A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies
Abstract
1. Introduction
1.1. Neuropathic Pain
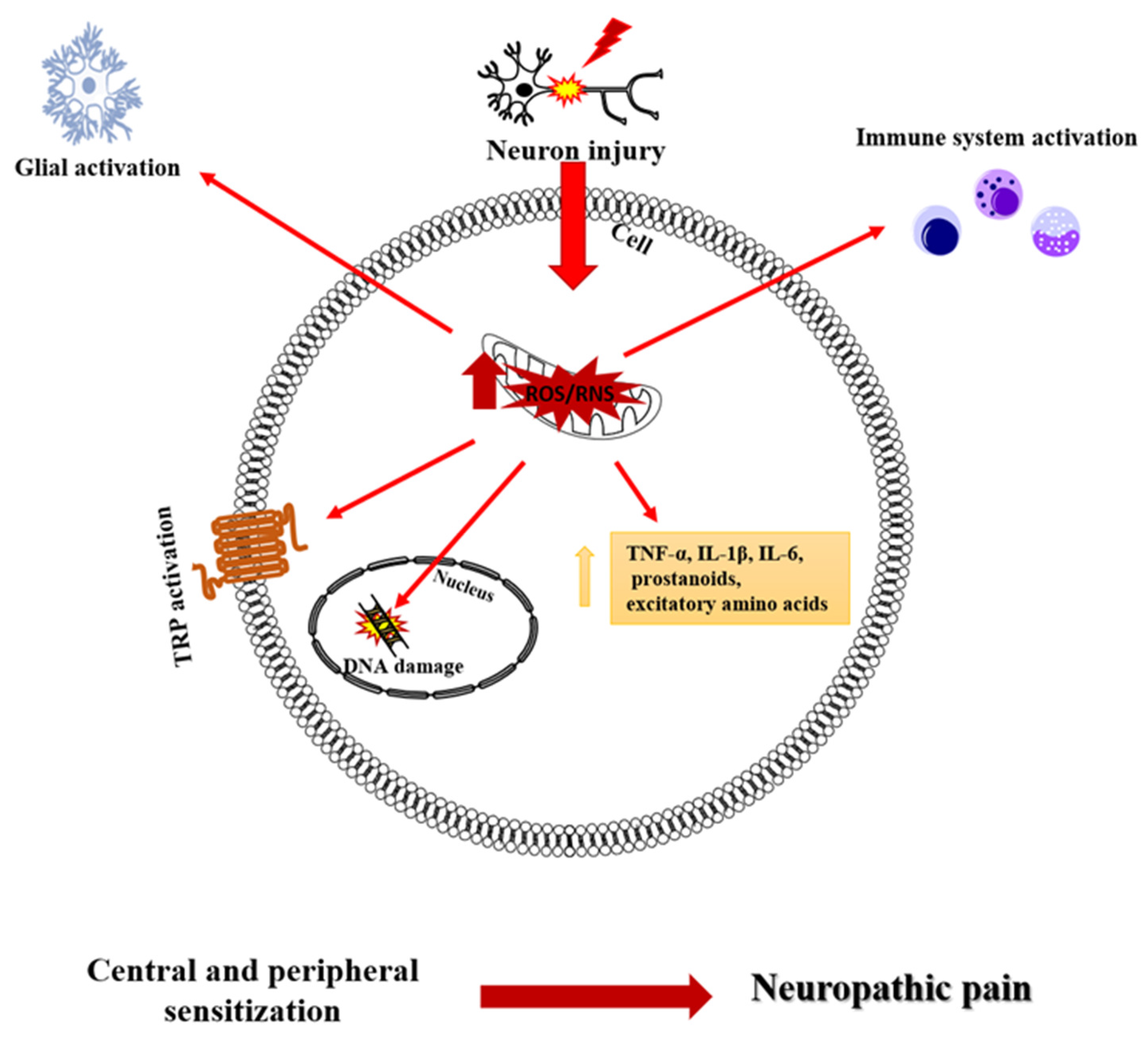
1.2. Oxidative Stress in Neuropathic Pain
1.3. Natural and Synthetic Antioxidants
2. Materials and Methods
2.1. Database Sources
2.2. Eligibility Criteria
- − Studies on animals (rats or mice) with neuropathic pain;
- − Study using any natural drug treatments for neuropathic pain.
- − Studies were excluded from the analysis for the following reasons:
- − use of non-natural drugs or synthetic substances;
- − non-pharmacological interventions for pain;
- − cellular studies;
- − human studies;
- − studies in animals other than rats and mice.
2.3. Study Outcomes
2.4. Statistical Analysis
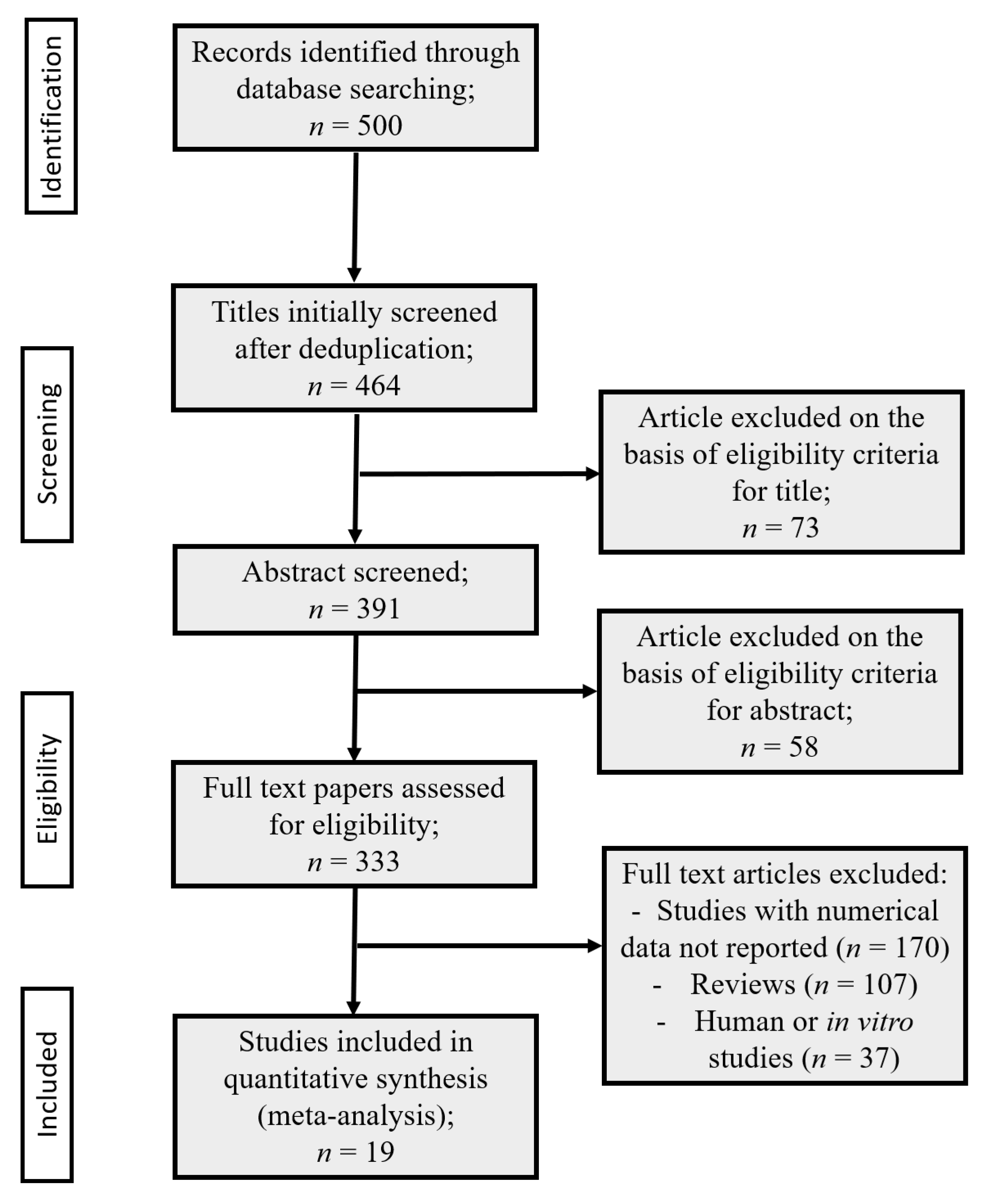
3. Results
3.1. Data Collection
3.2. Systematic Review on the Effect of Nutraceuticals in Neuropathic Pain
| References | Substances | Route of Administration | Gender Animals Strains | Pain Model | Behavioral Test | Results |
|---|---|---|---|---|---|---|
| KOMATSU et al., 2018 [69] | Essential oil of Bergamot (BEO) (20 µg/paw) | Subcutaneous injection (s.c) of BEO | Male Mice ddY | PSNL (partial sciatic nerve ligation) | Von Frey test | Attenuation of allodynia |
| ILARI et al., 2020 [3] | Polyphenol fraction of Bergamot (BPF) (50 mg/Kg) | Subcutaneous infusion of BPF by mini-pump | Male Rats Sprague–Dawley | Neuropathic pain induced by sciatic nerve injury (CCI) | -Von Frey test -Plantar test | Reduction in mechanical allodynia and thermal hyperalgesia |
| ILARI et al., 2021 [71] | Polyphenol fraction of Bergamot (BPF) (25 mg/Kg) | Intraperitoneal injection (i.p.) of BPF | Male Rats Sprague–Dawley | Neuropathic pain induced by administration of paclitaxel | -Von Frey test -Plantar test | Reduction in mechanical allodynia and thermal hyperalgesia |
| IANNOTTA et al., 2021 [72] | N-Palmitoyl-D-glucosamine (PGA) (20 mg/Kg) | Oral gavage | Male Mice CD1 | Chemotrapy-induced peripheral neuropathy by oxaliplatin | -Von Frey -Cold plate | Prevention of mechanical allodynia and hyperalgesia |
| TENCI et al., 2017 [73] | Lepidium meyenii Walp. (Maca) (10g/Kg) | Oral gavage | Male Rats Sprague–Dawley | Neuropathic pain induced by administration of paclitaxel and oxaliplatin | -Cold plate | Reduction in thermal hyperalgesia |
| BAGDAS et al., 2014 [74] | Chlorogenic Acid (5-caffeoylquinic acid, CGA) (100 mg/Kg) | Intraperitoneal injection (i.p.) | Male Rats Wistar | Streptozotocin-induced diabetic neuropathic pain | -Randall–Selitto test | Prevention of mechanical allodynia |
| LINHER-MELVILLE et al., 2020 [75] | Cannabil oil (CBD) (0.0833 mg/200gr rats) and Δ9-tetrahydrocannabinol (THC) (0.0167 mg/200 rats). | Oral gavage | Male Rats Sprague–Dawley | Neuropathic pain by sciatic nerve cuff surgery | Von Frey test | Reduction in mechanical allodynia |
| BELARDO et al., 2019 [77] | Cannabil oil (CBD) (30 μL of CBD dissolved in 10% hemp seed oil and natural tocopherols) | Oral gavage | Male Mice C57BL/6 | Traumatic brain injury (TBI) | Von Frey test | Reduction in mechanical allodynia |
| GUIDA et al., 2015 [80] | Palmitoylethanolmide (PEA) (10 mg/Kg) and Oleoylethanolamide (OEA) (10 mg/Kg) | Intraperitoneal injection (i.p.) | Male Mice CD1 | Neuropathic pain induced by spared nerve injury (SNI) | -Plantar test -Dynamic plantar aesthesiometer | Reduction in mechanical allodynia and hyperalgesia |
| RENNO et al., 2012 [81] | Epigallocatechin-3-Gallate(EGCG) (50 mg/Kg) | Intraperitoneal injection (i.p.) | Male Rats Wistar | Neuropathic pain induced by sciatic nerve crush injury | -Von Frey test -Randall–Selitto test -Hotplate test | Reduction in mechanical allodynia and hyperalgesia |
