Protein Glutathionylation and Glutaredoxin: Role in Neurodegenerative Diseases
Abstract
1. Introduction
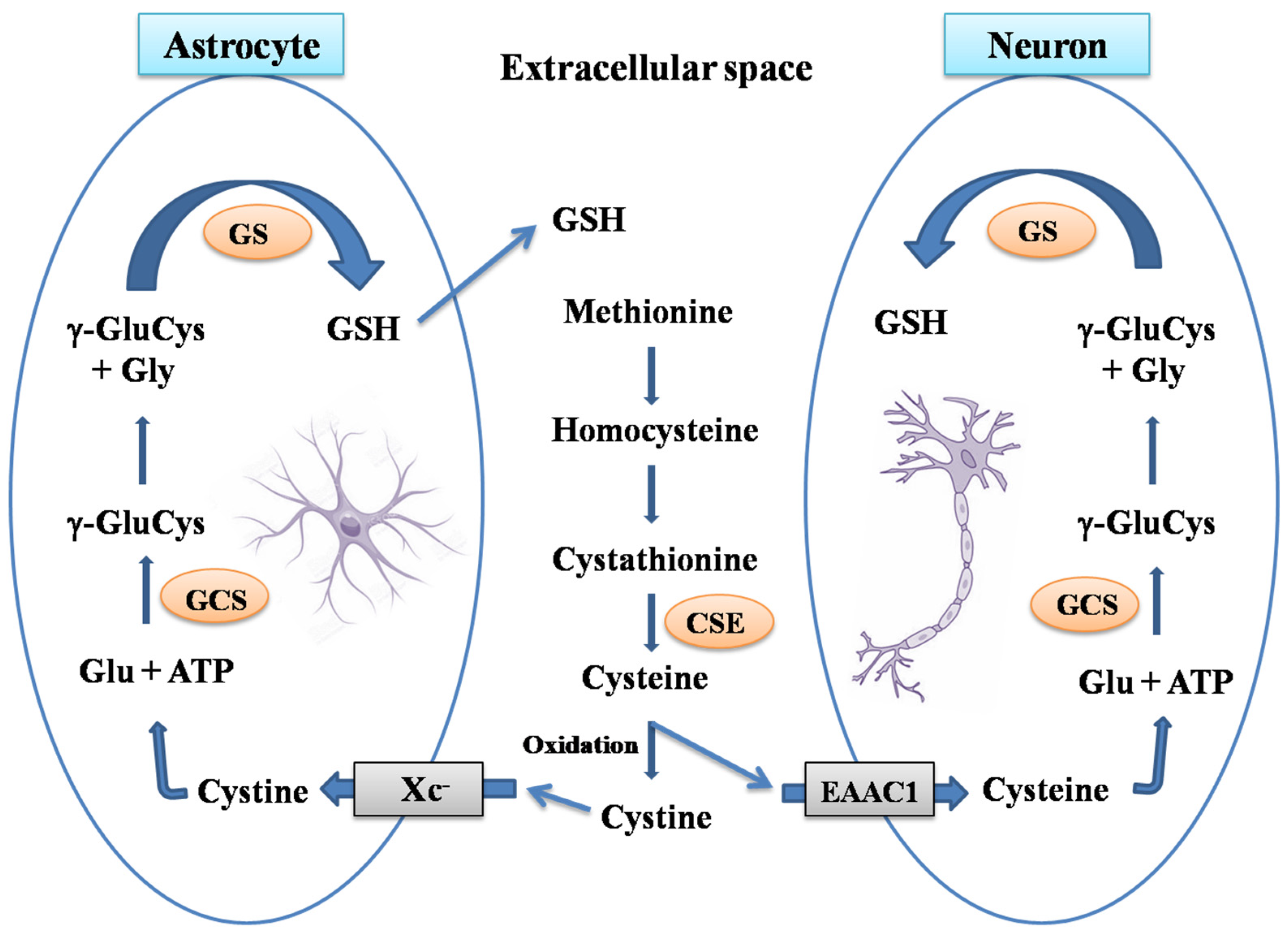
1.1. Brain Glutathione
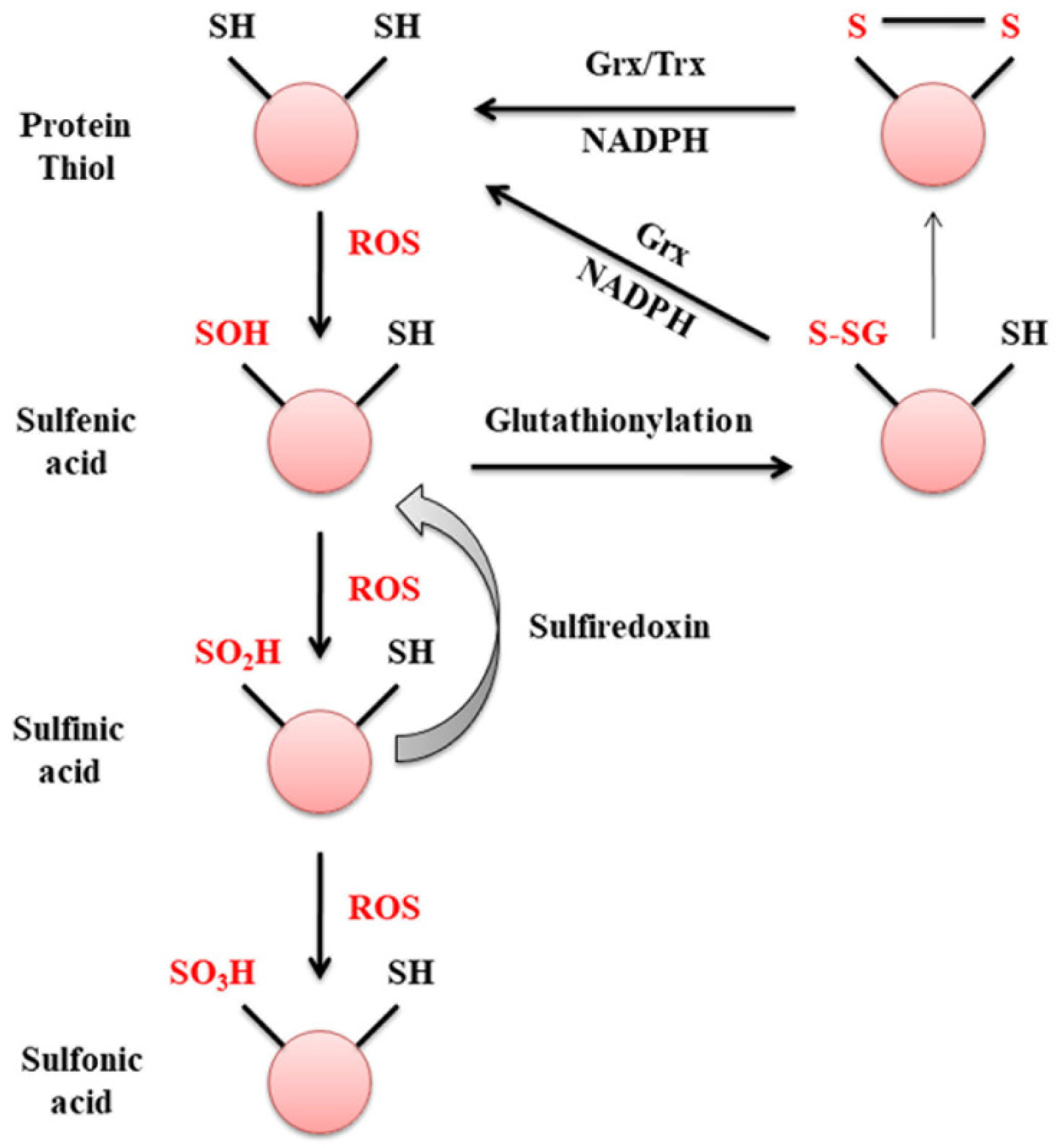
1.2. Glutaredoxin
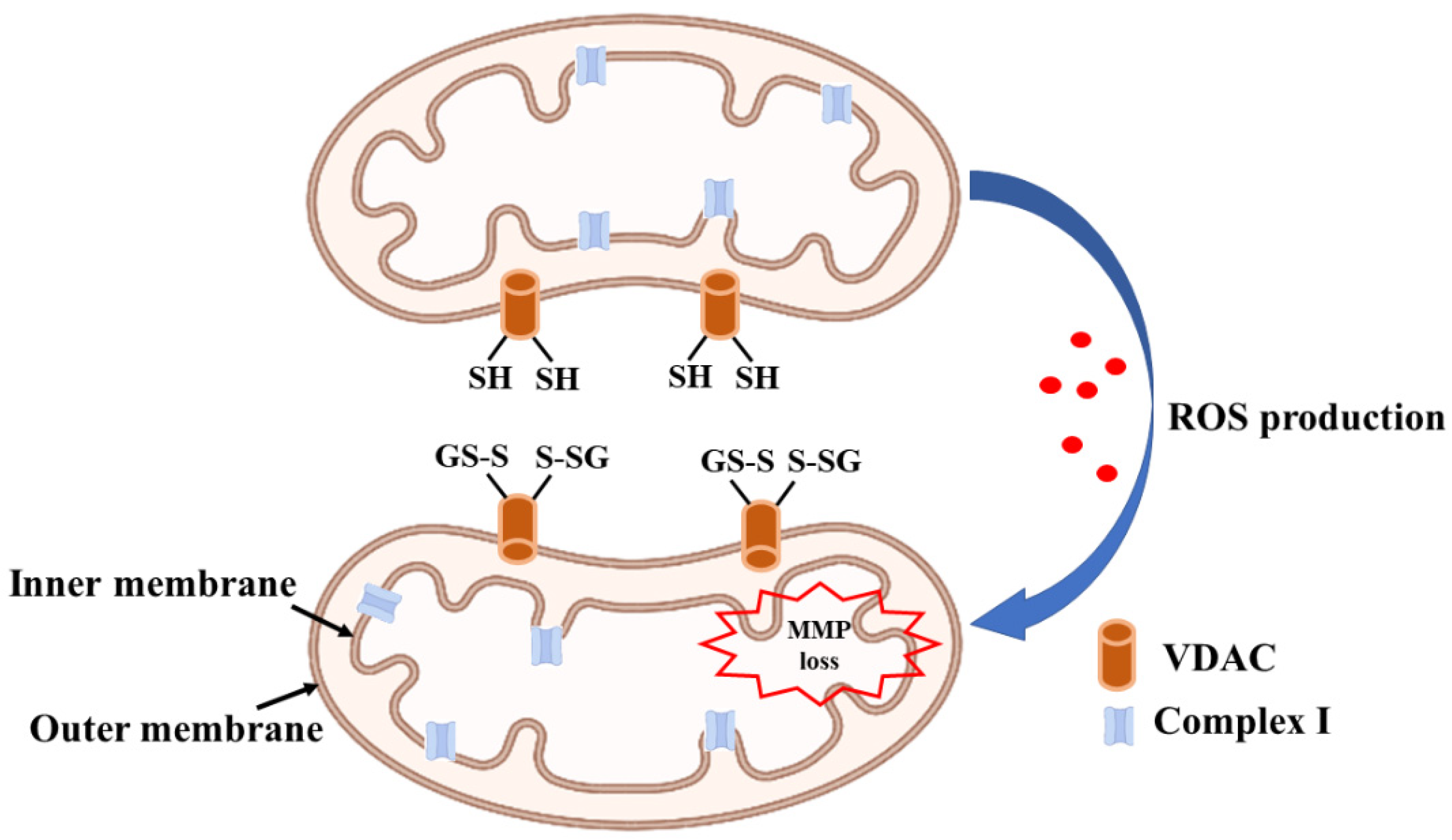
1.3. Glutaredoxin and Mitochondrial Dysfunction
1.4. Excitotoxicity and Glutaredoxin
1.5. Ischemic Reperfusion Injury
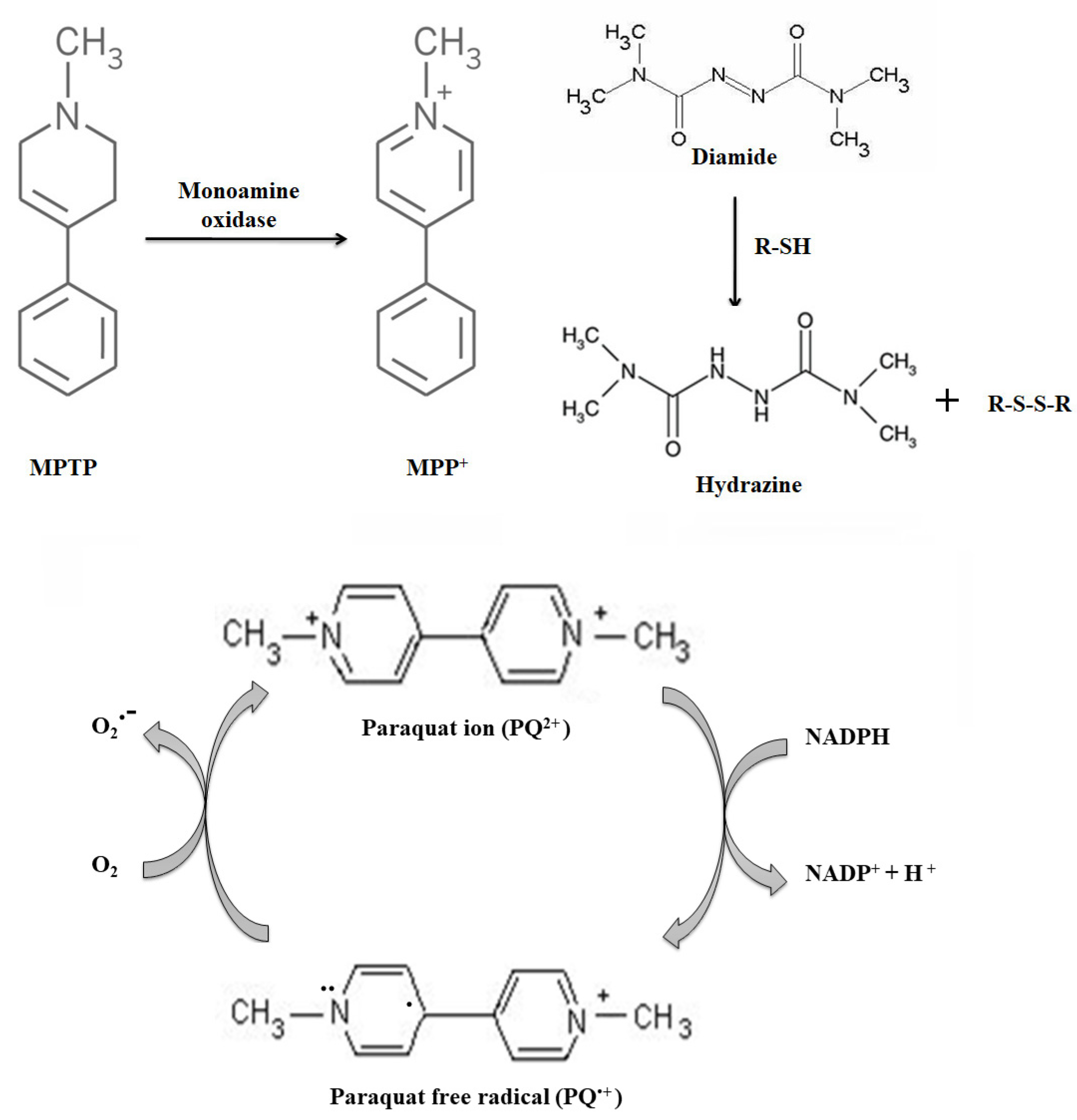
1.6. Parkinson’s Disease
1.7. Alzheimer’s Disease (AD)
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AKt | Intracellular serine/threonine kinase protein kinase B |
| ALA | α-lipoic acid |
| ANT | Adenosine nucleotide translocase |
| AP1 | Activator protein 1 |
| APP/PS1 | Amyloid Precursor Protein/Presenilin1 |
| ASK | Apoptosis signal-regulating kinase |
| BSO | Buthionine sulfoximine |
| cFC | Contextual fear conditioning |
| Cu, Zn SOD | Copper Zinc superoxide dismutase |
| Cys | Cysteine |
| DA | Dopamine |
| DJ1 | Protein Deglycase1 |
| EAAC1 | Excitatory amino-acid carrier 1 |
| EAAT | Excitatory amino acid transporter |
| FOXO | Forkhead box |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| Grx | Glutaredoxin |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| JNK | c-Jun N-terminal kinase |
| L-BOAA | βeta-oxalylamino-L-alanine |
| LRRK | Leucine-rich repeat kinase |
| MMP | Mitochondrial Membrane Potential |
| Mn-SOD | Manganese-superoxide dismutase |
| MPP+ | 1-Methyl-4-phenylpyridinium ion |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| mTOR | Mammalian target of rapamycin |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate Hydrogen |
| NFκB | Nuclear Factor-kappa B |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| P38-MAPK | p38 mitogen-activated protein kinases |
| PD | Parkinson’s disease |
| PINK | PTEN-induced putative kinase 1 |
| PrSH | Protein thiol |
| PrSSG | Protein disulfide |
| Pr-ss-Pr | Protein mixed disulfides |
| ROS | Reactive Oxygen Species |
| SNpc | Substantia Nigra par compacta |
| TH | Tyrosine hydroxylase |
| TNF-α | Tumor Necrosis Factor α |
| VDAC | Voltage-dependent anion-selective channel |
| VTA | Ventral tegmental area |
| Xc | Cystine/glutamate antiporter |
| γ-GCS | γ-glutamylcysteine synthetase |
| γ-glu | γ-glutamate |
References
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in biology and medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef]
- Guo, C.Y.; Sun, L.; Chen, X.P.; Zhang, D.S. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidatives Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive Oxygen Species and the Central Nervous System. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Okado-Matsumoto, A.; Fridovich, I. Subcellular Distribution of Superoxide Dismutases (SOD) in Rat Liver. Cu, Zn-SOD in Mitochondria. J. Biol. Chem. 2001, 276, 38388–38393. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, L.; Sosa-Peinado, A.; Hansberg, W. Catalase Evolved to Concentrate H2O2 at Its Active Site. Arch. Biochem. Biophys. 2010, 500, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Han, D.; Cadenas, E. Relative Contributions of Heart Mitochondria Glutathione Peroxidase and Catalase to H2O2 Detoxification in in Vivo Conditions. Free Radic. Biol. Med. 2002, 33, 1260–1267. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of Free Radical-Induced Damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Metabolism and Functions of Glutathione. Trends Biochem. Sci. 1981, 6, 231–234. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef] [PubMed]
