Neuroprotective Effect of Stearidonic Acid on Amyloid β-Induced Neurotoxicity in Rat Hippocampal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Fatty Acid Treatment
2.3. Analysis of EPA and DHA Content
2.4. MTT Assay
2.5. Total RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.6. Western Blot Analysis
2.7. Total Antioxidant Capacity (T-AOC) Assay
2.8. Catalase Activity Assay
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Statistical Analysis
3. Results
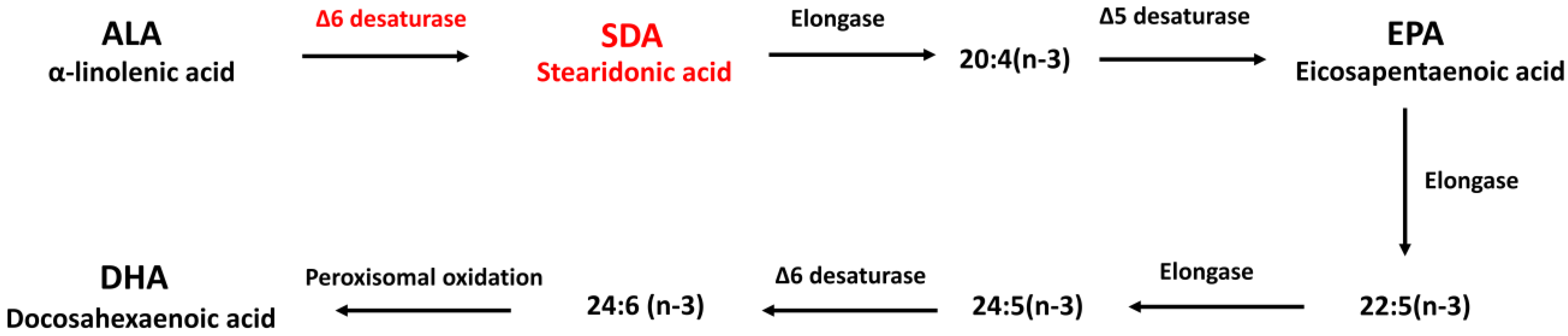
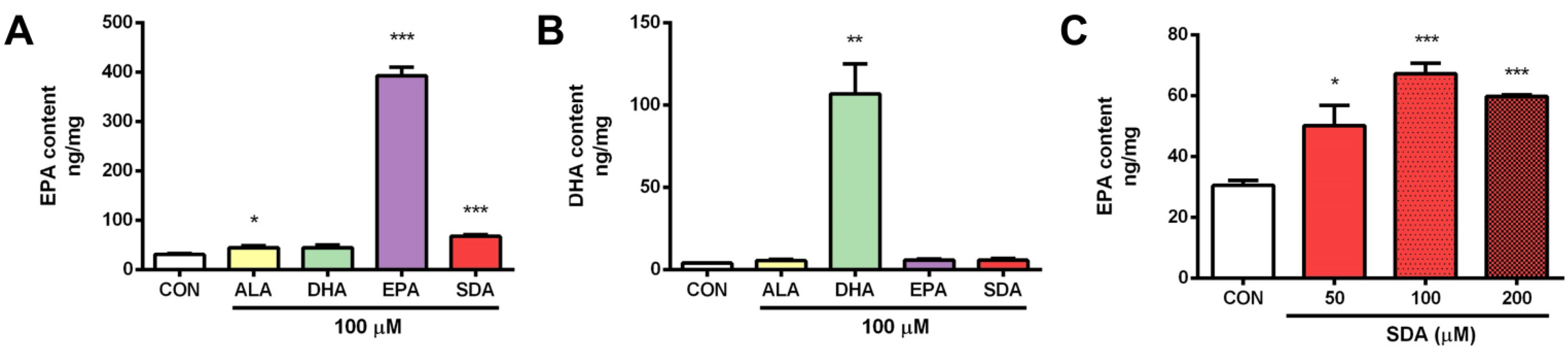
3.1. SDA Effectively Converted to EPA in Rat Hippocampal Cells
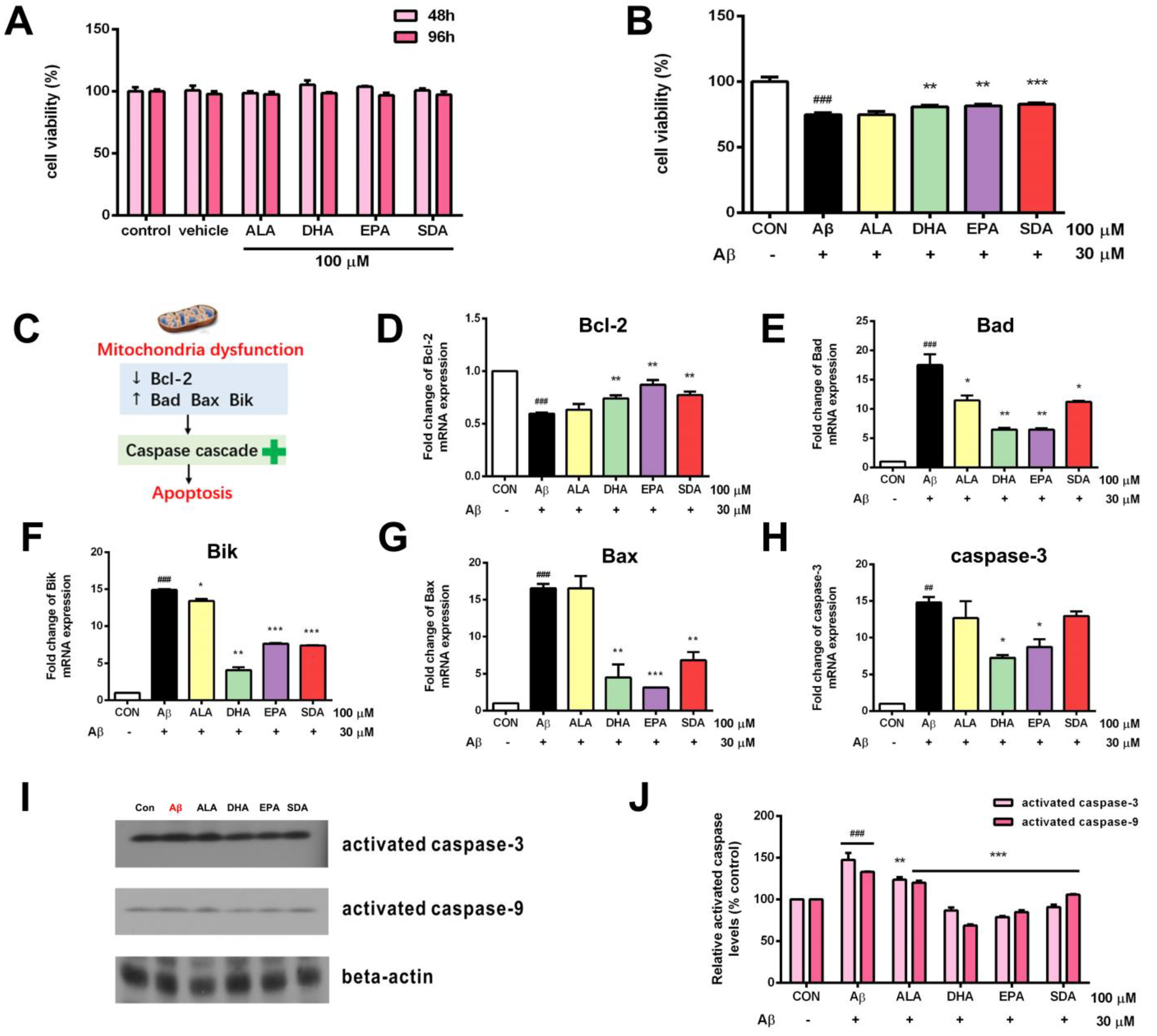
3.2. SDA Protects against Aβ-Induced Hippocampal Cell Death
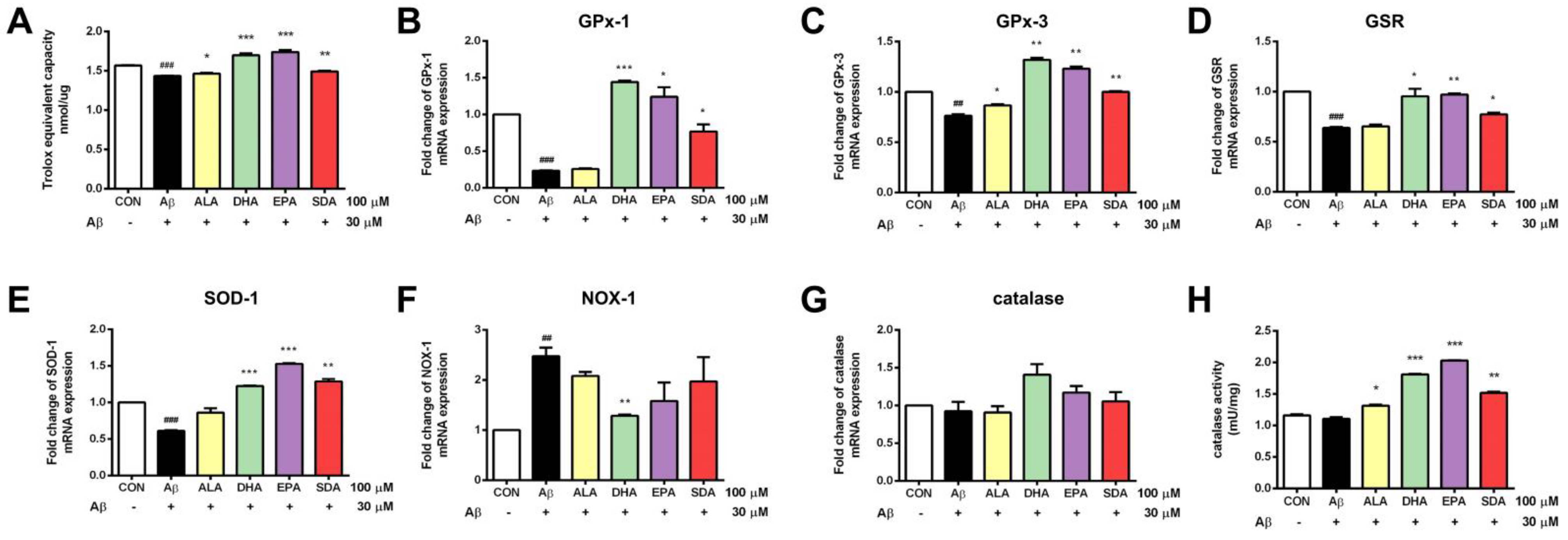
3.3. SDA Protects against Aβ-Induced Oxidative Stress in Rat Hippocampal Cells
3.4. SDA Protects against Aβ-Induced Inflammation in Rat Hippocampal Cells
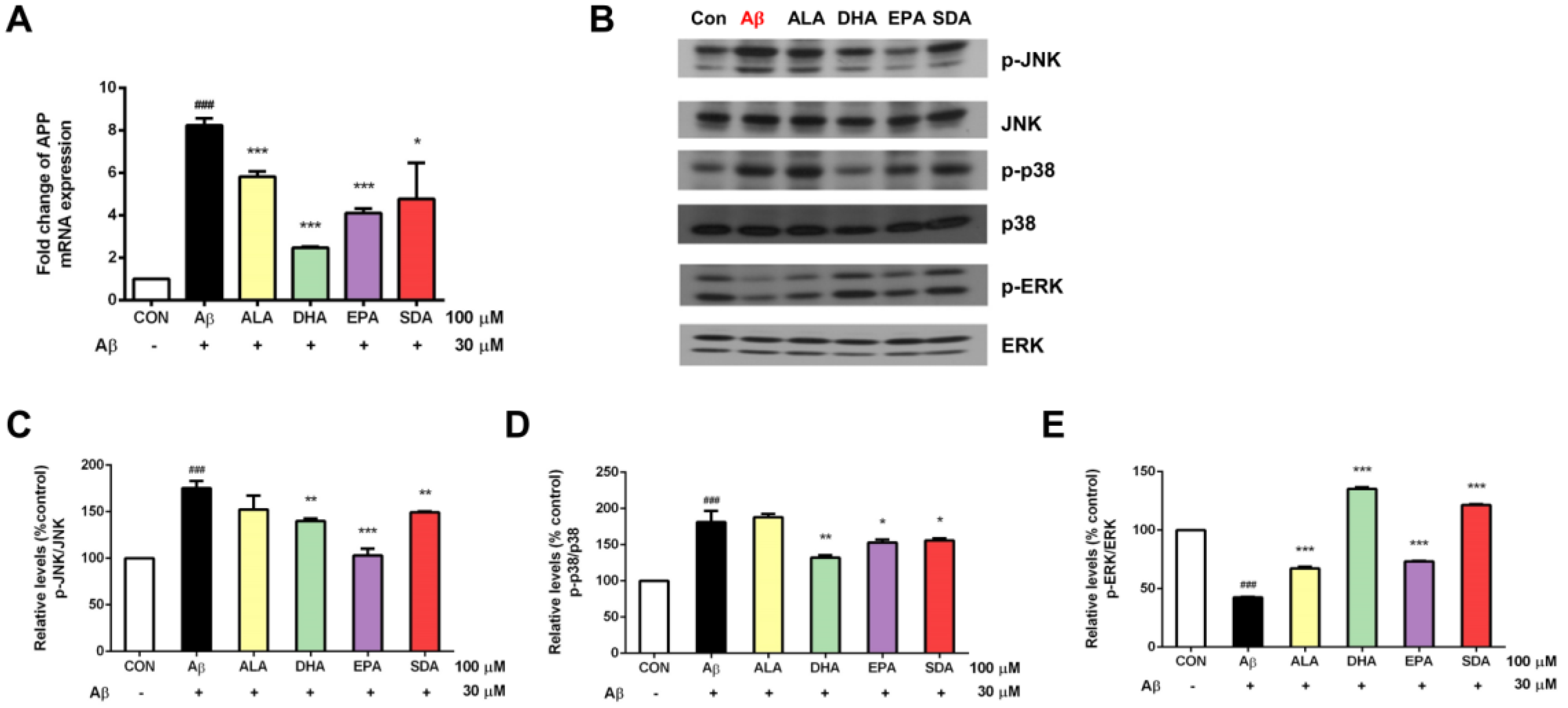
3.5. The Anti-Aβ Effect of SDA Involves Its Inhibition on APP Expression and Regulation on MAPK Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nedelec, T.; Couvy-Duchesne, B.; Monnet, F.; Daly, T.; Ansart, M.; Gantzer, L.; Lekens, B.; Epelbaum, S.; Dufouil, C.; Durrleman, S. Identifying health conditions associated with Alzheimer’s disease up to 15 years before diagnosis: An agnostic study of French and British health records. Lancet Digit. Health 2022, 4, e169–e178. [Google Scholar] [CrossRef]
- Yu, T.W.; Lane, H.Y.; Lin, C.E.H. Novel Therapeutic Approaches for Alzheimer’s Disease: An Updated Review. Int. J. Mol. Sci. 2021, 22, 8208. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.R.; Bäckman, K.; Scarmeas, N.; Stern, Y.; Manly, J.J.; Mayeux, R.; Gu, Y. Dietary fatty acids and risk of Alzheimer’s disease and related dementias: Observations from the Washington Heights-Hamilton Heights-Inwood Columbia Aging Project (WHICAP). Alzheimer’s Dement. 2020, 16, 1638–1649. [Google Scholar] [CrossRef]
- Yan, L.; Xie, Y.; Satyanarayanan, S.K.; Zeng, H.; Liu, H.; Huang, M.; Ma, Y.; Wan, J.-B.; Yao, X.; Su, K.-P.; et al. Omega-3 polyunsaturated fatty acids promote brain-to-blood clearance of β-Amyloid in a mouse model with Alzheimer’s disease. Brain Behav. Immun. 2020, 85, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Peinado, I.; Miles, W.; Koutsidis, G. Odour characteristics of seafood flavour formulations produced with fish by-products incorporating EPA, DHA and fish oil. Food Chem. 2016, 212, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.A.; Cheung, W.W.L.; Anticamara, J.A.; Sumaila, R.U.; Zeller, D.; Pauly, D. Global marine yield halved as fishing intensity redoubles. Fish Fish. 2012, 14, 493–503. [Google Scholar] [CrossRef]