| XIFRò et al., 2015 [82] | Epigallocatechin-3-Gallate (EGCG) (50 mg/Kg) | Intraperitoneal injection (i.p.) of EGCG | Female Mice Balb-c | Neuropathic pain induced by sciatic nerve injury (CCI) | Plantar test | Reduction in thermal hyperalgesia |
| BOSCH-MOLA et al., 2017 [83] | Epigallocatechin-3-Gallate (EGCG) (50 mg/Kg) | Intraperitoneal injection (i.p.) of EGCG | Female Mice Balb/c | Neuropathic pain induced by sciatic nerve injury (CCI) | Plantar test | Reduction in thermal hyperalgesia |
| ISHOLA et al., 2014 [84] | Annona muricata Linn. (Soursop) (200 mg/Kg) | Oral gavage | Male albino mice | Morphine induced pain | Hot plate test | Reduction in thermal hyperalgesia |
| HASANEIN et al., 2014 [88] | Rosmarinic Acid (RA) (30 mg/Kg) | Oral gavage (o.g.) of RA | Male Rats Wistar | Diabetic neuropathy induced by streptozotocin (STZ) | Tail flick latency | Reduction in hyperalgesia and allodynia |
| LEE et al., 2016 [89] | Agrimonia eupatoria L. (200 mg/Kg) | Oral gavage | Male Rats Sprague–Dawley | Cisplatin-induced neuropathic pain | -Pin-prick test -Randall–Selitto -Plantar test | Reduction in hyperalge |
| LEE et al., 2021 [104] | Ginseng (Panax ginseng C.A. Meyer) (saponin extract 50 mg/Kg; Rb1 12.5 mg/Kg) | Oral gavage | Male Rats Sprague–Dawley | Neuropathic pain induced by tail nerve injury (TNI) and spinal cord injury (SCI) | -Plantar test -Von Frey test | Reduction in allodynia and hyperalgesia |
| PARENTI et al., 2015[94] SHAHID et al., 2017[95] | Harpagophytum procumbens(400–800 mg/Kg) plus morphine (3–5 mg/Kg) Bacopa monnieri (Linn.) Pennell (80 mg/Kg) | Intraperitoneal injection (i.p.) Oral gavage | Male Rats ---- Male Rats Sprague–Dawley | Neuropathic pain induced by sciatic nerve injury (CCI) Neuropathic pain induced by sciatic nerve injury (CCI) | -Plantar test -Von Frey test -Von Frey test -Hot plate test | Reduction in allodynia and hyperalgesia Reduction in mechanical allodynia and hyperalgesia |
| HOLMES et al., 2015 [103] | -Menhaden oil(replacing 50% of the high fat diet) | Oral administration (containing in high fat diet) | Male Rats Sprague–Dawley | Diabetic neuropathy induced by streptozotocin (STZ) | Plantar test | Reduction in thermal hyperalgesia |
3.3. Meta-Analysis
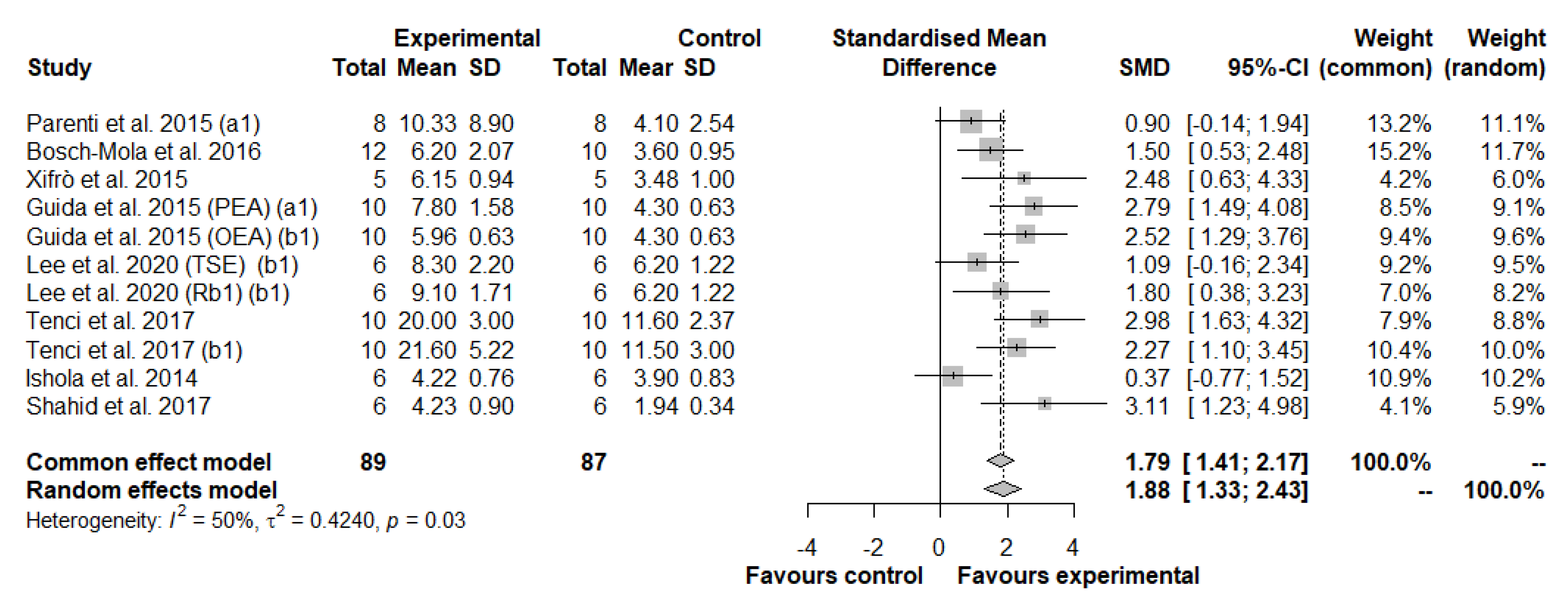
3.3.1. Meta-Analysis of Thermal Hyperalgesia
Multivariate Meta-Regression Analysis in Thermal Hyperalgesia
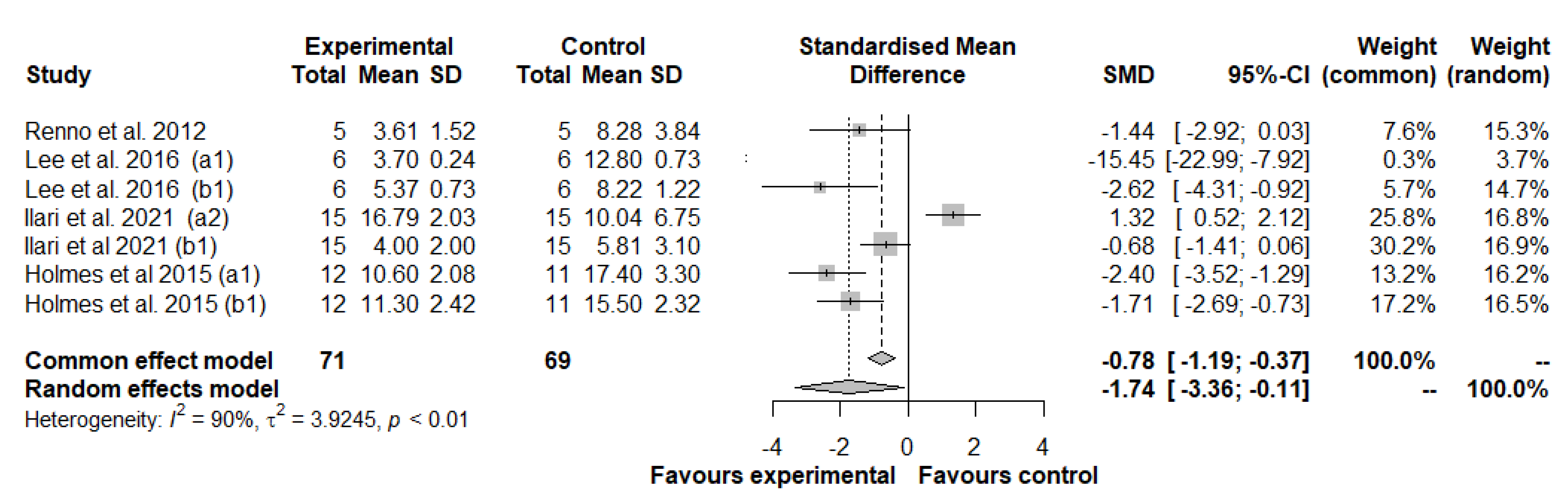
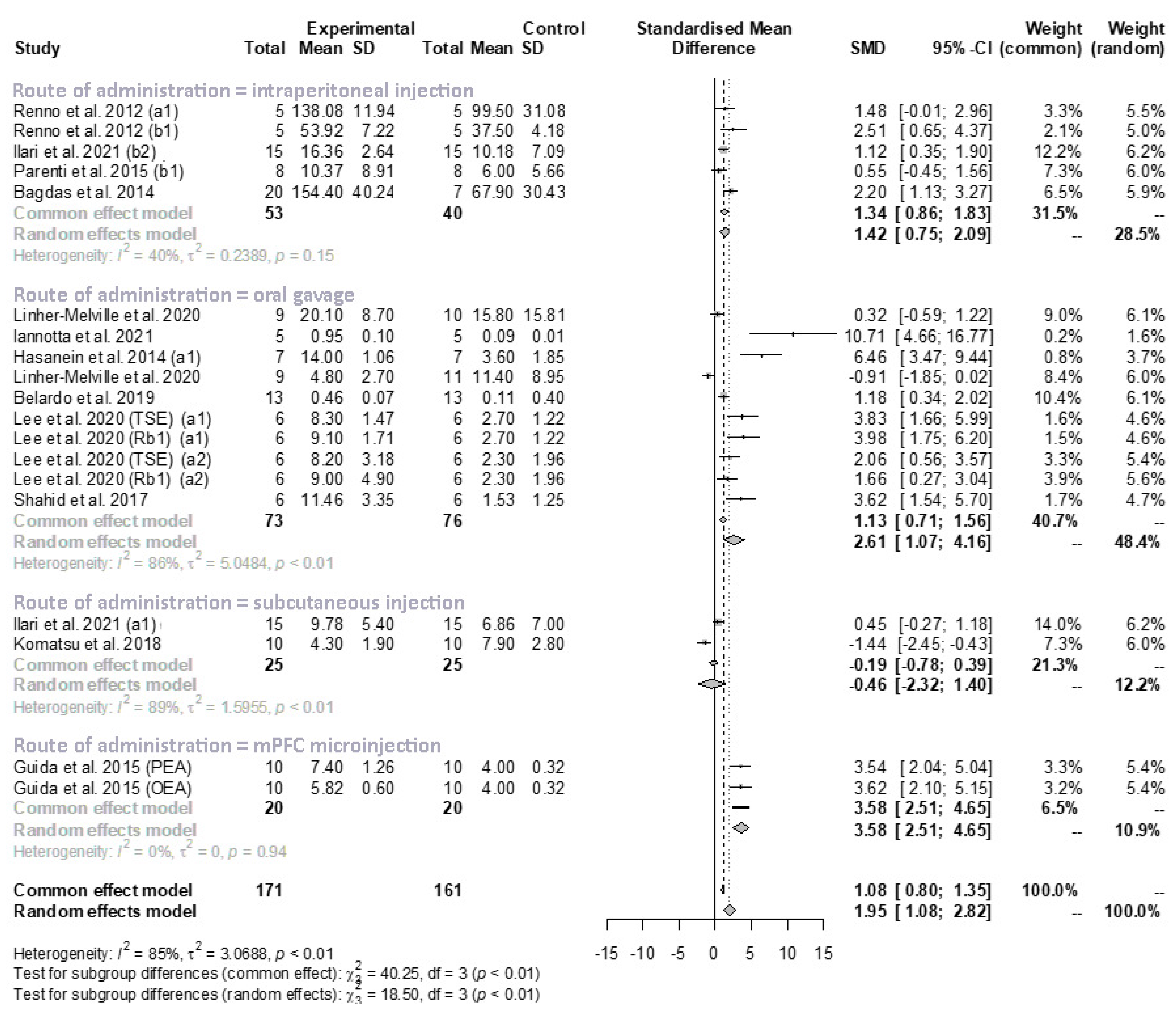
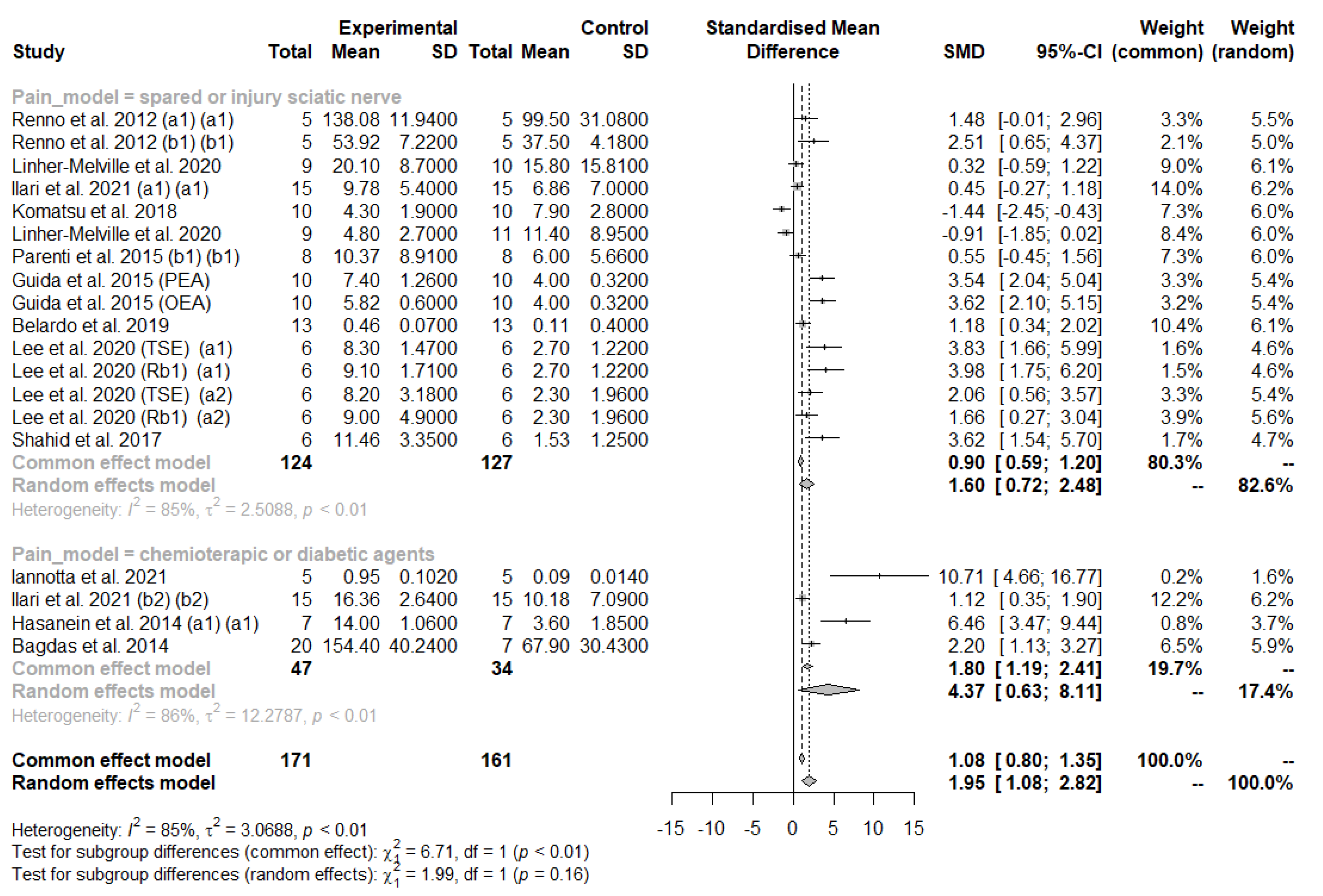
3.3.2. Meta-Analysis of Mechanical Allodynia/Hyperalgesia
Subgroup Analysis and Univariate Meta-Regression in Mechanical Allodynia/Hyperalgesia
4. Discussion
Limits and Strengths of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murnion, B.P. Neuropathic pain: Current definition and review of drug treatment. Aust. Prescr. 2018, 41, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Chen, Z.; Muscoli, C.; Bryant, L.; Esposito, E.; Cuzzocrea, S.; Dagostino, C.; Ryerse, J.; Rausaria, S.; Kamadulski, A.; et al. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J. Neurosci. 2012, 32, 6149–6160. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Tafani, M.; Russo, M.A.; Tomino, C.; Fini, M.; et al. Natural Antioxidant Control of Neuropathic Pain-Exploring the Role of Mitochondrial SIRT3 Pathway. Antioxidants 2020, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A. Neuropathic pain: Drug targets for current and future interventions. Drug News Perspect. 2004, 17, 5–17. [Google Scholar] [CrossRef]
- Raffaeli, W.; Tenti, M.; Corraro, A.; Malafoglia, V.; Ilari, S.; Balzani, E.; Bonci, A. Chronic Pain: What Does It Mean? A Review on the Use of the Term Chronic Pain in Clinical Practice. J. Pain Res. 2021, 14, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.N.; Meyer, R.A. Mechanisms of neuropathic pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Bouhassira, D.; Couturier, J.A.; Alchaar, H.; Conradi, S.; Delmotte, M.H.; Lanteri-Minet, M.; Lefaucheur, J.P.; Mick, G.; Piano, V.; et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev. Neurol. 2020, 176, 325–352. [Google Scholar] [CrossRef]