- Ralf, D. Metabolism and Functions of Glutathione in Brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar]
- Zhang, C.; Rodriguez, C.; Spaulding, J.; Aw, T.Y.; Feng, J. Age-Dependent and Tissue-Related Glutathione Redox Status in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2012, 28, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Van Bergen, L.A.H.; Roos, G.; De Proft, F. From Thiol to Sulfonic Acid: Modeling the Oxidation Pathway of Protein Thiols by Hydrogen Peroxide. J. Phys. Chem. A 2014, 118, 6078–6084. [Google Scholar] [CrossRef]
- Gallogly, M.M.; Mieyal, J.J. Mechanisms of Reversible Protein Glutathionylation in Redox Signaling and Oxidative Stress. Curr. Opin. Pharmacol. 2007, 7, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.K.; Lekli, I.; Ray, D.; Yodoi, J.; Das, D.K. Redox Regulation of Cell Survival by the Thioredoxin Superfamily: An Implication of Redox Gene Therapy in the Heart. Antioxid. Redox Signal. 2009, 11, 2741–2758. [Google Scholar] [CrossRef] [PubMed]
- Gravina, S.A.; Mieyal, J.J. Thioltransferase Is a Specific Glutathionyl Mixed Disulfide Oxidoreductase. Biochemistry 1993, 32, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Gorelenkova Miller, O.; Mieyal, J.J. Critical Roles of Glutaredoxin in Brain Cells—Implications for Parkinson’s Disease. Antioxid. Redox Signal. 2019, 30, 1352–1368. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, U.; Mieyal, P.A.; Mieyal, J.J. PH Profiles Indicative of Rate-Limiting Nucleophilic Displacement in Thioltransferase Catalysis. Biochemistry 1997, 36, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Fernandes, A.P.; Kumar, S.; Holmgren, A. Cellular and Plasma Levels of Human Glutaredoxin 1 and 2 Detected by Sensitive ELISA Systems. Biochem. Biophys. Res. Commun. 2004, 319, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; Pal, R.; Qu, Y.; Liu, X.; Yu, H.; Shiao, S.L.; Wang, X.; Cui, X.; Rodney, G.G.; Cheng, N.; et al. Nuclear glutaredoxin 3 is critical for protection against oxidative stress-induced cell death. Free Radic. Biol. Med. 2016, 197–206. [Google Scholar] [CrossRef]
- Andersen, J.K. Oxidative Stress in Neurodegeneration: Cause or Consequence? Nat. Rev. Neurosci. 2004, 10, S18. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Michaelis, E.K. Selective Neuronal Vulnerability to Oxidative Stress in the Brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of Dopamine Metabolism. Cell Commun. Signal. 2013, 11, 34. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Glutathione in Cellular Redox Homeostasis: Association with the Excitatory Amino Acid Carrier 1 (EAAC1). Molecules 2015, 20, 8742–8758. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Vali, S.; Mythri, R.B.; Jagatha, B.; Padiadpu, J.; Ramanujan, K.S.; Andersen, J.K.; Gorin, F.; Bharath, M.M.S. Integrating Glutathione Metabolism and Mitochondrial Dysfunction with Implications for Parkinson’s Disease: A Dynamic Model. Neuroscience 2007, 149, 917–930. [Google Scholar] [CrossRef]
- Wang, X.F.; Cynader, M.S. Astrocytes Provide Cysteine to Neurons by Releasing Glutathione. J. Neurochem. 2000, 74, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Watabe, M.; Nakaki, T. Modulation of Neuronal Glutathione Synthesis by EAAC1 and Its Interacting Protein GTRAP3-18. Amino Acids 2012, 42, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, L.; Ravindranath, V. Inhibition of Cystathionine-γ-Lyase Leads to Loss of Glutathione and Aggravation of Mitochondrial Dysfunction Mediated by Excitatory Amino Acid in the CNS. Neurochem. Int. 2007, 50, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.M.; Wilson-Delfosse, A.L.; Mieyal, J.J. Dysregulation of Glutathione Homeostasis in Neurodegenerative Diseases. Nutrients 2012, 4, 1399–1440. [Google Scholar] [CrossRef]
