochem. 2019, 120, 14285–14295. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Dietz, K.J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef]
- Marshall, J.A.; Hovenden, M.; Oda, T.; Hallegraeff, M.G. Photosynthesis does influence superoxide production in the ichthyotoxic alga Chattonella marina (Raphidophyceae). J. Plankton Res. 2002, 24, 1231–1236. [Google Scholar] [CrossRef]
- Yuasa, K.; Shikata, T.; Ichikawa, T.; Tamura, Y.; Nishiyama, Y. Nutrient deficiency stimulates the production of superoxide in the noxious red-tide-forming raphidophyte Chattonella antiqua. Harmful Algae 2020, 99, 101938. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.B.; Ingram, G.A. Noncellular nonspecific defence mechanisms of fish. Annu. Rev. Fish Dis. 1992, 2, 249–279. [Google Scholar] [CrossRef]
- Ingram, G.A. Substances involved in the natural resistance of fish to infection—A review. J. Fish. Biol. 1980, 16, 23–60. [Google Scholar] [CrossRef]
- Fletcher, T.C. The identification of nonspecific humoral factors in the plaice (Pleuronectes platessa L). Dev. Biol. Stand. 1981, 49, 321–327. [Google Scholar]
- Imai, I.; Yamaguchi, M.; Watanabe, M. Ecophysiology, life cycle, and bloom dynamics of Chattonella in the Seto Inland Sea, Japan. In NATO ASI Series, Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; Volume G41, pp. 95–112. [Google Scholar]
- Matsuoka, K.; Iwataki, M. Present status in study on a harmful unarmored dinoflagellate Cochlodinium polykrikoides Margalef. Bull. Plankton Soc. Jpn. 2004, 51, 38–45. [Google Scholar]
- Whyte, J.N.C.; Haigh, N.; Ginther, N.G.; Keddy, L.J. First record of blooms of Cochlodinium sp. (Gymnodiniales, Dinophyceae) causing mortality to aquacultured salmon on the west coast of Canada. Phycologia 2001, 40, 298–304. [Google Scholar] [CrossRef]
- Kudela, R.M.; Gobler, C.J. Harmful dinoflagellate blooms caused by Cochlodinium sp: Global expansion and ecological strategies facilitating bloom formation. Harmful Algae 2012, 14, 71–86. [Google Scholar] [CrossRef]
- Yamatogi, T.; Sakamoto, S.; Yamaguchi, M.; Murata, K.; Sakurada, K.; Takano, Y.; Matsuoka, K. Geographical distribution and growth characteristics of a harmful unarmored dinoflagellate Cochlodinium sp. type–Kasasa in west Kyushu, Japan. Bull. Jpn. Soc. Phycol. 2010, 58, 167–172. [Google Scholar]
- Mulholland, M.R.; Morse, R.E.; Boneillo, G.E.; Bernhardt, P.W.; Filippino, K.C.; Procise, L.A.; Blanco-Garcia, J.L.; Marshall, H.G.; Egerton, T.A.; Hunley, W.S.; et al. Understanding causes and impacts of the dinoflagellate, Cochlodinium polykrikoides blooms in the Chesapeake Bay. Estuar. Coasts 2009, 32, 734–747. [Google Scholar] [CrossRef]
- Richlen, M.L.; Morton, S.L.; Jamali, E.A.; Rajan, A.; Anderson, D.M. The catastrophic 2008–2009 red tide in the Arabian Gulf Region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 2010, 9, 163–172. [Google Scholar] [CrossRef]
- Gobler, C.J.; Berry, D.L.; Anderson, O.R.; Burson, A.; Koch, F.; Rodgers, B.S.; Moore, L.K.; Goleski, J.A.; Allam, B.; Bowser, P.; et al. Characterization, dynamics, and ecological impacts of harmful Cochlodinium polykrikoides blooms on eastern Long Island, NY, USA. Harmful Algae 2008, 7, 293–307. [Google Scholar] [CrossRef]
- Kim, H.G. Cochlodinium polykrikoides blooms in Korean coastal waters and their mitigation. In Harmful Algae; Reguera, B., Blanco, J., Fernandez, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela, Spain, 1998; pp. 227–228. [Google Scholar]