- Attal, N. Pharmacological treatments of neuropathic pain: The latest recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef]
- Szok, D.; Tajti, J.; Nyari, A.; Vecsei, L. Therapeutic Approaches for Peripheral and Central Neuropathic Pain. Behav. Neurol. 2019, 2019, 8685954. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.; Klose, P.; Welsch, P.; Petzke, F.; Hauser, W. Opioids for chronic non-cancer neuropathic pain. An updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Eur. J. Pain 2020, 24, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Tremont-Lukats, I.W.; Megeff, C.; Backonja, M.M. Anticonvulsants for neuropathic pain syndromes: Mechanisms of action and place in therapy. Drugs 2000, 60, 1029–1052. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Huang, Y. Advances in the Treatment of Neuropathic Pain. Adv. Exp. Med. Biol. 2016, 904, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Tverdohleb, T.; Nikibin, F.; Knezevic, I.; Candido, K.D. Management of chronic neuropathic pain with single and compounded topical analgesics. Pain Manag. 2017, 7, 537–558. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Kent, J.; Mackey, S.C.; Raja, S.N.; Stacey, B.R.; Levy, R.M.; Backonja, M.; Baron, R.; Harke, H.; et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 2013, 154, 2249–2261. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Kim, J.H.; Kismali, G.; Gupta, S.C. Natural Products for the Prevention and Treatment of Chronic Inflammatory Diseases: Integrating Traditional Medicine into Modern Chronic Diseases Care. Evid. Based Complement Alternat. Med. 2018, 2018, 9837863. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Ilari, S.; Dagostino, C.; Malafoglia, V.; Lauro, F.; Giancotti, L.A.; Spila, A.; Proietti, S.; Ventrice, D.; Rizzo, M.; Gliozzi, M.; et al. Protective Effect of Antioxidants in Nitric Oxide/COX-2 Interaction during Inflammatory Pain: The Role of Nitration. Antioxidants 2020, 9, 1284. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Giancotti, L.A.; Lauro, F.; Dagostino, C.; Gliozzi, M.; Malafoglia, V.; Sansone, L.; Palma, E.; Tafani, M.; Russo, M.A.; et al. Antioxidant modulation of sirtuin 3 during acute inflammatory pain: The ROS control. Pharmacol. Res. 2020, 157, 104851. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, C.; Cuzzocrea, S.; Riley, D.P.; Zweier, J.L.; Thiemermann, C.; Wang, Z.Q.; Salvemini, D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br. J. Pharmacol. 2003, 140, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, C.; Mollace, V.; Wheatley, J.; Masini, E.; Ndengele, M.; Wang, Z.Q.; Salvemini, D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: A novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain 2004, 111, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, C.; Dagostino, C.; Ilari, S.; Lauro, F.; Gliozzi, M.; Bardhi, E.; Palma, E.; Mollace, V.; Salvemini, D. Posttranslational nitration of tyrosine residues modulates glutamate transmission and contributes to N-methyl-D-aspartate-mediated thermal hyperalgesia. Mediat. Inflamm. 2013, 2013, 950947. [Google Scholar] [CrossRef] [PubMed]
- Lauro, F.; Giancotti, L.A.; Ilari, S.; Dagostino, C.; Gliozzi, M.; Morabito, C.; Malafoglia, V.; Raffaeli, W.; Muraca, M.; Goffredo, B.M.; et al. Inhibition of Spinal Oxidative Stress by Bergamot Polyphenolic Fraction Attenuates the Development of Morphine Induced Tolerance and Hyperalgesia in Mice. PLoS ONE 2016, 11, e0156039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lauro, F.; Ilari, S.; Giancotti, L.A.; Ventura, C.A.; Morabito, C.; Gliozzi, M.; Malafoglia, V.; Palma, E.; Paolino, D.; Mollace, V.; et al. Pharmacological effect of a new idebenone formulation in a model of carrageenan-induced inflammatory pain. Pharmacol. Res. 2016, 111, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Malafoglia, V.; Ilari, S.; Vitiello, L.; Tenti, M.; Balzani, E.; Muscoli, C.; Raffaeli, W.; Bonci, A. The Interplay between Chronic Pain, Opioids, and the Immune System. Neuroscientist 2021, 28, 613–627. [Google Scholar] [CrossRef]
