Preparation of an Antioxidant Assembly Based on a Copolymacrolactone Structure and Erythritol following an Eco-Friendly Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
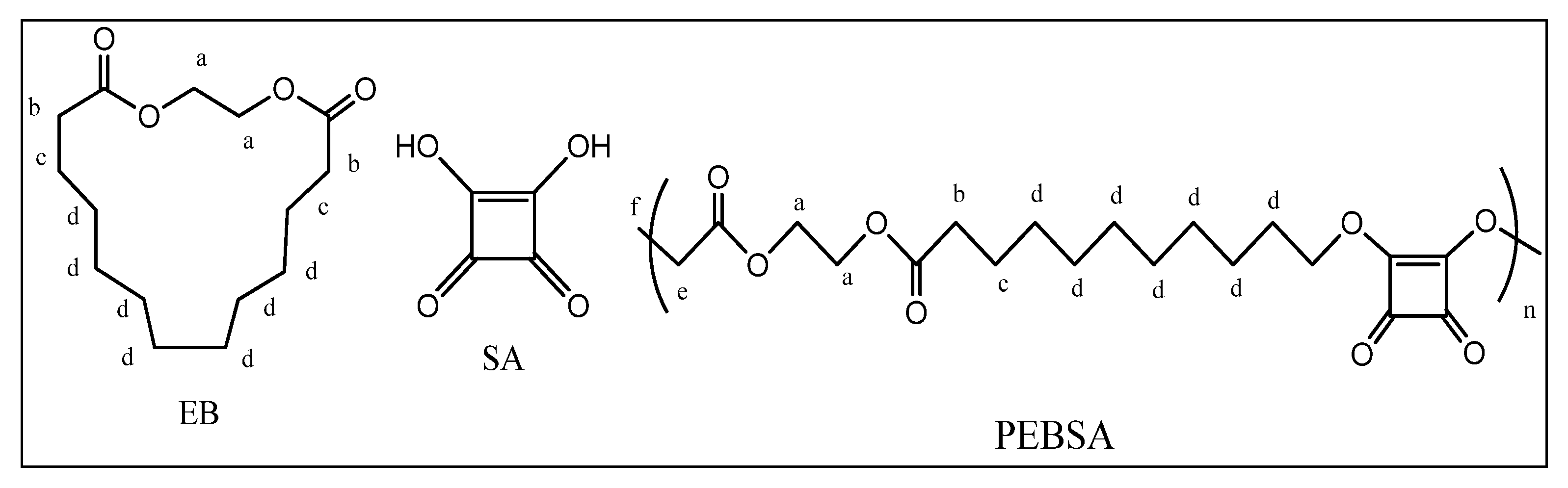
2.2.1. Synthesis of the Copolymacrolactone
2.2.2. Preparation of the Bioactive Compound
2.3. Sample Characterisation
2.3.1. Spectroscopic Measurements
FTIR Spectroscopic Analyses
1H-NMR Spectroscopic Analyses
2.3.2. Molecular Weights of the Copolymacrolactones
2.3.3. Thermogravimetric Analysis
2.3.4. Size Measurements
2.3.5. Dynamic Vapours Sorption Measurements
2.3.6. X-ray Diffraction Analysis
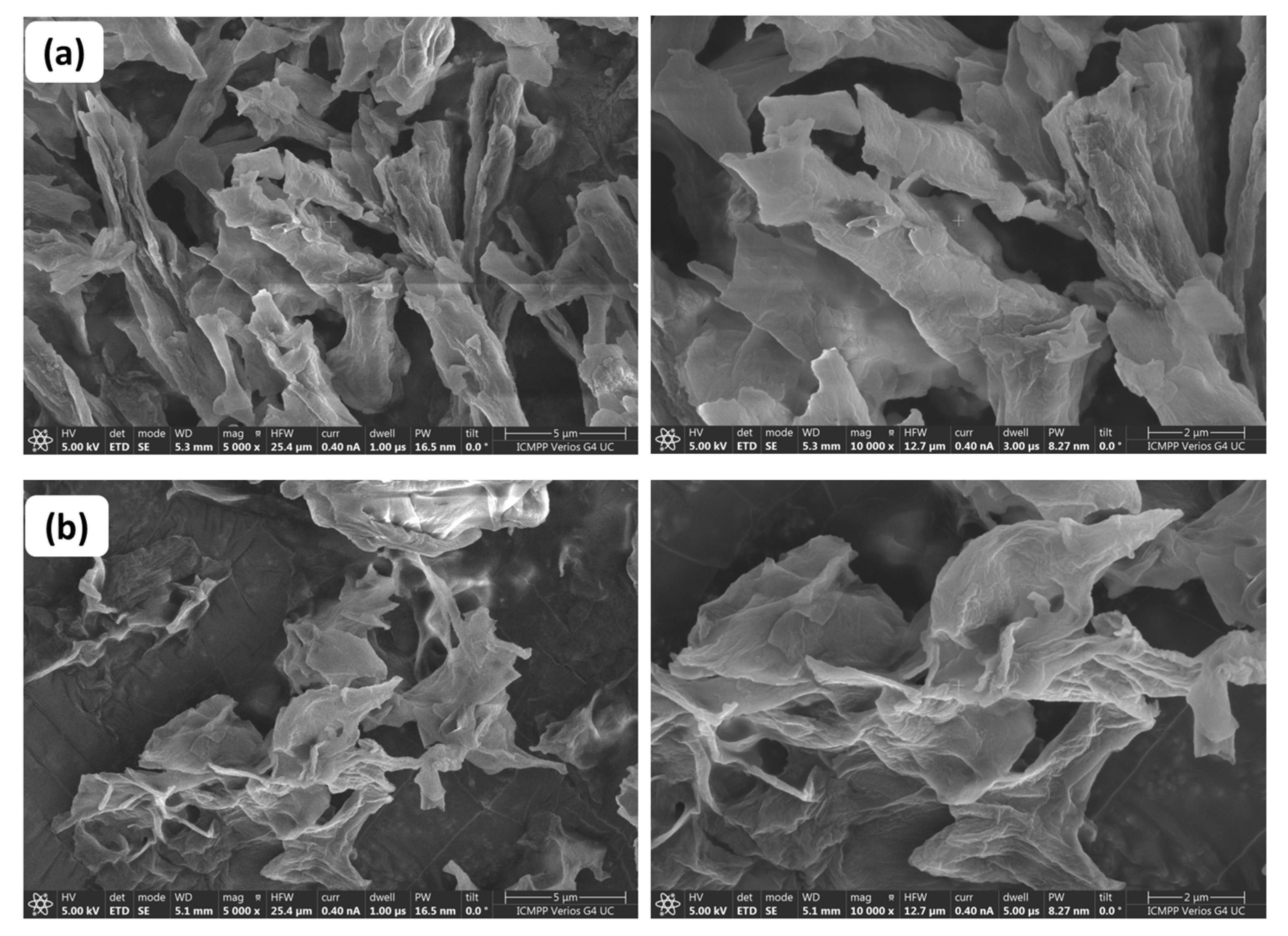
2.3.7. Morphological Characterisation
2.3.8. Antioxidant Behaviour of PEBSA–Eryt Bioactive Compound
2.3.9. Biocompatibility of PEBSA–Eryt Bioactive Compound
2.4. Statistical Analysis
3. Results and Discussion
3.1. FTIR Spectra
3.2. H-NMR Spectra
3.3. Thermal Degradation
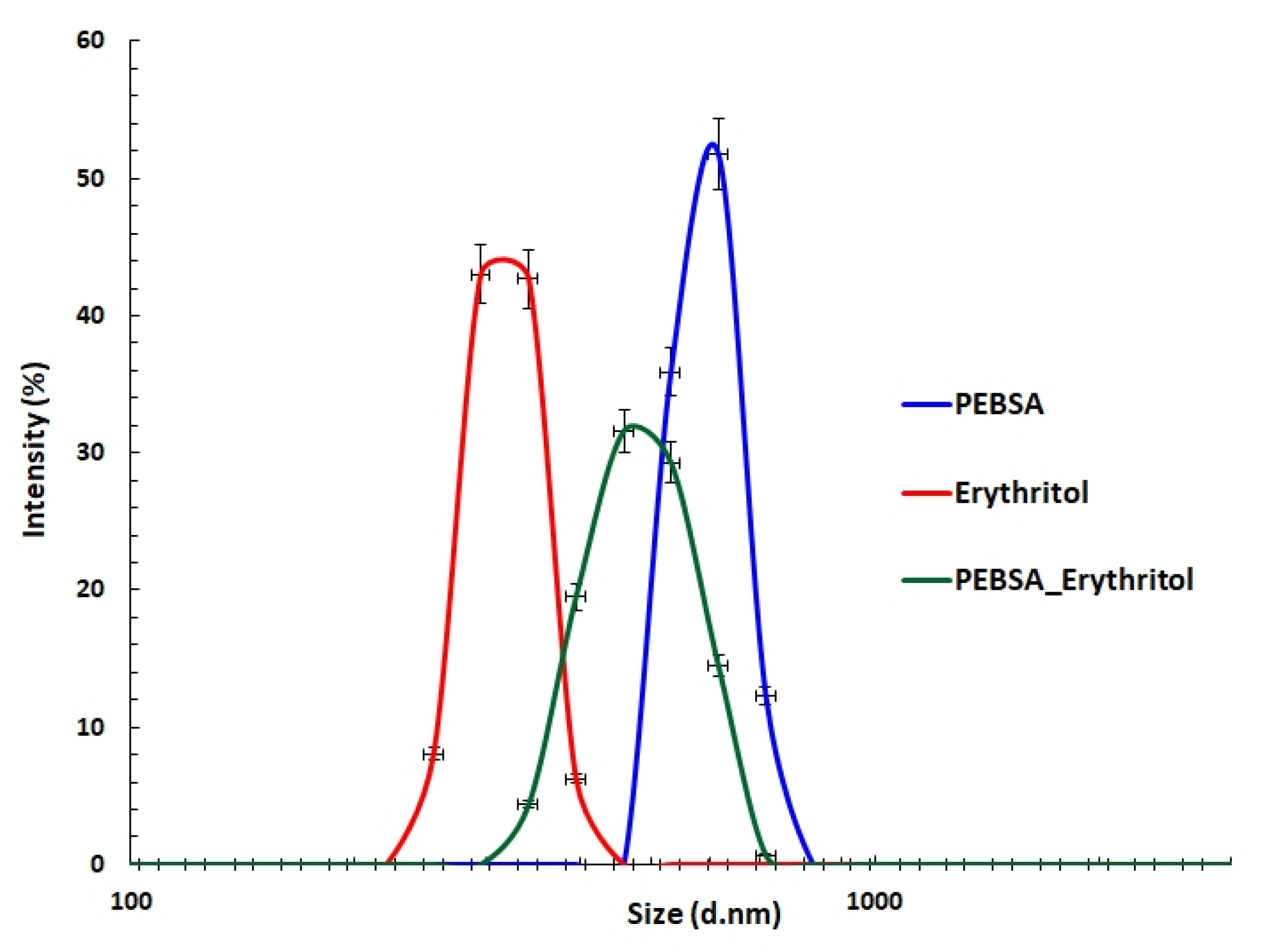
3.4. DLS Measurements
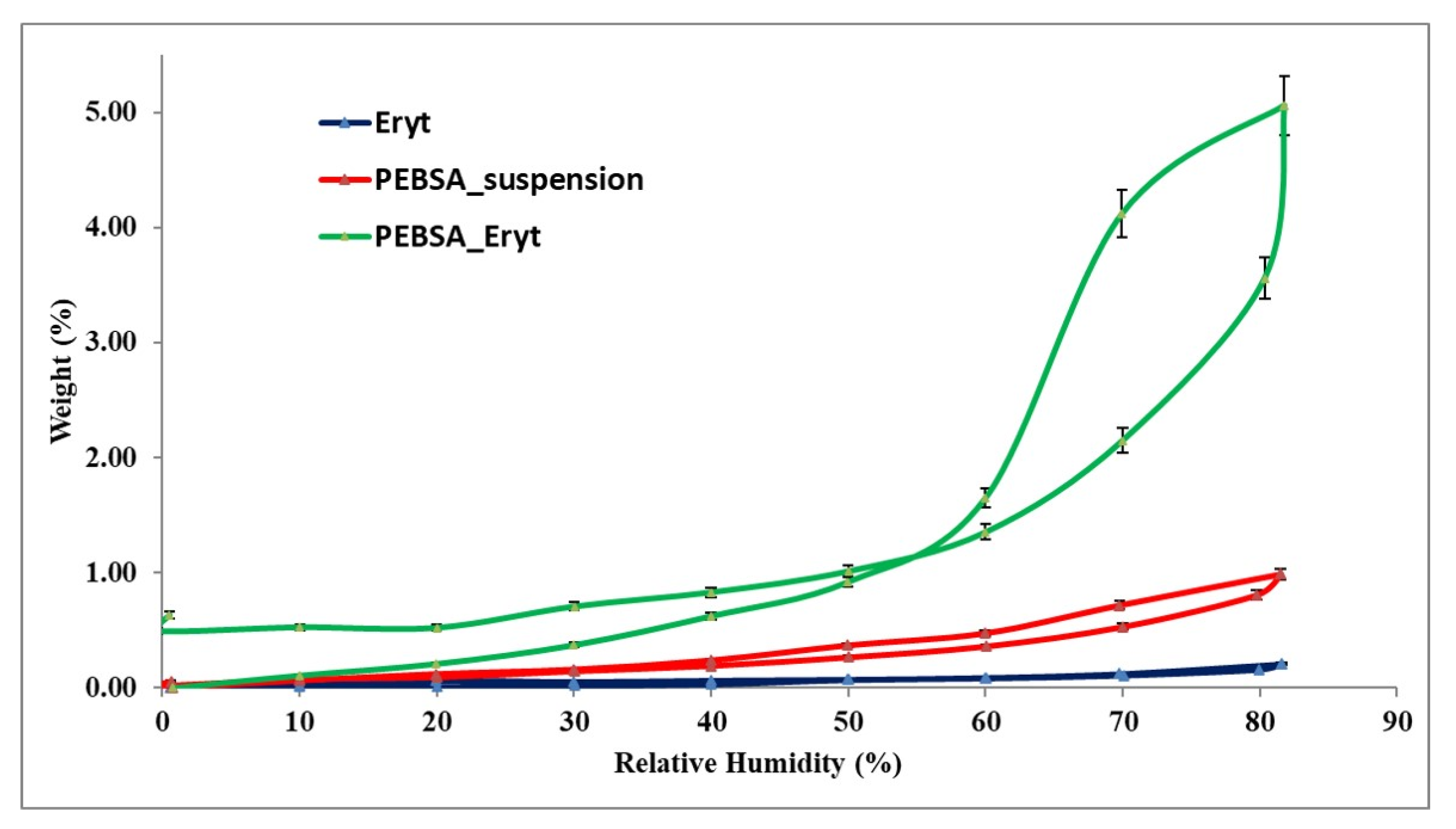
3.5. Dynamic Vapour Sorption Behaviour
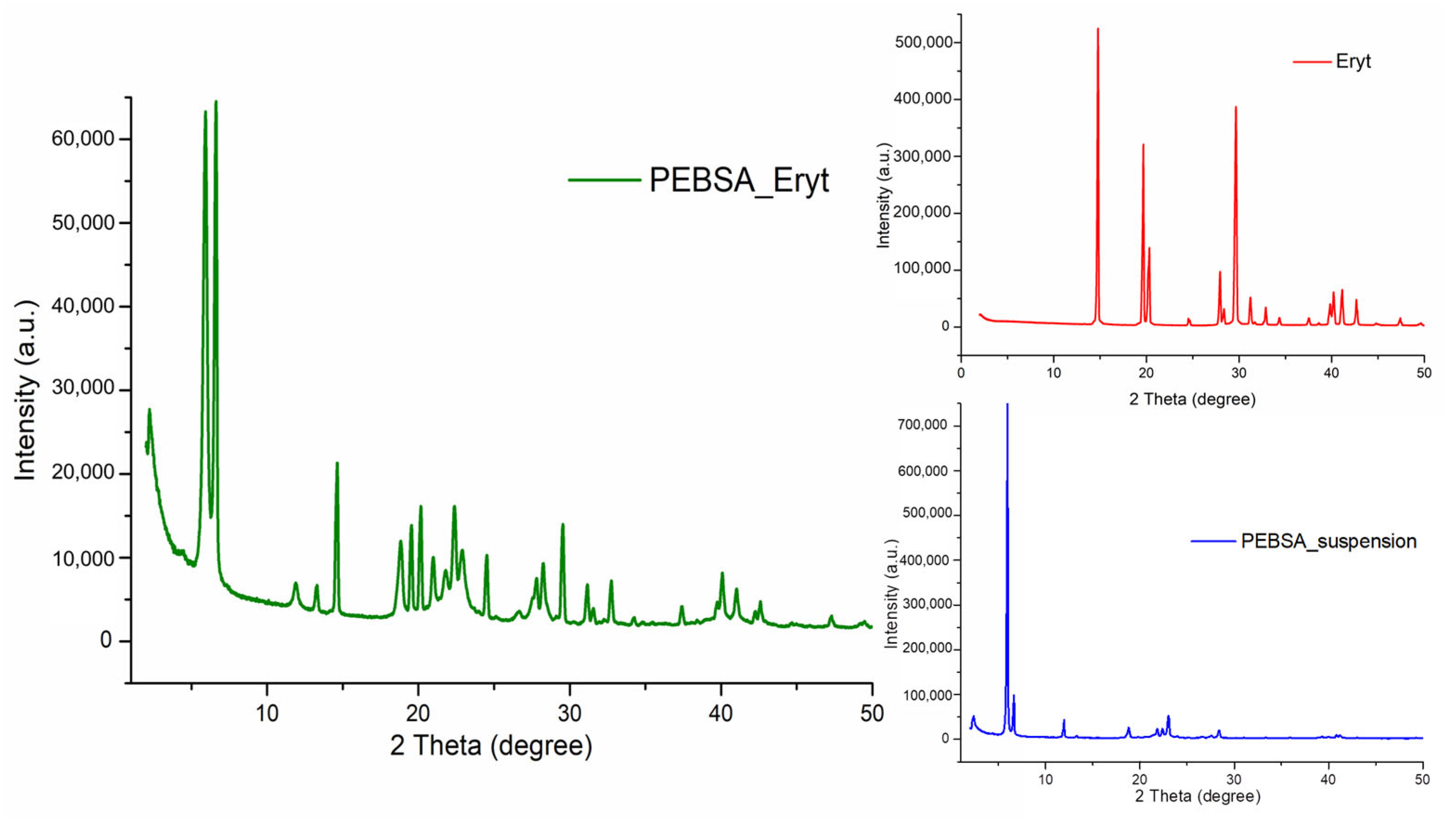
3.6. X-ray Diffraction Analysis
3.7. Morphological Characterisation
3.8. Antioxidant Behaviour of PEBSA–Eryt Bioactive Complex
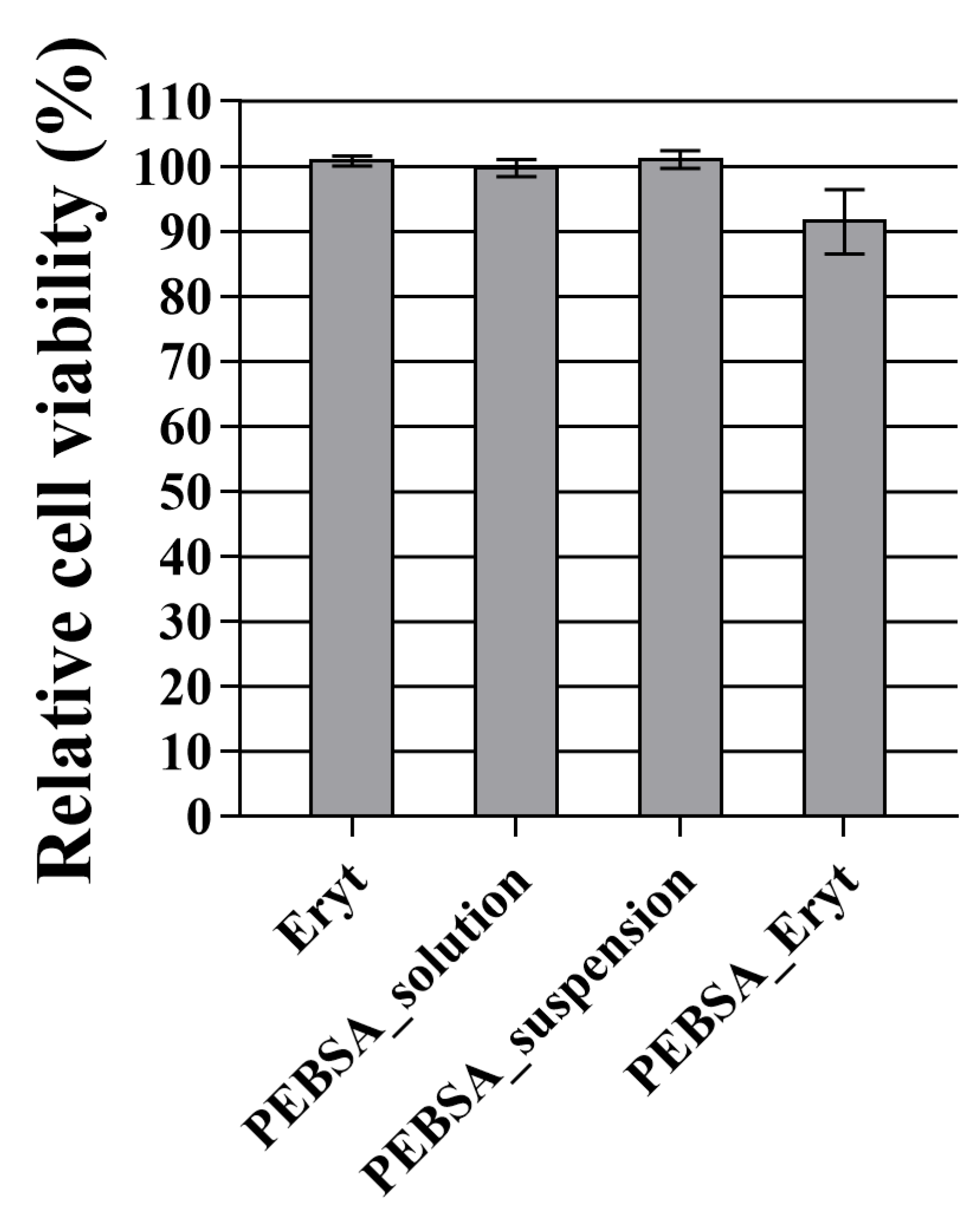
3.9. Biocompatibility of PEBSA–Eryt Bioactive Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiaradia, V.; Polloni, A.E.; de Oliveira, D.; de Oliveira, J.V.; Araújo, P.H.H.; Sayer, C. Polyester nanoparticles from macrolactones via miniemulsion enzymatic ring-opening polymerization. Colloid. Polym. Sci. 2018, 296, 861–869. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Asandulesa, M.; Stoica, I.; Tudorachi, N.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Timpu, D. Comparative study on the properties of a bio-based copolymacrolactone system. Polym. Test. 2022, 109, 107555. [Google Scholar] [CrossRef]