- Sioen, I.; De Henauw, S.; Van Camp, J. Evaluation of benefits and risks related to seafood consumption. Verh. K. Acad. Voor Geneeskd. Belg. 2007, 69, 249–289. [Google Scholar]
- Prasad, P.; Anjali, P.; Sreedhar, R.V. Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Crit. Rev. Food Sci. Nutr. 2020, 61, 1725–1737. [Google Scholar] [CrossRef]
- Li, Y.; Rong, Y.; Bao, L.; Nie, B.; Ren, G.; Zheng, C.; Amin, R.; Arnold, R.D.; Jeganathan, R.B.; Huggins, K.W. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipids Health Dis. 2017, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Gouffon, J.; Zhao, Y. Effects of Dietary Stearidonic Acid on Biomarkers of Lipid Metabolism. J. Nutr. 2012, 142, 630S–634S. [Google Scholar] [CrossRef]
- Lemke, S.L.; Vicini, J.L.; Su, H.; Goldstein, D.A.; Nemeth, M.A.; Krul, E.S.; Harris, W.S. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: Randomized, double-blind clinical study of efficacy and safety. Am. J. Clin. Nutr. 2010, 92, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, K.; Fuhrmann, C.; Köhler, M.; Kiehntopf, M.; Jahreis, G. Dietary Echium Oil Increases Long-Chain n–3 PUFAs, Including Docosapentaenoic Acid, in Blood Fractions and Alters Biochemical Markers for Cardiovascular Disease Independently of Age, Sex, and Metabolic Syndrome. J. Nutr. 2014, 144, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Surette, M.E.; Edens, M.; Chilton, F.H.; Tramposch, K.M. Dietary echium oil increases plasma and neutrophil long-chain (n-3) fatty acids and lowers serum triacylglycerols in hypertriglyceridemic humans. J. Nutr. 2004, 134, 1406–1411. [Google Scholar] [CrossRef]
- Casey, J.M.; Banz, W.J.; Krul, E.S.; Butteiger, D.N.; Goldstein, D.A.; Davis, J.E. Effect of stearidonic acid-enriched soybean oil on fatty acid profile and metabolic parameters in lean and obese Zucker rats. Lipids Health Dis. 2013, 12, 147. [Google Scholar] [CrossRef]
- James, M.J.; Ursin, V.M.; Cleland, L.G. Metabolism of stearidonic acid in human subjects: Comparison with the metabolism of other n−3 fatty acids. Am. J. Clin. Nutr. 2003, 77, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Krul, E.; Lemke, S.; Mukherjea, R.; Taylor, M.; Goldstein, D.; Su, H.; Liu, P.; Lawless, A.; Harris, W.; Maki, K. Effects of duration of treatment and dosage of eicosapentaenoic acid and stearidonic acid on red blood cell eicosapentaenoic acid content. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 51–59. [Google Scholar] [CrossRef]
- Lemke, S.L.; Maki, K.C.; Hughes, G.; Taylor, M.L.; Krul, E.S.; Goldstein, D.A.; Su, H.; Rains, T.M.; Mukherjea, R. Consumption of Stearidonic Acid−Rich Oil in Foods Increases Red Blood Cell Eicosapentaenoic Acid. J. Acad. Nutr. Diet. 2013, 113, 1044–1056. [Google Scholar] [CrossRef]