- Braidy, N.; Zarka, M.; Jugder, B.E.; Welch, J.; Jayasena, T.; Chan, D.K.Y.; Sachdev, P.; Bridge, W. The Precursor to Glutathione (GSH), γ-Glutamylcysteine (GGC), Can Ameliorate Oxidative Damage and Neuroinflammation Induced by Aβ40 Oligomers in Human Astrocytes. Front. Aging Neurosci. 2019, 10, 177. [Google Scholar] [CrossRef]
- Skvarc, D.R.; Dean, O.M.; Byrne, L.K.; Gray, L.; Lane, S.; Lewis, M.; Fernandes, B.S.; Berk, M.; Marriott, A. The Effect of N-Acetylcysteine (NAC) on Human Cognition—A Systematic Review. Neurosci. Biobehav. Rev. 2017, 78, 44–56. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Li, B.; Yao, H.; Zarka, M.; Welch, J.; Sachdev, P.; Bridge, W.; Braidy, N. Supplementation with γ-Glutamylcysteine (γ-GC) Lessens Oxidative Stress, Brain Inflammation and Amyloid Pathology and Improves Spatial Memory in a Murine Model of AD. Neurochem. Int. 2021, 144, 104931. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, C.; Kikuchi-Utsumi, K.; Aoyama, K.; Suzuki, R.; Okamoto, Y.; Matsumura, N.; Omata, D.; Maruyama, K.; Nakaki, T. Inhibition of MiR-96-5p in the Mouse Brain Increases Glutathione Levels by Altering NOVA1 Expression. Commun. Biol. 2021, 4, 1–12. [Google Scholar] [CrossRef]
- Shelton, D.M.; Chock, P.B.; Mieyal, J.J. S-Glutathionylation As a Mechanism of Redox Signal Transduction and Regulation of Protein Translocation, and the Central Role of Glutaredoxin. Antioxid. Redox Signal. 2005, 7, 348–367. [Google Scholar] [CrossRef]
- Mieyal, J.J.; Starke, D.W.; Chung, J.S.; Gravina, S.A.; Dothey, C. Thioltransferase in Human Red Blood Cells: Purification and Properties. Biochemistry 1991, 30, 6088–6097. [Google Scholar] [CrossRef] [PubMed]
- Chrestensen, C.A.; Eckman, C.B.; Starke, D.W.; Mieyal, J.J. Cloning, Expression and Characterization of Human Thioltransferase (Glutaredoxin) in E. coli. FEBS Lett. 1995, 374, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Balijepalli, S.; Tirumalai, P.S.; Swamy, K.V.; Boyd, M.R.; Mieyal, J.J.; Ravindranath, V. Rat Brain Thioltransferase: Regional Distribution, Immunological Characterization, and Localization by Fluorescent in Situ Hybridization. J. Neurochem. 1999, 72, 1170–1178. [Google Scholar] [CrossRef]
- Balijepalli, S.; Boyd, M.R.; Ravindranath, V. Human Brain Thioltransferase: Constitutive Expression and Localization by Fluorescence in Situ Hybridization. Mol. Brain Res. 2000, 85, 123–132. [Google Scholar] [CrossRef]
- Griffith, O.W.; Meister, A. Origin and Turnover of Mitochondrial Glutathione. Proc. Natl. Acad. Sci. USA 1985, 82, 4668–4672. [Google Scholar] [CrossRef]
- Ravindranath, V.; Reed, D.J. Glutathione Depletion and Formation of Glutathione-Protein Mixed Disulfide Following Exposure of Brain Mitochondria to Oxidative Stress. Biochem. Biophys. Res. Commun. 1990, 169. [Google Scholar] [CrossRef]
- Olafsdottir, K.; Reed, D.J. Retention of Oxidized Glutathione by Isolated Rat Liver Mitochondria during Hydroperoxide Treatment. BBA Gen. Subj. 1988, 964, 377–382. [Google Scholar] [CrossRef]
- Taylor, E.R.; Hurrell, F.; Shannon, R.J.; Lin, T.K.; Hirst, J.; Murphy, M.P. Reversible Glutathionylation of Complex I Increases Mitochondrial Superoxide Formation. J. Biol. Chem. 2003, 278, 19603–19610. [Google Scholar] [CrossRef]