- Kim, Y.; Mafy, N.N.; Maisonneuve, S.; Lin, C.; Tamaoki, N.; Xie, J. Glycomacrocycle-Based Azobenzene Derivatives as Chiral Dopants for Photoresponsive Cholesteric Liquid Crystals. ACS Appl. Mater. Interfaces 2020, 12, 52146–52155. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.K.; Diaz, M.A.N.; Diaz-Muñoz, G. Lactones: Classification, synthesis, biological activities, and industrial applications. Tetrahedron 2021, 84, 132001. [Google Scholar] [CrossRef]
- Van der Meulen, I.; de Geus, M.; Antheunis, H.; Deumens, R.; Joosten, E.A.J.; Koning, C.E.; Heise, A. Polymers from Functional Macrolactones as Potential Biomaterials: Enzymatic Ring Opening Polymerization, Biodegradation, and Biocompatibility. Biomacromolecules 2008, 9, 3404–3410. [Google Scholar] [CrossRef]
- Myers, D.; Witt, T.; Cyriac, A.; Bown, M.; Mecking, S.; Williams, C.K. Ring opening polymerization of macrolactones: High conversions and activities using an yttrium catalyst. Polym. Chem. 2017, 8, 5780–5785. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.; Slomkowski, S. Ring-opening polymerization. Chem. Teach. Int. 2021, 3, 33–57. [Google Scholar] [CrossRef]
- Müller-Hülstede, J.; Schäfer, H.; Schiffels, P.; Bottke, P.; Wark, M.; Koschek, K. Surface-initiated ring-opening polymerization of ɛ-caprolactone as a feasible approach to modify flax yarn. Compos. Appl. Sci. Manuf. 2022, 152, 106714. [Google Scholar] [CrossRef]
- Pascual, A.; Sardon, H.; Veloso, A.; Ruipérez, F.; Mecerreyes, D. Organocatalyzed Synthesis of Aliphatic Polyesters from Ethylene Brassylate: A Cheap and Renewable Macrolactone. ACS Macro Lett. 2014, 3, 849–853. [Google Scholar] [CrossRef]
- Chen, J.-C.; Li, J.-Z.; Liu, J.-H.; Xu, L.-Q. Amphiphilic poly(ethylene glycol)-b-poly(ethylene brassylate) copolymers: One-pot synthesis, self-assembly, and controlled drug release. Chin. Chem. Lett. 2015, 26, 1319–1321. [Google Scholar] [CrossRef]
- Jin, C.; Wei, Z.; Yu, Y.; Sui, M.; Leng, X.; Li, Y. Copolymerization of ethylene brassylate with δ-valerolactone towards iso-dimorphic random copolyesters with continuously tunable mechanical properties. Eur. Polym. J. 2018, 102, 90–100. [Google Scholar] [CrossRef]