- Harris, W.S. Stearidonic acid as a ‘pro-eicosapentaenoic acid’. Curr. Opin. Lipidol. 2012, 23, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Botelho, P.B.; Mariano, K.D.R.; Rogero, M.M.; de Castro, I.A. Effect of Echium oil compared with marine oils on lipid profile and inhibition of hepatic steatosis in LDLr knockout mice. Lipids Health Dis. 2013, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Forrest, L.M.; Boudyguina, E.; Wilson, M.D.; Parks, J.S. Echium oil reduces atherosclerosis in apoB100-only LDLrKO mice. Atherosclerosis 2012, 220, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Arm, J.P.; Boyce, J.A.; Wang, L.; Chhay, H.; Zahid, M.; Patil, V.; Govindarajulu, U.; Ivester, P.; Weaver, K.L.; Sergeant, S.; et al. Impact of botanical oils on polyunsaturated fatty acid metabolism and leukotriene generation in mild asthmatics. Lipids Health Dis. 2013, 12, 141. [Google Scholar] [CrossRef]
- Hsueh, H.W.; Zhou, Z.; Whelan, J.; Allen, K.G.D.; Moustaid-Moussa, N.; Kim, H.; Claycombe, K.J. Stearidonic and Eicosapentaenoic Acids Inhibit Interleukin-6 Expression in ob/ob Mouse Adipose Stem Cells via Toll-like Receptor-2–Mediated Pathways. J. Nutr. 2011, 141, 1260–1266. [Google Scholar] [CrossRef]
- Subedi, K.; Yu, H.-M.; Newell, M.; Weselake, R.J.; Meesapyodsuk, D.; Qiu, X.; Shah, S.; Field, C.J. Stearidonic acid-enriched flax oil reduces the growth of human breast cancer in vitro and in vivo. Breast Cancer Res. Treat. 2014, 149, 17–29. [Google Scholar] [CrossRef]
- Kotlęga, D.; Peda, B.; Palma, J.; Zembroń-Łacny, A.; Gołąb-Janowska, M.; Masztalewicz, M.; Nowacki, P.; Szczuko, M. Free Fatty Acids Are Associated with the Cognitive Functions in Stroke Survivors. Int. J. Environ. Res. Public Health 2021, 18, 6500. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.; Bottcher, M.; Geetha, T.; Broderick, T.; Babu, J. Neuroprotective effects of resveratrol against β-amyloid induced oxidative damage and memory loss in rat hippocampal (H19-7) cells (647.44). FASEB J. 2014, 28, 647.44. [Google Scholar] [CrossRef]
- Wood, A.H.R.; Chappell, H.F.; Zulyniak, M.A. Dietary and supplemental long-chain omega-3 fatty acids as moderators of cognitive impairment and Alzheimer’s disease. Eur. J. Nutr. 2021, 61, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.; Montine, T.J. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark. Med. 2010, 4, 27–36. [Google Scholar] [CrossRef]
- Bonda, D.J.; Wang, X.; Perry, G.; Nunomura, A.; Tabaton, M.; Zhu, X.; Smith, M.A. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology 2010, 59, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.; Geetha, T.; Broderick, T.L.; Babu, J.R. Resveratrol Protects β Amyloid-Induced Oxidative Damage and Memory Associated Proteins in H19-7 Hippocampal Neuronal Cells. Curr. Alzheimer Res. 2015, 12, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, Y.