- Chen, Z.; Muscoli, C.; Doyle, T.; Bryant, L.; Cuzzocrea, S.; Mollace, V.; Mastroianni, R.; Masini, E.; Salvemini, D. NMDA-receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain 2010, 149, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Malafoglia, V.; Traversetti, L.; del Grosso, F.; Scalici, M.; Lauro, F.; Russo, V.; Persichini, T.; Salvemini, D.; Mollace, V.; Fini, M.; et al. Transient Receptor Potential Melastatin-3 (TRPM3) Mediates Nociceptive-Like Responses in Hydra vulgaris. PLoS ONE 2016, 11, e0151386. [Google Scholar] [CrossRef] [PubMed]
- Sciskalska, M.; Oldakowska, M.; Marek, G.; Milnerowicz, H. Changes in the Activity and Concentration of Superoxide Dismutase Isoenzymes (Cu/Zn SOD, MnSOD) in the Blood of Healthy Subjects and Patients with Acute Pancreatitis. Antioxidants 2020, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell. Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Chovanova, K.; Bohmer, M.; Poljovka, A.; Budis, J.; Harichova, J.; Szemes, T.; Zamocky, M. Parallel Molecular Evolution of Catalases and Superoxide Dismutases-Focus on Thermophilic Fungal Genomes. Antioxidants 2020, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Muscoli, C.; Dagostino, C.; Giancotti, L.A.; Gliozzi, M.; Sacco, I.; Visalli, V.; Gratteri, S.; Palma, E.; Malara, N.; et al. The effect of peroxynitrite decomposition catalyst MnTBAP on aldehyde dehydrogenase-2 nitration by organic nitrates: Role in nitrate tolerance. Pharmacol. Res. 2014, 89, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Nistico, S.; Ventrice, D.; Dagostino, C.; Lauro, F.; Ilari, S.; Gliozzi, M.; Colica, C.; Musolino, V.; Carresi, C.; Strongoli, M.C.; et al. Effect of MN (III) tetrakis (4-benzoic acid) porphyrin by photodynamically generated free radicals on SODs keratinocytes. J. Biol. Regul. Homeost. Agents 2013, 27, 781–790. [Google Scholar] [PubMed]
- Zhou, Q.; Einert, M.; Schmitt, H.; Wang, Z.; Pankratz, F.; Olivier, C.B.; Bode, C.; Liao, J.K.; Moser, M. MnTBAP increases BMPR-II expression in endothelial cells and attenuates vascular inflammation. Vascul. Pharmacol. 2016, 84, 67–73. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Cuzzocrea, S.; Reboucas, J.S.; Ferrer-Sueta, G.; Mazzon, E.; di Paola, R.; Radi, R.; Spasojevic, I.; Benov, L.; Salvemini, D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: Comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic. Biol. Med. 2009, 46, 192–201. [Google Scholar] [CrossRef] [PubMed]
- El-Senousey, H.K.; Chen, B.; Wang, J.Y.; Atta, A.M.; Mohamed, F.R.; Nie, Q.H. Effects of dietary vitamin C, vitamin E, and alpha-lipoic acid supplementation on the antioxidant defense system and immune-related gene expression in broilers exposed to oxidative stress by dexamethasone. Poult. Sci. 2018, 97, 30–38. [Google Scholar] [CrossRef]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Gyuraszova, M.; Kovalcikova, A.; Jansakova, K.; Sebekova, K.; Celec, P.; Tothova, L. Markers of oxidative stress and antioxidant status in the plasma, urine and saliva of healthy mice. Physiol. Res. 2018, 67, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Tomino, C.; Ilari, S.; Solfrizzi, V.; Malafoglia, V.; Zilio, G.; Russo, P.; Proietti, S.; Marcolongo, F.; Scapagnini, G.; Muscoli, C.; et al. Mild Cognitive Impairment and Mild Dementia: The Role of Ginkgo biloba (EGb 761((R))). Pharmaceuticals 2021, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Li, S.