- Fernández, J.; Amestoy, H.; Sardon, H.; Aguirre, M.; Varga, A.L.; Sarasua, J.-R. Effect of molecular weight on the physical properties of poly(ethylene brassylate) homopolymers. J. Mech. Behav. Biomed. Mater. 2016, 64, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Larrañaga, A.; Etxeberria, A.; Sarasua, J.-R. Ethylene brassylate-co-δ-hexalactone biobased polymers for application in the medical field: Synthesis, characterization and cell culture studies. RSC Adv. 2016, 6, 22121–22136. [Google Scholar] [CrossRef]
- Hua, G.; Odelius, K. Exploiting Ring-Opening Aminolysis–Condensation as a Polymerization Pathway to Structurally Diverse Biobased Polyamides. Biomacromolecules 2018, 19, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Leng, X.; Zhang, M.; Wang, Y.; Wei, Z.; Li, Y. Fully biobased biodegradable poly(l-lactide)-b-poly(ethylene brassylate)-b-poly(l-lactide) triblock copolymers: Synthesis and investigation of relationship between crystallization morphology and thermal properties. Polym. Int. 2020, 69, 363–372. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Ko, W.-C.; Zhuang, Y.-N.; Wang, L.-Y.; Yu, T.-W.; Lee, S.-Y.; Rwei, S.-P. Development of Self-Healable Organic/Inorganic Hybrid Materials Containing a Biobased Copolymer via Diels–Alder Chemistry and Their Application in Electromagnetic Interference Shielding. Polymers 2019, 11, 1755. [Google Scholar] [CrossRef] [PubMed]
- Butron, A.; Llorente, O.; Fernandez, J.; Meaurio, E.; Sarasua, J.-R. Morphology and Mechanical Properties of Poly(ethylene brassylate)/Cellulose Nanocrystal composites. Carbohydr. Polym. 2019, 221, 137–145. [Google Scholar] [CrossRef]
- Wei, Z.; Jin, C.; Xu, Q.; Leng, X.; Wang, Y.; Li, Y. Synthesis, microstructure and mechanical properties of partially biobased biodegradable poly(ethylene brassylate-co-ε-caprolactone) copolyesters. J. Mech. Behav. Biomed. Mater. 2019, 91, 255–265. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Liu, J.; Weng, B.; Xu, L. Synthesis and self-assembly of four-armed star copolymer based on poly(ethylene brassylate) hydrophobic block as potential drug carries. J. Nanoparticle Res. 2016, 18, 134. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Macsim, A.-M.; Tudorachi, N.; Rosca, I.; Doroftei, F. Synthesis of Poly(Ethylene Brassylate-Co-squaric Acid) as Potential Essential Oil Carrier. Pharmaceutics 2021, 13, 477. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Stoleru, E.; Rosca, I.; Serban, A.; Nita, L.E.; Rusu, A.G.; Ghilan, A.; Macsim, A.-M.; Mititelu-Tartau, L. Development of a new polymer network system carrier of essential oils. Biomed. Pharmacother. 2022, 149, 112919. [Google Scholar] [CrossRef] [PubMed]
- Chasak, J.; Slachtov, V.; Urban, M.; Brulíkova, L. Squaric acid analogues in medicinal chemistry. Eur. J. Med. Chem. 2020, 209, 112872. [Google Scholar] [CrossRef] [PubMed]
- de Cock, P.; Mäkinen, K.; Honkala, E.; Saag, M.; Kennepohl, E.; Eapen, A. Erythritol Is More Effective Than Xylitol and Sorbitol in Managing Oral Health Endpoints. Int. J. Dent. 2016, 2016, 9868421. [Google Scholar] [CrossRef] [PubMed]
- de Cock, P. Erythritol Functional Roles in Oral-Systemic Health. Adv. Dent. Res. 2018, 29, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J. Erythritol attenuates the diabetic oxidative stress through modulating glucose metabolism and lipid peroxidation in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2002, 11, 5485–5489. [Google Scholar] [CrossRef]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Beglinger, C.; Islam, S. Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 12, 1986–1998. [Google Scholar] [CrossRef]
- Nistor, A.; Stiubianu, G.; Racles, C.; Cazacu, M. Evaluation of the Water Sorption Capacity of Some Polymeric Materials by Dynamic Vapour Sorptiop. Mater. Plast. 2011, 48, 33–37. [Google Scholar]