- Gárate-Lizárraga, I.; López-Cortes, D.J.; Bustillos-Guzmán, J.J.; Hernández-Sandoval, F. Blooms of Cochlodinium polykrikoides (Gymnodiniaceae) in the Gulf of California, Mexico. Rev. Biol. Trop. 2004, 52, 51–58. [Google Scholar]
- Kim, C.S.; Lee, S.G.; Kim, H.G. Biochemical responses of fish exposed to a harmful dinoflagellate Cochlodinium polykrikoides. J. Exp. Mar. Biol. Ecol. 2000, 254, 131–141. [Google Scholar] [CrossRef]
- Shin, Y.K.; Nam, S.-E.; Kim, W.J.; Seo, D.Y.; Kim, Y.-J.; Rhee, J.-S. Red tide dinoflagellate Cochlodinium polykrikoides induces significant oxidative stress and DNA damage in the gill tissue of the red seabream Pagrus major. Harmful Algae 2019, 86, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, T.; Lin, J.; Yu, R.; Zhou, M. Detrimental impacts of the dinoflagellate Karenia mikimotoi in Fujian coastal waters on typical marine organisms. Harmful Algae 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Koizumi, Y.; Uchida, T.; Honjo, T. Diurnal vertical migration of Gymnodinium mikimotoi during a red tide in Hoketsu Bay, Japan. J. Plankton Res. 1996, 18, 289–294. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Honjo, T. Effects of temperature, salinity and irradiance on the growth of the noxious red tide flagellate Gymnodinium nagasakiense (Dinophyceae). Nippon Suisan Gakkaishi 1989, 55, 2029–2036. [Google Scholar] [CrossRef]
- Lei, Q.Y.; Lu, S.H. Molecular ecological responses of the dinoflagellate Karenia mikimotoi to phosphate stress. Harmful Algae 2011, 12, 39–45. [Google Scholar] [CrossRef]
- Richardson, B.; Corcoran, A.A. Use of dissolved inorganic and organic phosphorus by axenic and nonaxenic clones of Karenia brevis and Karenia mikimotoi. Harmful Algae 2015, 48, 30–36. [Google Scholar] [CrossRef]
- Yoshimatsu, S. Long-term variation of phytoplankton in southern part of Harimanada. Bull. Plankton Soc. Jpn. 2008, 55, 41–44. [Google Scholar]
- Davidson, K.; Miller, P.; Wilding, T.A.; Shutler, J.; Bresnan, E.; Kennington, K.; Swan, S. A large and prolonged bloom of Karenia mikimotoi in Scottish waters in 2006. Harmful Algae 2009, 8, 349–361. [Google Scholar] [CrossRef]
- Lu, S.; Hodgkiss, I.J. Harmful algal bloom causative collected from Hong Kong waters. Hydrobiology 2004, 512, 231–238. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.D.; Song, S.Q. Selective feeding of Calanus sinicus on harmful algal blooms species in East China Sea in spring. J. Appl. Ecol. 2007, 18, 151–157. [Google Scholar]
- Yamaguchi, M. Physiological ecology of the red tide flagellate Gymnodinium nagasakiense (Dinophyceae). Mechanism of the red tide occurrence and its prediction. Bull. Nansei Natl. Fish. Res. Inst. 1994, 27, 251–394. [Google Scholar]
- Iizuka, S.; Irie, H. The hydrographic conditions and the fisheries damages by the red tide occurred in Omura Bay in summer 1965. Bull. Fac. Fish. Nagasaki Univ. 1966, 21, 67–101. [Google Scholar]
- Yasumoto, T.; Underdal, B.; Aune, T.; Hormazabal, V.; Skulberg, O.M.; Oshima, Y. Screening for hemolytic and ichthyotoxic components of Chrysochromulina polylepis and Gyrodinium aureolum from Norwegian coastal waters. In Toxic Marine Phytoplankton; Graneli, E., Sundstrom, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 436–440. [Google Scholar]