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.M.; Issaoui, M.; Chammem, N. Analysis of Main and Healthy Phenolic Compounds in Foods. J. AOAC Int. 2019, 102, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Martinez, Y.; Mas, D.; Betancur, C.; Gebeyew, K.; Adebowale, T.; Hussain, T.; Lan, W.; Ding, X. Role of the Phytochemical Compounds like Modulators in Gut Microbiota and Oxidative Stress. Curr. Pharm. Des. 2020, 26, 2642–2656. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Dos Santos, J.M.; Alexander, M.Y. Modulating Oxidative Stress in Drug-Induced Injury and Metabolic Disorders: The Role of Natural and Synthetic Antioxidants. Oxid. Med. Cell. Longev. 2019, 2019, 3206401. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Borgonetti, V.; Lopez, V.; Galeotti, N. Ylang-ylang (Cananga odorata (Lam.) Hook. f. & Thomson) essential oil reduced neuropathic-pain and associated anxiety symptoms in mice. J. Ethnopharmacol. 2022, 294, 115362. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Liu, H.F.; Sun, H.; Xiao, C.J.; Jiang, B.; Shen, L. Antinociceptive effect of ethanolic extract of Bauhinia brachycarpa Benth on neuropathic pain model induced by partial sciatic nerve ligation. J. Ethnopharmacol. 2022, 295, 115412. [Google Scholar] [CrossRef] [PubMed]
- Abed, D.Z.; Sadeghian, R.; Mohammadi, S.; Akram, M. Thymus persicus (Ronniger ex Rech. f.) Jalas alleviates nociceptive and neuropathic pain behavior in mice: Multiple mechanisms of action. J. Ethnopharmacol. 2022, 283, 114695. [Google Scholar] [CrossRef]
- Kwankaew, N.; Okuda, H.; Aye-Mon, A.; Ishikawa, T.; Hori, K.; Sonthi, P.; Kozakai, Y.; Ozaki, N. Antihypersensitivity effect of betanin (red beetroot extract) via modulation of microglial activation in a mouse model of neuropathic pain. Eur. J. Pain 2021, 25, 1788–1803. [Google Scholar] [CrossRef] [PubMed]
- Ahmadimoghaddam, D.; Zarei, M.; Mohammadi, S.; Izadidastenaei, Z.; Salehi, I. Bupleurum falcatum L. alleviates nociceptive and neuropathic pain: Potential mechanisms of action. J. Ethnopharmacol. 2021, 273, 113990. [Google Scholar] [CrossRef] [PubMed]
- Sukmawan, Y.P.; Anggadiredja, K.; Adnyana, I.K. Anti-Neuropathic Pain Activity of Ageratum conyzoides L due to the Essential Oil Components. CNS Neurol. Disord. Drug Targets 2021, 20, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Rouhollahi, E.; MacLeod, B.A.; Barr, A.M.; Puil, E. Cannabis Extract CT-921 Has a High Efficacy-Adverse Effect Profile in a Neuropathic Pain Model. Drug Des. Devel. Ther. 2020, 14, 3351–3361. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Biagi, M.; Pellati, F.; Galeotti, N. Zingiber officinale Roscoe rhizome extract alleviates neuropathic pain by inhibiting neuroinflammation in mice. Phytomedicine 2020, 78, 153307. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Pareek, A.; Bhardwaj, Y.R.; Sinha, S.K.; Gupta, M.M.; Singh, N. Punicalagin and ellagic acid containing Punica granatum L. fruit rind extract prevents vincristine-induced neuropathic pain in rats: An in silico and in vivo evidence of GABAergic action and cytokine inhibition. Nutr. Neurosci. 2022, 25, 2149–2166. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Rombola, L.; Amantea, D.; Russo, R.; Adornetto, A.; Berliocchi, L.; Tridico, L.; Corasaniti, M.T.; Sakurada, S.; Sakurada, T.; Bagetta, G.; et al. Rational Basis for the Use of Bergamot Essential Oil in Complementary Medicine to Treat Chronic Pain. Mini Rev. Med. Chem. 2016, 16, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Katsuyama, S.; Uezono, Y.; Sakurada, C.; Tsuzuki, M.; Hamamura, K.; Bagetta, G.; Sakurada, S.; Sakurada, T. Possible involvement of the peripheral Mu-opioid system in antinociception induced by bergamot essential oil to allodynia