- Beer, S.M.; Taylor, E.R.; Brown, S.E.; Dahm, C.C.; Costa, N.J.; Runswick, M.J.; Murphy, M.P. Glutaredoxin 2 Catalyzes the Reversible Oxidation and Glutathionylation of Mitochondrial Membrane Thiol Proteins: Implications for Mitochondrial Redox Regulation and Antioxidant Defense. J. Biol. Chem. 2004, 279, 47939–47951. [Google Scholar] [CrossRef]
- Kenchappa, R.S.; Ravindranath, V. Glutaredoxin Is Essential for Maintenance of Brain Mitochondrial Complex I: Studies with MPTP. FASEB J. 2003, 17, 717–719. [Google Scholar] [CrossRef][Green Version]
- Lundberg, M.; Johansson, C.; Chandra, J.; Enoksson, M.; Jacobsson, G.; Ljung, J.; Johansson, M.; Holmgren, A. Cloning and Expression of a Novel Human Glutaredoxin (Grx2) with Mitochondrial and Nuclear Isoforms. J. Biol. Chem. 2001, 276, 26269–26275. [Google Scholar] [CrossRef] [PubMed]
- Lönn, M.E.; Hudemann, C.; Berndt, C.; Cherkasov, V.; Capani, F.; Holmgren, A.; Lillig, C.H. Expression Pattern of Human Glutaredoxin 2 Isoforms: Identification and Characterization of Two Testis/Cancer Cell-Specific Isoforms. Antioxid. Redox Signal 2008, 10, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, S.; Saeed, U.; Ramakrishnan, S.; Koumar, R.C.; Ravindranath, V. Constitutive Expression and Functional Characterization of Mitochondrial Glutaredoxin (Grx2) in Mouse and Human Brain. Brain Res. 2007, 1185. [Google Scholar] [CrossRef] [PubMed]
- Enoksson, M.; Fernandes, A.P.; Prast, S.; Lillig, C.H.; Holmgren, A.; Orrenius, S. Overexpression of Glutaredoxin 2 Attenuates Apoptosis by Preventing Cytochrome c Release. Biochem. Biophys. Res. Commun. 2005, 327, 774–779. [Google Scholar] [CrossRef]
- Ferri, A.; Fiorenzo, P.; Nencini, M.; Cozzolino, M.; Pesaresi, M.G.; Valle, C.; Sepe, S.; Moreno, S.; Carrì, M.T. Glutaredoxin 2 Prevents Aggregation of Mutant SOD1 in Mitochondria and Abolishes Its Toxicity. Hum. Mol. Genet. 2010, 19, 4529–4542. [Google Scholar] [CrossRef]
- Petronilli, V.; Costantini, P.; Scorrano, L.; Colonna, R.; Passamonti, S.; Bernardi, P. The Voltage Sensor of the Mitochondrial Permeability Transition Pore Is Tuned by the Oxidation-Reduction State of Vicinal Thiols. Increase of the Gating Potential by Oxidants and Its Reversal by Reducing Agents. J. Biol. Chem. 1994, 269, 16638–16642. [Google Scholar] [CrossRef]
- Saeed, U.; Durgadoss, L.; Valli, R.K.; Joshi, D.C.; Joshi, P.G.; Ravindranath, V. Knockdown of Cytosolic Glutaredoxin 1 Leads to Loss of Mitochondrial Membrane Potential: Implication in Neurodegenerative Diseases. PLoS ONE 2008, 3, e2459. [Google Scholar] [CrossRef]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial Membrane Potential Regulates Matrix Configuration and Cytochrome c Release during Apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef]
- Ravindranath, V. Neurolathyrism: Mitochondrial Dysfunction in Excitotoxicity Mediated by L-β-Oxalyl Aminoalanine. Neurochem. Int. 2002, 40, 505–509. [Google Scholar] [CrossRef]
- Sadashiva Pai, K.; Ravindranath, V. L-BOAA Induces Selective Inhibition of Brain Mitochondrial Enzyme, NADH-Dehydrogenase. Brain Res. 1993, 621, 215–221. [Google Scholar] [CrossRef]
- Sriram, K.; Shankar, S.K.; Boyd, M.R.; Ravindranath, V. Thiol Oxidation and Loss of Mitochondrial Complex I Precede Excitatory Amino Acid-Mediated Neurodegeneration. J. Neurosci. 1998, 18, 10287–10296. [Google Scholar] [CrossRef] [PubMed]