- Jenkinson, I.R.; Arzul, G. Mitigation by cysteine compounds of rheotoxicity, cytotoxicity and fish mortality caused by the dinoflagellates, Gymnodinium mikimotoi and G. cf. maguelonnense. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; IOC: Paris, France, 2001; pp. 71–84. [Google Scholar]
- Neely, T.; Campbell, L. A modified assay to determine hemolytic toxin variability among Karenia clones isolated from the Gulf of Mexico. Harmful Algae 2006, 5, 592–598. [Google Scholar] [CrossRef]
- Mooney, B.D.; Nichols, P.D.; De Salas, M.F.; Hallegraeff, G.M. Lipid, fatty acid, and sterol composition of eight species of Kareniaceae (Dinophyta): Chemotaxonomy and putative lipid phycotoxins. J. Phycol. 2007, 43, 101–111. [Google Scholar] [CrossRef]
- Satake, M.; Shoji, M.; Oshima, Y.; Naoki, H.; Fujita, T.; Yasumoto, T. Gymnocin-A, a cytotoxic polyether from the notorious red tide dinoflagellate, Gymnodinium mikimotoi. Tetrahedron Lett. 2002, 43, 5829–5832. [Google Scholar] [CrossRef]
- Satake, M.; Tanaka, Y.; Ishikura, Y.; Oshima, Y.; Naoki, H.; Yasumoto, T. Gymnocin-B with the largest contiguous polyether rings from the red tide dinoflagellate, Karenia (formerly Gymnodinium) mikimotoi. Tetrahedron Lett. 2005, 46, 3537–3540. [Google Scholar] [CrossRef]
- Gentien, P.; Lunven, M.; Lazure, P.; Youenou, A.; Crassous, M.P. Motility and autotoxicity in Karenia mikimotoi (Dinophyceae). Philos. Trans. R. Soc. B 2007, 362, 1937–1946. [Google Scholar] [CrossRef]
- Matsuyama, Y. Effects of harmful dinoflagellates, Gymnodinium mikimotoi and Heterocapsa circularisquama, red-tide on filtering rate of bivalve mollusks. Fish. Sci. 1999, 65, 248–253. [Google Scholar] [CrossRef]
- Sellem, F.; Pesando, D.; Bodennec, G.; Abed, A.E.; Girard, J.P. Toxic effects of Gymnodinium cf. mikimotoi unsaturated fatty acids to gametes and embryos of the sea urchin Paracentrotus lividus. Water Res. 2000, 34, 550–556. [Google Scholar]
- Mitchell, S.; Rodger, H. Pathology of wild and cultured fish affected by a Karenia mikimotoi bloom in Ireland, 2005. Bull. Eur. Ass. Fish. Pathol. 2007, 27, 39–42. [Google Scholar]
- Basti, L.; Nagai, S.; Go, J.; Okano, S.; Nagai, K.; Watanabe, R.; Suzuki, T.; Tanaka, Y. Differential inimical effects of Alexandrium spp. and Karenia spp. on cleavage, hatching, and two larval stages of Japanese pearl oyster Pinctada fucata martensii. Harmful Algae 2015, 43, 1–12. [Google Scholar] [CrossRef]
- Cohen, M.S.; Metcalf, J.A.; Root, R.K. Regulation of oxygen metabolism in human granulocytes: Relationship between stimulus binding and oxidative response using plant lectins as probes. Blood 1980, 55, 1003–1009. [Google Scholar] [CrossRef]
- Kayashima, K.; Onoue, K.; Nakagawa, A.; Minakami, S. Superoxide anion-generating activities of macrophages as studied by using cytochalasin E and lectins as synergistic stimulants for superoxide release. Microbiol. Immun. 1980, 24, 449–461. [Google Scholar] [CrossRef]
- Zou, Y.; Yamasaki, Y.; Matsuyama, Y.; Yamaguchi, K.; Honjo, T.; Oda, T. Possible involvement of haemolytic activity in the contact-dependent lethal effects of the dinoflagellate Karenia mikimotoi on the rotifer Brachionus plicatilis. Harmful Algae 2010, 9, 367–373. [Google Scholar] [CrossRef]
- Zou, Y.; Kim, D.; Yagi, M.; Yamasaki, Y.; Kurita, J.; Iida, T.; Matsuyama, Y.; Yamaguchi, K.; Honjo, T.; Oda, T. Application of LDH-release assay to cellular-level evaluation of the toxic potential of harmful algal species. Biosci. Biotechnol. Biochem. 2013, 77, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Wang, X.Y.; Xu, M.E.; Jiang, Y.; Yan, T.; Wang, X.C. Progress on the usage of the rotifer Brachionus plicatilis in marine ecotoxicology: A review. Aquat. Toxicol. 2020, 229, 105678. [Google Scholar] [CrossRef]