after peripheral nerve injury. Neurosci. Lett. 2018, 686, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Parafati, M.; Lascala, A.; La Russa, D.; Mignogna, C.; Trimboli, F.; Morittu, V.M.; Riillo, C.; Macirella, R.; Mollace, V.; Brunelli, E.; et al. Bergamot Polyphenols Boost Therapeutic Effects of the Diet on Non-Alcoholic Steatohepatitis (NASH) Induced by “Junk Food”: Evidence for Anti-Inflammatory Activity. Nutrients 2018, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Lauro, F.; Giancotti, L.A.; Malafoglia, V.; Dagostino, C.; Gliozzi, M.; Condemi, A.; Maiuolo, J.; Oppedisano, F.; Palma, E.; et al. The Protective Effect of Bergamot Polyphenolic Fraction (BPF) on Chemotherapy-Induced Neuropathic Pain. Pharmaceuticals 2021, 14, 975. [Google Scholar] [CrossRef]
- Iannotta, M.; Belardo, C.; Trotta, M.C.; Iannotti, F.A.; Vitale, R.M.; Maisto, R.; Boccella, S.; Infantino, R.; Ricciardi, F.; Mirto, B.F.; et al. N-palmitoyl-D-glucosamine, a Natural Monosaccharide-Based Glycolipid, Inhibits TLR4 and Prevents LPS-Induced Inflammation and Neuropathic Pain in Mice. Int. J. Mol. Sci. 2021, 22, 1491. [Google Scholar] [CrossRef]
- Tenci, B.; di Mannelli, L.C.; Maresca, M.; Micheli, L.; Pieraccini, G.; Mulinacci, N.; Ghelardini, C. Effects of a water extract of Lepidium meyenii root in different models of persistent pain in rats. Z. Naturforsch. C J. Biosci. 2017, 72, 449–457. [Google Scholar] [CrossRef]
- Bagdas, D.; Ozboluk, H.Y.; Cinkilic, N.; Gurun, M.S. Antinociceptive effect of chlorogenic acid in rats with painful diabetic neuropathy. J. Med. Food 2014, 17, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Linher-Melville, K.; Zhu, Y.F.; Sidhu, J.; Parzei, N.; Shahid, A.; Seesankar, G.; Ma, D.; Wang, Z.; Zacal, N.; Sharma, M.; et al. Evaluation of the preclinical analgesic efficacy of naturally derived, orally administered oil forms of Delta9-tetrahydrocannabinol (THC), cannabidiol (CBD), and their 1:1 combination. PLoS ONE 2020, 15, e0234176. [Google Scholar] [CrossRef]
- Luongo, L.; Maione, S.; di Marzo, V. Endocannabinoids and neuropathic pain: Focus on neuron-glia and endocannabinoid-neurotrophin interactions. Eur. J. Neurosci. 2014, 39, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Belardo, C.; Iannotta, M.; Boccella, S.; Rubino, R.C.; Ricciardi, F.; Infantino, R.; Pieretti, G.; Stella, L.; Paino, S.; Marabese, I.; et al. Oral Cannabidiol Prevents Allodynia and Neurological Dysfunctions in a Mouse Model of Mild Traumatic Brain Injury. Front. Pharmacol. 2019, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- De Novellis, V.; Luongo, L.; Guida, F.; Cristino, L.; Palazzo, E.; Russo, R.; Marabese, I.; D’Agostino, G.; Calignano, A.; Rossi, F.; et al. Effects of intra-ventrolateral periaqueductal grey palmitoylethanolamide on thermoceptive threshold and rostral ventromedial medulla cell activity. Eur. J. Pharmacol. 2012, 676, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Luongo, L.; Marmo, F.; Romano, R.; Iannotta, M.; Napolitano, F.; Belardo, C.; Marabese, I.; D’Aniello, A.; de Gregorio, D.; et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol. Brain 2015, 8, 47. [Google Scholar] [CrossRef]
- Renno, W.M.; Al-Maghrebi, M.; Al-Banaw, A. (-)-Epigallocatechin-3-gallate (EGCG) attenuates functional deficits and morphological alterations by diminishing apoptotic gene overexpression in skeletal muscles after sciatic nerve crush injury. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Xifro, X.; Vidal-Sancho, L.; Boadas-Vaello, P.; Turrado, C.; Alberch, J.; Puig, T.; Verdu, E. Novel epigallocatechin-3-gallate (EGCG) derivative as a new therapeutic strategy for reducing neuropathic pain after chronic constriction nerve injury in mice. PLoS ONE 2015, 10, e0123122. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Mola, M.; Homs, J.; Alvarez-Perez, B.; Puig, T.; Reina, F.; Verdu, E.; Boadas-Vaello, P. (-)-Epigallocatechin-3-Gallate Antihyperalgesic Effect Associates With Reduced CX3CL1 Chemokine Expression in Spinal Cord. Phytother. Res. 2017, 31, 340–344. [Google Scholar] [CrossRef]