- Kenchappa, R.S.; Diwakar, L.; Boyd, M.R.; Ravindranath, V. Thioltransferase (Glutaredoxin) Mediates Recovery of Motor Neurons from Excitotoxic Mitochondrial Injury. J. Neurosci. 2002, 22, 8402–8410. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, L.; Kenchappa, R.S.; Annepu, J.; Ravindranath, V. Downregulation of Glutaredoxin but Not Glutathione Loss Leads to Mitochondrial Dysfunction in Female Mice CNS: Implications in Excitotoxicity. Neurochem. Int. 2007, 51. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion Injury and Reactive Oxygen Species: The Evolution of a Concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.; Sadguna, Y.; Shivakumar, B.R.; Kolluri, S.V.R.; Roy, S.; Packer, L.; Ravindranath, V. α-Lipoic Acid Protects against Reperfusion Injury Following Cerebral Ischemia in Rats. Brain Res. 1996, 717. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, B.R.; Kolluri, S.V.R.; Ravindranath, V. Glutathione Homeostasis in Brain during Reperfusion Following Bilateral Carotid Artery Occlusion in the Rat. Mol. Cell. Biochem. 1992, 111, 125–129. [Google Scholar] [CrossRef]
- Dickson, D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, a009258. [Google Scholar] [CrossRef]
- Hsu, L.J.; Sagara, Y.; Arroyo, A.; Rockenstein, E.; Sisk, A.; Mallory, M.; Wong, J.; Takenouchi, T.; Hashimoto, M.; Masliah, E. A-Synuclein Promotes Mitochondrial Deficit and Oxidative Stress. Am. J. Pathol. 2000, 157, 401–410. [Google Scholar] [CrossRef]
- Li, W.; Fu, Y.H.; Halliday, G.M.; Sue, C.M. PARK Genes Link Mitochondrial Dysfunction and Alpha-Synuclein Pathology in Sporadic Parkinson’s Disease. Front. Cell Dev. Biol. 2021, 9, 612476. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.B.; Lee, S.; Kim, Y.; Song, S.; Kim, S.; Bae, E.; Kim, J.; Shong, M.; Kim, J.M.; et al. Mitochondrial Dysfunction in Drosophila PINK1 Mutants Is Complemented by Parkin. Nature 2006, 441, 1157–1161. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Kostic, M.; Horne, A.; Gandhi, S.; Sekler, I.; Abramov, A.Y. LRRK2 deficiency induced mitochondrial Ca2+ efflux inhibition can be rescued by Na+/Ca2+/Li+ exchanger upregulation. Cell Death Dis. 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Zhi, L.; Zhang, H. LRRK2 and Mitochondria: Recent Advances and Current Views. Brain Res. 2019, 1702, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, M.; Double, K.L.; Ben-Shachar, D.; Zecca, L.; Youdim, M.B.H.; Riederer, P. Neuromelanin and Its Interaction with Iron as a Potential Risk Factor for Dopaminergic Neurodegeneration Underlying Parkinson’s Disease. Neurotox. Res. 2003, 5, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Gerlach, M.; Youdim, M.B.H.; Double, K.L.; Zecca, L.; Riederer, P.; Becker, G. Erratum: Brain Iron Pathways and Their Relevance to Parkinson’s Disease. J. Neurochem. 2002, 80, 719. [Google Scholar] [CrossRef]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron Homeostasis and Iron-Regulated ROS in Cell Death, Senescence and Human Diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Lu, H.; Zhou, M.; Chai, Z.; Hu, Y. Iron-Induced Generation of Mitochondrial ROS Depends on AMPK Activity. BioMetals 2017, 30, 623–628. [Google Scholar] [CrossRef]
- Read, A.D.; Bentley, R.E.; Archer, S.L.; Dunham-Snary, K.J. Mitochondrial Iron–Sulfur Clusters: Structure, Function, and an Emerging Role in Vascular Biology: Mitochondrial Fe-S Clusters—A Review. Redox Biol. 2021, 47, 102164. [Google Scholar] [CrossRef]