-J.; Son, I.H.; Yang, H.D. Inhibition of beta-amyloid (1-40) Peptide Aggregation and Neurotoxicity by Citrate. Korean J. Physiol. Pharmacol. 2009, 13, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Mazza, M.; Pomponi, M.; Janiri, L.; Bria, P.; Mazza, S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction, Oxidative Stress, and Neuroinflammation: Intertwined Roads to Neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-M.; Park, J.; Kim, S.-H.; Jung, Y.-K. Emerging perspectives on mitochondrial dysfunction and inflammation in Alzheimer’s disease. BMB Rep. 2020, 53, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, I.; Zimin, P.I.; Wulff, H.; Jin, L.-W. Amyloid-beta protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J. Biol. Chem. 2011, 286, 3693–3706. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Varga, E.; Tamási, K.; Pap, R.; Nagy, J.; Sipos, K. Effect of Inflammatory Mediators Lipopolysaccharide and Lipoteichoic Acid on Iron Metabolism of Differentiated SH-SY5Y Cells Alters in the Presence of BV-2 Microglia. Int. J. Mol. Sci. 2018, 20, 17. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek, M.; Jabłoński, M. Alzheimer’s mechanisms in ischemic brain degeneration. Anat. Rec. 2009, 292, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Probst, S.; Krüger, M.; Kägi, L.; Thöni, S.; Schuppli, D.; Nitsch, R.M.; Konietzko, U. Fe65 is the sole member of its family that mediates transcription regulated by the amyloid precursor protein. J. Cell Sci. 2020, 133, jcs242917. [Google Scholar] [CrossRef]
- Moir, R.D.; Lathe, R.; Tanzi, R.E. The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 1602–1614. [Google Scholar] [CrossRef]
- Priller, C.; Bauer, T.; Mitteregger, G.; Krebs, B.; Kretzschmar, H.A.; Herms, J. Synapse Formation and Function Is Modulated by the Amyloid Precursor Protein. J. Neurosci. 2006, 26, 7212–7221. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.R.; O’Connor, K.; Tate, W.P.; Abraham, W.C. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 2003, 70, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Sagare, A.P.; Coma, M.; Perlmutter, D.; Gelein, R.; Bell, R.D.; Deane, R.J.; Zhong, E.; Parisi, M.; Ciszewski, J.; et al. Low levels of copper disrupt brain amyloid-beta homeostasis by altering its production and clearance. Proc. Natl. Acad. Sci. USA 2013, 110, 14771–14776. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Fang, F.; Zhang, W.; Yan, S.; Fukuzaki, E.; Du, H.; Sosunov, A.; McKhann, G.; Funatsu, Y.; Nakamichi, N.; et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 20021–20026. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, G.; Dolatshahi, M.; Rahmani, F.; Rezaei, N. Role of p38/MAPKs in Alzheimer’s disease: Implications for amyloid beta toxicity targeted therapy. Rev. Neurosci. 2018, 30, 9–30. [Google Scholar] [CrossRef]