- Kim, D.; Li, W.; Matsuyama, Y.; Matsuo, A.; Yagi, M.; Cho, K.; Yamasaki, Y.; Takeshita, S.; Yamaguchi, K.; Oda, T. Strain-dependent lethal effects on abalone and haemolytic activities of the dinoflagellate Karenia mikimotoi. Aquaculture 2020, 520, 734953. [Google Scholar] [CrossRef]
- Kikuchi, K.; Nagano, T.; Hayakawa, H.; Hirata, Y.; Hirobe, M. Detection of nitric oxide production from perfused organ by luminol-H2O2 system. Anal. Chem. 1993, 65, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, S.; Leopold, E.; Hemmens, B.; Schmidt, K.; Werner, E.R.; Mayer, B. Interference of carboxy-PTIO with nitric oxide- and peroxynitrite-mediated reactions. Free Radic. Biol. Med. 1997, 22, 787–794. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and (15N) nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Kojima, H.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Hirata, Y.; Nagano, T. Fluorescent indicators for imaging nitric oxide production. Angew. Chem. Int. Ed. 1999, 38, 3209–3212. [Google Scholar] [CrossRef]
- Sanzen, I.; Imanishi, N.; Takamatsu, N.; Konosu, S.; Mantani, N.; Terasawa, K.; Tazawa, K.; Odaira, Y.; Watanabe, M.; Takeyama, M.; et al. Nitric oxide-mediated antitumor activity induced by the extract from Grifola frondosa (Maitake mushroom) in a macrophage cell line, RAW264.7. J. Exp. Clin. Caner Res. 2001, 20, 591–597. [Google Scholar]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Mallick, N.; Rai, L.C.; Mohn, F.H.; Soeder, C.J. Studies on nitric oxide (NO) formation by the green alga Scenedesmus obliquus and the diazotrophic cyanobacterium Anabaena doliolum. Chemosphere 1999, 39, 1601–1610. [Google Scholar] [CrossRef]
- Mallick, N.; Mohn, F.H.; Rai, L.C.; Soeder, C.J. Evidence for the non-involvement of nitric oxide synthase in nitric oxide production by the green alga Scenedesmus obliquus. J. Plant Physiol. 2000, 156, 423–426. [Google Scholar] [CrossRef]
- Sakihama, Y.; Nakamura, S.; Yamasaki, H. Nitric oxide production mediated by nitrate reductase in the green alga Chlamydomonas reinhardtii: An alternative NO production pathway in photosynthetic organisms. Plant Cell Physiol. 2002, 43, 290–297. [Google Scholar] [CrossRef]
- Kennedy, F.; Martin, A.; McMinn, A. Insights into the production and role of nitric oxide in the Antarctic sea-ice diatom Fragilariopsis cylindrus. J. Phycol. 2020, 56, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.S.; Puntarulo, S. Nitric oxide generation upon growth of Antarctic Chlorella sp. cells. Physiol. Plant. 2005, 125, 192–201. [Google Scholar] [CrossRef]
- Stuehr, D.J. Mammalian nitric oxide synthases. Biochim. Biophys. Acta—Bioenerg. 1999, 1411, 217–230. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Takahashi, S. An alternative pathway for nitric oxide production in plants: New features of an old enzyme. Trends Plant Sci. 1999, 4, 128–129. [Google Scholar] [CrossRef]
- Bethke, P.C.; Badger, M.R.; Jones, R.L. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 2004, 16, 332–341. [Google Scholar] [CrossRef]
- Tischner, R.; Planchet, E.; Kaiser, W.M. Mitochondrial electron transport as a source for nitric oxide in the unicellular green alga Chlorella sorokiniana. FEBS Lett. 2004, 576, 151–155. [Google Scholar] [CrossRef]