- Asanuma, M.; Miyazaki, I.; Ogawa, N. Dopamine- or L-DOPA-Induced Neurotoxicity: The Role of Dopamine Quinone Formation and Tyrosinase in a Model of Parkinson’s Disease. Neurotox. Res. 2003, 5, 165–176. [Google Scholar] [CrossRef]
- Rabinovic, A.D.; Hastings, T.G. Role of Endogenous Glutathione in the Oxidation of Dopamine. J. Neurochem. 1998, 71, 2071–2078. [Google Scholar] [CrossRef]
- Stoker, T.B.; Greenland, J.C. Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Brisbane, Australia, 2018; ISBN 9780994438164. [Google Scholar]
- Langston, J.W. The MPTP Story. J. Parkinsons. Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef] [PubMed]
- Tetrud, J.W.; Langston, J.W. MPTP-induced Parkinsonism as a Model for Parkinson’s Disease. Acta Neurol. Scand. 1989, 80, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Pai, K.S.; Boyd, M.R.; Ravindranath, V. Evidence for Generation of Oxidative Stress in Brain by MPTP: In Vitro and in Vivo Studies in Mice. Brain Res. 1997, 749, 44–52. [Google Scholar] [CrossRef]
- Nicklas, W.J.; Vyas, I.; Heikkila, R.E. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985, 36, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Caudle, W.M.; Guillot, T.S.; Watson, J.L.; Nakamaru-Ogiso, E.; Seo, B.B.; Sherer, T.B.; Greenamyre, J.T.; Yagi, T.; Matsuno-Yagi, A.; et al. Obligatory Role for Complex I Inhibition in the Dopaminergic Neurotoxicity of 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP). Toxicol. Sci. 2007, 95, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Beal, M.F. Mitochondrial Dysfunction in Parkinson’s Disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease—Cause or Consequence? Biology 2019, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Durgadoss, L.; Nidadavolu, P.; Valli, R.K.; Saeed, U.; Mishra, M.; Seth, P.; Ravindranath, V. Redox Modification of Akt Mediated by the Dopaminergic Neurotoxin MPTP, in Mouse Midbrain, Leads to Down-regulation of PAkt. FASEB J. 2012, 26, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Nidadavolu, P.; Durgadoss, L.; Ravindranath, V. Critical Cysteines in Akt1 Regulate Its Activity and Proteasomal Degradation: Implications for Neurodegenerative Diseases. Free Radic. Biol. Med. 2014, 74, 118–128. [Google Scholar] [CrossRef]
- Ray, A.; Kambali, M.; Ravindranath, V. Thiol Oxidation by Diamide Leads to Dopaminergic Degeneration and Parkinsonism Phenotype in Mice: A Model for Parkinson’s Disease. Antioxid. Redox Signal. 2016, 10, 252–267. [Google Scholar] [CrossRef]
- Ray, A.; Sehgal, N.; Karunakaran, S.; Rangarajan, G.; Ravindranath, V. MPTP Activates ASK1-P38 MAPK Signaling Pathway through TNF-Dependent Trx1 Oxidation in Parkinsonism Mouse Model. Free Radic. Biol. Med. 2015, 87, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Ray, A.; Bapat, D.; Diwakar, L.; Kommaddi, R.P.; Schneider, B.L.; Hirsch, E.C.; Ravindranath, V. Glutaredoxin 1 Downregulation in the Substantia Nigra Leads to Dopaminergic Degeneration in Mice. Mov. Disord. 2020, 35, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. Update on Typical and Atypical Antipsychotic Drugs. Annu. Rev. Med. 2013, 64, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, B.R.; Ravindranath, V. Oxidative Stress Induced by Administration of the Neuroleptic Drug Haloperidol Is Attenuated by Higher Doses of Haloperidol. Brain Res. 1992, 595, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, B.R.; Ravindranath, V. Oxidative stress and thiol modification induced by chronic administration of haloperidol. J. Pharmacol. Exp. Ther. 1993, 265, 1137–1141. [Google Scholar]