- Bar-Am, O.; Amit, T.; Weinred, O.; Youdim, M.B.H.; Mandel, S. Propargylamine containing compounds as modulators of proteolytic cleavage of amyloid-beta protein precursor: Involvement of MAPK and PKC activation. J. Alzheimer’s Dis. 2010, 21, 361–371. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet Nutrition and the Prevention of Chronic Diseases: Report of the WHO/FAO Joint Expert Consultation; Technical Report Series 916; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Verhagen, H. Review of labelling reference intake values—Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the review of labelling reference intake values for selected nutritional elements. EFSA J. 2009, 1008, 1–14. [Google Scholar]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- FAO; WHO. Report of the Joint Expert Consultation on the Risks and Benefits of Fish Consumption. In FAO Fisheries and Aquaculture; Report No. 978; FAO: Rome, Italy, 2011. [Google Scholar]
- Solfrizzi, V.; Panza, F.; Frisardi, V.; Seripa, D.; Logroscino, G.; Imbimbo, B.; Pilotto, A. Diet and Alzheimer’s disease risk factors or prevention: The current evidence. Expert Rev. Neurother. 2011, 11, 677–708. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, B.M.; Tijhuis, M.; Kalmihn, S.; Kromhout, D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen Elderly Study. Am. J. Clin. Nutr. 2007, 85, 1142–1147. [Google Scholar] [CrossRef]
- Chung, W.-L.; Chen, J.-J.; Su, H.-M. Fish Oil Supplementation of Control and (n-3) Fatty Acid-Deficient Male Rats Enhances Reference and Working Memory Performance and Increases Brain Regional Docosahexaenoic Acid Levels. J. Nutr. 2008, 138, 1165–1171. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Tanabe, Y.; Kawashima, A.; Harada, T.; Yano, T.; Mizuguchi, K.; Shido, O. The protective effect of dietary eicosapentaenoic acid against impairment of spatial cognition learning ability in rats infused with amyloid β(1-40). J. Nutr. Biochem. 2009, 20, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Tanabe, Y.; Fujii, Y.; Kikuta, T.; Shibata, H.; Shido, O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J. Nutr. 2005, 135, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Rijpma, A.; Meulenbroek, O.; van Hees, A.M.J.; Sijben, J.W.C.; Vellas, B.; Shah, R.C.; Bennett, D.A.; Scheltens, P.; Rikkert, M.G.M.O. Effects of Souvenaid on plasma micronutrient levels and fatty acid profiles in mild and mild-to-moderate Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Kaduce, T.L.; Chen, Y.; Hell, J.W.; Spector, A.A. Docosahexaenoic acid synthesis from n-3 fatty acid precursors in rat hippocampal neurons. J. Neurochem. 