- Guo, F.-Q.; Okamoto, M.; Crawford, N.M. Identification of a nitric oxide synthase gene involved in hormonal signaling. Science 2003, 302, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Chandok, M.R.; Ytterberg, A.J.; van Wijk, K.J.; Klessig, D.F. The pathogen-inducible nitric oxide synthase (iNOS) in plants is a variant of the P protein of the glycine decarboxylase complex. Cell 2003, 113, 469–482. [Google Scholar] [CrossRef]
- Brown, J.F.; Hanson, P.J.; Whittle, B.J.R. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur. J. Pharmacol. 1992, 223, 103–104. [Google Scholar] [CrossRef]
- Brown, J.F.; Keates, A.C.; Hanson, P.J.; Whittle, B.J.R. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am. J. Physiol. 1993, 265, G418–G422. [Google Scholar] [CrossRef]
- Ichikawa, T.; Ishihara, K.; Kusakabe, T.; Kawakami, T.; Hotta, K. Stimulant effect of nitric oxide generator and roxatidine on mucin biosynthesis of rat gastric oxyntic mucosa. Life Sci. 1999, 65, 41–46. [Google Scholar] [CrossRef]
- Shimada, M.; Shimono, R.; Imahayashi, T.; Ozaki, H.H.; Murakami, T.H. Diazo-reaction positive substance observed in the cortex of Chattonella antiqua. Histol. Histopath. 1986, 1, 327–333. [Google Scholar]
- Taylor, M.B.; Christian, K.G.; Patel, N.; Churchwell, K.B. Methemoglobinemia: Toxicity of inhaled nitric oxide therapy. Pediatr. Crit. Care Med. 2001, 2, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M.; Jensen, F.B. NO2− uptake and HCO3− excretion in the intestine of the European flounder (Platichthys flesus). J. Exp. Biol. 1999, 202, 2103–2110. [Google Scholar] [CrossRef]
- Vedel, N.E.; Korsgaard, B.; Jensen, F.B. Isolated and combined exposure to ammonia and nitrite in rainbow trout (Oncorhynchus mykiss): Effects on electrolyte status, blood respiratory properties and brain glutamine: Glutamate concentrations. Aquat. Toxicol. 1998, 41, 325–342. [Google Scholar] [CrossRef]
- Shen, M.; Xu, J.; Chiang, M.W.; Au, D.W. Unravelling the pathway of respiratory toxicity in goldlined seabream (Rhabdosargus sarba) induced by the harmful alga Chattonella marina. Aquat. Toxicol. 2011, 104, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Castellano, I.; Patti, F.P.; Palumbo, A.; Buia, M.C. Nitric oxide in marine photosynthetic organisms. Nitric Oxide 2015, 47, 34–39. [Google Scholar] [CrossRef]
- Kuroda, A.; Nakashima, T.; Yamaguchi, K.; Oda, T. Isolation and characterization of light-dependent hemolytic cytotoxin from harmful red tide phytoplankton Chattonella marina. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 141, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Radical scavengers in red tide plankton, Chattonella marina. Bull. Fac. Fish. Nagasaki Univ. 2001, 82, 93–97. [Google Scholar]
- Sato, E.; Niwano, Y.; Mokudai, T.; Kohno, M.; Matsuyama, Y.; Kim, D.; Oda, T. A discrepancy in superoxide scavenging activity between the ESR-spin trapping method and the luminol chemiluminescence method. Biosci. Biotechnol. Biochem. 2007, 71, 1505–1513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niwano, Y.; Sato, E.; Kohno, M.; Matsuyama, Y.; Kim, D.; Oda, T. Antioxidant properties of aqueous extracts from red tide plankton cultures. Biosci. Biotechnol. Biochem. 2007, 71, 1145. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, Y.; Mukai, K.; Takai, Y.; Qiu, X.; Oshima, Y. Recent progress in the study of peroxiredoxin in the harmful algal Bloom species Chattonella marina. Antioxidants 2021, 10, 162. [Google Scholar] [CrossRef] [PubMed]