- Garcia, E.J.; Cadorin Oldoni, T.L.; de Alencar, S.M.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant Activity by DPPH Assay of Potential Solutions to be Applied on Bleached Teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Diaconu, A.; Nita, L.E.; Chiriac, A.P.; Butnaru, M. Investigation of the magnetic field effect upon interpolymeric complexes formation based on bovine serum albumin and poly(aspartic acid). Int. J. Biol. Macromol. 2018, 119, 974–981. [Google Scholar] [CrossRef]
- Yazdan, M.R.; Etula, J.; Zimmerman, J.B.; Seppälä, A. Ionic cross-linked polyvinyl alcohol tunes vitrification and cold-crystallization of sugar alcohol for long-term thermal energy storage. Green Chem. 2020, 22, 5447–5462. [Google Scholar] [CrossRef]
- Lee, K.-S.; Kweon, J.J.; Oh, I.-H.; Lee, C.E. Polymorphic phase transition and thermal stability in squaric acid. J. Phys. Chem. Solids 2012, 73, 890–895. [Google Scholar] [CrossRef]
- Jakab, E.; Mészáros, E.; Omastová, M. Thermal decomposition of polypyrroles. J. Therm. Anal. Calorim. 2007, 88, 515–521. [Google Scholar] [CrossRef]
- Direksilp, C.; Sirivat, A. Synthesis and Characterization of Hollow-Sphered Poly(N-methyaniline) for Enhanced Electrical Conductivity Based on the Anionic Surfactant Templates and Doping. Polymers 2020, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Wang, B.; Wen, H.; Cui, B.; Zhang, Z.; Kong, X.; Wang, Y. Improvement of the textural characteristics of curdlan gel by the formation of hydrogen bonds with erythritol. Food Hydrocoll. 2021, 117, 106648. [Google Scholar] [CrossRef]
- Hashino, E.; Kuboniwa, M.; Alghamdi, S.A.; Yamaguchi, M.; Yamamoto, R.; Cho, H.; Amano, A. Erythritol alters microstructure and metabolomic profiles of biofilm composed of Streptococcus gordoniiand Porphyromonas gingivalis. Mol. Oral Microbiol. 2013, 28, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Loimaranta, V.; Mazurel, D.; Deng, D.; Söderling, E. Xylitol and erythritol inhibit real-time biofilm formation of Streptococcus mutans. BMC Microbiol. 2020, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Staszczyk, M.; Jurczak, A.; Magacz, M.; Ko´scielniak, D.; Gregorczyk-Maga, I.; Jamka-Kasprzyk, M.; Kepisty, M.; Kołodziej, I.; Kukurba-Setkowicz, M.; Krzy´sciak, W. Effect of Polyols and Selected Dental Materials on the Ability to Create a Cariogenic Biofilm–On Children Caries-Associated Streptococcus Mutans Isolates. Int. J. Environ. Res. Public Health 2020, 17, 3720. [Google Scholar] [CrossRef]