- Ishola, I.O.; Awodele, O.; Olusayero, A.M.; Ochieng, C.O. Mechanisms of analgesic and anti-inflammatory properties of Annona muricata Linn. (Annonaceae) fruit extract in rodents. J. Med. Food 2014, 17, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Rosmarinic acid sensitizes cell death through suppression of TNF-alpha-induced NF-kappaB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 2010, 288, 183–191. [Google Scholar] [CrossRef]
- Lucarini, R.; Bernardes, W.A.; Ferreira, D.S.; Tozatti, M.G.; Furtado, R.; Bastos, J.K.; Pauletti, P.M.; Januario, A.H.; Silva, M.L.; Cunha, W.R. In vivo analgesic and anti-inflammatory activities of Rosmarinus officinalis aqueous extracts, rosmarinic acid and its acetyl ester derivative. Pharm. Biol. 2013, 51, 1087–1090. [Google Scholar] [CrossRef]
- Chu, X.; Ci, X.; He, J.; Jiang, L.; Wei, M.; Cao, Q.; Guan, M.; Xie, X.; Deng, X.; He, J. Effects of a natural prolyl oligopeptidase inhibitor, rosmarinic acid, on lipopolysaccharide-induced acute lung injury in mice. Molecules 2012, 17, 3586–3598. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Zaheri, L.M. Effects of rosmarinic acid on an experimental model of painful diabetic neuropathy in rats. Pharm. Biol. 2014, 52, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Rhee, K.H. Anti-Nociceptive Effect of Agrimonia Eupatoria Extract on a Cisplatin-Induced Neuropathic Model. Afr. J. Tradit. Complement Altern. Med. 2016, 13, 139–144. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Park, C.S.; Kim, D.H.; Yune, T.Y. Total saponin extract, ginsenoside Rb1, and compound K alleviate peripheral and central neuropathic pain through estrogen receptors on rats. Phytother. Res. 2021, 35, 2119–2132. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, E.M.; Kim, D.H.; Jung, K.; Jung, J.S.; Lee, E.J.; Hyun, J.W.; Kang, J.L.; Kim, H.S. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J. Neuroimmunol. 2009, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, I.M.; Ojewole, J.A. Analgesic, antiinflammatory and antidiabetic properties of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract. Phytother. Res. 2004, 18, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Argentieri, M.P. Quality Assessment of Commercial Spagyric Tinctures of Harpagophytum procumbens and Their Antioxidant Properties. Molecules 2019, 24, 2251. [Google Scholar] [CrossRef] [PubMed]
- Parenti, C.; Arico, G.; Pennisi, M.; Venditti, A.; Scoto, G.M. Harpagophytum procumbens extract potentiates morphine antinociception in neuropathic rats. Nat. Prod. Res. 2016, 30, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Subhan, F.; Ahmad, N.; Ullah, I. A bacosides containing Bacopa monnieri extract alleviates allodynia and hyperalgesia in the chronic constriction injury model of neuropathic pain in rats. BMC Complement Altern. Med. 2017, 17, 293. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Seong, A.R.; Yoo, J.Y.; Jin, C.H.; Lee, Y.H.; Kim, Y.J.; Lee, J.; Jun, W.J.; Yoon, H.G. Gallic acid, a histone acetyltransferase inhibitor, suppresses beta-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol. Nutr. Food Res. 2011, 55, 1798–1808. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Trevisan, G.; Rossato, M.F.; Tonello, R.; Hoffmeister, C.; Klafke, J.Z.; Rosa, F.; Pinheiro, K.V.; Pinheiro, F.V.; Boligon, A.A.; Athayde, M.L.; et al. Gallic acid functions as a TRPA1 antagonist with relevant antinociceptive and antiedematogenic effects in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 679–689. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S100–S110. [Google Scholar] [CrossRef]