- Balijepalli, S.; Kenchappa, R.S.; Boyd, M.R.; Ravindranath, V. Protein thiol oxidation by haloperidol results in inhibition of mitochondrial complex I in brain regions: Comparison with atypical antipsychotics. Neurochem. Int. 2001, 38, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Pai, B.N.; Janakiramaiah, N.; Gangadhar, B.; Ravindranath, V. Depletion of gluththione and enhanced lipid peroxidation in the CSF of acute psychotics following haloperidol administration. Biol. Psychiatry 1994, 36, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Ethell, D.W. Maneb potentiates paraquat neurotoxicity by inducing key Bcl-2 family members. J. Neurochem. 2008, 105, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.L.; Atienza, J.G.; Johnston, L.C.; Andersen, J.K.; Vu, S.; Di Monte, D.A. Role of Oxidative Stress in Paraquat-Induced Dopaminergic Cell Degeneration. J. Neurochem. 2005, 93, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.L.; Thiruchelvam, M.; Manning-Bog, A.B.; Thiffault, C.; Langston, J.W.; Cory-Slechta, D.A.; Di Monte, D.A. Environmental Risk Factors and Parkinson’s Disease: Selective Degeneration of Nigral Dopaminergic Neurons Caused by the Herbicide Paraquat. Neurobiol. Dis. 2002, 10, 119–127. [Google Scholar] [CrossRef]
- Bonneh-Barkay, D.; Reaney, S.H.; Langston, W.J.; Di Monte, D.A. Redox Cycling of the Herbicide Paraquat in Microglial Cultures. Mol. Brain Res. 2005, 134, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Mao, X.O.; Stevenson, F.F.; Hsu, M.; Andersen, J.K. The Herbicide Paraquat Induces Dopaminergic Nigral Apoptosis through Sustained Activation of the JNK Pathway. J. Biol. Chem. 2004, 279, 32626–32632. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rocha, H.; Garcia, A.G.; Zavala-Flores, L.; Li, S.; Madayiputhiya, N.; Franco, R. Glutaredoxin 1 Protects Dopaminergic Cells by Increased Protein Glutathionylation in Experimental Parkinson’s Disease. Antioxid. Redox Signal. 2012, 17, 1676–1693. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, S.; Wang, H. α-Lipoic Acid Alleviates Ferroptosis in the MPP+-Induced PC12 Cells via Activating the PI3K/Akt/Nrf2 Pathway. Cell Biol. Int. 2021, 45, 422–431. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Di Domenico, F.; Barone, E. Elevated Risk of Type 2 Diabetes for Development of Alzheimer Disease: A Key Role for Oxidative Stress in Brain. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Meraz-Ríos, M.A.; Franco-Bocanegra, D.; Rios, D.T.; Campos-Peña, V. Early Onset Alzheimer’s Disease and Oxidative Stress. Oxidative Med. Cell. Longev. 2014. [Google Scholar] [CrossRef]
- Hensley, K.; Carney, J.M.; Mattson, M.P.; Aksenova, M.; Harris, M.; Wu, J.F.; Floyd, R.A.; Butterfield, D.A. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: Relevance to Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 3270–3274. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Hensley, K.; Harris, M.; Mattson, M.; Carney, J. β-Amyloid Peptide Free Radical Fragments Initiate Synaptosomal Lipoperoxidation in a Sequence-Specific Fashion: Implications to Alzheimer′s Disease. Biochem. Biophys. Res. Commun. 1994, 200, 710–715. [Google Scholar] [CrossRef]