- So¨derling, E.M.; Aija-Maaria Hietala-Lenkkeri, Æ. Xylitol and Erythritol Decrease Adherence of Polysaccharide-Producing Oral Streptococci. Curr. Microbiol. 2010, 60, 25–29. [Google Scholar] [CrossRef]
- Kõljalg, S.; Smidt, I.; Chakrabarti, A.; Bosscher, D.; Mändar, R. Exploration of singular and synergistic efect of xylitol and erythritol on causative agents of dental caries. Sci. Rep. 2020, 10, 6297. [Google Scholar] [CrossRef] [PubMed]
- Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour. Effic. Technol. 2017, 3, 506–515. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Nita, L.E.; Tudorachi, N.; Neamtu, I.; Balan, V.; Tartau, L. Upon synthesis of a polymeric matrix with pH and temperature responsiveness and antioxidant bioactivity based on poly(maleic anhydride-co-3,9-divinyl-2,4,8,10-tetraoxaspiro[5.5] undecane) derivatives. Mater. Sci. Eng. C. 2015, 50, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Björklund, S.; Kocherbitov, V. Water vapor sorption-desorption hysteresis in glassy surface films of mucins investigated by humidity scanning QCM-D. J. Colloid Interface Sci. 2019, 545, 289–300. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Szabo, P.; Daugaard, A.E.; Giacinti Baschetti, M. Effect of Crystallinity on Water Vapor Sorption, Diffusion, and Permeation of PLA-Based Nanocomposites. ACS Omega. 2020, 5, 15362–15369. [Google Scholar] [CrossRef]
- Sangroniz, A.; Chaos, A.; Iriarte, M.; del Río, J.; Sarasua, J.-R.; Etxeberria, A. Influence of the Rigid Amorphous Fraction and Crystallinity on Polylactide Transport Properties. Macromolecules 2018, 51, 3923–3931. [Google Scholar] [CrossRef]
- Gilli, G.; Bertolasi, V.; Gilli, P.; Ferretti, V. Associations of squaric acid and its anions as multiform building blocks of hydrogen-bonded molecular crystals. Acta Crystallogr. Sect. B Struct. Sci. 2001, 57, 859–865. [Google Scholar] [CrossRef]