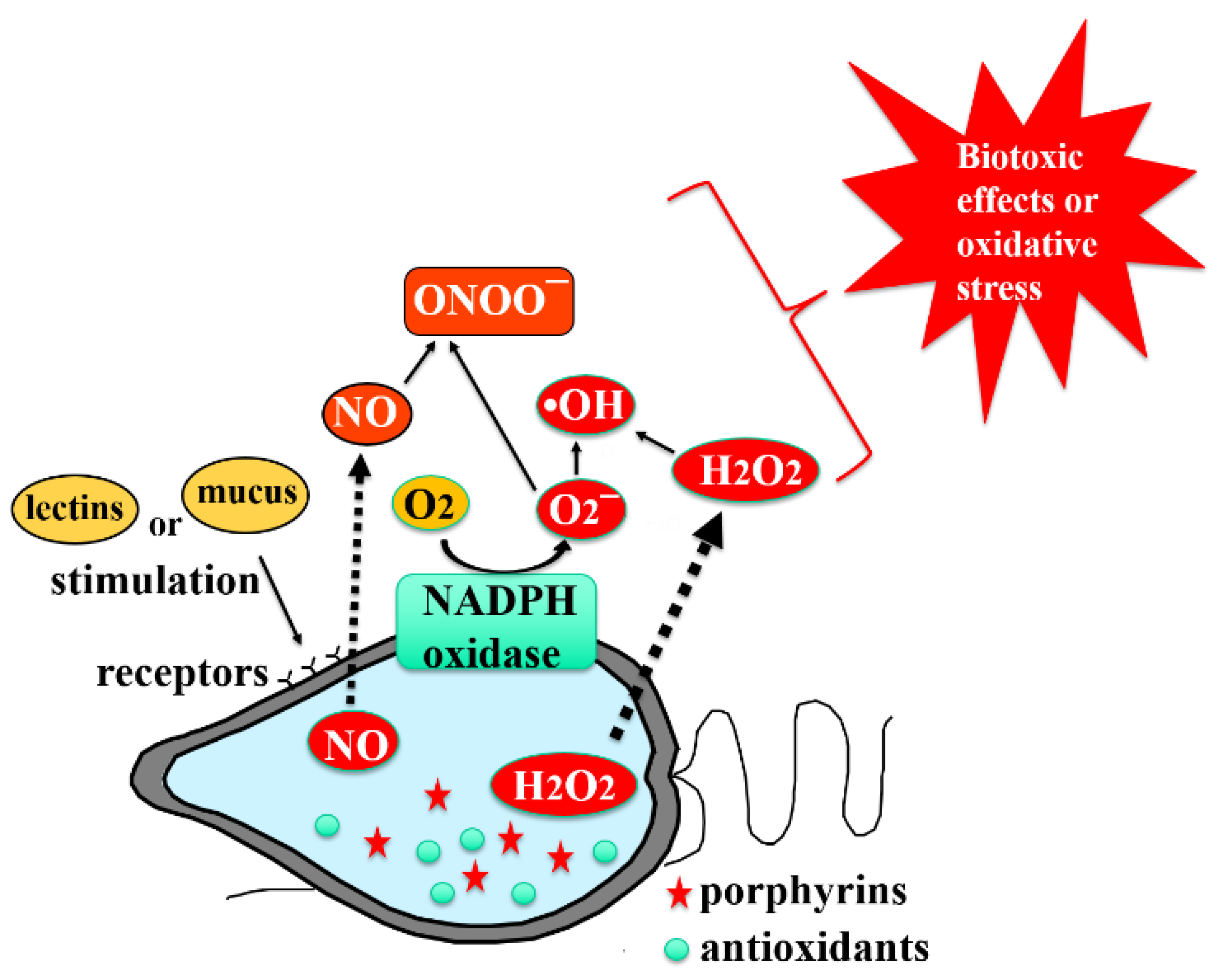
| Species | Main Toxic Factors | Event | References |
|---|---|---|---|
| Karenia mikimotoi | Hemolysin; Reactive oxygen species (ROS) | Ichthyotoxic; Toxic to invertebrates; Toxic to zooplankton; Antialgal | [48,49,50,51,52,53,54,55,56] |
| Cochlodinium polykrikoides | Hemolysin; ROS; Sulfated polysaccharides; Noxiustoxin | Antiviral; Ichthyotoxic; Molluscicidal | [57,58,59,60,61,62] |
| Eutreptiella gymnastica | ROS | - | [62] |
| Alexandrium tamarense | Saxitoxin; Neosaxitoxin; Gonyautoxin; N-sulfocarbomoyl toxins; Tetrodotoxin; Hemolysin; ROS | Neurotoxic; Paralytic shellfish poisoning; Toxic to marine organisms; Toxic to zooplankton; Cytotoxic | [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] |
| Heterosigma akashiwo (=Heterosigma carterae) | Brevetoxin-like toxin; ROS | Ichthyotoxic; Neurotoxic; Toxic to zooplankton | [84,85,86,87,88,89] |
| Chattonella marina | Brevetoxin-like toxin ROS; Hemolysin; Hemagglutinin | Ichthyotoxic; Neurotoxic | [9,88,90,91,92,93,94] |
| Chattonella ovata | ROS | Ichthyotoxic | [95] |
| Chattonella antiqua | Brevetoxin-like toxin; ROS | Ichthyotoxic; Neurotoxic | [96,97,98,99,100] |
| Chattonella subsalsa | ROS; Hemolysin | Ichthyotoxic; Toxic to zooplankton | [101] |
| Platymonas subcordiformis | ROS | - | [102] |
| Skeletonema costatum | ROS | Ichthyotoxic; Toxic to zooplankton; Antibacterial | [103,104,105,106] |
| Olisthodiscus luteus | ROS | Ichthyotoxic; Antimycotic; Toxic to phytoplankton | [88,98,107,108] |
| Fibrocapsa japonica | Brevetoxin-like toxin; ROS; Fibrocapsin | Ichthyotoxic; Toxic to marine mammals; Neurotoxic; Toxic to phytoplankton | [88,107,109] |
| Heterocapsa circularisquama | ROS; Hemolysin | Toxic to mollusks; Antialgal; Antiprotozoal; Toxic to zooplankton | [110,111,112,113,114,115] |
| Akashiwo sanguineum (=Gymnodinium sanguineum) | ROS | Ichthyotoxic; Toxic to mollusks; Antimycotic; Toxic to mice | [55,98,116,117] |
| Karlodinium veneficum | ROS | Ichthyotoxic; Toxic to zooplankton | [55,118,119] |
| Alexandrium catenella | Saxitoxin; Neosaxitoxin; Gonyautoxin; N-sulfocarbomoyl toxins; Hemolysin; ROS | Neurotoxic; Paralytic shellfish poisoning; Toxic to marine organisms | [73,82,120,121,122,123,124,125,126] |
| Prorocentrum minimum | Venerupin; Prorocentrin; ß-diketone; ROS | Venerupin shellfish poisoning; Toxic to marine organism; Neurotoxic | [127,128,129,130,131,132,133,134,135,136,137,138,139] |
| Prymnesium parvum | Prymnesin 1 and 2; Hemolysin | Ichthyotoxic; Toxic to invertebrates; Toxic to tadpoles; Toxic to zooplankton; Cytotoxic | [140,141,142,143,144,145] |
| Thalassiosira weissflogii | ROS | Toxic to zooplankton | [105] |
| Thalassiosira pseudonana | Apo-fucoxanthinoid pigments; ROS | Toxic to zooplankton | [146,147] |
| Coscinodiscus sp. | ROS | - | [148] |
| Pleurochrysis carterae | ROS | Toxic to zooplankton | [149] |
| Symbiodinium spp. | ROS | - | [150] |
| Trichodesmium erythraeum | ROS | Antibacterial; Toxic to marine organisms; Hepatotoxic; Neurotoxic; Ciguatoxin-like | [151,152,153,154] |
| Prorocentrum micans | ROS | Shellfish poisoning; Toxic to marine organisms; Antialgal | [62,155,156,157,158] |
| ROS | Studied Algal Species | Methods | References |
|---|---|---|---|