- Davidson, E.P.; Coppey, L.J.; Shevalye, H.; Obrosov, A.; Kardon, R.H.; Yorek, M.A. Impaired Corneal Sensation and Nerve Loss in a Type 2 Rat Model of Chronic Diabetes Is Reversible with Combination Therapy of Menhaden Oil, alpha-Lipoic Acid, and Enalapril. Cornea 2017, 36, 725–731. [Google Scholar] [CrossRef] [PubMed]
- de Cavanagh, E.M.; Inserra, F.; Toblli, J.; Stella, I.; Fraga, C.G.; Ferder, L. Enalapril attenuates oxidative stress in diabetic rats. Hypertension 2001, 38, 1130–1136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maglione, E.; Marrese, C.; Migliaro, E.; Marcuccio, F.; Panico, C.; Salvati, C.; Citro, G.; Quercio, M.; Roncagliolo, F.; Torello, C.; et al. Increasing bioavailability of (R)-alpha-lipoic acid to boost antioxidant activity in the treatment of neuropathic pain. Acta Biomed. 2015, 86, 226–233. [Google Scholar] [PubMed]
- Holmes, A.; Coppey, L.J.; Davidson, E.P.; Yorek, M.A. Rat Models of Diet-Induced Obesity and High Fat/Low Dose Streptozotocin Type 2 Diabetes: Effect of Reversal of High Fat Diet Compared to Treatment with Enalapril or Menhaden Oil on Glucose Utilization and Neuropathic Endpoints. J. Diabetes Res. 2015, 2015, 307285. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Clapham, H.E.; Tan, A.T.; Chia, W.N.; Tham, C.Y.L.; Lim, J.M.; Kunasegaran, K.; Tan, L.W.L.; Dutertre, C.A.; Shankar, N.; et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 2021, 218, e20202617. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, S.; Wang, J.; Wang, J.; Yan, Y.; Zhu, M.; Zhang, D.; Jiang, C.; Liu, T. Oxidative stress induced by NOX2 contributes to neuropathic pain via plasma membrane translocation of PKCepsilon in rat dorsal root ganglion neurons. J. Neuroinflamm. 2021, 18, 106. [Google Scholar] [CrossRef] [PubMed]


| Drugs | Mechanism of Action | Side Effect | Reference |
|---|---|---|---|
| Triciclic antidepressant (TCA) |
| somnolence, dizziness, suicide risk, urinary retention | [9] |
| Serotonin-noradrenalin reuptake inhibitors |
| nausea, hypertension, ataxia, lethargy | [14] |
| Anticonvulsant |
| sedation, dizziness, oedema | [11] |
| Local anesthetics - Licodaine |
| local erythema, rash | [13] |
| Opioids |
| nausea, vomiting, somnolence, dizziness | [13] |
| Polyphenols. | Flavonoids
| Flavones |
| Flavanols | ||
| Isoflavones | ||
| Flavanones | ||
| Anthocyanidis | ||
| Flavanols | ||
Phenolic Acid
| Hydroxycimamic Acids | |
| Hydrobenzoic Acids | ||
Lignans
| Secosolariciresinal diglucoside | |
Stilbenes
| Resveratrol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilari, S.; Proietti, S.; Russo, P.; Malafoglia, V.; Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Palma, E.; Tomino, C.; Fini, M.; et al. A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies. Antioxidants 2022, 11, 2361. https://doi.org/10.3390/antiox11122361
Ilari S, Proietti S, Russo P, Malafoglia V, Gliozzi M, Maiuolo J, Oppedisano F, Palma E, Tomino C, Fini M, et al. A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies. Antioxidants. 2022; 11(12):2361. https://doi.org/10.3390/antiox11122361
Chicago/Turabian StyleIlari, Sara, Stefania Proietti, Patrizia Russo, Valentina Malafoglia, Micaela Gliozzi, Jessica Maiuolo, Francesca Oppedisano, Ernesto Palma, Carlo Tomino, Massimo Fini, and et al. 2022. "A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies" Antioxidants 11, no. 12: 2361. https://doi.org/10.3390/antiox11122361
APA StyleIlari, S., Proietti, S., Russo, P., Malafoglia, V., Gliozzi, M., Maiuolo, J., Oppedisano, F., Palma, E., Tomino, C., Fini, M., Raffaeli, W., Mollace, V., Bonassi, S., & Muscoli, C. (2022). A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies. Antioxidants, 11(12), 2361. https://doi.org/10.3390/antiox11122361