- Cvikl, B.; Lussi, A. The Biocompatibility of a New Erythritol-and Xyltol-Containing Fluoride Toothpaste. Healthcare 2021, 9, 935. [Google Scholar] [CrossRef]
- Janus, M.M.; Volgenant, C.M.C.; Brandt, B.W.; Buijs, M.J.; Keijser, B.J.F.; Crielaard, W.; Krom, B.P. Effect of erythritol on microbial ecology of in vitro gingivitis biofilms. J. Oral. Microbiol. 2017, 9, 1337477. [Google Scholar] [CrossRef]
- Chung, Y.-S.; Lee, M. Genotoxicity Assessment of Erythritol by Using Short-term Assay. Toxicol. Res. 2013, 29, 249–255. [Google Scholar] [CrossRef]
- De Oliveira, F.C.S.; Olvera, D.; Sawkins, M.J.; Cryan, S.-A.; Kimmins, S.D.; da Silva, T.E.; Heise, A. Direct UV-Triggered Thiol–ene Cross-Linking of Electrospun Polyester Fibers from Unsaturated Poly(macrolactone)s and Their Drug Loading by Solvent Swelling. Biomacromolecules 2017, 18, 4292–4298. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.B.; Odeleye, A.O.O.; Chui, C.; Baudequin, T.; Cui, Z.; Ye, H. Development of thermo-responsive polycaprolactone macrocarriers conjugated with Poly(N-isopropyl acrylamide) for cell culture. Sci. Rep. 2019, 9, 3477. [Google Scholar] [CrossRef] [PubMed]






| Sample | Mp | Mn | Mw | Mz | Mz+1 | Mv | D |
|---|---|---|---|---|---|---|---|
| PEBSA_solution | 3785 ± 113 | 3977 ± 119 | 4006 ± 120 | 4076 ± 122 | 4137 ± 124 | 3992 ± 119 | 1.007 |
| PEBSA_suspension | 17,663 ± 529 | 22,703 ± 681 | 29,100 ± 873 | 42,848 ± 1285 | 62,605 ± 1878 | 27,081 ± 812 | 1.020 |
| Sample | Degradation Stage | Tonset °C | Tpeak °C | W % | Residue | T10 °C | T20 °C |
|---|---|---|---|---|---|---|---|
| PEBSA_solution | I | 278 | 338 | 70.96 | 0.04 | 287 | 310 |
| II | 421 | 454 | 29 | ||||
| PEBSA_suspension | I | 265 | 286 | 40.29 | 9.78 | 268 | 283 |
| II | 303 | 314 | 25.38 | ||||
| III | 398 | 420 | 9.89 | ||||
| IV | 442 | 456 | 14.66 |
| PEBSA_Solution | PEBSA_Suspension | |
|---|---|---|
| Tm (°C) | 104.4 | 114.1 |