| Superoxide (O2−) | Chattonella marina Chattonella antiqua Karenia mikimotoi Cochlodinium polykrikoides Chattonella ovata Olisthodiscus luteus | 1 MCLA-mediated chemiluminescence assay | [62,94,159,160,166,181,190,191,192] |
| Chattonella marina Chattonella antiqua Karenia mikimotoi Cochlodinium polykrikoides Chattonella ovata | 2 L012-mediated chemiluminescence assay | [164,193,194,195] | |
| Cochlodinium polykrikoides Heterosigma carterae Thalassiosira weissflogii Thalassiosira pseudonana | Cytochrome c-mediated spectrophotometric assay | [62,85,196] | |
| Chattonella marina Nostoc spongiaeforme | 3 DMPO-mediated spin trapping method using an ESR | [159,197,198] | |
| Trichodesmium erythraeum | 4 Red-CLA-mediated chemiluminescence | [199] | |
| Hydroxyl radical (OH−) | Chattonella marina | Phenol red assay | [161] |
| Hydrogen peroxide (H2O2) | Chattonella marina Cochlodinium polykrikoides | 3 DMPO-mediated spin trapping method using an ESR | [159,198] |
| Karenia mikimotoi Cochlodinium polykrikoides Chattonella ovata | 5 PHPA-mediated fluorescence spectrophotometric assay | [178,181,200] | |
| Cochlodinium polykrikoides | Scopoletin–peroxidase method | [62,201] | |
| Nitric oxide | Chattonella marina Cochlodinium polykrikoides | Phenol red assay | [178,202] |
| Chattonella marina | Luminol–H2O2-mediated luminescence assay | [185,203] | |
| Chattonella marina Heterosigma akashiwo Chatonella ovata Cochlodinium polykrikoides Alexandrium taylori Alexandrium tamarense Nannochloropsis oculata | 6 DAF-FM DA-mediated fluorometric assay | [204] | |
| Nitric oxide | Platymonas subcordiformis Skeletonema costatum Gymnodinium sp. | Nitric oxide detection microsensor | [102] |
| Algal Species | ROS | Estimated Production Mechanisms | References |
|---|---|---|---|
| Karenia mikimotoi | Superoxide Hydrogen peroxide | - | [181,182] |
| Cochlodinium polykrikoides | Superoxide Hydrogen peroxide Hydroxyl radical | • Auto-oxidation of an electron acceptor in photosystem I (superoxide) • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) | [159,178,198,217] |
| Eutreptiella gymnastica | Hydrogen peroxide | • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) | [62,184] |
| Prorocentrum micans | Hydrogen peroxide | • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) | [62] |
| Akashiwo sanguineum (=Gymnodinium sanguineum) | Hydrogen peroxide | • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) | [62] |
| Alexandrium tamarense | Hydrogen peroxide | • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) | [62] |
| Heterosigma akashiwo (=Heterosigma carterae) | Superoxide Hydrogen peroxide Hydroxyl radical Nitric oxide | • Glycocalyx-mediated ROS generation • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) | [62,85,162,218] |
| Chattonella marina | Superoxide Hydrogen peroxide Nitric oxide | • NAD(P)H oxidase located in cell surface-bounded glycocalyx (superoxide) • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) • Nitric oxide synthase-like enzyme-mediated mechanism (nitric oxide) | [94,162,185,203] |
| Chattonella ovata | Superoxide Hydrogen peroxide Nitric oxide | • NAD(P)H oxidase located in cell surface-bounded glycocalyx (superoxide) • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) | [162] |
| Chattonella antiqua | Superoxide Hydrogen peroxide | • NAD(P)H oxidase located in cell surface-bounded glycocalyx (superoxide) • Photosynthetic electron transport (superoxide) • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) | [193,219] |