- Wu, H.Y.; Hudry, E.; Hashimoto, T.; Kuchibhotla, K.; Rozkalne, A.; Fan, Z.; Spires-Jones, T.; Xie, H.; Arbel-Ornath, M.; Grosskreutz, C.L.; et al. Amyloid β Induces the Morphological Neurodegenerative Triad of Spine Loss, Dendritic Simplification, and Neuritic Dystrophies through Calcineurin Activation. J. Neurosci. 2010, 30, 2636–2649. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Singh, K.; Das, D.; Gowaikar, R.; Shaw, E.; Ramachandran, A.; Rupanagudi, K.V.; Kommaddi, R.P.; Bennett, D.A.; Ravindranath, V. Reactive Oxygen Species-Mediated Loss of Synaptic Akt1 Signaling Leads to Deficient Activity-Dependent Protein Translation Early in Alzheimer’s Disease. Antioxid. Redox Signal. 2017, 27, 1269–1280. [Google Scholar] [CrossRef]
- Borovac, J.; Bosch, M.; Okamoto, K. Regulation of Actin Dynamics during Structural Plasticity of Dendritic Spines: Signaling Messengers and Actin-Binding Proteins. Mol. Cell. Neurosci. 2018, 91, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Minamide, L.S.; Striegl, A.M.; Boyle, J.A.; Meberg, P.J.; Bamburg, J.R. Neurodegenerative Stimuli Induce Persistent ADF/Cofilin-Actin Rods That Disrupt Distal Neurite Function. Nat. Cell Biol. 2000, 2, 628–636. [Google Scholar] [CrossRef]
- Aksenov, M.Y.; Aksenova, M.V.; Butterfield, D.A.; Geddes, J.W.; Markesbery, W.R. Protein Oxidation in the Brain in Alzheimer’s Disease. Neuroscience 2001, 103, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; StClair, D.K.; Vore, M.; Sultana, R.; Cole, M.P.; Aluise, C.D.; Joshi, G. Alterations in Brain Antioxidant Enzymes and Redox Proteomic Identification of Oxidized Brain Proteins Induced by the Anti-Cancer Drug Adriamycin: Implications for Oxidative Stress-Mediated Chemobrain. Neuroscience 2010, 166, 796–807. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Colombo, R.; Milzani, A. Actin S-Glutathionylation: Evidence against a Thiol-Disulphide Exchange Mechanism. Free Radic. Biol. Med. 2003, 35, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Lundberg, M. Glutathionylation of Beta-Actin via a Cysteinyl Sulfenic Acid Intermediary. BMC Biochem. 2007, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Giustarini, D.; Rossi, R.; Colombo, R.; Milzani, A. Reversible S-Glutathionylation of Cys374 Regulates Actin Filament Formation by Inducing Structural Changes in the Actin Molecule. Free Radic. Biol. Med. 2003, 34, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kommaddi, R.P.; Tomar, D.S.; Karunakaran, S.; Bapat, D.; Nanguneri, S.; Ray, A.; Schneider, B.L.; Nair, D.; Ravindranath, V. Glutaredoxin1 Diminishes Amyloid Beta-Mediated Oxidation of F-Actin and Reverses Cognitive Deficits in an Alzheimer’s Disease Mouse Model. Antioxid. Redox Signal. 2019, 31, 1321–1338. [Google Scholar] [CrossRef]
- Wang, J.; Boja, E.S.; Tan, W.; Tekle, E.; Fales, H.M.; English, S.; Mieyal, J.J.; Chock, P.B. Reversible Glutathionylation Regulates Actin Polymerization in A431 Cells. J. Biol. Chem. 2001, 276, 47763–47766. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
A., H.P.; Diwakar, L.; Ravindranath, V. Protein Glutathionylation and Glutaredoxin: Role in Neurodegenerative Diseases. Antioxidants 2022, 11, 2334. https://doi.org/10.3390/antiox11122334
A. HP, Diwakar L, Ravindranath V. Protein Glutathionylation and Glutaredoxin: Role in Neurodegenerative Diseases. Antioxidants. 2022; 11(12):2334. https://doi.org/10.3390/antiox11122334
Chicago/Turabian StyleA., Haseena P., Latha Diwakar, and Vijayalakshmi Ravindranath. 2022. "Protein Glutathionylation and Glutaredoxin: Role in Neurodegenerative Diseases" Antioxidants 11, no. 12: 2334. https://doi.org/10.3390/antiox11122334
APA StyleA., H. P., Diwakar, L., & Ravindranath, V. (2022). Protein Glutathionylation and Glutaredoxin: Role in Neurodegenerative Diseases. Antioxidants, 11(12), 2334. https://doi.org/10.3390/antiox11122334