| ΔH (J/g) | 71.36 | 86.33 |
| ΔCp (J/g·K) | 0.819 | 0.522 |
| Sample | Peak (nm) | PDI | ZP (mV) |
|---|---|---|---|
| PEBSA_solution * | 420 ± 12.6 | 0.9 | |
| PEBSA_suspension | 596 ± 17.8 | 0.52 | −25 ± 0.75 |
| Eryt | 318 ± 9.5 | 0.67 | −3.42 ± 0.1 |
| PEBSA_Eryt | 487 ± 14.6 | 0.49 | −24.9 ± 0.74 |
| Sample | W (%) | rpm (nm) | BET Data * | |
|---|---|---|---|---|
| Area (m2/g) | Monolayer (g/g) | |||
| Erythritol | 0.2085 | 2.76 | 1.509 | 0.0004 |
| PEBSA | 0.9878 | 2.92 | 6.762 | 0.0019 |
| PEBSA_Eryt | 5.0588 | 3.54 | 28.575 | 0.0081 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiriac, A.P.; Ghilan, A.; Serban, A.-M.; Macsim, A.-M.; Bargan, A.; Doroftei, F.; Chiriac, V.M.; Nita, L.E.; Rusu, A.G.; Sandu, A.-I. Preparation of an Antioxidant Assembly Based on a Copolymacrolactone Structure and Erythritol following an Eco-Friendly Strategy. Antioxidants 2022, 11, 2471. https://doi.org/10.3390/antiox11122471
Chiriac AP, Ghilan A, Serban A-M, Macsim A-M, Bargan A, Doroftei F, Chiriac VM, Nita LE, Rusu AG, Sandu A-I. Preparation of an Antioxidant Assembly Based on a Copolymacrolactone Structure and Erythritol following an Eco-Friendly Strategy. Antioxidants. 2022; 11(12):2471. https://doi.org/10.3390/antiox11122471
Chicago/Turabian StyleChiriac, Aurica P., Alina Ghilan, Alexandru-Mihail Serban, Ana-Maria Macsim, Alexandra Bargan, Florica Doroftei, Vlad Mihai Chiriac, Loredana Elena Nita, Alina Gabriela Rusu, and Andreea-Isabela Sandu. 2022. "Preparation of an Antioxidant Assembly Based on a Copolymacrolactone Structure and Erythritol following an Eco-Friendly Strategy" Antioxidants 11, no. 12: 2471. https://doi.org/10.3390/antiox11122471
APA StyleChiriac, A. P., Ghilan, A., Serban, A.-M., Macsim, A.-M., Bargan, A., Doroftei, F., Chiriac, V. M., Nita, L. E., Rusu, A. G., & Sandu, A.-I. (2022). Preparation of an Antioxidant Assembly Based on a Copolymacrolactone Structure and Erythritol following an Eco-Friendly Strategy. Antioxidants, 11(12), 2471. https://doi.org/10.3390/antiox11122471