| Chattonella subsalsa | Superoxide | • Photosynthetic electron transport (superoxide) | [217] |
| Platymonas subcordiformis | Superoxide | - | [102] |
| Skeletonema costatum | Superoxide | - | [102] |
| Olisthodiscus luteus | Superoxide Hydrogen peroxide | • Cell surface redox enzyme-mediated mechanism (superoxide) • SOD catalyzed disproportionation of superoxide (hydrogen peroxide) | [108] |
| Fibrocapsa japonica | Superoxide Hydrogen peroxide | - | [174] |
| Heterocapsa circularisquama Karlodinium veneficum | Hydrogen peroxide Superoxide | - | [182,184] |
| - | |||
| Alexandrium catenella | Superoxide | - | [184] |
| Prorocentrum minimum | Hydrogen peroxide | - | [184] |
| Prymnesium parvum | Superoxide | - | [184] |
| Thalassiosira weissflogii | Superoxide Hydrogen peroxide | • NAD(P)H oxidase-related mechanism | [196,220] |
| Thalassiosira pseudonana | Superoxide Hydrogen peroxide | • NAD(P)H oxidase-related mechanism | [196] |
| Thalassiosira oceanica | Superoxide Hydrogen peroxide | • NAD(P)H oxidase-related mechanism | [221] |
| Coscinodiscus sp. | Superoxide | - | [184] |
| Pleurochrysis carterae | Hydrogen peroxide | - | [222] |
| Symbiodinium spp. | Superoxide | - | [150,223] |
| Trichodesmium erythraeum | Superoxide | - | [199] |
| Species | Main Toxic Factors Detected | Susceptible Organisms | Topics |
|---|---|---|---|
| Chattonella marina/antiqua (Raphidophyte) | 1 ROS (superoxide, hydrogen peroxide, and hydroxyl radical) 2 Nitric oxide (NO) | Fish | 1 NADPH oxidase is proposed as a mechanism of ROS production, which might be located on glycocalyx, a cell surface structure [94,162,193,219]. 2 The highest ROS generation rate among the species tested so far [184]. |
| Cochlodinium polykrikoides (Dinoflagellate) | 1 Hemolysin 2 ROS (superoxide and hydrogen peroxide) | Fish Shellfish | 1 Secretion of huge amount of highly viscous mucus-like substances [61,276]. |
| Karenia mikimotoi (Dinoflagellate) | 1 Hemolysin 2 ROS (superoxide and hydrogen peroxide) | Fish Shellfish | 1 Extremely toxic to both fish and shellfish, and HABs due to this dinoflagellate are often associated with mass mortality of both fish and shellfish [279,299]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.; Ueno, M.; Liang, Y.; Kim, D.; Oda, T. Generation of Reactive Oxygen Species (ROS) by Harmful Algal Bloom (HAB)-Forming Phytoplankton and Their Potential Impact on Surrounding Living Organisms. Antioxidants 2022, 11, 206. https://doi.org/10.3390/antiox11020206
Cho K, Ueno M, Liang Y, Kim D, Oda T. Generation of Reactive Oxygen Species (ROS) by Harmful Algal Bloom (HAB)-Forming Phytoplankton and Their Potential Impact on Surrounding Living Organisms. Antioxidants. 2022; 11(2):206. https://doi.org/10.3390/antiox11020206
Chicago/Turabian StyleCho, Kichul, Mikinori Ueno, Yan Liang, Daekyung Kim, and Tatsuya Oda. 2022. "Generation of Reactive Oxygen Species (ROS) by Harmful Algal Bloom (HAB)-Forming Phytoplankton and Their Potential Impact on Surrounding Living Organisms" Antioxidants 11, no. 2: 206. https://doi.org/10.3390/antiox11020206
APA StyleCho, K., Ueno, M., Liang, Y., Kim, D., & Oda, T. (2022). Generation of Reactive Oxygen Species (ROS) by Harmful Algal Bloom (HAB)-Forming Phytoplankton and Their Potential Impact on Surrounding Living Organisms. Antioxidants, 11(2), 206. https://doi.org/10.3390/antiox11020206