2008, 105, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Sawangjit, A.; Oyanedel, C.N.; Niethard, N.; Salazar, C.; Born, J.; Inostroza, M. The hippocampus is crucial for forming non-hippocampal long-term memory during sleep. Nature 2018, 564, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Henninger, N.; Feldmann, R.E.; Fütterer, C.D.; Schrempp, C.; Maurer, M.H.; Waschke, K.F.; Kuschinsky, W.; Schwab, S. Spatial learning induces predominant downregulation of cytosolic proteins in the rat hippocampus. Genes Brain Behav. 2006, 6, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, G.V.; Hasselmo, M.E.; Eichenbaum, H. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998, 21, 317–323. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Guo, Z. Alzheimer’s A42 and A40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Liu, Q.; Chen, Y.-X.; Zhao, Y.-F.; Li, Y.-M. Aβ42 and Aβ40: Similarities and differences. J. Pept. Sci. 2015, 21, 522–529. [Google Scholar] [CrossRef]
- Sánchez, L.; Madurga, S.; Pukala, T.; Vilaseca, M.; López-Iglesias, C.; Robinson, C.V.; Giralt, E.; Carulla, N. Aβ40 and Aβ42 Amyloid Fibrils Exhibit Distinct Molecular Recycling Properties. J. Am. Chem. Soc. 2011, 133, 6505–6508. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, C. Abeta40 promotes neuronal cell fate in neural progenitor cells. Cell Death Differ. 2009, 16, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.-I.; Sato, N.; Yamamoto, A.; Ikegame, Y.; Nakashima, S.; Ogihara, T.; Morishita, R. Alzheimer Disease–Associated Peptide, Amyloid β40, Inhibits Vascular Regeneration with Induction of Endothelial Autophagy. Arter. Thromb. Vasc. Biol. 2009, 29, 1909–1915. [Google Scholar] [CrossRef]
- Florent, S.; Malaplate-Armand, C.; Youssef, I.; Kriem, B.; Koziel, V.; Escanyé, M.-C.; Fifre, A.; Sponne, I.; Leininger-Muller, B.; Olivier, J.-L.; et al. Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-beta oligomers. J. Neurochem. 2006, 96, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Casañas-Sánchez, V.; Pérez, J.A.; Fabelo, N.; Quinto-Alemany, D.; Díaz, M.L. Docosahexaenoic (DHA) modulates phospholipidhydroperoxide glutathione peroxidase (Gpx4) gene expression to ensure self-protection from oxidative damage in hippocampal cells. Front. Physiol. 2015, 6, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, M.; Meng, T.; Fei, J. Hippocampal microglial activation triggers a neurotoxic-specific astrocyte response and mediates etomidate-induced long-term synaptic inhibition. J. Neuroinflammation 2020, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflammation 2019, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-C.; Lathia, J.D.; Selvaraj, P.K.; Jo, D.-G.; Mughal, M.R.; Cheng, A.; Siler, D.A.; Markesbery, W.R.; Arumugam, T.V.; Mattson, M.P. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp. Neurol. 2008, 213, 114–121. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Thangnipon, W.; Puangmalai, N.; Chinchalongporn, V.; Jantrachotechatchawan, C.; Kitiyanant, N.; Soi-ampornkul, R.; Tuchinda, P.; Nobsathian, S. N-benzylcinnamide protects rat cultured cortical neurons from β-amyloid peptide-induced neurotoxicity. Neurosci. Lett. 2013, 556, 20–25. [Google Scholar] [CrossRef]
- Bastianetto, S.; Krantic, S.; Chabot, J.-G.; Quirion, R. Possible Involvement of Programmed Cell Death Pathways in the Neuroprotective Action of Polyphenols. Curr. Alzheimer Res. 2011, 8, 445–451. [Google Scholar] [CrossRef]
- Ma, B.; Meng, X.; Wang, J.; Sun, J.; Ren, X.; Qin, M.; Sun, J.; Sun, G.; Sun, X. Notoginsenoside R1 attenuates amyloid-β-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int. Immunopharmacol. 2014, 22, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, L.; Sun, M.; Zhou, T.; Zhong, K.; Wang, H.; Liu, Y.; Liu, X.; Xiao, R.; Ge, J.; et al. Xylocoside G reduces amyloid-β induced neurotoxicity by inhibiting NF-κB signaling pathway in neuronal cells. J. Alzheimer’s Dis. 2012, 30, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Li, S.-P.; Westermarck, J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008, 22, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ichikawa, S.; Tani, C.; Zhu, B.; Tada, M.; Shimoishi, Y.; Murata, Y.; Nakamura, Y. Docosahexaenoic acid induces ERK1/2 activation and neuritogenesis via intracellular reactive oxygen species production in human neuroblastoma SH-SY5Y cells. Biochim. Biophys. Acta 2009, 1791, 8–16. [Google Scholar] [CrossRef]
- Turkson, J.; Bowman, T.; Adnane, J.; Zhang, Y.; Djeu, J.Y.; Sekharam, M.; Frank, D.A.; Holzman, L.B.; Wu, J.; Sebti, S.; et al. Requirement for Ras/Rac1-Mediated p38 and c-Jun N-Terminal Kinase Signaling in Stat3 Transcriptional Activity Induced by the Src Oncoprotein. Mol. Cell. Biol. 1999, 19, 7519–7528. [Google Scholar] [CrossRef]
- Martín, V.; Almansa, E.; Fabelo, N.; Díaz, M.; Martín, M.V. Selective polyunsaturated fatty acids enrichment in phospholipids from neuronal-derived cell lines. J. Neurosci. Methods 2006, 153, 230–238. [Google Scholar] [CrossRef]
- Freund Levi, Y.; Vedin, I.; Cederholm, T.; Basun, H.; Faxén Irving, G.; Eriksdotter, M.; Hjorth, E.; Schultzberg, M.; Vessby, B.; Wahlund, L.-O.; et al. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer’s disease: The OmegAD study. J. Intern. Med. 2014, 275, 428–436. [Google Scholar] [CrossRef]
- Ouellet, M.; Emond, V.; Chen, C.T.; Julien, C.; Bourasset, F.; Oddo, S.; LaFerla, F.; Bazinet, R.P.; Calon, F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood–brain barrier: An in situ cerebral perfusion study. Neurochem. Int. 2009, 55, 476–482. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer | Accession Number |
|---|---|---|---|
| APP | 5′-TCAGATTGCGATGTTCTGTGG-3′ | 5′-CTGGCTGGTTTGCTTCCATCA-3′ | NM_019288.2 |
| Bad | 5′-AAGTCCGATCCCGGAATCC-3′ | 5′-GCTCACTCGGCTCAAACTCT-3′ | NM_022698.2 |
| Bax | 5′-TGAAGACAGGGGCCTTTTTG-3′ | 5′-AATTCGCCGGAGACACTCG-3′ | NM_017059.2 |
| Bcl-2 | 5′-GTCGCTACCGTCGTGACTTC-3′ | 5′-CAGACATGCACCTACCCAGC-3′ | NM_016993.2 |
| Bik | 5′-ACTGTTCCACACGACCAGG-3′ | 5′-CACAGGACTAAGGTTTTCCCC-3′ | NM_053704.2 |
| Caspase-3 | 5′-ATGGAGAACAACAAAACCTCAGT-3′ | 5′-TTGCTCCCATGTATGGTCTTTAC-3′ | NM_012922.2 |
| Catalase | 5′-AGCGACCAGATGAAGCAGTG-3′ | 5′-TCCGCTCTCTGTCAAAGTGTG-3′ | NM_012520.2 |
| COX-2 | 5′-TGAGCAACTATTCCAAACCAGC-3′ | 5′-GCACGTAGTCTTCGATCACTATC-3′ | NM_017232.4 |
| GPx-1 | 5′-AGTCCACCGTGTATGCCTTCT-3′ | 5′-GAGACGCGACATTCTCAATGA-3′ | NM_030826.4 |
| GPx-3 | 5′-TCACACTTTCTCCAGGTTCCCGTT-3′ | 5′-TCATGTGGGCATATGGGAGATGCT-3′ | NM_022525.4 |
| GSR | 5′-GACACCTCTTCCTTCGACTACC-3′ | 5′-CCCAGCTTGTGACTCTCCAC-3′ | NM_053906.2 |
| IL-1β | 5′-GCAACTGTTCCTGAACTCAACT-3′ | 5′-ATCTTTTGGGGTCCGTCAACT-3′ | NM_031512.2 |
| IL-6 | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 5′-TTGGTCCTTAGCCACTCCTTC-3′ | NM_012589.2 |
| MCP-1 | 5′-TTAAAAACCTGGATCGGAACCAA-3′ | 5′-GCATTAGCTTCAGATTTACGGGT-3′ | NM_031530.1 |
| NOX-1 | 5′-GGTTGGGGCTGAACATTTTTC-3′ | 5′-TCGACACACAGGAATCAGGAT-3′ | NM_053683.2 |
| SOD-1 | 5′-AACCAGTTGTGTTGTCAGGAC-3′ | 5′-CCACCATGTTTCTTAGAGTGAGG-3′ | NM_017050.1 |
| TLR4 | 5′-GCCTTTCAGGGAATTAAGCTCC-3′ | 5′-AGATCAACCGATGGACGTGTAA-3′ | NM_019178.2 |
| TNFα | 5′-CCCTCACACTCAGATCATCTTCT-3′ | 5′-GCTACGACGTGGGCTACAG-3′ | NM_012675.3 |
| β-actin | 5′-GGCTGTATTCCCCTCCATCG -3′ | 5′-CCAGTTGGTAACAATGCCATGT-3′ | NM_031144.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lai, W.; Zheng, C.; Babu, J.R.; Xue, C.; Ai, Q.; Huggins, K.W. Neuroprotective Effect of Stearidonic Acid on Amyloid β-Induced Neurotoxicity in Rat Hippocampal Cells. Antioxidants 2022, 11, 2357. https://doi.org/10.3390/antiox11122357
Li Y, Lai W, Zheng C, Babu JR, Xue C, Ai Q, Huggins KW. Neuroprotective Effect of Stearidonic Acid on Amyloid β-Induced Neurotoxicity in Rat Hippocampal Cells. Antioxidants. 2022; 11(12):2357. https://doi.org/10.3390/antiox11122357
Chicago/Turabian StyleLi, Yueru, Wencong Lai, Chen Zheng, Jeganathan Ramesh Babu, Changhu Xue, Qinghui Ai, and Kevin W. Huggins. 2022. "Neuroprotective Effect of Stearidonic Acid on Amyloid β-Induced Neurotoxicity in Rat Hippocampal Cells" Antioxidants 11, no. 12: 2357. https://doi.org/10.3390/antiox11122357
APA StyleLi, Y., Lai, W., Zheng, C., Babu, J. R., Xue, C., Ai, Q., & Huggins, K. W. (2022). Neuroprotective Effect of Stearidonic Acid on Amyloid β-Induced Neurotoxicity in Rat Hippocampal Cells. Antioxidants, 11(12), 2357. https://doi.org/10.3390/antiox11122357






